Abstract
The impact of single-nucleotide polymorphisms (SNPs) of the DNA repair gene XPC on DNA repair capacity (DRC) and genotoxicity has not been comprehensively determined. We constructed a comprehensive haplotype map encompassing all common XPC SNPs and evaluated the effect of Bayesian-inferred haplotypes on DNA damage associated with tobacco smoking, using chromosome aberrations (CA) as a biomarker. We also used the mutagen-sensitivity assay, in which mutagen-induced CA in cultured lymphocytes are determined, to evaluate the haplotype effects on DRC. We hypothesized that if certain XPC haplotypes have functional effects, a correlation between these haplotypes and baseline and/or mutagen-induced CA would exist. Using HapMap and single nucleotide polymorphism (dbSNP) databases, we identified 92 SNPs, of which 35 had minor allele frequencies ≥ 0.05. Bayesian inference and subsequent phylogenetic analysis identified 21 unique haplotypes, which segregated into six distinct phylogenetically grouped haplotypes (PGHs A–F). A SNP tagging approach used identified 11 tagSNPs representing these 35 SNPs (r2 = 0.80). We utilized these tagSNPs to genotype a population of smokers matched to nonsmokers (n = 123). Haplotypes for each individual were reconstituted and PGH designations were assigned. Relationships between XPC haplotypes and baseline and/or mutagen-induced CA were then evaluated. We observed significant interaction among smoking and PGH-C (p = 0.046) for baseline CA where baseline CA was 3.5 times higher in smokers compared to nonsmokers. Significant interactions among smoking and PGH-D (p = 0.023) and PGH-F (p = 0.007) for mutagen-induced CA frequencies were also observed. These data indicate that certain XPC haplotypes significantly alter CA and DRC in smokers and, thus, can contribute to cancer risk.
Keywords: DNA nucleotide excision repair, XPC gene, polymorphism, haplotypes, biomarkers, chromosome, smoking, cancer
Smoking is associated with a high risk of cancer at many organs (IARC, 1986). Not all smokers, however, develop cancer, which clearly indicates a significant interindividual variation in metabolism of tobacco carcinogens and in repair of the resulting genetic damage (Liu et al., 2005). In fact, studies have consistently shown a significant association between reduced DNA repair capacity (DRC) and increased risk of tobacco-related cancers (Shen et al., 2003; Zhu et al., 2007). The nucleotide excision repair (NER) is the major DNA repair pathway that removes genetic damage resulting from exposure to many tobacco carcinogens (Friedberg, 2001). An important protein in this pathway is the xeroderma pigmentosum complementation group C (XPC) protein, which plays a key role as a part of the DNA damage–recognition complex (Araki et al., 2001). XPC is the only protein in this complex that directly binds to the damaged DNA (Park and Choi, 2006) to initiate the NER process through the recruitment of other proteins, including xeroderma pigmentosum complementation group A (XPA), transcription factor II H (TFIIH), xeroderma pigmentosum complementation group G (XPG), and replication protein A (RPA) (Bunick et al., 2006).
The XPC gene spans 33 kb and encodes a 940 amino acid protein (Genbank accession No. AC090645). XPC is highly polymorphic, with many single-nucleotide polymorphisms (SNPs) in the exonic region and the intronic, 3′ and 5′ untranslated regions (UTRs), including the promoter region. Only a few of these SNPs, namely the exon 16 variant K939Q (rs2228001), exon 8 variant A499V (rs2228000), intron 11–5 splice site C/A (rs3729587), and intron 9 PolyAT insertion, have been studied as potential modifiers of cancer risk in humans. Many epidemiological studies have shown associations between these SNPs and risk for human cancer for many organs (e.g., An et al., 2007; Guo et al., 2008; Hansen et al., 2007; Zhu et al., 2007).
Over 90 SNPs in the XPC gene have been reported in the International HapMap Project (www.hapmap.org) and the National Center for Biotechnology Information (NCBI) single nucleotide polymorphism (dbSNP) (www.ncbi.nlm.nih.gov/projects/SNP) databases. The phenotypic and/or functional effects of these SNPs have not yet been characterized, including their impact on DNA damage response and DRC. Analysis of the potential effect of each of these SNPs on disease risk, or evaluation of their individual phenotypic effects, is certainly impractical. However, it is well known that genetic variation in human populations is not arrayed simply as independent SNPs but, rather, as various combinations of SNPs or “haplotypes”. This is because some of the individual SNPs, often those located in close proximity to one another, are correlated and exist in degrees of linkage disequilibrium (LD). This creates identifiable haplotypes, comprising several SNPs (Gabriel et al., 2002). Therefore, the phenotypic effects of haplotypes, rather than that of individual SNPs, should be examined in studies designed to determine the role of genetic variability in relation to disease outcome. A practical approach to achieve this goal would be to identify subsets of SNPs that accurately identify haplotypes. Such SNPs could be identified using a “tagging SNPs (tagSNPs)” strategy (Johnson et al., 2001). Thus, a subset of all SNPs (i.e., tagSNPs) in a given gene region, highly correlated with other SNPs, could then be selected for analysis, significantly reducing the volume of genotyping needed. This approach is biologically more plausible, and more comprehensive, since it involves the evaluation of effects of multiple SNPs that could jointly influence disease outcome.
To our knowledge, a comprehensive haplotype analysis of the entire XPC genomic sequence has not been conducted. Furthermore, an evaluation of the functional effects of the XPC haplotypes, with regard to their effect on DRC, has not yet been pursued. In the current study, we constructed a comprehensive haplotype map encompassing SNPs of the XPC gene that are reported to exist with a minor allele frequency (MAF) ≥ 0.05 in the general population. We hypothesized that if certain XPC haplotypes have phenotypic or functional effects, there would be a correlation between these haplotypes and genetic damage in individuals exposed to environmental carcinogens, such as those found in tobacco smoke. Genetic damage was evaluated in our study population using chromosome aberrations (CA) as a biomarker since increased frequency of CA in circulating peripheral blood lymphocytes (PBLs) is considered an indication of increased cancer risk (Bonassi et al., 2000; Hagmar et al., 1998). In addition, we used the mutagen-sensitivity assay, in which CA frequency is determined following exposure of cultured PBLs to a known mutagen. This is a biomarker that serves as an indirect measure for DRC and as an intermediate phenotype for cancer risk (Hsu et al., 1991; Spitz et al., 1995).
MATERIALS AND METHODS
Study subjects and blood collection.
The study protocol was approved by the University of Texas Medical Branch (UTMB) Institutional Review Board. All study subjects signed a written consent form that described the purpose of the study. A total of 123 White non-Hispanic subjects participated in this study. They were subjects who were a subset of a larger cohort recruited without regard to age, sex, or ethnicity from the smoking and nonsmoking staff and student population of UTMB in Galveston, TX. This cohort is composed of individuals who had responded to posted notices and advertisements requesting volunteers for studies aimed at understanding the functional and biological significance of sequence variability in DNA repair genes. Participation in this study was open to White non-Hispanics only to avoid potential problems with admixtures when developing the tagSNPs analysis. TagSNPs are not applicable to all races/ethnicities, and separate sets of tagSNPs would need to be developed for each ethnic/racial group. White non-Hispanics are accurately represented in HapMap by the CEPH population (Utah residents with ancestry from northern and western Europe; abbreviated and thereafter referred to as CEU).
