Abstract
Purpose
Combination estrogen+progestin therapy has been associated with increased breast cancer risk in postmenopausal women. Selective estrogen receptor modulators (SERMs) are potential alternatives to progestins, although endometrial safety of estrogen+SERM co-therapies is not known. The goal of this study was to evaluate the endometrial profile of low-dose estradiol and the SERM tamoxifen alone and in combination.
Experimental design
Twenty-four postmenopausal female cynomolgus macaques were randomized by social group to receive placebo, low-dose micronized estradiol (E2, 0.25 mg/1800 kcal), the SERM tamoxifen (Tam, 20 mg/1800 kcal), or E2+Tam for 4 months in a parallel-arm design.
Results
Tamoxifen alone resulted in overlapping but distinct effects compared to E2. Both E2 and Tam increased uterine weight and endometrial thickness, while only E2 increased endometrial proliferation. Morphologic effects were similar for Tam and E2+Tam, which both induced stromal fibrosis and cystic change. Tamoxifen inhibited E2-induced proliferation and expression of genes related to cell cycle progression while exhibiting mixed agonist and antagonist effects on gene markers of estrogen receptor activity. The gene expression profile for E2+Tam was distinct from either E2 or Tam alone but dominated by the Tam effect for estrogen-regulated genes. Tam also attenuated E2 effects on both vaginal maturation and cervical epithelial height.
Conclusions
These findings characterize a novel phenotype resulting from estrogen+SERM co-therapy. The predominance of Tam effects on endometrial proliferation, morphology, and transcriptional profiles suggests that endometrial risks for E2+Tam may be similar to Tam alone.
Keywords: endometrium, tamoxifen, estradiol, estrogen receptor, SERM
Introduction
Estrogen exposure is an important risk factor for endometrial cancer (1, 2). Many key risk factors for endometrial cancer risk relate to lifetime exposure to endogenous estrogens (2), and long-term unopposed estrogen therapy (ET) results in markedly increased risk of endometrial hyperplasia and cancer in postmenopausal women (1). Progestogens protect the endometrium from adverse estrogen-induced effects (1) and for this reason are given with estrogen in combined postmenopausal hormone therapy (HT) regimens. Progestogens lack similar protective effects against breast cancer, however. Results from the Women’s Health Initiative randomized clinical trials (3, 4) and other observational studies (5) indicate that long-term use of estrogen+progestin therapy (EPT) results in a modest but significant increase in invasive breast cancer incidence among postmenopausal women, above that seen with ET. This evidence has generated interest in progestin alternatives that selectively block estrogen actions in both the endometrium and breast but not in other tissues such as bone, urogenital tract, and brain.
The most promising candidates for this application are selective estrogen receptor modulators (SERMs), which exhibit tissue-specific estrogen agonist and antagonist effects (6). Tamoxifen (Tam) is a first-generation SERM widely used in the treatment and prevention of estrogen-responsive breast cancer. Tam metabolites competitively bind estrogen receptors (ERs) and inhibit the growth-promoting activity of endogenous estrogens in the breast (6). In clinical trials, Tam treatment decreases the incidence of ER-positive breast cancer by 30–60% over 5+ years in women at high risk for the disease (7, 8). However, Tam is also associated with adverse side effects related to estrogen deficiency, most notably menopausal symptoms and urogenital atrophy (9–11), which negatively impact quality of life in breast cancer survivors and many other postmenopausal women. These observations have contributed to the idea that the combination of low-dose estrogen and Tam may provide a unique safety and therapeutic profile, particularly for women at high risk of breast cancer (12–15). Data from observational studies of Tam-treated women provide support for this idea, suggesting that Tam may reduce breast cancer risk even when given alongside HT (16, 17).
Tamoxifen and certain other SERMs elicit diverse and poorly understood effects in the human endometrium. Tam-associated changes include increased endometrial thickening, stromal fibrosis, cystic change, and polyp formation (18, 19). In addition, Tam increases incidence of endometrial carcinoma from ~1 to 2 cases per 1,000 women per year (7, 20, 21) and uterine sarcoma from 0.04 to 0.17 cases per 1,000 women per year (22). Tam also induces certain markers of ER activity in the endometrium (23, 24), suggesting that estrogen agonist activity may contribute to Tam-associated cancer risk (25). Endometrial profiles of Tam and other SERMs given with ET are not well-known, despite recent interest in these co-therapies (12–15, 26–31). The purpose of this study was to evaluate the endometrial phenotype of low-dose oral estradiol and Tam in the postmenopausal endometrium.
Materials and Methods
Study design and treatments
This study followed a parallel-arm design in which 24 ovariectomized female cynomolgus macaques (Macaca fascicularis) with a mean age of 14.7 ± 0.7 years were randomized to receive one of the following four treatments for 4 months: (1) placebo (control) (n = 6); (2) micronized 17β-estradiol (E2) (Estrace, Mylan Pharmaceuticals; Morgantown, WV) at a dose of 16.7 μg/kg body weight (0.25 mg/1800 kcal) (n = 6); (3) the SERM tamoxifen (Tam) (Nolvadex, AstraZeneca Pharmaceuticals LP; Wilmington, DE) at a dose of 1.3 mg/kg body weight (20 mg/1800 kcal) (n = 6); or (4) E2+Tam (n = 6). Dose equivalents approximated a low ET dose of oral E2 in postmenopausal women (32) (standard dose is 1.0 mg/day) and a standard maintenance dose of Tam following breast cancer diagnosis (7). In a previous study in this model, serum concentrations of 4-hydroxytamoxifen (one of the primary active metabolites of Tam) for the 20 mg/1800 kcal dose were 5 ± 1 ng/ml, similar to those reported in women (33).
Hormone treatments were administered in standard control diets with casein+lactalbumin as the protein source and macronutrient composition based on a typical North American human diet. Other than E2 and/or Tam treatments, group diets were the same in macronutrients, cholesterol, calcium, and phosphorus. Animals were fed 60 kcal/kg body weight (+10% extra to account for waste) twice daily. Daily E2 and Tam doses were scaled to 1800 kcal of diet (the estimated daily intake for a U.S. woman) to account for differences in metabolic rates between monkeys and human subjects. All animals were originally imported from the Institut Pertanian Bogor in Bogor, Indonesia, and housed in stable social groups of 3–4 animals each. All animals were considered multiparous based on historical data from the original breeding colony, in which >90% of the adult females have had 2+ live births, and on myometrial evidence of prior pregnancy (expansion of venous adventitia).
Macaques are anthropoid primates with a high overall genetic coding sequence identity to humans, including important genes related to cancer susceptibility (34). Prior work from our lab and others has shown similarities between macaque and human endometrial biology, including responses to exogenous estrogen and SERMs, sex steroid receptor expression, and the presence of hyperplastic lesions (35, 36).
All procedures involving these animals were conducted in compliance with State and Federal laws, standards of the U.S. Department of Health and Human Services, and guidelines established by the Wake Forest University Animal Care and Use Committee. The facilities and laboratory animal program of Wake Forest University are fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care.
Serum estradiol concentrations
To confirm dietary intake of treatments, serum E2 concentrations were measured in blood samples collected by femoral venipuncture. Estradiol concentrations were measured during each month of treatment by radioimmunoassay using a commercially available kit and protocol from Diagnostic Systems Laboratories (E2, DSL-4800 ultra-sensitive; Webster, TX). Assays were performed at the Yerkes National Primate Research Center Endocrinology Laboratory. Calibration standards ranged from 5 to 750 pg/ml.
