Abstract
The membrane-anchored form of the chemokine fractalkine (CX3CL1) has been identified as a novel adhesion molecule that interacts with its specific receptor (CX3CR1) expressed in monocytes, T cells and natural killer cells to induce adhesion. In addition, CX3CL1 can be cleaved from the cell membrane to induce chemotaxis of CX3CR1-expressing leucocytes. Recently, marked variations in CX3CR1 monocyte expression have been observed during several pathological conditions. Regulation of CX3CR1 in monocytes during basal or inflammatory/anti-inflammatory conditions is poorly understood. The aim of this study was therefore to examine CX3CR1 expression during monocyte maturation and the effect of soluble mediators on this process. We found that basal expression of CX3CR1 in fresh monocytes was reduced during culture, and that lipopolysacchairde accelerated this effect. In contrast, interleukin-10 and interferon-γ treatment abrogated CX3CR1 down-modulation, through a phosphatidylinositol 3 kinase-dependent pathway. Most importantly, CX3CR1 membrane expression correlated with monocyte CX3CL1-dependent function. Taken together, our data demonstrate that CX3CR1 expression in monocytes can be modulated, and suggest that alterations in their environment are able to influence CX3CL1-dependent functions, such as chemotaxis and adhesion, leading to changes in the kinetics, composition and/or functional status of the leucocyte infiltrate.
Keywords: chemokine receptors, cytokines, human macrophages/monocytes, innate immunity, signalling/signal transduction
Introduction
Within the chemokine family, fractalkine (CX3CL1) is expressed on neurons, macrophages and also on endothelial, epithelial and dendritic cells.1,2 This chemokine has been identified as a transmembrane molecule that induces adhesion by interaction with its specific receptor (CX3CR1) expressed in monocytes (Mo), T cells, mast cells and natural killer cells.3 Adhesion mediated by CX3CL1–CX3CR1 does not require receptor signalling, is resistant to physiological shear flow and is independent of extracellular calcium.4 Besides its activity as an adhesion molecule, CX3CL1 can be cleaved from cell membrane to generate a soluble 80 000 molecular weight molecule, which induces chemotaxis of CX3CR1-expressing leucocytes.5
Expression of CX3CR1 in Mo is significantly modulated during different pathological conditions.6–8 We have previously reported the drastic decrease of fractalkine receptor expression on both CD16− and CD16+ Mo subpopulations, in children with haemolytic uraemic syndrome (HUS).9 This finding was especially striking because the loss of CX3CR1 correlated with the severity of renal failure.9 Similarly, in septic patients a higher down-expression was associated with poor evolution.8 The reduction of CX3CR1 expression in Mo could be related to different regulatory mechanisms at the cellular level, which implies down-regulation of either its membrane exposure or its synthesis. In an effort to address this issue, we have studied cellular and molecular mechanisms involved in the regulation of CX3CR1 expression in Mo, under basal or stimulated conditions. The stimuli evaluated included lipopolysaccharide (LPS) and Shiga toxin-1 (Stx1) as the main pathogenic factors in sepsis and HUS,10 and tumour necrosis factor-α (TNF-α) as a common inflammatory mediator. On the other hand, as deactivating factors we studied interleukin-4 (IL-4), IL-10 and transforming growth factor-β (TGF-β), all present at elevated concentration in HUS and sepsis.11,12 Moreover, we analysed the interferon-γ (IFN-γ) effect, which is constitutively expressed in Mo and plays a central role promoting its maturation and activation.13
We found that CX3CR1 membrane expression decreased in Mo during culture. Moreover, while LPS accelerated this process, IL-10 and IFN-γ prevented it through a phosphatidylinositol 3-kinase (PI3K) -dependent pathway. Protein synthesis inhibitors impaired the down-modulation during culture, but this effect was not additive with the inhibiting effect induced by IL-10. Most importantly, CX3CR1 membrane expression correlated with Mo CX3CL1-dependent function. In addition, phorbol 12-myristate 13-acetate (PMA) maturation of THP-1 monocytic cells reproduced the CX3CR1-down-regulation and allowed us to study the signal transduction pathways involved in cytokine-mediated effects.
This study demonstrates that locally produced cytokines or bacterial products are able to regulate CX3CR1 expression in Mo. In addition, it suggests that environment can influence the kinetics, composition and functional status of the leucocyte infiltrate by affecting both CX3CL1 expression on endothelial cells and CX3CR1 expression in Mo.
Materials and methods
Reagents and antibodies
Propidium iodide (PI), fluorescein isothiocyanate (FITC) -conjugated annexin V, cycloheximide (CHX), actinomycin D (Act D), aprotinin, leupeptin, pepstatin A, sodium orthovanadate (NaVO4), sodium fluoride (NaF), phenylmethylsulphonyl fluoride (PMSF), TNF-α, LPS, TGF-β and IFN-γ were obtained from Sigma (St Louis, MO). The mitogen-activated protein kinase (MAPK) inhibitors SB203580 and PD98059, the PI3K inhibitor LY294002, PMA, anisomycin and, Wortmannin were purchased from Calbiochem-Novabiochem (La Jolla, CA). Purified Stx1 holotoxin was purchased from Denka Seiken Co., Ltd. (Chuo-Ku, Tokyo, Japan). Human IL-4, IL-10, granulocyte–macrophage colony-stimuilating factor (GM-CSF) and soluble CX3CL1 (sCX3CL1) sCX3CL1 were from Preprotech (Preprotech Mexico, DF, Mexico). Unless stated otherwise, all other reagents were obtained from Sigma Chemical Co. All tissue culture flasks, dishes and multiwell plates were Falcon (Orange Scientific, Graignette Business Park, Belgium).
Purification of monocytes
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood collected from adult normal volunteers by centrifugation over a Ficoll–Hypaque (Ficoll Pharmacia, Uppsala; Hypaque, Wintthrop Products, Buenos Aires, Argentina) gradient. Monocytes were further isolated from PBMC using Percoll (Amersham Pharmacia Biotech, Uppsala, Sweden) gradient centrifugation as previously described.14 Viability of Mo was > 96% as determined by trypan blue exclusion test and CD14 staining of Mo revealed that their purity was > 90%. Finally, Mo were suspended and cultured at 106/ml in RPMI-1640 (Hyclone Laboratories Inc., Logan, Utah) supplemented with 10% heat-inactivated fetal calf serum (Natocor, Córdoba, Argentina), and Antibiotic–Antimycotic liquid (Gibco, Invitrogen, San Diego, CA).
Cell cultures
The THP-1 human monocytic cell line (American Type Culture Collection, Manassas, VA) was cultured at 37° and 5% CO2 in RPMI-1640 medium containing 10% fetal calf serum and antibiotics and were maturated with 5 ng/ml PMA (Sigma).
