Abstract
Gene loss has been proposed to play a major role in adaptive evolution, and recent studies are beginning to reveal its importance in human evolution. However, the potential consequence of a single gene-loss event upon the fates of functionally interrelated genes is poorly understood. Here, we use the purine metabolic pathway as a model system in which to explore this important question. The loss of urate oxidase (UOX) activity, a necessary step in this pathway, has occurred independently in the hominoid and bird/reptile lineages. Because the loss of UOX would have removed the functional constraint upon downstream genes in this pathway, these downstream genes are generally assumed to have subsequently deteriorated. In this study, we used a comparative genomics approach to empirically determine the fate of UOX itself and the downstream genes in five hominoids, two birds, and a reptile. Although we found that the loss of UOX likely triggered the genetic deterioration of the immediate downstream genes in the hominoids, surprisingly in the birds and reptiles, the UOX locus itself and some of the downstream genes were present in the genome and predicted to encode proteins. To account for the variable pattern of gene retention and loss after the inactivation of UOX, we hypothesize that although gene loss is a common fate for genes that have been rendered obsolete due to the upstream loss of an enzyme a metabolic pathway, it is also possible that same lack of constraint will foster the evolution of new functions or allow the optimization of preexisting alternative functions in the downstream genes, thereby resulting in gene retention. Thus, adaptive single-gene losses have the potential to influence the long-term evolutionary fate of functionally interrelated genes.
Keywords: adaptive evolution, gene loss, purine metabolism, urate oxidase, uric acid
Introduction
The potential contribution of gene duplication to the evolutionary process has long been appreciated (Ohno 1970), and though somewhat counterintuitive, gene loss has also been recognized as having the potential to contribute to the evolutionary process (Ohno 1970). More recently, gene loss has been hypothesized as a common molecular mechanism in adaptive evolution (Olson 1999). Specifically, in the less-is-more hypothesis, Olson proposes that loss of function alleles can arise frequently in a population and can be advantageous in certain environments: thus, natural selection for loss-of-function alleles can lead to the loss of an active copy of a particular gene from an entire population. Beyond the examples of advantageous gene-loss events described by Olson (1999) across taxa, there are now numerous examples of gene loss in the human lineage and contemporary populations that could have contributed to the evolution of our species. For instance, null alleles for several genes are segregating in the human population at high frequencies, some of which either show signatures of positive selection or have been associated with a beneficial fitness effect (Novembre et al. 2005; Xue et al. 2006, 2008; Perry et al. 2007; Seixas et al. 2007). Moreover, genome-wide scans for gene loss along with targeted studies of specific genes have uncovered a number of recent gene inactivation events during the course of human evolution (Varki 2001; Stedman et al. 2004; Wang et al. 2006; Zhu et al. 2007) and each of these could represent beneficial gene-loss events that occurred at various times in the distant past.
The central focus in terms of the evolutionary potential of such gene loss is the immediate benefit the lack of gene activity might confer on the individual. Yet the loss of a single gene can also have long-term consequences on the evolutionary fate of other genes as well. For example, if a metabolic pathway is disrupted by the loss of a specific enzymatic activity, then the genes that encode the enzymes that function downstream in that pathway will be rendered obsolete, and as a consequence, the downstream genes will no longer be subject to the evolutionary constraint previously required to maintain their functional role in the pathway. This loss of constraint triggered by a single gene inactivation event may then eventually lead to the loss of the downstream genes (Tanaka et al. 2005).
A classic example of gene loss in a metabolic pathway is the loss of urate oxidase (UOX) activity (EC 1.7.3.3) in the hominoid and bird/reptile lineages (Keilin 1959; Urich 1994). UOX is a peroxisomal enzyme in the purine catabolic pathway that functions primarily in the liver and converts uric acid to 5-hydroxyisourate (HIU; Hayashi et al. 2000; Ramazzina et al. 2006) (fig. 1). Loss of UOX activity was inferred in humans and great apes early in the 20th century via biochemical studies that revealed uric acid as the end product of purine metabolism in hominoids (Wiechowski 1909; Wells and Caldwell 1914) and subsequently by direct assays for UOX activity (e.g., see Friedman et al. 1985). Similarly, birds and reptiles were also inferred to lack UOX activity and direct assays for UOX activity have confirmed this result (Remy et al. 1951; Keilin 1959; Urich 1994). Because the lack of UOX activity leads to increased serum uric acid levels and makes humans particularly susceptible to gout, the loss of this enzyme has been considered an “evolutionary accident” (Walker et al. 1954). However, a series of adaptive hypotheses have been put forward to explain the potential beneficial effects the increased serum uric acid levels may have provided our ancient hominoid ancestors. Specifically, due to uric acid's structural similarity to caffeine, increased serum uric acid levels have been proposed to have resulted in increased cognitive abilities in hominoids as a consequence of uric acid's potential stimulatory effects (Haldane 1955; Orowan 1955). A second hypothesis is that because uric acid is an effective antioxidant, increased serum uric acid levels could result in a longer life span and reduce the incidence of age-related cancer (Ames et al. 1981). More recently, a third hypothesis has been proposed in which the loss of UOX provided a mechanism to maintain optimal blood pressure in ancestral hominoids that subsisted on a low-sodium, low-purine diet (Watanabe et al. 2002). Independent adaptive hypotheses related to water conservation and/or embryonic environment have also been proposed to account for the loss of UOX activity in birds and reptiles (Campbell et al. 1987).
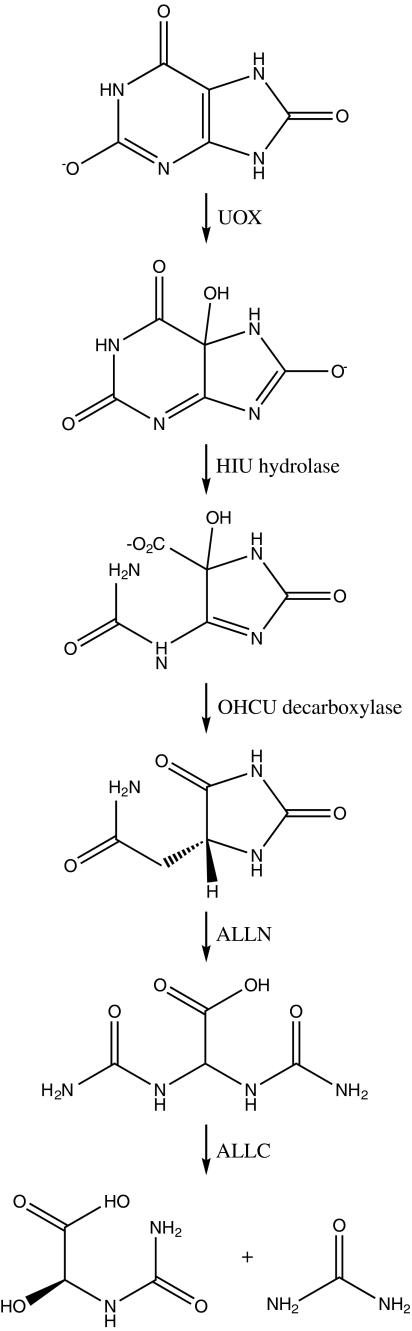
FIG. 1.
