Abstract
Objective: To understand the function of nicotinamide N-methyltransferase (NNMT) protein as tumor biomarker in renal carcinoma. Methods: Recombinant NNMT protein was used to prepare monoclonal antibodies by hybridoma technique. The diagnostic and prognostic function of NNMT protein in renal carcinoma was evaluated by analyzing 74 renal cancer tissues through immunohistochemical staining for NNMT by using the prepared antibodies. Results: Two hybridomas named 2F8 and 1E7 stably secreting the monoclonal antibodies were isolated successfully, and characters such as isotypes and specificity were determined. NNMT protein was significantly up-regulated in renal cancer and significantly associated with tumor histology and ages. The univariate survival analysis demonstrated that the pT-status, high levels of NNMT, and distant metastasis were significant prognosticators. Conclusion: NNMT is over-expressed in a large proportion in renal cell cancers. High NNMT expression is significantly associated with unfavorable prognosis. However, the prognostic value of NNMT needs further verification in larger sample sizes.
Keywords: Nicotinamide N-methyltransferase, Monoclonal antibody, Tumor biomarker, Renal cell cancer
1. Introduction
Renal cell cancer (RCC) is one of the most common genitourinary malignancies and accounts for around 3% of cancers worldwide (McLaughlin and Lipworth, 2000; Jemal et al., 2007). The tumor-node-metastasis (TNM) staging system is the most often used factorial system to assess the prognosis of patients. There is an urgent need to identify new biomarkers, which are warranted to provide more information on the tumor biology, chemotherapeutic effects, allowing a better prognostic and possibly predictive stratification of patients (Fritzsche et al., 2008).
Nicotinamide N-methyltransferase (NNMT, EC 2.1.1.1) catalyzes the N-methylation of nicotinamide, pyridines, and other structural analogs by using S-adenosylmethionine as methyl donor, playing a pivotal role in the biotransformation and detoxification of many xenobiotics (Rini et al., 1990; Aksoy et al., 1994). NNMT is predominantly expressed in the liver in which drug and other xenobiotic compounds are mainly metabolized by N-methylation method, and weakly expressed in other tissues such as the kidney, lung, skeletal muscle, placenta, heart, and brain (Yan et al., 1999). Recently, abnormal expression of NNMT has been reported in tumors such as papillary thyroid cancers (Xu et al., 2003; 2005), glioblastoma, stomach adenocarcinoma (Jang et al., 2004; Lim et al., 2006), colon cancer (Roessler et al., 2005), renal carcinoma (Yao et al., 2005; Sartini et al., 2006), oral squamous cell carcinoma (Sartini et al., 2007), lung cancer (Tomida et al., 2009), and liver cancer (Kim et al., 2009). Those results indicate that NNMT may become a potential biomarker for diagnosis of tumor and may have a potential role for predicting response to radiation therapy or chemotherapy.
Although NNMT mRNA was reported to be highly expressed in RCC (Yao et al., 2005; Sartini et al., 2006), so far there has been no report on a direct relationship between the level of NNMT protein expression or tissue distribution pattern and development and/or progression of RCC. The aim of the present investigation was to evaluate the diagnostic and prognostic function of NNMT protein in renal carcinoma. We used recombinant NNMT protein to prepare monoclonal antibodies against NNMT, and two hybridomas, named 2F8 and 1E7, were obtained. Immunohistochemical staining for NNMT using the prepared antibodies was performed. The results show that NNMT protein was significantly up-regulated in renal cancer, and NNMT level was significantly associated with tumor histology and ages of the patients. High NNMT expression is significantly associated with unfavorable prognosis. However, the prognostic value of NNMT should be further verified in larger sample sizes.
2. Materials and methods
2.1. Patients
Seventy-four Chinese patients (26 women, 48 men) diagnosed for renal cancer at the Pathology Department, Sir Run Run Shaw Hospital (SRRSH), Hangzhou, China, between 2005 and 2006 were enrolled in this study. The study has been approved by the SRRSH Ethics Committee.
