Abstract
Versican is highly expressed in developing joint interzones during limb morphogenesis. The present study was undertaken to examine whether proteolytic cleavage of versican occurs that could potentially impact its function during the process of embryonic synovial joint formation. Using an antibody to the DPEAAE neoepitope generated by ADAMTS proteolysis, versican amino terminal cleavage fragments were detected in joint interzones at 12–16 days post coitum (dpc). ADAMTS-1 localization overlapped that of DPEAAE-reactive versican fragments suggesting it as one possible protease activity involved in processing of versican in the interzone. Results show that increased cleavage of versican in the interzone accompanies cavitation and suggests that proteolytic modification of versican may be important during the process of synovial joint maturation
Keywords: versican, ECM, synovial joint development, limb development, joint interzone
Introduction
Development of the synovial joint has been an active focus of investigation, particularly during recent years. Much of this effort has concentrated on the joint interzone, an area comprised of a non-chondrogenic central lamina between two chondrogenic zones (Mitrovic 1978) which subsequently cavitates to form the synovial cavity and from which many structures of the synovial joint are derived (Archer et al., 1994, Pacifici et al., 2005). Studies have suggested the importance of numerous signaling molecules as well as transcription factors to interzone and synovial joint formation (reviewed in Pacifici et al., 2005), however, less is known regarding the role of the extracellular matrix (ECM). Hyaluronan and its receptor, CD44, have been shown previously to be necessary for cavitation of the joint interzone (Craig et al, 1990; Pitsillides et al., 1995) and type I collagen (Craig et al., 1987) and tenascin C (Pacifici et al., 1993) have also been implicated in interzone function. Moreover, down regulation of α5β1 integrin may be necessary in sites of joint formation (Garciadiego-Cazares et al., 2004). These studies have led to the recognition that cell-ECM interactions are likely requisite for interzone formation and function (Pacific et al., 2005); hence further investigation of matrix molecules and their interactions will be important for complete understanding of synovial joint morphogenesis.
The hyalectin versican has been shown to be important in early events such as precartilage mesenchymal aggregation during limb skeletogenesis (Williams et al., 2005; Kamiya et al., 2006; Shepard et al., 2008) and, although versican expression diminishes from most of the maturing limb cartilage, it is maintained in the epiphyseal ends of long bones (Yamamura et al., 1997; Shibata et al., 2003) and presumptive joints of mouse (Snow et al., 2005) and chick (Shepard et al., 2007), suggesting a conserved role for versican in the ECM during joint morphogenesis.
Versican is comprised of globular amino (G1) and carboxy (G3) domains separated by two chondroitin sulfate glycosaminoglycan (GAG) attachment regions, α and β (Kimata et al., 1986; Zimmerman and Rouslahti, 1989). In addition to effects on cell migration and adhesion attributed to the GAG complement, the G1 domain binds hyaluronan, an interaction stabilized by link protein (Matsumoto et al., 2003) and also impacts adhesion (Ang et al., 1999; Yang et al., 1999; Zhang et al., 1999). G1 and G3 domains both increase proliferation and, in addition, G3 participates in multiple cellular and matrix interactions (Shinomura et al., 1993; Yang et al., 1999; Zhang et al., 1998b, 1999). Four versican isoforms (V0–V3) have been identified in tissue specific locations due to splicing in or out of GAG-α and -β domains and may regulate differing cellular behaviors during migration and adhesion (Kimata et al., 1986; Zimmermann and Rouslahti, 1989; Zako et al., 1995).
Studies have demonstrated possible regulation of versican activity through proteolytic cleavage by several matrix metalloproteinases (MMPs) and, more recently, ADAMTS family members (a disintegrin and metalloproteinase with thrombospondin motifs; Sandy et al., 2001; Russell et al., 2003; Kern et al., 2006; Longpre et al., 2009; McCullough et al., 2009). ADAMTS-1, -4, and -5 cleavage of the V1 versican isoform yield an approximately 70 kilodalton (kDa) amino terminal fragment bearing the neoepitope sequence DPEAAE (Sandy et al., 2001; Longpre et al., 2009). ADAMTS-5 expression overlaps that of versican in several areas of the embryonic mouse limb, including perichondrium, and interdigital mesenchyme surrounding digit primordia (McCullough et al., 2009). ADAMTS-1 been localized to articular chondrocytes in the human adult (Wachsmuth et al., 2004) and neonatal mice (Little et al., 2005), but to date nothing is known regarding potential regulation of versican activity by proteolytic turnover during the process of joint morphogenesis. The present study was undertaken in order to investigate whether proteolytic processing of versican occurred that might potentially regulate its function during synovial joint formation in the embryonic limb.
