Abstract
Cutaneous squamous cell cancer (SCC) affects up to 30% of kidney transplant recipients (KTRs) within 10 years of transplantation. There are no reliable clinical tests that predict those who will develop multiple skin cancers. High numbers of regulatory T cells associate with poor prognosis for patients with cancer in the general population, suggesting their potential as a predictive marker of cutaneous SCC in KTRs. We matched KTRs with (n = 65) and without (n = 51) cutaneous SCC for gender, age, and duration of immunosuppression and assessed several risk factors for incident SCC during a median follow-up of 340 days. Greater than 35 peripheral FOXP3+CD4+CD127low regulatory T cells/μl, <100 natural killer cells/μl, and previous SCC each significantly associated with increased risk for new cutaneous SCC development (hazard ratio [HR] 2.48 [95% confidence interval (CI) 1.04 to 5.98], HR 5.6 [95% CI 1.31 to 24], and HR 1.33 [95% CI 1.15 to 1.53], respectively). In addition, the ratio of CD8/FOXP3 expression was significantly lower in cutaneous SCC excised from KTRs (n = 25) compared with matched SCC from non-KTRs (n = 25) and associated with development of new cutaneous SCCs. In summary, monitoring components of the immune system can predict development of cutaneous SCC among KTRs.
Organ transplantation is the treatment of choice for individuals with organ failure. Immunosuppressive regimens have become more potent, resulting in transplant recipients experiencing fewer acute rejection episodes and improved 1-year graft survival.1 Concurrently, there has been an increased incidence of malignancy.2,3 Organ transplant recipients (OTRs) have a cancer prevalence four to six times higher than the general population.4,5
Patients on long-term immunosuppression have an increased risk for cutaneous squamous cell cancer (SCC). Patients who require regular oral steroid therapy and rheumatoid arthritis patients who take disease-modifying agents have a two to four times increased risk for SCC compared with the general population.6,7 In comparison, OTRs have up to a 200 times increased risk for SCC compared with the general population.8 Three percent of OTRs will require extensive plastic surgical procedures per year.9 Age at transplantation and duration and dosage of immunosuppression are major determinants of SCC development.10,11 Furthermore, dosage of immunosuppression is a major determinant of the risk for SCC metastasis, which has an incidence of 1 to 4%.9,12 The median survival after a diagnosis of poor-prognosis SCC is approximately 2 years.13,14 Although previous SCC is a major determinant of new SCC development,15,16 approximately 30% of OTRs with one SCC will not develop another SCC.
Attempts have been made to define those at risk for cancer after transplantation by measuring lymphocyte subsets. In two studies, a low CD4 count predicted those at risk for any cancer after transplantation, including SCC.17,18 These studies were performed within the first 10 years of transplantation, when skin cancer incidence is relatively low, and although predictive, CD4 T cell count in this population had limited clinical utility. If a particular immune profile could accurately predict new SCC development in those at risk, then it would be a valuable tool for posttransplantation clinical management and could allow targeted manipulations in immunosuppressive therapy and skin surveillance.
Candidate cell types that may predict cancer in OTRs could be similar to those found in the general population with cancer. In the general population, the presence of increased numbers of regulatory T cells (Tregs; CD4+CD25highFOXP3+ and CD8+CD28− cells) within the tumor and peripheral circulation is associated with poor prognosis.19–22 Under physiologic conditions, Tregs control immune responses, preventing excessive tissue damage and autoimmunity.23,24 In the tumor microenvironment, Tregs may act by a variety of mechanisms, impairing antitumor functions of CD8+ T cells and natural killer (NK) cells.25–27
In contrast, in OTRs, Tregs have been shown to control or prevent rejection28,29 and may lead to improved long-term outcomes.30 Importantly, immunosuppressive drugs have differential effects on Tregs.31 The addition of sirolimus to CD4+ T cells in vitro increases the number of FOXP3+ cells,32 whereas cyclosporine decreases the number of Tregs.33
Despite the effect of immunosuppression on Treg number and the relationship of immunosuppression with the development of cancer in OTRs, Tregs have not been assessed in relation to cancer after transplantation. We therefore investigated the hypothesis that SCC in kidney transplant recipients (KTRs) would be associated with an increased number of Tregs by determining the immune phenotype of leukocytes present in the peripheral blood and at the site of the SCC lesion.
We phenotyped peripheral blood from KTRs with (n = 60) and without SCC (n = 50), matched for age, gender, and duration of immunosuppression, using flow cytometry. We investigated the impact of clinical and immunosuppressant variables using conditional logistic regression and quantified lymphocyte populations (FOXP3+, CD8+, and CD56+) within the SCC from KTRs (n = 25) and matched SCC from non-KTRs (n = 25). Finally, we assessed the prognostic value of immune phenotype in predicting new SCC development in a multivariate Cox regression.
Results
A total of 110 patients were enrolled into the study (n = 60 KTRs with SCC; n = 50 matched KTRs without SCC). Three (2.6%) died during follow-up (one death from metastatic SCC). No patients were lost to follow-up. The demographics and drug history of KTRs with and without SCC were similar (Table 1). No KTRs were currently taking sirolimus or everolimus.
Table 1.
Clinical characteristics of KTRs with and without cutaneous SCC
| Characteristic | KTRs with SCC (n = 60) | KTRs without SCC (n = 50) |
|---|---|---|
| Age at assessment (years; median [range]) | 63.2 (45.5 to 81.9) | 61.8 (39.2 to 81.5) |
| Age at first transplantation (years; median [range]) | 47 (20 to 65) | 46 (18 to 66) |
| Male gender (n [%]) | 52 (86.7) | 45 (90.0) |
| Years of immunosuppression | 17.6 (4.8 to 32.4) | 16.7 (4.8 to 27.4) |
| Total dosage of cyclosporin (mg/kg; median [range]) | 3183 (997 to 7297) | 3231 (497 to 7923) |
| Total dosage of azathioprine (mg/kg; median [range]) | 1207 (279 to 3856) | 1008 (103 to 4300) |
| Total dosage of prednisolone (mg/kg; median [range]) | 71.0 (2.3 to 474.0) | 23.0 (1.8 to 232.0) |
| Time-averaged C0 trough level (median [range]) | 128 (69 to 207) | 133 (35 to 204) |
| Current azathioprine use (n [%]) | 46 (77) | 43 (86) |
| Current CNI use (n [%]) | 43 (72) | 37 (74) |
| Current prednisolone use (n [%]) | 34 (57) | 16 (32) |
| No rejection episodes (n [%]) | 26 (43.3) | 24 (48.0) |
| GN or vasculitis as cause of ESRF (n [%]) | 22 (37) | 12 (24) |
| Creatinine (μmol/L; median [range]) | 138 (54 to 427) | 139 (66 to 366) |
| HLA mismatch (median [range]) | 2 (0 to 6) | 3 (0 to 5) |
| % CD4+ cells FOXP3+ (median [range]) | 4 (1 to 12) | 3 (1 to 9) |
| CD4+ FOXP3+ cells/μl | 21 (3 to 91) | 16 (1 to 32) |
| % CD8+ cells CD28− (median [range]) | 55 (0 to 96) | 25 (0 to 97) |
| CD8+CD28− cells/μl (median [range]) | 140 (0 to 1180) | 72 (0 to 1300) |
| CD4+ cells/μl (median [range]) | 620 (50 to 1980) | 510 (60 to 1600) |
| CD8+ cells/μl (median [range]) | 330 (30 to 1340) | 280 (30 to 1360) |
| Central memory CD8+ cells/μl (median [range]) | 32 (1 to 230) | 53 (2 to 550) |
| % central memory CD8+ (median [range]) | 10.5 (2 to 40) | 14 (1 to 66) |
| NK cells/μl (median [range]) | 65 (1 to 580) | 36 (1 to 740) |
Total drug dosage was calculated by recording all alterations in immunosuppressive medication from 12 months after transplantation until October 2008. Time-averaged C0 level was determined by calculating the duration of time a given C0 level was maintained. GN or vasculitis included all glomerulonephritis excluding IgA and FSGS. Vasculitis includes ANCA-positive vasculitis, lupus nephritis, and microscopic polyarteritis nodosa. ESRF, end-stage renal failure.
