Abstract
BACKGROUND
Multiple studies have suggested that resection of the primary tumor improves survival in patients with stage IV breast cancer, yet in the era of targeted therapy, the relation between surgery and tumor molecular subtype is unknown. The objective of the current study was to identify subsets of patients who may benefit from primary tumor treatment and assess the frequency of local disease progression.
METHODS
Patients presenting with stage IV breast cancer and intact primary tumors (n = 186) were identified from a prospectively maintained clinical database (2000-2004) and clinical data were abstracted (grading determined according to the American Joint Committee on Cancer staging system).
RESULTS
Surgery was performed in 69 (37%) patients: 34 (49%) patients with unknown metastatic disease at the time of surgery, 15 (22%) patients for local control, 14 (20%) patients for palliation, and in 6 (9%) patients to obtain tissue. Surgical patients were more likely to be HER-2/neu negative for HER-2/neu (P = .001), and to have smaller tumors (P = .05) and solitary metastasis (P <.001). Local therapy included axillary lymph node clearance in 33 (48%) patients and postoperative radiotherapy in 9 (13%) patients. The median survival was 35 months. Cox regression analysis identified estrogen receptor (ER) positivity (hazard ratio [HR], 0.47; 95% confidence interval [95% CI], 0.29-0.76), progesterone receptor positivity (HR, 0.57; 95% CI, 0.36-0.90), and HER-2/neu amplification (HR, 0.51; 95% CI, 0.34-0.77) as being predictive of improved survival. There was a trend toward improved survival with surgery (HR, 0.71; 95% CI, 0.47-1.06). On exploratory analyses, surgery was found to be associated with improved survival in patients with ER/PR positive or HER-2/neu-amplified disease (P = .004). No survival benefit was observed in patients with triple-negative disease.
CONCLUSIONS
Although a trend toward improved survival with surgery was observed, it was noted most strongly in patients with ER/PR positive and/or HER-2/neu-amplified disease. This suggests that the impact of local control is greatest in the presence of effective targeted therapy, and supports the need for further study to define patient subsets that will benefit most.
Keywords: metastatic breast cancer, surgery, primary tumor, local control, survival
The prognosis of patients presenting with stage IV breast cancer has improved over the last decade.1 Recent data suggest that patients are being diagnosed with a lower burden of disease because of improved diagnostic modalities.1 Along with these trends, systemic treatment options have proliferated rapidly and now include several molecularly targeted therapies. As a result, the survival of patients with stage IV breast cancer has improved to a median of 29 months.1 For many patients, the treatment paradigm for stage IV disease is changing and can be viewed as chronic, maintenance therapy.
Traditionally, local treatment of stage IV breast cancer, either through surgery or radiotherapy, has been reserved for palliation of advanced local disease.2 However, recent reviews of population and institutional databases suggest that a significant percentage of women (approximately 40-60%) are receiving surgical treatment of their primary tumor as a component of therapy for stage IV disease,3-6 a rate that is considerably higher than what would be expected for palliative purposes alone. Although the clinical rationale for the inclusion of surgery in the treatment of many of these patients with stage IV disease is unclear, these retrospective studies have suggested that surgical resection of the primary tumor is associated with a survival benefit.3-5,7-11 However, in the absence of prospective data, the subset of patients presenting with stage IV disease who are most likely to benefit from surgical resection of the primary tumor is unknown.
Although the population database studies addressing the role of surgery in the management of patients with stage IV breast cancer incorporate large cohorts of patients and provide the most generalizable conclusions, they are inherently limited by the clinical variables recorded.12 This is especially significant when it applies to hormone receptor or HER-2/neu status. In addition, clinical details such as indications for the procedure, the specific procedure performed, surgical margin status, and local radiotherapy are unable to be considered. In contrast, single-institution studies may be limited by institutional biases and smaller numbers of evaluable patients. Therefore, any conclusions reached from these series should be considered exploratory. However, the strengths of these single-institution datasets are the degree of clinical detail available. Institution-based studies have the potential to contribute significantly to the literature regarding surgical resection in patients with stage IV breast cancer by identifying those subsets of patients with the greatest potential to benefit from surgical resection—information critical to the design of the prospective clinical trial needed to definitively address this question. In addition, single-institution studies are needed to clarify the magnitude of the problem of uncontrolled local disease in this patient population in the modern era of systemic therapy. Therefore, we sought, in a single-institution study, to explore the role of local control of the breast primary tumor in patients presenting with stage IV disease in the era of molecularly targeted therapy. The objective of the current study was to identify subsets of patients who may benefit from primary tumor treatment and assess the frequency of local disease progression.
