Abstract
Background
Cyclophosphamide, an alkylating agent, is widely used for the treatment of many adult and pediatric malignancies. The stability of cyclophosphamide in aqueous- and methylcellulose-based oral suspending vehicles is currently unknown.
Objectives
The goals of this study were (1) to develop and validate a stability-indicating HPLC method to measure cyclophosphamide concentrations in simple syrup and Ora-Plus, and (2) to assess the 56-day chemical stability and physical appearance of cyclophosphamide in these suspensions at both room temperature and 4°C.
Methods
The i.v. formulation of cyclophosphamide was diluted to 20 mg/mL in normal saline, compounded 1:1 with either suspending vehicle, and stored in the dark in 3mL amber polypropylene oral syringes at 4°C and 22°C. Aliquots from each syringe were obtained on days 0, 3, 7, 14, 21, 28, 35, 42, 49, and 56 and assayed using the validated stability-indicating HPLC-UV method. A C18 analytical column was used to separate cyclophosphamide from the internal standard, ifosfamide, with a mobile phase of 21% acetonitrile in 79% sodium phosphate buffer. The suspension was examined for odor change, visually examined under normal fluorescent light for color change, and examined under a light microscope for evidence of microbial growth.
Results
Samples of cyclophosphamide in both simple syrup and Ora-Plus were stable when kept at 4°C for at least 56 days. At room temperature, cyclophosphamide in simple syrup and Ora-Plus had a shelf life of 8 and 3 days, respectively. No changes in color or odor or evidence of microbial growth were observed.
Conclusion
Cyclophosphamide can be extemporaneously prepared in simple syrup or Ora-Plus and stored at least 2 months under refrigeration without significant degradation.
Keywords: Ora-Plus, simple syrup, oral suspension, cyclophosphamide
INTRODUCTION
The alkylating agent cyclophosphamide is a widely administered cancer chemotherapeutic used to treat a broad spectrum of malignancies.1, 2 After activation of cyclophosphamide by hepatic enzymes, the active metabolite forms covalent bonds with DNA and proteins, inducing cell death.2–4 Cyclophosphamide is often administered intravenously as induction therapy.5 Significant dose-dependent toxicities, particularly myelosuppression, occur in both children and adults.6, 7
Tumor growth is largely supported by angiogenesis, the development of new blood vessels from existing ones.8 High-dose cyclophosphamide has been shown to have an anti-angiogenic effect in tumor cells.9 However, administering continuous low-dose oral cyclophosphamide (i.e., metronomic therapy) also prevents angiogenesis with less risk of toxicity.7, 9–11 Metronomic cyclophosphamide delayed progression of prostate cancer, breast cancer, and erythroleukemia in mouse models, with minimal toxicity.12, 13 In adult metastatic breast cancer patients, Colleoni and colleagues found metronomically administered cyclophosphamide achieves significant long-term disease stability with minimal leucopenia.11 In pediatric studies, low-dose metronomic cyclophosphamide was well tolerated and showed promising response with little toxicity in heavily pretreated solid tumor patients.14, 15
Due to daily drug administration during maintenance therapy, it is preferable to give cyclophosphamide orally, and in the case of young children, to administer it as an oral suspension. A previous oral suspension of cyclophosphamide was prepared in Aromatic Elixir; however, this vehicle is no longer commercially available. Although it was reported that cyclophosphamide is stable for at least 2 weeks at 5°C in Aromatic Elixir, its stability in modern suspending agents is unknown.16 In this study, we validated and implemented a stability-indicating high performance liquid chromatography (HPLC) method to determine the stability of cyclophosphamide over 56 days in both simple syrup and Ora-Plus, two common oral suspending vehicles.