Flow cytometry
Measurement of CD14, and CX3CR1 surface expression on Mo or THP-1 cells (3 × 105) was performed by direct immunofluorescence flow cytometry on purified cells using the following conjugated anti-human monoclonal antibodies: CD14-phycoerythrin cyanine-5 (PE-Cy5) [mouse immunoglobulin G2a (IgG2a); Immunotech, Marseille, France], and CX3CR1-FITC (rat IgG2b; Medical & Biological Laboratories Co, Woburn, MA). In all cases, isotype-matched antibodies were assayed in parallel. Monocytes were analysed for membrane and total CX3CR1 expression by indirect immunofluorescence using rabbit anti-human CX3CR1 IgG (Torrey Pines Biolabs, Inc, East Orange, NJ) followed by the FITC-conjugated secondary antibody (Sigma-Aldrich) as previously reported.8 Briefly, total expression was determined with paraformaldehyde-fixed cells after permeabilization with Permeabilizing Solution#2 (Becton Dickinson, San Jose, CA) for 10 min at room temperature. Membrane expression was assayed on fixed intact cells without permeabilization. Intracellular expression was determined by the difference between total and surface expression. The mean CX3CR1 specific fluorescence was corrected for background, determined with non-specific rabbit IgG as the primary antibody. Fluorescence was measured with a Becton Dickinson FACScan. The analysis was made on 10 000 events on each sample by using the Cell Quest program (Becton Dickinson). The Mo were identified and gated according to their forward and side scattering (FSC/SSC) dot-plot profiles and positivity for CD14.
Apoptosis assay
The proportion of apoptotic Mo was determined by using PI (50 μg/ml) and Annexin V-FITC. The percentage of Annexin V-positive cells, which includes early apoptotic cells (single positive) and late apoptotic cells (double positive), was quantified using a flow cytometer (Becton Dickinson FACScan).
Calcium mobilization
For the detection of intracellular calcium, the PBMC (5 × 106 cells/ml) were suspended in incubation buffer (RPMI-1640 + 1 mm CaCl2, pH 7·4) containing the Ca2+ indicator fluo3-AM (4 μm; Sigma). The cells were incubated for 30 min at 37°, washed twice with fluo3-AM-free incubation buffer and analysed by flow cytometry.
Measurement of surface expression of CD11b
CD11b expression was measured as an indicator of CX3CL1-dependent signalling in a manner similar to that previously described.8 Briefly, Mo (1 × 105/0·1 ml) under different culture conditions were incubated with sCX3CL1 (0·2 and 2 ng/ml) for 15 min at 37°. The incubation was terminated by the addition of ice-cold phosphate-buffered saline and centrifugation. The cells were stained with anti-CD11b PE-conjugated (mouse IgG1; DAKO, Carpinteria, CA) and anti-CD14-PECy5. After cell washing, CD11b expression was analysed by flow cytometry on CD14+ Mo.
Western blot of THP-1 cell lysates
The PMA-maturated THP-1 cells (1 × 106 cells/ml) under different treatments were lysed by incubation on ice for 15 min in 0·4 ml 100 mm Tris–HCl (pH 8·0), 100 mm NaCl, 2 mm ethylenediaminetetraacetic acid, 1% Nonidet P-40 (RIPA buffer), 1 mm Na3VO4, 50 mm NaF, 0·3 U/ml aprotinin, 2 mm PMSF and 1 μg/ml each of leupeptin and pepstatin A. Lysates were centrifuged for 15 min at 14 000 g. Protein concentration was determined using a micro Bradford assay (Pierce, Rockford, IL). The supernatants were prepared for sodium dodecyl sulphate–polyacrylamide gel electrophoresis under reducing conditions.
The sodium dodecyl sulphate–polyacrylamide gele electrophoresis was run on 10% minigels using standard Tris–glycine buffers. Proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (BioRad, Hercules, CA) for 1.5 hr at 300 mA and blocked with phosphate-buffered saline 3% non-fat dried milk for 30 min. The membrane was probed with primary rabbit antibody anti-Phospho-Akt (Cell Signaling Technology, Beverly, MA) or anti β-actin (Cell Signaling Technology) overnight. After washing, blots were incubated for 2 hr with a horseradish peroxidase-conjugated goat anti-rabbit IgG (Caltag, Burlingame, CA). Immunoreactivity was detected using the ECL Western blotting detection reagent (Pierce Biotechnology, Rockford, IL).
Statistics
When required, the significance of differences between groups were evaluated using Student’s paired t-test.
Results
Mononcytes down-modulated the expression of CX3CR1 but not that of CD14 after culture
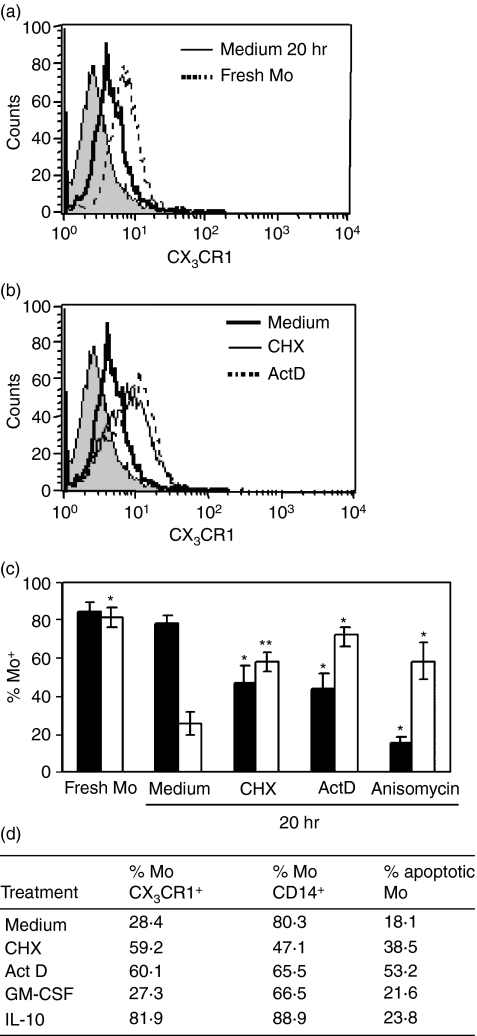
Human Mo were purified as described in the Materials and methods (purity > 95%; 88–92% with CD14+ CD16− CX3CR1+ phenotype) and incubated in complete medium for 20 hr. Although expression of CD14 was not modified during this time, CX3CR1 expression was significantly down-modulated (Fig. 1a,c).
Figure 1.