The median age of the patients was 54 years old, ranging from 33 to 74. Cases were not stratified for any known preoperative or pathological prognostic factor and were selected according to tissue availability. Histological diagnosis and tumor stage were established according to the guidelines of the World Health Organization (Eble et al., 2004). Fifty-eight (78.4%) patients had a clear cell RCC (ccRCC), and 16 (21.6%) a chromophobe RCC. Clinical follow-up data and the survival time assessed every six months to one year were available for all patients. The follow-up time of all cases ranged from 3 to 40 months with the median of 30 months. Three patients died from renal cancer. Fifty-five (74.3%) patients were at pT1, 17 (23.0%) at pT2, 2 (2.7%) at pT3, and 0 (0%) at pT4. The occult blood (OB) test results of urinanalysis and hemoglobin (Hb) of blood before nephrectomy were also recorded as clinico-pathological parameters.
2.2. Development of monoclonal antibodies against NNMT
The recombinant glutathione S-transferase (GST)-NNMT and NNMT were produced and purified as we reported previously (Ma et al., 2006), and were used for immunization of mice and making hybridomas secreting Ag-specific monoclonal antibodies. The conventional hybridoma technique was performed for producing monoclonal antibodies with some modification described by Zhang et al. (2008). In brief, 6 to 8 week-old female BALB/c mice were immunized subcutaneously with 120 μg of GST-NNMT that was emulsified with Freund’s complete adjuvant (Sigma, St. Louis, MO, USA). After three weeks, the mice were boosted with the antigen in incomplete Freund’s adjuvant at a 10-d interval. Finally, those that were selected for hybridization received a boost of 80 μg NNMT in saline 3 d prior to removal of the spleen. Then the immunized splenocytes were fused with SP2/0 myeloma cells in the presence of 50% (w/v) polyethylene glycol (PEG)-4000 (Sigma). Fusion products were re-suspended in RPMI 1640 medium (Gibco Invitrogen Corp., Grand Island, New York, USA) supplemented with 20% (v/v) fetal calf serum, hypoxantin-aminopterin-thymidine (HAT) (Sigma), and 100 U/ml penicillin/100 μg/ml streptomycin, and seeded in 96-well plates containing feeder cells and cultured in a CO2 incubator.
Hybridomas secreting anti-NNMT monoclonal antibodies were screened with the indirect enzyme-linked immunosorbent assay (ELISA). The supernatants of each well were screened by analyzing their reactivity to NNMT and GST (Sigma) separately, and the hybridomas secreting antibodies only reactive to NNMT were selected. To get stable and monoclonal antibody-secreting hybridomas, positive clones were sub-cloned successively at least six times by limiting dilution.
To prepare ascetic fluid containing anti-NNMT antibody, the selected hybridomas were injected into the peritoneal cavity of BALB/c mice primed with liquid paraffin. Immunoglobulins were purified by caprylic acid/saturated ammonium sulfate precipitation, and the concentration was determined by spectrophotometry. The monoclonal antibodies were characterized for titers, isotypes, and specificity using ELISA and immunoblotting.
2.3. Immunohistochemistry (IHC) and evaluation of the immunohistochemical staining
The paraffin-fixed samples of renal cancer were sliced into 4-μm sections and subjected to immunohistochemical staining using Dako Elivision plus two-step system (Dako, Hamburg, Germany). Briefly, paraffin sections were de-waxed in xylene, rinsed in alcohol, and further dehydrated in graded alcohol. The sections were then subjected to antigen retrieval treatment by boiling in 0.01 mol/L citric acid, pH 6.0, for 5 min in a pressure cooker and then were treated with 0.3% (v/v) hydrogen peroxide for 30 min in absolute methanol to inhibit endogenous peroxidase. After treatment, the sections were blocked with diluted normal calf serum for 10 min at room temperature and incubated with anti-NNMT (2F8, 1:2000) at room temperature for 60 min. Then sections were incubated with rabbit anti-mouse horseradish peroxidase (HRP)-conjugated antibody (1:5000) at room temperature for 40 min and then washed in phosphate buffered saline (PBS). The color reaction was carried out using 3,3′-diamino-benzidine-tetrahydrochloride/H2O2 for 3–10 min. Sections were then counterstained with hematoxylin. Negative control reactions were performed in parallel without primary antibodies.