MATERIALS AND METHODS
This project was approved by the Institutional Review Board. Two institutional databases were screened to identify all patients evaluated at the Memorial Sloan-Kettering Cancer Center who presented with an intact primary tumor and stage IV disease from January 1, 2000 through December 31, 2004. The cohort was limited to this time period to ensure homogeneity in available treatment options, specifically molecularly targeted systemic therapy. Patients were classified as having stage IV disease based on the sixth edition of the American Joint Committee on Cancer (AJCC) staging manual13; patients with supraclavicular disease only were excluded. Patients undergoing surgery for the primary tumor >30 months from the time of the initial diagnosis of breast cancer also were excluded.
Demographic, tumor, treatment, and survival data were retrospectively abstracted from the chart. Breast tumor size; histology; and estrogen receptor (ER), progesterone receptor (PR), and HER-2/neu status were recorded. ER and PR status was available for all patients; in 2 patients, the HER-2/neu status was unknown. Site(s) of metastases at the time of initial presentation were classified as soft tissue, bone, visceral disease, or a combination of disease sites. The number of metastases at the time of diagnosis was categorized into 1 or >1 site. Indications for surgical resection were classified into 1 of 4 categories: palliation of symptoms, local control in the setting of good systemic response or minimal metastatic disease, to obtain tissue (for additional receptor status testing, protocols, etc), or the presence of metastatic disease unknown at time of surgery. Surgical procedure performed, margin status, and the use of local radiotherapy were recorded. Local disease recurrence after surgical resection (chest wall or axillary) was also abstracted; only those patients undergoing axillary lymph node clearance were evaluated for axillary disease recurrence. It was not possible from retrospective chart review to accurately determine why patients were not selected for surgery. In addition, local control of the chest wall or axilla for patients not selected for surgical resection was not able to be evaluated accurately in a retrospective manner.
All statistical analyses were performed using Stata statistical software (version 9.2; StataCorp, College Station, Tex). Baseline characteristics of patients selected or not selected for surgery were compared using the Student t test or chi-square test when appropriate. Survival time was defined from the time of the diagnosis of breast cancer until the time of death or last follow-up. Unadjusted survival curves were calculated using the Kaplan-Meier method, and differences in survival were evaluated with a log-rank statistic. Multivariate assessment of prognostic factors associated with survival was performed using Cox regression analysis. Factors evaluated in the model included surgical resection, ER/PR status, HER-2/neu amplification, age at the time of the diagnosis of breast cancer, the presence of solitary metastasis, and site(s) of initial metastatic disease. Age was regarded as a continuous variable.
RESULTS
A total of 186 patients meeting the inclusion criteria were identified from the institutional databases. Overall demographics are presented in Table 1. The median age at the time of diagnosis was 56 years. The majority of women (64%) had T1 or T2 breast tumors. At the time of diagnosis, 31% had visceral-only disease, 37% had bone-only disease, and 29% had both; 3% of patients had soft tissue disease alone. Most patients received a variety of systemic treatments, including chemotherapy in 88%, hormonal therapy in 65%, and trastuzumab in 29% of patients. Overall, 89% of patients with hormone receptor-positive (113 of 127 patients) and 90% of patients with HER-2/neu-amplified (53 of 59 patients) disease received hormonal therapy and trastuzumab, respectively.
Table 1.