Materials and Methods
In order to investigate versican proteolysis during early murine joint development, adjacent or neighboring sections of ethanol/formaldehyde fixed embryonic fore- and hindlimbs were immunostained after low pH citrate unmasking (Vector Labs) according to Snow et al. (2005). Primary antibodies utilized in this study included: anti-versican V0/V1 isoforms (anti-GAG-β peptide; Chemicon), anti-C-terminal neoepitope DPEAAE sequence of proteolytically cleaved versican V0/V1 (Affinity Bioreagents; the V0/V1 GAG-β epitope is not present in the versican cleavage fragment recognized by anti-DPEAAE), anti-ADAMTS-1, -4, and MMP-2 (Santa Cruz Biotechnology), and anti-link protein (Developmental Studies Hybridoma Bank, University of Iowa). Histochemical detection of hyaluronan was performed utilizing biotinylated hyaluronic acid binding protein (HABP; Associates of Cape Cod). Incubation with primary reagents was followed by relevant fluorescein- or rhodamine-coupled secondary antibodies (Cappel) or fluorescein-strepavidin (Vector Labs). Primary reagents were omitted from control specimens. Sections receiving anti-versican, -DPEAAE, -link protein or HABP were treated with 0.25U/ml chondroitinase ABC (Sigma) and those receiving anti-ADAMTS-1, -4, or MMP-2 with 0.1 mg/ml proteinase K (Sigma; modification of Kern et al, 2006) prior to blocking with 3% bovine serum albumen and 1% normal goat serum. Wild type and heterozygous hdf (heart defect) specimens, which bear an insertional mutation within the Cspg2 gene encoding versican (Mjaatvedt et al., 1998), but no embryonic limb phenotype (Snow et al., 2005), were tested with no differences in staining patterns noted. Immunoblotting of wild type embryonic extract was performed to confirm that the appropriately sized anti-DPEAAE-reactive fragment of ~70 kDa, representing the versican cleavage product described by Sandy et al. (2001), was present (data not shown).
Results
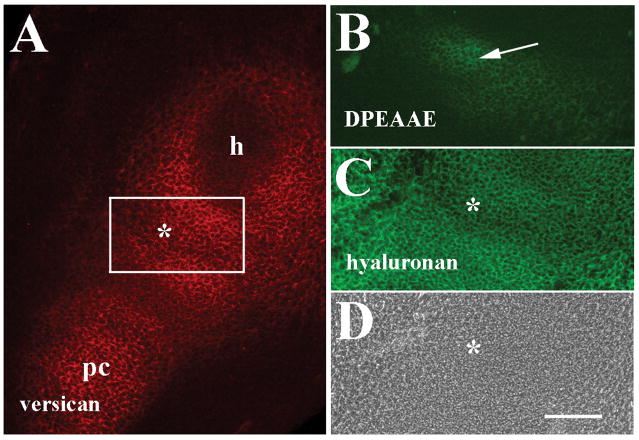
Immunohistochemical staining of forelimb sections at 12 days post coitum (dpc) was initially performed to localize versican and anti-DPEAAE-reactive versican cleavage products in presumptive joint regions at an early stage of limb skeletogenesis (Fig. 1). V0/V1 versican, detected in this study by an antibody directed to a peptide sequence within the GAG-β domain, was prevalent in the ECM surrounding the differentiated humeral cartilage and within precartilage condensations of the forelimb as well as the interzone of the presumptive elbow joint (Fig. 1A) as described previously (Snow et al., 2005). Double labeling with HABP also revealed wide ranging expression of hyaluronan in the limb that co-distributed with versican in the future joint interzone (Fig. 1C). At 12 dpc, only low levels of anti-DPEAAE reactivity could be detected in the forming joint interzone in adjacent sections (Fig. 1B). Interestingly, localization of anti-DPEAAE staining corresponded to a site of the nascent interzone in which V0/V1 versican signal was slightly reduced, suggesting that limited levels of versican proteolysis were occurring at this early phase of joint development and within restricted areas. Little anti-DPEAAE reactivity was noted in other areas of the developing limb at this stage (data not shown).
Figure 1.
Localization of versican, anti-DPEAAE-reactive versican proteolytic fragments and hyaluronan in the 12 dpc forelimb. A: Versican is localized in the future joint interzone (asterisks in A, C and D) between the humeral primordium (h), and precartilage condensation (pc) of the zeugopod. B: Anti-DPEAAE staining shows low levels of reactivity in the interzone (arrow). C: Double labeling of A shows widespread hyaluronan localization. D: Phase contrast image of A and C. Scale bar = 100 μm for all panels.