The purine catabolic pathway. The substrates, products, and enzymes of UOX and downstream enzymes in the vertebrate purine catabolic pathway. UOX (substrate uric acid), HIU hydrolase (EC 3.5.2.17, substrate HIU), OHCU decarboxylase (EC 4.1.1.–, substrate OHCU), ALLN (EC 3.5.2.5; substrate is allantoin), ALLC (EC 3.5.3.4; substrate is allantoate and products are ureidoglycolate and urea).
Although the evolutionary history of the loss of UOX function in the hominoids has been well characterized (Wu et al. 1989, 1992; Yeldandi et al. 1991; Oda et al. 2002), the impact this gene-loss event had on the downstream genes in the purine catabolic pathway have only been cursorily examined (Ramazzina et al. 2006; Keebaugh and Thomas 2009). Furthermore, there are conflicting reports as to the fate of UOX in birds and reptiles (Przylecki and Rogalski 1927; Remy et al. 1951; Varela-Echavarria et al. 1988; Ito et al. 1989; Andersen et al. 2006) as well as the status of the downstream genes (Ramazzina et al. 2006; Keebaugh and Thomas 2009). Here we report a detailed study of genes that encode the purine catabolic enzymes in hominoids, birds, and reptiles designed to characterize and compare the evolutionary fate of these genes in those three lineages and propose a formal model articulating the potential indirect effects that a single gene-loss event can have on the long-term evolution of functionally interrelated genes.
Materials and Methods
Identification of Orthologs Encoding the Purine Catabolic Pathway
Orthologs of the terminal enzymes in the purine catabolic pathway (UOX, HIUase hydrolase, 2-oxo-4-hydroxy-4-carboxy-5-ureidoimidazoline [OHCU] decarboxylase, allantoinase [ALLN], and allantoicase [ALLC]) were compiled from a previously published data set (Keebaugh and Thomas 2009). In particular, the TreeFam database of precomputed gene families derived from whole-genome gene annotations (Li et al. 2006) was used to extract orthologous gene sets from four teleosts (fugu [Takifugu rubripes], tetraodon [Tetraodon nigroviridis], zebrafish [Danio rerio], and stickleback [Gasterosteus aculeatus]), an amphibian (frog [Xenopus tropicalis]), a bird [chicken (Gallus gallus)], four non-hominoid placental mammals (cow [Bos taurus], dog [Canis familiaris], mouse [Mus musculus], rat (Rattus norvegicus)], and a hominoid [human (Homo sapiens)]. Orthologs from an additional bird (zebra finch [Taeniopygia guttata]), reptile (lizard [Anolis carolinesis]), two nonhuman primates (marmoset [Callithrix jacchus] and rhesus monkey [Macaca mulatta]), and hominoid (chimpanzee [Pan troglodytes]) were identified by a combination of Blast searches (best hit, minimum e value of <1 × 10−20) (Altschul et al. 1997) and use of genome-wide multiple-species alignments (Karolchick et al. 2008) of publicly available genome assemblies for those species and were required to map to the predicted orthologous genomic interval compared with the known genes (i.e., display conserved synteny). For three additional hominoids (gorilla [Gorilla gorilla], orangutan [Pongo pygmaeus abelii], and gibbon [Nomascus leucogenys]), orthologs were compiled from published UOX sequences and Blast searches (minimum e value of <1 × 10−50) of unassembled genome sequences generated at The Genome Sequencing Center at Washington University (http://genome.wustl.edu/), The Baylor College of Medicine Human Genome Sequencing Center (http://www.hgsc.bcm.tmc.edu/), The Wellcome Trust Sanger Institute (http://www.sanger.ac.uk/), and the Broad Institute (http://www.broadinstitute.org/). Consensus sequences for each exon based on alignments to the whole-genome shotgun reads were assembled into a predicted cDNA, and orthology of these sequences was confirmed by requiring that the predicted cDNAs were the reciprocal best matches to the human genome and by constructing a gene tree (see Supplementary fig. 1, Supplementary Material online). Although differences between the established species phylogeny and the trees of the individual genes were observed in the gene trees, these discrepancies are most likely the result of a lack of power to accurately resolve the true phylogeny of the genes due to the small sample size of sites available for each gene and were particularly apparent among the closely related primates. Indeed, the bootstrap values provided in Supplementary figure 1 (Supplementary Material online) show that the discrepancies between the gene and species trees were not strongly supported. Sequence identifiers and manually annotated sequences are provided in Supplementary table 1 and Supplementary data file 1 (Supplementary Material online). The complete sets of orthologs were aligned with ClustalX (Jeanmougin et al. 1998) and the resulting alignments manually edited.
Classification of Orthologs
Orthologs were classified as a gene if it had an open-reading frame, was evolving under purifying selection, and had a conserved intron/exon structure; as a pseudogene if the protein-coding sequence was truncated by early nonsense mutations and/or evolving neutrally; or absent if homology searches of the assembled genome (and when available expressed sequence tag [EST] databases) and direct analysis of the predicted syntenic genomic location failed to detect the gene of interest.
Sequence Evolution
Alignments of the protein-coding sequence of each gene were generated, and a maximum likelihood method was implemented using the “codeml” program in PAML (Yang 1997) to estimate the ratio of nonsynonymous (Ka) to synonymous (Ks) substitutions, that is, Ka/Ks. The following phylogeny was used in the estimation and comparison of Ka/Ks in the hominoids to the other vertebrates: ((((Marmoset, (Rhesus, (Gibbon, (Orangutan, (Gorilla, (Chimp, Human)))))), (Rat, Mouse)), (Dog, Cow)), Xenopus, (((Fugu, Tetraodon), Stickleback), Zebrafish)), with the exception of HIU hydrolase and ALLC for which the marmoset sequence was not included due to limited sequence availability or because the marmoset gene was potentially a pseudogene, respectively. The following phylogeny was used in the estimation and comparison of Ka/Ks in the birds and reptile to the other vertebrates: (((((Marmoset, Rhesus), (Rat, Mouse)), (Dog, Cow)), ((Chicken, Zebra finch), Anolis lizard)), Xenopus, (((Fugu, Tetraodon), Stickleback), Zebrafish)) again excluding marmoset from the HIU hydrolase and ALLC analyses, and the chicken and zebra finch sequences when they were unavailable (i.e., ALLN and ALLC). For each set of orthologs, we tested for significant variation in Ka/Ks within the hominoids by comparing a pair of models using the likelihood ratio test (LRT; Yang 1998). Specifically, in the first model, two Ka/Ks values were estimated across the phylogeny: one for the hominoid branches (which are labeled in fig. 2) and one for the rest of the tree (M2: two rate). This model was compared with one in which a Ka/Ks was estimated for each hominoid branch and a single Ka/Ks was estimated for the rest of the tree (M2: free rate among hominoids). Note that for all genes the LRT test of the M2: free rate among the hominoid branches did not detect a significant difference (i.e., all P values > 0.01) between these models, and thus, for each gene, a single Ka/Ks was assigned and reported for all hominoid branches, including the terminal branches. To test if the estimated hominoid Ka/Ks was different than the rest of the tree, the M2: two-rate model described above was compared with a model in which a single Ka/Ks was estimated for the entire tree (M0) using the LRT. To test if the estimated hominoid Ka/Ks was different than a value of 1, the M2: two-rate model was compared with a model in which the Ka/Ks of the hominoid branches was set equal to 1 and a single Ka/Ks was estimated for the rest of the tree (M2: two-rate with hominoid set equal to 1) using the LRT. In addition, a z-test (Suzuki and Gojobori 2003) was also applied to the Ka and Ks values estimated using the M2: free rate among the hominoids model to test if the Ka and Ks values were equal (i.e., Ka/Ks = 1). Note that in all cases, the results of the z-test were consistent with the LRT test. Note that because a focal point of this study was on the evolution of these genes in the hominoids since the inactivation of UOX, only branches in the hominoid lineage since their most recent common ancestor were grouped together for the Ka/Ks analyses (see fig. 2). In other words, because UOX was presumably active in the branch leading to the most recent common ancestor of hominoids from the node representing the most recent common ancestor of the hominoids and Old World monkeys, this lineage was included with the “rest” of the tree and not grouped with other hominoid branches. Analogous models and statistical tests were applied to the birds and reptile data sets.