Individuals were defined as nonsmokers if they had smoked less than 100 cigarettes during their lifetime. Individuals were defined as current smokers if they had smoked at least five cigarettes per day for at least 1 year prior to enrollment in the study. Smokers (n = 62) were matched to nonsmokers (n = 61) based on age (± 5 years) and sex. Participants were asked to fill out a questionnaire that provided demographic, occupational, and medical information. Also collected was information regarding smoking habits, including number of cigarettes per day, preferred brand, duration of smoking, former tobacco use, and use of other tobacco products. Exclusion criteria for all volunteers included a recent acute viral or bacterial infection; a major chronic illness, such as cancer or an autoimmune disorder; a recent blood transfusion; treatment with mutagenic agents, such as chemotherapeutic drugs or radiation; excessive alcohol consumption, defined as more than a 10 g serving per day (as determined by nationwide standard practices); and employment involving exposure to potentially mutagenic agents. Because of these criteria, only apparently healthy volunteers were included in the study to control for potential confounders. A blood sample (10 ml) was obtained from each volunteer for genotype analysis and cytogenetic cultures.
Identification of tagSNPs.
The HapMap Data Release 22 phase II assembly (HapMap online database at www.hapmap.org data release 22 phase II NCBI assembly B36 dbSNP b126) was used as the source of genotypes for this study. Genotypes for all SNPs reported in the genomic region encompassing XPC were obtained from the International HapMap Project database representing the CEU population. The CEU population sample is the one that is the most ethnically similar to our sample of self-reported White non-Hispanic subjects from UTMB who were evaluated in this study. We examined 2 kb of the 5′ UTR of XPC since this region contains elements controlling XPC gene expression, and we also examined the entire gene region and 2 kb of the 3′ UTR. Genotypes for the CEU population were screened using Haploview ver. 4.1 to ensure that only SNPs with a MAF of 0.05 or greater were used in the subsequent haplotype inference. Next, we used Tagger software (www.broad.mit.edu/mpg/tagger) to identify tagSNPs for assay design and subsequent haplotype determination. Specifically, we used an aggressive multimarker approach (up to six markers) restricted to SNPs with a MAF ≥ 0.05. We conservatively set the r2 threshold to ≥ 0.8 (mean value 0.971) and used a logarithm of odds score for estimating a recombination frequency heterogeneity threshold of 2.
Genotyping of tagSNPs.
Custom-designed real-time PCR-based assay kits using the TaqMan chemistry from Applied Biosystems (Foster City, CA) were used for genotyping tagSNPs. Each kit was developed to our specifications using fluorescent probes that were designed to anneal to the designated SNP, dependent on its sequence as determined from the reference SNP (rs) number designated for that SNP in the NCBI dbSNP database (http://www.ncbi.nlm.nih.gov/SNP/). Allele-specific probes were labeled with either the FAM or the VIC fluorophore and an appropriate quencher. The PCR consisted of TaqMan universal master mix, template DNA, and target-assay mix in a total reaction volume of 12 μl at concentrations recommended by Applied Biosystems. Thermal cycling was carried out in our laboratory on an MJ Research DNA Engine thermocycler (from a subsidiary of BioRad Labs) equipped with a computerized BioRad Chromo4 real-time PCR detection system (Hercules, CA), under recommended conditions (50°C, 2 min; 95°C, 10 min; and 40 cycles at 95°C for 15 s and 58–61°C for 1 min). Designation of referent and polymorphic forms was determined by the FAM to VIC ratio. For quality control, all PCRs were run in duplicate, and, along with no-template negative controls, positive controls for each possible genotypic combination were included when possible. Samples were coded for case-control status so that the operator interpreting the results was blinded to the smoking status of the subject. Samples from smokers and nonsmokers were run together in mixed batches, and 10% of the samples were randomly selected and subjected to repeat analysis, as another quality-control measure for verification of genotyping results. Additionally, genotypes for all tagSNPs were analyzed for deviations from Hardy-Weinberg equilibrium (HWE) on a locus-by-locus basis using two methods implemented in LD Analyzer ver. 1.0. The first method is a standard two-sided Pearson chi-squared test and is rapid and computationally simple. The second method relies on a Monte Carlo permutation–based exact test to estimate deviations from HWE. Any SNP failing the test was excluded from the study as an added quality-control measure.
Construction of XPC haplotypes and phylogenetic analysis.
Our strategy consisted of first using the HapMap data on the CEU population as a resource to infer possible XPC haplotypes. These inferred haplotypes were then used to develop a tagSNPs panel. These tagSNPs were subsequently used to genotype our study participants. Based on the genotyping results, individuals were then assigned to haplotypes corresponding to those inferred from the CEU population. Haplotypes were inferred from the CEU population, using Bayesian statistics implemented in PHASE ver. 2.1 software (www.stat.washington.edu/stephens/phase.html), formatting the input file to account for the family trios comprising the CEU sample. The number of iterations was increased to 10,000, the thinning interval was increased to 10, and the burn-in was increased to 200 to improve the accuracy of the inferred haplotypes. The default setting was selected with an output posterior probability threshold of 0.9. To ensure accuracy of reported results, individuals lacking defined genotype data for more than one SNP were excluded from the analysis. In addition, individuals lacking identification of a single SNP that prevented the accurate assignment of full haplotypes were also excluded from further analysis.
Because a substantial number of inferred haplotypes were expected, making the number of potential statistical comparisons problematic, a phylogenetic grouping approach was used to group or cluster evolutionarily related haplotypes from the CEU population. Genetic distances were computed among haplotypes using the maximum likelihood composite model implemented in MEGA 4 (http://www.megasoftware.net/). Distances among haplotypes were then phylogenetically clustered using the neighbor-joining method in MEGA 4. Phylogenetically related haplotypes were given group designations for further statistical comparisons and analysis. The use of the tagSNPs panel derived from the CEU population allowed us to assign haplotypes to our population corresponding to CEU-inferred haplotypes and subsequently to groups based on the phylogenetic analysis of the complete SNP panel from the CEU population. Grouping of haplotypes, based on genealogical or phenotypic relationships, previously has been used successfully by many other investigators (Bardel et al., 2009; Rieder et al., 2005; Veenstra et al., 2005). Phylogenetically grouped haplotypes (PGHs), which share strong genealogical similarities, serve to substantially increase the statistical power of analyses by reducing the number of groups to be evaluated.
Cytogenetic cultures and the mutagen-sensitivity assay.
Cultures for cytogenetic assays were established according to standard procedures (Evans and O'Riordan, 1975), as routinely done in our laboratory (Abdel-Rahman and El-Zein, 2000; Affatato et al., 2004). Briefly, aliquots of 1 ml of PBLs were cultured with 9 ml of RPMI 1640 medium supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 10% fetal bovine serum, and 2mM L-glutamine (Invitrogen, Carlsbad, CA). Stimulation of PBLs was accomplished by the addition of 0.18 mg/ml phytohemagglutinin (reagent grade; Remel, Lenexa, KS). Two cultures were set up for each subject: one culture was not treated to give a baseline in vivo CA frequency and the second culture was used for the mutagen-sensitivity assay. After 46 h, the suspended cells in the second culture were centrifuged and the growth medium reserved. The PBLs were then resuspended in 5 ml serum-free RPMI 1640 supplemented with 0.24mM of the mutagen and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) (CAS#64091-91-4, National Cancer Institute, Midwest Carcinogen Repository, Kansas City, MO) and incubated at 37°C in the presence of 5% CO2 for 1 h. Following NNK treatment, the PBLs were washed twice with serum-free RPMI 1640, transferred to clean tubes and resuspended in the original growth medium until harvested. Harvesting was performed 72 h after NNK treatment. The mutagen concentration and harvest times had been established from our previous studies and have been shown to produce measurable levels of genetic damage and low levels of toxicity over a period of time that allows the effects of DNA repair to be manifest (Abdel-Rahman and El-Zein, 2000; Affatato et al., 2004).