Endometrial tissue collection and processing
At the end of the treatment period, animals were sedated with ketamine and euthanized using sodium pentobarbital (100 mg/kg, intravenous), as recommended by the Panel on Euthanasia of the American Veterinary Medical Association. Euthanasia was performed for data collection related to cardiovascular and brain endpoints, to be described elsewhere. Uteri were collected and weighed. Samples of endometrium were divided into two portions; the first part was snap-frozen in liquid nitrogen and stored at −70°C for gene expression analyses, while the remaining part was fixed for histology and immunohistochemistry. Fixed tissues were placed in fresh 4% paraformaldehyde solution at 4°C, transferred to 70% ethanol 24 hours later, and then sectioned transversely immediately proximal to the uterotubal junction. All fixed samples were paraffin-embedded, sectioned at 5 μm, and stained with hematoxylin and eosin (H&E) using standard histologic procedures.
Histomorphometry and histology
Endometrial thickness, stromal collagen, glandular area, cervical epithelial cell height, vaginal epithelial thickness, and vaginal keratin thickness were quantified by histomorphometric methods similar to those described previously (37). Briefly, H&E slides were digitized using a Labophot 3 light microscope (Nikon Instruments, Melville, NY) and Infinity 3 digital camera (Lumenera, Ottawa, ON), and measurements were taken with Image Pro-Plus software (Media Cybernetics, Bethesda, MD). For each measure six microscopic fields were randomly selected and examined at 200x magnification. Endometrial collagen content was determined from slides stained with Masson’s trichrome (containing Weigert’s iron hematoxylin, Crocein Scarlet MOO, 5% aqueous phosphomolybdic acid, and aniline blue) (Fisher Scientific, Atlanta, GA, and Sigma, St. Louis, MO); pale blue-stained areas (representing collagen and/or ground substance) were digitally measured using selective color-based analysis on Image Pro-Plus and values were averaged for each animal. Endometrial edema was quantified in a similar manner by digitally selecting and measuring clear or white areas within endometrial stroma. Cystic space was measured by manually tracing luminal area of endometrial glands. Epithelial area was determined by digital quantification of red positive staining for the glandular epithelial marker cytokeratin (CK) 18 (see below). Endometrial sections stained with H&E were evaluated for evidence of complex hyperplasia, neoplasia, and other histologic lesions by two board-certified veterinary pathologists (CEW, JMC). All histomorphometric measures were made blinded to treatment group.
Immunohistochemistry
Fixed endometrial sections were immunostained using commercially-available primary monoclonal antibodies for the proliferation marker Ki67 (Ki67/MIB1; Dako, Carpinteria, CA), the apoptosis marker cleaved caspase 3 (CC3) (Cell Signaling Technologies, Beverly, MA), the glandular epithelial marker cytokeratin 18 (CK18) (clone DC10; Lab Vision, Fremont, CA), and estrogen receptor-alpha (ESR1) (NCL-ER-6F11; Novocastra, Newcastle–upon–Tyne, UK). Antibodies were diluted 1:50 for Ki67 and CC3 and 1:100 for CK18 and ESR1 in 1X Automation Buffer (Biomeda, Foster City, CA) containing 0.5% casein (Sigma). Immunostaining procedures included antigen-retrieval with citrate buffer (pH 6.0), biotinylated rabbit anti-mouse Fc antibody as a linking reagent, alkaline phosphatase-conjugated streptavidin as the label, and Vector Red as the chromogen (Vector Laboratories, Burlingame, CA). Negative control slides were run for each immunostain using the same protocol as for study slides except with non-immune serum (from the same species as primary antibody) in place of the primary antibody. Nuclear cell labeling for Ki67 and ESR1 was quantified by a computer-assisted counting technique using a grid filter to select cells for counting and our modified procedure of cell selection, described previously (38). For endometrial glands and stroma, 200 cells were counted in both superficial and basal compartments. Immunolabeling counts were conducted blinded to experimental treatment and analyzed as a percentage of the total number of cells examined. Cytoplasmic CK18 labeling was quantified digitally as % positive area across 6 microscopic fields per slide and values were averaged for each individual.
Gene microarray analyses
Endometrial total RNA was extracted from frozen samples using Tri Reagent (Molecular Research Center, Cincinnati, OH), purified using RNeasy Mini kit (QIAGEN, Valencia, CA), and quantitated using a NanoDrop ND-1000 UV-vis spectrophotometer (NanoDrop, Wilmington, DE). Nucleic acid intactness and quality were confirmed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Wilmington, DE). Biotinylated cRNA samples were prepared according to the standard Enzo Bioarray™ protocol (Enzo Life Sciences, Farmingdale, NY) and hybridized using the standard Affymetrix (Santa Clara, CA) protocol for eukaryotic samples. Biotinylated cRNA from each sample was hybridized to Affymetrix GeneChip Rhesus Macaque Genome Arrays, washed and stained in an Affymetrix GeneChip Fluidics Station, and scanned with an Affymetrix GeneChip® Scanner 3000. Intensity data were extracted from scanned images and checked for quality using Affymetrix GeneChip Operating Software and Expression Console (MAS5 algorithm). Microarray assays were performed at Cogenics®, a Division of Clinical Data (Morrisville, NC). Microarray data are publicly available on the NCBI Gene Expression Omnibus database (accession number GSE14518).
Microarray data analyses were performed using the GeneSifter® software program (Geospiza, Seattle, WA). Intensity data were RMA-normalized, converted to a log2 scale, screened for heterogeneity among samples and groups, and evaluated using supervised analysis of variance (ANOVA) and pairwise comparisons between treatments. Principal components analysis (PCA), pattern navigation, cluster analysis, heatmapping, and KEGG pathway analyses were performed on filtered data subsets, as described in results. Differences in gene numbers altered by each treatment were compared using either Fisher’s Exact Test or Chi-Square Test. Euclidean distances (representing the numeric difference between treatment vectors) were calculated as part of hierarchical clustering dendrograms using average linkage. Pathways related to cell proliferation were evaluated using z-scores generated in KEGG analyses; a z-score > 2.0 was considered significant overrepresentation of genes in a particular pathway. All P values were corrected when possible for multiple comparisons using the Benjamini and Hochberg method (Padj) (39), which derives a false discovery rate estimate from the raw P values (40). Representation of differentially expressed genes within specific functional categories was evaluated using Ingenuity Pathway Analysis (IPA) software v6 (Ingenuity Systems, Redwood City, CA). Significance of gene numbers represented within a given category was determined in IPA using a Fisher’s Exact Test with Benjamini and Hochberg correction and expressed as -log10 (P value) for gene numbers within each treatment group.
Quantitative gene expression
Expression of genes associated with proliferation (MKI67, Ki67 antigen), matrix remodeling (OVOS2, ovostatin 2), and ER activity (ESR1, estrogen receptor; TFF1, trefoil factor 1, also known as pS2; STC2, stanniocalcin 2; IGFBP2, insulin-like binding protein 2; PGR, progesterone receptor; and CXCL12, chemokine (C-X-C motif) ligand 12, also known as SDF1) were measured in endometrial samples using quantitative real-time reverse transcriptase polymerase chain reaction (qRT-PCR). Macaque-specific qRT-PCR primer-probe sets for internal control genes (GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ACTB, beta-actin) were generated through the Applied Biosystems (ABI) Taqman Assay-by-Design service (Foster City, CA). Sources for target primer/probe sets are given in Supplementary Table 1. All probes spanned an exon-exon junction to eliminate genomic DNA contamination. qRT-PCR reactions (20 μl volume) were performed on an ABI Prism 7000 Sequence Detection System using standard Taqman reagents and thermocycling protocol (41). Relative expression was determined using the ΔΔCt method described in ABI User Bulletin #2 (available online). The Ct values for the control genes GAPDH and ACTB were averaged for use in internal calibration, while reference premenopausal breast tissue RNA was run in parallel for plate-to-plate calibration. Calculations were performed using ABI Relative Quantification SDS Software v1.1.
