Abstract
Tumor necrosis factor-alpha (TNF-α) derived from activated Schwann cells (SCs) plays a critical role as an inflammatory mediator in the peripheral nervous system disease. TNF-α could act as an autocrine mediator in SC activation. In this study, we found knockdown Src-suppressed protein kinase C substrate (SSeCKS) expression suppressed TNF-α production induced by TNF-α, overexpression of SSeCKS could promoted TNF-α autocrine in SCs. Such effects might be resulted in SSeCKS promoted p38 and JNK activation in SCs treated by TNF-α. Thus present data show that while SCs activation, SSeCKS may plays an important role in the release of inflammatory mediators.
Keywords: Schwann cells, Tumor Necrosis Factor-alpha, Autocrine, p38, JNK
Introduction
Schwann cells (SCs) are the main glia of the peripheral nervous system (PNS). Besides their roles in myelination, trophic support, and regeneration of axons, SCs exhibit potential for some immune functions, similarly to non-myelinating glia of the CNS. Recently, interest has focused on the association between glia activation and the effect, because peripheral nerve injury that produces hyperalgesia and allodynia activates glial cells in the dorsal root ganglion. The activation of glial cells is thought to be involved in the pathogenesis of neuropathic pain (Campana 2007).
Tumor necrosis factor-alpha (TNF-α) is synthesized and released in several PNS diseases with diverse etiology and pathological manifestation such as trauma injury, degeneration diseases, and inflammation (Raine et al. 1998). In the PNS, activated SCs are one of the main sources of TNF-α. Our groups’ previous studies have shown that in the SCs activation, TNF-α production by SCs was partially mediated by TNF-α produced by SCs (Cheng et al. 2007). The positive autocrine loop may exist to promote SCs various biological functions. But the mechanism in the autocrine loop has not been studied. In SCs, two receptors, tumor necrosis factor receptor-1 (TNFR1) and TNFR2 mediate a wide variety of TNF-α biological functions (McFarlane et al. 2002). Studies have demonstrated that TNF-α can activate the mitogen-activated protein kinases (MAPKs) and nuclear factor kappa B (NF-κB) signal pathways that induce expression of many genes (Maeng et al. 2006; Zhu et al. 2007). But the signal pathway involved in the TNF-α-induced gene expression in SCs has not been clarified.
Src-suppressed protein kinase C substrate (SSeCKS) was originally identified as a major substrate of PKC both in vitro and in vivo (Lin and Gelman 2002). Upon PKC activation, SSeCKS translocates from the cell cortex to perinuclear sites (Nelson et al. 1999). Over-expression of SSeCKS results in the increase of ERK1/2 and protein kinase B (PKB, Akt) activation, but a decrease of intergrin-independent focal adhesion kinase (FAK) phosphorylation and growth arrest (Akakura et al. 2008; Xia and Gelman 2002). Our group has also demonstrated that SSeCKS may up-regulate lipopolysaccharide (LPS)-induced TNF-α expression in astrocytes (Sun et al. 2007). These observations have led to the proposition that SSeCKS might be a regulator in PKC-mediated MAPKs activation and cytokine production. Recently, we have also found the expression of SSeCKS was significantly increased in TNF-α-treated SCs. But whether SSeCKS plays a role in the TNF-α-induced autocrine or the mechanism is still unknown. Our studies aim to determine whether SSeCKS is required or SSeCKS affects MAPKs signal pathway in the TNF-α autocrine regulation.
In the study, we have determined that SSeCKS plays a positive role in the regulation of TNF-α-induced TNF-α secretion. SSeCKS appears to promote the activation of p38 and JNK which subsequently enhances TNF-α secretion in TNF-α-treated SCs. These investigations indicate that SSeCKS may play an essential role in the SCs activation.
Materials and Methods
SCs Culture and Treatment
SCs were cultured from excised DRG, brachial plexus, and sciatic nerves from SD rat as described previously (Brockes et al. 1979). Briefly, cells were cultured with DMEM containing 10% FCS. In order to reduce the number of dividing fibroblasts, SCs cultures were treated with monoclonal anti-thy 1.1 antibody and rabbit complement (BD PharMingen). SCs cultures were primed for indicated times or indicated concentrations with recombined TNF-α (50 ng/ml, Sigma) for 24 h. In some experiments, SB202190 (10 μM/ml, Sigma) and SP600125 (20 μM/ml, Sigma) were applied 40 min prior to TNF-α stimulation.
siRNAs and Transfection
The SSeCKS expression vector was gift by Pro. Gelman (Mount Sinai School of Medicine), and then sub-cloned to the EGFP vector. The rat SSeCKS siRNA expression vector was constructed which targeted the nucleotide residues 5′-AAGGAGATGTCCATGTCCAAG-3′. For transient transfection, the SSeCKS expression vector, siRNA vector, and the non-specific vector were carried out using lipofectamine 2000 (Invitrogen) and plus reagent in OptiMEM (Invitrogen) as suggested by the manufacturer. Transfected cells were used for the subsequent experiments 48 h after transfection.
