Abstract
Limited success has been achieved in extending the survival of patients with metastatic and hormone-refractory prostate cancer (HRPC). There is a strong need for novel agents in the treatment and prevention of HRPC. We have shown that flavokawain B (FKB), a kava chalcone, is about 4 to 12 fold more effective in reducing the cell viabilities of androgen receptor (AR)-negative, HRPC cell lines DU145 and PC-3 than AR–positive, hormone-sensitive prostate cancer cell lines LAPC4 and LNCaP, with minimal effect on normal prostatic epithelial and stromal cells. FKB induces apoptosis with an associated increased expression of proapoptotic proteins: death receptor-5, Bim and Puma, and a decreased expression of inhibitors of apoptosis protein: XIAP and survivin. Among them, Bim expression was significantly induced by FKB as early as four hours of the treatment. Knockdown of Bim expression by short-hairpin RNAs attenuates the inhibitory effect on anchorage-dependent and-independent growth and caspase cleavages induced by FKB. These findings suggesting the effect of FKB, at least in part, requires Bim expression. In addition, FKB synergize with TRAIL for markedly enhanced induction of apoptosis. Furthermore, FKB treatment of mice bearing DU145 xenograft tumors results in tumor growth inhibition and increases Bim expression in tumor tissues. Together, these results suggest robust mechanisms for FKB induction of apoptosis preferentially for HRPC and the potential usefulness of FKB for prevention and treatment of HRPC in an adjuvant setting.
Introduction
A significant portion of prostate cancer (PCa) patients are curable either by surgery or by radiotherapy when detected early (1, 2), and many patients require no active therapy but are managed by expectant management. For some patients progression and metastasis require androgen-deprivation therapies (ADT), including orchiectomy or administration of leutinizing hormone-releasing hormone agonists/antagonists (1, 2). The majority of these PCa is initially androgen-sensitive and responds to ADT (1, 2). However, ADT rarely cures patients, but will eventually result in a resistant phenotype to androgen blockade with more aggressive disease (1, 2), which represents a major clinical challenge (1, 2). Recently, docetaxel-based chemotherapy has shown minor survival benefit and has become the remaining treatment option for hormone-refractory PCa (HRPC) (3). As effective treatment for HRPC is still not available, new agents that are particularly designed for the prevention and treatment of HRPC are highly desired.
Multiple mechanisms have been reported to account for progression of PCa to a hormone-refractory stage (4–12). In the majority of these hormone refractory cases, AR signaling is aberrantly activated (4). The reported mechanisms for the aberrant AR activation include AR amplification, mutation, splicing and/or co-regulator interactions, as well as through peptide growth factors and ligands for G-protein-coupled receptors (4–6). But there are also reports about extensive loss of AR expression through its promoter hypermethylation in about 20–30% of HRPC. In addition, HRPC has been associated with neuroendocrine differentiation, with some neuroendocrine differentiated PCa cells being AR-negative (9). Furthermore, PCa initiating cells may consist of a very small subpopulation of AR-negative, stem/progenitor cells. These PCa initiating cells are resistant to current anti-hormonal therapy, radiotherapy, and/or chemotherapies and therefore contribute to the recurrence of PCa (10). Taken together, we can argue that both AR-negative and -positive PCa cells should be targeted in order to develop more effective treatment and prevention approaches for HRPC.
Many phytochemicals have shown promising anticancer results with little or no toxicity to normal cells (13). In addition, most of these phytochemicals are constituents of the human diet or are taken as dietary supplements (13–17). Therefore, some of these phytochemicals may have the potential for supplementation of traditional main therapies for treatment and prevention of HRPC. Kava (Piper methysticum) is an ancient crop of the western Pacific. The root extract of kava has been part of the Pacific Islanders’ culture for thousands of years, serving as a beverage, medicine and in soci-religious functions similar to wine in Western cultures (18). Consumption of traditional aqueous kava preparation correlates with low and uncustomary sex ratios (more cancer in women and men) of cancer incidences in three kava-drinking countries: Fiji, Vanuatu, and Western Samoa (19). We recently demonstrated that flavokawains from kava extracts are strong apoptosis inducers against bladder cancer cells and that flavokawain A partially requires Bax activation for its apoptotic effect (20). However, the mechanism for Bax activation induced by flavokawains and the potential role of flavokawains as anti-cancer agents in PCa is not known. In addition, there is very little reported data about agents that can particularly target HRPC cell lines.
In this study, we demonstrate for the first time that flavokawain B (FKB) is significantly more effective in inhibiting the growth of AR-negative, HRPC cell lines (DU145 and PC-3) than AR-positive, hormone-sensitive cell lines (LNCaP and LAPC4), with a minimal effect on the growth of primary prostatic epithelial and stromal cells derived from normal prostates. In addition, we show that FKB up-regulated expression of up-stream Bax activators, including Bim, Puma and DR5; and down-regulated expression of survivin and XIAP for induction of apoptosis. Furthermore, FKB demonstrated in vivo anti-tumor efficacy and induced Bim expression in tumor tissues.
