Abstract
Background
Currently platelet concentrates (PC) are collected using different synthetic materials and different centrifugation/leucocyte-removal processes. Upon exposure to artificial surfaces and high centrifugation forces, blood cells can undergo various levels of stress-induced, cellular activation/fragmentation and release reactions which may not only influence the extent of the platelet storage lesion but may also contribute to poor clinical effectiveness of the PC and transfusion reactions.
Materials and methods.
An array of assays, used for quality control of PC, was performed in two different groups of PC prepared from random donor plasma on days 1, 3 and 5 of storage. The group 1 PC were not leucoreduced while the group 2 PC underwent prestorage leucoreduction using a PL50E filter. As current recommendations for the evaluation of PC include the measurement of platelet activation, in this study CD62P on platelet membrane was measured. Furthermore, in vitro studies indicate that sHLA antigens may modulate immune competent cell function so, the presence of sHLA-1 in blood components is considered a marker of immunological reactivity and this, too, was measured.
Results
The levels of CD62P and sHLA-1 were significantly lower in leucoreduced PC than in non-leucoreduced ones. However, the overall rate of increase of sHLA-1 during storage was faster in the leucoreduced group of PC. No significant differences were detected regarding other assays of quality.
Conclusion
Based on our findings, leucoreduced PC differ from non-leucoreduced ones in terms of some specific markers such as CD62P as a marker of platelet activation and sHLA-1 as a marker of immunological reactivity. Pre-storage leucofiltration, followed by storage in currently used plastic bags is a safe procedure for PC for up to 5 days. The available leucoreduction technologies are not, however, sufficiently robust to completely abrogate transfusions reactions, and improvements are required to reach the goal of optimised yield and minimal transfusion reactions with platelet therapy.
Keywords: platelet concentrate, platelet storage lesion, CD62P, sHLA-1
Introduction
Platelet concentrates (PC) are widely used to support patients who receive intensive therapies for haematological malignancies and solid tumours1. Platelets in PC undergo a number of events during collection, processing, and storage that adversely affect their structure and function, resulting in reduced post-transfusion recovery2. The overall changes that platelets undergo following collection, processing and storage prior to transfusion are defined as the platelet storage lesion. This lesion results in loss of integrity of platelet function, changes in aggregation and release, re-arrangement of the platelet cytoskeleton, exposure of phosphatidyl serine on the outer membrane surface and microvesiculation. Some of these changes are reversible, others not3.
Currently, PC are collected using different synthetic materials and different centrifugation/leucocyte-removal processes and stored in different types of bags. Upon exposure to artificial surfaces and high centrifugation forces, blood cells can undergo various levels of stress-induced, cellular activation/fragmentation and release reactions which may not only influence the extent of the platelet storage lesion but may also contribute to poor clinical effectiveness of the PC and transfusion reactions4,5.
The use of leucoreduced PC has been associated with a number of clinical advantages, such as decreases in HLA alloimmunisation, transmission of infectious diseases, and immunomodulation6. Three main types of synthetic materials are currently in use to produce white blood cell (WBC)-reduction filters for PC: negatively charged polyester, positively charged polyester, and non-charged polyurethane. These filters also have different structures: non-woven mesh for polyester filters and multiple-layer sponge for polyurethane filters5.
No single laboratory test on stored PC correlates with the in vivo haemostatic function of platelets, but it has been recognised that a battery of in vitro tests, which examine different aspects of platelet physiology, provide some indication of platelet quality4,7,8. In addition to platelet count and swirling, in vitro assays commonly used to assess platelet quality are metabolic tests, such as pH, pO2, pCO2, rate of lactate production, and oxygen consumption rate. Correlations of the results of the various assays with in vivo platelet recovery and survival have been reviewed by Murphy and collaborators7.