Statistical analysis
Data were analyzed using the SAS statistical package (version 8, SAS Institute; Cary, NC). All data were evaluated for normal distribution and homogeneity of variances among groups. A general linear model was used to determine mean values and calculate group differences for body weight, age, serum E2, uterine weight, endometrial morphometric measures, Ki67 immunolabeling, and qRT-PCR expression data. Immunolabeling data for ESR1 and CC3 was evaluated using a nonparametric Kruskal-Wallis test followed by two-sided Wilcoxon Rank Sum pairwise analysis. Gene expression data were log-transformed to improve distribution, and data were then retransformed to original scale and reported as fold-change of control with 90% confidence interval. All other data are reported as mean ± standard error. One animal randomized to the Tam group was excluded from all analyses based on repeated baseline serum E2 values >30 pg/ml, indicating ectopic and/or remnant ovarian tissue. Final group sizes were thus n = 6 for Con, E2, and E2+Tam and n = 5 for Tam for all endpoints. All pairwise P-values were adjusted for the number of pairwise tests using a Bonferroni correction. A two-tailed significance level of 0.05 was chosen for all comparisons.
Results
Treatment group characteristics
No treatment group differences were noted in age or body weight at baseline (P > 0.1 for both) (Supplementary Table 2). During 4 months of treatment, no significant differences in body weight or body weight changes among groups were noted. Serum E2 was higher in the E2 and E2+Tam groups compared to control in each month of treatment (P < 0.01 for all), while no significant differences were observed between E2 and E2+Tam groups.
Tamoxifen and estradiol effects on endometrial thickness and proliferation
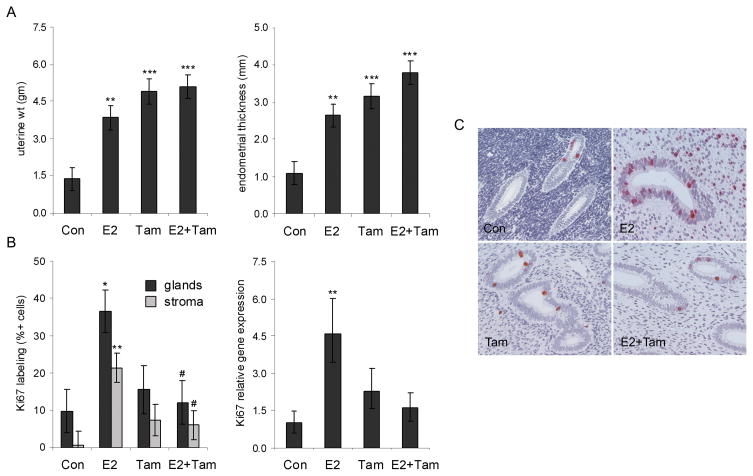
Uterine weight and endometrial thickness were at least two-fold higher in E2, Tam, and E2+Tam groups compared to placebo (P < 0.01 for all) (Figure 1A). Endometrial thickness was marginally higher in the E2+Tam group compared to E2 alone (P = 0.06). In contrast, endometrial proliferation, indicated by MKI67 gene expression and Ki67 immunolabeling within glandular and stromal compartments, was greater only in the E2 alone group (P < 0.05 for all) (Figure 1B–1C). The addition of Tam significantly abolished E2-induced stromal and epithelial Ki67 expression (P < 0.05 for both for E2+Tam compared to E2) (Figure 1B). No significant treatment effects were seen on endometrial apoptosis, measured by expression of the CC3 marker (data not shown). No neoplastic or complex/atypical hyperplastic lesions were noted on histology.
Fig. 1.
Tamoxifen (Tam) increases uterine weight and endometrial thickness while antagonizing estradiol (E2)-induced proliferation. A, Uterine weight and endometrial thickness were higher in all treatment groups. B-C, Endometrial proliferation, determined by immunolabeling and relative gene expression for the Ki67 marker, was greater only in the E2 group. The addition of Tam antagonized E2-induced proliferation (B). Images of Ki67 labeling (C) were taken at 200x magnification. Gene expression values were measured by qRT-PCR, corrected for internal control gene expression, and expressed relative to control group values. Vertical bars indicate 90% confidence intervals (gene expression) or standard errors (other measures). * P < 0.05, ** P < 0.01, and *** P < 0.001 compared to respective control group values; # P < 0.05 compared to E2 group.
Tamoxifen and estradiol effects on endometrial gene expression profiles
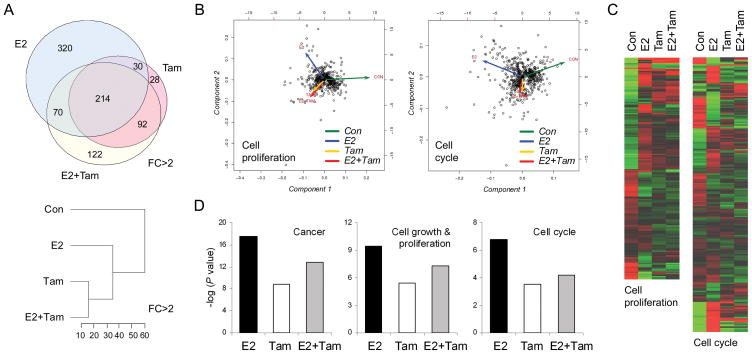
Gene microarrays were used to further investigate treatment effects on endometrial proliferation. Global expression profiles showed greater numbers of genes altered by E2 compared to Tam but greater percent overlap between Tam and E2+Tam. For example, among significantly altered (named) genes with FC>2 (ANOVA Padj < 0.05), E2 (n = 634) had 38% overlap with Tam (n = 364) and 45% overlap with E2+Tam (n = 498) while Tam had 67% overlap with E2 and 84% overlap with E2+Tam (Figure 2A). Supervised hierarchical clustering also indicated that Tam and E2+Tam were the most closely associated groups with a Euclidean distance of ~15 for genes significantly altered at FC>2 (Figure 2A). The number of genes significantly altered only in the E2 group was greater than that for Tam and E2+Tam groups at all FC values <5 (P < 0.001 by Chi-square test). This divergence of E2 from Tam and E2+Tam was evident qualitatively from PCA vectors and heatmaps for both overall altered genes and altered genes specifically related to cell proliferation and cell cycle (based on ontology classification) (Figure 2B, 2C). A similar pattern was seen when altered genes were sorted by functional category, which showed significant overrepresentation of genes (-log P-value > 1.2) related to cancer, cell cycle, and cell proliferation functions in all groups, with the greatest representation in the E2 group (Figure 2D).
Fig. 2.
Tamoxifen (Tam) and estradiol (E2) exhibit divergent effects on gene expression profiles in the endometrium related to cell proliferation and cell cycle. A, Venn diagram (upper) and hierarchical clustering dendrogram (lower) show greater percent overlap and tighter clustering between Tam and E2+Tam groups for all differentially expressed genes at fold-change (FC) >2. Dendrogram axis values represent Euclidean distances between groups. B, C, Principal component analyses (B) and heatmaps (C) of differentially regulated genes related to cell proliferation (n = 461) and cell cycle (n = 563) show a distinct pattern for E2 with close overlap of vectors for Tam and E2+Tam groups. D, Functional categories with significant overrepresentation of genes included cancer, cell growth and proliferation, and cell cycle. Treatment with E2 resulted in the largest number of differentially altered genes among these categories, while Tam and E2+Tam resulted in lesser number of genes represented. Diagrams in B and C correspond to significantly altered genes (ANOVA P < 0.05) within cell proliferation and cell cycle ontology categories. Diagrams in A and D correspond to the following gene filter: FC >2 in at least one group versus control, adjusted ANOVA P < 0.05, and quality > 2.