Overall Demographic and Clinical Features of Patients Presenting With Stage IV Breast Cancer and an Intact Primary Tumor
| Total | Surgery | No Surgery | P | |
|---|---|---|---|---|
| No. | 186 | 69 (37%) | 117 (63%) | |
| Median age, y | 56 (26-90) | 53 (26-90) | 58 (27-86) | .28 |
| Median size of breast-primary, cm | 4 (0-20) | 3 (1-20) | 5 (0-20) | .05 |
| Invasive ductal histology | 154 (86%) | 57 (83%) | 97 (87%) | .38 |
| ER positive | 127 (68%) | 48 (70%) | 79 (68%) | .77 |
| PR positive | 74 (40%) | 32 (46%) | 42 (36%) | .16 |
| HER-2/neu amplified | 59 (32%) | 12 (17%) | 47 (41%) | .001 |
| Triple negative | 35 (19%) | 18 (26%) | 35 (19%) | .05 |
| Solitary metastasis | 24 (13%) | 20 (29%) | 4 (3%) | <.001 |
| Bone metastases at initial stage IV diagnosis | 122 (66%) | 43 (62%) | 79 (68%) | .47 |
| Visceral metastases at initial stage IV diagnosis | 111 (60%) | 36 (52%) | 75 (64%) | .11 |
ER indicates estrogen receptor; PR, progesterone receptor.
Surgery was performed in 69 (37%) patients. When the clinical features of patients selected for surgery were evaluated (Table 1), surgical patients were more likely to be negative for HER-2/neu (P = .001), have smaller tumors (P = .05), and have a solitary metastasis (P <.0005). In 34 patients (49%), surgery was performed before the extent of disease workup that demonstrated metastatic disease (Table 2). Other reasons included palliation in 14 (20%) patients, local control in 15 (22%) patients, and to obtain tissue in 6 (9%) patients. The median time to surgery in this cohort of patients was 8 months (range, 0-25 months) after diagnosis. Among patients selected for surgery, 29 (42%) had bone-only disease at the time of diagnosis, 10 of whom had a solitary bony metastasis. Seven of the 22 patients with visceral-only disease and 3 of the 4 patients with soft tissue-only disease had a solitary metastasis.
Table 2.
Characteristics and Outcomes of Patients Presenting With Stage IV Breast Cancer and An Intact Primary Tumor Who Were Selected for Surgical Resection
| Characteristic | N=69 |
|---|---|
| Reason for surgery | |
| Unknown metastatic disease at the time of surgery | 34 (49%) |
| Palliation | 14 (20%) |
| Local control | 15 (22%) |
| To obtain tissue | 6 (9%) |
| Median time from stage IV diagnosis until surgerya mo, (range) | 8 (0-25) |
| Procedure performed | |
| Mastectomy | 28 |
| With axillary lymph node clearance | 21/28 |
| Surgical margin positive | 7/28 |
| With radiotherapy | 6/28 |
| Local excision | 41 |
| With axillary lymph node clearance | 12/41 |
| Surgical margin positive | 22/41 |
| With radiotherapy | 3/41 |
| Clinical chest wall recurrence after surgery | 8/69 (12%)b |
| Clinical axillary lymph node recurrence after axillary lymph node clearance | 3/33 (9%)b |
| Site of metastases at diagnosis | |
| Soft tissue | 4 (6%) |
| Visceral only | 22 (32%) |
| Bone only | 29 (42%) |
| Both bone and visceral | 14 (20%) |
Excluding patients with unknown metastatic disease at time of surgery.
One patient with both axillary lymph node and chest wall disease recurrence.
Local excision was performed in 59% (41 of 69) of patients undergoing surgery, and axillary lymph node clearance was performed in 48% (33 of 69 patients). A minority of patients received radiotherapy to the chest wall (9 of 69 patients). The median follow-up of survivors who underwent surgery was 52 months (range, 12-87 months). At a median of 8.5 months (range, 4-27 months) from surgery, 12% (8 of 69) of patients experienced a clinical disease recurrence in the chest wall (n = 5 patients) or breast (n = 3 patients), and 9% (3 of 33) of patients undergoing axillary lymph node clearance developed a clinical disease recurrence in the axilla. No clinical factors (tumor size, surgical margin status, use of postoperative radiotherapy, reason for surgery) were found to be associated with disease recurrence. A trend toward increased locoregional disease recurrence was observed in patients who were ER/PR negative and HER-2/neu negative. However, the number of patients in each subset was too small to draw any definitive conclusions.