FIG. 2.
Gene content of the purine catabolic pathway in vertebrates. The tree on the left represents the accepted vertebrate phylogeny with divergence dates (Ma) (Kumar and Hedges 1998). The species included in each category are teleosts (stickleback, fugu and tetraodon, and zebrafish), an amphibian (Xenopus), a reptile (Anolis lizard), two birds (chicken and zebra finch), and placental mammals, including the hominoids (gibbon, gorilla, orangutan, human, and chimpanzee) and other placental mammals (mouse, rat, dog, and cow). The gene content and classification of the purine catabolic pathway for each group of species is summarized on the right. Genes were defined as having an open-reading frame, were evolving under purifying selection, and had a conserved intron/exon structure. Predicted pseudogenes were identified by protein-coding sequences interrupted by truncating mutations and/or a Ka/Ks not significantly different than 1. Note that in the case of the marmoset ALLC gene, two potential frameshift mutations were used to classify it as a predicted pseudogene. When no ortholog could be identified, the gene was classified as absent. “*” Indicates the hominoid branches and “**” the birds and reptile branches used to calculate the Ka/Ks for those clades (see Materials and Methods).
RNA Isolation and cDNA Sequencing in the Zebra Finch
Total RNA was isolated from ∼100 mg of frozen zebra finch liver by homogenization with TRIzol (Invitrogen), and first-strand cDNA synthesis was performed with the SuperScript First Strand Synthesis Kit (Invitrogen). The region spanning the predicted exon 2 to the 3′ untranslated region was amplified by reverse transcription polymerase chain reaction (RT-PCR) using primers 5′-TGA TAC CTA CGG ACA CCA TAA AG-3′ and 5′-TGA CAT TTC CTC TCA TCT CTC ATC-3′. To amplify the 5′ end of the gene, 5′ rapid amplification of cDNA ends (RACE) was carried out using the GeneRacer Core Kit (Invitrogen). The Gene Racer 5′ primer and UOX-specific reverse primer 5′-GAT GTG GGG AAC GCC ATC CTC ATG-3′ were used in the initial RT-PCR (RT-PCR). Cycling conditions for the 5′ RACE were initial denaturation of 1 cycle at 94 °C for 2 min, 5 cycles at 94 °C for 30 s followed by 72 °C for 1 min, 5 cycles at 94 °C for 30 s followed by 70 °C for 1 min, 25 cycles with a denaturation temperature of 94 °C for 30 s, an annealing temperature of 65 °C for 30 s, and an elongation temperature for 72 °C for 1 min. The final extension was at 72 °C for 10 min. One-fifth (5 μl) of the initial PCR reaction was used as the template for nested PCR using the GeneRacer 5′ nested primer and the UOX-specific nested reverse primer 5′-GAC GTA CGC CAC TTG GCA AAA TGT TGT C-3′. The same cycle parameters were used as for the initial RT-PCR. The PCR products were treated with Exonuclease I and Shrimp Alkaline Phosphatase and directly sequenced using the following internal primers: 5′-TGA TAC CTA CGG ACA CCA TAA AG-3′, 5′-TGA CAT TTC CTC TCA TCT CTC ATC-3′, 5′-ACA CTG ACA TGG ACT GAA GGA-3′, and 5′-TGG TGT CCG TAC GTA TCA CAA-3′. The resulting partial cDNA sequences for the zebra finch UOX gene have been deposited in GenBank (EU068002 and EU068003).
Results
Predicted Fate of the Hominoid and Bird/Reptile Purine Catabolic Genes
Previously, we reported the characterization of UOX and four downstream genes (HIU hydrolase, OHCU decarboxylase, ALLN, and ALLC) in a broad sampling of vertebrates, including two hominoids, a bird, and a reptile (Keebaugh and Thomas 2009). In order to rigorously determine the evolutionary fate of these genes in hominoids and birds/reptiles, we reexamined our previous data set and expanded our study to include a total of five hominoids (human, chimpanzee, gorilla, orangutan, and gibbon), two birds (chicken and zebra finch), and a reptile (Anolis lizard). We then classified each of the purine catabolic genes in the hominoids, birds, and reptile as either present or absent in the genome, and if present, whether the genes were likely to be functional or to represent pseudogenes (see Materials and Methods for functional classification criteria as well as details regarding the sequence evolution analyses). A summary of the results of these analyses is presented in figure 2. Note that previous work has established that teleosts and amphibians have complete purine catabolic pathways (Urich 1994) and were included here as a control set of genes as a point of comparison for the hominoids, birds, and reptiles. Similarly, the other placental mammals included in the study have a functional purine catabolic pathway that can be inferred to include UOX, HIU hydrolase, and OHCU decarboxylase (Urich 1994) and are also known to encode the gene for ALLC (Keebaugh and Thomas 2009) were also included as a control set of genes.
UOX
UOX enzymatic activity is absent in hominoids, and inactivating mutations for this gene have already been described in the five species included in this study (Wu et al. 1989, 1992; Oda et al. 2002). In addition, we were able to confirm that as expected for a pseudogene the hominoid UOX loci were found to be evolving neutrally (i.e., a Ka/Ks = 1.38 which was not significantly different from 1, table 1). The hominoid UOX genes therefore met both criteria for being a pseudogene (Ka/Ks = 1 and the presence of one or more mutations that disrupt the open reading frame) and were classified as such (fig. 2). In contrast, no obvious inactivating mutations were detected in the bird and reptile UOX genes. Moreover, the Ka/Ks for the bird and reptile clade was 0.26, which though significantly higher than for the control set of vertebrates was significantly less than 1 (table 1). These results suggest that the UOX locus in birds and reptiles continues to encode a protein that is evolving under purifying selection despite presumably having lost its activity as a purine catabolic enzyme in the most recent common ancestor of these species which dates back some ∼200 million years ago (Ma) (Kumar and Hedges 1998).