Cell culture harvest and cytogenetic analysis.
Prior to harvest, cells from all cultures were treated with 0.1 μg/ml colcemid (Gibco-Invitrogen) for 1 h to arrest the cells in metaphase. The cultures of PBLs were centrifuged and the cells resuspended in hypotonic solution (0.075M potassium chloride), fixed with Carnoy's fixative (three parts methanol/one part acetic acid, vol/vol), and stored at 4°C. Slides for cytogenetic analysis were then prepared in duplicate by spreading the fixed cells on the slides and staining them with Giemsa. One hundred metaphase cells on each slide were scored for CAs using a Nikon 400 light microscope, according to standard procedures (ISCN, 1985). Aberrations were recorded as chromosome breaks or frank chromatid breaks. Chromatid breaks were counted as one break and chromosome breaks as two breaks. Total aberrant cells were recorded as a percentage of aberrant cells (breaks per 100 cells). For quality control, slides were coded before scoring to protect against scorer bias. Cells from slides prepared from both smokers and nonsmokers were scored blindly in mixed batches. To ensure quality control, 20% of the slides were randomly selected for blind rescoring. Agreement between the original data and rescored data was measured using the Cohen's kappa statistical test. A statistically significant value of p < 0.001 was obtained for both baseline and mutagen-induced CA, indicating that the agreement between the original and rescored data was not attributable to random chance.
Statistical analysis.
Each individual was coded for the presence (+) or absence (−) of each PGH. We used descriptive statistical analyses [mean (± standard errors of the mean; SEM)] for continuous variables and frequencies for categorical variables to characterize the study population. We compared mean CA frequencies for each PGH (present vs. absent) using preliminary Student's two-sample t-tests. In order to account for the fact that we were performing multiple tests in our comparison of baseline and mutagen-induced CA frequencies within each PGH group separately, we completed a permutation test with 1000 replicates (PGH present/absent status was randomly permuted within each replicate) to calculate empirical p values, respectively, for each PGH comparison. Permutations test corrections are known to be robust and have the benefit that the empirical p value is constructed directly from the experimental data at hand (Cheverud, 2001). We completed the same procedure upon stratification by smoking status (nonsmokers and smokers). Guided by these preliminary results, a general linear statistical model that included the final parameters estimated from the exploratory analysis was then fit to evaluate differences in CA frequency involving interactions between each PGH and smoking, separately for each PGH, adjusted for age and gender. We constructed error-bar plots (depicting mean and 95% confidence interval limits) to graphically visualize statistically significant interactions.
RESULTS
Characteristics of the Study Population
The study population consisted of 123 White non-Hispanic subjects. We were able to obtain full haplotype data on 99 of the 123 individuals, and therefore, only those 99 subjects were included in all subsequent analyses. Of these individuals, 78 were females (78.8%) and 21 were males (21.2%). There were 50 smokers and 49 nonsmokers, who were matched with respect to age (± 5 years) and sex. The smokers had smoked between 5 and 50 cigarettes per day (mean ± SD: 17.4 ± 1.26) for a minimum of 1 year (mean ± SD: 19.8 ± 1.62 years) before participating in the study. The age of the participants ranged from 20 to 72 years, with a median of 37 years and a mean (± SD) of 39.0 (± 1.30) years. There was no significant difference in the smoking habits (total number of smoking years, number of cigarettes smoked per day, and pack years, defined as packs smoked per day × the number of smoking years) between males and females. The mean ± SEM frequency of baseline CA frequency for the study population was 0.79 ± 0.10. After mutagen challenge, the mean ± SEM frequency of mutagen-induced CA was 5.24 ± 0.29.
Identification of tagSNPs, Structures of XPC Haplotypes, and Phylogenetic Analysis
At the time of the analysis, using the information available on the CEU population from HapMap, we identified 92 SNPs encompassing the entire coding region, the introns, and 2 kb upstream and 2 kb downstream of the coding region of the XPC gene. Of these, 35 SNPs were predicted to occur with a MAF ≥ 0.05. We identified 11 tagSNPs (the bolded and underlined rs numbers in Table 1), which tagged the 35 SNPs with a correlation coefficient of r2 = 0.8. These 11 tagSNPs were used for subsequent genotyping of the study population. This linkage-based genotyping significantly reduced the volume of unique genotyping assays and concurrently reduced the effort and time required to evaluate the effect of all 35 SNPs on genetic damage. The 35 SNPs tagged by these 11 tagSNPs and their position on the XPC gene are presented in Table 1.
TABLE 1.
SNPs Existing with a MAF ≥ 0.05 in the XPC Gene
| rsa | Alleles | Ancestral allele | Haplotype position | Variation site |
| 8516 | C/T | T | 9 | 3′ UTR |
| 10468 | C/T | T | 9 | 3′ UTR |
| 1126547 | C/G | G | 1 | 3′ UTR |
| 2470352 | A/T | A | 2 | 3′ UTR |
| 2229090 | C/G | C | 9 | 3′ UTR |
| 2228001 | A/C | C | 3 | Exon 16b |
| 2733532 | C/T | T | 3 | Intron 15 |
| 2733533 | A/C | C | 11 | Intron 15 |
| 2733534 | C/G | G | 11 | Intron 15 |
| 2279017 | G/T | T | 3 | Intron 12 |
| 2470353 | C/G | G | 11 | Intron 12 |
| 2607734 | A/G | A | 3 | Intron 11 |
| 2607736 | A/G | A | 3 | Intron 11 |
| 2607737 | C/T | C | 11 | Intron 11 |
| 3731149 | A/C | A | 8 | Intron 10 |
| 3731146 | G/T | T | 8 | Intron 10 |
| 9653966 | G/T | T | 4 | Intron 10 |
| 1124303 | G/T | T | 5 | Intron 10 |
| 3731143 | C/T | T | 6 | Intron 10 |
| 2228000 | C/T | C | 9 | Exon 9c |
| 2227999 | A/G | G | 6 | Exon 9d |
| 3731127 | C/T | C | 7 | Intron 8 |
| 3731125 | A/G | A | 4 | Intron 7 |
| 3731124 | A/C | A | 8 | Intron 7 |
| 13099160 | A/G | A | 7 | Intron 7 |
| 1106087 | G/T | G | 9 | Intron 5 |
| 3731108 | C/T | C | 8 | Intron 5 |
| 3731106 | A/G | A | 8 | Intron 5 |
| 3729587 | C/G | C | 8 | Intron 5 |
| 3731093 | C/T | T | 4 | Intron 3 |
| 2733537 | A/G | A | 10 | Intron 3 |
| 3731081 | G/T | G | 8 | Intron 3 |
| 3731068 | A/C | C | 8 | Intron 2 |
| 1350344 | A/G | G | 11 | Intron 1 |
| 2607775 | C/G | C | 11 | 5′ UTR |
Reference SNP (rs) numbers are those designated by the dbSNP database of the NCBI (http://www.ncbi.nlm.nih.gov/SNP/). Bold and underlined rs numbers correspond to the 11 tagSNPs used in genotyping analysis of the 35 SNPs identified with MAF > 0.05 in the XPC gene.