The overall median survival for the entire cohort was 35 months, with a 1-year survival rate of 88% and a 5-year survival rate of 30%. A total of 128 deaths were observed in the cohort during the follow-up period, and the median follow-up of survivors was 53 months (range, 6-88 months). On univariate analysis, patients selected for surgical resection were found to have improved survival compared with those patients undergoing systemic therapy alone; the median survival times were 40 months and 33 months, respectively. No survival difference was observed in patients who underwent surgical resection based on margin status or the timing of the resection (data not shown). On Cox regression, ER status, PR status, HER-2/neu amplification, size and site of metastatic disease predicted survival (Table 3).
Table 3.
Predictors of Survival of Patients Presenting With Stage IV Breast Cancer and an Intact Primary Tumor
| No. | Hazard Ratio | 95% CI | P | |
|---|---|---|---|---|
| Surgery | ||||
| Resection | 69 (37%) | 0.71 | 0.47-1.1 | .10 |
| No resection | Reference | |||
| ER status | ||||
| Positive | 127 (68%) | 0.47 | 0.29-0.76 | .002 |
| Negative | Reference | |||
| PR status | ||||
| Positive | 74 (40%) | 0.57 | 0.37-0.90 | .02 |
| Negative | Reference | |||
| HER-2/neu | ||||
| Amplified | 59 (32%) | 0.51 | 0.34-0.77 | .001 |
| Not amplified | Reference | |||
| Age at stage IV diagnosis | 1.0 | 0.98-1.01 | .73 | |
| Solitary metastasis | ||||
| Yes | 24 (13%) | 1.2 | 0.62-2.4 | .57 |
| No | Reference | |||
| Bone metastasesa | ||||
| Yes | 122 (66%) | 1.7 | 1.1-2.8 | .02 |
| No | Reference | |||
| Visceral metastasesa | ||||
| Yes | 111 (60%) | 2.3 | 1.4-3.6 | <.001 |
| No | Reference |
95%CI indicates 95% confidence interval; ER, estrogen receptor; PR, progesterone receptor.
At time of diagnosis.
Several exploratory analyses were performed to attempt to identify a subset of patients with stage IV disease and an intact primary tumor who may most benefit from the incorporation of surgery as a component of multidisciplinary treatment. In our cohort, tumor molecular subtype was found to be the most significant prognostic factor (Table 3). When considering surgery in the context of tumor molecular subtype, surgery was associated with improved survival in patients with either hormone receptor-positive disease or HER-2/neu amplification; surgery was not found to be associated with improved survival in patients with triple-negative disease (Fig. 1). This correlation was maintained on multivariate analysis after adjusting for age, site of metastasis, and number of metastases (Table 4).
Figure 1.
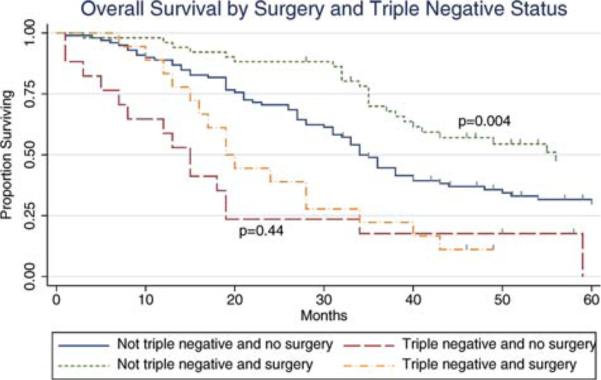
The overall survival of patients with stage IV breast cancer and an intact primary tumor, with and without triple-negative disease, is shown by surgical status.
Table 4.
Predictors of Survival in Patients Presenting With Stage IV Breast Cancer and an Intact Primary Tumor Based on Triple-Negative Disease
| No. | Hazard Ratio | 95% CI | P | |
|---|---|---|---|---|
| Surgery | ||||
| Resection | 69 (37%) | 0.68 | 0.46-1.0 | .05 |
| Noresection | Reference | |||
| Triple negative | ||||
| Yes | 35 (19%) | 3.5 | 2.2-5.4 | <.001 |
| No | Reference | |||
| Age at stage IV diagnosis | 1.0 | 0.98-1.0 | .97 | |
| Solitary metastasis | ||||
| Yes | 24 (13%) | 1.2 | 0.64-2.4 | .52 |
| No | Reference | |||
| Bone metastasesa | ||||
| Yes | 122 (66%) | 1.6 | 1.0-2.5 | .03 |
| No | Reference | |||
| Visceral metastasesa | ||||
| Yes | 111 (60%) | 2.1 | 1.3-3.2 | .001 |
| No | Reference |
95% CI indicates 95% confidence interval.