Detection of TNF-α Secretion by ELISA
The concentrations of TNF-α released in the culture supernatants were measured by specific rat TNF-α enzyme-linked immunosorbent assay (ELISA) according to the instruction of the manufacturer (BD PharMingen). Briefly, cells in 100 μl of medium were seeded onto 96-well plates, and treated under different conditions. At the appropriate time, 100 μl of supernatants were harvested for ELISA assay. The amount of TNF-α added for stimulation was subtracted from the results in order to show the real TNF-α production from SCs.
RNA Isolation and Reverse Transcriptase PCR (RT-PCR) Analysis
Total RNA of SCs was extracted using Trizol extraction kit according to the manufacturer’s protocol, and reverse-transcribed using ThermoScript RT-PCR system (Invitrogen). Primer sequences used in this report were listed as follows: SSeCKS (GenBank Accession Number: U23146): forward, 5′-AAGAATGGCCAGCTGTCTAC-3′; reverse, 5′-GCTTTGGAACTGTCTGTCACT-3′; TNF-α (GenBank Accession Number: NM_012675): forward, 5′-CGTCGTAGCAAACCACCAAG-3′; reverse, 5′-CACAGAGCAATGACTCCAAAG-3′. The GAPDH was used as an internal control. PCR amplification was carried out with an initial denaturing step at 94°C for 5 min, then 20 cycles at 94°C for 45 s, at 58°C for 45 s, and at 72°C for 45 s, and a further extension at 72°C for 10 min. The signal intensities of RT–PCR products were quantified by calculating the integrated volume of the band with a computing laser densitometer.
Western Blot Analysis
Cell lysates were obtained by scratching cells in a lysisbuffer. Proteins were loaded into wells of acryl/bisacrylamide gel, and after separation, proteins were transferred to a Polyvinylidene fluoride (PVDF) membrane. After saturation in TBST containing 5% milk, primary antibodies and horseradish peroxidase-conjugated secondary antibodies were sequentially added. Revelation was obtained by enhanced chemiluminescence.
Statistical Analysis
All experiments were repeated at least three times. The relative difference between the control and treatment groups was calculated and expressed as relative ratio over the control (the control was set as 1). All numerical data were described as mean ± SEM. Data were analyzed using the two-tailed t test. A probability value of 0.05 or less was considered significant.
Results
SSeCKS Expression Promote TNF-α-induced TNF-α Secretion in SCs
TNF-α Induces TNF-α Secretion and SSeCKS Expression
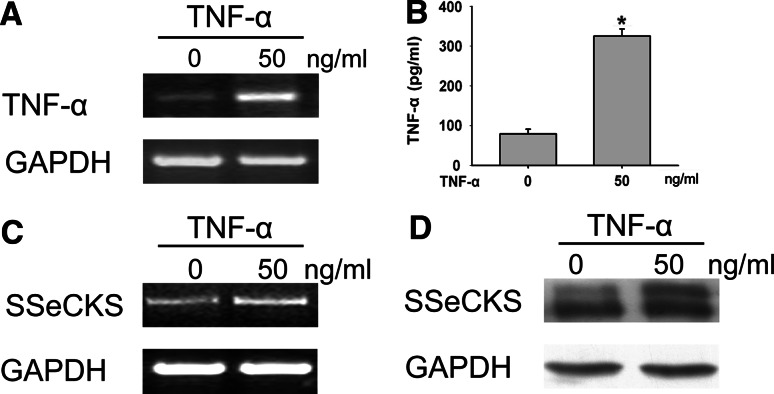
When rat primary SCs were stimulated with recombinant TNF-α for 24 h in vitro, the induction of TNF-α mRNA and protein expression was observed and reached high level. However, the cultured SCs without TNF-α stimulation produced minimal TNF-α (Fig. 1a, b). Next we investigated the expression of SSeCKS with 50 ng/ml TNF-α treatment for 24 h. Significant SSeCKS expression was observed in both mRNA and protein levels (Fig. 1c, d). These results encouraged us to focus on the function of SSeCKS in the regulation of TNF-α autocrine following TNF-α treatment.