We assessed clinical and immunologic variables in a conditional logistic regression and corrected them for age and gender (Table 2). Only current prednisolone use and no other immunosuppressive variables were associated with SCC development. Immunosuppression before transplantation to treat vasculitis or glomerulonephritis associated with an increased risk for developing SCC after transplantation (odds ratio [OR] 1.83 [95% confidence interval (CI) 0.8 to 4.22]) but did not reach statistical significance (P = 0.155). Additional immunosuppression after transplantation to treat rejection was not associated with an increased risk for SCC.
Table 2.
ORs for SCC development using a conditional logistic regression
| Parameter | Univariate Analysis |
Multivariate Analysisa |
||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Age at transplantation | 1.05 (0.99 to 1.11) | 0.695 | ||
| Gender (male versus female) | 0.72 (0.22 to 2.37) | 0.591 | ||
| Immunosuppression (years) | 1.05 (1.00 to 1.11) | 0.064 | 1.10 (1.02 to 1.18) | 0.011 |
| Total CNI (mg/kg) | 1.00 (0.99 to 1.02) | 0.91 | ||
| Total C0 (ng/ml) | 1.00 (1.00 to 1.00) | 0.77 | ||
| Total azathioprine (mg/kg) | 0.99 (0.97 to 1.02) | 0.60 | ||
| Total steroid (mg/kg) | 1.33 (0.83 to 2.14) | 0.23 | ||
| Components of drug regimen | ||||
| azathioprine | 0.53 (0.20 to 1.45) | 0.219 | ||
| calcineurin | 0.88 (0.38 to 2.07) | 0.784 | ||
| prednisolone | 2.77 (1.27 to 6.08) | 0.011 | 3.18 (1.39 to 7.28) | 0.006 |
| Immunosuppression before Tx | 1.83 (0.80 to 4.22) | 0.155 | ||
| Required therapy for rejection | ||||
| steroid versus no rejection | 0.91 (0.45 to 1.81) | 0.79 | ||
| OKT-3/ATG versus no rejection | 1.07 (0.32 to 3.66) | 0.91 | ||
| % CD4+ cells FOXP3+ | 1.24 (1.01 to 1.52) | 0.042 | 1.26 (1.02 to 1.55) | 0.035 |
| CD4+ FOXP3+ cells/μl | 1.06 (1.02 to 1.10) | 0.003 | 1.07 (1.03 to 1.12) | 0.001 |
| % CD8+ cell CD28− | 1.02 (1.01 to 1.03) | 0.006 | 1.02 (1.01 to 1.03) | 0.005 |
| CD8+CD28− cells/μl | 2.18 (1.01 to 4.70) | 0.047 | 2.15 (0.99 to 4.71) | 0.054 |
| CD4+ cells/μl | 1.10 (0.97 to 1.24) | 0.127 | ||
| CD8+ cells/μl | 0.99 (0.88 to 1.12) | 0.896 | ||
| Central memory CD8+ cells/μl | 0.96 (0.92 to 0.99) | 0.030 | 0.96 (0.92 to 0.99) | 0.028 |
| % central memory CD8+ | 0.92 (0.85 to 0.99) | 0.027 | 0.92 (0.85 to 0.99) | 0.027 |
| NK cell (per 100 cells/μl) | 1.00 (1.00 to 1.03) | 0.566 | ||
Tx, transplantation.
aAdjusted for age/gender.
There were no differences in the number of CD4, CD8, and NK cells (see Table 1 and corresponding ORs in Table 2). There were, however, differences in some lymphocyte subsets. Increasing numbers of Tregs, both FOXP3+CD127lowCD69−CD4+ and CD28−CD8+ cells, and a decreasing number of central memory CD8 T cells in the peripheral blood were found to be associated with SCC in KTRs (Table 2).
The major predictors of FOXP3+CD127lowCD69−CD4+ were CD4+ T cell count and history of SCC (P < 0.001 and P = 0.006, respectively). A calcineurin inhibitor (CNI)-based immunosuppressive regimen reduced the number of FOXP3+ cells/μl (P = 0.032). The number of KTRs who did and did not have SCC and were treated with CNIs was similar, as were the dosages given and the cyclosporine trough levels.
Regarding CD8+CD28− T cells, it is known that increasing age,34 chronic viral infections such as cytomegalovirus (CMV),35 and cancer status20,36 can increase the number of these cells. Although age was not an independent predictor of CD8+CD28− T cells in this study, CMV seropositivity and SCC history associated with increased CD8+CD28− T cells (OR 2.28 [95% CI 1.04 to 5.42; P = 0.039] and OR 2.63 [95% CI 1.11 to 6.23; P = 0.027], respectively). We found no association with immunosuppressive regimen. Increasing numbers of CD8+CD28− T cells was associated with reduced lymphocyte proliferative capacity to phytohemagluttinin (PHA) in vitro (OR 0.72 [95% CI 0.52 to 0.94]; P = 0.014; Figure 1), so although KTRs with SCC had poorer proliferative responses compared with KTRs without SCC (Figure 1), part of this observation was explained by CD8+CD28− T cell number.
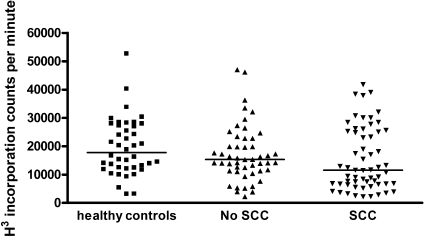
Figure 1.