The reactivity to human liver tissue analyzed by IHC was used as positive control, and adsorption experiment using NNMT antigen (the prepared antibodies adsorbed by NNMT protein at 4 °C overnight) was also performed to further verify the specific positivity of the antibody.
Immunostaining was evaluated by two pathologists and a four-tier grading system was used to describe the staining intensity (0=negative, ≤5%; 1+=weak, 6%–25%; 2+=moderate, 26%–50%; and 3+=strong, >50%). A 5% threshold was used to determine positivity irrespective of the intensity grade; thus tumors without any NNMT immunoreactivity or with staining of less than 5% of the tumor cells were considered to be negative.
2.4. Statistical analysis
Statistical analysis was performed using SPSS, version 16.0. χ 2-test, Fisher’s exact test, and two-independent-samples t-test were applied to assess the statistical significance of the associations between clinico-pathological parameters and NNMT expression. Kaplan-Meier and the log rank tests were used to evaluate univariate survival analysis. P values <0.05 were considered to be statistically significant.
3. Results
3.1. Preparation of monoclonal antibodies against NNMT
Two stable hybridoma cell lines, 2F8 and 1E7, which could continuously produce specific monoclonal antibodies against NNMT, were successfully established. The titers of antibodies from 2F8 and 1E7 were 1:2.56×104 and 1:6.4×103 in culture supernatant, respectively, and 1:8.192×106 and 1:1.024×106 in ascitic fluids, respectively. The antibodies secreted by 2F8 were captured by anti-IgG2b heavy chain and anti-κ light chain and thus were IgG2b-κ. The antibodies secreted by 1E7 were captured by anti-IgG2a heavy chain and anti-κ light chain and thus were IgG2a-κ. The specificity of the antibodies was further investigated by Western-blot analysis using GST-NNMT fusion protein, NNMT, GST proteins and Escherichia coli (E. coli) BL21 (DE3) cell lysate. Both 2F8- and 1E7-produced antibodies recognized GST-NNMT fusion protein (55 kDa) and NNMT protein (29 kDa), but did not recognize GST protein or E. coli BL21 (DE3) cell lysate (Fig. 1).
Fig. 1.
Western blot analysis of specificity of antibodies secreted by 2F8 (a) and 1E7 (b) against NNMT
M: protein marker, pre-stained; Lane 1: GST-NNMT fusion protein; Lane 2: NNMT; Lane 3: GST; Lane 4: E. coli BL21 (DE3) cell lysate
3.2. NNMT expression in renal cell cancer
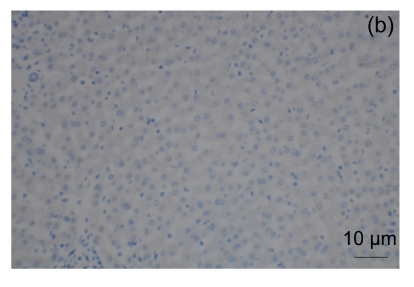
Strong staining of NNMT was observed in the cytoplasm in human liver cell (positive control, Fig. 2a) and in most RCC cells (Fig. 3). The reactivity to human liver cells can be eliminated when the antibody was previously adsorbed by NNMT antigen (Fig. 2b). Moderate nucleus staining of NNMT was also observed in RCC cells: negative, 20 (27.0%); 1+, 22 (29.7%); 2+, 12 (16.2%); and 3+, 20 (27.1%). NNMT positivity was significantly higher in ccRCC cells when compared with the chromophobe RCC cells (Table 1, Fig. 3).
Fig. 2.
NNMT immunohistochemistry in normal liver
NNMT expression in the cytoplasma of liver cells was strongly positive (a), and the reactivity to human liver tissue can be eliminated when the antibody previously adsorbed by NNMT antigen (b)
Fig. 3.
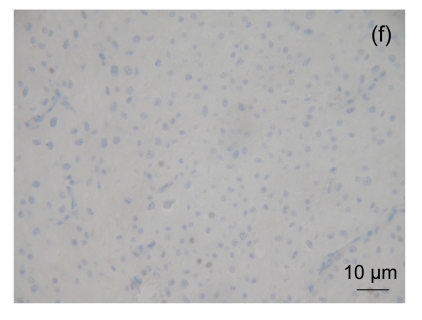
NNMT immunohistochemistry in renal cell carcinomas
NNMT expression in the vast majority of clear cell RCC was strongly positive (a), and in the minority of clear cell RCC was negative (b). NNMT expression in matching normal renal tissues was negative (c) and positive (d). NNMT expression in a chromophobe RCC was positive (e) and in most of chromophobe RCC was negative (f)
Table 1.