Table 1.
Estimations of Ka/Ks.
| Hominoid |
Birds and Reptile |
|||||||
| Gene | Non | Syn | Hominoid Ka/Ks | Other Vertebrates Ka/Ks | Non | Syn | Bird and Reptile Ka/Ks | Other Vertebrates Ka/Ks |
| UOX | 574 | 197 | 1.38a,b | 0.09 | 576 | 195 | 0.26a | 0.10 |
| HIU hydrolase | 134 | 37 | 0.27 | 0.11 | 112 | 32 | 0.16 | 0.16 |
| OHCU decarboxylase | 315 | 105 | 0.83a,b | 0.18 | NA | NA | NA | NA |
| ALLN | NA | NA | NA | NA | 849 | 327 | 0.10c | 0.09 |
| ALLC | 705 | 267 | 0.31 | 0.14 | 685 | 248 | 0.30c | 0.14 |
NOTE.—Non, nonsynonymous sites; Syn, synonymous sites; NA, not available.
Indicates a hominoid or bird and reptile Ka/Ks not significantly different (P < 0.01) from 1 based on LRT and z-test.
Indicates a Ka/Ks significantly different (P < 0.01) from the other vertebrates based on LRT test.
Indicates lizard only.
HIU Hydrolase
Mixed results were observed for the gene immediately downstream of UOX, HIU hydrolase, in the hominoids (fig. 2). Specifically, potential inactivating mutations were detected in the human, chimpanzee, and gibbon but not the gorilla or orangutan HIU hydrolases genes (table 2). In addition, the Ka/Ks ratio in the hominoids was 0.27, which was significantly different than 1 and not different from the value observed for the other vertebrates (table 1). Thus, by virtue of a lack of inactivating mutations and a Ka/Ks ratio consistent with functional constraint, the orangutan and gorilla HIU hydrolase genes were classified as a gene in that pair of species, whereas the presence of inactivating mutations in the three other hominoids in this gene led us to classify HIU hydrolase in those species as a pseudogene. In the bird/reptile clade, no inactivating mutations were detected in HIU hydrolase and the calculated Ka/Ks = 0.16 indicated that this gene has been evolving under functional constraint comparable to that of other vertebrates in which this gene encodes a functional enzyme (table 1). Based on those results, HIU hydrolase was classified as gene in the birds and reptile (fig. 2). Thus, a mixed pattern of functional constraint and pseduogenization was observed in the hominoid HIU hydrolase genes, while this gene has been actively retained in the bird and reptile genomes.
Table 2.
Inactivating mutations in the hominoid HIU hydrolase genes.
| Mutationa |
|||||
| Species | c.1A>G | c.2T>C | c.163_179delb | c.234_235delc | c.315_331deld |
| Human | − | + | + | − | + |
| Chimp | + | + | − | − | − |
| Orangutan | − | − | − | − | − |
| Gorilla | − | − | − | − | − |
| Gibbon | − | + | − | + | − |
NOTE.—(+), mutation present; (−), mutation absent.
aMutations based on coordinates from mouse HIU hydrolase coding sequence (GenBank accession number NM_029821).
Fourteen base pair frameshift deletion leads to an early stop at codon 67.
Leads to early stop at codon 107.
Fourteen base pair frameshift deletion.
OHCU Decarboxylase
Two inactivating mutations, a 1-bp deletion at position 244 and a 4-bp deletion at positions 431–434 (relative to the mouse protein-coding sequence in GenBank accession number NM_001039678), were detected in the gibbon OHCU decarboxylase gene, strongly suggesting this is a pseudogene (fig. 2). Although inactivating mutations were absent in the other hominoids, the hominoid Ka/Ks of 0.83, which was not significantly different from 1 (table 1), was consistent with OHCU decarboxylase being a pseudogene in the other species in this clade as well (fig. 2). The OHCU decarboxylase locus could not be detected in the genome assemblies or EST resources available for either bird or the Anolis lizard; thus, it was classified as absent in those species (fig. 2).
ALLN
Prior to the loss of UOX in the hominoid lineage, the purine catabolic pathway was truncated by the loss of ALLN in the most recent common ancestor of all placental mammals (Keebaugh and Thomas 2009). As a result, though ALLN is absent from the hominoid genomes, the fate of this gene was already determined prior to the inactivation of UOX and should not have been influenced by that event (fig. 2). ALLN was absent in chicken and zebra finch but was detected in the Anolis lizard (fig. 2). The terminal branch leading to the Anolis lizard ALLN gene had a Ka/Ks indicative of purifying selection (Ka/Ks = 0.1), which was not significantly different from the Ka/Ks ratio observed in species in which ALLN is known to be active (i.e., the frog and teleosts, table 1 and fig. 2). Thus, the ALLN gene has been selectively maintained in the reptile lineage but presumably lost in birds.
ALLC
As mentioned above, ALLN was lost in the common ancestor of eutherians and the next gene downstream in the pathway, ALLC, would thus have already been rendered obsolete as a purine catabolic enzyme when UOX was inactivated in the hominoid lineage. As has been shown previously for humans and other eutherians (Keebaugh and Thomas 2009), ALLC was present in all the hominoids and like other vertebrates had a Ka/Ks ratio that was significantly less than 1 (Ka/Ks = 0.31, table 1 and fig. 2). Thus, ALLC was classified as encoding a gene in the hominoids. ALLC was also present in the Anolis lizard and was classified as a gene in this species based on a Ka/Ks = 0.3, which was similar to that seen in other vertebrates and significantly less than 1 (table 1). In contrast, we could not detect ALLC in either the chicken or zebra finch. The variability of the status of ALLC within the birds and reptile was reminiscent of what we observed for ALLN and could reflect a true difference between these species or it could be the result of incomplete EST and genomic sequence data for those species.