Monocytes down-regulated CX3CR1 but not CD14 during culture. Purified Mo (1 × 106/ml) were cultured for 20 hr with medium or with the following protein synthesis inhibitors: cycloheximide (CHX; 1 μg/ml), actinomycin D (ActD; 0·5 μg/ml) or anisomycin (0·1 μg/ml). After washing, cells were stained with fluorescein isothiocyanate-conjugated anti-CX3CR1, phycoerythrin-Cy5-conjugated anti-CD14 monoclonal antibodies, or isotype-matched control antibody and analysed by flow cytometry. (a) Representative histograms of surface CX3CR1-expression in fresh Mo (broken line) or after culture 20 hr (solid line), filled histogram represents the isotype control. (b) Representative histograms of surface CX3CR1 expression upon incubation for 20 hr with medium (black line), or with CHX (thin line) or with ActD (broken line). (c) The results are expressed as the percentage of CX3CR1-positive (white bars) or CD14-positive (black bars) cells into the gate for Mo defined by forward (FSC) and side (SSC) scatter parameters. Each bar represents the mean ± SEM of 17 healthy donors for culture in medium, or six donors for experiments with protein synthesis inhibitors. *P < 0·05; **P < 0·01 statistically different compared with the same parameter of Mo cultured in medium for 20 hr. (d) Purified Mo (1 × 106/ml) were cultured for 20 hr with medium or with the following agents: CHX (1 μg/ml), ActD (0·5 μg/ml), granulocyte–macrophage colony-stimulating factor (GM-CSF; 50 ng/ml), interleukin-10 (IL-10; 10 ng/ml). The table shows the percentage of CX3CR1, CD14 and AnnexinV positive Mo calculated by flow cytometry, of one experiment representative of three.
To test the involvement of protein synthesis in the regulation of CX3CR1 membrane expression, Mo were incubated in the presence of CHX (1 μg/ml), ActD (0·5 μg/ml) or anisomycin (0·1 μg/ml). We found that while all inhibitors decreased CD14 expression, they significantly prevented the CX3CR1 down-modulation from Mo membrane upon overnight incubation (Fig. 1b,c). The sustained expression of CX3CR1 after incubation in the presence of protein inhibitors could be interpreted as the inhibition of some active mechanism involved in the down-modulation of CX3CR1 dependent on protein synthesis (Fig. 1c).
To rule out that CX3CR1 down-modulation is induced by cellular death signals, Mo were cultured overnight in the presence of GM-CSF (50 ng/ml). This survival factor did not modify CD14 or CX3CR1 expression, as compared with Mo cultured without this factor (data not shown). In addition, apoptosis was evaluated in parallel with receptor expression. We found that Mo cultured in medium either with or without GM-CSF presented less than 25% of Annexin V-positive cells. When Mo apoptotic rate was increased by protein inhibitors the decrease in the CX3CR1 membrane expression was significantly blocked, suggesting that the down-regulation of CX3CR1 membrane expression is not associated with apoptosis (Fig. 1d).
Effect of bacterial factors and cytokines on CX3CR1 membrane expression
Next, we examined whether bacterial factors and cytokines were able to modify the basal CX3CR1 expression and modulation in culture. Both LPS and Stx1 slightly reduced CX3CR1 expression after 4 hr in culture, although only LPS, either alone or together with Stx1 had a significant effect (medium: 100%; LPS = 72·6 ± 5·3%*; Stx1 = 82·2 ± 7·4; LPS + Stx1 = 77·4 ± 7·0*; TNF-α = 92·1 ± 7·5; n = 7, *P < 0·01). However, LPS only accelerated CX3CR1 down-modulation because the expression of CX3CR1 at 20 hr was similar in the presence or absence of this agonist (data not shown).
On the other hand, neither inflammatory (TNF-α) nor anti-inflammatory (IL-4 and TGF-β) mediators modified CX3CR1 surface expression, at least at the time and experimental conditions assayed. In contrast, IL-10 significantly prevented CX3CR1 down-modulation at 20 hr of incubation (medium = 48·1 ± 5·1%; IL-10 = 78·3 ± 5·8%*; n = 17, *P < 0·0001). This effect was specific for CX3CR1 because IL-10 simultaneously enhanced CD14 membrane expression at 20 hr, as previously reported.15,16
These data suggest that the CX3CR1 down-modulation observed in Mo after overnight culture is a consequence of an active process, which could be modulated by IL-10.
CX3CR1 modulation by IL-10 was dose- and time-dependent
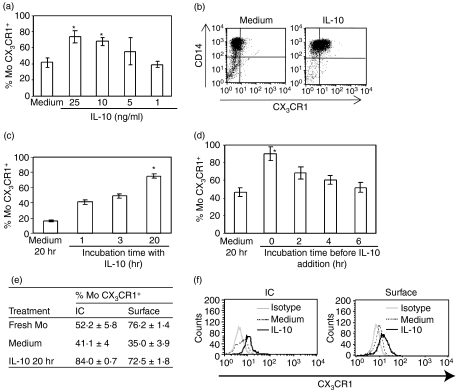
We further investigated the IL-10 effect on the modulation of CX3CR1 expression in Mo. We found that IL-10 prevented CX3CR1 down-modulation in a dose-dependent manner (Fig. 2a,b). As a consistent effect on CX3CR1 membrane expression was obtained with 10 ng/ml IL-10, subsequent experiments were carried out at this concentration. When IL-10 was added for increasing periods of time and then washed and incubated in medium up to 20 hr, CX3CR1 expression increased in a time-dependent manner (Fig. 2c).
Figure 2.
Interleukin-10 (IL-10) avoided down-regulation of CX3CR1 during culture. Purified monocytes (Mo; 1 × 106/ml) were incubated for 20 hr with medium or with IL-10 at different doses and times, followed by flow cytometry analysis of CX3CR1 expression. (a) Purified Mo (1 × 106/ml) were incubated for 20 hr with IL-10 at the concentrations indicated below each bar. (b) Representative dot-plots for double CD14/CX3CR1-staining showing the up-regulation of CX3CR1 expression in Mo upon 20 hr culture with IL-10 (10 ng/ml). (c) Purified Mo (1 × 106/ml) were incubated with medium or IL-10 (10 ng/ml) added during different periods, washed and then cultured in medium for up to 20 hr (or IL-10 over all 20 hr). (d) Purified Mo (1 × 106/ml) were incubated with medium for different periods before adding IL-10 (time 0 represents IL-10 over all 20 hr). Each bar represents the mean ± SEM of 16 healthy donors for culture in medium or IL-10 (10 ng/ml), and three donors for each point on the time and dose curves. *P < 0·05 statistically different compared with the same parameter of Mo in medium. (e) Table showing the CX3CR1-surface expression and the intracellular content (IC) after Mo permeabilization as detailed in the Materials and methods. Data are expressed as the mean ± SEM of three independent experiments. (f) Representative histograms showing CX3CR1-surface and intracellular (IC) expression in Mo only upon 20 hr culture with medium or IL-10 (10 ng/ml).
On the other hand, when Mo were incubated with medium and IL-10 was added at different times and the incubation was continued up to 20 hr, the restraining effect of IL-10 on CX3CR1 expression was time dependent (Fig. 2d). These results suggest that the CX3CR1 down-modulation upon culture is not reversible and that the IL-10 modulatory effect is proportional to the incubation time.