Fig. 1.
TNF-α induces the expression of TNF-α and SSeCKS in cultured SCs. Cultures were untreated or treated with TNF-α (50 ng/ml) for 24 h. The expression of TNF-α mRNA (a) and SSeCKS mRNA (c) were detected by RT-PCR. At the same time, the secretion of TNF-α was detected by ELISA (b), and the change of SSeCKS protein level was analyzed by immunoblot (d). GAPDH was used as an internal control. Statistical differences compared with the controls (untreated) were given as * P < 0.05
SSeCKS Promote TNF-α Secretion Induced by TNF-α
To further assess the importance of SSeCKS in TNF-α autocrine, we transfected SCs with SSeCKS siRNA or a non-specific siRNA vector. As predicted, SSeCKS mRNA expression was considerably decreased in SSeCKS siRNA-transfected SCs with or without 50 ng/ml TNF-α treatment for 24 h, although only 80% of SSeCKS was knocked down because of the limitation of transient transfection efficiency (Fig. 2a). After treating SSeCKS-knockdowned SCs with TNF-α for 24 h, the ELISA assay showed about half of the TNF-α secretion relative to the non-specific siRNA-transfected SCs. However, this was not observed for the cells without TNF-α treatment (Fig. 2b). And we have also used the SSeCKS expression vector to transfected SCs. Just as shown in Fig. 2c, the EGFP–SSeCKS were expressed in SCs. ELISA assay showed the TNF-α secretion was increased about twofolds when treating SSeCKS overexpressed SCs with TNF-α for 24 h compared with non-treated SSeCKS overexpressed SCs. However, there were no differences in over-expression cells without TNF-α treatment (Fig. 2d). These results implied that SSeCKS might play an essential role in TNF-α secretion induced by TNF-α.
Fig. 2.
SSeCKS expression impaired TNF-α secretion induced by TNF-α. SCs were transfected with a non-specific siRNA or SSeCKS siRNA and then treated with TNF-α (50 ng/ml) for 24 h. a RT-PCR was used to assess the efficiency of the siRNA vector transfection. b ELISA was used to detect the secretion of TNF-α. c Light microscope shown EGFP–SSeCKS were expressed in SCs. d ELISA shown the secretion of TNF-α in SSeCKS-overexpressed SCs. Data were expressed as mean ± SEM of the maximum response observed. Statistical differences compared with the normal SCs were given as * P < 0.05 and with the TNF-α treated normal SCs group as # P < 0.05
SSeCKS Is Involved in TNF-α-induced p38 and JNK Signal Pathway Activation
p38 and JNK Signal Pathway Are Involved in TNF-α-induced TNF-α Production
p38 and JNK pathways are initially involved in TNF-α-induced expression of various inflammatory mediators, including cytokines, chemokines, and adhesion molecules. As illustrated in Fig. 3a, the activities of p38 and JNK were significantly increased in the TNF-α treated SCs at 24 h. To detect the effect of these pathways in the autocrine of TNF-α, the inhibitor SB202190 and SP600125 which may inhabited the phosphorylated p38 and phosphorylated JNK activity were used. SCs were pretreated with the SB202190 and SP600125 for 40 min followed by stimulation with TNF-α for 24 h. As shown in Fig. 3b, TNF-α secretion was decreased significantly when inhabiting either the p38 or the JNK pathway in TNF-α-treated SCs.
Fig. 3.
Effect of p38 and JNK signal pathways in TNF-α-induced TNF-α production. a Primary SCs were treated with TNF-α (50 ng/ml) for 24 h, and then cells were lysed and proceeded for analysis of the expression of phosphorylated p38 and JNK (p-p38 and p-JNK) and total p38 and JNK (t-p38 and t-JNK). b Cells were pretreated with SB202190 (SB) and SP600125 (SP) for 40 min, and then stimulated with TNF-α for 24 h. Culture mediums were harvested and ELISA was used to assay the level of TNF-α. These data were means ± SEM. * P < 0.05 comparing with the untreated groups. # P < 0.05 comparing with the TNF-α-treated group
SSeCKS Promotes p38 and JNK Activation in TNF-α-treated SCs
To assess whether SSeCKS is related to TNF-α-induced TNF-α secretion, SSeCKS siRNA was applied. As shown in Fig. 4a, the activation of p38 and JNK did not show any differences in SSeCKS siRNA-transfected SCs compared with non-specific siRNA-transfected SCs in the absence of TNF-α stimulation. But in the presence of TNF-α stimulation, the activation of p38 and JNK were significantly decreased in SSeCKS siRNA transfected-SCs in comparing with the non-specific siRNA transfected-SCs. In further, SSeCKS overexpressed SCs were pretreated with the SB202190 and SP600125 for 40 min followed by stimulation with TNF-α for 24 h. As shown in Fig. 4b, TNF-α autocrine was decreased significantly when inhabiting either the p38 or the JNK pathway in SSeCKS-overexpressed SCs (Fig. 4b).