Timing of the Gene Inactivation Events in the Hominoid Lineage
Loss of UOX activity is believed to have occurred twice in the hominoid lineage, once in the gibbon lineage ∼10 Ma and once in the lineage leading to humans and the great apes ∼15 Ma (Oda et al. 2002). Based on the assumption that the loss of UOX was the evolutionary force that precipitated the potential inactivation of some hominoid HIU hydrolase and OHCU decarboxylase genes, we predicted that those genes would have begun to evolve neutrally at a time point that postdates the inactivation of UOX. To test this hypothesis, we first re-estimated the timing of the UOX inactivation events using the method described in Chou et al. (2002) and calibrated the date according to the accepted divergence between hominoids and Old World monkeys (23 Ma) (Raaum et al. 2005). In keeping with what has been reported previously, we estimated the inactivation of UOX occurred ∼13.1 Ma in the gibbon lineage and ∼12.9 Ma in the human and great ape lineage. The lack of a common inactivating mutation in the HIU hydrolase gene among the hominoids (table 2) and a Ka/Ks estimate typical of genes evolving under purifying selection are qualitatively consistent with either very recent or no loss of functional constraint on this gene. Application of the method to estimate the date of gene inactivation to HIU hydrolase produced a broad range of estimates from ∼2 to 16 Ma. However, it should be noted that these estimates are based on a very small sample size (total of 171 synonymous and nonsynonymous sites, table 1) and very few substitutions and, therefore, are not expected to be accurate. In the case of OHCU decarboxylase, the presence of just one inactivating in gibbon and none of the other hominoids was compatible with recent inactivations, and the dating method predicted that the protein-coding sequence for these genes began to evolve neutrally in the hominoids ∼4.8–12.6 Ma. Thus, the combined qualitative and quantitative estimates of the potential timing of the predicted inactivation of the HIU hydrolase and OHCU decarboxylase genes suggest that they occurred after the inactivation of UOX.
Molecular Characterization of the Bird and Reptile UOX Loci
The lack of UOX activity in birds and reptiles is consistent with this enzyme having been inactivated in the most recent common ancestor of those species some ∼200 Ma. Though there have been previous reports of UOX transcripts and protein expression in the chicken embryo (Ito et al. 1989), we were still quite surprised to find putatively functional UOX loci in the birds and the Anolis lizard given the clear evidence that pseudogenization was observed in a much shorter time frame (∼10–15 My) in the hominoid lineage (fig. 2). We therefore carried out a more detailed characterization of the predicted bird and Anolis lizard UOX loci to confirm that these genes are indeed transcribed and to determine whether there were any notable differences in the genomic structure of the UOX loci in these species versus other vertebrates.
Spliced ESTs corresponding to the chicken (GenBank accession numbers BU465474 and CV037459) and Anolis lizard (GenBank accession numbers FG720659, FG802163, FG682219, FG720611, FG802116, and FG671812) UOX loci were readily detected, indicating that these genes are indeed transcribed in those species. To directly validate transcription of UOX, we performed RT-PCR and 5′ RACE on total RNA extracted from the adult liver of a zebra finch. By sequencing the amplified cDNA products, we were able to confirm that the zebra finch UOX locus was also transcribed. Note that in the process of sequencing the partial zebra finch UOX cDNAs, we identified five single-nucleotide polymorphisms in the protein-coding portion of this gene. As would be expected for a functional gene evolving under purifying selection, the majority (4/5) of these polymorphisms were synonymous substitutions.
The UOX gene in other vertebrates has a conserved intron–exon structure consisting of eight exons (fig. 3A). In order to compare the intron–exon structure of the bird and reptile UOX loci to other vertebrates, the predicted cDNA sequences and ESTs were used to infer the intron–exon structure of UOX in those species (fig. 3). The intron–exon structure of the interior exons (exons 2–7, mouse gene as a reference) and the splice site of the 3′ terminal coding exon (exon 8) were conserved between the birds/reptile and the other vertebrates. However, the protein-coding regions of the 3′ terminal exons in the birds/reptile displayed no obvious homology to the other vertebrate proteins across its entire length and were 21–23 amino acids longer than the other vertebrate proteins. In addition, the first protein-coding exon in birds mapped to a distinct location in chicken and zebra finch compared with the other vertebrates (compare fig. 3A and B). Due to the partial nature of the predicted cDNA in the Anolis lizard, we were unable to establish where the first protein-coding exon was in that species. However, we were able detect a protein-coding exon upstream of the conserved exon 2 that did not display any conservation with the bird or any other vertebrate genome (fig. 3C). Thus, overall, our analyses were consistent with a functional protein being encoded by the UOX locus in chicken, zebra finch, and Anolis lizard, although the encoded proteins have divergent N- and C-terminal ends.
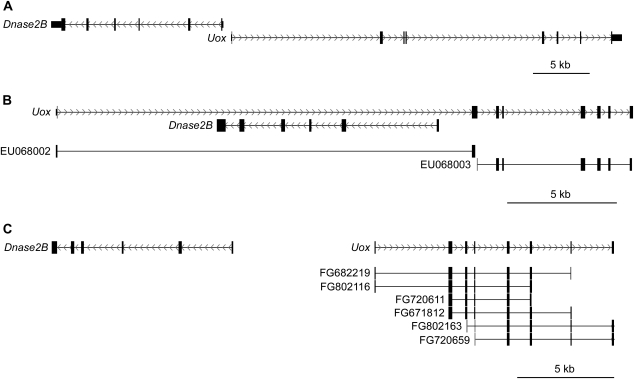
FIG. 3.
Intron–exon structure of the UOX gene. The intron–exon structures of UOX along with the neighboring DNASE2B gene and supporting ESTs and partial mRNAs are shown relative to their chromosomal locations for mouse (chr3:146244338–146294447, mm9) (A), zebra finch (chr8:13247864-13271492, taeGut1) (B), and Anolis lizard (scaffold_16:5170254-5205217, anoCar1) (C). Boxes indicate the location of exons and arrows indicate the direction of transcription for each gene. ESTs are labeled with their GenBank accession number. Note the differences in scale between the panels.
Discussion
It was generally assumed that after the inactivation of UOX, the downstream genes in the purine catabolic pathway would have been rendered obsolete and thus lost (Keilin 1959). Here we have shown that there is mixed evidence that this assumption is valid in the hominoid lineage for the two immediate downstream genes, HIU hydrolase and OCHU decarboxylase. In the case of both HIU hydrolase and OCHU decarboxylase, inactivating mutations were found in a least one hominoid, consistent with their inactivation. On the other hand, the lack of inactivating mutations in HIU hydrolase and OHCU decarboxylase in all hominoids may indicate that in some species they still encode functional proteins or, perhaps, are in the initial phase of genetic degeneration. In particular, for a number of reasons, the pattern of protein evolution inferred from the Ka/Ks values is by no means a definitive indicator of functional versus nonfunctional genes and is especially true for a gene that has only just recently been inactivated. In addition, the small number of sites available to estimate Ka/Ks, particularly in HIU hydrolase, makes it especially difficult to generate reliable predictions based solely on that value for that gene. Therefore, only those genes we found to contain inactivating mutations can be confidently considered pseudogenes, whereas the few genes classified as pseudogenes based solely on a Ka/Ks not different than 1 should be considered provisional until functional assays can be performed. Within the bird/reptile lineage, the UOX gene itself and some of the downstream genes (one of four genes in birds and three of four genes in the Anolis lizard) have been retained in the genome of these species, despite the fact that they are no longer required to encode functional purine catabolic enzymes. Below we discuss the specific results of our study with respect to the evolution of the purine catabolic pathway and then articulate a synthetic model for the long-term consequences of a gene-loss event on functionally interrelated genes.