At time of diagnosis.
DISCUSSION
In this study, we observed a trend toward improved survival in patients presenting with stage IV breast cancer who underwent surgical resection of the primary tumor. This observation is in keeping with the remarkably consistent findings of other retrospective studies evaluating the role of surgery in the treatment of patients with stage IV disease (Table 5),3-11 and similar outcomes also have been observed in patients receiving radiotherapy for local control in the breast.14 As in prior studies, patients selected for surgery in our cohort represented a somewhat better prognostic group: they tended to have smaller primary tumors and less metastatic disease, and were less likely to have HER-2/neu amplification. However, we found that the survival benefit of surgery was present not only in the group of patients with the more favorable hormone receptor-positive status, but also in patients with HER-2/neu-amplified disease when targeted systemic therapy was used.
Table 5.
Summary of Studies Assessing the Impact of Local Control of the Breast Primary Tumor on Survival in Patients With Stage IV Breast Cancer
| Study | Study Type | Years Conducted | Overall No. | Percentage Undergoing Surgery | Median Overall Survival, Months | HR (95% CI) | |
|---|---|---|---|---|---|---|---|
| Surgery | No Surgery | ||||||
| Khan 20023 | NCDB | 1990-1993 | 16,024 | 57% | 0.61 (0.58-0.65) | ||
| Rapiti 20065 | Geneva Cancer Registry | 1977-1996 | 300 | 42% | 0.6 (0.3-0.7) | ||
| Babiera 20067 | Institutional database | 1997-2002 | 244 | 34% | 0.50 (0.21-1.19) | ||
| Gnerlich 20074 | SEER | 1988-2003 | 9734 | 47% | 0.62 (0.59-0.66) | ||
| Fields 200710 | Institutional database | 1996-2005 | 409 | 46% | 26.8 | 12.6 | 0.53 (0.42-0.67) |
| Blanchard 20089 | Institutional database | 1973-1991 | 395 | 65% | 27.1 | 16.8 | 0.7 (0.56-0.91) |
| Bafford 20088 | Institutional database | 1998-2005 | 147 | 41% | 42 | 28 | 0.47 |
| Cady 20086 | Tumor registry | 1970-2002 | 622 | 38% | NR | ||
| Hazard 200811 | Institutional database | 1995-2005 | 111 | 42% | 26.3 | 29.2 | 0.80 (0.4-1.52) |
| Current study | Institutional database | 2000-2004 | 186 | 37% | 40 | 33 | 0.71 (0.47-1.06) |
HR indicates hazard ratio; 95% CI, 95% confidence interval; NCDB, National Cancer Data Base; SEER, Surveillance, Epidemiology, and End Results; NR, not reported.
Although a trend toward improved survival after surgical resection of the primary tumor was observed in our overall study cohort, on exploratory analyses this effect was limited to patients with ER/PR positive or HER-2/neu-amplified cancers; patients with triple-negative disease did not experience any differential improvement in survival. To the best of our knowledge, the current study is the first to recognize that the molecular subtype of the primary tumor influences the outcomes of patients with stage IV disease who undergo surgical resection of the primary tumor. However, other recent studies have suggested that the molecular subtype of the primary tumor has a significant influence on outcome after local treatment, including the likelihood of local disease recurrence after breast conservation or mastectomy, and even overall survival.15-18 In a study by Kyndi et al, the molecular subtypes of the tumors in a subset of patients randomized as part of the Danish Breast Cancer Cooperative Group trials evaluating postmastectomy radiotherapy were reviewed.16 Overall survival in this trial was found to be improved with postmastectomy radiotherapy; however, on subset analyses, this effect was found to be limited to patients who were ER/PR positive and HER-2/neu negative who received tamoxifen. The impact of radiotherapy on locoregional control also was found to be greatest in these subgroups. Similarly, Nguyen et al reported that the molecular subtype impacted the likelihood of local and distant disease recurrence after breast-conserving therapy.17 Patients with ER/PR negative tumors, regardless of HER-2/neu amplification status, were found to have the highest likelihood of disease recurrence, whereas patients with ER/PR positive tumors who received tamoxifen had a 5-year risk of local disease recurrence of <2%.