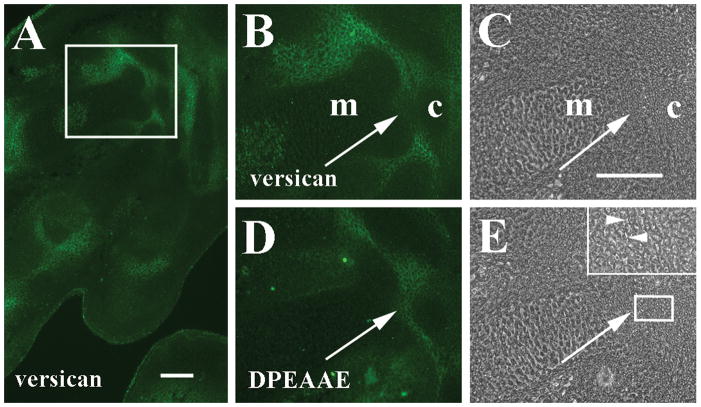
At 15 dpc, the stage at which many limb skeletal and joint primordia are well designated (Kaufman, 1995), anti-V0/V1 versican immunoreactivity was widespread in the interzone ECM of multiple articulations of the forelimb autopod, including carpal and carpometacarpal joints (Fig. 2A and B). Anti-DPEAAE-reactive versican overlapped with V0/V1 versican in areas along the proximal edges of the carpometacarpal interzone (Fig. 2D), but anti-DPEAAE staining was clearly present in the matrix within the central portion of the joint interzone, an area in which anti-GAG-β-reactive versican was much reduced at this stage (Fig. 2B), suggesting that versican proteolysis had also occurred in this location. At 15 dpc, slight separation of some cells suggestive of early cavitation of the carpometacarpal interzone could be detected in regions in which anti-DPEAAE-positive versican fragments were located (Fig. 2E). Interestingly, low levels of anti-V0/V1 versican immunoreactivity were often associated with hypertrophic chondrocytes within some long bone templates (Fig. 2A and B, Fig. 4A).
Figure 2.
Localization of versican and anti-DPEAAE-reactive versican proteolytic fragments in 15 dpc autopod. A: Versican is localized in interzones of carpometacarpal (boxed area) and metacarpophalangeal joints. B: Higher magnification of boxed area in panel A shows versican surrounding interzone cells and around metacarpal edges (m). Note reduction of versican between carpal (c) and metacarpal. C: Phase contrast image of B. D: Anti-DPEAAE cleavage product has overlapping distribution with versican but is also prevalent where versican staining is reduced (arrows B–E). E: Phase contrast image of D. Inset shows higher magnification of boxed area including edge of carpal template and adjacent interzone. Arrowheads indicate separation of some interzone cells. Scale bars = 100 μm in A and C (applies to panels B–E).
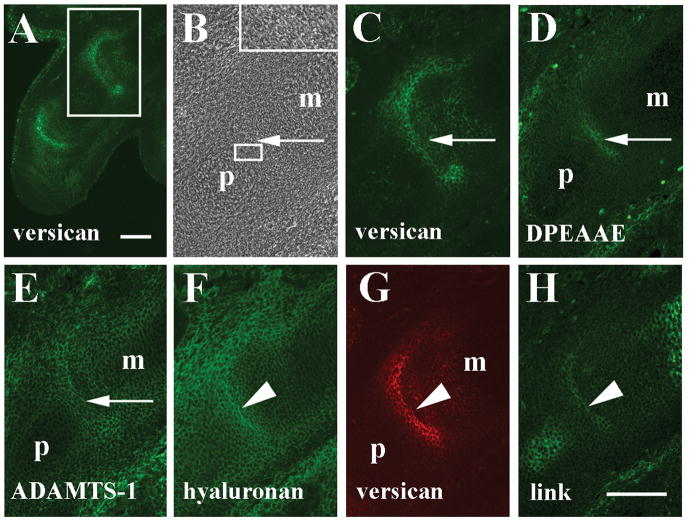
Figure 4.
Localization of versican, anti-DPEAAE-reactive versican proteolytic fragments, ADAMTS-1, and hyaluronan in the 16 dpc metatarsophalangeal joint. A: Versican staining in numerous joints of the hindlimb autopod. Staining is reduced in the metatarsophalangeal joint of digit 1 (larger boxed area) relative to digit 4 (smaller boxed area). Arrowheads, tarsometatarsal and interphalangeal joints. B: Higher magnification phase contrast image of metatarso- and interphalangeal joints (arrows in panels B–G) of digit 1 in A. Cavitation is readily evident. C: Higher magnification of versican staining in A. Versican is reduced in metatarsophalalangeal and interphalangeal interzones. D: Anti-DPEAAE reactivity is localized in the central joint interzone where versican staining is largely absent (asterisks in panels C and D). E: Low level ADAMTS-1 staining in interzones. F: Hyaluronan localization in joint interzones and surrounding tissues. G: Little or no anti-link protein reactivity is observed. H: Higher magnification phase contrast image of metatarsophalangeal joint of digit 4 in A (arrowheads in H–K). I: Higher magnification of versican localization shows strong signal in the interzone. J: Weak anti-DPEAAE reactivity in the joint interzone. K: ADAMTS-1 reactivity in the interzone. m, metatarsal; p and p′, phalanges. Scale bars = 100 μm in A and B (applies to panels B–K).