Associations (χ 2-test) between NNMT protein expression in renal cell cancer and clinico-pathological parameters*
| Total | NNMT expression |
NNMT expression |
|||||
| Negative | Positive | P value | Low | High | P value | ||
| All cases | 74 (100) | 20 (27.0) | 54 (73.0) | 42 (56.8) | 32 (43.2) | ||
| Gender | 0.169 | 0.143 | |||||
| Men | 48 (64.9) | 10 (20.8) | 38 (79.2) | 24 (50.0) | 24 (50.0) | ||
| Women | 26 (35.1) | 10 (38.5) | 16 (61.5) | 18 (69.2) | 8 (30.8) | ||
| Age | 0.017 | 0.002 | |||||
| >54 years | 34 (45.9) | 14 (41.2) | 20 (58.8) | 26 (76.5) | 8 (23.5) | ||
| ≤54 years | 40 (54.1) | 6 (15.0) | 34 (85.0) | 16 (40.0) | 24 (60.0) | ||
| pT-status | 0.034 | 0.313 | |||||
| 1 | 55 (74.3) | 12 (21.8) | 43 (78.2) | 29 (52.7) | 26 (47.3) | ||
| 2 | 17 (23.0) | 6 (35.3) | 11 (64.7) | 11 (64.7) | 6 (35.3) | ||
| 3 | 2 (2.7) | 2 (100.0) | 0 (0.0) | 2 (100.0) | 0 (0.0) | ||
| pN status | 0.070 | 0.502 | |||||
| 0/x | 72 (97.3) | 18 (25.0) | 54 (75.0) | 40 (55.6) | 32 (44.4) | ||
| 1 | 2 (2.7) | 0 (0.0) | 2 (100.0) | 2 (100.0) | 0 (0.0) | ||
| Metastasis | 0.294 | 1.000 | |||||
| M0 | 70 (94.6) | 18 (25.7) | 52 (74.3) | 40 (57.1) | 30 (42.9) | ||
| M1 | 4 (5.4) | 2 (50.0) | 2 (50.0) | 2 (50.0) | 2 (50.0) | ||
| Histology | 0.001 | 0.009 | |||||
| Clear cell RCC | 58 (78.4) | 10 (17.2) | 48 (82.8) | 28 (48.3) | 30 (51.7) | ||
| Chromophobe RCC | 16 (21.6) | 10 (62.5) | 6 (37.5) | 14 (87.5) | 2 (12.5) | ||
| OB | 0.016 | 0.198 | |||||
| Negative | 54 (73.0) | 10 (18.5) | 44 (81.5) | 28 (51.9) | 26 (48.1) | ||
| Positive | 20 (27.0) | 10 (50.0) | 10 (50.0) | 14 (70.0) | 6 (30.0) | ||
| Hb (mg/dl)# | 12.28±1.76 | 13.6±1.62 | 0.006 | 12.76±1.73 | 13.86±1.59 | 0.003 | |
Data are expressed as number (percentage)
Concentrations of Hb are expressed as mean±SD
OB: occult blood in urine before nephrectomy; Hb: hemoglobin of blood before nephrectomy. Two-independent-samples t-test was used for statistical analysis
Ages of the patients and pT status were significantly correlated to the expression of NNMT. The younger patients had higher positivity and higher level of NNMT expression than older ones, and the positivity of NNMT expression is significantly correlated inversely with pT status. The blood Hb level before nephrectomy was significantly higher in patients who have high or positive NNMT level than in ones who have low or negative NNMT (Table 1). No other correlations or associations with clinico-pathological parameters were detected.
A total of 37 cases matching normal renal tissues were evaluated, 12 scored at 1+ and 2 at 2+ for NNMT while the remaining 23 were negative. To eliminate the interference from the background expression of NNMT, tumors with none to weak staining intensity and tumors with moderate to strong NNMT expression were lumped to delineate between low and high levels of NNMT expression. Histology and age remained significantly correlated to the expression of NNMT protein (Table 1).