Fig. 4.
Knockdown SSeCKS expression reduces phosphorylation of p38 and JNK induced by TNF-α. a SCs were transfected with a non-specific siRNA or SSeCKS siRNA and then exposed to 50 ng/ml TNF-α for 24 h. Whole-cell lysates were prepared and immunoblotted with the antibodies against the p-p38, p-JNK, t-p38, t-JNK. Densitometric values were plotted and values represented the means ± SEM. * P < 0.05 vs. TNF-α untreated non-specific siRNA-transfected SCs. # P < 0.05 comparing with the TNF-α-treated non-specific siRNA-transfected SCs. b SCs were transfected with EGFP or EGFP–SSeCKS overexpression vectors and pretreated with SB202190 (SB) and SP600125 (SP) for 40 min following by TNF-α treatment for 24 h. Densitometric values were plotted and values represented the means ± SEM. * P < 0.05 vs. TNF-α untreated SCs. # P < 0.05 comparing with the TNF-α treated SSeCKS over-expressed SCs
Discussion
In the present study, we are the first to demonstrate that SSeCKS increased TNF-α-induced TNF-α secretion in SCs. SSeCKS conferred its effect by promoting p38 and JNK activation induced by TNF-α which leading to the induction of TNF-α secretion.
While in many PNS degeneration and injury diseases, TNF-α has been described as one of the central injury-induced cytokines responsible for a series of disease defining events during Wallerian degeneration (Hartung et al. 1992). TNF-α generation and secretion are also central to setting the cytokine network through the process (Schafers et al. 2003). In the PNS, SCs and neurons are highly vulnerable to TNF-α. Suppression of TNF-α signaling rescues toxicity in inflammatory, injury, and degenerative processes (Merrill et al. 1993; Selmaj et al. 1991). Long-term SC activation is present proximal to demyelinated areas in the chronic stages of MS (Nishie et al. 2004) or around degenerating neurons in disorders such as Alzheimer disease (Melton et al. 2003). These results suggest the existence of a mechanism chronically activating SC, leading to the extended survival of SC cells. TNF-α induced the increased production of TNF-α, as well as additional inflammatory mediators, including interleukin-6 (IL-6), IL-1β, and nitric oxide (Cheng et al. 2007). However, the physiological and pathological significance of the TNF-α-mediated autocrine loop is still unclear.
Our previous study has demonstrated that TNF-α binds to TNFR1 in SCs (Qin et al. 2008). When binding with TNF-α, the receptor trimerization and recruited several signaling proteins to the cytoplasmic domains of the receptors such as TNFR1-associated death domain protein (TRADD), receptor-interacting protein 1 (RIP1), Fas-associated death domain protein (FADD), and TNF-receptor-associated factor 2 (TRAF2). They may induce the activation of several MAPKs, most notably JNK and p38 (Liu et al. 1996; Natoli et al. 1997). Various studies have shown that the activation of MAPKs may induce the biological effects such as the secretion of TNF-α.
SSeCKS was originally identified as a transcriptionally suppressed gene in v-src and ras-transformed rodent fibroblast cells (Lin et al. 1995). It is the ortholog of the human AKAP12 gene that encodes a kinase-scaffolding protein (Nauert et al. 1997).