The proportion of activated platelets has also been suggested to be a predictor of platelet quality. There has been considerable interest in the potential use of platelet surface P-selectin as a marker for the detection of circulating degranulated platelets in clinical settings (e.g., acute coronary artery syndromes, transfusion of PC)9. The expression of P-selectin (CD62P) has been shown to be inversely correlated with the platelet count increment10 and recovery of platelets11–13. CD62 exposure on the platelet surface during PC storage triggers fast CD62-mediated platelet clearance14. Of interest, soluble HLA-I (sHLA-I) and soluble Fas ligand (sFasL) in blood components are functional and may exert immunoregulatory effects in vitro as shown by the inhibition of mixed lymphocyte responses and antigen-specific cytotoxic T-cell activity and by the induction of apoptosis in Fas-expressing cells15. In vitro studies indicate that sHLA may modulate immune competent cell function in at least two ways: (i) sHLA-I and sHLA-II molecules may bind their physiological ligands and inhibit T-cell function by receptor blockade and/or induction of apoptosis16,17; and (ii) sHLA-I and sHLA-II can be phagocytosed by antigen-presenting cells, degraded to peptides, and presented to CD4 T cells in the context of membrane HLA class II antigens. This latter process is known as indirect presentation18 and may lead to either immune tolerance or activation depending on the tolerogenic or stimulating capacity of the HLA-derived peptides presented by HLA class II antigens. The presence of soluble HLA in blood components is considered as a marker of immunological reactivity as it has been shown that it can modulate the activity of cytotoxic T cells19. sHLA-class 1 molecules can, therefore, be used as an indicator of the potential performance of leucodepleted PC.
It is important to define the impact of the leucocyte-removal process on the acceleration of platelet storage lesion in terms of some essential markers of cellular activation and fragmentation. This can be done using P-selectin as a marker of platelet activation and sHLA as a marker of immunological reactivity.
This study was carried out to establish whether leucoreduced and non-leucoreduced platelets obtained from random donor plasma are equivalent in terms of various factors that may accelerate the platelet storage lesion i.e. cellular activation and cellular fragmentation.
Materials and methods
A. Blood collection
Units of whole blood (500 ± 50 mL) were collected into triple blood bags (Baxter, Fenwal Division, USA) containing 70 mL of CPDA-1 as anticoagulant (PL 1240 containers). Each 100 ml solution of CPD-A1 contains: 2.63 g sodium citrate, 0.30 g citric acid, 0.22 g sodium biphosphate, 3.19 g dextrose and 27.5 mg adenine. Criteria for excluding potential blood donors include low platelet count (< 150 × 103/μL) and use of any drug known to affect platelet functions in the 72 hours prior to the planned donation (e.g. aspirin, oral anticoagulants or non-steroidal anti-inflammatory drugs).
B. Preparation of the PC
Manual technique (random donor method)20. Collected whole blood was left to stand for 30–45 minutes at room temperature, then centrifuged at 280 × g for 10 minutes at 22ºC to form platelet-rich plasma (PRP). The PRP was expressed into one of the satellite bag (PL 1240), then centrifuged at 1,760 × g, for 20 minutes to obtain platelet-poor plasma (PPP). PPP was returned to the concentrated red cells in the primary bag, leaving 40–60 mL in the prepared PC unit which was left undisturbed for 1–2 hours. The PC were divided into two groups:
Group 1: 10 units of PC that did not undergo WBC reduction.
Group 2: 10 units of PC that underwent pre-storage WBC reduction using a PL 50E filter (Pall Biomedical, Portsmouth, UK). This filter is composed of a synthetic, negatively-charged polyester in the form of a mesh of non-woven fibres. All the PC units were then placed on a horizontal shaker (Forma Scientific, Marietta, OH, USA) at 22 ºC for 5 days. At the end of this storage period, samples from all the PC units were cultured to evaluate the presence of bacterial contamination.
C. Laboratory tests for evaluation of PC
Samples from all PC were taken immediately after preparation (day 1) and on days 3 and 5 during storage in order to determine the following: platelet count, WBC count, swirling score, pH, pO2, pCO2 and bicarbonate levels, CD62P expression and concentration of sHLA.