EXPERIMENTAL
Chemicals and reagents
Cyclophosphamide used for the preparation of standards and quality control samples and ifosfamide used as the internal standard were supplied by Sigma-Aldrich (St. Louis, MO, USA). Vials of Cyclophosphamide 2 g Injection, USP and 0.9% normal saline used to prepare the suspensions for stability studies were supplied by Baxter (Deerfield, IL, USA). Simple syrup and Ora-Plus were supplied by Humco (Texarkana, TX, USA) and Paddock Laboratories (Minneapolis, MN, USA), respectively. HPLC grade acetonitrile and methanol were purchased from Burdick & Jackson (Muskegon, MI, USA). Sodium phosphate monobasic monohydrate, phosphoric acid, and sodium hydroxide were obtained from Fisher Scientific (Fairlawn, NJ, USA). All water was distilled, deionized and purified with a Millipore Milli-Q UV plus and Ultra-Pure Water System (Tokyo, Japan).
Equipment and chromatographic conditions
The HPLC system consisted of Shimadzu LC-20AD pump, a Shimadzu SPD-10AVP UV-VIS detector with a detection wavelength of 190 nm and a Shimadzu SIL-10ADVP autoinjector set to 4°C with an injection volume of 50 µL. Chromatographic data were analyzed using Shimadzu ClassVP Integrating Software v. 7.3. Our preliminary conditions were based on previously reported methods.17, 18 All chromatographic separations were obtained using a Phenomenex® Luna, 5µm, C18, 100Å, 125 × 4 mm column (Torrance, CA, USA) with a Phenomenex® Security Guard, C18, 4 × 2.0 mm guard column. Analytes were eluted using an acetonitrile-phosphate buffer (21:79) mobile phase flowing at 1.0 mL/minute. The 0.2 M sodium phosphate monobasic monohydrate mobile phase buffer, prepared in distilled, deionized water (DDH2O), was adjusted to pH 4.0 with phosphoric acid and sodium hydroxide using an Orion 250A pH meter (Beverly, MA, USA). The phosphate buffer was vacuum filtered through a 0.2 µm PALL membrane (Port Washington, NY, USA).
Preparation of stock and working solutions
A 200 mg/mL stock solution was prepared by dissolving cyclophosphamide powder into 80% methanol/20% DDH2O. Aliquots of the stock solution were stored in 4 mL glass amber vials at −20°C. Appropriate dilutions of the stock solution were made in 80% methanol/20% DDH2O to obtain working stock solutions of 10 and 40 mg/mL, which were also stored at −20°C. A 100 mg/mL internal standard stock solution was prepared by dissolving ifosfamide into 80% methanol/20% DDH2O. Aliquots of the internal standard stock solution were stored in 4 mL glass amber vials at −20°C. A dilution of the internal standard stock solution was made in 80% methanol/20% DDH2O to obtain an internal standard working stock solution of 15 mg/mL and these stocks were lso stored at −20°C.
Calibration curves, sample preparation, and quality controls
Simple syrup and Ora-Plus calibration standards were prepared by adding cyclophosphamide working stock solutions into either syrup suspension to achieve the following concentrations: 5, 7, 10, 15 and 20 mg/mL. Ifosfamide internal standard working stock solution was added to each standard to create a final concentration of 1.5 mg/mL. The lower limit of quantification of cyclophosphamide in simple syrup and Ora-Plus was defined as the first calibration curve standard, which had a signal to noise ratio <5. Quality control samples were prepared by adding cyclophosphamide working stock solutions into simple syrup and Ora-Plus to obtain final concentrations of 8, 12, and 18 mg/mL. Before all analytic runs, all standards, quality controls and cyclophosphamide stability samples were diluted to a 20x final dilution in 20% methanol/80% DDH2O. Two calibration curves of both simple syrup and Ora-Plus were obtained by performing linear regression on the analyte/internal standard peak height ratio and the analyte/internal standard quantity ratio. Prior to measurement of each weekly stability sample, three quality controls (low, middle, and high) were prepared and analyzed to ensure values were accurate within 10% of the nominal value. If controls were not within 10% of their nominal value, a new calibration curve was prepared and analyzed.
Method validation
Intra-day and Inter-day precision and accuracy
Intra-day precision (expressed as % coefficient of variation (CV)) and accuracy were evaluated by preparing and analyzing ten low (8 mg/mL) and ten high (18 mg/mL) replicates within one day. Inter-day precision and accuracy was evaluated by preparing and analyzing three low (8 mg/mL), three middle (12 mg/mL), and three high (18 mg/mL) replicates for four consecutive days. The criteria for acceptability of the method included accuracies within 10% of the nominal value and precisions with < 10% CV.