Though previous studies have reported evidence for the transcription of UOX and the expression of a UOX protein in the chicken embryo (Przylecki and Rogalski 1927; Ito et al. 1989), UOX activity is widely accepted to have been lost in the bird and reptile lineage over 200 Ma (Urich 1994). Why then can transcripts corresponding to the UOX locus in the birds and reptiles be detected if there is a general lack of UOX enzymatic activity (Varela-Echavarria et al. 1988)? One possibility is that in the common ancestor of birds and reptiles, the UOX gene acquired one or more mutations that led to the loss of the enzymatic activity associated with purine catabolism (Andersen et al. 2006) but escaped the fate of becoming a pseudogene. Because there is one report of UOX activity in chicken embryos (Przylecki and Rogalski 1927), we cannot rule out that the mutations accounting for the loss of enzymatic activity may be further restricted to the protein isoforms expressed in the adults: for example, by alterations in the intracellular localization of the protein caused by the divergent C- or N-terminal regions of the protein (Andersen et al. 2006). It is also possible that, although UOX transcripts are present in the adult liver, they may never be translated. Future experiments that characterize the bird and reptile UOX proteins will be needed to explore these possibilities.
The inferred evolutionary fate of the genes downstream of UOX varied both among genes, as well as between the birds and Anolis lizard. Specifically, we observed strong evidence that, like the UOX locus, the HIU hydrolase locus in the birds and the Anolis lizard encodes a functional gene that has persisted in both the bird and reptile lineages despite having presumably been rendered obsolete ∼200 Ma. Additionally, although ALLN and ALLC appear to have been lost in the bird lineage, these two genes have also been actively retained in the Anolis lizard lineage. The retention of these downstream genes in one or both lineages is reminiscent of the fate of ALLC in eutherian genomes which has also been actively maintained in the mammalian genome for an extended period of time despite the inactivation of the upstream enzyme (ALLN) (Vigetti et al. 2002, 2003; Keebaugh and Thomas 2009). On the other hand, our data suggest that the gene-encoding OHCU decarboxylase was either lost in the most recent common ancestor of the birds and reptiles or was lost independently in both lineages.
Our observation that some of the downstream genes in the purine catabolic pathway have been retained in the genome, despite becoming dispensable millions of years ago, is not unprecedented (Tamir and Ratner 1963; Vigetti et al. 2003; Tanaka et al. 2005). For example, birds have been classified as lacking a urea cycle, and the chicken is devoid of the enzymatic activity of carbamoyl phosphate synthetase 1 (CPS1) that initiates this metabolic pathway (Tamir and Ratner 1963). However, like UOX, the gene that encodes CPS1 is present in the chicken genome and expressed in some tissues (Hillier et al. 2004). Furthermore, the chicken genome also contains all the genes necessary to encode a complete urea cycle (Hillier et al. 2004), though as was the case with the downstream purine catabolic enzymes, those genes were presumably rendered dispensable after the loss of CPS1 activity. Indeed, vestigial enzymatic activity has been detected for all the enzymes in the urea cycle in the chicken (except CPS1), although not in the liver which is the primary site of activity for this pathway in mammals (Tamir and Ratner 1963).
What potential mechanisms of natural selection could therefore be responsible for the long-term persistence of genes in a population once they are no longer required to function in a particular biochemical pathway? To formally address this question, we propose the following general model that incorporates accepted theories related to the fate of newly duplicated genes and previous hypotheses related to the persistence of seemingly obsolete genes in the genome (Vigetti et al. 2003; Tanaka et al. 2005; Andersen et al. 2006). Inactivation is the most likely fate for a duplicate gene due to the loss of functional constraint caused by genetic redundancy (Lynch and Conery 2003). Similarly, the most probable fate of a gene that has been rendered obsolete either directly or indirectly by an inactivating mutation is genetic degeneration and loss from the genome (Tanaka et al. 2005). However, some duplicate genes are retained in the genome due to the acquisition of a new function (neofunctionalization) (Ohno 1970), the partitioning of the ancestral functions between both members of the pair (subfunctionalization) (Hughes 1994; Force et al. 1999), or both (He and Zhang 2005). Analogously, it is thus also possible that once a gene acquires an inactivating mutation or is rendered obsolete by a proximal mutation in a pathway, it can acquire previously “forbidden” (Ohno 1970) mutations that could result in the acquisition of a new beneficial function, thereby resulting in the selection for the retention of this new allele (and thus the “old” gene) in the genome (Vigetti et al. 2003). Alternatively, in cases where a gene has more than one function, a mutation may abrogate one but not all those functions. Such “partially” inactivated genes could thus be retained in the genome simply because of selection for the remaining functions. Likewise, genes that are rendered obsolete by a proximal mutation in one pathway may be retained merely because they have one or more other functions (Tanaka et al. 2005). In addition, the potential relaxation in functional constraint caused by a gene having been freed from performing a previously required activity could also foster the acquisition of secondary mutations that enhance the remaining functions of that gene. And finally, note that while the above text refers explicitly to the potential evolutionary fates of downstream genes in a metabolic pathway, gene loss would also trigger a similar loss of functional constraint on other proteins that previously had direct physical interactions with the protein that is no longer expressed. Thus, gene loss could alter the evolutionary potential and fate of any type of functionally interrelated gene.
In this context, we propose that the genes that have been retained since the loss of UOX activity in the birds and reptiles have persisted because they either had one or more function outside purine catabolism before UOX enzyme activity was lost or they gained a novel function in the time interval after UOX activity was lost but before they genetically deteriorated. Because there are many well-documented examples of proteins, even those in ancient biochemical pathways, performing more than one function (i.e., “moonlighting”) (Jeffery 1999; Sriram et al. 2005), we view the existence of one or more functions that predate the loss of UOX as the most likely basis for the retention of the genes that encode the downstream purine catabolic enzymes. Note that it is also strictly possible that the surviving genes may at one point in their history have become inactivated pseudogenes, which were then resurrected to their current functional form (Brosius and Gould 1992; Bekpen et al. 2009). However, we view this possibility as unlikely given that the Ka/Ks for most of the retained genes were similar to those observed in known functional orthologs.
In conclusion, the less-is-more hypothesis posits that under certain environmental conditions, gene loss can be beneficial and therefore contribute to the evolutionary process (Olson 1999). When beneficial, gene loss will thus have an immediate impact on the fitness of an individual, and the inactivated allele will spread rapidly through a small population. Though Olson (1999) reviewed the potential of an inactivated allele to revert to a functional allele, the less-is-more hypothesis does not take into account the long-term adaptive consequences the loss of a single gene can have on functionally linked genes. The results presented here highlight the possibility that the loss of a single gene can have long-term consequences on the adaptive potential of many genes, and we have articulated a model whereby in addition to the direct benefits associated with a gene inactivation event, a single-gene loss may indirectly allow downstream genes to optimize a secondary function or evolve a completely novel function. With the development of complete protein networks that map out the protein–protein interactions and all functional connections between proteins, in the future, it should be possible to quantify the influence gene loss has had on the adaptive evolution of other proteins.