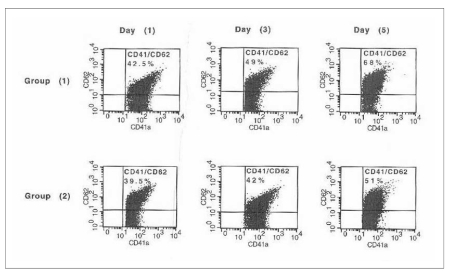
Platelets were counted using a Cell Dyne 1700 instrument (ABBOT Diagnostics, USA) only in day 1 samples. WBC were counted using a Nageotte chamber (Assistant, Sondheim, Germany) only for day 1 samples of group 2 PC21. The swirling score, advocated as a rapid inexpensive quality control measure, was assessed22. Swirling is induced by gently rotating or tapping a platelet bag in front of a light source. Functional platelets have a discoid morphology, which allows them to align with flow and gives the appearance of a wave or swirl to the naked eye. The degree of swirling was scored from 0 (no swirling) to 3 (optimal swirling). pH, pO2, pCO2 and bicarbonate levels were measured at 37°C using a blood gas analyser (Rapid Point 400, Bayer Diagnostics, UK). CD62P expression, a marker of platelet activation, was evaluated by a flow cytometer (FACScaliber, Becton Dickinson, Belgium) equipped with Cell Quest software. Fluorescein isothiocyanate (FITC)-conjugated anti-CD41 was used to identify the platelets. Phycoerythrin (PE)-conjugated anti-CD62P was used as a marker for platelet activation (Immunocytometry System, Becton Dickinson, Belgium). Unstained samples and negative controls containing FITC-IgG1 and PE-IgG2α were included with each analysis to estimate autoflourescence and non-specific binding, respectively. The instrument setting was adjusted to a log scale of 25,000. Platelets, identified according to their position in forward angle and right angle light scatter, were acquired from each sample. The percentages of events with positive staining for CD41 and CD62P were calculated (Figure 1). sHLA, a result of cellular fragmentation and a marker of immunological reactivity, was assayed by quantitative measurements of the soluble form of human leukocyte antigen-G (sHLA-G) using an enzyme-linked immunosorbent assay (ELISA) (BioVender Laboratory Medicine, Inc. Czech Republic).
Figure 1.
CD62P expression as a marker of platelet activation in the two studied groups of platelet concentrates (PC) on days 1, 3 and 5 of storage. Group 1: PC that did not undergo WBC reduction; group 2: PC that underwent pre-storage WBC reduction
Statistical analysis
The data collected were analysed using SPSS version 15 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 5. Data are expressed as means ± SD. Friedman’s test (analysis of variance for non-parametric variables) was used to test the significance of differences between repeated measures of each parameter in each group. The Mann-Whitney test was used to test the significance of differences between unmatched pairs of readings.
Results
All PC, after preparation, fulfilled the quality control requirements23. Platelet counts were in the range of 6.5–7.6 ×1010/unit. Plasma volumes were in the range of 42–63 mL. pH varied between 6.92 and 7.17. The mean post-filtration residual WBC count/unit in group 2 PC was 6.5 × 106 ± 3.4 (Table I).
Table I.
Comparison of mean values of measured analytes, during day 1, 3 and 5 in group 1 to that of group 2
| Analyte | Day | Group 1 (n= 10 ) | Group 2 (n= 10 ) |
|---|---|---|---|
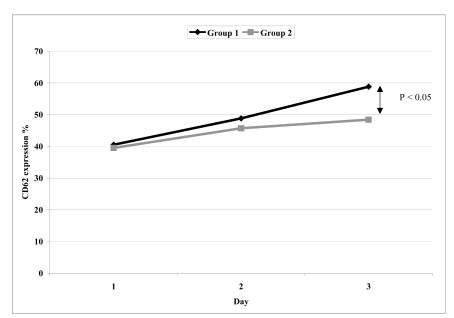