Maximal proliferative response of peripheral blood leukocytes to PHA in healthy control subjects (n = 32), KTRs with SCC, and matched KTRs without SCC. Poor proliferative response, defined as a count of <10,000 cpm (10th centile of healthy control subjects), was more common in KTRs with a history of SCC (OR 3.7 [95% 1.5 to 9.8]; P = 0.004).
Other differences in the CD8 compartment included a difference in memory cell subsets. Two subsets of memory T cells exist: Secondary lymphoid tissue–homing CD62L+ central memory (TCM) T cells and peripheral tissue–homing CD62L− effector memory (TEM) T cells with immediate effector function.37,38 Increasing numbers of CD8 TCM in the peripheral blood was associated with not developing SCC after renal transplantation (P = 0.027; Table 2). Prednisolone use, irrespective of SCC history, reduced the number of CD8 TCM, and there was significant interaction of these variables in the conditional logistic regression analysis (Table 3). Steroid did not statistically significantly affect the subsets of CD4+ T cells (data not shown).
Table 3.
Conditional logistic regression multivariate models: OR of developing cutaneous SCC in matched kidney transplant recipients
| Parameter | Full Model |
Model Removing Variable “Immunosuppression” |
Model Removing Variable “Steroid” |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Immunosuppression (years) | 1.06 (0.83 to 1.36) | 0.620 | ||||
| FOXP3+ cells/μl (per cell increase) | 1.04 (1.01 to 1.07) | 0.033 | 1.04 (1.01 to 1.07) | 0.003 | 1.04 (1.02 to 1.07) | 0.002 |
| % CD8+CD28− of CD8 T cells | 1.02 (1.00 to 1.04) | 0.015 | 1.02 (1.01 to 1.04) | 0.039 | 1.02 (1.00 to 1.03) | 0.020 |
| Steroid regimen | 2.32 (0.97 to 5.55) | 0.059 | 2.44 (1.05 to 5.70) | 0.011 | ||
| TCM CD8 cells/μl (per cell increase) | 0.99 (0.98 to 1.00) | 0.07 | 0.99 (0.98 to 1.00) | 0.067 | 0.99 (0.98 to 0.99) | 0.016 |
Only variables that were significant after correction for age and gender were included in the multivariate model. Duration of immunosuppression was initially included to ensure the matching was valid and was subsequently removed from the model. Steroid use predicted TCM cell numbers and was also removed from the model.
We also investigated immune infiltrates into excised SCC. Twenty-seven matched pairs of excised SCC from KTRs and non-KTRs identified by the dermatopathologist were stained by triple immunohistochemistry for the presence of FOXP3+, CD8+, and CD56+ cells. Unfortunately, two of the 54 slides did not stain correctly (one slide from two matched pairs), and therefore the data set contained 25 matched pairs. The differences in peritumor cell densities comparing SCC from KTRs and non-KTRs are shown in Table 4. The only significant difference in peritumor cell infiltrates between KTR and non-KTR SCC was the CD8-FOXP3 ratio. Because three independent variables were assessed in this analysis, the appropriate Bonferroni correction set the levels of significance at P < 0.017; therefore, the P value for FOXP3+ does not reach significance.
Table 4.
Peritumor lymphocyte density in SCCs from KTRs and matched SCCs from non-KTRs
| Parameter | Nontransplant SCC (n = 25) | Transplant SCC (n = 25) | P |
|---|---|---|---|
| FOXP3+ cells (0.25 mm2; median [range]) | 63 (32 to 280) | 102 (16 to 353) | 0.030 |
| CD8+ cells (0.25 mm2; median [range]) | 157 (54 to 421) | 117 (36 to 625) | 0.353 |
| CD8/FOXP3 (median [range]) | 2.1 (0.8 to 6.1) | 1.4 (0.4 to 5.5) | 0.013 |
| CD56+ cells (0.25 mm2; median [range]) | 10 (0 to 115) | 16 (0 to 60) | 0.476 |
| CD8+ FOXP3+ cells (n [%]) | 15 (65) | 13 (57) | 0.627a |
All cancers were matched for grade and depth ±0.2 mm. One 9-mm poorly differentiated tumor in a transplant recipient could be matched only to a 13-mm tumor in the nontransplant population. All tumors were well differentiated apart from five moderately and two poorly differentiated tumors. Differences were assessed by Wilcoxon sign rank test (two-tailed).
aFisher exact test (two tailed).
When a given SCC had both intratumor and peritumor cell infiltrate, these cell densities were compared within individual SCC. Because there were differences in the CD8-FOXP3 ratio between KTRs and non-KTRs, these two groups were analyzed separately, as shown in Table 5. Intratumor cell infiltrates were invariably less dense than peritumor infiltrates. The median change in FOXP3+ for SCC from KTRs, comparing intra and peritumor areas, was 10 cells/0.25 mm2. Every SCC from KTRs had less FOXP3+ in peritumor areas compared with intratumor areas. This observation probably explains why the P value was 0.011 with only 14 observations. The intratumor CD8-FOXP3 ratio was inverted in KTR and non-KTR SCC when compared with peritumor CD8-FOXP3 ratio (Figure 2). A total of 53.6% of KTRs developed a new SCC within 1 year of the SCC that was stained by immunohistochemistry. No non-KTR developed a new SCC during the follow-up period.
Table 5.
Comparison of intra- and peritumor lymphocyte populations within SCCs from KTRs and non-KTRs
| Parameter | Peritumor Infiltrate (Median [Range]) | Intratumor Infiltrate (Median [Range]) | P |
|---|---|---|---|
| KTR SCC (n = 14) | |||
| FOXP3+ cells (0.25 mm2) | 70 (27 to 264) | 60 (13 to 155) | 0.011 |
| CD8+ cells (0.25 mm2) | 102 (50 to 449) | 24 (2 to 153) | <0.001 |
| CD8/FOXP3 | 1.46 (0.70 to 4.00) | 0.54 (0.01 to 2.50) | <0.001 |
| CD56+ cells (0.25 mm2) | 13 (0 to 43) | 5 (1 to 14) | 0.003 |
| Non-KTR SCC (n = 11) | |||
| FOXP3+ cells (0.25 mm2) | 65 (34 to 280) | 67 (25 to 434) | 0.637 |
| CD8+ cells (0.25 mm2) | 148 (54 to 377) | 53 (17 to 191) | 0.001 |
| CD8/FOXP3 | 1.57 (0.80 to 6.10) | 0.89 (0.30 to 2.60) | 0.001 |
| CD56+ cells (0.25 mm2) | 14 (2 to 115) | 4 (1 to 60) | 0.001 |
Fourteen KTR SCCs and 11 non-KTR SCCs had both intra- and peritumor infiltrate as defined in the Concise Methods section. Comparison of the median density of infiltrate for each cell type between the two regions was compared for each individual tumor by Wilcoxon sign rank test (two-tailed).