The rs2228001 (A/C) SNP in exon 16 results in a lysine to glutamine amino acid change in codon 939 (K939Q).
The rs2228000 (C/T) SNP in exon 9 results in a valine to arginine amino acid change in codon 499 (V499R).
The rs 2227999 (A/G) SNP in exon 9 results in a histidine to arginine amino acid change at codon 492 (R492H).
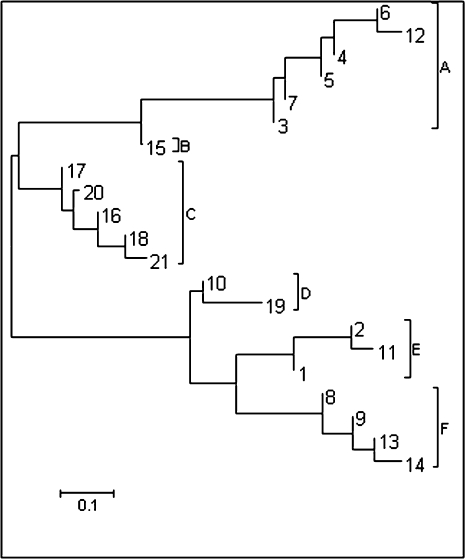
Using the information available on the CEU population, we utilized the Bayesian-inference analysis implemented in PHASE ver. 2.1 software (www.stat.washington.edu/stephens/software/html), which revealed 21 unique haplotypes. Table 2 shows the full 21 haplotypes, as generated by the PHASE analysis used in this study. Phylogenetic analysis of these haplotypes was used to assess genealogical relationships among these 21 haplotypes, utilizing the maximum likelihood model implemented in MEGA 4 software (http://www.megasoftware.net/). Haplotypes were grouped based on clade formation and percent sequence divergence. Six clades were apparent in the midpoint-rooted cluster analysis corresponding to the PGHs A–F. Percent sequence divergence within groups ranged from 4.8% in PGH-F to 8.6% in PGH-D. Percent sequence divergence between groups ranged from 18.6% (PGH-A and PGH-B) to 57.3% (PGH-A and PGH-E). Since there is no firm objective metric that exists for deciding what should constitute acceptable levels of within- and between-group or clade percent sequence divergence for this type of analysis, the apparently “natural” groups and divisions in this case were used, based on the phylogenetic structuring in the tree. A bootstrap analysis (data not shown), using 10,000 replicates, strongly supported such groupings. There was ≥ 90% clade support for the selected PGHs. As shown in Figure 1, PGH-A consisted of six haplotypes, PGH-B of one haplotype, PGH-C of five haplotypes, PGH-D of two haplotypes, PGH-E of three haplotypes, and PGH-F consisted of four haplotypes.
TABLE 2.
Individual Haplotypes Determined by PHASE Analysis for the CEU Population (Utah Residents with Ancestry from Northern and Western Europe) of HapMapa
| 1 | TTCACACCGGGGGCATGTTCGCGAAGCACCGGCGC |
| 2 | TTCACACCGGGGGCATGTTCGTGAGGCACCGGCGC |
| 3 | TTCACACACGCGGTCGTTTCGCACAGTGGTATCAG |
| 4 | TTCACACACGCGGTCGTTTCGCACAGTGGTATAAG |
| 5 | TTCACACACGCGGTCGTTTTGCACAGTGGTATAAG |
| 6 | TTCACACACGCGGTCGTGTCGCACAGTGGTATAAG |
| 7 | TTCACCCACGCGGTCGTTTCGCACAGTGGTATCAG |
| 8 | TTCACCTCGTGAGCATTTTCGCAAAGCACTAGCGC |
| 9 | TTCACCTCGTGAACATTTTCGCAAAGCACTAGCGC |
| 10 | TTCAGACCGGGGGCATTTTTGCAAATCACTGGCGC |
| 11 | TTCTCACCGGGGGCATGTTCGTGAGGCACCGGCGC |
| 12 | TTCTCACACGCGGTCGTGTCGCACAGTGGTATAAG |
| 13 | TTGACCCCGTGAACATTTTCGCAAAGCACTAGCGC |
| 14 | TTGACCTCGTGAACATTTTCGCAAAGCACTAGCGC |
| 15 | TCCACACACGCGGTAGTTTCGCAAAGCGGTAGCAG |
| 16 | CCCACACACGCGGTATTTCTACAAATCACTGGCAG |
| 17 | CCCAGACACGCGGTATTTTTGCAAATCACTGGCAG |
| 18 | CCCAGACACGCGGTATTTCTACAAATCACTGGCAG |
| 19 | CCCTGACCGGGGGCATTTTTGCAAATCACTGGCGC |
| 20 | CCCTGACACGCGGTATTTTTGCAAATCACTGGCAG |
| 21 | CCCTGACACGCGGTATTTCTACAAATCACTGGCAG |
A total of 21 unique haplotypes were identified using Bayesian inference implemented in PHASE v2.1.1. The 21 haplotypes presented in the table represent the specific combinations of the 35 SNPs evaluated in the study.
FIG. 1.
Haplotype structure of the XPC gene. A total of 21 unique haplotypes were identified using Bayesian inference implemented in PHASE v2.1.1. A maximum likelihood composite model of phylogenetic analysis in MEGA 4 was conducted on these 21 haplotypes resulting in six PGH (PGH-A, PGH-B, PGH-C, PGH-D, PGH-E, and PGH-F) based on genetic distances, as indicated by the brackets. These six PGHs were used as individual units in further analyses.
Genotype Analysis of the Study Population
After all individuals were genotyped for the 11 tagSNPs, the genotype data were analyzed for HWE. In this analysis, only 10 of the 11 SNPs passed. As a result of this analysis, we subsequently excluded rs2470352, which was determined not to be in LD with any of the other SNPs under study. We then reconstituted haplotypes for each individual in our study population using genotype data generated with the remaining 10 SNPs, which were compared to the CEU haplotypes. All SNP genotyping reactions were performed with more than 95% success rate. We excluded 24 subjects from the study who lacked genotype data for one (n = 18) or more (n = 6) SNPs due to repeated PCRs failure since this prevented accurate haplotype assignment for these individuals. Subsequently, we reconstituted haplotypes for the remaining 99 individuals, using the genotype data we generated and the CEU haplotypes as a reference. For accuracy purposes, these 24 individuals were also excluded from further analysis. All the subjects excluded were not different in any other aspect from the rest of the study population.
A PGH designation was assigned to each individual evaluated. The descriptive statistical results indicated that the most common PGH in the study population was PGH-F (40.4%), while the least common PGH was PGH-B (3.0%). The frequencies of each of the PGHs are presented in Table 3.
TABLE 3.
Frequencies of the PGH of the XPC Gene in the Study Population
| PGH status | n (%) |
| A | |
| + | 26 (26.3) |
| − | 73 (73.7) |
| Ba | |
| + | 3 (3.0) |
| − | 96 (97.0) |
| C | |
| + | 20 (20.2) |
| − | 79 (79.8) |
| D | |
| + | 4 (4.0) |
| − | 95 (96.0) |
| E | |
| + | 7 (7.0) |
| − | 92 (92.9) |
| Fb | |
| + | 40 (40.4) |
| − | 59 (59.6) |
+, presence; −, absence.