Cellular mechanisms involved in CX3CR1 modulation by IL-10
To understand the cellular mechanism involved in the regulation of CX3CR1 by culture or IL-10, we examined the intracellular receptor pool by analysing total and surface expression by flow cytometry in permeabilized and intact cells, respectively. We observed that after overnight culture there was a significant decrease in both CX3CR1 membrane expression and intracellular content compared with fresh Mo. In contrast, after 20 hr of culture in the presence of IL-10, the membrane expression was preserved and the intracellular CX3CR1 pool was markedly increased as compared with fresh Mo (Fig. 2e,f). In addition, we evaluated the effect of this cytokine in the presence of the protein synthesis inhibitor CHX. The simultaneous treatment of Mo with IL-10 and CHX did not modify the pattern of CX3CR1 down-modulation obtained with each agent when individually assayed (%CX3CR1+ Mo: Medium = 24·1 ± 7·5; IL-10 = 57·6 ± 3·1*; Medium + CHX = 49·6 ± 8·4*; IL-10 + CHX = 52·1 ± 1·5*; *P < 0·05). In contrast, CHX decreased the expression of CD14 and completely counteracted the IL-10-induced enhancement (data not shown).
CX3CR1 modulation by IL-10 did not involve MAPK, but rather PI3K activation
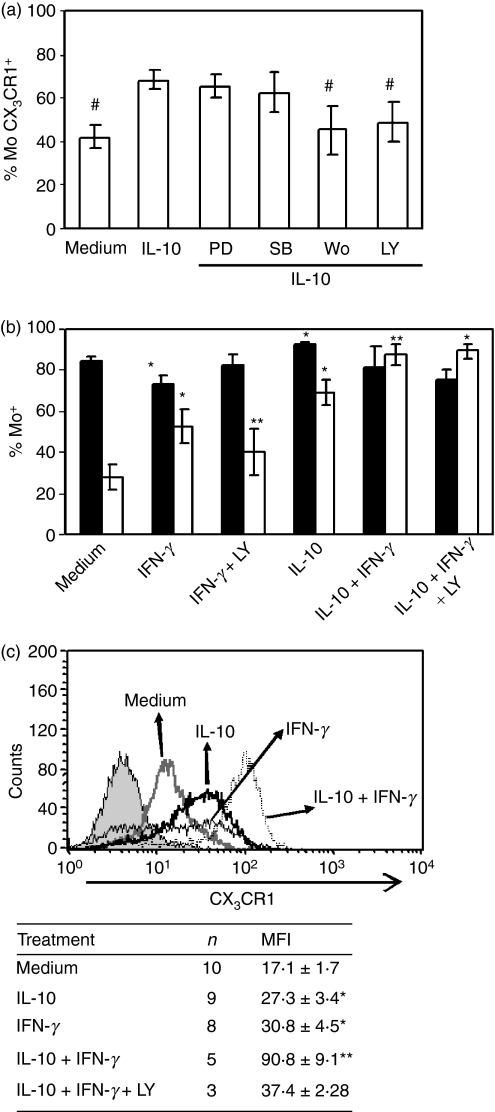
Since MAPK and PI3K pathways play a major role in IL-10-mediated induction of several cell surface receptors,17–19 Mo were treated with IL-10 in the presence of MAPK or PI3K inhibitors for 20 hr. We found that none of the MAPK inhibitors impaired IL-10 regulatory effect (Fig. 3a). However, PI3K inhibitors Wortmanin (Wo, 100 nm), or the more specific LY294002 (25 μm), impaired the effect of IL-10 on CX3CR1 expression (Fig. 3b). It may be noted that treatment of cells with LY294002 alone did not affect cell viability and did not induce any variation in basal CX3CR1 and CD14 expression when incubated alone (data not shown).
Figure 3.
Influence of mitogen-activated protein kinase (MAPK) or phosphatidylinositol 3-kinase (PI3K) chemical inhibitors on cytokine-mediated CX3CR1 regulation. (a) Purified Mo (1 × 106/ml) were pre-incubated for 1 hr with the corresponding kinase-inhibitors (PD98059, 20 μm; SB203580, 30 μm; Wortmannin, 100 nm; LY294002, 25 μm) before interleukin-10 (IL-10; 10 ng/ml) addition, and cultured for 20 hr. The results are expressed as the percentage of CX3CR1-positive cells. Each bar represents the mean ± SEM of five to seven healthy donors. #P < 0·05 statistically different compared with IL-10 alone. (b) Purified Mo (1 × 106/ml) were cultured for 20 hr with medium or interferon-γ (IFN-γ; 240 U/ml) alone or with LY294002 (25 μm); and/or IL-10 (10 ng/ml). Then, CD14 and CX3CR1 expression was analysed by flow cytometry. The results are expressed as the percentage of CX3CR1-positive (white bars) or CD14-positive (black bars) cells into the gate of Mo. Each bar represents the mean ± SEM of five to seven healthy donors, except for medium and IFN-γ bars which each correspond to 11 blood samples. *P < 0·05; statistically different compared with the same parameter of Mo in medium; **P < 0.05 statistically different compared with IFN-γ alone. (c) Representative histograms of CX3CR1 expression after culture with medium, IL-10, IFN-γ or both cytokines for 20 hr. Filled histogram corresponds to isotype-control. Data in the table are the mean ± SEM of CX3CR1 mean fluorescence intensity (MFI) of n experiments. *P < 0·05 statistically different compared with medium; **P < 0·05 statistically different compared with IFN-γ alone.
IFN-γ prevented CX3CR1 down-modulation during Mo culture
Interferon-γ and IL-10 usually have antagonistic effects on Mo so we investigated IFN-γ action on CX3CR1 modulation in Mo. Surprisingly, overnight incubation of Mo in the presence of 240 U/ml IFN-γ blocked the down-modulation of CX3CR1, through a PI3K-dependent mechanism similar to that of IL-10 modulation (Fig. 3b). In contrast, and in line with previous reports, IFN-γ significantly down-modulated CD14 expression (Fig. 3b).When Mo were incubated with both IFN-γ and IL-10, we observed a synergistic effect, which was revealed not only by a higher percentage of CX3CR1+ cells but also by a significant increase in the mean fluorescence intensity (MFI) (Fig. 3c). When LY294002 was assayed in Mo treated with IFN-γ and IL-10, a partial blocking effect was observed in the increase in CX3CR1 MFI (Fig. 3b,c).
IL-10 blocked CX3CR1 down-modulation in mature THP-1 cells
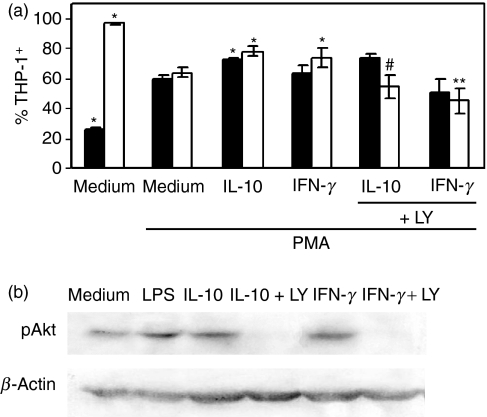
To study intracellular signals in a pure cell population we examined the regulation of CX3CR1 expression in THP-1 cells. The treatment of these cells with 5 ng/ml PKC-activating phorbol ester (PMA) for 48 hr is a widely accepted procedure for maturation of Mo.20 Hence, PMA-treated THP-1 cells significantly enhanced CD14 (approximately 20–60%) and decreased CX3CR1 cell surface expression (approximately 90–60%) (Fig. 4a). These results reproduce the phenotype alterations observed in fresh Mo cultured overnight. Subsequently, we demonstrated that IL-10 and IFN-γ increased CX3CR1 expression in maturated THP-1 cells, in a way similar to that observed in human Mo. Moreover, the specific PI3K inhibitor LY294002 counteracted IL-10 and IFN-γ modulatory effects on CX3CR1 expression, whereas MAPK inhibitors had no effect (Fig. 4a).