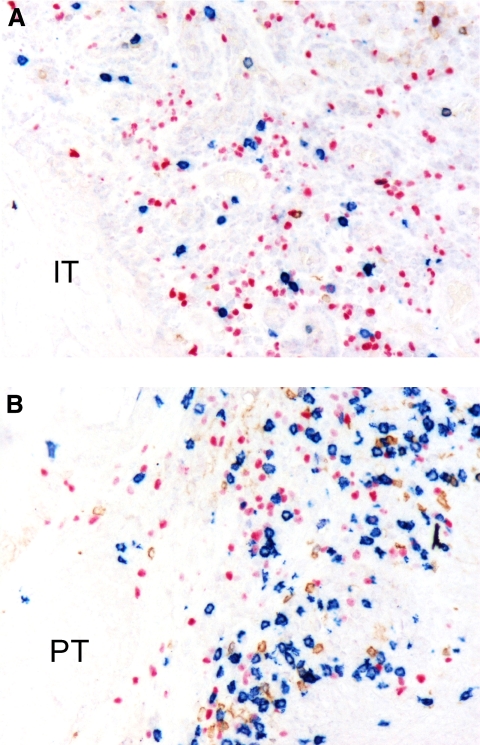
Figure 2.
Immunophenotype of infiltrating lymphocytes in cutaneous SCC is shown. (A and B) Paraffin-embedded tissue sections of cutaneous SCC were investigated by triple immunoenzymatic labeling for detection of CD56 (brown), CD8 (blue), and FOXP3 (pink) antigens (no counterstain). Within each tumor, two areas were identified: One showing intratumor lymphocyte infiltration (IT; A) and the second characterized by a peritumor infiltrate (PT; B). IT areas were composed mainly of FOXP3+ cells (pink), contrasting with the PT areas showing a predominance of CD8+ cells (blue).
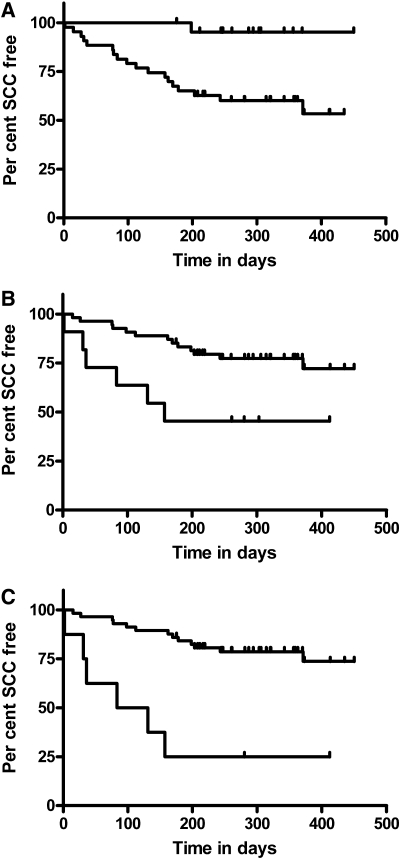
For the prospective follow-up study, we followed the entire cohort of KTRs with and without SCC (n = 116; median follow-up after enrollment 340 days [range 167 to 907 days]). Only KTRs with a previous SCC developed a new SCC in this period (n = 23). Univariate Cox regression for time to next tumor and for immune phenotype of circulating peripheral blood lymphocytes therefore included the 65 KTRs with a previous SCC and incorporated previous published factors involved in new tumor development39,40: Age; thickness and grade of index tumor (complete histologic data, thickness, and grade, available on 39 tumors), and previous number of SCCs. The number of previous SCCs was the only significant factor previously reported to predict the time to next tumor.15,16 Hazard ratios (HRs) for new SCC development were per FOXP3+ cell/μl whole blood (HR 1.01 [95% CI 0.99 to 1.03]; P = 0.22) and per NK cell/μl (HR 0.99 [95% CI 0.98 to 1.00]; P = 0.06); however, risk for SCC with FOXP3+CD4+CD127low and NK cells was not constant when spline plots were inspected. These variables were dichotomized at the point at which risk for SCC changed: >35 FOXP3+ cells/μl (HR 2.48 [95% CI 1.04 to 5.98]; P = 0.041) and NK <100 cells/μl (HR 5.6 [95% CI 1.31 to 24]; P = 0.02). The relative effects of these three predictors are shown in Figure 3.
Figure 3.
(A) Kaplan-Meier curve shows time to next SCC in 65 KTRs with a previous SCC. Top line represents KTRs with only one previous SCC; bottom line represents KTRs with more than two SCCs (HR 12.5 [95% CI 1.7 to 94.0]; P = 0.014). Tick marks represent censored observation for time of follow-up. (B) Kaplan-Meier curve of time to next tumor when KTRs are categorized as having >35 FOXP3+ cells/μl and <100 NK cells/μl. Bottom line represents those with high FOXP3+ cell number and low NK cell number (high-risk phenotype; n = 11); top line represents those with low FOXP3+ cell number and high NK cell number (HR 3.77 [95% CI 1.42 to 10.00]; P = 0.008 for high-risk phenotype). (C) Kaplan-Meier curve combining high-risk phenotype and single or multiple previous SCCs. Bottom line represents KTRs (n = 8) with high-risk phenotype and multiple previous SCCs (HR 6.13 [95% CI 2.30 to 16.00]; P < 0.001 for new SCC development).
Discussion
This is the largest study to analyze the immune phenotype of peripheral blood leukocytes present in long-term KTRs (>5 years after transplantation) and is the first report that the immune phenotype of peripheral blood leukocytes is different between KTRs with and without SCC. KTRs with previous SCC have a higher number of FOXP3+CD4+CD127low and CD8+CD28− T cells present in the peripheral blood than KTRs without SCC. Moreover, we found an overrepresentation of FOXP3+ cells within SCC removed from KTRs compared with matched SCC from patients who were not taking immunosuppression. A low CD8-FOXP3 ratio therefore associated with new tumor development, because only KTRs with previous SCC developed subsequent new SCC during follow-up in this study. During prospective follow-up of the KTRs enrolled in the study, high numbers of FOXP3+CD4+CD127low and low numbers of NK cells predicted KTRs who had a previous SCC and went on to develop a new SCC.
First, because FOXP3 is transiently expressed in activated human T cells, which do not subsequently have the ability to regulate,23 we excluded these recently activated cells by ensuring FOXP3+ cells were CD69− and CD127low. As has been found previously, we noted that a CNI-based immunosuppressive regimen resulted in reduced numbers of FOXP3+ cells in the peripheral blood41; however, independent of CNI use, FOXP3+ cell number was still an important independent risk factor for development of SCC after transplantation and for predicting new SCC development in KTRs with a previous SCC.
