Abstract
Inflammation associated with rheumatoid arthritis is stoked by platelets, expanding their roles into the immune system.
Platelets are small anucleate cells that circulate in the blood of mammals and are critical effectors of hemostasis, blood clotting, and wound repair. Rheumatoid arthritis is a chronic inflammatory disease of humans that involves the innate and adaptive limbs of the host immune system, and is thought to be autoimmune in nature. Traditionally, platelets and rheumatoid arthritis don’t go together. On page 580 of this issue, Boilard et al. (1) report that they do—microparticles released by platelets may be incendiary devices in the conflagration of a hot, swollen, and painful rheumatoid joint.
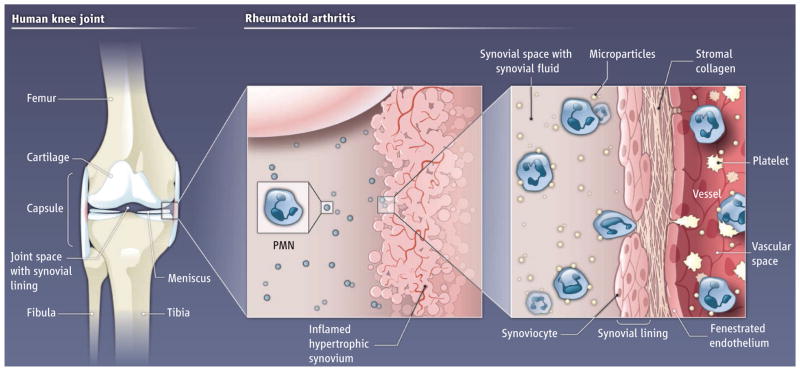
Although rheumatoid arthritis affects several tissues, destructive joint inflammation is a central feature (see the figure). The normal knee is a synovial joint that encloses a space containing a clear, viscous, largely acellular fluid filtrate of plasma and is bordered by synovium, a tissue consisting of lining cells, stromal matrix molecules, and blood vessels. Boilard et al. have discovered that platelet microparticles—vesicles shed by activated platelets (2)—are present in knee joint fluid in rheumatoid arthritis and other inflammatory arthritic conditions. The authors show, using a mouse model, that platelets are critical in the development of inflammatory arthritis. Activation of glycoprotein VI, a platelet-specific receptor for collagen, induces microparticle shedding. In addition, fibroblast-like cells that line the synovial Cavity of the joint can also trigger microparticle release. Because these fibroblast-like synoviocytes and collagen are present in the inflamed synovium, platelet interactions in this milieu could lead to local release of microparticles and their translocation into the joint space.
Figure. Perpetrators in a painful joint.
In rheumatoid arthritis, the joint synovium becomes inflamed with dilated blood vessels and immune cells. Polymorphonuclear leukocytes (PMN), cytokines, chemokines, and other mediators of inflammation, accumulate in the synovial fluid. Local activation of platelets by collagen and synoviocytes may trigger the release of microparticles from platelets. These microparticles then enter the joint space and further amplify inflammation by producing interleukin-1. This then activates synoviocytes, thus supporting a cycle of inflammation.
Boilard et al. also determined that microparticles from the joint fluid of patients with rheumatoid arthritis can reciprocally activate fibroblast-like synoviocytes, and this interaction induces synoviocytes to secrete inflammatory chemokines and cytokines. Interleukin-1—a pleiotropic cytokine that is rapidly synthesized by activated human platelets (3) and is packaged into microparticles (1)—accounted for much of this stimulatory activity. Thus, a vicious cycle ensues: Fibroblast-like synoviocytes induce formation of platelet-derived microparticles. The microparticles then deliver interleukin-1, which triggers synoviocytes to synthesize other cytokines and chemokines, some of which attract polymorphonuclear leukocytes and thereby fan the fire of inflammation.