| CD62 expression % | 1 | 40.5 ± 4.1 | 39.5 ± 2.5 |
| 3 | 48.8 ± 1.3 | 45.7 ± 5.1 | |
| 5 | 58.8 ± 9.3 | 48.4 ± 3.4* | |
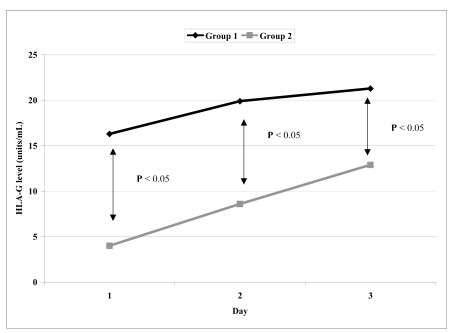
| HLA-G level unit/mL | 1 | 16.3 ± 0.9 | 4.0 ± 0.4* |
| 3 | 19.9 ± 1.1 | 8.6 ± 1.1* | |
| 5 | 21.3 ± 1.4 | 12.9 ± 0.8* | |
| pH | 1 | 7.02 ± 0.04 | 7.04 ± 0.03 |
| 3 | 7.10 ± 0.07 | 7.08 ± 0.04 | |
| 5 | 6.98 ± 0.05 | 7.02 ± 0.03 | |
| pO2 (mmHg) | 1 | 16.8 ± 1.0 | 17.0 ± 0.3 |
| 3 | 17.7 ± 1.7 | 16.8 ± 1.2 | |
| 5 | 18.1 ± 0.8 | 18.0 ± 0.3 | |
| pCO2 (mmHg) | 1 | 8.0 ± 0.3 | 7.4 ± 0.3 |
| 3 | 4.1 ± 0.2 | 4.0 ± 0.7 | |
| 5 | 3.6 ± 0.0 | 3.1 ± 0.8 | |
| Bicarbonate (mmol/L) | 1 | 16.2 ± 0.0 | 15.8 ± 0.4 |
| 3 | 12.3 ± 1.0 | 11.9 ± 0.1 | |
| 5 | 7.1 ± 0.3 | 6.8 ± 0.5 | |
| Post-filtration WBCs/unit | 1 | — | 6.5× 106± 3.4 |
| 3 | — | — | |
| 5 | — | — |
Group 1: platelet concentrates without WBC reduction
Group 2: platelet concentrates that underwent prestorage WBC reduction
Statistical significant difference p < 0.05 (p-value for Mann-Whitney test)
The swirling score was 2 in two PC from group 2. Platelet morphology was well preserved (swirling score 3) in all the other PC.
The comparisons of mean values of CD62P expression and sHLA-G levels between group 1 and group 2 PC on days 1, 3 and 5 are presented in table I. For CD62P, there was significant difference only on day 5 (Figure 2), whereas for sHLA-G, differences were statistically significant on days 1, 3 and 5 (Figure 3).
Figure 2.
The significance of difference of CD62 expression % between group1 and group 2 at day 1, 3 and 5
Figure 3.
The significance of difference of HLA-G level (units/mL) between group1 and group 2 at day 1, 3 and 5
The mean values of pH, pO2, pCO2 and bicarbonate on the same day did not differ between the two groups of PC (Table I).
Tables II and III show the significance of differences between repeated measures of each analyte in both groups of PC. For CD62P, the differences were statistically significant at each assessment in group 1 PC, whereas in group 2 PC, the difference was significant only between day 1 and day 5. For sHLA-G, differences were statistically significant between day 1 and day 3 and between day 1 and day 5 in group 1 PC, while in group 2 PC, the differences were significant between all the repeated measures. Differences between repeated measures for other analytes (pH, pO2, pCO2 and bicarbonate) in the two groups of PC were not statistically significant.
Table II.
The significance of difference between repeated measures of each analyte in group 1
| Day | Group 1 (n=10) | F-value | p-value | |
|---|---|---|---|---|
| CD62 expression % | 1 | 40.5±4.1a* | 23.99 | 0.00* |
| 3 | 48.8±1.3b* | |||
| 5 | 58.8±9.3c* | |||
| HLA-G level (units/mL) | 1 | 16.3±0.9a | 50.15 | 0.00* |
| 3 | 19.9±1.1b | |||
| 5 | 21.3±1.4c* | |||
| pH | 1 | 7.02±0.04a | 12.44 | 0.00* |
| 3 | 7.1±0.07b* | |||
| 5 | 6.98±0.05c | |||
| pO2 (mmHg) | 1 | 16.8±1 | 2.94 | 0.07 |
| 3 | 17.7±1.7 | |||
| 5 | 18.1±0.8 | |||
| pCO2 (mmHg) | 1 | 8±0.3a* | 339.23 | 0.00* |
| 3 | 4.1±0.2b | |||
| 5 | 3.6±0c* | |||
| Bicarbonate (mmol/L) | 1 | 16.2±0a* | 573.67 | 0.00* |
| 3 | 12.3±1b* | |||
| 5 | 7.1±0.3c* |
Group 1: platelet concentrates without WBC reduction.