Second, our data on CD8+CD28− T cells agree with previous published data. We found that higher levels of CD8+CD28− T cells were associated with a history of SCC and also previous CMV infection. Data suggest that CD8+CD28− T cells from patients with head and neck SCC have impaired cytotoxic activity,42 and CD8+CD28− T cells can inhibit the proliferation of effectors cells.20 Indeed, we found that lymphocytes from KTRs with SCC had a poorer proliferative response to PHA than did matched KTRs without SCC (Figure 1). This poor proliferative response was explained partly by the number of CD8+CD28− T cells in peripheral blood mononuclear cells (PBMCs). Unlike previous reports, we found no correlation with age in this cohort, but this may be due to the relatively greater age of this patient population compared with previous reports.34 Importantly, we found no effect of drug regimen on the number of CD8+CD28− T cells.
Finally, we found that low numbers of CD8+ TCM present in the peripheral blood associate with a history of SCC. The only independent predictors of CD8+ TCM were a history of SCC and a steroid-based immunosuppressive regimen, both reducing the number of CD8+ TCM. Importantly, we also found steroid continuation after transplantation increased the risk for SCC. Given that steroids can induce the same immune phenotype as SCC, the data support a possible mechanism by which steroids mediate an increased risk for SCC in KTRs. The contribution of CD8+ TCM and TEM subsets to antitumor responses43,44 has not yet been fully elucidated. CD8+ TCM are superior to TEM in clearing cutaneous viral infections,45,46 which may be important given that human papillomavirus and cutaneous warts are risk factors for SCC development in KTRs.10,47
The interaction of steroid therapy and the presence of TCM identified in this study also has implications for the interpretation of previous data that CD8+ TCM are markers of tolerance. In the study by Brouard and colleagues,48 the immune phenotype of tolerant KTRs was compared with KTRs with chronic rejection (n = 14). High numbers of CD8 TCM were found in tolerant patients compared with those with chronic rejection; however, the tolerant KTRs were no longer taking steroids, whereas the control subjects were maintained on steroid therapy at the time of the analysis.
Our observation that steroid cessation in KTRs is protective against the development of SCC has been suggested in Australian KTRs with certain GST genotypes49 but has not been found in UK cohorts.10 Steroid cessation or avoidance has been associated with reduced cancer incidence, but this has not reached statistical significance in meta-analysis.50 Regarding other immunosuppressive drugs, we found no association of CNIs with SCC development in this cohort. This is in contrast to the article by Dantal et al.51 in which low-dosage CNI regimens protected from SCC development within 5 years of transplantation. The following points are potential reasons for why we found no association with SCC and CNI.
First in the article by Dantal et al.,51 steroids were withdrawn within 90 days of transplantation, whereas the majority of patients in our cohort continued prednisolone. Second, the CNI dosages in our cohort were approximately halfway between the low- and standard-dosage CNI trough levels used by Dantal et al.51 Finally, our cohort had been immunosuppressed for, on average, 17 years, compared with 6 years in the study by Dantal et al.51; therefore, in the white cohort presented here, with the relevant caveats of immunosuppressive regimen and clinical variables, increased number of circulating FOXP3+CD4+CD127low and CD8+CD28− and decreased numbers of CD8 TCM associated with a history of SCC.
Similar immune phenotypes present within the tumor itself, in particular increased FOXP3 and reduced numbers of CD8 cells, have been associated with poor prognosis in other cancers.52 Because KTRs with previous SCC develop new tumors within months39 and SCC in non-KTRs are usually singular events, we hypothesized that FOXP3+ cells would be overrepresented in SCC from KTRs compared with non-KTRs. The only difference between SCC from KTRs matched for thickness and grade to SCCs removed from nonimmunosuppressed individuals was the ratio of CD8 to FOXP3 cells present in the infiltrate (Table 4). Importantly, 13 (53.6%) of 25 of the KTRs from whom SCCs were stained developed a new SCC within 1 year, whereas the non-KTR population did not develop new SCC. This finding suggests that a combination of a lower number of CD8+ and a higher number of FOXP3+ cells within an index tumor may predict new tumor development. This is the first time it has been associated with new SCC development in KTRs. The finding that site of infiltration of leukocytes within a tumor can have profound effects on CD8 and FOXP3 cell ratios (Table 5, Figure 2) has important implication for the validity of future reports in which total cells extracted from a tumor or total FOXP3 mRNA from a tumor are used as readouts. Our results suggest tumor location of lymphocytes can profoundly affect data regarding FOXP3.
Ultimately, we wished to test whether immune phenotyping could predict KTRs who are at increased risk for SCC development. Twenty-three of 65 KTRs with previous SCC developed new SCC, and this predominantly occurred in KTRs with more than two previous SCCs (Figure 3A), which is consistent with previous reports.15,16 In this select group of KTRs who had previous SCC and therefore were at high risk for new SCC development, we found that high numbers of FOXP3+ and low numbers of NK cells improved the accuracy of predicting a more than six-fold increased risk for developing a new SCC within 200 days (Figure 3, A versus C).
We accept that this defines a small proportion (eight [12.4%] of 65) of KTRs with SCC; however, combining immune phenotype and SCC history has a 95% confidence of predicting at least a doubling in the risk for developing a new SCC during a relatively short time frame. This provides clinicians and patients with a new parameter to consider when making alterations in immunosuppressive regimen in high-risk individuals. Immunosuppression dosage reduction can reduce the number of new tumors in KTRs without a previous SCC51 and reduce new SCC development in KTRs with poor-prognosis SCC.13,53 An important caveat is that these results may relate to the ultraviolet exposure and immunosuppression regimen peculiar to this cohort. Additional prospective monitoring of KTR populations with differing immunosuppressive regimens, ultraviolet exposure, and tumor accrual rates are required. Currently, we are determining whether these parameters are predictive in cohorts of patients who take sirolimus and in those with higher tumor accrual rates.
Conversely, the combination of immune phenotype and SCC history can also place some individuals in a lower risk category and could prevent unnecessary reductions in immunosuppression, which can have a concomitant risk for inducing rejection. Defining lower risk categories could save resources by allowing clinicians to reduce the frequency of dermatologic review. Indeed, given these data, even KTRs with a previous SCC may not need annual review, as suggested by the American Society of Transplantation.54
In summary, high numbers of Treg within the peripheral circulation and within the tumors associates with new tumor development in KTRs. If similar immune phenotypes are predictive in other KTR populations, then immune phenotype method has the potential to inform immunosuppressive regimen manipulation in KTRs at high risk for developing multiple SCCs.
Concise Methods
The study groups consisted of white KTRs with a functioning transplant and histologically diagnosed SCC and KTRs without SCC matched to the KTRs with SCC by gender, current age (±5 years), and total duration of immunosuppression (±5 years). Patients with second or subsequent grafts had the duration of immunosuppression summed. KTRs without SCC could have SCC in situ, basal carcinoma, or keratoacanthomas. White patients were selected because SCC invariably predominantly affects fair-skinned populations.