Complementary pathway analyses evaluated representation of altered genes at FC>2 in nine preselected KEGG pathways related to cell proliferation. Cell cycle was the only one of these pathways to have a significant z-score (Table 1A). Fourteen of the 18 cell cycle genes identified were upregulated in the E2 group; of these, E2 had a greater FC effect than Tam and E2+Tam in 13/14 (the exception being cyclin D1) (Table 1B). Three of the 4 downregulated genes were CDK inhibitors involved in negative regulation of cell cycle progression. Expanding the filter to genes significantly altered at FC>1.2 provided a cell cycle z-scores of 5.27 (53 genes represented out of 89 on array) on KEGG analysis and 7.75 on ontology analysis (308 genes represented out of 686 on array). Of the KEGG genes, 37/53 were upregulated in the E2 group and 34/37 of these had the greatest magnitude of change in the E2 group (P < 0.0001 compared to Tam and E2+Tam by Fisher’s exact test), indicating partial or full antagonism of Tam on E2 effects related to cell cycle gene expression. A complete list of all significantly altered genes related to cell cycle (KEGG and ontology) and proliferation (ontology) is provided in Supplementary Table 3A–3C.
Table 1.
| Table 1A. Representation of significantly altered genes in preselected pathways related to cell proliferation.a | |||
|---|---|---|---|
| KEGG Pathway | List | Array | z-score |
| Cell cycle | 18 | 89 | 3.90 |
| mTOR signaling pathway | 5 | 35 | 1.17 |
| TGF-beta signaling pathway | 5 | 46 | 0.52 |
| Jak-STAT signaling pathway | 10 | 103 | 0.36 |
| ErbB signaling pathway | 6 | 68 | 0.03 |
| Insulin signaling pathway | 9 | 105 | −0.06 |
| Wnt signaling pathway | 9 | 106 | −0.09 |
| VEGF signaling pathway | 4 | 49 | −0.14 |
| Notch signaling pathway | 2 | 34 | −0.59 |
| MAPK signaling pathway | 9 | 156 | −1.35 |
| Table 1B. Treatment effects on relative expression (fold-change vs control) of individual genes involved in cell cycle regulation on microarray analysis.b | ||||
|---|---|---|---|---|
| Gene ID | GenBank ID | E2 | Tam | E2+Tam |
| BUB1 (+/−) | AF043294 | 4.3 | 1.4 | 1.5 |
| BUB1B (+/−) | NM_001211 | 5.3 | 1.4 | 1.6 |
| CCNB1 (+) | BE407516 | 3.9 | 1.4 | 1.5 |
| CCNB2 (+) | NM_004701 | 5.1 | 1.5 | 1.8 |
| CCND1 (+) | BC000076 | 1.8 | 2.2 | 2.7 |
| CDC14B (+/−) | AK024886 | 0.5 | 0.6 | 0.5 |
| CDC2 (+) | NM_001786 | 3.9 | 1.1 | 1.1 |
| CDC20 (+) | NM_001255 | 2.0 | 1.0 | 1.2 |
| CDC6 (+) | NM_001254 | 3.8 | 1.2 | 1.3 |
| CDK6 (+) | AW274756 | 2.6 | 2.1 | 2.4 |
| CDKN1B (−) | BC001971 | 0.4 | 0.5 | 0.5 |
| CDKN1C (−) | R78668 | 0.4 | 0.5 | 0.5 |
| CDKN2B (−) | AW444761 | 0.3 | 0.4 | 0.5 |
| CHEK1 (−) | NM_001274 | 3.4 | 1.8 | 2.1 |
| MAD2L1 (+/−) | AF394735 | 2.2 | 1.4 | 1.2 |
| MCM4 (+) | AI936566 | 2.3 | 1.2 | 1.2 |
| PTTG1 (+) | NM_004219 | 3.5 | 1.1 | 1.2 |
| SKP2 (+) | BC001441 | 2.2 | 1.6 | 1.4 |
Microarray gene expression data were screened using a threshold fold-change (FC) > 2.0 (in at least one group), Benjamini and Hochberg-adjusted ANOVA P value < 0.05, and quality setting > 2. The resulting gene set (n = 2065) was then subjected to KEGG pathway analysis in the Genesifter software program.
E2, low-dose estradiol; Tam, tamoxifen; (+) and (−) indicate respective effects on cell cycle progression.
To examine genes altered specifically in the E2+Tam group, differentially expressed transcripts (ANOVA Padj < 0.05) were screened for a pattern of FC>2.0 in E2+Tam but not E2 or Tam groups. A total of 169 transcripts (with GenBank accession numbers) were identified (Supplementary Table 3D). Notable genes in this list included androgen receptor (AR) (↓ 2.0x), RAR-related orphan receptor B (RORB) (↓ 2.2x), V-erb-a erythroblastic leukemia viral oncogene homolog 4 (ERBB4) (↑ 2.6x), trefoil factor 3 (TFF3) (↑ 2.3x), tumor protein D52 (TPD52) (↑ 2.3x), myosin heavy chains 1 (MYH1) (↑ 3.0x) and 2 (MYH2) (↑ 7.1x), and breast carcinoma amplified sequence 1 (BCAS1) (↑ 2.2x).
Divergent effects of estradiol and tamoxifen on endometrial morphology
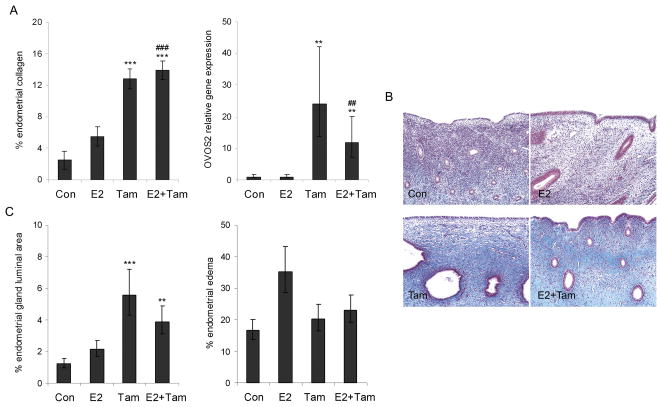
Endometrial morphometric measures were used to evaluate changes contributing to the increased uterine weight and endometrial thickness. In the Tam and E2+Tam groups these effects were due in part to greater endometrial fibrosis (P < 0.001 for both groups compared to control), which was evident on histology and confirmed using morphometry on sections stained with Masson’s trichrome for collagen (Figure 3A, 3B). This change has been noted previously in the human endometrium in response to Tam and other SERMs and may contribute to formation of polyps (18, 19).
Fig. 3.
Effects of tamoxifen (Tam) + estradiol (E2) on endometrial morphology are dominated by Tam. A, Increased endometrial fibrosis in Tam-treated groups corresponded with increased gene expression of the proteinase inhibitor ovostatin 2 (OVOS2). B, Representative images of superficial endometrium stained with Masson’s trichrome show increased stromal collagen (pale blue) in Tam and E2+Tam groups; images were taken at 100x magnification. C, Tam and E2+Tam groups showed greater luminal area within endometrial glands, indicative of cystic change noted on histology, while stromal edema was marginally higher only in the E2 group. Morphometric measures are expressed as percent total endometrial area measured. Vertical lines indicate 90% confidence intervals (gene expression) or standard errors (other measures). ** P < 0.01, and *** P < 0.001 compared to respective control group values; ## P < 0.01 and ### P < 0.001 compared to E2 group.