The least common PGH.
The most common PGH.
Relationship between XPC Haplotypes and the Background (Baseline) CA Frequency
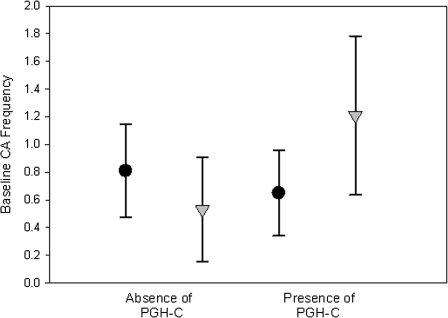
The background (baseline) and mutagen-induced CA frequencies observed in the presence (+) and absence (−) of haplotypes from the different PGHs identified in this study are presented in Table 4. The PGH groups were first coded (PGH-A to PGH-F) and then analyzed based on “haplotype group copy” (HGC) using a dominant genetic model (0 HGCs = 0, 1 or 2 HGCs = 1). When the general linear model, adjusted for age and sex, was fit to investigate interactions between each PGH and smoking on baseline CA frequencies, we observed a significant interaction between smoking and PGH-C (p = 0.046) (Fig. 2). Nonsmokers who were negative for PGH-C had the lowest level of baseline CA (mean ± SEM = 0.53 ± 0.192), while smokers who were positive for PGH-C had significantly higher baseline CA frequencies (mean ± SEM = 1.21 ± 0.29). Among those positive for PGH-C, the baseline CA frequency was 3.5 times higher in smokers compared to nonsmokers. In contrast, we observed no significant interactions between smoking and PGH-A and PGH-F on baseline CA (data not shown). Because of the small sample sizes of PGHs B, D, and E, their interaction effect with smoking on baseline CA could not be evaluated in the current study.
TABLE 4.
Effect of XPC Haplotype Groups on Background (Baseline) and Mutagen-Induced CA Frequencies
| aPGH | bStatus | Nonsmokers | Smokers |
| Baseline CA frequency | |||
| A | + | 0.69 (0.17c) | 0.78 (0.21) |
| − | 0.76 (0.21) | 0.91 (0.21) | |
| B | + | 0.25 (0.25) | 0.00 (0.00) |
| − | 0.77 (0.14) | 0.9 (0.16) | |
| C | + | 0.53 (0.19) | 1.21 (0.29) |
| − | 0.81 (0.17) | 0.65 (0.16) | |
| D | + | 0.75 (0.48) | 1.75 (0.63) |
| − | 0.72 (0.14) | 0.78 (0.15) | |
| E | + | 0.67 (0.33) | 0.43 (0.20) |
| − | 0.73 (0.14) | 0.93 (0.17) | |
| F | + | 0.92 (0.21) | 0.82 (0.18) |
| − | 0.48 (0.12) | 1.00 (0.25) | |
| Mutagen-induced CA frequency | |||
| A | + | 5.11 (0.51) | 4.67 (0.72) |
| − | 4.73 (0.51) | 6.03 (0.57) | |
| B | + | 5.25 (1.65) | 2.50 (1.50) |
| − | 4.91 (0.37) | 5.67 (0.46) | |
| C | + | 5.81 (0.48) | 5.68 (0.69) |
| − | 4.52 (0.47) | 5.45 (0.60) | |
| D | + | 3.75 (0.85) | 8.75 (2.43) |
| − | 5.04 (0.38) | 5.26 (0.43) | |
| E | + | 4.57 (1.23) | 4.29 (1.25) |
| − | 5.00 (0.37) | 5.74 (0.48) | |
| F | + | 4.63 (0.47) | 6.03 (0.51) |
| − | 5.32 (0.56) | 4.00 (0.86) | |
+, presence and −, absence.
PGH: phylogenetically-grouped haplotype.
Status: presence (+) or absence (−) of the haplotype group.
SEM = standard error of the mean.
FIG. 2.
Interaction between PGH-C and smoking, as related to CA frequency. A general linear model adjusted for age and gender was fit to investigate interactions between PGH-C and smoking on CA. A permutation test with 1000 replicates was used to calculate empirical p values to account for multiple testing. Error-bar plots depict mean and 95% confidence interval limits. The round symbols indicate nonsmokers and the triangular symbols indicate smokers. The interaction between smoking and PGH-C was significant (p = 0.046).
Relationship between XPC Haplotypes and Mutagen Sensitivity
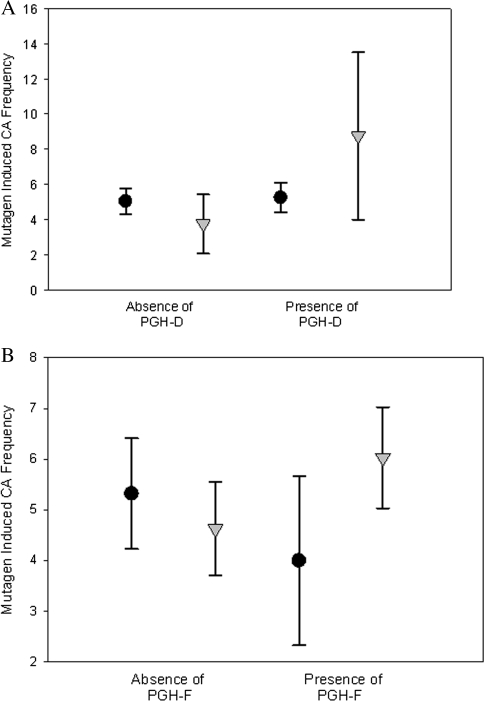
Using the general linear statistical model, adjusted for age and sex, to investigate interactions between each PGH and smoking on mutagen-induced CA frequency, we observed no significant interactions between smoking and PGHs A, B, C, and E (data not shown). However, we observed significant interactions between smoking and PGH-D (p = 0.023) and PGH-F (p = 0.031) (Fig. 3). Nonsmokers who were positive for PGH-D had a significantly lower level of mutagen-induced CA frequencies (mean ± SEM = 3.75 ± 0.85) than smokers who were positive for PGH-D (8.75 ± 2.43). Among those positive for PGH-D, the mutagen-induced CA frequency was 2.3 times higher in smokers compared to nonsmokers, whereas among those who were negative for PGH-D, this difference in response in smokers compared to nonsmokers was not observed. Likewise, nonsmokers who were positive for PGH-F had a lower frequency of mutagen-induced CA (4.63 ± 0.47) compared to smokers who were positive for PGH-F (6.03 ± 0.51). Among those positive for PGH-F, the mutagen-induced CA frequency was 1.3 times (24%) higher in smokers compared to nonsmokers.
FIG. 3.
Interaction between PGH-D and PGH-F and smoking as related to mutagen-induced CA frequency. A general linear model adjusted for age and gender was fit to investigate interactions between PGH-D (A) and PGH-F (B) and smoking as related to mutagen-induced CA. A permutation test with 1000 replicates was used to calculate empirical p values, respectively, for each outcome, to account for multiple testing. Error-bar plots depict mean and 95% confidence interval limits. The round symbols indicate nonsmokers and the triangular symbols indicate smokers. The interaction between smoking and PGH-D and PGH-F was significant (p values = 0.023 and 0.031 for PGH-D and PGH-F, respectively).