Materials and methods
Cell lines, compounds and reagents
The LNCaP, LAPC4, DU145, and PC-3 cell lines were obtained from ATCC and cultured in RPMI 1640 medium with 10%FBS. Normal prostate epithelial (PrECs) and stromal cells (PrSCs) were obtained from Clonetics Inc. and maintained in PrEBM and SCBM medium, respectively (Cambrex). Pure flavokawain A and B (99%) were from LKT Laboratories, Inc. Flavokawain C was purchased from INDOFINE Chemical Company, Inc.. Flavokawain A, B and C were dissolved in DMSO, aliquoted, and stored at −80°C. Antibodies for DR5, DR3, DR4, XIAP and survivin were from Cell Signaling Technology, Inc. Antibodies against BAX, Bcl2, Bclx/l and beta-actin were from Santa Cruz Biotechnology, Inc. Bim antibody was purchased from Calbiochem. Inc. Anti-Bax 6A7 antibody, which recognizes only the open form of Bax, was from Sigma. Thymidine, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), cycloheximide and propidium iodide were from Sigma. RNAazol B was purchased from Tel-Test. The Reverse Transcription System kit was from Promega.
MTT assay and combination studies
The MTT assays were performed as previously described (20). Dose–response curves for growth inhibition were generated as a percentage of vehicle-treated control. The combination index (CI) was calculated to determine whether the flavokawains interact synergistically, additively, or antagonistically (21). The nature of the interaction between flavokawain A, B and C was analyzed using the median effect/combination index analysis described by Chou and Talaly (21). Mixtures of flavokawain A and B, flavokawain A and C, and flavokawain B and C were made so that two compounds were equipotent. Three sets of experiments for each compound combination were carried out. The CI was calculated as follows: CIAB = ICA,B/ICA + ICB,A/ICB+ICA,B*ICB,A/ICA*ICB, or CIAC = ICA,C/ICA+ICC,A/ICC+ ICA,C*ICC,A/ICA*ICc, or CIBC = ICC,B/ICc + ICB,C/ICB+ICC,B* ICB,C/ICC*ICB. ICA, ICB and ICc are the concentrations of flavokawain A, B, and C, respectively, needed to produce a given level of growth inhibition when used alone, whereas ICA,B, ICB,A, ICA,C, ICC,A, ICC,B, and ICB,C are the concentration needed to produce the same effect when used in combination. The concentration needed to produce a given level of growth inhibition (% effect) was determined by non-linear least square regression (GraphPad PRISM). The CI values were computed automatically to indicate the degree of synergy or antagonism.
Fluorescence-activated cell sorting (FACS) analysis of apoptosis
Cells were treated with 0.1% DMSO, 8.8 μM FKB, 100 ng/ml TRAIL, or 100ng/mol TRAIL plus 8.8 μM FKB for 24 hours. After stated treatments, cells were stained with FITC-conjugated Annexin V and propidium iodide in PBS according to the manufacturer’s protocol (PharMingen). All analyses of cells were done using appropriate scatter gates to exclude cellular debris and aggregated cells. Ten thousand events were collected for each sample stained with Annexin V.
Quantification of Apoptosis by ELISA
The Cell Death Detection ELISA kit (Roche) was used to detect apoptosis. Cells seeded in six-well plates were treated with 0.1% DMSO or FKB (4.4, 8.8 and 16.7 μmol/L) for 24 hours. The cells were lysed and centrifuged. Then, the supernatant was transferred into anti-histone-coated microtiter plate and incubated with anti-DNA peroxidase antibody. After removal of the unbound antibodies, the nucleosomes were quantified by the peroxidase reaction using 2,2′-azino-di(3-ethylbenzthiazolin-sulfonat) as substrate. A microtiter plate reader at 492 nm read the color intensity.
Caspase Activity Assay
Apoptosis was confirmed by using the Caspase-Glo® 3/7, Caspase-Glo® 8 and Caspase-Glo® 9 Assay (Promega) according to the manufacturer’s instructions. Cells were plated in a 24-well plate and treated with 0.1% DMSO or FKB (4.4, 8.8 and 16.7 μmol/L) for 24 hours. Then 100 μL of Caspase-Glo® 3/7, Caspase-Glo® 8 or Caspase-Glo® 9 reagent was added on to each well and the luminescence of each sample was measured in a luminometer (GloMax ®-MultiDetection System).