A role for platelets has previously been described in inflammatory joint diseases, yet this is not commonly mentioned in summaries of their pathogenesis (4, 5). It’s worth noting that platelets are previous suspects in rheumatoid arthritis: They accumulate in the joints of affected patients; platelet thrombi were observed in synovial vessels of patients with rheumatoid arthritis; and increased numbers of platelets in synovial fluid and of microparticles in blood are associated with the condition (6, 7–9). Why the lack of mechanistic study? Platelets have generally been viewed as cells with limited short-term hemostatic activities that exclusively take place in the vascular compartment. Recently discovered “new biology” of platelets is changing these perceptions, however. For example, they have an intricate transcriptome (entire repertoire of RNAs produced), activation-dependent posttranscriptional pathways, influences on extravascular events, and, perhaps, longer life spans than previously appreciated (10). Furthermore, there is a wealth of information indicating that platelets have inflammatory functions and a substantial arsenal of factors that confer the ability to signal to monocytes, dendritic cells, and other immune effector cells. There is also evidence that platelet microparticles activate adaptive immune cells in specific tissue compartments in response to cues that trigger antibody synthesis and alter lymphocyte activities (11).
A perplexing issue is that Boilard et al. did not detect intact platelets in the synovial fluid of patients with rheumatoid arthritis, although other investigators have found them (7, 8). How, then, did the incendiary microparticles gain access to the joint space? One possibility is that microparticles associate with transmigrating leukocytes and are then carried into the synovial fluid, a mechanism supported by microscopic and flow cytometric observations of Boilard et al. This could happen in synovial blood vessels, where the numbers of microparticles may be much higher than reported in peripheral blood (9). It could also occur in extravascular synovial tissue if release of microparticles occurs as a result of platelet interactions with collagen and/or fibroblast-like synoviocytes in this compartment (1). Platelets adhere to activated polymorphonuclear leukocytes and monocytes in the circulation in many inflammatory conditions (12), including rheumatoid arthritis (13). Thus, leukocytes may deliver platelets and/or microparticles to extravascular sites by this, or other, mechanisms (14). Polymorphonuclear leukocytes may well be accomplices that deliver microparticles to the rheumatoid arthritis joint space and, conceivably, facilitate their local formation in transit through the synovium.
Acknowledgments
We thank D. Lim for assistance with the figure.
References and Notes
- 1.Boilard E, et al. Science. 2010;327:580. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perez-Pujol S, et al. Cytometry A. 2007;71:38. doi: 10.1002/cyto.a.20354. [DOI] [PubMed] [Google Scholar]
- 3.Denis MM, et al. Cell. 2005;122:379. doi: 10.1016/j.cell.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg AE. In: Robbins and Cotran Pathologic Basis of Disease. 8. Kumar V, Abbas AK, Favsto N, Aster JC, editors. Saunders; Philadelphia: 2010. pp. 1205–1256. [Google Scholar]
- 5.Choy EH, Panayi GS. N Engl J Med. 2001;344:907. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 6.Diaz-Gonzalez F, Ginsberg MH. In: Kelly’s Textbook of Rheumatology. Harris ED Jr, et al., editors. Vol. 1. Elsevier Saunders; Philadelphia: 2005. pp. 252–259. [Google Scholar]
- 7.Herd CM, Page CP. In: Immunopharmacology of Platelets. Joseph M, editor. Academic Press; London: 1995. [Google Scholar]
- 8.Yaron M, Djaldetti M. Arthritis Rheum. 1978;21:607. doi: 10.1002/art.1780210509. [DOI] [PubMed] [Google Scholar]
- 9.Knijff-Dutmer EAJ, et al. Arthritis Rheum. 2002;46:1498. doi: 10.1002/art.10312. [DOI] [PubMed] [Google Scholar]
- 10.Zimmerman GA, Weyrich AS. Arterioscler Thromb Vasc Biol. 2008;28:s17. doi: 10.1161/ATVBAHA.107.160218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sprague DL, et al. Blood. 2008;111:5028. doi: 10.1182/blood-2007-06-097410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weyrich AS, Zimmerman GA. Trends Immunol. 2004;25:489. doi: 10.1016/j.it.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Joseph JE, et al. Br J Haematol. 2001;115:451. doi: 10.1046/j.1365-2141.2001.03101.x. [DOI] [PubMed] [Google Scholar]
- 14.Weissmuller T, et al. J Clin Invest. 2008;118:3682. doi: 10.1172/JCI35874. [DOI] [PMC free article] [PubMed] [Google Scholar]