DISCUSSION
To our knowledge, this is the first study to provide a comprehensive evaluation of the relationship between XPC haplotypes and genetic damage associated with tobacco smoking. Rather than addressing the effect of a few individual SNPs, we determined the relationship between genetic damage and haplotypes that comprise the common SNPs in the entire genomic region of the XPC gene. This approach is comprehensive and biologically more plausible since it allows for the evaluation of the effect of multiple SNPs that could jointly influence outcome. The relationship between XPC haplotypes and genetic damage was evaluated using CA as a biomarker because of the well-established strong association between increased CA frequency and cancer risk. Of all biomarkers available for human studies, CA is the only biomarker that has been adequately validated in many independent prospective studies as a risk factor for cancer (Bonassi et al., 1995, 2000; Hagmar et al., 1994, 1998). Our data indicate a significant XPC haplotype–smoking interaction, which was observed between smoking and PGH-C on frequencies of CA. Our data provide support for results from previous association studies linking certain XPC polymorphisms to smoking-associated cancer risk (An et al., 2007; Guo et al., 2008; Hansen et al., 2007). Our results suggest that certain XPC haplotypes could affect the repair of genetic damage caused by tobacco-smoke carcinogens. Our findings also suggest that certain smokers may be at greater risk than others for the development of genomic instability, a critical step in the carcinogenic process, as evidenced by the increase in CA in PBLs from individuals with PGH-C.
Previous studies addressed associations between only four XPC polymorphisms and cancer risk, and these studies produced inconsistent results. For example, positive associations between the rs2228000 SNP (A499V) and cancer risk were reported in some studies (An et al., 2007; Sak et al., 2006; Shen et al., 2005) but not in others (Guo et al., 2008; Weiss et al., 2006). Similarly, an association between the rs2279017 in intron 12 of XPC and bladder cancer risk was reported (Sak et al., 2006); however, this association remains to be confirmed. The rs2228001 SNP in exon 16 (K939Q) was associated with esophageal, colorectal, and lung cancers in some studies (Guo et al., 2008; Hansen et al., 2007) but not in others (An et al., 2007; Weiss et al., 2006; Zhu et al., 2008). Inconsistencies between studies are not surprising and have been reported before with polymorphisms of other genes. Possible explanations for such inconsistencies were often discussed and included differences in study design and ethnicities of the studied populations (Au et al., 2004; Manuguerra et al., 2006). Another possible explanation we propose for such discrepancies is that the XPC polymorphisms evaluated exist in variable degrees of LD with others that were not evaluated in these investigations. Differences in sampling procedures, coupled with incomplete LD in some cases, may capture SNPs with functional effects that are not being directly investigated, but in other cases, such SNPs may not be captured. Such sampling inconsistencies, possibly influenced by an inadequate number of studied subjects, may explain these disparate results. It is also conceivable that the polymorphisms previously studied have little or no biological effect independently, but when present as part of a specific haplotype, they exert a phenotypic effect. This hypothesis is supported by recent findings from our laboratory indicating that the ss74800505 SNP that we discovered in the NEIL2 gene had no effect on expression levels when evaluated independently, yet when evaluated as part of a haplotype, a significant reduction (69%) in NEIL2 expression was observed (Kinslow et al., 2008). Another possible reason for inconsistencies could be that the phenotypic effect observed with a certain SNP was, in fact, due to the effects evoked by other SNPs that exist in LD with the studied SNP. Because of the variability in the degree of LD existing in different populations, the effects observed for a certain SNP in one study may not be the same in other studies because of the population effect. Future research based on our current study, addressing the effect of haplotypes rather than the effects of individual SNPs, may clarify these issues and may significantly reduce inconsistencies in the results currently observed between different investigations.
Our findings with PGH-C are consistent with reports indicating that certain XPC SNPs belonging to this phylogenetic group of haplotypes are associated with increased cancer risk. For example, the rs2228000 (V499R) SNP, uniformly present in PGH-C, was associated with increased risk of head and neck, bladder, and lung cancers (An et al., 2007; Sak et al., 2006; Shen et al., 2005). Our data are also consistent with a recent report indicating that the rs2228000 (V499R) SNP is associated with decreased DRC (Zhu et al., 2008). Whether the effect observed is related to the particular SNP evaluated in these earlier investigations or to other SNPs in PGH-C remains to be determined.
We found a significant difference in mutagen sensitivity between smokers who were positive compared to those who were negative for PGH-D and PGH-F. Smokers with these PGHs exhibited significantly higher mutagen sensitivity than smokers who did not have one of these PGHs. This suggests that smokers with PGH-D or PGH-F could be predisposed to a greater risk for developing cancer, given the well-established association between reduced DRC, as determined by mutagen sensitivity, and cancer risk (An et al., 2007; Cheng et al., 1998; Spitz et al., 1995; Wang et al., 2007). The haplotype-smoking interaction is not surprising since reduced repair would only be important in presence of genotoxic exposure. This gene-smoking interaction is consistent with previous reports with other polymorphisms in other DNA repair genes (e.g., Abdel-Rahman et al., 2000; Affatato et al., 2004). A plausible biological explanation for such interaction is that, in smokers, continuous exposure to tobacco smoke mutagens could overwhelm the DNA repair machinery, making the effect of the polymorphisms that reduce repair capacity more pronounced. Thus, the inheritance of polymorphisms that result in even a slight decrease in DNA repair could lead to more noticeable genetic damage in such individuals compared to nonsmokers. It is noteworthy that while PGH-C was associated with differences in baseline CA, it was not associated with mutagen-induced genetic damage. This could likely be due to differences in the mechanism(s) by which certain PGHs exert their effects with respect to chronic and acute exposures. For example, in response to chronic tobacco carcinogens exposure, haplotypes belonging to PGH-C could possibly affect XPC binding to the DNA lesion, thus reducing overall DNA repair over time, which would manifest as an increase in CA in smokers. Conversely, PGH-D and PGH-F may exert their effect primarily in the presence of an acute exposure to a mutagen, suggesting that XPC haplotypes belonging to these PGHs could affect protein stability and/or turnover at the translational and/or transcriptional levels. Additional studies are warranted to support or refute these potential mechanisms. It should be noted, however, that while the exact mechanisms by which SNPs belonging to PGH-C, -D, and -F influence genetic damage are not fully understood, some of the previously studied SNPs belonging to these PGHs (e.g., rs2228000, rs2279017) might have potentially significant effects on protein structure and/or function. For example, the rs2228000 (A499V) SNP of PGH-C is located at the 5′ end of the hHR23B-binding region of the gene and may, thus, alter the function of XPC by altering its binding with the hHR23B protein that is necessary for XPC function. However, other SNPs in other regions of the gene, which exist in LD with rs2228000, may also contribute to the observed phenotypic effect. For example, an SNP in the 3′ UTR can affect posttranscriptional activity, such as messenger RNA (mRNA) folding–directed rates of translation or mRNA half-life stability (George Priya Doss et al., 2008). Similarly, intronic SNPs that are at, or near, exonic boundaries can affect mRNA translation through exon skipping and/or aberrant mRNA folding (Cheng et al., 2006; Duan et al., 2007; Kinslow et al., 2008; Law et al., 2007), and SNPs in the 5′ UTR can affect XPC gene expression via promoter modulation (Cheng et al., 2006). Taken together, our findings suggest that SNPs, in coding as well as noncoding regions of the XPC gene, that are in LD with each other as part of a given haplotype may act in a collective manner to influence the phenotype. Mechanistic studies examining the effects of haplotypes, rather than the effects of individual SNPs, on XPC function are warranted to clarify the role of XPC polymorphisms.