To explore gene expression changes related to the Tam effect on endometrial collagen we evaluated pathways involved in matrix remodeling. Among significantly altered genes with FC>2 in Tam and E2+Tam groups (but not E2) on microarray analysis, no significant ontology or KEGG pathways directly related to extracellular matrix or collagen remodeling were identified. Similarly, no effects specific to Tam and E2+Tam groups were observed for genes within related classes such as collagens, matrix metalloproteinases, fibrogenic cytokines, and tissue inhibitors of matrix metalloproteinases. However, markedly increased gene expression of the protease inhibitor ovostatin 2 (OVOS2) was noted in Tam (24x) and E2+Tam (12x) groups (P < 0.01 for both compared to control group) (Figure 3A). While the exact role of ovostatin 2 is undetermined, the highly similar ovostatin 1 protein is a potent inhibitor of MMPs, including collagenase (42). An incidental but potentially related change noted on histology was distinctive thickening of glandular basement membranes in Tam and E2+Tam groups.
A second contributing factor to increased endometrial thickness in Tam and E2+Tam groups was cystic dilation of glands, indicated by greater glandular luminal area (P < 0.01 for both compared to control group) (Figure 3C). The addition of E2 to Tam had modest if any abrogating effects on fibrosis or cystic changes. In contrast to Tam, E2 effects on endometrial thickness were due largely to superficial stromal edema, which was marginally higher in the E2 but not Tam or E2+Tam groups (ANOVA P = 0.06) (Figure 3C). Glandular epithelium measured by CK18 expression was higher in E2, Tam, and E2+Tam groups for deep but not superficial endometrial glands but did not contribute substantially to overall endometrial thickness, occupying <3% of the sectional area (data not shown).
Estrogen agonist and antagonist effects of tamoxifen on endometrium
Nuclear expression of ESR1 protein was detected within endometrial glands and stroma. No significant treatment effects were seen for ESR1 immunolabeling (Supplementary Figure 1A) or gene expression (data not shown). Two major patterns of expression were seen for gene markers of ER activity. In the first pattern, E2-induced genes such as TFF1, STC2, and IGFBP2 were higher in all treatment groups (P < 0.01 for all compared to control) (Supplementary Figure 1B), consistent with an ER agonist effect of Tam. Other ER-driven genes such as PGR and CXCL12 showed a similar pattern, although these changes were not significant on qRT-PCR (ANOVA P > 0.05) (data not shown). In the second pattern, E2-induced genes were higher only in the E2 group, with full or partial antagonism of E2 by Tam (Table 2). As with MKI67 and many of the cell cycle targets (Table 1B), E2-induced gene expression for E2+Tam appeared to be dominated by Tam rather than E2.
Table 2.
Treatment effects on relative expression (fold-change vs control) of selected estrogen-induced genes on microarray analysis.a,b
| Gene ID | GenBank ID | E2 | Tam | E2+Tam |
|---|---|---|---|---|
| Tamoxifen agonist pattern | ||||
| STC2 | BC000658 | 2.9 | 2.1 | 2.4 |
| IGFBP2 | NM_000597 | 2.9 | 2.8 | 3.4 |
| CDH1 | L08599 | 2.2 | 2.5 | 2.0 |
| EGFR | S75916 | 3.0 | 2.3 | 2.4 |
| MUC1 | AI610869 | 2.0 | 2.8 | 3.6 |
| Tamoxifen antagonist pattern | ||||
| MKI67 | AU132185 | 2.6 | 1.0 | 1.1 |
| PCNA | NM_002592 | 1.4 | 1.0 | 1.0 |
| TOP2A | AL561834 | 5.3 | 1.5 | 1.8 |
| PTEN | U96180 | 1.6 | 1.3 | 1.1 |
| PTTG1 | NM_004219 | 3.5 | 1.1 | 1.2 |
Microarray gene expression data were screened using a threshold fold-change (FC) > 2.0 (in at least one group), Benjamini and Hochberg-adjusted ANOVA P value < 0.05, and quality setting > 2. Known estrogen-induced genes shown were selected from the resulting gene set.
E2, low-dose estradiol; Tam, tamoxifen.
Antagonism of estradiol effects by tamoxifen in the genital tract
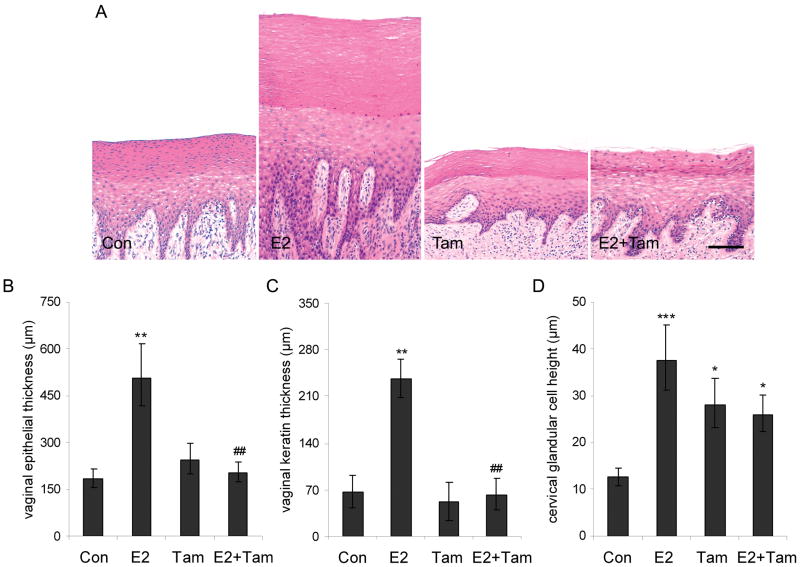
Treatment with E2 resulted in ~3-fold greater vaginal epithelial thickness (Figure 4A, 4B), vaginal keratin thickness (Figure 4C), and cervical gland height (Figure 4D) (P < 0.01 for all compared to control). Tam had no effects on any of these measures when given alone, completely antagonized E2 effects on vaginal maturation, and partially antagonized E2 effects on cervical epithelial height (Figure 4A–4D).
Fig. 4.
Tamoxifen (Tam) antagonizes estradiol (E2) effects on lower reproductive tract measures. A, Representative images of vaginal epithelium show increased overall epithelial maturation and keratinization (superficial laminar zone) following E2 and antagonism of this effect by Tam. Images were taken at 100x magnification; H&E stain. B-D, Tam fully antagonized E2 effects on overall epithelial thickness (B) and keratin thickness (C) and partially antagonized stimulatory E2 effects on cervical glandular height (D). Vertical lines indicate standard errors. * P < 0.05, ** P < 0.01, and *** P < 0.001 compared to respective control group values; ## P < 0.01 compared to E2 group.
Discussion
Estrogen+SERM co-therapy is an emerging alternative to traditional EPT, especially for postmenopausal women concerned about the promotional effects of progestins on breast cancer. Endometrial safety of estrogen+SERM combinations is not known, however. The primary goal of this study was to evaluate the endometrial profile of low-dose E2 and Tam alone and in combination. Our findings reveal an endometrial phenotype for E2+Tam characterized by increased endometrial thickness, stromal fibrosis, and cystic change with reduced epithelial and stromal proliferation compared to E2 alone. A divergent effect of Tam was observed for specific ER activity markers and endometrial proliferation, suggesting that Tam effects on endometrial cancer risk may not relate exclusively to partial estrogen agonist activity. Despite clear effects of low-dose E2 alone, the profile for E2+Tam based on endometrial morphology, proliferation, and transcriptional profile was dominated by Tam, suggesting that long-term risks associated with this combination may be similar to those seen with Tam alone.