Figure 4.
Phorbol 12-myristate 13-acetate (PMA) -maturated THP-1 cells down-modulated CX3CR1 expression. (a) THP-1 cells (1 × 106/ml) were differentiated with PMA (5 ng/ml) for 48 hr, then they were incubated with medium, interleukin-10 (IL-10; 10 ng/ml) or interferon-γ (IFN-γ; 240 U/ml) during 20 hr. Some cell samples were pre-incubated for 1 hr with LY294002 (25 μm). All samples were analysed for CX3CR1 and CD14 expression by flow cytometry. The results are expressed as the percentage of CX3CR1-positive (white bars) or CD14-positive (black bars) THP-1 cells. Each bar represents the mean ± SEM of five independent experiments. *P < 0·05 statistically different compared with the same parameter of THP-1 cells in medium + PMA; **P < 0·05 statistically different compared with IFN-γ alone; #P < 0·02 statistically different compared with IL-10 alone. (b) LY294002 impaired IL-10- or IFN-γ-dependent Akt activation. PMA-maturated THP-1 cells (1 × 106/ml) were pre-incubated during 60 min with medium or LY294002 (25 μM), and 60 min with IL-10 (10 ng/ml) or IFN-γ (240 U/ml). Then protein cell extracts were assayed for Akt phosphorylation by immunoblotting as described in the Materials and methods. Cells incubated with lipopolysaccharide (1 μg/ml) during 60 min were assayed as a positive control.
Then, we evaluated Akt activation upon IL-10 and IFN-γ treatment by western blot (WB) to corroborate that PI3K was involved in cytokine up-regulation of CX3CR1.20 The results shown in Fig. 4(b) indicate that both IL-10 and IFN-γ induced a strong phosphorylation of Akt in THP-1 cells at 30 min post-stimulation. In addition, this effect was blocked by LY294002.
CX3CR1 membrane expression correlated with CX3CL1-dependent functionality
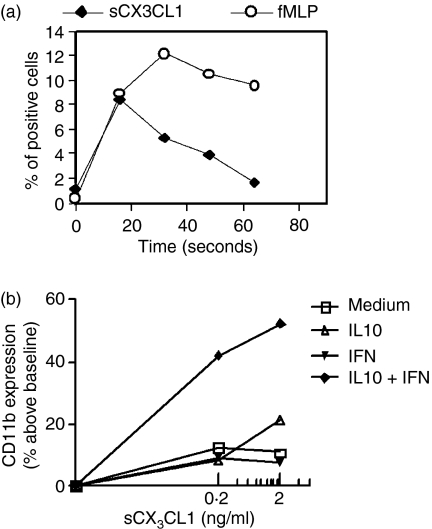
A common feature of chemokine receptors is their capacity to mobilize calcium upon interaction with their specific ligand. As a consequence, incubating Fluo3-AM PBMC with 100 ng/ml sCX3CL1 resulted in a rapid increase in [Ca2+]i, reaching peak values within 10–20 seconds and declining thereafter to baseline levels (Fig. 5a). The increase was concentration dependent in the 10–100 ng/ml range, and reached a maximum at 100 ng/ml. However, [Ca2+] mobilization could not be tested in purified Mo treated with cytokines to correlate the CX3CR1-dependent function with changes in its membrane expression, probably as the result of a previous [Ca2+] mobilization during the purification and cytokine incubation periods.
Figure 5.
CX3CL1-dependent functionality. (a) Peripheral blood mononuclear cells (PBMC) were loaded with Fluo3-AM, as indicated in the Materials and methods. Then cells were treated with sCX3CL1 (100 ng/ml) or fMLP (10−7m). Changes in [Ca2+]i were followed by monitoring the change in the percentage of positive cells inside the monocyte (Mo) gate. The figure shows one representative experiment out of three. (b) Purified Mo (1 × 106 cells/ml) cultured with medium, interleukin-10 (IL-10; 10 ng/ml), interferon-γ (IFN-γ; 240 U/ml) or IL-10 + IFN-γ for 20 hr, were incubated with CX3CL1 (0·2; 2 ng/ml) for 15 min at 37°. Mo were gated out based on forward and side scatter parameters. CD11b expression, measured as mean fluorescence intensity (MFI), was expressed as percentage above baseline (i.e. incubation with each cytokine without sCX3CL1). The figure shows one representative experiment out of three.
Then, to assess whether variations in CX3CR1 expression might impact Mo functionality, we investigated the up-regulation of CD11b, which has been previously demonstrated to be a sensitive marker of sCX3CL1-mediated Mo activation.8 We observed a slight increase in CD11b-expression with 2 ng/ml sCX3CL1 in Mo incubated for 20 hr with IL-10, as compared with Mo cultured in medium (Fig. 5b). However, when Mo were incubated for 20 hr in the presence of both cytokines (IL-10 + IFN-γ) simultaneously, there was a clear increase in CD11b compared with all the other treatments. These data suggest that up-regulation of CX3CR1 was accompanied by a significant increase in the sCX3CL1-dependent response.
Discussion
Emerging evidence shows that the regulation of chemokine receptor expression during cell activation or deactivation is as important as the regulation of chemokine production for tuning the chemokine system.21
In this study, we addressed the regulation of Mo CX3CR1 membrane expression during maturation and the influence of bacterial factors and cytokines on this process. We observed the selective down-modulation of CX3CR1 during overnight culture of Mo, which was not associated with apoptosis or cellular death and which resulted from an active process dependent on protein synthesis. In addition, PMA-maturated THP-1 cells mimic the down-modulation of CX3CR1 observed in cultured human Mo. Similarly, it has been reported that the maturation process itself is the main factor in the selective loss of CCR2 gene expression during Mo maturation using the PMA-differentiated THP-1 cell line model.22–24.
Previous studies on mononuclear phagocytes have shown that exposure to microbial agents or inflammatory mediators (e.g. LPS, IL-1 and TNF-α) induces specifically the down-modulation of certain CC chemokine receptors (CCR1, CCR2, CCR5),22,25,26 and that anti-inflammatory agents, such as IL-10, have an opposite effect.21 In line with these reports, we found that LPS accelerated the down-regulation of CX3CR1 during Mo culture, and that IL-10 strongly prevented it. Moreover, IL-10 increased CX3CR1 expression in PMA-maturated THP-1 cells. The IL-10 effect was time- and dose-dependent, and specific for IL-10 because other Mo deactivating factors such as IL-4 and TGF-β did not affect CX3CR1 expression. In addition, IFN-γ, which promotes Mo maturation,24,27 also prevented CX3CR1 down-modulation in Mo and increased CX3CR1 expression in PMA-maturated THP-1 cells. Moreover, IFN-γ and IL-10 together showed a synergistic effect, leading Mo to reach a maximum CX3CR1 expression that was even higher than the level in fresh Mo. These results suggest that, besides sharing some intracellular signalling pathways, both cytokines may be acting through alternative and additional signalling or by sensitizing Mo to the other cytokine-mediated signalling.