Group 2: platelet concentrates that underwent prestorage WBC reduction
= significance of difference between 1, 3 day,
= significance of difference between 3, 5 day,
= significance of difference between 1, 5day,
Statistical significant difference p < 0.05 (p-value for Friedman test)
Table III.
The significance of difference between repeated measures of each analyte in group 2
| Day | Group 2 (n=10) | F-value | p-value | |
|---|---|---|---|---|
| CD62 expression % | 1 | 39.5±2.5a | 14.26 | 0.00* |
| 3 | 45.7±5.1b | |||
| 5 | 48.4±3.4c* | |||
| HLA-G level (units/mL) | 1 | 4±0.4a* | 295.67 | 0.00* |
| 3 | 8.6±1.1b* | |||
| 5 | 12.9±0.8c* | |||
| pH | 1 | 7.04±0.03a* | 8.24 | 0.00* |
| 3 | 7.08±0.04b* | |||
| 5 | 7.02±0.03c | |||
| pO2 (mmHg) | 1 | 17±0.3a | 7.65 | 0.00* |
| 3 | 16.8±1.2b* | |||
| 5 | 18±0.3c* | |||
| pCO2 (mmHg) | 1 | 7.4±0.3a* | 126.48 | 0.00* |
| 3 | 4±0.7b* | |||
| 5 | 3.1±0.8c* | |||
| Bicarbonate (mmol/L) | 1 | 15.8±0.4a* | 455.00 | 0.00* |
| 3 | 11.9±0.1b* | |||
| 5 | 6.8±0.5c* |
Group 1: platelet concentrates without WBC reduction.
Group 2: platelet concentrates that underwent prestorage WBC reduction
= significance of difference between 1, 3 day,
= significance of difference between 3, 5 day,
= significance of difference between 1, 5 day,
Statistical significant difference p < 0.05 (p-value for Friedman test)
Discussion
The demand for platelet transfusions continues to grow. Platelet transfusions are a crucial component of support for patients with severe thrombocytopenia. However, platelets undergo lesions during collection, preparation and storage which can affect their function and possibly their clinical efficacy. Some of these lesions are reversible, others are not1.
The purpose of this study was to investigate the 5-day storage stability of leucoreduced versus non-leucoreduced PC obtained from random donor plasma, in terms of some parameters which may accelerate the platelet storage lesion.
An array of assays, used for quality control of PC, was performed in two different groups of PC, prepared from random donor plasma, on days 1, 3 and 5 of storage. The group 1 PC were not leucoreduced while the group 2 PC underwent pre-storage leucoreduction using a PL50E filter. The quality control tests included platelet yield, WBC count and metabolic tests (pH, pO2, and pCO2) and bicarbonate. As current recommendations for the evaluation of PC include the measurement of platelet activation, in this study CD62P expression on platelet membrane was measured. Furthermore, since the activity of cytotoxic T cells has been shown to be modulated by soluble HLA class 1 antigen, the presence of sHLA-1 in blood components is considered a marker of immunological reactivity. It is, therefore, important to define whether current methods of leucoreduction of PC have any influence on the generation/retention of sHLA-1, so its level was measured in the two studied groups.
This study showed that CD62P expression was significantly lower in the filtered PC than in the non-leucoreduced PC. Similar findings have been reported by others4,24. This implies that filtration did not activate platelets and/or that activated platelets expressing CD62P were removed by the filter25 and hence no net effect was seen. Similar activation levels have been reported12,26 although lower levels may occur with other processing and storage conditions27. Recently, considerable variability was found between leucoreduced and non-leucoreduced PC from the same well-established donors28. Indeed, the role of P-selectin in post-transfusion clearance of platelets is unclear1. Michelson et al. demonstrated that circulating degranulated platelets rapidly lose surface P-selectin to the plasma pool, indicating that platelet surface P-selectin is not an ideal marker for the detection of circulating degranulated platelets although it may still be a useful marker of platelet degranulation if there is continuous activation of platelets29. Previous studies suggested that the plasma concentration of soluble P-selectin could be used as a marker of platelet activation in clinical settings, although an increase in the plasma concentration may also reflect the release of P-selectin from activated and/or damaged endothelial cells30.