Previous studies evaluating ET or EPT alongside Tam are limited. In a recent small clinical trial, Tam at a low dose of 5 mg/day increased endometrial thickness but not Ki67 expression when given with EPT; no significant increases in vasomotor symptoms were noted compared to EPT alone (14). In a second larger trial, Tam at 20 mg/day tended to attenuate ET/EPT effects on vasomotor symptoms in women at increased risk of breast cancer (15). A more recent study also found no benefit on vasomotor symptoms from adding ET or EPT to Tam at 20 mg/day (43). Findings from the current study indicate that Tam may also inhibit beneficial estrogen effects on urogenital atrophy. Collectively, these data indicate that Tam may override two of the primary indications for ET and EPT and is thus not a suitable SERM co-therapy for estrogen.
Other SERMs investigated as estrogen co-therapies include raloxifene and bazedoxifene. A small clinical trial reported that raloxifene at 60 mg/day given with oral E2 at 1 mg/day improved menopausal symptoms but increased endometrial thickness compared to baseline and treatment with raloxifene alone (28). Similar findings were noted in an earlier pilot study using transdermal estradiol (27). One additional study examining vaginal atrophy found no adverse attenuating effects of raloxifene on the efficacy of an estradiol-releasing vaginal ring in postmenopausal women (26). Preclinical data indicate that the third-generation SERM bazedoxifene may antagonize estrogen effects on both mammary gland and uterine measures while maintaining estrogen effects on vaginal maturation (29–31). These latter effects include dose-dependent attenuation of estrogen-induced proliferation of ER-positive MCF-7 breast cancer cells in culture (29) and uterine weight gain in mice (30–31).
Endometrial safety is an important concern in the development and long-term use of SERMs. Preclinical and clinical evaluation of endometrial SERM effects is often based on markers of estrogen agonist activity. Previous studies have shown that Tam induces estrogen-responsive markers in the endometrium (23, 24) and increases endometrial thickness and cancer risk in postmenopausal women (7, 20, 21), supporting the idea that carcinogenic effects of Tam are due to ER agonist signaling. In this study Tam induced a subset of ER-driven genes in the endometrium while antagonizing others, indicating that Tam is not simply acting as a weak ER agonist relative to E2 but instead exhibiting more complex mixed patterns of ER transactivation. Given the heterogeneity of tissue samples used for gene expression analyses, it is possible that Tam estrogen agonist/antagonist effects may even differ within specific compartments (e.g. stroma and epithelium) of the same tissue. Of note, Tam has also been shown to induce certain estrogen-responsive markers in normal mammary gland (44) and breast cancer cells (45) despite having well-documented ER-antagonist effects. This information suggests that individual estrogen response markers are not necessarily the best predictors of SERM effects or risk profile.
Increased proliferation is associated with the development of many cancers and provides a useful biomarker of potential cancer-promoting effects. In the uterus, epithelial cell proliferation serves as an important prognostic marker in human endometrial cancers (46) and may help predict risk associated with different hormone therapies (47). In this study Tam decreased E2-induced Ki67 labeling and inhibited or partially inhibited expression of numerous E2-induced genes related to proliferation. Many of the proliferation-related genes with this pattern directly involve cell cycle progression. These findings, in combination with phenotypic features dominated by Tam, suggest that E2+Tam may be associated with cancer risk estimates more similar to Tam than E2 alone.
Numerous studies have shown that standard doses of unopposed ET (for oral E2, 1 or 2 mg/d) increases endometrial hyperplasia and cancer risk in postmenopausal women (1, 2). Endometrial effects of newer low-dose ETs are less clear, though. A small clinical trial previously reported increased endometrial thickness followingoral E2 doses of 0.5 and 1.0 mg/d but not 0.25 mg/d (32), while a separate trial using oral conjugated equine estrogens noted a dose-related increase in endometrial hyperplasia incidence from 3% for 0.3 mg/d to 27% for the standard 0.625 mg/d dose after 2 years (48). While no neoplastic or complex hyperplastic lesions were observed in the current study, our results suggest that lower doses of oral E2 (≤0.5 mg/d) may still exert a clear stimulatory effect, increasing endometrial weight, thickness, glandular area, and proliferation. It is also worth noting that the peak serum E2 concentrations in the current study (40–80 pg/ml) were comparable to steady-state concentrations reported in postmenopausal women receiving E2 via vaginal ring at 150 μg/d (49) or transdermal patch at 50 μg/d (50). Serum E2 concentrations (and pharmacodynamics) vary widely for different E2 formulations and routes of administration, however, and it is unclear whether Tam or other SERMs may exert similar effects to those seen in the present study when given alongside lower doses of parenteral E2. Dose-dependent endometrial effects may also be seen with Tam (19), and additional studies are needed to determine whether lower Tam doses than those used here (<20 mg/d human equivalent) would similarly influence E2 effects.
The ideal postmenopausal HT would provide estrogen agonist effects in tissues such as bone and urogenital tract while minimizing risk of breast and endometrial cancer. Estrogen+SERM combinations have been proposed recently as a potential way to achieve this profile. Data from this study provide an initial step in profiling uterine effects of these therapies. Our results demonstrate that a SERM given at a standard dose may dominate the estrogen phenotype in the endometrium, at least for lower doses of oral E2. In the case of Tam, this profile may still be associated with adverse long-term effects, including cancer risk. Nevertheless, the dominance of the SERM signature suggests that other SERMs with more favorable profiles in the endometrium and elsewhere may be used as progestin alternatives in future postmenopausal therapies. Further studies directly comparing different SERM-estrogen combinations are needed to identify the safest SERM for this purpose.
Supplementary Material
Tamoxifen (Tam) and estradiol (E2) induce specific gene markers of estrogen receptor activity in the endometrium. A, Abundant ESR1 immunolabeling was present in endometrial glandular epithelium and stromal cells. No treatment effects were noted. B, E2 and Tam, alone and in combination, resulted in greater expression of the estrogen-induced genes TFF1, STC2, and IGFBP2. Gene expression values were corrected for internal control gene expression and expressed relative to control group values (fold-change). Vertical lines indicate 90% confidence intervals. * P < 0.05, ** P < 0.01, and *** P < 0.001 compared to respective control values.
Acknowledgments
Sponsored by the National Institutes of Health (K01 RR 021322-04 and R01 HL 49085).
The authors thank Lisa O’Donnell, Joseph Finley, Hermina Borgerink, Jean Gardin, Diana Swaim, Dewayne Cairnes, Jamie Fox, and Brian McCollough for their technical contributions. This work was supported by grants from the National Institutes of Health (NIH) National Center for Research Resources (NCRR) (K01 RR 021322-04) and National Heart, Lung, and Blood Institute (NHLBI) (R01 HL 49085). The contents are solely the responsibility of the authors and do not necessarily represent the view of the NCRR, NHLBI, or NIH.