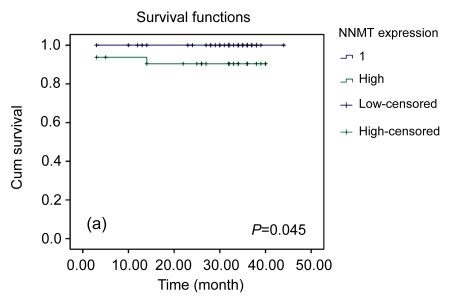
The univariate survival analysis demonstrated that pT-status, distant metastasis, and high levels of NNMT were significant prognosticators, whereas age, gender, and histology were not (Table 2). For NNMT, the prognostic value for patient survival was not clear, although in the Kaplan-Meier curve a trend for longer survival time of patients with low NNMT tumors was observed (Fig. 4).
Table 2.
Univariate survival analysis (Kaplan-Meier)
| Characteristic | No. of cases | No. of events | Two-year survival rate (%)* | P |
| NNMT expression | 0.045 | |||
| Low | 42 | 0 | ||
| High | 32 | 3 | 90.4±0.053 | |
| NNMT expression | 0.301 | |||
| Negative | 20 | 0 | ||
| Positive | 54 | 3 | 94.4±0.032 | |
| pT-status | 0.003 | |||
| 1 | 55 | 0 | ||
| 2/3 | 19 | 3 | 83.9±0.085 | |
| pN-status | 0.767 | |||
| pN0 | 72 | 3 | 95.7±0.024 | |
| pN1+ | 2 | 0 | ||
| Metastasis | <0.001 | |||
| M0 | 70 | 1 | 98.4±0.016 | |
| M1 | 4 | 2 | 50.0±0.250 | |
| Histology | 0.356 | |||
| Clear cell RCC | 58 | 3 | 94.7±0.030 | |
| Chromophobe RCC | 16 | 0 | ||
| Gender | 0.201 | |||
| Men | 48 | 3 | 93.6±0.036 | |
| Women | 26 | 0 | ||
| Age | 0.113 | |||
| >54 years | 34 | 0 | ||
| ≤54 years | 40 | 3 | 92.4±0.042 |
Mean±SE
Fig. 4.
Kaplan-Meier survival curve for NNMT
Tumors with high NNMT expression revealed a significant trend for shortened survival time of patients compared to those with low NNMT expression (a). There was no significant trend between NNMT positive and negative tumors (b)
4. Discussion
Proteomic technologies and DNA microarray are providing the tools needed to discover and identify biomarkers associated with diverse diseases and biological processes (Hanash, 2003; Eun et al., 2004; Yao et al., 2005). NNMT was one of the potential tumor biomarkers identified recently by those technologies, in which NNMT in both mRNA and protein levels was found over-expressed in a wide range of tumors. For better understanding the function of NNMT as a tumor biomarker, and to develop immunodiagnostic kits (ELISA or Immunohistochemical staining, IHC), high quality and stable antigen and monoclonal antibodies against NNMT are needed.
Because the recombinant GST-NNMT contains GST-tag, which may cause false-positive results, choosing the GST as the negative screening antigen could effectively eliminate the false-positive antibodies to GST-tag. The Western blot analysis showed that the two antibodies produced in the present study had no cross reactivity with GST-tag or host proteins (cell lysate of E. coli BL21 (DE3) containing pGEX-4T-2).
To confirm the specificity of the positive signals of NNMT, we used human liver tissue as positive control in immunohistochemistry studies, and strong staining of NNMT was observed in the cytoplasm. The reactivity to human liver tissue can be weakened or eliminated when the antibody was previously adsorbed by NNMT antigen, further confirming the specificity of the antibodies. Besides the strong staining in the cytoplasma of RCC cells, moderate nucleus staining was also observed in this study. While NNMT is cytoplasmic protein, its nucleus staining has also been found in normal mucosa, normal thyroid cells, goiter, and thyroid adenomas and papillary carcinomas by IHC (Xu et al., 2003; Sartini et al., 2007). The nature of the nuclear staining of NNMT needs to be further studied.