As far as regards sHLA-1, we found that the levels of these antigens were significantly higher in non-leucoreduced PC than in the leucoreduced PC, but that the overall rate of increase of the level during storage was faster in the latter. This indicates that combined preparation and filtration processes have considerable effects on the release/retention of sHLA-1. We hypothesise that HLA class I antigens are released from residual donor blood leucocyte membranes during storage. This hypothesis is supported by the finding that the amount of sHLA-1 is related to the duration of refrigerated storage, which leads to damage of leucocyte membrane and to cell death31. The release of HLA class I antigens adhered on residual donor platelet membrane might also contribute to increase the concentration of sHLA-I molecules in some blood components32. In this respect, Segatchian reported that leucocyte filtration did not lead to significant cellular fragmentation28. However, more comprehensive data show that some leucoreduction processes do cause a relative decrease in the level of cellular fragments33. Indeed, the fact that current technologies for the production and storage of PC often generate different levels of sHLA-1 during 5-day storage, implies the need for in-depth analyses, as they may account for the residual side effects of some blood components19. Our findings are in agreement with some published data reporting that sHLA-I molecules are detectable in blood components34,35. For example, Ghio et al. reported that sHLA-I and FasL levels in red blood cells stored for up to 30 days and in random-donor platelets are significantly higher than in other blood components and their amounts are proportional to the number of residual donor leucocytes and to the duration of storage. They also found that blood components with high sHLA-I and sFasL levels play immunoregulatory roles in vitro in allogeneic mixed lymphocyte responses and antigen-specific cytotoxic T-cell activity, and also induce apoptosis in Fas-positive cells15. If these effects also occur in vivo, they should be taken into account in transfusion practice. Blood components that can cause immunosuppression should be chosen to induce transplantation tolerance, whereas blood components that lack immunosuppressive effects should be preferred to reduce the risk of postoperative complications and cancer recurrence.
Conclusion
Based on our findings, it can be stated that leucoreduced PC differ in terms of some specific markers of platelet activation (CD62P) and immunological reactivity (sHLA-1) and that pre-storage leucofiltration, followed by storage in the currently used plastic bags is a safe procedure for at least 5 days. The available leucoreduction technologies are not, however, sufficiently robust to completely abrogate transfusions reactions, and their ability to reach the goal of optimized yield and minimal transfusion reactions with platelet therapy still needs to improved.
References
- 1.Wallace EL, Churchill WH, Surgenor DM, Cho GS. Collection and transfusion of blood and blood components in the United States, 1994. Transfusion. 1998;38:625–36. doi: 10.1046/j.1537-2995.1998.38798346630.x. [DOI] [PubMed] [Google Scholar]
- 2.Klinger MH. The storage lesion of ultrastructural and functional aspects. Ann Hematol. 1996;73:103–12. doi: 10.1007/s002770050210. [DOI] [PubMed] [Google Scholar]
- 3.Seghatchian J, Krailadsiri P.Platelet storage lesion and apoptosis: are they related? Transfus Apheresis Sci 200124103–5.[Review] [DOI] [PubMed] [Google Scholar]
- 4.Seghatchian J, Krailadsiri P. The platelet storage lesion. Transfus Med Rev. 1997;11:130–44. doi: 10.1053/tm.1997.0110130. [DOI] [PubMed] [Google Scholar]