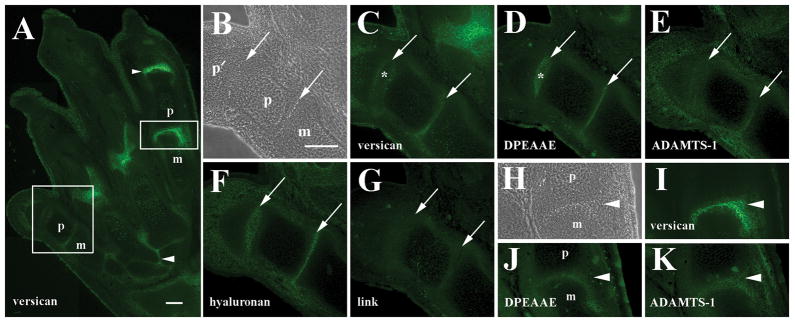
In addition to expression in the wrist at 15 dpc, strong versican immunoreactivity was also observed in metacarpophalangeal as well as interphalangeal joints (Fig. 3A) in agreement with Shibata et al. (2003). Versican was localized not only in joint interzones, but extended around the expanding distal edges of the metacarpal cartilage primordium (Fig. 3C). In the metacarpophalangeal joint little evidence of overt cavitation was observed at this stage (Fig. 3B). Anti-DPEAAE-reactive versican fragments (Fig. 3D) were localized in the matrix surrounding cells toward the center of the metacarpophalangeal interzone, proximal to strongest V0/V1 versican signal in an area where versican staining was noticeably weaker (arrows in Fig. 3B–D), suggesting that versican turnover in the ECM was again occurring in restricted and specific sites within the joint interzone. Low levels of anti-DPEAAE reactivity were also noted around the distal edge of the metacarpal element where strong versican staining remained. Anti-DPEAAE-positive versican was particularly wide-ranging in the developing dermis of the limb (Fig. 3D).
Figure 3.
Localization of versican, anti-DPEAAE-reactive versican proteolytic fragments, ADAMTS-1, hyaluronan, and link protein in the 15 dpc metacarpophalangeal joint. A: Versican is highly expressed in the interzone of metacarpophalangeal (boxed area) and interphalangeal joints. B: Higher magnification phase contrast image of boxed area in A. Inset shows higher magnification of boxed area including edge of proximal phalanx and adjacent interzone. C: Higher magnification of versican staining in A. D: Anti-DPEAAE reactivity is localized immediately proximal to versican (arrows in B–D). E: ADAMTS-1 staining overlaps that of anti- DPEAAE (arrow). F: Hyaluronan localization in interzone and surrounding tissues. G: Double label of F shows co-distribution of versican with hyaluronan. H: Link protein co-localizes with hyaluronan and versican in the central interzone lamina (arrowheads in F–H). m, metacarpal; p, phalanx. Scale bars = 100μm in A and H (applies to panels B–H).
In order to determine if relevant protease localization could be demonstrated in interzone areas where DPEAAE-positive versican fragments were found, immunostaining with ADAMTS-1, ADAMTS-4, and MMP2 antibodies was performed. Of the proteases investigated only ADAMTS-1 immunoreactivity was observed in direct association with developing joints overlapping regions where anti-DPEAAE-reactive versican was located (arrow in Fig. 3E). Little or no ADAMTS-4 or MMP2 staining was seen in developing joints at any stage examined, although ADAMTS-4 and MMP2 reactivity could be localized in other tissues of the trunk (data not shown). Anti-ADAMTS-1 reactivity was widespread within the ECM of the metacarpophalangeal joint interzone with staining extended around the distal edges of the metacarpal element, as seen with V0/V1 versican. ADAMTS-1 was also observed in the dermis and surrounding differentiated epiphyseal chondrocytes within metacarpal and phalangeal cartilages, consistent with its role also as an aggrecanase (Rodriguez-Manzaneque et al., 2002).
Double labeling of sections through the 15 dpc metacapophalangeal joint with HABP and anti-GAG-β versican showed widespread hyaluronan accumulation in autopod cartilages, joint primordia, and dermal tissues. Strong HABP signal was particularly distributed in the metacarpophalangeal interzone, extending around the expanding distal edge of the metacarpus similar to that of V0/V1 versican (Fig. 3F and G). Moreover, the interzone region that stained most strongly for hyaluronan and versican was also coincident with localization of link protein in adjacent sections (Fig. 3H). Localization of these matrix components in the central interzone lamina occurred just distal to that of anti-DPEAAE positive versican fragments (Fig. 3D). As expected, link protein was also expressed in the chondrocyte matrix within developing skeletal elements.