Real-time RT-PCR
Total RNA was isolated from PCa cells using the RNAazol B method as described (22). Real-time quantitative PCR amplification reactions for DR5 mRNA levels were carried out using MyiQ system (Bio-Rad) as described previously (22) and the sequences of primer sets are available upon request. Data were analyzed by using the comparative Ct method, where Ct is the cycle number at which fluorescence first exceeds the threshold. The Ct values from each sample were obtained by subtracting the values for β-actin Ct from the DR5 Ct value. The variation of β-actin Ct values is <0.5 among different samples. One difference of Ct value represents a 2-fold difference in the level of mRNA. Specificity of resulting PCR products was confirmed by melting curves.
Western blot analysis
Clarified protein lysates (20–80 μg) were denatured and resolved by 8–16% SDS-PAGE. Proteins were transferred to nitrocellulose membranes, and probed with antibodies and visualized by an enhanced chemiluminescence detection system.
Immunoprecipitation
Total protein (200μg) was precleared with protein G plus-agarose and then precipitated with 2 μg anti-Bim or IgG antibody overnight at 4°C. Agarose beads were washed four times with lysis buffer and resuspended in SDS-PAGE 2x sample buffer. Proteins were eluted by boiling the beads and subjected to immunoblotting of active Bax protein.
RNA interference
Two different Bim targeting sequences were designed using the RNAi Designer program (Invitrogen), and then subcloned into a pENTR/U6 vector according to the Gateway protocol. After that, these RNAi cassettes were transferred into a pBLOCK-iT 3-DEST vector using Clonase™ to catalyze a recombination reaction (Invitrogen). Negative controls used shRNA targeting sequences complementary to the LacZ gene without any match to human genome sequences. These shRNAs can effectively knock down transfected LacZ genes. All constructs were transfected using Lipofectamine 2000 (Invitrogen) and selected with G418. The pooled stable transfectants were examined.
In vivo tumor model
FKB was formulated in 10% grain alcohol (is pure alcohol made from fermented grains and used as a solvent in commercial kava root extracts) in 0.9% saline and given by gavage. NCR-nu/nu (nude) mice were obtained from Taconic. DU145 cells were concentrated to 1× 106 per 100 μL PBS and injected s.c. into the right flank of each mouse. After seven days tumors were approximately the size of 120 mm3, the mice bearing DU145 tumors were randomly divided and pair matched into treatment and control groups of 10 mice each. Daily dosing was begun with vehicle or 50 mg/kg FKB. Because there were no in vivo data regarding FKB before this study, the dose of FKB (50 mg/kg/d) was used according to a pharmacologically effective and non-toxic dose of flavokawain A (a close analogue of FKB in kava extracts) (20). Body weight, diet, and water consumption were recorded thrice weekly throughout the study. Once xenografts started growing, their sizes were measured every three day. The tumor volume was calculated by the formula: 0.5236 L1(L2)2, where L1 is the long axis and L2 is the short axis of the tumor. At the end of the experiment, tumors were excised and weighed, blood was collected and all were stored at −80°C until additional analysis.
Statistics
Comparisons of caspase activities, OD values for cell death and cell viabilities between treatment and control were conducted using Student’s t test. For tumor growth experiments, repeated-measures ANOVA was used to examine the differences in tumor sizes among treatments, time points, and treatment-time interactions. Additional post-tests were done to examine the differences in tumor sizes between control and FKB treatment at each time point by using conservative Bonferroni method. All statistical tests were two sided.
Results
FKB differentially inhibits AR-positive and -negative PCa cell lines and has minimal effects on normal primary prostate epithelial and stromal cells
Flavokawain A, B and C constitute about 0.46%, 0.015% and 0.012% of kava extracts, respectively (23). To determine which flavokawain(s) or their combination(s) could be the most potent agent against HRPC, we first examined the effect of individual flavokawains and the 1:1/31:1/38 mixture of flavokawain A:B:C (The ratio of flavokawains in the mixture is similar to that naturally occurred in kava extracts) on the growth of an AR-negative DU145 cells. Figure 1A shows that FKB is the most potent growth-inhibitory agent among the individual flavokawains and the flavokawain mixture. Figure 1B shows that combination indexes for different flavokawain combinations on cell growth inhibition are more than 1.0, suggesting there is no synergistic effect.
Figure 1.
FKB is the most potent agent among flavokawains and their combinations against the growth of PCa cell lines and exhibits a differential effect on prostatic cells. Cells were treated with indicated treatment in the figure for 48 hours and cell densities were measured by MTT assay. The combination indexes (CIs) were calculated as described in Materials and Methods. Points, mean of four independent plates; bars, SE. (A) Growth inhibitory effects of flavokawain A, B, C and the 1:1/31:1/38 mixture of flavokawain A, B, and C on DU145 cells. (B) Graphical presentation of the CI with respect to the fraction of cell growth inhibition by mixtures of flavokawain A, B and C in DU145 cells. (C) Growth inhibitory effects of FKB on AR-positive and –negative PCa cell lines, as well as on normal prostatic cells. (D) & (E) time-dependent inhibitory effect of FKB on the growth of DU145 and PC-3 cells.