Two important mechanisms of chemokine-receptor regulation have been described. One of them involves intracellular storage and its rapid mobilization and internalization.28 Although almost no information is available about CX3CR1 regulation in Mo, a recent study has shown that MCP-1 induces a transient increase in CX3CR1 expression on Mo surface at 15 min, which suggests mobilization from intracellular pools to the plasma membrane rather than de novo synthesis of receptor protein.29 Following this enhancement a marked and rapid down-regulation is observed at 60 min, which is an indirect evidence of the existence of an active degradation mechanism. Under our experimental conditions, we did not observe such early increase with IL-10 or IFN-γ treatment (data not shown). However, through the evaluation of surface and intracellular CX3CR1 expression, we concluded that during culture of non-stimulated Mo the rate of CX3CR1 membrane appearance is lower than the rate of degradation or loss. In contrast, in IL-10-stimulated Mo the rate of formation is higher than the rate of degradation, regardless of the mechanism involved.
The second mechanism involved in chemokine receptor regulation is at messenger RNA (mRNA) level, through both transcription induction and stability enhancement of the corresponding mRNA.30 In particular, mRNA for CX3CR1 has been reported to be regulated in different cells and under different situations. In this regard, Koziolek et al.31 have shown a strong mRNA induction in human fibroblasts by H2O2, a mediator of oxidative stress. Similar results have been reported in microglia after ischaemia.32 Furthermore, recent reverse transcription–polymerase chain reaction assays have demonstrated that IL-10 treatment induces high levels of CX3CR1 mRNA in Mo.33 On the other hand, other authors have reported that LPS reduces CX3CR1 mRNA in PBMC, while IL-10 has no effect.8 In this context, when protein synthesis inhibitors were used simultaneously with IL-10 no additive effects were observed, leading us to hypothesize that CHX could be abrogating the de novo synthesis necessary for IL-10 modulation of CX3CR1. Interleukin-10 could directly induce the de novo synthesis of either CX3CR1, or some protein involved in its degradation step, and so interfere with the circulating circuit of this receptor from the intracellular pool to the plasma membrane. However, further studies with IL-10 and IFN-γ treatment should be carried out on CX3CR1 mRNA in Mo to achieve a final conclusion.
Interleukin-10 is secreted by T helper type 2 (Th2) cells and plays a major role in the inhibition of Mo/macrophage function.34 In contrast, IFN-γ is produced by natural killer and T cells, and plays an important role in orienting responses toward a Th1 pattern through macrophage activation.35,36 Consequently, IL-10 and IFN-γ generally have divergent effects on monocytic function. In fact, in the presence of IFN-γ, IL-10 is less effective at suppressing cytokine and chemokine production, and down-regulating major histocompatibility complex class II expression.37 The reciprocal is equally and often reported, i.e. IL-10 inhibits IFN-γ-mediated induction of early response genes.38 Therefore, the results reported in this manuscript showing that IL-10 and IFN-γ have similar and even synergistic effects on Mo-CX3CR1 regulation have scarce antecedents.39,40 Most importantly, the synergistic effect between IL-10 and IFN-γ observed in CX3CR1 membrane expression correlated with the enhancement in CX3CR1 functionality.
Interferon-γ and IL-10 bind to their cognate receptors and initiate a signal that results in the activation of janus kinase (JAK) and signal transducer and activator of transcription (STAT) proteins, leading to transcription of early response genes.41,42 In addition, it has been shown that JAK proteins can also activate other signalling molecules, specially from the PI3K family.18,43 In this regard, we demonstrated the activation of the survival enzyme Akt-1, the signalling molecule downstream of PI3K by IL-10 and IFN-γ treatment. Moreover, chemical inhibition of PI3K abrogated IL-10 and IFN-γ-mediated up-regulation of CX3CR1 in both Mo and PMA-maturated THP-1 cells, supporting the conclusion that Akt is involved in the signalling pathway that regulates CX3CR1 expression by IL-10 and IFN-γ. A number of phosphorylation targets for Akt are now emerging,43 providing alternative routes by which IL-10 and IFN-γ can potentially act. These include the activation of nuclear factor-κB,44 and the Akt capability to translocate to the nucleus where it may influence protein transcription and the cell cycle.45 Moreover, several reports agree that Akt may be responsible for serine-phosphorylation of STAT1 in response to IFN-γ or IL-10.46 The simultaneous serine-phosphorylation of these transcription factors by Akt seems to be necessary for the complete or enhanced transcriptional activity of STATs on the CX3CR1 promoter. Further studies are necessary to completely elucidate which are the signalling molecules and the transcription factors involved in the up-regulation of CX3CR1 by these cytokines.
Although the biological role of the variations in CX3CR1 levels in the Mo membrane is still poorly understood, it has been recently reported that a high expression of CX3CR1 correlates with an increased functional capacity, assayed as adherent or chemotactic responses.29 Here, we have similarly shown that CX3CR1 expression correlates with CD11b up-regulation secondary to sCX3CL1 incubation. This effect may have biological consequences because the recruitment of Mo subsets into tissues requires firm arrest and attachment onto vascular endothelia under shear stress conditions. This recruitment is mediated by specific combinations of adhesion molecules and chemokine receptors.47
In conclusion, this study further reinforces the concept that locally produced cytokines or bacterial products may regulate the kinetics, composition and functional status of the leucocyte infiltrate by affecting both chemokine production and receptor expression.48
Acknowledgments
The authors thank Marta Felippo, Nora Galassi and Norma Riera for their excellent technical assistance. The authors also thank Fundación de la Hemofilia and Academia Nacional de Medicina for the use of the FACScan flow cytometer, and the Department of Hemotherapy of CEMIC for normal blood samples. This work was supported by grants from Fundación Alberto J. Roemmers (to M.V.R. and V.I.L.), Consejo Nacional de Investigaciones Científicas y Tecnológicas, CONICET) and Agencia Nacional de Promoción Científica y Tecnológica, Argentina (to M.S.P. and M.A.I.).