In the 16 dpc hindlimb differences in V0/V1 versican expression were evident between anterior and posterior sides of the autopod (Fig. 4A). Moderate levels of anti-GAG-β versican reactivity were present in tarsal and tarsometatarsal joint interzones at this stage. Lower levels of staining were seen in metatarsophalangeal or interphalangeal joints of digit 1, but strong anti-versican staining was noted in joint interzones associated with digit 4. Strong staining was also observed at the distal ends of metatarsals 2 and 3. Versican was also present in the epidermis and hypertrophic cartilages within the core of long bone templates as seen at 15 dpc (Fig. 2A and 3A). Cavitation of metatarsophalangeal and interphalangeal joints was clearly observed at 16 dpc with cell separation appearing at intervals across the width of the interzone (Fig. 4B). In cavitating interzones of digit 1 where limited anti-V0/V1 versican immunoreactivity was noted, moderate staining was restricted primarily to the outer aspect of the metatarsophalangeal joint and along the future articular surfaces of the proximal interphalangeal joint (Fig. 4C), but much reduced within the central lamina of the interzone. In contrast to V0/V1 versican immunolocalization, anti-DPEAAE-reactive versican fragments in adjacent sections were detected throughout the central lamina of metatarsophalangeal and interphalangeal interzones of digit 1 (Fig. 4D). Strongest anti-DPEAAE signal was in a largely non-overlapping pattern with anti-GAG-β-positive versican. Anti-ADAMTS-1 reactivity could be observed in these interzones as well (Fig. 4E). Hyaluronan was detected in interzone tissues of digit 1 in a pattern very similar to that of DPEAAE-reactive versican (Fig. 4F); however, little or no anti-link protein was observed in interzones at this stage (Fig. 4G). In contrast, toward the posterior aspect of the autopod, V0/V1 versican was expressed more strongly in metatarsophalangeal and interphalangeal joints (Fig. 4A and I). In a distribution similar to that observed in metacarpophalangeal joints of the forelimb, anti-versican staining was spread throughout the metatarsophalangeal joint interzone and extended around the enlarging distal edge of the metatarsal. Slightly lower levels of anti-DPEAAE (Fig. 4J) and anti-ADAMTS-1 (Fig. 4K) staining in the metatarsophalangeal interzone tissue of digit 4 were detected.
Discussion
Results from the present study provide evidence that increases in proteolytic processing of versican occur during the process of synovial joint formation in the developing mouse limb. Intact versican was widely expressed in early joint interzone tissues along with hyaluronan as described previously (Shibata et al., 2003; Snow et al., 2005; Shepard et al., 2007). The interzone region that stained most strongly for hyaluronan and versican was also coincident with localization of link protein in adjacent sections of the metacarpophalangeal joint at 15 dpc. Localization of these matrix components in the central interzone lamina raises the possibility that link protein may serve to stabilize hyaluronan-versican interactions (Matsumoto et al., 2003) in the interzone of some joint primordia prior to extensive cavitation and formation of the synovial cavity. Intact versican expression was also noted around the expanding distal edges of the metacarpal cartilage primordium at 15 dpc, an area in which cell proliferation has been suggested to aid in morphogenesis of the convex surface of distal skeletal elements (Pacifici et al., 2005). As versican domains have been shown to increase cellular proliferation (Zhang et al., 1998b, 1999), it is possible that its expression along the enlarging edge of the distal metacarpal could facilitate expansion of that skeletal element.
During early stages relatively weak and spatially restricted expression of anti-DPEAAE reactive versican was noted. As joint formation progressed, V0/V1 versican and increased levels of anti-DPEAAE-positive fragments were often found in non-overlapping locations within the interzone, particularly within the central lamina from which the synovial cavity will form during the process of cavitation (Archer et al., 1994, Pacifici et al., 2005). Differences observed in relative staining intensity of V0/V1 versican and anti-DPEAAE-reactive versican fragments between digits at 16 dpc could reflect differences in versican processing among joints of the hindlimb autopod, but may also be indicative of differences in developmental maturation among its various joints (Pacifici et al., 2005).