We subsequently examined the effect of FKB on the growth of different PCa cell lines and prostatic cells derived from a normal prostate by primary culturing. Figure 1C shows that FKB at a concentration of 17.6 μM inhibits the growth of AR-negative, hormone refractory PCa cell lines (DU145 and PC3) by about 90%, and partially reduces the growth of AR-positive, hormone sensitive PCa cell lines (LNCaP and LAPC4) by about 32–50% (Students’ t test, Ps<0.01). At the same concentration, FKB has minimal effect on the growth of prostatic epithelial and fibroblastic cells from a normal prostate (less than 6%, Ps>0.05). The IC50 of FKB treatment for 48 hours on different PCa cell lines are estimated to be 32 (LAPC4), 48.3 (LNCaP), 6.2 (PC-3) and 3.9 μM (DU145). AR-negative cell lines (PC-3 and DU145) are approximately 4 to 12 fold more sensitive to FKB’s effect than AR-positive cell lines (LAPC4 and LNCaP). This selective killing effect of FKB on AR-negative, hormone-refractory PCa cell lines suggest a potentially novel strategy for prevention and treatment of HRPC.
Figure 1D & E shows that treatment of AR negative DU145 and PC-3 cells grown in 10% FBS with FKB results in a highly significant to complete inhibition of their growth in a time-dependent manner. An inhibitory effect of FKB was evident at 1 day, but a more profound effect was observed during 3–5 days of treatment. The 1.1- and 2.2-μM concentrations of FKB shows 34 and 80% inhibition in growth of DU145 cells and 12 and 64% inhibition in growth of PC-3 cells, respectively. Cells treated with 8.8 and 17.6 μM of FKB shows complete growth inhibition, respectively (Figure 1D &E). At these concentrations of FKB, cells stopped growing as early as 3 days, with a small reduction in initial cell density (Figure 1D & E).
FKB induces a typical apoptosis via activation of capase-3,-8 and -9 activities in AR-negative PCa cell lines DU145 and PC-3
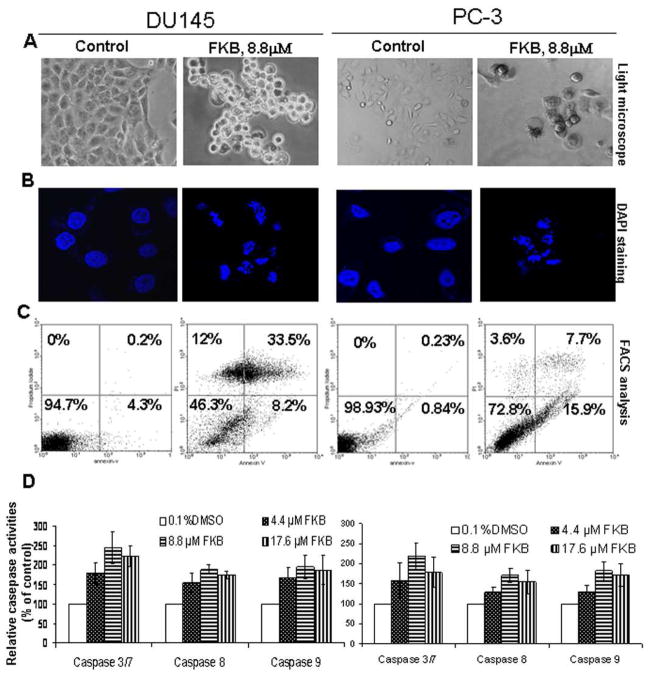
To determine whether the growth inhibitory effect of FKB is through the induction of apoptosis, we examined the apoptotic morphology of control- and FKB-treated cells under light- and fluorescence microscopes. Figure 2A&B shows typical apoptotic morphologies in FKB-treated cells treated but not with control- treatment. The apoptotic morphologies include cell shrinkage and rounding up, cell membrane blebbing, as well as nuclear fragmentation and condensation. In addition, FKB resulted in a significant increase in both early (Annexin V staining only, right-lower panels) and late apoptosis (Annexin V and PI staining, right-upper panels) populations when compared to control treatment (Figure 2C, 41.7 ± 4% and 23.6 ± 2% apoptotic cells in FKB treated DU145 and PC-3 cells, respectively, vs. 4.5 ± 0.3% and 1.07 ± 0.2% in control treated DU145 and PC-3 cells; Students’ t test, P<0.01). Cell death ELISA experiments further confirmed that FKB causes cell death in a dose-dependent manner (data not shown). In addition, FKB causes PARP cleavage in both DU145 and PC3 cells (data not shown). Together, these results provide a firm conclusion that FKB induces apoptosis in DU145 and PC3 cells.
Figure 2.