Supplementary Material
Supplementary figure 1, table 1, and data file 1 are available at Molecular Biology and Evolution online (http://www.mbe.oxfordjournals.org/).
Supplementary Material
Acknowledgments
The authors would like to thank the members of A.C.K.’s thesis committee, D.H. Ledbetter, T. Schlenke, S.V. Yi, and M.E. Zwick for their comments on the manuscript and the technical writing edits by C. Strauss. The authors were supported by a grant from the National Institutes of Health (1R21NS060935).
References
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA. 1981;78:6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O, Aas TS, Skugor S, Takle H, van Nes S, Grisdale-Helland B, Helland SJ, Terjesen BF. Purine-induced expression of urate oxidase and enzyme activity in Atlantic salmon (Salmo salar). Cloning of urate oxidase liver cDNA from three teleost species and the African lungfish Protopterus annectens. FEBS J. 2006;273:2839–2850. doi: 10.1111/j.1742-4658.2006.05288.x. [DOI] [PubMed] [Google Scholar]
- Bekpen C, Marques-Bonet T, Alkan C, Antonacci F, Legrande MB, Ventura M, Kidd JM, Siswara P, Howard JC, Eichler EE. Death and resurrection of the human IRGM gene. PLoS Genet. 2009;5:e1000403. doi: 10.1371/journal.pgen.1000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J, Gould SJ. On “genomenclature”: a comprehensive (and respectful) taxonomy for pseudogenes and other “junk DNA”. Proc Natl Acad Sci USA. 1992;89:10706–10710. doi: 10.1073/pnas.89.22.10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JW, Vorhaben JE, Smith DD., Jr Uricoteley: its nature and origin during the evolution of tetrapod vertebrates. J Exp Zool. 1987;243:349–363. doi: 10.1002/jez.1402430302. [DOI] [PubMed] [Google Scholar]
- Chou HH, Hayakawa T, Diaz S, Krings M, Indriati E, Leakey M, Paabo S, Satta Y, Takahata N, Varki A. Inactivation of CMP-N-acetylneuraminic acid hydroxylase occurred prior to brain expansion during human evolution. Proc Natl Acad Sci USA. 2002;99:11736–41. doi: 10.1073/pnas.182257399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Force A, Lynch M, Pickett FB, Amores A, Yan YL, Postlethwait J. Preservation of duplicate genes by complementary, degenerative mutations. Genetics. 1999;151:1531–1545. doi: 10.1093/genetics/151.4.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman TB, Polanco GE, Appold JC, Mayle JE. On the loss of uricolytic activity during primate evolution-I. Silencing of urate oxidase in a hominoid ancestor. Comp Biochem Physiol B. 1985;3:653–659. doi: 10.1016/0305-0491(85)90381-5. [DOI] [PubMed] [Google Scholar]
- Haldane JB. Origin of man. Nature. 1955;176:169–170. doi: 10.1038/176169a0. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Fujiwara S, Noguchi T. Evolution of urate-degrading enzymes in animal peroxisomes. Cell Biochem Biophys. 2000;32:123–129. doi: 10.1385/cbb:32:1-3:123. [DOI] [PubMed] [Google Scholar]
- He X, Zhang J. Rapid subfunctionalization accompanied by prolonged and substantial neofunctionalization in duplicate gene evolution. Genetics. 2005;169:1157–1164. doi: 10.1534/genetics.104.037051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier LW, Miller W, Birney E, et al. (185 co-authors) Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature. 2004;432:695–716. doi: 10.1038/nature03154. [DOI] [PubMed] [Google Scholar]
- Hughes AL. The evolution of functionally novel proteins after gene duplication. Proc Biol Sci. 1994;256:119–124. doi: 10.1098/rspb.1994.0058. [DOI] [PubMed] [Google Scholar]
- Ito M, Nakamura M, Ogawa H, Takagi Y. Sequence analysis of rat liver uricase-cDNA and the possible presence of the homologous cDNA sequences in chicken embryo. Adv Exp Med Biol. 1989;253A:507–510. doi: 10.1007/978-1-4684-5673-8_82. [DOI] [PubMed] [Google Scholar]
- Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ. Multiple sequence alignment with Clustal X. Trends Biochem Sci. 1998;23:403–405. doi: 10.1016/s0968-0004(98)01285-7. [DOI] [PubMed] [Google Scholar]
- Jeffery CJ. Moonlighting proteins. Trends Biochem Sci. 1999;24:8–11. doi: 10.1016/s0968-0004(98)01335-8. [DOI] [PubMed] [Google Scholar]
- Karolchick D, Kuhn RM, Baertsch R, et al. (26 co-authors) The UCSC Genome Browser Database: 2008 update. Nucleic Acids Res. 2008;36:D773–D779. doi: 10.1093/nar/gkm966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keebaugh AC, Thomas JW. The genomes of the South American opossum (Monodelphis domestica) and platypus (Ornithorhynchus anatinus) encode a more complete purine catabolic pathway than placental mammals. Comp Biochem Physiol. Part D Genomics Proteomics. 2009;4:174–178. doi: 10.1016/j.cbd.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilin J. The biological significance of uric acid and guanine excretion. Biol Rev Camb Philos Soc. 1959;34:265–296. [Google Scholar]
- Kumar S, Hedges SB. A molecular timescale for vertebrate evolution. Nature. 1998;392:917–920. doi: 10.1038/31927. [DOI] [PubMed] [Google Scholar]
- Li H, Coghlan A, Ruan J, et al. (15 co-authors) TreeFam: a curated database of phylogenetic trees of animal gene families. Nucleic Acids Res. 2006;34:D572–D580. doi: 10.1093/nar/gkj118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M, Conery JS. The evolutionary demography of duplicate genes. J Struct Funct Genomics. 2003;3:35–44. [PubMed] [Google Scholar]
- Novembre J, Galvani AP, Slatkin M. The geographic spread of the CCR5 Delta32 HIV-resistance allele. PLoS Biol. 2005;3:e339. doi: 10.1371/journal.pbio.0030339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda M, Satta Y, Takenaka O, Takahata N. Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol Biol Evol. 2002;19:640–653. doi: 10.1093/oxfordjournals.molbev.a004123. [DOI] [PubMed] [Google Scholar]
- Ohno S. Evolution by gene duplication. Springer: Berlin (Germany); 1970. [Google Scholar]
- Olson MV. When less is more: gene loss as an engine of evolutionary change. Am J Hum Genet. 1999;64:18–23. doi: 10.1086/302219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orowan E. The origin of man. Nature. 1955;175:683–684. doi: 10.1038/175683a0. [DOI] [PubMed] [Google Scholar]