In this study, we investigated the expression of the NNMT protein in tumor tissues of 74 patients with RCC, and 37 were found to match normal renal tissue. We demonstrated that NNMT protein was over-expressed in RCC, especially in clear cell RCC (82.8%). We also found NNMT positive staining in the matched normal tissue. However, compared with normal tissue, the positivity and the positive staining grade were significantly higher in tumor tissues. Over-expression of NNMT in the majority of ccRCC was observed, consistent with NNMT mRNA level reported (Sartini et al., 2006). In the matched normal tissue, the predominant positive grade was scored at 1+. To eliminate the interference from the background expression of NNMT, tumors were divided into two groups, the high and low levels of NNMT expression. Histology and age were found significantly correlated with the expression of NNMT protein level. The NNMT expression is significantly correlated inversely with tumor size (pT status), but no significant difference between the high and low NNMT expression was found. Interestingly, it was noted that younger patients had higher positivity and higher level of NNMT expression than older ones. While this was not reported in other tumors, it was in accordance with the finding of Aoyama et al. (2001) that the NNMT protein in Parkinson’s disease patients was significantly affected by aging.
In the Kaplan-Meier curve a trend for longer survival time was observed in the patients who had a lower NNMT level, suggesting the possibility for a high level of NNMT to be a prognostic factor, and suggesting a role of NNMT in tumor growth. However, the prognostic value for patient survival was not clear, because there was insignificant difference in the Kaplan-Meier curve between the patients with positive and negative NNMT expression. In addition to the tumor size and the presence of distant metastasis (T and M within the TNM staging system), the grade of malignancy, gender, and microscopic tumor necrosis are also considered to be prognostic markers in RCC (Cohen and McGovern, 2005; Ficarra et al., 2007). In the present study, the gender was not found to be a prognostic marker. Since this study was of medium size, the reliability of the NNMT protein level as an independent prognostic marker needs further confirmation in a larger study.
This is the first IHC study of NNMT in RCC patients in a Chinese population. While, no such IHC study in other races has been reported, it was observed that NNMT mRNA was up-regulated in RCC patients in other races as well (Yao et al., 2005; Sartini et al., 2006). We therefore speculate that IHC of NNMT may be applicable to all races, not just Asian population.
Mechanisms for the enhanced NNMT expression in RCC are not clear. In previous studies, high level of NNMT mRNA in clear cell RCC was reported (Yao et al., 2005; Sartini et al., 2006). In the current study, we demonstrated a high level of NNMT protein expression in RCC. Thus, transcriptional regulation of NNMT is likely to be an important regulatory mechanism in ccRCC cells. Tomida et al. (2008) reported that NNMT is a novel Stat3-regulated gene. Because constitutive activation of Stat3 has been detected in a wide variety of primary human tumors including kidney cancer (Horiguchi et al., 2002), we thus also speculate that constitutive activation of Stat3 may be one of the mechanisms for the enhanced NNMT expression in RCC.
In the current study we found that the blood Hb level was significantly higher in tumor patients with a high NNMT level than in ones with a low expression. This is the first time correlating NNMT with blood Hb level. This could not be explained by the occult blood loss and may not be resulted from high expression of erythropoietin (EPO) in RCC (Papworth et al., 2009). In fact, the effect of NNMT over-expression seems to be diversiform because the enzyme NNMT could play a fundamental role in the regulation of those cellular events such as energy production, cellular resistance to stress or injury, and longevity, relating to nicotinamide metabolism using S-adenosylmethionine (Zhang, 2003; Sartini et al., 2007). Further characterization of biological functions of NNMT is needed to make the enzyme as a biomarker for tumor diagnosis, prognosis, and therapy.
5. Acknowledgement
We are grateful for the expertise and advice of Tao ZHU (Department of Pathology, Sir Run Run Show Hospital, School of Medicine, Zhejiang University, China) who helped to do IHC. We also thank Drs. Xiao-tong HU (Key Laboratory of Biotherapy of Zhejiang Province, China) and Gong-xiang CHEN (Department of Clinical Laboratory, the Second Hospital, School of Medicine, Zhejiang University, China) for their advice in the study.