FKB induces apoptosis and activates caspase 3/8/9. (A) Live cell morphology under phase-contrast light microscope (Magnification: X100). A representative picture was shown from a random field. (B) DAPI staining of nuclear morphology under fluorescence microscope (Magnification: X400). A representative picture was shown from a random field. (C) Cells were stained by Annexin V and PI and analyzed by flowcytometry. Data represent the means from three independent experiments. Standard errors are less than 5%. (D) Caspase activation was determined with a caspase-3/7, caspase-9 or caspase-8 activity assay. Bars are means ± SE of three independent quantitative measures.
Apoptosis can be induced by the extrinsic pathway associated with death receptor stimulation on the cell surface (25), and by the intrinsic pathway characterized by the involvement of mitochondrial dysfunction (26). While caspase 3 is an effecter caspase, initiator caspase 8 and 9 are activated by death receptors and mitochondrial releasing factors, respectively (24). Figure 2D shows that FKB increases caspase 8, 9 and 3 by about 30 to 143% compared to vehicle control treatment, indicating that both death receptor- and mitochondrial- mediated apoptotic pathways are activated (24).
FKB increases the protein and mRNA expression of death receptor 5 (DR5) and enhances TRAIL ligand induced apoptosis
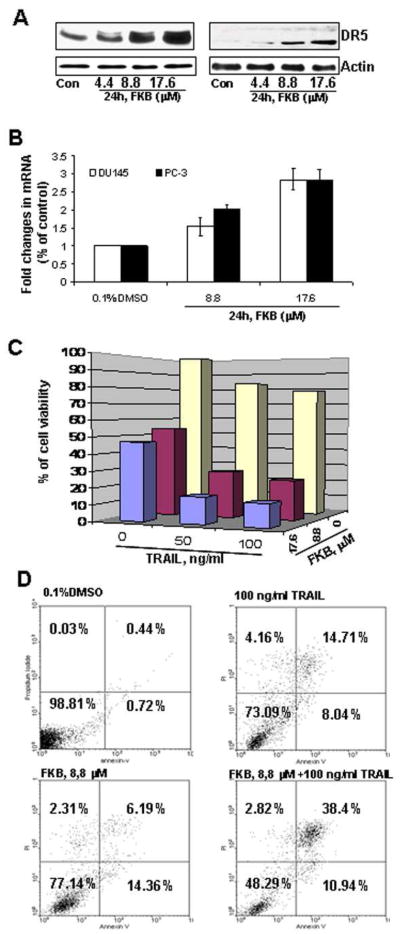
Figure 3A&B shows that FKB specifically increases the protein and mRNA expression of DR5, without affecting the expression of DR4 and DR3 (data not shown). Additionally, PC-3 cells treated with 8.8 μM FKB and 100 ng/ml TRAIL reduces cell viabilities by 46 % and 22 %, respectively, while combination of both agents leads to a marked decrease of cell viability by 76% (Figure 3C, Students’ t test, Ps<0.01). Similarly, Annexin V staining shows that either 8.8μM FKB or 100 ng/ml TRAIL treatment alone results in about 22% or 23% of PC-3 cells undergoing apoptosis, whereas combination of both agents increase the percentage of apoptotic cells to 49% (Figure 3D, Students’ t test, P<0.01). Together, these results suggest that FKB potentiates the apoptotic effect of TRAIL ligand via induction of DR5.
Figure 3.
FKB induces mRNA and protein expression of DR5 and synergizes with TRAIL for enhanced cell growth inhibitory and apoptotic effect. (A) The expression of DR5 after indicated treatments for 24 hours was analyzed by Western blot. β-Actin was detected as a loading control. A representative blot was shown from three independent experiments. (B) Real-time RT-PCR analysis of DR5 mRNA expression. Bars are means ± SE of three independent quantitative measures. (C) The combined effect of FKB and TRAIL on PC-3 cell viability. Columns, mean for percentage of cell viability relative to control (n = 4); bars, SE. (D) The combined effect of FKB and TRAIL on apoptosis of PC3 cells. Cells were stained by Annexin V and PI and analyzed by flowcytometry. Data represent the means from three independent experiments. SEs are less than 5%.