Although these studies support the current study findings that locoregional therapy has a positive impact on outcome in patients with ER/PR positive disease, they differ with respect to the influence of HER-2/neu status. This is likely attributable to differences in the patient cohorts. We limited the current study cohort to a relatively modern time period during which homogenous treatment options were available. As a result, nearly 90% of the patients in our cohort who were eligible for hormonal therapy or trastuzumab received the targeted therapy. In contrast, although the majority of patients in both the studies by Kyndi et al16 and Nguyen et al17 received hormonal therapy, none received trastuzumab. Therefore, we believe the current study represents a unique cohort in which to observe the impact of a local therapy in women optimally treated with targeted systemic therapy. In addition, it has been postulated by others that the likelihood of local therapy improving survival in patients with stage I to stage III breast cancer is correlated with the effectiveness of adjuvant systemic therapy.19 The results of the current study support this concept for patients with stage IV disease by demonstrating a survival benefit for both a traditionally good-risk group (patients with tumors that are ER/PR positive) and a high-risk group (patients with HER-2/neu-amplified disease) in the setting of effective targeted therapy.
Survival is an important outcome when considering treatment modalities in the setting of metastatic disease. However, when contemplating the role of surgical resection of the breast primary tumor in patients with stage IV disease, factors beyond survival must be considered. Retrospective analyses suggest that most patients in the modern era do not undergo surgery for palliation of symptoms.2,7,10,11,20 The possibility that uncontrolled local disease in the breast will become a concern increases as survival time increases, and a desire by clinicians to avoid “toilet” mastectomy, may be behind the high rates of breast surgery observed in the patient population with de novo stage IV disease. However, to the best of our knowledge, few studies to date have evaluated the effectiveness of surgical local control in this setting. Current data are limited to 2 relatively small retrospective series (20 patients and 47 patients, respectively) in which chest wall local control was maintained after surgical resection of the primary tumor in 82% to 85% of patients (follow-up of a mean of 20 months21 and a median of 26.9 months11). In what to our knowledge is the largest series, surgical resection was found to be protective against the development of symptomatic chest wall disease when compared with a cohort not selected for surgical resection (64 patients).11 Although 66% of the patients undergoing surgery in this study also underwent axillary lymph node dissection, no comment was made regarding local control of axillary disease.
Data from the current study confirm that surgery can provide durable control of in-breast disease, even when a limited surgical approach is used. Although approximately 42% of patients had positive surgical margins, and radiotherapy was used infrequently, only 12% of patients developed disease recurrence in the chest wall or breast. The axillary lymph node recurrence rate of 9% is somewhat higher than that reported after axillary lymph node dissection in the nonmetastatic setting, but represents a small group of patients and may be a reflection of the failure to use radiotherapy in patients with a heavy disease burden. The molecular subtype of the tumor did not appear to impact the likelihood of durable local control after surgical resection; however, the number of patients included in this exploratory analysis was very small. It was not possible from our retrospective chart review to determine how many patients who were not selected for surgery may have benefited from preemptive local treatment for symptom control. However, the true benefit of surgery with regard to local control in the breast or the axilla can only be answered within the setting of a prospective trial.
The current study is limited by its retrospective design and cannot provide definitive causation between surgical resection of the primary tumor and the improved survival observed; for this reason, it should be considered an exploratory study intended to identify subsets of patients in whom a stronger association between surgery and survival is present. We were unable to evaluate some clinically relevant data points, such as why some patients were not selected for surgery and the incidence of locoregional disease progression in those patients. In addition, our study population was derived from a single tertiary care hospital, and the results may be less generalizable than those from larger population-based studies. In particular, a high percentage of these patients had asymptomatic metastatic disease detected on postoperative staging workup, and their clinical course may not reflect that of patients with overt metastatic disease and a higher tumor burden. However, the strength of the current study lies in the relative homogeneity of the study cohort and the completeness of data regarding prognostic factors. In contrast to population database studies,12 ER/PR status was available for all patients, HER-2/neu status was available for 99% of patients, and we used a single definition of stage IV disease (the sixth edition of the AJCC staging manual).6,13 By limiting the time period of the study, we were able to ensure homogeneity in available treatment options1 and confirm the receipt of molecularly targeted therapies when appropriate. Because of the detailed clinical data available, we were able to perform exploratory analyses to identify those patients who may benefit the most from surgical intervention to the primary tumor.