In summary, despite the small sample size of the current study, a limitation that we acknowledge and which limited our ability to conclusively evaluate the effect of some PGHs, our data indicate that haplotypes belonging to PGH-C, -D, and -F appear to confer sensitivity to the mutagenic effects of tobacco carcinogens. Larger studies are needed to confirm our initial findings, and mechanistic research investigating the effect of XPC haplotypes on NER capacity and on the risk of developing diseases is clearly warranted. These studies are currently in progress in our laboratory.
FUNDING
National Institute of Environmental Health Science (NIEHS) Center award (ES06676), by a John Sealy Memorial Endowment Foundation grant to S.A.-R.; a predoctoral fellowship to C.M.R. from the NIEHS (T32-07454), a cancer prevention fellowship funded by the National Cancer Institute (K07CA093592) to C.J.E.; National Cancer Institute (CA123208) to C.J.E.; CA129050 and CA098549 to R.E.-Z. and by the National Institute of Neurological Disorders and Stroke NS065392-01 to S.A.-R.; studies were conducted with the assistance of the Institute for Translational Sciences—Clinical Research Center at UTMB funded by a 1UL1RR029876-01 grant from the National Center for Research Resources, National Institutes of Health.
Acknowledgments
We thank Dr Marinel M. Ammenheuser for her critical review of the manuscript.
References
- Abdel-Rahman SZ, El-Zein RA. The 399Gln polymorphism in the DNA repair gene XRCC1 modulates the genotoxic response induced in human lymphocytes by the tobacco-specific nitrosamine NNK. Cancer Lett. 2000;159:63–71. doi: 10.1016/s0304-3835(00)00532-2. [DOI] [PubMed] [Google Scholar]
- Abdel-Rahman SZ, Salama SA, Au WW, Hamada FA. Role of polymorphic CYP2E1 and CYP2D6 genes in NNK-induced chromosome aberrations in cultured human lymphocytes. Pharmacogenetics. 2000;10:239–249. doi: 10.1097/00008571-200004000-00005. [DOI] [PubMed] [Google Scholar]
- Affatato AA, Wolfe KJ, Lopez MS, Hallberg C, Ammenheuser MM, Abdel-Rahman SZ. Effect of XPD/ERCC2 polymorphisms on chromosome aberration frequencies in smokers and on sensitivity to the mutagenic tobacco-specific nitrosamine NNK. Environ. Mol. Mutagen. 2004;44:65–73. doi: 10.1002/em.20032. [DOI] [PubMed] [Google Scholar]
- An J, Liu Z, Hu Z, Li G, Wang LE, Sturgis EM, El-Naggar AK, Spitz MR, Wei Q. Potentially functional single nucleotide polymorphisms in the core nucleotide excision repair genes and risk of squamous cell carcinoma of the head and neck. Cancer Epidemiol. Biomarkers Prev. 2007;16:1633–1638. doi: 10.1158/1055-9965.EPI-07-0252. [DOI] [PubMed] [Google Scholar]
- Araki M, Masutani C, Takemura M, Uchida A, Sugasawa K, Kondoh J, Ohkuma Y, Hanaoka F. Centrosome protein centrin2/caltracin1 is part of the xeroderma pigmentosum group c complex that initiates global genome nucleotide excision repair. J. Bio. Chem. 2001;276:18665–18672. doi: 10.1074/jbc.M100855200. [DOI] [PubMed] [Google Scholar]
- Au WW, Navasumrit P, Ruchirawat M. Use of biomarkers to characterize functions of polymorphic DNA repair genotypes. Int. J. Hyg. Environ. Health. 2004;207:301–313. doi: 10.1078/1438-4639-00294. [DOI] [PubMed] [Google Scholar]
- Bardel C, Danjean V, Morange P, Génin E, Darlu P. On the use of phylogeny-based tests to detect association between quantitative traits and haplotypes. Genet Epidemiol. 2009;33:729–739. doi: 10.1002/gepi.20425. [DOI] [PubMed] [Google Scholar]
- Bonassi S, Abbondandolo A, Camurri L, Dal Pra L, De Ferrari M, Degrassi F, Forni A, Lamberti L, Lando C, Padovani P. Are chromosome aberrations in circulating lymphocytes predictive of future cancer onset in humans? Preliminary results of an Italian cohort study. Cancer Genet. Cytogenet. 1995;79:133–135. doi: 10.1016/0165-4608(94)00131-t. [DOI] [PubMed] [Google Scholar]
- Bonassi S, Hagmar L, Stromberg U, Montagud AH, Tinnerberg H, Forni A, Heikkila P, Wanders S, Wilhardt P, Hansteen IL, et al. Chromosomal aberrations in lymphocytes predict human cancer independently of exposure to carcinogens. European Study Group on Cytogenetic Biomarkers and Health. Cancer Res. 2000;60:1619–1625. [PubMed] [Google Scholar]
- Bunick CG, Miller MR, Fuller BE, Fanning E, Chazin WJ. Biochemical and structural domain analysis of xeroderma pigmentosum complementation group C protein. Biochemistry. 2006;45:14965–14979. doi: 10.1021/bi061370o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AJ, Mao YM, Cui RZ. The effect of gene polymorphism in promoter and intron 1 on human Apo A I expression. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2006;23:610–613. [PubMed] [Google Scholar]
- Cheng L, Eicher SA, Guo Z, Hong WK, Spitz MR, Wei Q. Reduced DNA repair capacity in head and neck cancer patients. Cancer Epidemiol. Biomarkers Prev. 1998;7:465–468. [PubMed] [Google Scholar]
- Cheverud JM. A simple correction for multiple comparisons in interval mapping genome scans. Heredity. 2001;87:52–58. doi: 10.1046/j.1365-2540.2001.00901.x. [DOI] [PubMed] [Google Scholar]
- Duan ZX, Zhu PF, Dong H, Gu W, Yang C, Liu Q, Wang ZG, Jiang JX. Functional significance of the TLR4/11367 polymorphism identified in Chinese Han population. Shock. 2007;160:160–164. doi: 10.1097/SHK.0b013e31803df782. [DOI] [PubMed] [Google Scholar]
- Evans HJ, O'Riordan ML. Human peripheral blood lymphocytes for the analysis of chromosome aberrations in mutagen tests. Mutat. Res. 1975;31:135–148. doi: 10.1016/0165-1161(75)90082-5. [DOI] [PubMed] [Google Scholar]
- Friedberg EC. How nucleotide excision repair protects against cancer. Nat. Rev. Cancer. 2001;1:22–33. doi: 10.1038/35094000. [DOI] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, et al. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- George Priya Doss C, Sundandiradoss R, Rajasekaran R, Choudhury P, Sinha P, Hota P, Batra UP, Rao S. Applications of computational algorithm tools to identify functional SNPs. Funct. Integr. Genomics. 2008;9:309–316. doi: 10.1007/s10142-008-0086-7. [DOI] [PubMed] [Google Scholar]