Specificity, conversion and carry-over
No peaks co-eluted with cyclophosphamide or ifosfamide in either simple syrup or Ora-Plus. To assess conversion to degradation products and possible interference, neat aliquots of the middle quality control were prepared in 20% methanol/80% DDH2O without internal standard. These aliquots were heated at 45°C and periodically analyzed in order to observe the appearance of secondary peaks in the chromatogram as well as peak shifting and asymmetry. To assess carry-over, cyclophosphamide samples (20 mg/mL) in simple syrup and Ora-Plus were injected and followed by one wash run to determine if any residual cyclophosphamide would be detected in the wash.
Cyclophosphamide stability experiments
Cyclophosphamide samples were prepared in the Central Pharmacy at St. Jude Children’s Research Hospital in a manner consistent with how they are prepared for patients. Vials of Cyclophosphamide 2g Injection, USP were reconstituted with 100 mL of 0.9% normal saline to a concentration of 20 mg/mL prior to compounding. This cyclophosphamide injectable solution was then mixed in a 1:1 ratio with either simple syrup or Ora-Plus for a final concentration of 10 mg/mL. Aliquots of 2.4 mL were added to 3 mL amber polypropylene oral dispensing syringes (Becton Dickinson Company, Franklin Lanes, NJ, USA). One syringe of each suspension was stored in a plastic bag inside a closed box in a 4°C cold room (range: 3.8–4.2°C). Another syringe of each suspension was stored in a plastic bag in a drawer at approximately 22°C (range: 21.3–22.5°C). Cyclophosphamide concentrations in all samples were analyzed immediately after compounding and after 3, 7, 14, 21, 28, 35, 42, 49, and 56 days. On each day of the stability study, samples were spiked with internal standard and diluted as outlined in the calibration curve and method validation sections, and were assayed in triplicate. The color was examined under normal fluorescent lighting, the odor was examined, and an aliquot of the suspension was observed under a light microscope for evidence of microbial growth. Linear regression of all concentration data was performed using GraphPad Prism version 5.02 for Windows (GraphPad Software, San Diego, CA). Samples were considered stable if the 95% lower confidence limit of the estimated regression line was within 10% of the initial concentration.
RESULTS
Cyclophophosphamide assay development
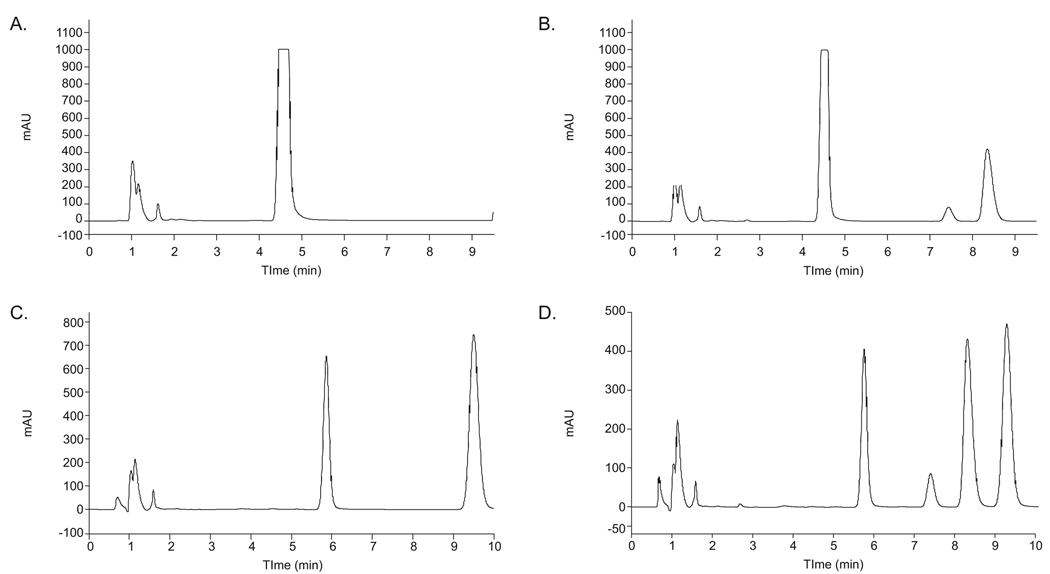
Because sample extraction was not necessary, little optimization was performed to improve experimental conditions. As shown in Figure 1A, the chromatogram of blank simple syrup shows a single peak at 4.5 min, whereas two peaks, eluting at 5.9 and 9.4 min, are present in the blank Ora-Plus chromatogram (Figure 1C). The percent acetonitrile in the mobile phase was adjusted to ensure adequate resolution between the analyte and the internal standard peaks, and between the analyte and the late eluting peak in Ora-Plus (Figure 1D), and secondly to minimize the run time. With our final chromatographic conditions, the resolution (R) value was ≥1.09 between these two peaks. In simple syrup, the retention times of cyclophosphamide and ifosfamide were 8.4 min and 7.5 min, respectively, with a total run time of 9.5 minutes (Figure 1B). Retention times were similar with Ora-Plus, except the total run time was 10 minutes (Figure 1D).