Conclusions
The results of the current study contribute to the growing body of literature addressing the question of whether surgical resection of the primary tumor in patients presenting de novo with stage IV disease improves survival. It represents the outcomes of a modern cohort of patients treated entirely in the era of molecularly targeted therapy. Although a trend toward improved survival with surgery was observed in the overall study population, this was most strongly noted in the subset of patients with ER/PR positive and/or HER-2/neu-amplified disease, suggesting that the impact of local control is most evident in the presence of effective targeted therapy. It is interesting to note that patients with HER-2/neu-amplified disease, who are in the subset of patients most likely to benefit from local therapy, are also the patients least likely to be selected for this treatment modality.
It is conceivable that the observations of the current study and others may be attributable to selection bias that is not controlled for in the analyses; the true impact on survival can only be definitely answered in a prospective fashion. However, a biological rationale for the improved survival observed with surgical resection of the primary tumor in patients with stage IV disease does exist. New concepts of the metastatic dissemination of tumors support the possibility that resection of the primary tumor may impact survival. Under the “self-seeding” theory, tumor cells have the propensity to escape from the primary tumor and seed distant sites, but also may metastasize back to the site of the primary tumor22; resection of the primary tumor in this scenario would have clinical relevance. Thus, clinical observations, viewed in parallel with new theories of cancer biology, mandate that further exploration into the role of surgery for local control, as well as the potential survival implications, be performed. The strong preferences for management of the breast among women diagnosed with breast cancer also dictate that quality of life be a part of any future study evaluating the role of surgical resection of the primary tumor in patients with stage IV breast cancer.
As in any multidisciplinary treatment plan, some patients will benefit more from therapy than others, and treatments should be tailored toward those patients who will derive the most benefit. The same should hold true for surgical resection of the primary tumor in patients with stage IV breast cancer. The current study was intended as an exploration to identify those patients who may benefit most from surgical resection of the primary tumor in the modern era of targeted systemic therapy. The prognostic factors identified should be considered in the patient stratification phase of any future prospective study.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.Andre F, Slimane K, Bachelot T, et al. Breast cancer with synchronous metastases: trends in survival during a 14-year period. J Clin Oncol. 2004;22:3302–3308. doi: 10.1200/JCO.2004.08.095. [DOI] [PubMed] [Google Scholar]
- 2.Morrogh M, Park A, Norton L, King TA. Changing indications for surgery in patients with stage IV breast cancer: a current perspective. Cancer. 2008;112:1445–1454. doi: 10.1002/cncr.23319. [DOI] [PubMed] [Google Scholar]
- 3.Khan SA, Stewart AK, Morrow M. Does aggressive local therapy improve survival in metastatic breast cancer? Surgery. 2002;132:620–626. doi: 10.1067/msy.2002.127544. discussion 626-627. [DOI] [PubMed] [Google Scholar]
- 4.Gnerlich J, Jeffe DB, Deshpande AD, Beers C, Zander C, Margenthaler JA. Surgical removal of the primary tumor increases overall survival in patients with metastatic breast cancer: analysis of the 1988-2003 SEER data. Ann Surg Oncol. 2007;14:2187–2194. doi: 10.1245/s10434-007-9438-0. [DOI] [PubMed] [Google Scholar]