- 5.Krailadsiri P, Seghatchian J, Williamson LM. Platelet storage lesion of WBC-reduced pooled buffy coat-derived PC prepared in three in-process filtered/storage bag combinations. Transfusion. 2001;41:243–50. doi: 10.1046/j.1537-2995.2001.41020243.x. [DOI] [PubMed] [Google Scholar]
- 6.Bordin JO, Heddle NM, Blajchman MA. Biological effects of leukocytes present in transfused cellular blood products. Blood. 1994;84:1703–21. [PubMed] [Google Scholar]
- 7.Murphy S, Rebulla P, Bertolini F. In vitro assessment of the quality of stored platelet concentrates. The BEST (Biomedical Excellence for Safer Transfusion) Task Force of the International Society of Blood Transfusion. Transfus Med Rev. 1994;8:29–36. doi: 10.1016/s0887-7963(94)70095-x. [DOI] [PubMed] [Google Scholar]
- 8.Vostal JG. FDA guidance for industry: for platelet testing and evaluation of platelet substitute products. Presented at the 18th meeting of the BEST Working Party of the International Society of Blood Transfusion; San Francisco. November 5, 1999. [Google Scholar]
- 9.Michelson AD. Flow cytometry: a clinical test of platelet function. Blood. 1996;87:4925–36. [PubMed] [Google Scholar]
- 10.Triulzi DJ, Kickler TS, Braine HG. Detection and significance of alpha granule membrane protein 140 expression on platelets collected by apheresis. Transfusion. 1992;32:529–33. doi: 10.1046/j.1537-2995.1992.32692367196.x. [DOI] [PubMed] [Google Scholar]
- 11.Rinder HM, Murphy M, Mitchell JG. Progressive platelet activation with storage: evidence for shortened survival of activated platelets after transfusion. Transfusion. 1991;31:409–14. doi: 10.1046/j.1537-2995.1991.31591263195.x. [DOI] [PubMed] [Google Scholar]
- 12.Dumont LJ, AuBuchon JP, Whitley P. Seven-day storage of single-donor platelets: recovery and survival in an autologous transfusion study. Transfusion. 2002;42:847–54. doi: 10.1046/j.1537-2995.2002.00147.x. [DOI] [PubMed] [Google Scholar]
- 13.Rinder HM, Smith BR. In vitro evaluation of platelets: is there hope for predicting posttransfusion platelet survival and function? Transfusion. 2003;43:2–6. doi: 10.1046/j.1537-2995.2003.00261.x. [DOI] [PubMed] [Google Scholar]
- 14.Leytin V, Allen DJ, Gwozdz A, Garvey B. Role of platelet surface glycoprotein Ibá and P-selectin in the clearance of transfused platelet concentrates. Transfusion. 2004;44:1486–95. doi: 10.1111/j.1537-2995.2004.04042.x. [DOI] [PubMed] [Google Scholar]
- 15.Ghio M, Contini P, Mazzei C. Soluble HLA class 1, HLA class 11 and Fas ligand in blood components: a possible key to explain the immunomodulatory effects of allogenic blood transfusion. Blood. 1999;93:1770–7. [PubMed] [Google Scholar]
- 16.Nicolle MW, Nag B, Sharma SD, et al. Specific tolerance to an acetylcholine receptor epitope induced in vitro in myasthenia gravis CD41 lymphocytes by soluble major histocompatibility complex class II-peptide complexes. J Clin Invest. 1994;93:1361–9. doi: 10.1172/JCI117112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zavazava N, Kronke M. Soluble HLA class I molecules induce apoptosis in alloreactive cytotoxic T lymphocytes. Nature Med. 1996;2:1005–10. doi: 10.1038/nm0996-1005. [DOI] [PubMed] [Google Scholar]
- 18.Sayegh MH, Carpenter CB. Role of indirect allorecognition in allograft rejection. Int Rev Immunol. 1996;13:221–9. doi: 10.3109/08830189609061749. [DOI] [PubMed] [Google Scholar]
- 19.Seghatchian J. Universal leucodepletion: an overview of some unresolved issues and the highlights of lesson learned. Transfus Apheresis Sci. 2003;20:105–17. doi: 10.1016/S1473-0502(03)00112-5. [DOI] [PubMed] [Google Scholar]