Figure 1.
Example HPLC chromatograms of cyclophosphamide assay. Chromatograms of blank simple syrup (A) and simple syrup spiked with 10 mg/mL cyclophosphamide and internal standard (B). Chromatograms of blank Ora-Plus (C) and Ora-Plus spiked with 10 mg/mL cyclophosphamide and internal standard (D).
Cyclophosphamide assay validation
Simple syrup calibration curves for cyclophosphamide were linear over a concentration range of 5 mg/mL to 20 mg/mL with correlation coefficient (r2) equal to 0.994 (y=1.5646x – 1.1006). The Ora-Plus calibration curve for cyclophosphamide was linear over a concentration range of 5 mg/mL to 20 mg/mL with an r2 value of 0.999 (y=1.7125x – 0.8341). The intra-day precision (% CV) in simple syrup was ≤ 3.4 with an accuracy (% change from nominal value) ≤ 5.4%, and in Ora-Plus the intra-day precision was ≤3.7% with an accuracy ≤3.2%. As shown in Table 1, inter-day precision of the cycophosphamide assay in both simple syrup and Ora-Plus was within 5.5% and accuracy was within 4.3%. The LLOQ was 5 mg/mL, which was acceptable for the concentration range over which samples were measured. Although lower concentrations were detected on the column, 5 mg/mL was found as the lowest concentration that fit well with the calibration curve, as a slight deviation from linearity was noted below this concentration. No secondary peaks indicating detectable degradation products appeared in the chromatograms analyzed during the conversion study. Furthermore, the cyclophosphamide peak did not shift or lose symmetry throughout the study implying that possible degradation products did not co-elute with the cyclophosphamide peak. No residual peaks appeared in carryover studies.
Table 1.
Inter-day accuracy and precision of cyclophosphamide assay.
| Accuracy (% nominal) |
Precision (% CV) |
|||||
|---|---|---|---|---|---|---|
| Control 1 | Control 2 | Control 3 | Control 1 | Control 2 | Control 3 | |
| Simple syrup | 95.64 | 99.03 | 99.55 | 2.96 | 5.52 | 3.60 |
| Ora-Plus | 98.06 | 99.55 | 99.17 | 4.85 | 5.24 | 4.18 |
Stability of cyclophosphamide in oral suspending vehicles
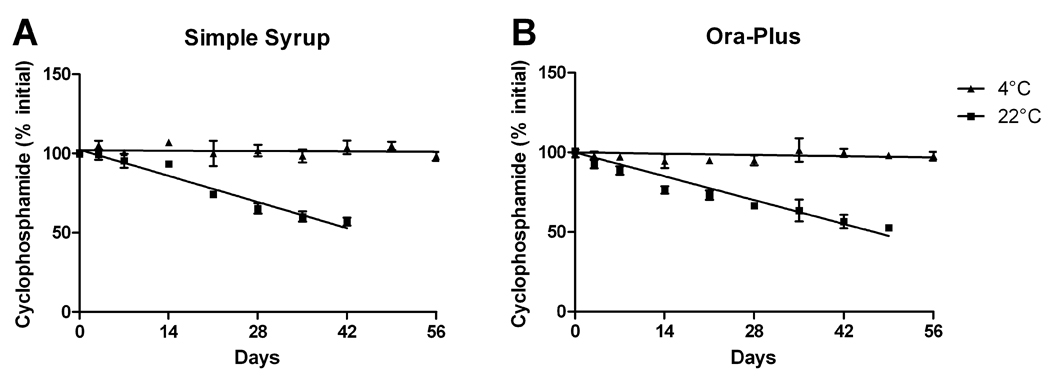
Cyclophosphamide suspended in simple syrup and stored at 4°C retained its concentration within 96% of the baseline value at 56 days after compounding (Figure 2A, Table 2). Cyclophosphamide suspended in Ora-Plus and stored at 4°C retained its concentration within 98% of the baseline value at 56 days after compounding (Figure 2B, Table 2). Because there was nearly no degradation within 56 days, linear regression analysis could not be used to assess when