Glossary
Abbreviations:
- CHX
cycloheximide
- FITC
fluorescein isothiocyanate
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- HUS
haemolytic uraemic syndrome
- IFN
interferon
- IgG
immunoglobulin G
- IL
interleukin
- JAK
Janus kinase
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- Mo
monocytes
- mRNA
messenger RNA
- PBMC
peripheral blood mononuclear cells
- PI
propidium iodide
- PI3K
phosphatidylinositol 3 kinase
- PMA
phorbol 12-myristate 13-acetate
- PMSF
phenylmethylsulphonyl fluoride
- STAT
signal transducer and activator of transcription
- Stx-1
Shiga toxin 1
- TGF
transforming growth factor
- Th2
T helper type 2
- TNF
tumour necrosis factor
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Lucas AD, Chadwick N, Warren BF, Jewell DP, Gordon S, Powrie F, Greaves DR. The transmembrane form of the CX3CL1 chemokine fractalkine is expressed predominantly by epithelial cells in vivo. Am J Pathol. 2001;158:855–66. doi: 10.1016/S0002-9440(10)64034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bazan JF, Bacon KB, Hardiman G, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–4. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 3.Umehara H, Tanaka M, Sawaki T, et al. Fractalkine in rheumatoid arthritis and allied conditions. Mod Rheumatol. 2006;16:124–30. doi: 10.1007/s10165-006-0471-9. [DOI] [PubMed] [Google Scholar]
- 4.Goda S, Imai T, Yoshie O, et al. CX3C-chemokine, fractalkine-enhanced adhesion of THP-1 cells to endothelial cells through integrin-dependent and -independent mechanisms. J Immunol. 2000;164:4313–20. doi: 10.4049/jimmunol.164.8.4313. [DOI] [PubMed] [Google Scholar]
- 5.Hundhausen C, Misztela D, Berkhout TA, et al. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell–cell adhesion. Blood. 2003;102:1186–95. doi: 10.1182/blood-2002-12-3775. [DOI] [PubMed] [Google Scholar]
- 6.Umehara H, Bloom ET, Okazaki T, Nagano Y, Yoshie O, Imai T. Fractalkine in vascular biology: from basic research to clinical disease. Arterioscler Thromb Vasc Biol. 2004;24:34–40. doi: 10.1161/01.ATV.0000095360.62479.1F. [DOI] [PubMed] [Google Scholar]
- 7.Bjerkeli V, Damas JK, Fevang B, Holter JC, Aukrust P, Froland SS. Increased expression of fractalkine (CX3CL1) and its receptor, CX3CR1, in Wegener’s granulomatosis – possible role in vascular inflammation. Rheumatology (Oxford) 2007;46:422–7. doi: 10.1093/rheumatology/kem168. [DOI] [PubMed] [Google Scholar]
- 8.Pachot A, Cazalis MA, Venet F, et al. Decreased expression of the fractalkine receptor CX3CR1 on circulating monocytes as new feature of sepsis-induced immunosuppression. J Immunol. 2008;180:6421–9. doi: 10.4049/jimmunol.180.9.6421. [DOI] [PubMed] [Google Scholar]
- 9.Ramos MV, Fernandez GC, Patey N, et al. Involvement of the fractalkine pathway in the pathogenesis of childhood hemolytic uremic syndrome. Blood. 2007;109:2438–45. doi: 10.1182/blood-2006-06-026997. [DOI] [PubMed] [Google Scholar]
- 10.Sakiri R, Ramegowda B, Tesh VL. Shiga toxin type 1 activates tumor necrosis factor-alpha gene transcription and nuclear translocation of the transcriptional activators nuclear factor-kappaB and activator protein-1. Blood. 1998;92:558–66. [PubMed] [Google Scholar]
- 11.Yamamoto T, Nagayama K, Satomura K, Honda T, Okada S. Increased serum IL-10 and endothelin levels in hemolytic uremic syndrome caused by Escherichia coli O157. Nephron. 2000;84:326–32. doi: 10.1159/000045607. [DOI] [PubMed] [Google Scholar]
- 12.Kellum JA, Kong L, Fink MP, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167:1655–63. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheibenbogen C, Andreesen R. Developmental regulation of the cytokine repertoire in human macrophages: IL-1, IL-6, TNF-alpha, and M-CSF. J Leukoc Biol. 1991;50:35–42. doi: 10.1002/jlb.50.1.35. [DOI] [PubMed] [Google Scholar]
- 14.Hardin JA, Downs JT. Isolation of human monocytes on re-orienting gradients of Percoll. J Immunol Methods. 1981;40:1–6. doi: 10.1016/0022-1759(81)90074-0. [DOI] [PubMed] [Google Scholar]
- 15.Rahimi AA, Gee K, Mishra S, Lim W, Kumar A. STAT-1 mediates the stimulatory effect of IL-10 on CD14 expression in human monocytic cells. J Immunol. 2005;174:7823–32. doi: 10.4049/jimmunol.174.12.7823. [DOI] [PubMed] [Google Scholar]
- 16.Lingnau M, Hoflich C, Volk HD, Sabat R, Docke WD. Interleukin-10 enhances the CD14-dependent phagocytosis of bacteria and apoptotic cells by human monocytes. Hum Immunol. 2007;68:730–8. doi: 10.1016/j.humimm.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- 18.Zhou JH, Broussard SR, Strle K, Freund GG, Johnson RW, Dantzer R, Kelley KW. IL-10 inhibits apoptosis of promyeloid cells by activating insulin receptor substrate-2 and phosphatidylinositol 3′-kinase. J Immunol. 2001;167:4436–42. doi: 10.4049/jimmunol.167.8.4436. [DOI] [PubMed] [Google Scholar]
- 19.Crawley JB, Williams LM, Mander T, Brennan FM, Foxwell BM. Interleukin-10 stimulation of phosphatidylinositol 3-kinase and p70 S6 kinase is required for the proliferative but not the antiinflammatory effects of the cytokine. J Biol Chem. 1996;271:16357–62. doi: 10.1074/jbc.271.27.16357. [DOI] [PubMed] [Google Scholar]
- 20.Rovera G, Santoli D, Damsky C. Human promyelocytic leukemia cells in culture differentiate into macrophage-like cells when treated with a phorbol diester. Proc Natl Acad Sci USA. 1979;76:2779–83. doi: 10.1073/pnas.76.6.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sozzani S, Ghezzi S, Iannolo G, et al. Interleukin 10 increases CCR5 expression and HIV infection in human monocytes. J Exp Med. 1998;187:439–44. doi: 10.1084/jem.187.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tangirala RK, Murao K, Quehenberger O. Regulation of expression of the human monocyte chemotactic protein-1 receptor (hCCR2) by cytokines. J Biol Chem. 1997;272:8050–6. doi: 10.1074/jbc.272.12.8050. [DOI] [PubMed] [Google Scholar]
- 23.Fantuzzi L, Borghi P, Ciolli V, Pavlakis G, Belardelli F, Gessani S. Loss of CCR2 expression and functional response to monocyte chemotactic protein (MCP-1) during the differentiation of human monocytes: role of secreted MCP-1 in the regulation of the chemotactic response. Blood. 1999;94:875–83. [PubMed] [Google Scholar]