ADAMTS-1 immunostaining was also noted at 15–16 dpc in areas containing DPEAAE-positive versican fragments, suggesting that it may be one protease involved in versican cleavage. ADAMTS-1 is produced by both adult (Wachsmuth et al., 2004) and neonatal (Little et al., 2005) articular chondrocytes and results from this study suggest that ADAMTS-1 may play a role in proteolytically processing versican during embryonic joint development as well. The ADAMTS-1 immunoreagent used in this study detects both inactive precursor and activated forms of ADAMTS-1, therefore ADAMTS-1 protease activity may be more limited in scope than suggested by its staining pattern in the autopod, however, it is interesting to note that areas of stronger ADAMTS-1 immunostaining correlated with that of anti-DPEAAE-positive versican fragments. The amino terminal fragment containing the DPEAAE neoepitope was generated from versican V0/V1 isoforms by recombinant ADAMTS-1 and ADAMTS-4 activities in vitro (Sandy et al., 2001), suggesting that versican may be a substrate for ADAMTS-1 proteolysis in the embryonic mouse limb as reported in the atrioventricular cushions of the developing heart (Kern et al., 2006). It is probable that other ADAMTS- or perhaps MMP-mediated proteolytic activities may also generate DPEAAE-versican fragments in the embryonic limb. Indeed, ADAMTS-5 has been localized in interdigital mesenchyme and perichondrial ltissues (McCulloch et al., 2009) which are in close proximity to the developing joint, but not reported within the interzone.
What is the significance of versican proteolysis to its possible function during joint morphogenesis? Versican has been documented in tissues during early phases of development, and its expression often wanes as tissue maturation occurs (e.g., Shibata et al., 2003; Snow et al., 2005). Versican may play a role at early stages of differentiation, perhaps by contributing to a provisional matrix that aids in maintenance of cells in an embryonic or immature state that then must be cleared for subsequent development to continue (Zhang et al., 1998a). Perhaps this is the case in developing joints where versican is highly expressed during early stages of interzone formation and then must be degraded by proteolytic activity as joint maturation proceeds. On the other hand, an increasing number of studies have demonstrated that versican domains may function independently of the full length proteoglycan with differing effects on cellular behavior (Ang et al., 1999; Yang et al., 1999; Zhang et al., 1999; Kern et al., 2007). Since ADAMTS cleavage of versican generates the anti-DPEAAE-positive versican fragment containing the hyaluronan-binding G1 domain (Sandy et al., 2001), it is possible that proteolysis leaves versican G1 fragments in the ECM that continue to function during joint morphogenesis. Co-localization of versican and hyaluronan in the early interzone suggests that versican may interact with hyaluronan via its G1 domain (Matsumoto et al., 2003). Versican’s G3 domain may also tether hyaluronan to other matrix proteins localized in the interzone such as tenascin C or type I collagen as well as to the cell surface (Craig et al., 1987; Pacifici et al., 1993; Wu et al., 2005). Combined with its ability to bind hyaluronan, intact V0/V1 versican could provide a mechanism to aid localization of hyaluronan in close association with the cell membrane, forming a stable pericellular matrix (Wu et al., 2005) that promotes cell adhesion or interzone stability during early stages of joint formation. Since increased hyaluronan accumulation in the joint interzone is coupled with loss of tissue stability and cell separation, steps necessary for the process of cavitation (Craig et al, 1990; Pitsillides et al., 1995; Pacifici et al. 2005), perhaps increased versican cleavage releases hyaluronan from its indirect association with other matrix molecules or the cell surface. The DPEAAE-versican fragments that remain might then facilitate reduction of cell adhesion as demonstrated for ectopic expression of the G1 domain in other cells and tissues (Ang et al., 1999; Yang et al., 1999; Zhang et al., 1999; Kern et al., 2007), thus enabling G1-versican to function cooperatively with hyaluronan to promote cavitation or other events during joint morphogenesis.
Acknowledgments
Appreciation is extended to Holly Snow for preparation of paraffin sections and to the anonymous referees for thoughtful comments on the manuscript.