Footnotes
Translational Relevance
The addition of a progestin to estrogen therapy has been associated with increased breast cancer risk in postmenopausal women. Recently, selective estrogen receptor modulators (SERMs) have been proposed as progestin alternatives. Endometrial safety is a major concern, however, for both SERM and estrogen therapies. In this preclinical study we investigate for the first time the endometrial profile of an estrogen+SERM co-therapy. Our findings demonstrate a dominant effect of the SERM tamoxifen (Tam) over oral estradiol (E2) on measures of endometrial morphology, proliferation, and transcriptional profiles, suggesting that long-term risks associated with E2+Tam may be similar to those seen with Tam alone rather than E2 alone. This information should be useful for postmenopausal women who are taking or considering estrogen+progestin therapy for menopausal symptoms and for future trials of estrogen+SERM co-therapies.
References
- 1.Weiderpass E, Adami HO, Baron JA, et al. Risk of endometrial cancer following estrogen replacement with and without progestins. J Natl Cancer Inst. 1999;91:1131–7. doi: 10.1093/jnci/91.13.1131. [DOI] [PubMed] [Google Scholar]
- 2.Purdie DM, Green AC. Epidemiology of endometrial cancer. Best Pract Res Clin Obstet Gynaecol. 2001;15:341–54. doi: 10.1053/beog.2000.0180. [DOI] [PubMed] [Google Scholar]
- 3.Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative Randomized Trial. JAMA. 2003;289:3243–53. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 4.Stefanick ML, Anderson GL, Margolis KL, et al. Effects of conjugated equine estrogens on breast cancer and mammography screening in postmenopausal women with hysterectomy. JAMA. 2006;295:1647–57. doi: 10.1001/jama.295.14.1647. [DOI] [PubMed] [Google Scholar]
- 5.Schairer C, Lubin J, Troisi R, Sturgeon S, Brinton L, Hoover R. Estrogen-progestin replacement and risk of breast cancer. JAMA. 2000;284:691–4. [PubMed] [Google Scholar]
- 6.Shiau AK, Barstad D, Loria PM, et al. The Structural Basis of Estrogen Receptor/Coactivator Recognition and the Antagonism of this Interaction by Tamoxifen. Cell. 1998;95:927–37. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- 7.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 8.Cuzick J, Powles T, Veronesi U, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361:296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- 9.Mortimer JE, Boucher L, Baty J, Knapp DL, Ryan E, Rowland JH. Effect of tamoxifen on sexual functioning in patients with breast cancer. J Clin Oncol. 1999;17:1488–92. doi: 10.1200/JCO.1999.17.5.1488. [DOI] [PubMed] [Google Scholar]
- 10.Day R, Ganz PA, Costantino JP, Cronin WM, Wickerham DL, Fisher B. Health-related quality of life and tamoxifen in breast cancer prevention: a report from the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Clin Oncol. 1999;17:2659–69. doi: 10.1200/JCO.1999.17.9.2659. [DOI] [PubMed] [Google Scholar]
- 11.Land SR, Wickerham DL, Costantino JP, et al. Patient-reported symptoms and quality of life during treatment with tamoxifen or raloxifene for breast cancer prevention: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2742–51. doi: 10.1001/jama.295.23.joc60075. [DOI] [PubMed] [Google Scholar]
- 12.Fabian CJ. Low-dose tamoxifen for combination hormone replacement therapy users. J Clin Oncol. 2007;25:4162–4. doi: 10.1200/JCO.2007.11.9743. [DOI] [PubMed] [Google Scholar]
- 13.Decensi A, Galli A, Veronesi U. HRT opposed to low-dose tamoxifen (HOT study): rationale and design. Recent Results Cancer Res. 2003;163:104–11. doi: 10.1007/978-3-642-55647-0_10. [DOI] [PubMed] [Google Scholar]
- 14.Decensi A, Gandini S, Serrano D, et al. Randomized dose-ranging trial of tamoxifen at low doses in hormone replacement therapy users. J Clin Oncol. 2007;25:4201–9. doi: 10.1200/JCO.2006.09.4318. [DOI] [PubMed] [Google Scholar]
- 15.Sestak I, Kealy R, Edwards R, Forbes J, Cuzick J. Influence of hormone replacement therapy on tamoxifen-induced vasomotor symptoms. J Clin Oncol. 2006;24:3991–6. doi: 10.1200/JCO.2005.04.3745. [DOI] [PubMed] [Google Scholar]
- 16.Veronesi U, Maisonneuve P, Rotmensz N, et al. Italian randomized trial among women with hysterectomy: Tamoxifen and hormone-dependent breast cancer in high-risk women. J Natl Cancer Inst. 2003;95:160–5. doi: 10.1093/jnci/95.2.160. [DOI] [PubMed] [Google Scholar]
- 17.Powles TJ, Ashley S, Tidy A, Smith IE, Dowsett M. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst. 2007;99:283–90. doi: 10.1093/jnci/djk050. [DOI] [PubMed] [Google Scholar]
- 18.Deligdisch L, Kalir T, Cohen CJ, de Latour M, Le Bouedec G, Penault-Llorca F. Endometrial histopathology in 700 patients treated with tamoxifen for breast cancer. Gynecol Oncol. 2000;78:181–6. doi: 10.1006/gyno.2000.5859. [DOI] [PubMed] [Google Scholar]
- 19.Cohen I. Endometrial pathologies associated with postmenopausal tamoxifen treatment. Gynecol Oncol. 2004;94:256–66. doi: 10.1016/j.ygyno.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 20.Fisher B, Costantino JP, Redmond CK, Fisher ER, Wickerham DL, Cronin WM. Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst. 1994;86:527–37. doi: 10.1093/jnci/86.7.527. [DOI] [PubMed] [Google Scholar]
- 21.Bernstein L, Deapen D, Cerhan JR, et al. Tamoxifen therapy for breast cancer and endometrial cancer risk. J Natl Cancer Inst. 1999;91:1654–62. doi: 10.1093/jnci/91.19.1654. [DOI] [PubMed] [Google Scholar]
- 22.Wickerham DL, Fisher B, Wolmark N, et al. Association of tamoxifen and uterine sarcoma. J Clin Oncol. 2002;20:2758–60. doi: 10.1200/JCO.2002.20.11.2758. [DOI] [PubMed] [Google Scholar]
- 23.Satyaswaroop PG, Zaino RJ, Mortel R. Estrogen-like effects of tamoxifen on human endometrial carcinoma transplanted into nude mice. Cancer Res. 1984;44:4006–10. [PubMed] [Google Scholar]
- 24.Mourits MJ, Ten Hoor KA, van der Zee AG, Willemse PH, de Vries EG, Hollema H. The effects of tamoxifen on proliferation and steroid receptor expression in postmenopausal endometrium. J Clin Pathol. 2002;55:514–9. doi: 10.1136/jcp.55.7.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jordan VC, Gottardis MM, Satyaswaroop PG. Tamoxifen-stimulated growth of human endometrial carcinoma. Ann N Y Acad Sci. 1991;622:439–46. doi: 10.1111/j.1749-6632.1991.tb37886.x. [DOI] [PubMed] [Google Scholar]
- 26.Pinkerton JV, Shifren JL, La Valleur J, Rosen A, Roesinger M, Siddhanti S. Influence of raloxifene on the efficacy of an estradiol-releasing ring for treating vaginal atrophy in postmenopausal women. Menopause. 2003;10:45–52. doi: 10.1097/00042192-200310010-00008. [DOI] [PubMed] [Google Scholar]
- 27.Davis SR, O’Neill SM, Eden J, et al. Transition from estrogen therapy to raloxifene in postmenopausal women: effects on treatment satisfaction and the endometrium-a pilot study. Menopause. 2004;11:167–75. doi: 10.1097/01.gme.0000087981.28957.cf. [DOI] [PubMed] [Google Scholar]