FKB activates the Bax and mitochondrial- mediated apoptotic pathway by upregulation of Bim and Puma; and down-regulation of XIAP and survivin
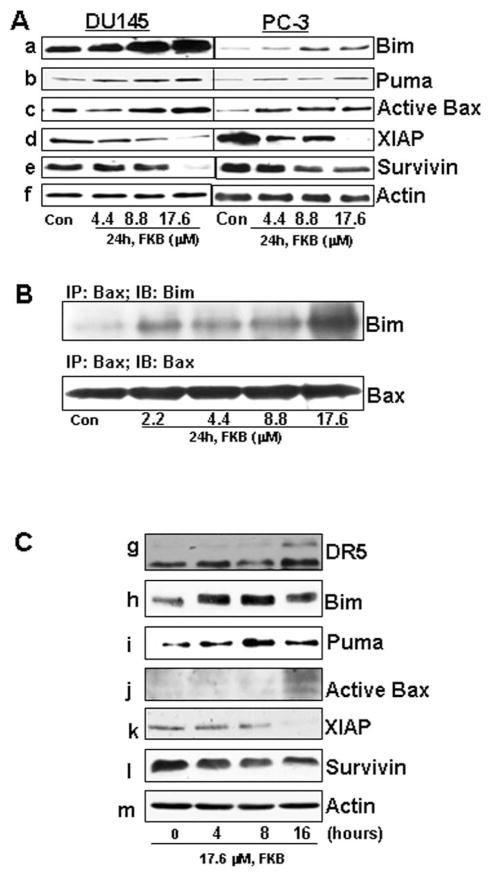
The BH3-only proteins, including Bad, Bim/Bod, Bid, Bmf, Noxa, and Puma, are immediate upstream triggers for Bax activation (26). We investigated which BH3-only proteins are responsible for FKB-induced activation of Bax. FKB treatment significantly increases the protein expression of Bim and Puma (Figure 4, panel a and b) without affecting the expression of other BH3-only proteins, including Bad, Bid and Noxa, in DU145 and PC3 cells (data not shown). Consistently, the increase in Bim and Puma by FKB treatment is associated with an increase in active Bax (Figure 4, panel c). In addition, FKB decreases the expression of inhibitors of apoptotic proteins: XIAP and survivin (Figure 4, panel d and e). Furthermore, FKB treatment causes an increased formation of a Bax/Bim immunocomplex in DU145 cells. Together, these results indicate that FKB activates the mitochondrial-mediated apoptotic pathway by changing the balance between proapoptotic and antiapoptotic proteins.
Figure 4.
FKB increases the expression of Bim, Puma and active Bax, decreases the expression of XIAP and survivin and induces a formation of Bim/Bax immunocomplex. (A) The protein levels of Puma, Bim, Active Bax, XIAP, and survivin after indicated treatments for 24 hours were analyzed by Western blotting. β-Actin was detected as a loading control. A representative blot was shown from three independent experiments. (B) Immunoprecipitation assay of the Bim/Bax complex. DU145 cell lysates after indicated treatments for 24 hours were incubated with conformation-specific anti-Bax 6A7 antibody and protein A-agarose beads and probed with anti-Bim antibody. A representative blot was shown from three independent experiments. (C) The protein levels of DR5, Puma, Bim, active Bax, XIAP, and survivin after 17.6 μM for indicated times were analyzed by Western blotting. β-Actin was detected as a loading control. A representative blot was shown from three independent experiments.
Figure 4C shows that FKB treatment of DU145 cells exhibits a time-dependent effect on the expression of apoptotic and inhibitors of apoptotic proteins. Bim and Puma expression were induced as early as 4 hours of FKB treatment. At 8 hours of FKB treatment, a decrease in XIAP and survivin were observed. The induction of DR5 and active Bax were only shown at 16 hours of FKB treatment. These results suggest that Bim is an early and up-stream event for FKB activation of apoptosis in DU145 cells.
Knock-down of Bim expression by ShRNAs attenuates the inhibitory effects of FKB on anchorage-dependent and –independent growth and its apoptotic effect
We next examined whether Bim is, at least in part, required for the growth inhibitory and apoptotic effect of FKB. Figure 5A shows that Bim protein expression was inhibited up to 75–95% by stable transfection of Bim ShRNA plasmids compared with a control LacZ ShRNA transfection. The control-transfected DU145 and PC3 cells did not show any difference in cell growth compared with their parental cells (data not shown). Figure 5B shows that cells with stable suppression of Bim expression are more resistant to the growth inhibitory effects of FKB than those transfected with LacZ ShRNA (Student’s t test, Ps < 0.05). Knockdown of Bim expression was also shown to attenuate the inhibitory effects of FKB on colony formation in soft agar (Student’s t test, Ps < 0.05; Figure 5C and Supplementary Figure 1). In addition, the degree to which FKB attenuated cell viability was associated with the level of Bim expression (Figure 5B&C). Furthermore, in control-transfected PC3 cells, 8.8 μM FKB treatment results in about 180 to 240% increase of cell death and caspase 3/7/9 activities compared to 0.1% DMSO treatment, respectively (Student’s t test, Ps < 0.05; Figure 5D), whereas in Bim ShRNA-transfected PC3 cell lines, FKB at the same concentration does not cause significant cell death nor caspase 3/7/9 activations (Student’s t test, Ps >0.05; Figure 5D). This result indicates the FKB-induced cell death and caspase 3/7/9 activation in PC-3 cells is blocked by Bim suppression. Together, these results provide strong evidence that Bim is a critical target for the apoptotic and growth inhibitory effects of FKB.
Figure 5.