Seventy white KTRs with a history of SCC were identified, and all white KTRs without a known SCC at the Oxford Transplant Centre were potential matches (n = 416). Matching for gender, ±5 years of current age, and duration of immunosuppression defined 74 matches. Only KTRs who were >5 years after transplantation developed SCC in this study, so matches were identified from KTRs who were >5 years after transplantation.
The study was approved by a multicenter ethics committee and performed according to Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for observational studies.55 Written informed consent was obtained from all patients who participated. Two KTRs without SCC declined to be assessed, and one KTR with SCC was not competent to provide informed consent. At the end of recruitment in August 2008, 65 KTRs with SCC and 51 KTRs without SCC had been recruited. For five KTRs with SCC and one KTR without SCC, the corresponding matches had not been recruited. Ultimately, this left 60 KTRs with SCC to be matched to 50 KTRs without SCC.
Case records and the hospital histopathology database were reviewed, and demographic, transplantation, and histopathologic variables were recorded. In January 2009, the same records and databases were reviewed to determine whether new histologically confirmed SCC had developed. Tumors that were defined as re-excision or as arising from scar tissue were excluded.
Immune Phenotyping
Peripheral blood was collected from KTRs during trough levels of CNIs. PBMCs were separated from whole blood by standard centrifugation and Ficoll techniques within a median of 40 minutes (range 15 to 150 minutes) of venesection. Lymphocytes were stained as described previously and analyzed via flow cytometry.56 Absolute counts for cell populations identified were calculated using total lymphocyte count from routine hematology laboratory results and the proportion of each cell type in the lymph gate from flow cytometry data.
Immune phenotyping was postponed when the following were present: Fever >37.8°C or oral or intravenous antibiotic or antiviral therapy current or completed with 2 weeks. Patients who were admitted 1 week after immune phenotyping for suspected or confirmed infection were reassessed during a clinically quiescent period as defined already. This scenario occurred for only three patients during the recruitment period.
Immune Function
To determine whether immune phenotype had an effect on immune function, we tested the proliferative capacity of lymphocytes to the pan cell mitogen PHA, in an assay based on that described previously.57 In summary, the modified protocol was as follows: quadruplicates of 20,000 PBMCs were stimulated with varying concentration of PHA over 72 hours in 96-well plates in tissue culture medium. Maximal proliferative response was measured by the incorporation of tritiated thymidine during an additional 16-hour incubation period. Previously, a low proliferative response was defined as a proliferative response <10th centile of healthy control subjects.57
Immunohistochemistry
Biopsies from 58 SCCs from consenting KTRs were available to be reassessed by a consultant dermatopathologist to reconfirm a diagnosis of SCC. Once SCCs from KTRs were defined, SCCs from non-KTRs were matched for grade (well, moderately, and poorly differentiated) and thickness by sequentially searching the hospital histopathology database for an SCC that matched the given grade and thickness.
Matched SCC pairs were then randomly selected and anonymized (n = 27 SCC pairs). Paraffin-embedded tissue sections of the aforementioned tumors were stained by triple immunoenzymatic labeling techniques using FOXP3 (clone 236A/E7; Abcam, Cambridge, UK), CD8 (clone C8/144B; DAKO UK Ltd., Ely, UK), and CD56 (clone BC56C04; A. Menarini Diagnostics UK, Wokingham, UK) antibodies and following a previously published protocol.58
All cells were counted in the peritumor infiltrate that surrounds all SCCs. The total number of graticule areas was recorded in addition to cell numbers and therefore cell density. Lymphocyte populations that were not contiguous with the peritumor infiltrate but were entirely surrounded by tumor were recorded separately as intratumor infiltrate.
All counting of positive cells was performed in a blinded manner by a single observer. Twenty randomly selected areas were also independently counted by a second independent blinded observer. The average variability between these two independent blinded observations was 6.5%.
Statistical Analysis
The relationship of KTRs with and without SCC and demographic, transplant, and immunosuppression parameters and phenotypes was assessed using conditional logistic regression models. These models yielded ORs (and their SEs) that were adjusted for confounders (age and gender) and accounted for the matched design. The multivariate models were formulated using only variables with a statistical significance of P < 0.05 from their univariate models and then removing variables with P > 0.05 from the full model. Because there was limited power to test for interactions, we did not explicitly fit those in the models. Because of the nonsymmetric nature of histopathologic data, we used a paired nonparametric Wilcoxon test.
In a subanalysis, the predictors of Treg number were also assessed. This included a linear regression of log-transformed FOXP3+ cells/μl, and because the distribution of CD28−CD8+ T cell was bimodal with nadir at 40%, predictors were assessed in a binary logistic regression with this as the dichotomization. Only parameters previously published as predictors were used in these analyses.
Univariate time-to-event analyses on continuous variables were performed using Cox regression, and categorical variables were analyzed using the Kaplan-Meier and the log-rank methods.59,60 Both univariate and multivariate HRs were calculated by Cox regression. The proportionality assumption of the Cox regression model was assessed by looking at Schoenfeld partial residuals. In the multivariate Cox models, only variables previously published as predictors of subsequent SCC were assessed together with a priori hypothesis of high Treg and low CD8 and NK cells. The linearity (or more complex forms) of the effect of the continuous factors FOXP3+CD4+CD127low and NK cells was assessed using fractional polynomials and splines.61 As a result, these variables were dichotomized to >35 FOXP3+CD4+CD127low cells/μl and <100 NK cells/μl, respectively, and incorporated into the Cox regression.
Performing multiple statistical tests leads to an inflation in the occurrence of false-positive results, and it is required that the P value significance threshold (usually 5%) be adjusted to account for the number of independent tests. Because of the high correlation between the variables considered, a Bonferroni correction would be too conservative. By considering the number of independent variables and tests, it is possible to interpret P less than approximately 0.01 to be statistically significant. All statistical analysis was performed using the R statistical software (http://www.r-project.org) and SPSS 16 (SPSS, Chicago, IL).
Disclosures
None.
Supplementary Material
Acknowledgments
This study was supported by grants from the Roche Organ Transplant Research Foundation, Oxford Renal Unit Trust Fund, Oxford Biomedical Research Centre, Oxfordshire Health Services Research Committee, and The Wellcome Trust. D.S. is a recipient of a Lopez-Albo grant (Fundación Marqués de Valdecilla-IFIMAV).