- 20.Slichter SJ, Harker LA. Preparation and storage of platelet concentrates. 1. Factors influencing the harvest of viable platelet from whole blood. Br J Haematol. 1976;39:395–402. doi: 10.1111/j.1365-2141.1976.tb03586.x. [DOI] [PubMed] [Google Scholar]
- 21.Moroff G, Dich J, Dabay M. Validation of use of the Nogeotte hemocytometer to count low levels of white cells reduced platelet components. Transfusion. 1994;34:35–8. doi: 10.1046/j.1537-2995.1994.34194098600.x. [DOI] [PubMed] [Google Scholar]
- 22.Bertolini F. Murphy S. A multicenter evaluation of reproducibility of swirling in platelet concentrates. Biomedical Excellence for Safer Transfusion (BEST) Working Party of the International Society of Blood Transfusion. Transfusion. 1994;34:796–801. doi: 10.1046/j.1537-2995.1994.34994378282.x. [DOI] [PubMed] [Google Scholar]
- 23.Slichter SJ. Controversies in platelet transfusion therapy. Ann Rev Med. 1980;31:509–40. doi: 10.1146/annurev.me.31.020180.002453. [DOI] [PubMed] [Google Scholar]
- 24.Krailadsiri P, Seghatchian J, Amiral J. Annexin V. A maker of platelet storage lesion: correlation with dMPV. Transfus Science. 1997;18:223–6. doi: 10.1016/s0955-3886(97)00013-1. [DOI] [PubMed] [Google Scholar]
- 25.Pedigo M, Wun T, Paglieroni T. Removal by white cell-reduction filters of activated platelets expressing CD62. Transfusion. 1993;11:930–5. doi: 10.1046/j.1537-2995.1993.331194082385.x. [DOI] [PubMed] [Google Scholar]
- 26.Curvers J, van Pampus EC, Feijge MA, et al. Decreased responsiveness and development of activated markers of PLTs stored in plasma. Transfusion. 2004;44:49–58. doi: 10.1111/j.0041-1132.2004.00628.x. [DOI] [PubMed] [Google Scholar]
- 27.Perrotta PL, Perrotta CL, Synder EL. Apoptotic activity in stored human platelets. Transfusion. 2003;43:526–35. doi: 10.1046/j.1537-2995.2003.00349.x. [DOI] [PubMed] [Google Scholar]
- 28.Seghatchian J. Platelet storage lesion: an update on the impact of various leukoreduction processes on the biological response modifiers. Transfus Apheresis Sci. 2006;34:125–30. doi: 10.1016/j.transci.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Michelson AD, Barnard MR, Hechtmant HB. In vivo tracking of platelets: circulating degranulated platelets rapidly lose surface P-selectin but continue to circulate and function. Proc Natl Acad Sci USA. 1996;93:11877–82. doi: 10.1073/pnas.93.21.11877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chong BH, Murray B, Berndt MC, et al. Plasma P-selectin is increased in thrombotic consumptive platelet disorders. Blood. 1994;83:1535–41. [PubMed] [Google Scholar]
- 31.Mincheff M. Changes in donor leukocytes during blood storage. Implications on post-transfusion immunomodulation and transfusion-associated GVHD. Vox Sang. 1998;74(suppl 2):189–200. doi: 10.1111/j.1423-0410.1998.tb05420.x. [DOI] [PubMed] [Google Scholar]
- 32.Kao KJ, Scornik JC, Riley WJ, McQueen CF. Association between HLA phenotype and HLA concentration in plasma or platelets. Hum Immunol. 1988;21:115–24. doi: 10.1016/0198-8859(88)90086-9. [DOI] [PubMed] [Google Scholar]
- 33.Krailadsiri P, Seghatchian J, Macgregor I, et al. The effects of leukodepletion on the generation and removal of microvesicles and prion-protein in blood components. Transfusion. 2006;46:407–17. doi: 10.1111/j.1537-2995.2006.00737.x. [DOI] [PubMed] [Google Scholar]
- 34.Westhoff U, Grosse Wilde H. Soluble HLA class I and class II concentrations in factor VIII and PCC preparations. Vox Sang. 1995;68:73–6. doi: 10.1111/j.1423-0410.1995.tb02556.x. [DOI] [PubMed] [Google Scholar]
- 35.Dzik S, Szuflad P, Eaves S. HLA antigens on leukocyte fragments and plasma proteins: prestorage leukoreduction by filtration. Vox Sang. 1994;66:104–11. doi: 10.1111/j.1423-0410.1994.tb00290.x. [DOI] [PubMed] [Google Scholar]