- Guo W, Zhou RM, Wan LL, Wang N, Li Y, Zhang XJ, Dong XJ. Polymorphisms of the DNA repair gene xeroderma pigmentosum groups A and C and risk of esophageal cell carcinoma in a population of high incidence region of North China. J. Cancer Res. Clin. Oncol. 2008;134:267–270. doi: 10.1007/s00432-007-0283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmar L, Bonassi S, Stromberg U, Brogger A, Knudsen LE, Norppa H, Reuterwall C. Chromosomal aberrations in lymphocytes predict human cancer: a report from the European Study Group on Cytogenetic Biomarkers and Health (ESCH) Cancer Res. 1998;58:4117–4121. [PubMed] [Google Scholar]
- Hagmar L, Brogger A, Hansteen IL, Heim S, Hogstedt B, Knudsen L, Lambert B, Linnainmaa K, Mitelman F, Nordenson I. Cancer risk in humans predicted by increased levels of chromosomal aberrations in lymphocytes: Nordic study group on the health risk of chromosome damage. Cancer Res. 1994;54:2919–2922. [PubMed] [Google Scholar]
- Hansen RD, Sorensen M, Tjonneland A, Overvad K, Walling H, Raaschou-Nielsen O, Vogel U. XPA A23G, XPC Lys939Gln, XPD Lys751Gln and XPD Asp312Asn polymorphisms, interactions with smoking, alcohol and dietary factors, and risk of colorectal cancer. Mutat. Res. 2007;619:68–80. doi: 10.1016/j.mrfmmm.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Hsu TC, Spitz MR, Schantz SP. Mutagen sensitivity: a biologic marker of cancer susceptibility. Cancer Epidemiol. Biomarkers Prev. 1991;1:83–89. [PubMed] [Google Scholar]
- IARC. IARC Monographs for the Evaluation of the Carcinogenic Risk of Chemicals to Humans (IARC) 1986. Tobacco smoking. (World Health Organization, Ed.), pp. 312–314. IARC, Lyon, France. [Google Scholar]
- ISCN. An International System for Human Cytogenetic Nomenclature. Report of the Standing Committee on Human Cytogenetic Nomenclature. Birth Defects Orig. Artic. Ser. 1985;21:1–117. [PubMed] [Google Scholar]
- Johnson GC, Esposito L, Barratt BJ, Smith AN, Heward J, Di Genova G, Ueda H, Cordell HJ, Eaves IA, Dudbridge F, et al. Haplotype tagging for the identification of common disease genes. Nat. Genet. 2001;29:233–237. doi: 10.1038/ng1001-233. [DOI] [PubMed] [Google Scholar]
- Kinslow CJ, El-Zein RA, Hill CE, Wickliffe JK, Abdel-Rahman SZ. Single nucleotide polymorphisms 5’ upstream the coding region of the NEIL2 gene influence gene transcription levels and alter levels of genetic damage. Genes Chromosomes Cancer. 2008;47:923–932. doi: 10.1002/gcc.20594. [DOI] [PubMed] [Google Scholar]
- Law AJ, Kleinman JE, Weinberger DR, Weickert CS. Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. Hum. Mol. Genet. 2007;16:129–141. doi: 10.1093/hmg/ddl449. [DOI] [PubMed] [Google Scholar]
- Liu G, Zhou W, Christiani DC. Molecular epidemiology of non-small cell lung cancer. Semin. Respir. Crit. Care Med. 2005;26:265–272. doi: 10.1055/s-2005-871983. [DOI] [PubMed] [Google Scholar]
- Manuguerra M, Saletta F, Karagas MR, Berwick M, Veglia F, Vineis P, Matullo G. XRCC3 and XPD/ERCC2 single nucleotide polymorphisms and the risk of cancer: a HuGE review. Am. J. Epidemiol. 2006;164:297–302. doi: 10.1093/aje/kwj189. [DOI] [PubMed] [Google Scholar]
- Park CJ, Choi BS. The protein shuffle: sequential interactions among components of the human nucleotide excision repair pathway. FEBS J. 2006;273:1600–1608. doi: 10.1111/j.1742-4658.2006.05189.x. [DOI] [PubMed] [Google Scholar]
- Rieder MJ, Reiner AP, Gage BF, Nickerson DA, Eby CS, McLeod HL, Blough DK, Thummel KE, Veenstra DL, Rettie AE. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N. Engl. J. Med. 2005;352:2285–2293. doi: 10.1056/NEJMoa044503. [DOI] [PubMed] [Google Scholar]
- Sak SC, Barrett JH, Paul AB, Bishop TD, Kiltie AE. Comprehensive analysis of 22 XPC polymorphisms and bladder cancer risk. Cancer Epidemiol. Biomarkers Prev. 2006;15:2537–2541. doi: 10.1158/1055-9965.EPI-06-0288. [DOI] [PubMed] [Google Scholar]
- Shen H, Spitz MR, Qiao Y, Guo Z, Wang LE, Bosken CH, Amos CI, Wei Q. Smoking, DNA repair capacity and risk of nonsmall cell lung cancer. Int. J. Cancer. 2003;107:84–88. doi: 10.1002/ijc.11346. [DOI] [PubMed] [Google Scholar]
- Shen M, Berndt SI, Rothman N, DeMarini DM, Mumford JL, He X, Bonner MR, Tian L, Yeager M, Welch R, et al. Polymorphisms in the DNA nucleotide excision repair gene and lung cancer risk in Xuan Wei, China. Int. J. Cancer. 2005;116:768–773. doi: 10.1002/ijc.21117. [DOI] [PubMed] [Google Scholar]
- Spitz MR, Hsu TC, Wu XF, Fueger JJ, Amos CI, Roth JA. Mutagen sensitivity as a biologic marker of lung cancer risk in African Americans. Cancer Epidemiol. Biomarkers Prev. 1995;4:99–103. [PubMed] [Google Scholar]
- Veenstra DL, You JH, Rieder MJ, Farin FM, Wilkerson HW, Blough DK, Cheng G, Rettie AE. Association of Vitamin K epoxide reductase complex 1 (VKORC1) variants with warfarin dose in a Hong Kong Chinese patient population. Pharmacogenet. Genomics. 2005;15:687–691. doi: 10.1097/01.fpc.0000174789.77614.68. [DOI] [PubMed] [Google Scholar]
- Wang Y, Spitz MR, Lee JJ, Huang M, Lippman SM, Wu X. Nucleotide excision repair pathway genes and oral premalignant lesions. Clin. Cancer Res. 2007;13:3753–3758. doi: 10.1158/1078-0432.CCR-06-1911. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Weiss NS, Ulrich CM, Doherty JA, Chen C. Nucleotide excision repair genotype and the incidence of endometrial cancer: effect of other risk factors on the association. Gynecol. Oncol. 2006;103:891–896. doi: 10.1016/j.ygyno.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Lai M, Yang H, Lin J, Huang M, Grossman HB, Dinney CP, Wu X. Genotypes, haplotypes, and diplotypes of XPC and risk of bladder cancer. Carcinogenesis. 2007;28:698–703. doi: 10.1093/carcin/bgl201. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Yang H, Chen Q, Lin J, Grossman HB, Dinney CP, Wu X, Gu J. Modulation of DNA damage/DNA repair capacity by XPC polymorphisms. DNA Repair. 2008;7:141–148. doi: 10.1016/j.dnarep.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]