Footnotes
Project supported by the Science Foundation of Health Bureau of Zhejiang Province (Nos. 2005A055 and 2008B114) and the Science Foundation of Education Bureau of Zhejiang Province (No. 20061271), China
References
- 1.Aksoy S, Szumlanski CL, Weinshilboum RM. Human liver nicotinamide N-methyltransferase: cDNA cloning, expression, and biochemical characterization. J Biol Chem. 1994;269:14835–14840. [PubMed] [Google Scholar]
- 2.Aoyama K, Matsubara K, Kondo M, Murakawa Y, Suno M, Yamashita K, Yamaguchi S, Kobayashi S. Nicotinamide N-methyltransferase is higher in the lumbar cerebrospinal fluid of patients with Parkinson’s disease. Neurosci Lett. 2001;298(1):78–80. doi: 10.1016/S0304-3940(00)01723-7. [DOI] [PubMed] [Google Scholar]
- 3.Cohen H, McGovern F. Renal-cell carcinoma. N Engl J Med. 2005;353(23):2477–2490. doi: 10.1056/NEJMra043172. [DOI] [PubMed] [Google Scholar]
- 4.Eble JN, Sauter G, Epstein JI, et al. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Lyon: IARC Press; 2004. pp. 10–32. [Google Scholar]
- 5.Eun JP, Choi HY, Kwak YG. Proteomic analysis of human cerebral cortex in epileptic patients. Exp Mol Med. 2004;36:185–191. doi: 10.1038/emm.2004.26. [DOI] [PubMed] [Google Scholar]
- 6.Ficarra V, Galfano A, Mancini M, Martignoni G, Artibani W. TNM staging system for renal-cell carcinoma: current status and future perspectives. Lancet Oncol. 2007;8(6):554–558. doi: 10.1016/S1470-2045507)70173-0. [DOI] [PubMed] [Google Scholar]
- 7.Fritzsche FR, Riener MO, Dietel M, Moch H, Jung K, Kristiansen G. GOLPH2 expression in renal cell cancer. BMC Urology. 2008;8(1):15. doi: 10.1186/1471-2490-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanash S. Disease proteomics. Nature. 2003;422(6928):226–232. doi: 10.1038/nature01514. [DOI] [PubMed] [Google Scholar]
- 9.Horiguchi A, Oya M, Shimada T, Uchida A, Marumo K, Murai M. Activation of signal transducer and activator of transcription 3 in renal cell carcinoma: a study of incidence and its association with pathological features and clinical outcome. J Urol. 2002;168(2):762–765. doi: 10.1016/S0022-5347(05)64741-6. [DOI] [PubMed] [Google Scholar]
- 10.Jang JS, Cho HY, Lee YJ, Ha WS, Kim HW. The differential proteome profile of stomach cancer: identification of the biomarker candidates. Oncol Res. 2004;14:491–499. doi: 10.3727/0965040042380441. [DOI] [PubMed] [Google Scholar]
- 11.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA: A Cancer Journal for Clinicians. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 12.Kim J, Hong SJ, Lim EK, Yu YS, Kim SW, Roh JH, Do IG, Joh JW, Kim DS. Expression of nicotinamide N-methyltransferase in hepatocellular carcinoma is associated with poor prognosis. J Exp Clin Cancer Res. 2009;28(1):20. doi: 10.1186/1756-9966-28-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim BH, Cho BI, Kim YN, Kim JW, Park ST, Lee CW. Overexpression of nicotinamide N-methyltransferase in gastric cancer tissues and its potential post-translational modification. Exp Mol Med. 2006;38:455–465. doi: 10.1038/emm.2006.54. [DOI] [PubMed] [Google Scholar]
- 14.Ma CF, Zhang J, Xie XY. Construction of recombinant plasmid nicotinamide N-methyltransferase gene and expression in E. coli . J Zhejiang Med. 2006;28(1):10–12. (in Chinese) [Google Scholar]
- 15.McLaughlin JK, Lipworth L. Epidemiologic aspects of renal cell cancer. Seminars in Oncology. 2000;27:115–123. [PubMed] [Google Scholar]