We thank Dr. P. Trzonkowski, Dr. J. Wieckiewicz, and J. Paterson for advice and technical support.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1. Zand MS: Immunosuppression and immune monitoring after renal transplantation. Semin Dial 18: 511– 519, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Kauffman HM, Cherikh WS, McBride MA, Cheng Y, Hanto DW: Post-transplant de novo malignancies in renal transplant recipients: The past and present. Transpl Int 19: 607– 620, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Faull RJ, Hollett P, McDonald SP: Lymphoproliferative disease after renal transplantation in Australia and New Zealand. Transplantation 80: 193– 197, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Webster AC, Wong G, Craig JC, Chapman JR: Managing cancer risk and decision making after kidney transplantation. Am J Transplant 8: 2185– 2191, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Kasiske BL, Snyder JJ, Gilbertson DT, Wang C: Cancer after kidney transplantation in the United States. Am J Transplant 4: 905– 913, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Karagas MR, Cushing GL, Jr, Greenberg ER, Mott LA, Spencer SK, Nierenberg DW: Non-melanoma skin cancers and glucocorticoid therapy. Br J Cancer 85: 683– 686, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buchbinder R, Barber M, Heuzenroeder L, Wluka AE, Giles G, Hall S, Harkness A, Lewis D, Littlejohn G, Miller MH, Ryan PF, Jolley D: Incidence of melanoma and other malignancies among rheumatoid arthritis patients treated with methotrexate. Arthritis Rheum 59: 794– 799, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, Chapman JR, Webster AC, Kaldor JM, Grulich AE: Cancer incidence before and after kidney transplantation. JAMA 296: 2823– 2831, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Carroll RP, Ramsay HM, Fryer AA, Hawley CM, Nicol DL, Harden PN: Incidence and prediction of nonmelanoma skin cancer post-renal transplantation: A prospective study in Queensland, Australia. Am J Kidney Dis 41: 676– 683, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Ramsay HM, Fryer AA, Reece S, Smith AG, Harden PN: Clinical risk factors associated with nonmelanoma skin cancer in renal transplant recipients. Am J Kidney Dis 36: 167– 176, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Euvrard S, Kanitakis J, Decullier E, Butnaru AC, Lefrancois N, Boissonnat P, Sebbag L, Garnier JL, Pouteil-Noble C, Cahen R, Morelon E, Touraine JL, Claudy A, Chapuis F: Subsequent skin cancers in kidney and heart transplant recipients after the first squamous cell carcinoma. Transplantation 81: 1093– 1100, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Veness MJ, Quinn DI, Ong CS, Keogh AM, Macdonald PS, Cooper SG, Morgan GW: Aggressive cutaneous malignancies following cardiothoracic transplantation: The Australian experience. Cancer 85: 1758– 1764, 1999 [PubMed] [Google Scholar]
- 13. Moloney FJ, Kelly PO, Kay EW, Conlon P, Murphy GM: Maintenance versus reduction of immunosuppression in renal transplant recipients with aggressive squamous cell carcinoma. Dermatol Surg 30: 674– 678, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Martinez JC, Otley CC, Stasko T, Euvrard S, Brown C, Schanbacher CF, Weaver AL: Defining the clinical course of metastatic skin cancer in organ transplant recipients: A multicenter collaborative study. Arch Dermatol 139: 301– 306, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Lindelof B, Sigurgeirsson B, Gabel H, Stern RS: Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol 143: 513– 519, 2000 [PubMed] [Google Scholar]
- 16. Liddington M, Richardson AJ, Higgins RM, Endre ZH, Venning VA, Murie JA, Morris PJ: Skin cancer in renal transplant recipients. Br J Surg 76: 1002– 1005, 1989 [DOI] [PubMed] [Google Scholar]
- 17. Thibaudin D, Alamartine E, Mariat C, Absi L, Berthoux F: Long-term kinetic of T-lymphocyte subsets in kidney-transplant recipients: Influence of anti-T-cell antibodies and association with posttransplant malignancies. Transplantation 80: 1514– 1517, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Ducloux D, Carron PL, Rebibou JM, Aubin F, Fournier V, Bresson-Vautrin C, Blanc D, Humbert P, Chalopin JM: CD4 lymphocytopenia as a risk factor for skin cancers in renal transplant recipients. Transplantation 65: 1270– 1272, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Fenoglio D, Ferrera F, Fravega M, Balestra P, Battaglia F, Proietti M, Andrei C, Olive D, Antonio LC, Indiveri F, Filaci G: Advancements on phenotypic and functional characterization of non-antigen-specific CD8+CD28− regulatory T cells. Hum Immunol 69: 745– 750, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Filaci G, Fenoglio D, Fravega M, Ansaldo G, Borgonovo G, Traverso P, Villaggio B, Ferrera A, Kunkl A, Rizzi M, Ferrera F, Balestra P, Ghio M, Contini P, Setti M, Olive D, Azzarone B, Carmignani G, Ravetti JL, Torre G, Indiveri F: CD8+ CD28− T regulatory lymphocytes inhibiting T cell proliferative and cytotoxic functions infiltrate human cancers. J Immunol 179: 4323– 4334, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Viguier M, Lemaitre F, Verola O, Cho MS, Gorochov G, Dubertret L, Bachelez H, Kourilsky P, Ferradini L: Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol 173: 1444– 1453, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH: Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 24: 5373– 5380, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Bacchetta R, Passerini L, Gambineri E, Dai M, Allan SE, Perroni L, Dagna-Bricarelli F, Sartirana C, Matthes-Martin S, Lawitschka A, Azzari C, Ziegler SF, Levings MK, Roncarolo MG: Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J Clin Invest 116: 1713– 1722, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wildin RS, Smyk-Pearson S, Filipovich AH: Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet 39: 537– 545, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N, Puig PE, Novault S, Escudier B, Vivier E, Lecesne A, Robert C, Blay JY, Bernard J, Caillat-Zucman S, Freitas A, Tursz T, Wagner-Ballon O, Capron C, Vainchencker W, Martin F, Zitvogel L: CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med 202: 1075– 1085, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen ML, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, von Boehmer H, Khazaie K: Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci U S A 102: 419– 424, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trzonkowski P, Szmit E, Mysliwska J, Dobyszuk A, Mysliwski A: CD4+CD25+ T regulatory cells inhibit cytotoxic activity of T CD8+ and NK lymphocytes in the direct cell-to-cell interaction. Clin Immunol 112: 258– 267, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Wood KJ, Sakaguchi S: Regulatory T cells in transplantation tolerance. Nat Rev Immunol 3: 199– 210, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Dijke IE, Velthuis JH, Caliskan K, Korevaar SS, Maat AP, Zondervan PE, Balk AH, Weimar W, Baan CC: Intragraft FOXP3 mRNA expression reflects antidonor immune reactivity in cardiac allograft patients. Transplantation 83: 1477– 1484, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Velthuis JH, Mol WM, Weimar W, Baan CC: CD4+CD25bright+ regulatory T cells can mediate donor nonreactivity in long-term immunosuppressed kidney allograft patients. Am J Transplant 6: 2955– 2964, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Gao