Knock-down of Bim expression by ShRNAs attenuates the anchorage-dependent and -independent growth inhibitory effects and caspase cleavage induced by FKB. PC-3 and DU145 cells were transfected with ShLacZ, ShBIM117, and ShBIM 459. Stable clones were obtained via G418 selection, and pooled clones for each line were used. (A) Western blotting verified knockdown of Bim expression. (B). PC3 or DU145/ShLacZ, PC3 or DU145/ShBim117, and PC3 or DU145/ShBim459 cells were treated with 0.1% DMSO or indicated concentrations of FKB for 48 hours. Cell viabilities were measured by MTT assays. Points: mean of four independent plates; bars: SE. Each sample was counted in duplicate. (C) PC3 or DU145/ShLacZ, PC3 or DU145/ShBim117, and PC3 or DU145/ShBim459 cells were grown in soft agar in six-well plates and treated with 0.1% DMSO or FKB at the indicated concentrations for 30 days. The number of colonies was determined by counting them under an inverted phase-contrast microscope at ×100 magnification; a group of >10 cells was counted as a colony. Columns: mean of four independent wells at 30 days after the start of cell seeding; bars: SE. (D) PC3/ShLacZ, PC3/ShBim117, and PC3/ShBim459 cells were treated with 0.1% DMSO or indicated concentrations of FKB for 24 hours. Cell deaths were measured by cell death ELISA kit. Caspase activation was determined with a caspase-3/7, caspase-9 or caspase-8 activity assay. Each result is expressed as a percentage relative to control. Bars are means ± SE of three independent quantitative measures.
FKB inhibits tumor growth in vivo in a DU145 xenograft model and induces Bim expression in tumor tissues
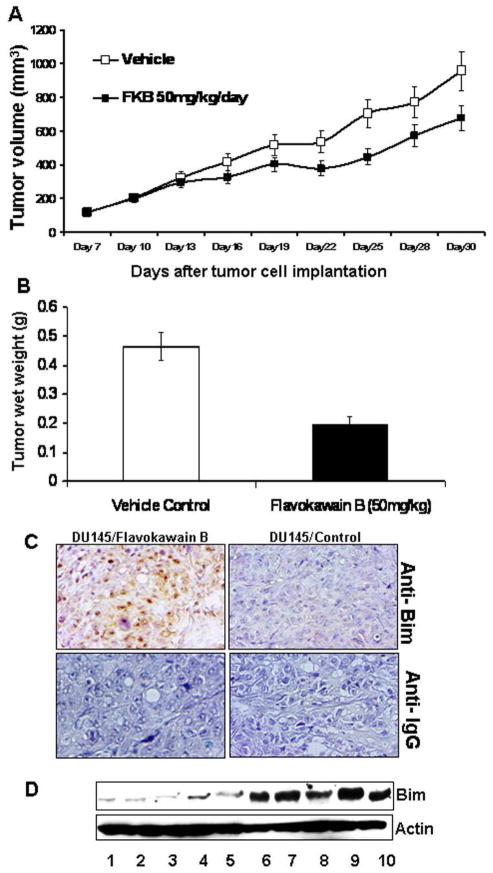
Finally, Figure 6A demonstrates the effects of oral administration of 50 mg/kg FKB daily for 24 days on mice bearing established DU145 tumors resulted in a significant decrease in the growth rate of tumors compared with control group (P < 0.05, ANOVA). The wet tumor weights in control- and FKB–treated group recorded at the end of the treatment are 465 ± 49 and 199 ± 25 mg, respectively (Figure 6B; mean ± SE; P = 0.045, Student’s t test). FKB treatment reduced tumor growth by 67%. The body weight gain and diet and water consumption of the FKB–treated mice were similar to the control group of mice (Supplementary figure S2). In addition, the mice did not show any gross abnormalities on necropsy at the end of the treatment. Immunohistochemical analysis shows that there is a strong increase of Bim expression in tumor tissues in the FKB group compared to the control group. Consistently, western blotting analysis of tumor lysates revealed higher levels of Bim protein in the FKB-treated tumor tissues than those treated by control. Together, FKB demonstrated in vivo antitumor activity, which was accompanied by increased expression of Bim.
Figure 6.
FKB reduces tumor growth of DU145 cells in a xenograft model and decreases Bim expression in tumor tissues. (A) The mice bearing DU145 tumors were randomly divided, pair-matched into treatment and control groups of ten mice each, and daily dosing was begun with vehicle or FKB at 50 mg/kg. Tumor volumes were recorded, and presented as mean ± SE. (B) At the termination of the study, tumors were excised from each mouse in different groups and weighed. Wet weight of tumors is represented as mean of 10 tumors from individual mouse in each group. Bars, ± SE. (C) Immunostainings of Bim protein in DU145 xenograft tumors. Control immunostaining was performed with IgG isotype alone; Slides were counterstained with hematoxylin and photographed using a light microscope. Original magnification: X200 and X40 (insert). (D) Western blotting analysis of Bim expression in control (lanes, 1, 2, 3, 4, and 5) – or FKB (lanes 6, 7, 8, 9, and 10) - treated DU145 xenograft tumors.