- 28.Stovall DW, Utian WH, Gass ML, et al. The effects of combined raloxifene and oral estrogen on vasomotor symptoms and endometrial safety. Menopause. 2007;14:510–7. doi: 10.1097/GME.0b013e318031a83d. [DOI] [PubMed] [Google Scholar]
- 29.Komm BS, Kharode YP, Bodine PV, Harris HA, Miller CP, Lyttle CR. Bazedoxifene acetate: a selective estrogen receptor modulator with improved selectivity. Endocrinology. 2005;146:3999–4008. doi: 10.1210/en.2005-0030. [DOI] [PubMed] [Google Scholar]
- 30.Kharode Y, Bodine PV, Miller CP, Lyttle CR, Komm BS. The pairing of a selective estrogen receptor modulator, bazedoxifene, with conjugated estrogens as a new paradigm for the treatment of menopausal symptoms and osteoporosis prevention. Endocrinology. 2008;149:6084–91. doi: 10.1210/en.2008-0817. [DOI] [PubMed] [Google Scholar]
- 31.Crabtree JS, Peano BJ, Zhang X, Komm BS, Winneker RC, Harris HA. Activity of three selective estrogen receptor modulators on hormone-dependent responses in the mouse uterus and mammary gland. Mol Cell Endocrinol. 2008;287:40–6. doi: 10.1016/j.mce.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 32.Prestwood KM, Kenny AM, Kleppinger A, Kulldorff M. Ultralow-dose micronized 17beta-estradiol and bone density and bone metabolism in older women: a randomized controlled trial. JAMA. 2003;290:1042–8. doi: 10.1001/jama.290.8.1042. [DOI] [PubMed] [Google Scholar]
- 33.Williams JK, Wagner JD, Li Z, Golden DL, Adams MR. Tamoxifen inhibits arterial accumulation of LDL degradation products and progression of coronary artery atherosclerosis in monkeys. Arterioscler Thromb Vasc Biol. 1997;17:403–8. doi: 10.1161/01.atv.17.2.403. [DOI] [PubMed] [Google Scholar]
- 34.Pavlicek A, Noskov VN, Kouprina N, Barrett JC, Jurka J, Larionov V. Evolution of the tumor suppressor BRCA1 locus in primates: implications for cancer predisposition. Hum Mol Genet. 2004;13:2737–51. doi: 10.1093/hmg/ddh301. [DOI] [PubMed] [Google Scholar]
- 35.Cline JM, Soderqvist G, Register TC, Williams JK, Adams MR, Von Schoultz B. Assessment of hormonally active agents in the reproductive tract of female nonhuman primates. Toxicol Pathol. 2001;29:84–90. doi: 10.1080/019262301301418883. [DOI] [PubMed] [Google Scholar]
- 36.Van Esch E, Cline JM, Buse E, Weinbauer G. The macaque endometrium, with special reference to the cynomolgus monkey (Macaca fascicularis) Toxicol Pathol. 2008;36:67S–100S. [Google Scholar]
- 37.Wood CE, Register TC, Anthony MS, Kock ND, Cline JM. Breast and uterine effects of soy isoflavones and conjugated equine estrogens in postmenopausal female monkeys. J Clin Endocrinol Metab. 2004;89:3462–8. doi: 10.1210/jc.2003-032067. [DOI] [PubMed] [Google Scholar]
- 38.Cline JM. Assessing the mammary gland of nonhuman primates: effects of endogenous hormones and exogenous hormonal agents and growth factors. Birth Defects Res B Dev Reprod Toxicol. 2007;80:126–46. doi: 10.1002/bdrb.20112. [DOI] [PubMed] [Google Scholar]
- 39.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc Ser B M1. 1995;57:289–300. [Google Scholar]
- 40.Reiner A, Yekutieli D, Benjamini Y. Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics. 2003;19:368–75. doi: 10.1093/bioinformatics/btf877. [DOI] [PubMed] [Google Scholar]
- 41.Wood CE, Register TC, Franke AA, Anthony MS, Cline JM. Dietary soy isoflavones inhibit estrogen effects in the postmenopausal breast. Cancer Res. 2006;66:1241–9. doi: 10.1158/0008-5472.CAN-05-2067. [DOI] [PubMed] [Google Scholar]
- 42.Nagase H, Harris ED., Jr Ovostatin: a novel proteinase inhibitor from chicken egg white. II. Mechanism of inhibition studied with collagenase and thermolysin. J Biol Chem. 1983;258:7490–98. [PubMed] [Google Scholar]
- 43.Osborne CR, Duncan A, Sedlacek S, et al. The addition of hormone therapy to tamoxifen does not prevent hot flashes in women at high risk for developing breast cancer. Breast Cancer Res Treat. 2009;116:521–7. doi: 10.1007/s10549-008-0284-y. [DOI] [PubMed] [Google Scholar]
- 44.Isaksson E, Wang H, Sahlin L, von Schoultz B, Cline JM, von Schoultz E. Effects of long-term HRT and tamoxifen on the expression of progesterone receptors A and B in breast tissue from surgically postmenopausal cynomolgus macaques. Breast Cancer Res Treat. 2003;79:233–9. doi: 10.1023/a:1023925906199. [DOI] [PubMed] [Google Scholar]
- 45.May FE, Westley BR. Expression of human intestinal trefoil factor in malignant cells and its regulation by oestrogen in breast cancer cells. J Pathol. 1997;182:404–13. doi: 10.1002/(SICI)1096-9896(199708)182:4<404::AID-PATH875>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 46.Salvesen HB, Iversen OE, Akslen LA. Identification of high-risk patients by assessment of nuclear Ki-67 expression in a prospective study of endometrial carcinomas. Clin Cancer Res. 1998;4:2779–85. [PubMed] [Google Scholar]
- 47.Dowsett M, Howell R, Salter J, Thomas NM, Thomas EJ. Effects of the pure anti-oestrogen ICI 182780 on oestrogen receptors, progesterone receptors and Ki67 antigen in human endometrium in vivo. Hum Reprod. 1995;10:262–7. doi: 10.1093/oxfordjournals.humrep.a135926. [DOI] [PubMed] [Google Scholar]
- 48.Pickar JH, Yeh IT, Wheeler JE, Cunnane MF, Speroff L. Endometrial effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate: two-year substudy results. Fertil Steril. 2003;80:1234–40. doi: 10.1016/s0015-0282(03)01167-1. [DOI] [PubMed] [Google Scholar]
- 49.Maruo T, Mishell DR, Ben-Chetrit A, Hochner-Celnikier D, Hamada AL, Nash HA. Vaginal rings delivering progesterone and estradiol may be a new method of hormone replacement therapy. Fertil Steril. 2002;78:1010–6. doi: 10.1016/s0015-0282(02)03365-4. [DOI] [PubMed] [Google Scholar]
- 50.Stanosz S, Zochowska E, Safranow K, Sieja K, Stanosz M. Influence of modified transdermal hormone replacement therapy on the concentrations of hormones, growth factors, and bone mineral density in women with osteopenia. Metabolism. 2009;58:1–7. doi: 10.1016/j.metabol.2008.07.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tamoxifen (Tam) and estradiol (E2) induce specific gene markers of estrogen receptor activity in the endometrium. A, Abundant ESR1 immunolabeling was present in endometrial glandular epithelium and stromal cells. No treatment effects were noted. B, E2 and Tam, alone and in combination, resulted in greater expression of the estrogen-induced genes TFF1, STC2, and IGFBP2. Gene expression values were corrected for internal control gene expression and expressed relative to control group values (fold-change). Vertical lines indicate 90% confidence intervals. * P < 0.05, ** P < 0.01, and *** P < 0.001 compared to respective control values.