SSeCKS is defined as a PKC substrate in vitro and in vivo. The coding sequence of SSeCKS contains four domains of overlapping PKC phosphorylation motifs. Each of these sites can bind PKC in a phosphatidylserine-dependent manner (Lin et al. 1996). Myristoylated alanine-rich C kinase substrate (MARCKS), another widely studied PKC substrate, sharing biochemical and structural characteristics with SSeCKS, has been demonstrated to be closely linked to glial activation processes in both LPS or amyloid β-induced inflammation (Nakai et al. 2001). Considering that SSeCKS binds PKC in a phosphatidyl serine-dependent manner and is also a major PKC substrate in vitro and in vivo. Cellular TNF-α production depends on PKC activity as well as the availability of p38 and JNK pathway, which is controlled by enzymes such as MEK (Maeng et al. 2006). Recent work has linked activation of the MAPKS pathway with the PKC pathway in astrocytes (Maeng et al. 2006). Thus, it is possible that enhanced TNF-α production in TNF-α-stimulated astrocytes also involved in PKC–SSeCKS-mediated MAPKs activation. An important question was whether TNF-α-induced activation of p38 and JNK were related to SSeCKS expression. Our results demonstrate for the first time that SSeCKS gene silencing aborted p38 and JNK activation in SCs. Expression of SSeCKS has been studied by others as well. The ability of its regulatory subunit to bind PKC, protein kinase A, calmodulin, and β2-adrenergic receptors suggests its function to assemble a multiprotein signaling complex at the appropriate cell response to stimuli (Lin and Gelman 2002; Lin et al. 1996; Xia and Gelman 2002).
In conclusion, our results demonstrate that TNF-α induces TNF-α secretion via p38 and JNK activation in SCs. SSeCKS is likely a transducer of MAPKs p38- and JNK-mediated signals. This raises the possibility that specific targeting of such upstream signaling pathways may represent a more effective strategy than blocking TNF-α, a downstream product of the inflammatory cascades.
Acknowledgments
The authors wish to thank Dr. Yuxiang Hu for helpful criticism and linguistic revision of the manuscript. This work was supported by the National Natural Science Foundation of China (No. 30300099, No. 30770488, and No. 30870320); Natural Science Foundation of Jiangsu province (No. BK2009156, No. BK2009157); Natural Science Foundation of Jiangsu Colleges and Universities Grant (09KJD310005); Special Research Grant (No. XK200723) for the Key Laboratory from the Department of Health, Jiangsu Province; The Society and Technology Grew Project of Nantong City (No. S2008020); Postgraduate Scientific Innovation Program of Nantong University (YKC09024).
Footnotes
Zhengming Zhou and Tao Tao contributed equally to this work.
Contributor Information
Aiguo Shen, Email: shen_aiguo@yahoo.com.cn.
Xiang Lu, Email: nanjingluxiang@gmail.com.
References
- Akakura S, Huang C, Nelson PJ, Foster B, Gelman IH (2008) Loss of the SSeCKS/Gravin/AKAP12 gene results in prostatic hyperplasia. Cancer Res 68:5096–5103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockes JP, Fields KL, Raff MC (1979) Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res 165:105–118 [DOI] [PubMed] [Google Scholar]
- Campana WM (2007) Schwann cells: activated peripheral glia and their role in neuropathic pain. Brain Behav Immun 21:522–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Qin Y, Shao X, Wang H, Gao Y, Cheng M, Shen A (2007) Induction of TNF-alpha by LPS in Schwann cell is regulated by MAPK activation signals. Cell Mol Neurobiol 27:909–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung HP, Jung S, Stoll G, Zielasek J, Schmidt B, Archelos JJ, Toyka KV (1992) Inflammatory mediators in demyelinating disorders of the CNS and PNS. J Neuroimmunol 40:197–210 [DOI] [PubMed] [Google Scholar]
- Lin X, Gelman IH (2002) Calmodulin and cyclin D anchoring sites on the Src-suppressed C kinase substrate, SSeCKS. Biochem Biophys Res Commun 290:1368–1375 [DOI] [PubMed] [Google Scholar]
- Lin X, Nelson PJ, Frankfort B, Tombler E, Johnson R, Gelman IH (1995) Isolation and characterization of a novel mitogenic regulatory gene, 322, which is transcriptionally suppressed in cells transformed by src and ras. Mol Cell Biol 15:2754–2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Tombler E, Nelson PJ, Ross M, Gelman IH (1996) A novel src- and ras-suppressed protein kinase C substrate associated with cytoskeletal architecture. J Biol Chem 271:28430–28438 [DOI] [PubMed] [Google Scholar]