- 5.Rapiti E, Verkooijen HM, Vlastos G, et al. Complete excision of primary breast tumor improves survival of patients with metastatic breast cancer at diagnosis. J Clin Oncol. 2006;24:2743–2749. doi: 10.1200/JCO.2005.04.2226. [DOI] [PubMed] [Google Scholar]
- 6.Cady B, Nathan NR, Michaelson JS, Golshan M, Smith BL. Matched pair analyses of stage IV breast cancer with or without resection of primary breast site. Ann Surg Oncol. 2008;15:3384–3395. doi: 10.1245/s10434-008-0085-x. [DOI] [PubMed] [Google Scholar]
- 7.Babiera GV, Rao R, Feng L, et al. Effect of primary tumor extirpation in breast cancer patients who present with stage IV disease and an intact primary tumor. Ann Surg Oncol. 2006;13:776–782. doi: 10.1245/ASO.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 8.Bafford AC, Burstein HJ, Barkley CR, et al. Breast surgery in stage IV breast cancer: impact of staging and patient selection on overall survival. Breast Cancer Res Treat. 2009;115:7–12. doi: 10.1007/s10549-008-0101-7. [DOI] [PubMed] [Google Scholar]
- 9.Blanchard DK, Shetty PB, Hilsenbeck SG, Elledge RM. Association of surgery with improved survival in stage IV breast cancer patients. Ann Surg. 2008;247:732–738. doi: 10.1097/SLA.0b013e3181656d32. [DOI] [PubMed] [Google Scholar]
- 10.Fields RC, Jeffe DB, Trinkaus K, et al. Surgical resection of the primary tumor is associated with increased long-term survival in patients with stage IV breast cancer after controlling for site of metastasis. Ann Surg Oncol. 2007;14:3345–3351. doi: 10.1245/s10434-007-9527-0. [DOI] [PubMed] [Google Scholar]
- 11.Hazard HW, Gorla SR, Scholtens D, Kiel K, Gradishar WJ, Khan SA. Surgical resection of the primary tumor, chest wall control, and survival in women with metastatic breast cancer. Cancer. 2008;113:2011–2019. doi: 10.1002/cncr.23870. [DOI] [PubMed] [Google Scholar]
- 12.Nathan H, Pawlik TM. Limitations of claims and registry data in surgical oncology research. Ann Surg Oncol. 2008;15:415–423. doi: 10.1245/s10434-007-9658-3. [DOI] [PubMed] [Google Scholar]
- 13.Greene FL, Page DL, Fleming ID, et al., editors. American Joint Committee on Cancer Cancer Staging Manual. 6th ed. Springer-Verlag; New York: 2002. [Google Scholar]
- 14.Le Scodan R, Stevens D, Brain E, et al. Breast cancer with synchronous metastases: survival impact of exclusive locoregional radiotherapy. J Clin Oncol. 2009;27:1375–1381. doi: 10.1200/JCO.2008.19.5396. [DOI] [PubMed] [Google Scholar]
- 15.Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 16.Kyndi M, Sorensen FB, Knudsen H, Overgaard M, Nielsen HM, Overgaard J. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J Clin Oncol. 2008;26:1419–1426. doi: 10.1200/JCO.2007.14.5565. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen PL, Taghian AG, Katz MS, et al. Breast cancer sub-type approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26:2373–2378. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 18.Le Scodan R, Stevens D, Brain E, et al. Breast cancer with synchronous metastases: survival impact of exclusive locoregional radiotherapy. J Clin Oncol. 2009;27:1375–1381. doi: 10.1200/JCO.2008.19.5396. [DOI] [PubMed] [Google Scholar]
- 19.Punglia RS, Morrow M, Winer EP, Harris JR. Local therapy and survival in breast cancer. N Engl J Med. 2007;356:2399–2405. doi: 10.1056/NEJMra065241. [DOI] [PubMed] [Google Scholar]
- 20.Rao R, Feng L, Kuerer HM, et al. Timing of surgical intervention for the intact primary in stage IV breast cancer patients. Ann Surg Oncol. 2008;15:1696–1702. doi: 10.1245/s10434-008-9830-4. [DOI] [PubMed] [Google Scholar]
- 21.Carmichael AR, Anderson ED, Chetty U, Dixon JM. Does local surgery have a role in the management of stage IV breast cancer? Eur J Surg Oncol. 2003;29:17–19. doi: 10.1053/ejso.2002.1339. [DOI] [PubMed] [Google Scholar]
- 22.Norton L, Massague J. Is cancer a disease of self-seeding? Nat Med. 2006;12:875–878. doi: 10.1038/nm0806-875. [DOI] [PubMed] [Google Scholar]