W, Lu Y, El Essawy B, Oukka M, Kuchroo VK, Strom TB: Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am J Transplant 7: 1722– 1732, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Battaglia M, Stabilini A, Roncarolo MG: Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood 105: 4743– 4748, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Baan CC, van der Mast BJ, Klepper M, Mol WM, Peeters AM, Korevaar SS, Balk AH, Weimar W: Differential effect of calcineurin inhibitors, anti-CD25 antibodies and rapamycin on the induction of FOXP3 in human T cells. Transplantation 80: 110– 117, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Merino J, Martinez-Gonzalez MA, Rubio M, Inoges S, Sanchez-Ibarrola A, Subira ML: Progressive decrease of CD8high+ CD28+ CD57− cells with ageing. Clin Exp Immunol 112: 48– 51, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gamadia LE, Rentenaar RJ, Baars PA, Remmerswaal EB, Surachno S, Weel JF, Toebes M, Schumacher TN, ten Berge IJ, van Lier RA: Differentiation of cytomegalovirus-specific CD8(+) T cells in healthy and immunosuppressed virus carriers. Blood 98: 754– 761, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Montes CL, Chapoval AI, Nelson J, Orhue V, Zhang X, Schulze DH, Strome SE, Gastman BR: Tumor-induced senescent T cells with suppressor function: A potential form of tumor immune evasion. Cancer Res 68: 870– 879, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Tussey L, Speller S, Gallimore A, Vessey R: Functionally distinct CD8+ memory T cell subsets in persistent EBV infection are differentiated by migratory receptor expression. Eur J Immunol 30: 1823– 1829, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A: Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401: 708– 712, 1999 [DOI] [PubMed] [Google Scholar]
- 39. Lindelof B, Jarnvik J, Ternesten-Bratel A, Granath F, Hedblad MA: Mortality and clinicopathological features of cutaneous squamous cell carcinoma in organ transplant recipients: A study of the Swedish cohort. Acta Derm Venereol 86: 219– 222, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Brantsch KD, Meisner C, Schonfisch B, Trilling B, Wehner-Caroli J, Rocken M, Breuninger H: Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: A prospective study. Lancet Oncol 9: 713– 720, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Segundo DS, Ruiz JC, Izquierdo M, Fernandez-Fresnedo G, Gomez-Alamillo C, Merino R, Benito MJ, Cacho E, Rodrigo E, Palomar R, Lopez-Hoyos M, Arias M: Calcineurin inhibitors, but not rapamycin, reduce percentages of CD4+CD25+FOXP3+ regulatory T cells in renal transplant recipients. Transplantation 82: 550– 557, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Tsukishiro T, Donnenberg AD, Whiteside TL: Rapid turnover of the CD8(+)CD28(−) T-cell subset of effector cells in the circulation of patients with head and neck cancer. Cancer Immunol Immunother 52: 599– 607, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Perret R, Ronchese F: Memory T cells in cancer immunotherapy: Which CD8 T-cell population provides the best protection against tumours? Tissue Antigens 72: 187– 194, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, Waldmann TA, Restifo NP: Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A 102: 9571– 9576, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Klonowski KD, Marzo AL, Williams KJ, Lee SJ, Pham QM, Lefrancois L: CD8 T cell recall responses are regulated by the tissue tropism of the memory cell and pathogen. J Immunol 177: 6738– 6746, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sallusto F, Geginat J, Lanzavecchia A: Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu Rev Immunol 22: 745– 763, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Bouwes Bavinck JN, Euvrard S, Naldi L, Nindl I, Proby CM, Neale R, Abeni D, Tessari GP, Feltkamp MC, Claudy A, Stockfleth E, Harwood CA: Keratotic skin lesions and other risk factors are associated with skin cancer in organ-transplant recipients: A case-control study in the Netherlands, United Kingdom, Germany, France, and Italy. J Invest Dermatol 127: 1647– 1656, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Baeten D, Louis S, Braud C, Braudeau C, Ballet C, Moizant F, Pallier A, Giral M, Brouard S, Soulillou JP: Phenotypically and functionally distinct CD8+ lymphocyte populations in long-term drug-free tolerance and chronic rejection in human kidney graft recipients. J Am Soc Nephrol 17: 294– 304, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Fryer AA, Ramsay HM, Lovatt TJ, Jones PW, Hawley CM, Nicol DL, Strange RC, Harden PN: Polymorphisms in glutathione S-transferases and non-melanoma skin cancer risk in Australian renal transplant recipients. Carcinogenesis 26: 185– 191, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Pascual J, Zamora J, Galeano C, Royuela A, Quereda C: Steroid avoidance or withdrawal for kidney transplant recipients. Cochrane Database Syst Rev CD005632, 2009 [DOI] [PubMed] [Google Scholar]
- 51. Dantal J, Hourmant M, Cantarovich D, Giral M, Blancho G, Dreno B, Soulillou JP: Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: Randomised comparison of two cyclosporin regimens. Lancet 351: 623– 628, 1998 [DOI] [PubMed] [Google Scholar]
- 52. Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K: Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 102: 18538– 18543, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Otley CC, Coldiron BM, Stasko T, Goldman GD: Decreased skin cancer after cessation of therapy with transplant-associated immunosuppressants. Arch Dermatol 137: 459– 463, 2001 [PubMed] [Google Scholar]
- 54. Kasiske BL, Vazquez MA, Harmon WE, Brown RS, Danovitch GM, Gaston RS, Roth D, Scandling JD, Singer GG: Recommendations for the outpatient surveillance of renal transplant recipients. American Society of Transplantation. J Am Soc Nephrol 11[ Suppl 15]: S1– S86, 2000 [PubMed] [Google Scholar]
- 55. Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M: Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Ann Intern Med 147: W163– W194, 2007 [DOI] [PubMed] [Google Scholar]
- 56. Trzonkowski P, Zilvetti M, Friend P, Wood KJ: Recipient memory-like lymphocytes remain unresponsive to graft antigens after CAMPATH-1H induction with reduced maintenance immunosuppression. Transplantation 82: 1342– 1351, 2006 [DOI] [PubMed] [Google Scholar]
- 57. Hutchinson P, Chadban SJ, Atkins RC, Holdsworth SR: Laboratory assessment of immune function in renal transplant patients. Nephrol Dial Transplant 18: 983– 989, 2003 [DOI] [PubMed] [Google Scholar]
- 58. Paterson JC, Ballabio E, Mattsson G, Turner SH, Mason DY, Marafioti T: Labeling of multiple cell markers and mRNA using automated apparatus. Appl Immunohistochem Mol Morphol 16: 371– 381, 2008 [DOI] [PubMed] [Google Scholar]
- 59. Bradburn MJ, Clark TG, Love SB, Altman DG: Survival analysis part II: Multivariate data analysis—An introduction to concepts and methods. Br J Cancer 89: 431– 436, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Clark TG, Bradburn MJ, Love SB, Altman DG: Survival analysis part I: Basic concepts and first analyses. Br J Cancer 89: 232– 238, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Royston P, Altman DG: Regression using fractional polynomials of continuous covariates: parsimonious parametric modelling. Appl Stat 43: 429– 467, 1994 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.