the cyclophosphamide would be degraded by 10%. Based on the linear regression analysis of cyclophosphamide concentrations expressed as a percent of the initial values, cyclophosphamide in simple syrup is degraded by 10% after 10.6 days (90% confidence interval: 8.7–12.2 days) and cyclophosphamide in Ora-Plus is degraded by 10% after 6.0 days (90% confidence interval: 3.4–8.2 days) when stored at 22°C (Figure 2). Upon visual observation with a light microscope, no microbial growth was seen. No color or odor change occurred in any samples.
Figure 2.
Chemical stability of cyclophosphamide in oral suspending agents. Cyclophosphamide (10 mg/mL) was extemporaneously prepared in simple syrup (A) and Ora-Plus (B) and stored at either 22°C or 4°C. Aliquots of the suspension were assayed for cyclophosphamide concentrations in triplicate at the indicated time-points for up to 56 days.
Table 2.
Cyclophosphamide stability in simple syrup and Ora-Plus
| Cyclophosphamide concentration (mg/mL) ± SD |
||||
|---|---|---|---|---|
| 4°C |
22°C |
|||
| Time (days) | Simple syrup | Ora-Plus | Simple syrup | Ora-Plus |
| 0 | 9.74 ± 0.01 | 10.15 ± 0.24 | 9.81 ± 0.09 | 10.31 ± 0.17 |
| 3 | 10.28 ± 0.26 | 10.00 ± 0.27 | 9.70 ± 0.31 | 9.49 ± 0.30 |
| 7 | 9.82 ± 0.16 | 9.92 ± 0.12 | 9.31 ± 0.44 | 9.07 ± 0.27 |
| 14 | 10.45 ± 0.04 | 9.67 ± 0.45 | 9.11 ± 0.12 | 7.81 ± 0.24 |
| 21 | 9.77 ± 0.77 | 9.70 ± 0.04 | 7.26 ± 0.19 | 7.48 ± 0.29 |
| 28 | 9.97 ± 0.37 | 9.71 ± 0.32 | 6.39 ± 0.33 | 6.81 ± 0.14 |
| 35 | 9.61 ± 0.39 | 10.37 ± 0.75 | 5.88 ± 0.32 | 6.50 ± 0.70 |
| 42 | 10.16 ± 0.42 | 10.19 ± 0.26 | 5.57 ± 0.23 | 5.78 ± 0.43 |
| 49 | 10.24 ± 0.25 | 10.01 ± 0.20 | ||
| 56 | 9.60 ± 0.27 | 9.98 ± 0.27 | ||
Discussion
The HPLC assay that we developed and validated for measuring cyclophosphamide in suspending agents was specific, precise, and accurate. Our preliminary conditions for the HPLC method were based on several previous studies, which used a mobile phase comprised of 20% acetonitrile and 80% phosphate buffer.17, 18 We found that with our initial assay conditions, substances in Ora-Plus caused peaks that overlapped with the cyclophosphamide and ifosfamide peaks. Changing these conditions yielded acceptable resolution of cyclophosphamide and ifosfamide peaks. For assays of plasma cyclophosphamide, we have used a Phenomenex Gemini C18 100×2mm column.19 For this stability assay, we chose a Phenomenex Luna C18 125×4mm column, which provided better resolution of peaks, due to greater column length and volume. The forced-degradation experiment shows that this assay is stability-indicating.