- 24.Phillips RJ, Lutz M, Premack B. Differential signaling mechanisms regulate expression of CC chemokine receptor-2 during monocyte maturation. J Inflamm (Lond) 2005;2:14. doi: 10.1186/1476-9255-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sica A, Saccani A, Borsatti A, et al. Bacterial lipopolysaccharide rapidly inhibits expression of C–C chemokine receptors in human monocytes. J Exp Med. 1997;185:969–74. doi: 10.1084/jem.185.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juffermans NP, Weijer S, Verbon A, Speelman P, van der Poll T. Expression of human immunodeficiency virus coreceptors CXC chemokine receptor 4 and CC chemokine receptor 5 on monocytes is down-regulated during human endotoxemia. J Infect Dis. 2002;185:986–9. doi: 10.1086/339606. [DOI] [PubMed] [Google Scholar]
- 27.Andreesen R, Scheibenbogen C, Brugger W, et al. A new approach to adoptive immunotherapy of cancer using tumor cytotoxic macrophages grown from peripheral blood monocytes. Cancer Detect Prev. 1991;15:413–21. [PubMed] [Google Scholar]
- 28.Le Y, Oppenheim JJ, Wang JM. Pleiotropic roles of formyl peptide receptors. Cytokine Growth Factor Rev. 2001;12:91–105. doi: 10.1016/s1359-6101(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 29.Green SR, Han KH, Chen Y, Almazan F, Charo IF, Miller YI, Quehenberger O. The CC chemokine MCP-1 stimulates surface expression of CX3CR1 and enhances the adhesion of monocytes to fractalkine/CX3CL1 via p38 MAPK. J Immunol. 2006;176:7412–20. doi: 10.4049/jimmunol.176.12.7412. [DOI] [PubMed] [Google Scholar]
- 30.Xu L, Rahimpour R, Ran L, et al. Regulation of CCR2 chemokine receptor mRNA stability. J Leukoc Biol. 1997;62:653–60. doi: 10.1002/jlb.62.5.653. [DOI] [PubMed] [Google Scholar]
- 31.Koziolek MJ, Schmid H, Cohen CD, Blaschke S, Hemmerlein B, Zapf A, Muller GA, Strutz F. Potential role of fractalkine receptor expression in human renal fibrogenesis. Kidney Int. 2007;72:599–607. doi: 10.1038/sj.ki.5002368. [DOI] [PubMed] [Google Scholar]
- 32.Tarozzo G, Bortolazzi S, Crochemore C, Chen SC, Lira AS, Abrams JS, Beltramo M. Fractalkine protein localization and gene expression in mouse brain. J Neurosci Res. 2003;73:81–8. doi: 10.1002/jnr.10645. [DOI] [PubMed] [Google Scholar]
- 33.Jung M, Sabat R, Krätzschmar J, et al. Expression profiling of IL-10-regulated genes in human monocytes and peripheral blood mononuclear cells from psoriatic patients during IL-10 therapy. Eur J Immunol. 2004;34:481–93. doi: 10.1002/eji.200324323. [DOI] [PubMed] [Google Scholar]
- 34.Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991;174:1549–55. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young HA, Hardy KJ. Role of interferon-gamma in immune cell regulation. J Leukoc Biol. 1995;58:373–81. [PubMed] [Google Scholar]
- 36.Penton-Rol G, Polentarutti N, Luini W, Borsatti A, Mancinelli R, Sica A, Sozzani S, Mantovani A. Selective inhibition of expression of the chemokine receptor CCR2 in human monocytes by IFN-gamma. J Immunol. 1998;160:3869–73. [PubMed] [Google Scholar]
- 37.Herrero C, Hu X, Li WP, Samuels S, Sharif MN, Kotenko S, Ivashkiv LB. Reprogramming of IL-10 activity and signaling by IFN-gamma. J Immunol. 2003;171:5034–41. doi: 10.4049/jimmunol.171.10.5034. [DOI] [PubMed] [Google Scholar]
- 38.Ito S, Ansari P, Sakatsume M, Dickensheets H, Vazquez N, Donnelly RP, Larner AC, Finbloom DS. Interleukin-10 inhibits expression of both interferon alpha- and interferon gamma- induced genes by suppressing tyrosine phosphorylation of STAT1. Blood. 1999;93:1456–63. [PubMed] [Google Scholar]
- 39.Liu Y, Masuda E, Blank MC, Kirou KA, Gao X, Park MS, Pricop L. Cytokine-mediated regulation of activating and inhibitory Fc gamma receptors in human monocytes. J Leukoc Biol. 2005;77:767–76. doi: 10.1189/jlb.0904532. [DOI] [PubMed] [Google Scholar]
- 40.Comber PG, Lentz V, Schreiber AD. Modulation of the transcriptional rate of Fc gamma receptor mRNA in human mononuclear phagocytes. Cell Immunol. 1992;145:324–38. doi: 10.1016/0008-8749(92)90335-m. [DOI] [PubMed] [Google Scholar]
- 41.Riley JK, Takeda K, Akira S, Schreiber RD. Interleukin-10 receptor signaling through the JAK-STAT pathway. Requirement for two distinct receptor-derived signals for anti-inflammatory action. J Biol Chem. 1999;274:16513–21. doi: 10.1074/jbc.274.23.16513. [DOI] [PubMed] [Google Scholar]
- 42.Ramana CV, Gil MP, Schreiber RD, Stark GR. Stat1-dependent and -independent pathways in IFN-gamma-dependent signaling. Trends Immunol. 2002;23:96–101. doi: 10.1016/s1471-4906(01)02118-4. [DOI] [PubMed] [Google Scholar]
- 43.Curnock AP, Logan MK, Ward SG. Chemokine signalling: pivoting around multiple phosphoinositide 3-kinases. Immunology. 2002;105:125–36. doi: 10.1046/j.1365-2567.2002.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kane LP, Shapiro VS, Stokoe D, Weiss A. Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol. 1999;9:601–4. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- 45.Klippel A, Escobedo MA, Wachowicz MS, Apell G, Brown TW, Giedlin MA, Kavanaugh WM, Williams LT. Activation of phosphatidylinositol 3-kinase is sufficient for cell cycle entry and promotes cellular changes characteristic of oncogenic transformation. Mol Cell Biol. 1998;18:5699–711. doi: 10.1128/mcb.18.10.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen H, Ramana CV, Bayes J, Stark GR. Roles of phosphatidylinositol 3-kinase in interferon-gamma-dependent phosphorylation of STAT1 on serine 727 and activation of gene expression. J Biol Chem. 2001;276:33361–8. doi: 10.1074/jbc.M105070200. [DOI] [PubMed] [Google Scholar]
- 47.Schulz C, Schafer A, Stolla M, et al. Chemokine fractalkine mediates leukocyte recruitment to inflammatory endothelial cells in flowing whole blood: a critical role for P-selectin expressed on activated platelets. Circulation. 2007;116:764–73. doi: 10.1161/CIRCULATIONAHA.107.695189. [DOI] [PubMed] [Google Scholar]
- 48.Ancuta P, Wang J, Gabuzda D. CD16+ monocytes produce IL-6, CCL2, and matrix metalloproteinase-9 upon interaction with CX3CL1-expressing endothelial cells. J Leukoc Biol. 2006;80:1156–64. doi: 10.1189/jlb.0206125. [DOI] [PubMed] [Google Scholar]