Grant Sponsor: NIH; grant number: HD040846
References
- Ang LC, Zhang Y, Cao L, Yang BL, Young B, Kiani C, Lee V, Allan K, Yang BB. Versican enhances locomotion of astrocytoma cells and reduces cell adhesion through its G1 domain. J Neuropathol Exp Neurol. 1999;58:597–605. doi: 10.1097/00005072-199906000-00004. [DOI] [PubMed] [Google Scholar]
- Archer CW, Morrison H, Pitsillides AA. Cellular aspects of the development of diarthroidal joints and articular cartilage. J Anat. 1994;184:447–456. [PMC free article] [PubMed] [Google Scholar]
- Craig FM, Bentley G, Archer CW. The spatial and temporal pattern of collagens I and II and keratin sulphate in the developing chick metatarsophalangeal joint. Development. 1987;99:383–391. doi: 10.1242/dev.99.3.383. [DOI] [PubMed] [Google Scholar]
- Craig FM, Bayliss MT, Bentley G, Archer CW. A role for hyaluronan in joint development. J Anat. 1990;171:17–23. [PMC free article] [PubMed] [Google Scholar]
- Garciadiego-Cazares D, Rosales C, Katoh M, Cimal-Monroy J. Coordination of chondrocyte differentiation and joint formation by α5β1 integrin in the developing appendicular skeleton. Development. 2004;131:4735–4742. doi: 10.1242/dev.01345. [DOI] [PubMed] [Google Scholar]
- Kamiya N, Watanabe H, Habuchi H, Takagi H, Shinomura T, Shimizu K, Kimata K. Versican/PG-M regulates chondrogenesis as an extracellular matrix molecule crucial for mesenchymal condensation. J Biol Chem. 2006;281:2390–2400. doi: 10.1074/jbc.M509341200. [DOI] [PubMed] [Google Scholar]
- Kaufman MH. The atlas of mouse development. Academic Press; London: 1995. [Google Scholar]
- Kern CB, Twal WO, Mjaatvedt CH, Fairey SE, Toole BP, Iruela-Arispe M, Argraves WS. Proteolytic cleavage of versican during cardiac cushion morphogenesis. Dev Dyn. 2006;235:2238–2247. doi: 10.1002/dvdy.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern CB, Norris RA, Thompson RP, Argraves WS, Fairey SE, Reyes L, Hoffman S, Markwald RR, Mjaatvedt CH. Versican proteolysis mediates myocardial regression during outflow tract development. Dev Dyn. 2007;236:671–683. doi: 10.1002/dvdy.21059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata K, Oike Y, Tani K, Shinomura T, Yamagata M, Uritani M, Suzuki S. A large chondroitin sulfate proteoglycan (PG-M) synthesized before chondrogenesis in the limb bud of chick embryo. J Biol Chem. 1986;261:13517–13525. [PubMed] [Google Scholar]
- Little CB, Mittaz L, Belluoccio D, Rogerson FM, Campbell IK, Meeker CT, Bateman JF, Pritchard MA, Fosang AJ. ADAMTS-1 knockout mice do not exhibit abnormalities in aggrecan turnover in vitro or in vivo. Arthritis Rheum. 2005;52:1461–1472. doi: 10.1002/art.21022. [DOI] [PubMed] [Google Scholar]
- Longpre JM, McCulloch DR, Koo BH, Alexander JP, Apte SS, Leduc R. Characterization of ADAMTS5 processing by proprotein convertases. Int J Biochem Cell Biol. 2009;41:1116–1126. doi: 10.1016/j.biocel.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Shionyu M, Go M, Shimizu K, Shinomura T, Kimata K, Watanabe H. Distinct interaction of versican/PG-M with hyaluronan and link protein. J Biol Chem. 2003;278:41205–41212. doi: 10.1074/jbc.M305060200. [DOI] [PubMed] [Google Scholar]
- McCulloch DR, Goff CL, Bhatt S, Dixon LJ, Sandy JD, Apte SS. Adamts5, the gene encoding a proteoglycan-degrading metalloprotease is expressed by specific cell lineages during mouse embryonic development and in adult tissues. Gene Exp Patterns. 2009;9:314–323. doi: 10.1016/j.gep.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrovic D. Development of the diarthroidal joints in the rat embryo. Am J Anat. 1978;151:475–485. doi: 10.1002/aja.1001510403. [DOI] [PubMed] [Google Scholar]
- Mjaatvedt CH, Yamamura H, Capehart AA, Turner D, Markwald RR. The Cspg2 gene, disrupted in the hdf mutant, is required for right cardiac chamber and endocardial cushion formation. Dev Biol. 1998;202:56–66. doi: 10.1006/dbio.1998.9001. [DOI] [PubMed] [Google Scholar]
- Pacifici M, Iwamoto M, Golden EB, Leatherman JL, Lee YS, Chuong CM. Tenascin is associated with articular cartilage development. Dev Dyn. 1993;198:123–134. doi: 10.1002/aja.1001980206. [DOI] [PubMed] [Google Scholar]
- Pacifici M, Koyama E, Iwamoto M. Mechanisms of synovial joint and articular cartilage formation: recent advances, but many lingering mysteries. Birth Defects Res. 2005;75:237–248. doi: 10.1002/bdrc.20050. [DOI] [PubMed] [Google Scholar]