Low-dose cyclophosphamide is increasingly being used for maintenance therapy in children with solid tumors.14, 15, 20 Daily administration of maintenance therapy necessitates that the drug be administered orally. Although cyclophosphamide is available in tablet form, an oral suspension is a better option for infants and young children. Since there are no commercially available pediatric formulations of cyclophosphamide, suspensions have been extemporaneously prepared in aromatic elixir. Because aromatic elixir is alcohol-based, it is less palatable to children. It was shown that suspensions of cyclophosphamide in aromatic elixir were stable for two weeks at 5°C.16 Although it is likely stable beyond this time, because there were no data to support this, pharmacists would prepare only a two week supply at one time.
Aromatic elixir is no longer commercially available. Thus, a new suspending agent is needed for administration of oral cyclophosphamide to young children. We chose to examine cyclophosphamide in two suspending agents: simple syrup and Ora-Plus. Ora-plus has the advantage of preservatives, which reduce the possibility of microbial contamination. Cyclophosphamide has high solubility in neutral and acidic aqueous solutions, and thus it was possible to compound a 10 mg/mL solution.
Cyclophosphamide was stable at 4°C in both simple syrup and Ora-Plus for 56 days. At day 56, the concentration had not changed significantly from the starting concentration of 10 mg/mL. Thus, it is likely to be stable for longer, but we did not examine later time-points. We did not examine whether the rate of degradation was affected by light; therefore, it is recommended that the oral syringes be kept in the dark. With this information, pharmacists can prepare a supply of oral cyclophosphamide suspension for two 28-day cycles at one time, compared to the two week supply that was prepared previously. This is more convenient for patient’s families, and more time- and cost-efficient for the pharmacy.
When stored at room temperature, cyclophosphamide in the oral suspensions degraded by 10%much more rapidly than at 4°C.. The lower 95% confidence limit of when the concentration reached 90% of the initial value was 8.7 days for simple syrup, and 3.4 days for Ora-Plus. We therefore consider the shelf life of these suspensions to be 8 days and 3 days, respectively. This is similar to what was reported for a parenteral formulation of cyclophosphamide in infusion fluids at 20–22°C.21 Cyclophosphamide was reported to be degraded by >80% after 4 days at room temperature when mixed with mesna in 5% dextrose in polyethylene infusion bags.22 Although it is recommended that the suspension is stored in the refrigerator, if a syringe is left out for a day, it should not result in significant loss of potency. Due to possible photodegradation, we recommend the use of amber syringes. This study did not examine what the degradation products of cyclophosphamide are in these oral suspensions. However, a previous study showed that in buffered aqueous solutions, multiple pH-dependent hydrolysis products were observed, none of which had anti-tumor activity.23 We expect that simple syrup and Ora-Plus solutions are in the pH range in which there is little effect of pH on the rate of degradation (pH 3.4 to 8.6), although the rate of degradation is increased at lower pH.23
During the time that aromatic elixir has been unavailable, some pediatric oncology sites have been administering 20 mg/mL intravenous cyclophosphamide via oral syringes to young children. Dispensing an injectable medication without modifying it into an oral formulation could lead to wrong site administration if the container is not properly labeled as for oral use only. Cyclophosphamide for injection would also be less palatable than a properly compounded oral formulation with simple syrup or Ora-Plus. This could lead families to dilute it in beverages or vehicles of varying pH with potential differences in bioavailability or stability. Simple syrup and Ora-Plus also contain preservatives to help prevent microbial growth during storage. Dosages of oral cyclophosphamide are low enough that the 10 mg/mL concentrations results in a dose volume that is reasonable for infants and young children to swallow. A 10 mg/mL concentration is also easy to accurately measure in oral syringes which could result in fewer dosing errors.
This study has shown that simple syrup and Ora-Plus are suitable for extemporaneously preparing suspensions of parenteral cyclophosphamide. Thus, we recommend using these suspending agents, in which cyclophosphamide is now documented to be stable for eight weeks, compared to a documented stability of only two weeks in aromatic elixir. The pharmacokinetics of cyclophosphamide when compounded in simple syrup are now being examined in a clinical study of infants and young children with brain tumors.