- 16.Papworth K, Bergh A, Grankvist K, Ljungberg B, Rasmuson T. Expression of erythropoietin and its receptor in human renal cell carcinoma. Tumor Biol. 2009;30(2):86–92. doi: 10.1159/000216844. [DOI] [PubMed] [Google Scholar]
- 17.Rini J, Szumlanski C, Guerciolini R, Weinshilboum RM. Human liver nicotinamide N-methyltransferase: ion-pairing radiochemical assay, biochemical properties and individual variation. Clin Chim Acta. 1990;186(3):359–374. doi: 10.1016/0009-8981(90)90322-J. [DOI] [PubMed] [Google Scholar]
- 18.Roessler M, Rollinger W, Palme S, Hagmann ML, Berndt P, Engel AM, Schneidinger B, Pfeffer M, Andres H, Karl J, et al. Identification of nicotinamide N-methyltransferase as a novel serum tumor marker for colorectal cancer. Clin Cancer Res. 2005;11(18):6550–6557. doi: 10.1158/1078-0432.CCR-05-0983. [DOI] [PubMed] [Google Scholar]
- 19.Sartini D, Muzzonigro G, Milanese G, Pierella F, Rossi V, Emanuelli M. Identification of nicotinamide N-methyltransferase as a novel tumor marker for renal clear cell carcinoma. J Urol. 2006;176(5):2248–2254. doi: 10.1016/j.juro.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 20.Sartini D, Santarelli A, Rossi V, Goteri G, Rubini C, Ciavarella D, Muzio L, Emanuelli M. Nicotinamide N-methyltransferase upregulation inversely correlates with lymph node metastasis in oral squamous cell carcinoma. Mol Med. 2007;13(7-8):415–421. doi: 10.2119/2007-00035.Sartini. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomida M, Ohtake H, Yokota T, Kobayashi Y, Kurosumi M. Stat3 up-regulates expression of nicotinamide N-methyltransferase in human cancer cells. J Cancer Res Clin Oncol. 2008;134(5):551–559. doi: 10.1007/s00432-007-0318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomida M, Mikami I, Takeuchi S, Nishimura H, Akiyama H. Serum levels of nicotinamide N-methyltransferase in patients with lung cancer. J Cancer Res Clin Oncol. 2009;135(9):123–1229. doi: 10.1007/s00432-009-0563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu J, Moatamed F, Caldwell JS, Walker JR, Kraiem Z, Taki K, Brent GA, Hershman JM. Enhanced expression of nicotinamide N-methyltransferase in human papillary thyroid carcinoma cells. J Clin Endocrinol Metab. 2003;88(10):4990–4996. doi: 10.1210/jc.2002-021843. [DOI] [PubMed] [Google Scholar]
- 24.Xu J, Capezzone M, Xu X, Hershman JM. Activation of nicotinamide N-methyltransferase gene promoter by hepatocyte nuclear factor-1beta in human papillary thyroid cancer cells. Mol Endocrinol. 2005;19(2):527–539. doi: 10.1210/me.2004-0215. [DOI] [PubMed] [Google Scholar]
- 25.Yan L, Otterness DM, Weinshilboum RM. Human nicotinamide N-methyltransferase pharmacogenetics: gene sequence analysis and promoter characterization. Pharmacogenetics. 1999;9(3):307–316. doi: 10.1097/00008571-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Yao M, Tabuchi H, Nagashima Y, Baba M, Nakaigawa N, Ishiguro H, Hamada K, Inayama Y, Kishida T, Hattori K, et al. Gene expression analysis of renal carcinoma: adipose differentiation-related protein as a potential diagnostic and prognostic biomarker for clear-cell renal carcinoma. J Pathol. 2005;205(3):377–387. doi: 10.1002/path.1693. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J. Are poly(ADP-ribosyl)ation by PARP-1 and deacetylation by Sir2 linked? BioEssays. 2003;25(8):808–814. doi: 10.1002/bies.10317. [DOI] [PubMed] [Google Scholar]
- 28.Zhang JB, Lu XM, Wei HP, Cheng LY, He J, Zhang ZP, Zhang XE, Yu ZN. Production and characterization of monoclonal antibodies to nucleoprotein of Marburg virus. Hybridoma. 2008;27(6):423–429. doi: 10.1089/hyb.2008.0044. [DOI] [PubMed] [Google Scholar]