- Pitsillides AA, Archer CW, Prehm P, Bayliss MT, Edwards JC. Alterations in hyaluronan synthesis during developing joint cavitation. J Histochem Cytochem. 1995;43:263–273. doi: 10.1177/43.3.7868856. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Manzaneque JC, Westling J, Thai SN, Luque A, Knauper V, Murphy G, Sandy JD, Iruela-Arispe ML. ADAMTS-1 cleaves aggrecan at multiple sites and is differentially inhibited by metalloproteinase inhibitors. Biochem Biophys Res Commun. 2002;293:501–508. doi: 10.1016/S0006-291X(02)00254-1. [DOI] [PubMed] [Google Scholar]
- Russell DL, Doyle KM, Ochsner SA, Sandy JD, Richards JS. Processing and localization of ADAMTS-1 and proteolytic cleavage of versican during cumulus matrix expansion and ovulation. J Biol Chem. 2003;278:42330–42339. doi: 10.1074/jbc.M300519200. [DOI] [PubMed] [Google Scholar]
- Sandy JD, Westling J, Kenagy RD, Iruela-Arispe ML, Verscharen C, Rodriguez-Mazaneque JC, Zimmermann DR, Lemire JM, Fischer JW, Wight TN, Clowes AW. Versican V1 proteolysis in human aorta in vivo occurs at the Glu441-Ala442 bond, a site that is cleaved by recombinant ADAMTS-1 and ADAMTS-4. J Biol Chem. 2001;276:13372–13378. doi: 10.1074/jbc.M009737200. [DOI] [PubMed] [Google Scholar]
- Shepard JB, Krug HA, LaFoon BA, Hoffman S, Capehart AA. Versican expression during synovial joint morphogenesis. Int J Biol Sci. 2007;3:380–384. doi: 10.7150/ijbs.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard JB, Gliga DA, Morrow AP, Hoffman S, Capehart AA. Versican knock-down compromises chondrogenesis in the embryonic chick limb. Anat Rec. 2008;291:19–27. doi: 10.1002/ar.20627. [DOI] [PubMed] [Google Scholar]
- Shibata S, Fukada K, Imai H, Abe T, Yamashita Y. In situ hybridization and immunohistochemistry of versican, aggrecan, and link protein, and histochemistry of hyaluronan in the developing mouse limb bud cartilage. J Anat. 2003;203:425–432. doi: 10.1046/j.1469-7580.2003.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomura T, Nishida Y, Ito K, Kimata K. DNA cloning of PG-M, a large chondroitin sulfate proteoglycan expressed during chondrogenesis in chick limb buds. Alternative spliced multiforms of PG-M and their relationships to versican. J Biol Chem. 1993;268:14461–9. [PubMed] [Google Scholar]
- Snow HE, Riccio LM, Hoffman S, Mjaatvedt CH, Capehart AA. Versican expression during skeletal/joint morphogenesis and patterning of muscle and nerve in the embryonic mouse limb. Anat Rec. 2005;282:95–105. doi: 10.1002/ar.a.20151. [DOI] [PubMed] [Google Scholar]
- Wachsmuth L, Bau B, Fan Z, Pecht A, Gerwin N, Aigner T. ADAMTS-1, a gene product of articular chondrocytes in vivo and in vitro, is downregulated by interleukin 1beta. J Rheumatol. 2004;31:315–320. [PubMed] [Google Scholar]
- Williams DR, Presar AR, Richmond AT, Mjaatvedt CH, Hoffman S, Capehart AA. Limb chondrogenesis is compromised in the versican deficient hdf mouse. Biochem Biophys Res Comm. 2005;334:960–966. doi: 10.1016/j.bbrc.2005.06.189. [DOI] [PubMed] [Google Scholar]
- Wu YJ, La Pierre DP, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res. 2005;15:483–494. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]
- Yamamura H, Zhang M, Markwald RR, Mjaatvedt CH. A heart segmental defect in the anterior-posterior axis of a transgenic mutant mouse. Dev Biol. 1997;186:58–72. doi: 10.1006/dbio.1997.8559. [DOI] [PubMed] [Google Scholar]
- Yang BL, Zhang Y, Cao L, Yang BB. Cell adhesion and proliferation mediated through the G1 domain of versican. J Cell Biochem. 1999;72:210–220. doi: 10.1002/(sici)1097-4644(19990201)72:2<210::aid-jcb5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Zako M, Shinomura T, Ujita M, Ito K, Kimata K. Expression of PG-M (V3), an alternatively spliced form of PG-M without a chondroitin sulfate attachment region in mouse and human tissues. J Biol Chem. 1995;270:3914–3918. doi: 10.1074/jbc.270.8.3914. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cao L, Kiani BL, Yang BL, Yang BB. The G3 domain of versican inhibits mesenchymal chondrogenesis via the epidermal growth factor-like motifs. J Biol Chem. 1998a;273:33054–33063. doi: 10.1074/jbc.273.49.33054. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cao L, Yang BL, Yang BB. The G3 domain of versican enhances cell proliferation via epidermal growth factor-like motifs. J Biol Chem. 1998b;273:21342–21351. doi: 10.1074/jbc.273.33.21342. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cao L, Kiana C, Yang BL, Hu W, Yang BB. Promotion of chondrocyte proliferation by versican mediated by G1 domain and EGF-like motifs. J Cell Biochem. 1999;73:445–457. [PubMed] [Google Scholar]
- Zimmermann DR, Ruoslahti E. Multiple domains of the large fibroblast proteoglycan, versican. EMBO J. 1989;8:2975–2981. doi: 10.1002/j.1460-2075.1989.tb08447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]