Acknowledgments
This work was supported in part by the National Institutes of Health Cancer Center Support [CORE] Grant [P30 CA21765], The Noyes Brain Tumor Foundation, Musicians Against Childhood Cancer (MACC), the National Cancer Institute Pediatric Oncology Education Program [5R25CA023944], and the American Lebanese Syrian Associated Charities (ALSAC).
Contributor Information
Rachel Kennedy, Department of Pharmaceutical Sciences, St. Jude Children’s Research Hospital, Memphis, TN.
Daniel Groepper, Department of Pharmaceutical Sciences, St. Jude Children’s Research Hospital.
Michael Tagen, Department of Pharmaceutical Sciences, St. Jude Children’s Research Hospital.
Robbin Christensen, Department of Pharmaceutical Services, St. Jude Children’s Research Hospital.
Fariba Navid, Department of Oncology, St. Jude Children’s Research Hospital; Department of Pediatrics, College of Medicine, University of Tennessee Health Science Center.
Amar Gajjar, Department of Oncology, St. Jude Children’s Research Hospital; Professor, Department of Pediatrics, College of Medicine, U.
Clinton F. Stewart, Department of Pharmaceutical Sciences, St. Jude Children's Research Hospital; College of Pharmacy, University of Tennessee Health Sciences Center.
References
- 1.Ahmed AR, Hombal SM. Cyclophosphamide (Cytoxan). A review on relevant pharmacology and clinical uses. J Am Acad Dermatol. 1984;11:1115–1126. doi: 10.1016/s0190-9622(84)80193-0. [DOI] [PubMed] [Google Scholar]
- 2.Moore MJ. Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet. 1991;20:194–208. doi: 10.2165/00003088-199120030-00002. [DOI] [PubMed] [Google Scholar]
- 3.Fleming RA. An overview of cyclophosphamide and ifosfamide pharmacology. Pharmacotherapy. 1997;17:146S–154S. [PubMed] [Google Scholar]
- 4.Singh N, Nigam M, Ranjan V, Sharma R, Balapure AK, Rath SK. Caspase mediated enhanced apoptotic action of cyclophosphamide- and resveratrol-treated MCF-7 cells. J Pharmacol Sci. 2009;109:473–485. doi: 10.1254/jphs.08173fp. [DOI] [PubMed] [Google Scholar]
- 5.Sweetenham JW, Carella AM, Taghipour G, et al. High-dose therapy and autologous stem-cell transplantation for adult patients with Hodgkin's disease who do not enter remission after induction chemotherapy: results in 175 patients reported to the European Group for Blood and Marrow Transplantation. Lymphoma Working Party. J Clin Oncol. 1999;17:3101–3109. doi: 10.1200/JCO.1999.17.10.3101. [DOI] [PubMed] [Google Scholar]
- 6.Baumann F, Preiss R. Cyclophosphamide and related anticancer drugs. J Chromatogr B Biomed Sci Appl. 2001;764:173–192. doi: 10.1016/s0378-4347(01)00279-1. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D, Bergers G, Bergsland E. Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Invest. 2000;105:1045–1047. doi: 10.1172/JCI9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukumura D, Jain RK. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res. 2007;74:72–84. doi: 10.1016/j.mvr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Browder T, Butterfield CE, Kraling BMe, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–1886. [PubMed] [Google Scholar]
- 10.Kerbel RS, Klement G, Pritchard KI, Kamen B. Continuous low-dose anti-angiogenic/ metronomic chemotherapy: from the research laboratory into the oncology clinic. Ann Oncol. 2002;13:12–15. doi: 10.1093/annonc/mdf093. [DOI] [PubMed] [Google Scholar]