- Liu ZG, Hsu H, Goeddel DV, Karin M (1996) Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell 87:565–576 [DOI] [PubMed] [Google Scholar]
- Maeng YS, Min JK, Kim JH, Yamagishi A, Mochizuki N, Kwon JY, Park YW, Kim YM, Kwon YG (2006) ERK is an anti-inflammatory signal that suppresses expression of NF-kappaB-dependent inflammatory genes by inhibiting IKK activity in endothelial cells. Cell Signal 18:994–1005 [DOI] [PubMed] [Google Scholar]
- McFarlane SM, Pashmi G, Connell MC, Littlejohn AF, Tucker SJ, Vandenabeele P, MacEwan DJ (2002) Differential activation of nuclear factor-kappaB by tumour necrosis factor receptor subtypes. TNFR1 predominates whereas TNFR2 activates transcription poorly. FEBS Lett 515:119–126 [DOI] [PubMed] [Google Scholar]
- Melton LM, Keith AB, Davis S, Oakley AE, Edwardson JA, Morris CM (2003) Chronic glial activation, neurodegeneration, and APP immunoreactive deposits following acute administration of double-stranded RNA. Glia 44:1–12 [DOI] [PubMed] [Google Scholar]
- Merrill JE, Ignarro LJ, Sherman MP, Melinek J, Lane TE (1993) Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J Immunol 151:2132–2141 [PubMed] [Google Scholar]
- Nakai M, Tanimukai S, Yagi K, Saito N, Taniguchi T, Terashima A, Kawamata T, Yamamoto H, Fukunaga K, Miyamoto E, Tanaka C (2001) Amyloid beta protein activates PKC-delta and induces translocation of myristoylated alanine-rich C kinase substrate (MARCKS) in microglia. Neurochem Int 38:593–600 [DOI] [PubMed] [Google Scholar]
- Natoli G, Costanzo A, Ianni A, Templeton DJ, Woodgett JR, Balsano C, Levrero M (1997) Activation of SAPK/JNK by TNF receptor 1 through a noncytotoxic TRAF2-dependent pathway. Science 275:200–203 [DOI] [PubMed] [Google Scholar]
- Nauert JB, Klauck TM, Langeberg LK, Scott JD (1997) Gravin, an autoantigen recognized by serum from myasthenia gravis patients, is a kinase scaffold protein. Curr Biol 7:52–62 [DOI] [PubMed] [Google Scholar]
- Nelson PJ, Moissoglu K, Vargas J Jr, Klotman PE, Gelman IH (1999) Involvement of the protein kinase C substrate, SSeCKS, in the actin-based stellate morphology of mesangial cells. J Cell Sci 112(Pt 3):361–370 [DOI] [PubMed] [Google Scholar]
- Nishie M, Mori F, Yoshimoto M, Takahashi H, Wakabayashi K (2004) A quantitative investigation of neuronal cytoplasmic and intranuclear inclusions in the pontine and inferior olivary nuclei in multiple system atrophy. Neuropathol Appl Neurobiol 30:546–554 [DOI] [PubMed] [Google Scholar]
- Qin Y, Cheng C, Wang H, Shao X, Gao Y, Shen A (2008) TNF-alpha as an autocrine mediator and its role in the activation of Schwann cells. Neurochem Res 33:1077–1084 [DOI] [PubMed] [Google Scholar]
- Raine CS, Bonetti B, Cannella B (1998) Multiple sclerosis: expression of molecules of the tumor necrosis factor ligand and receptor families in relationship to the demyelinated plaque. Rev Neurol (Paris) 154:577–585 [PubMed] [Google Scholar]
- Schafers M, Svensson CI, Sommer C, Sorkin LS (2003) Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci 23:2517–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmaj K, Shafit-Zagardo B, Aquino DA, Farooq M, Raine CS, Norton WT, Brosnan CF (1991) Tumor necrosis factor-induced proliferation of astrocytes from mature brain is associated with down-regulation of glial fibrillary acidic protein mRNA. J Neurochem 57:823–830 [DOI] [PubMed] [Google Scholar]
- Sun LL, Cheng C, Liu HO, Shen CC, Xiao F, Qin J, Yang JL, Shen AG (2007) Src suppressed C kinase substrate regulates the lipopolysaccharide-induced TNF-alpha biosecretion in rat astrocytes. J Mol Neurosci 32:16–24 [DOI] [PubMed] [Google Scholar]
- Xia W, Gelman IH (2002) Mitogen-induced, FAK-dependent tyrosine phosphorylation of the SSeCKS scaffolding protein. Exp Cell Res 277:139–151 [DOI] [PubMed] [Google Scholar]
- Zhu G, Cai J, Zhang J, Zhao Y, Xu B (2007) Abnormal nuclear factor (NF)-kappaB signal pathway and aspirin inhibits tumor necrosis factor alpha-induced NF-kappaB activation in keloid fibroblasts. Dermatol Surg 33:697–708 [DOI] [PubMed] [Google Scholar]