- Perry GH, Dominy NJ, Claw KG, et al. (13 co-authors) Diet and the evolution of human amylase gene copy number variation. Nat Genet. 2007;39:1256–1260. doi: 10.1038/ng2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przylecki SJ, Rogalski L. La loi biogenetique et les fonctions des organisms vivants. I. La presence de l'uricase chez les embryons des oiseaux. Arch Int Physiol. 1927;28:423–430. [Google Scholar]
- Raaum RL, Sterner KN, Noviello CM, Stewart CB, Disotell TR. Catarrhine primate divergence dates estimated from complete mitochondrial genomes: concordance with fossil and nuclear DNA evidence. J Hum Evol. 2005;48:237–257. doi: 10.1016/j.jhevol.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Ramazzina I, Folli C, Secchi A, Berni R, Percudani R. Completing the uric acid degradation pathway through phylogenetic comparison of whole genomes. Nat Chem Biol. 2006;2:144–148. doi: 10.1038/nchembio768. [DOI] [PubMed] [Google Scholar]
- Remy C, Richert DA, Westerfeld WW. The determination of xanthine dehydrogenase in chicken tissues. J Biol Chem. 1951;193:649–657. [PubMed] [Google Scholar]
- Seixas S, Suriano G, Carvalho F, Seruca R, Rocha J, Di Rienzo A. Sequence diversity at the proximal 14q32.1 SERPIN subcluster: evidence for natural selection favoring the pseudogenization of SERPINA2. Mol Biol Evol. 2007;24:587–598. doi: 10.1093/molbev/msl187. [DOI] [PubMed] [Google Scholar]
- Sriram G, Martinez JA, McCabe ER, Liao JC, Dipple KM. Single-gene disorders: what role could moonlighting enzymes play? Am J Hum Genet. 2005;76:911–924. doi: 10.1086/430799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman HH, Kozyak BW, Nelson A, Thesier DM, Su LT, Low DW, Bridges CR, Shrager JP, Minugh-Purvis N, Mitchell MA. Myosin gene mutation correlates with anatomical changes in the human lineage. Nature. 2004;428:415–418. doi: 10.1038/nature02358. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Gojobori T. Analysis of coding sequences. In: Salemi M, Vandamme AM, editors. The phylogenetic handbook: a practical approach to DNA and protein phylogeny. Cambridge University Press: Cambridge; 2003. [Google Scholar]
- Tamir H, Ratner S. Enzymes of arginine metabolism in chicks. Arch Biochem Biophys. 1963;102:249–258. doi: 10.1016/0003-9861(63)90178-4. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Tateno Y, Gojobori T. Evolution of vitamin B6 (pyridoxine) metabolism by gain and loss of genes. Mol Biol Evol. 2005;22:243–250. doi: 10.1093/molbev/msi011. [DOI] [PubMed] [Google Scholar]
- Urich K. Comparative animal biochemistry. 1994. Berlin (Germany): Springer-Verlag. [Google Scholar]
- Varela-Echavarria A, Montes de Oca-Luna R, Barrera-Saldana HA. Uricase protein sequences: conserved during vertebrate evolution but absent in humans. FASEB J. 1988;2:3092–3096. doi: 10.1096/fasebj.2.15.3192041. [DOI] [PubMed] [Google Scholar]
- Varki A. Loss of N-glycolylneuraminic acid in humans: mechanisms, consequences, and implications for hominid evolution. Am J Phys Anthropol. 2001;33(Suppl):54–69. doi: 10.1002/ajpa.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigetti D, Binelli G, Monetti C, Prati M, Bernardini G, Gornati R. Selective pressure on the allantoicase gene during vertebrate evolution. J Mol Evol. 2003;57:650–658. doi: 10.1007/s00239-003-2515-5. [DOI] [PubMed] [Google Scholar]
- Vigetti D, Pollegioni L, Monetti C, Prati M, Bernardini G, Gornati R. Property comparison of recombinant amphibian and mammalian allantoicases. FEBS Lett. 2002;512:323–328. doi: 10.1016/s0014-5793(02)02264-0. [DOI] [PubMed] [Google Scholar]
- Walker BS, Boyd WC, Asimov I. Biochemistry and human metabolism. 1954. London: Baillere, Tindall and Cox. [Google Scholar]
- Wang X, Grus WE, Zhang J. Gene losses during human origins. PLoS Biol. 2006;4:e52. doi: 10.1371/journal.pbio.0040052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Kang DH, Feng L, Nakagawa T, Kanellis J, Lan H, Mazzali M, Johnson RJ. Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension. 2002;40:355–360. doi: 10.1161/01.hyp.0000028589.66335.aa. [DOI] [PubMed] [Google Scholar]
- Wells HG, Caldwell GT. The purine enzymes of the orang-utan (Simia satyrus) and chimpanzee (Anthropopithecus troglodytes) J Biol Chem. 1914;18:157–165. [Google Scholar]
- Wiechowski W. Das Vorhandensein van Allantoin im normalen Menschenharn und seine Bedeutung fur die Beurteilung des menschlichen Harnsaurestoffwechsels. Biochem Z. 1909;19:368–383. [Google Scholar]
- Wu XW, Lee CC, Muzny DM, Caskey CT. Urate oxidase: primary structure and evolutionary implications. Proc Natl Acad Sci USA. 1989;86:9412–9416. doi: 10.1073/pnas.86.23.9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XW, Muzny DM, Lee CC, Caskey CT. Two independent mutational events in the loss of urate oxidase during hominoid evolution. J Mol Evol. 1992;34:78–84. doi: 10.1007/BF00163854. [DOI] [PubMed] [Google Scholar]
- Xue Y, Daly A, Yngvadottir B, et al. (14 co-authors) Spread of an inactive form of caspase-12 in humans is due to recent positive selection. Am J Hum Genet. 2006;78:659–670. doi: 10.1086/503116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Sun D, Daly A, et al. (12 co-authors) Adaptive evolution of UGT2B17 copy-number variation. Am J Hum Genet. 2008;83:337–346. doi: 10.1016/j.ajhg.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. PAML: a program package for phylogenetic analysis by maximum likelihood. Comput Appl Biosci. 1997;13:555–556. doi: 10.1093/bioinformatics/13.5.555. [DOI] [PubMed] [Google Scholar]
- Yang Z. Likelihood ratio tests for detecting positive selection and application to primate lysozyme data. Mol Biol Evol. 1998;15:568–573. doi: 10.1093/oxfordjournals.molbev.a025957. [DOI] [PubMed] [Google Scholar]
- Yeldandi AV, Yeldandi V, Kumar S, Murthy CV, Wang XD, Alvares K, Rao MS, Reddy JK. Molecular evolution of the urate oxidase-encoding gene in hominoid primates: nonsense mutations. Gene. 1991;109:281–284. doi: 10.1016/0378-1119(91)90622-i. [DOI] [PubMed] [Google Scholar]
- Zhu J, Sanborn JZ, Diekhans M, Lowe CB, Pringle TH, Haussler D. Comparative genomics search for losses of long-established genes on the human lineage. PLoS Comput Biol. 2007;3:e247. doi: 10.1371/journal.pcbi.0030247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.