- 11.Colleoni M, Rocca A, Sandri MT, et al. Low-dose oral methotrexate and cyclophosphamide in metastatic breast cancer: antitumor activity and correlation with vascular endothelial growth factor levels. Ann Oncol. 2002;13:73–80. doi: 10.1093/annonc/mdf013. [DOI] [PubMed] [Google Scholar]
- 12.Emmenegger U, Man S, Shaked Y, et al. A comparative analysis of low-dose metronomic cyclophosphamide reveals absent or low-grade toxicity on tissues highly sensitive to the toxic effects of maximum tolerated dose regimens. Cancer Res. 2004;64:3994–4000. doi: 10.1158/0008-5472.CAN-04-0580. [DOI] [PubMed] [Google Scholar]
- 13.Shaked Y, Emmenegger U, Francia G, et al. Low-dose metronomic combined with intermittent bolus-dose cyclophosphamide is an effective long-term chemotherapy treatment strategy. Cancer Res. 2005;65:7045–7051. doi: 10.1158/0008-5472.CAN-05-0765. [DOI] [PubMed] [Google Scholar]
- 14.Bowers DC, Aquino VM, Leavey PJ, et al. Phase I study of oral cyclophosphamide and oral topotecan for children with recurrent or refractory solid tumors. Pediatr Blood Cancer. 2004;42:93–98. doi: 10.1002/pbc.10456. [DOI] [PubMed] [Google Scholar]
- 15.Kieran MW, Turner CD, Rubin JB, et al. A feasibility trial of antiangiogenic (metronomic) chemotherapy in pediatric patients with recurrent or progressive cancer. J Pediatr Hematol Oncol. 2005;27:573–581. doi: 10.1097/01.mph.0000183863.10792.d4. [DOI] [PubMed] [Google Scholar]
- 16.Brooke D, Davis RE, Bequette RJ. Chemical stability of cyclophosphamide in aromatic elixir USP. Am J Hosp Pharm. 1973;30:618–620. [PubMed] [Google Scholar]
- 17.Hassan M, Ljungman P, Ringden O, et al. The effect of busulphan on the pharmacokinetics of cyclophosphamide and its 4-hydroxy metabolite: time interval influence on therapeutic efficacy and therapy-related toxicity. Bone Marrow Transplant. 2000;25:915–924. doi: 10.1038/sj.bmt.1702377. [DOI] [PubMed] [Google Scholar]
- 18.Rustum AM, Hoffman NE. Determination of cyclophosphamide in whole blood and plasma by reversed-phase high-performance liquid chromatography. J Chromatogr. 1987;422:125–134. doi: 10.1016/0378-4347(87)80445-0. [DOI] [PubMed] [Google Scholar]
- 19.Bai F, Fraga CH, Tagen M, Schaiquevich P, Hagedorn N, Stewart CF. Simultaneous determination of cyclophosphamide and carboxyethylphosphoramide mustard in human plasma using online extraction and electrospray tandem mass spectrometry (HTLC-ESI-MS/MS) J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:1709–1715. doi: 10.1016/j.jchromb.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stempak D, Gammon J, Halton J, Moghrabi A, Koren G, Baruchel S. A pilot pharmacokinetic and antiangiogenic biomarker study of celecoxib and low-dose metronomic vinblastine or cyclophosphamide in pediatric recurrent solid tumors. J Pediatr Hematol Oncol. 2006;28:720–728. doi: 10.1097/01.mph.0000243657.64056.c3. [DOI] [PubMed] [Google Scholar]
- 21.Beijnen JH, van Gijn R, Challa EE, Kaijser GP, Underberg WJ. Chemical stability of two sterile, parenteral formulations of cyclophosphamide (Endoxan) after reconstitution and dilution in commonly used infusion fluids. J Parenter Sci Technol. 1992;46:111–116. [PubMed] [Google Scholar]
- 22.Menard C, Bourguignon C, Schlatter J, Vermerie N. Stability of cyclophosphamide and mesna admixtures in polyethylene infusion bags. Ann Pharmacother. 2003;37:1789–1792. doi: 10.1345/aph.1D200. [DOI] [PubMed] [Google Scholar]
- 23.Gilard V, Martino R, Malet-Martino MC, et al. Chemical and biological evaluation of hydrolysis products of cyclophosphamide. J Med Chem. 1994;37:3986–3993. doi: 10.1021/jm00049a018. [DOI] [PubMed] [Google Scholar]