Discussion
Because of lower cell proliferation rates in clinical PCa compared to most other cancers (27), induction of apoptosis could be a more effective mechanism for elimination of PCa cells (28–31). We demonstrated that FKB has robust mechanisms in induction of apoptosis via targeting both death receptors and the balance of pro- and anti-apototic proteins. FKB increases DR5 expression, leading to activation of the death receptor mediated apoptotic pathway. The TRAIL, a DR5 ligand, is considered an effective anticancer agent, as it selectively induces apoptosis in a variety of tumor cells, yet is relatively nontoxic to normal cells (25). We further demonstrated that FKB exerts a synergistic apoptotic effect when combined with TRAIL. The mechanism for FKB mediated DR5 expression remains unclear. In addition to up-regulation of DR5 mRNA expression, FKB was found to increase the mRNA expression of Puma and p21/WAF1 in PC3 cells (data not shown). DR5, Puma and P21/WAF1 are common p53 target genes (32). Because the PC3 cell line harbors a p53 gene deletion, it is unlikely that FKB activates the p53 for its effect on DR5 and Puma expression. The apoptotic effect of FKB is also not dependent on PTEN as there is no significant difference for the apoptotic effect observed in PTEN-wild type DU145 vs -mutant PC3 cells. In addition, FKB did not change the PTEN expression in DU145 cells (data not shown). Instead, we recently reported that flavokawain A regulated the expression of DR5 and Puma mRNA via increased expression of p73, a p53 family member, in bladder cancer cell lines (33). Further experiments are therefore in progress to determine whether FKB can stabilize p73 protein in p53 mutant PCa cells to explain its effect on gene transcription of DR5 and Puma. Altogether, these data suggest that combination of TRAIL and FKB may represent a novel therapeutic strategy against HRPC.
Bim directly initiates the BAX-mediated mitochondrial apoptosis via binding to stabilized α-helix of BCL-2 domains of BAX (34). We have shown that Bim forms an immunocomplex with active Bax protein, which presumably allows permeabilization of mitochondria with subsequent release of cytochrome C and others for activation of the caspase 9/3 cascade. In addition, knockdown of Bim was shown to markedly attenuate anchorage-dependent and -independent cell growth inhibitory effects and caspase apoptotic effects of FKB.
Bim does play a role in tumorigenesis. In mice, the loss of Bim can accelerate c-myc oncogene-driven lymphomagenesis (35, 36). In patients with mantle cell lymphoma and renal cell carcinoma, the loss or reduced expression of Bim has also been found (37, 38). Studies suggest Bim to be an important convergent point for both Akt and ERK regulating apoptotic signaling (39–42). Combination of an mTOR inhibitor rapamycin and an ERK inhibitor PD0325901 resulted in substantially enhanced antitumor effects particularly for hormone-refractory prostate tumors in the mouse model and led to increased expression of Bim in tumor tissues (43). We also demonstrated that FKB significantly induces Bim expression in tumor tissues while exhibiting antitumor effects. Together, these results suggest a role for Bim expression in antitumor mechanisms, and the potential usefulness of Bim protein as a surrogate biomarker for monitoring the apoptotic and anti-tumor effects of FKB in future studies.
In summary, we have demonstrated the strong inhibitory effect of FKB on the growth of PCa cell lines with more potency to AR negative, HRPC cell lines. FKB induces apoptosis via robust mechanisms; including (i) increases the expression of DR5 leading to activation of the death receptor pathway and (ii) upregulates Bim and Puma expression and down-regulates XIAP and survivin expression resulting in activation of the BAX-initiated mitochondria pathway. Additionally shown, FKB reduces tumor growth in vivo and induces the expression of Bim in tumor tissues. It appears that the growth inhibitory effect of FKB, at least in part, requires Bim expression. Based on these findings, we propose that FKB, either its derivatives or in combination with TRAIL may be an effective treatment modality for those PCa patients with hormone-refractory disease.
Acknowledgments
This work was supported by AICR grant 41493, NIH grants CA129793 and CA122558 (to X. Zi).
References
- 1.Hsing AW, Devesa SS. Trends and patterns of prostate cancer: what do they suggest? Epidemiol Rev. 2001;23:3–13. doi: 10.1093/oxfordjournals.epirev.a000792. [DOI] [PubMed] [Google Scholar]
- 2.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 3.Dagher R, Li N, Abraham S, Rahman A, Sridhara R, Pazdur R. Approval summary: Docetaxel in combination with prednisone for the treatment of androgen-independent hormone-refractory prostate cancer. Clin Cancer Res. 2004;10:8147–51. doi: 10.1158/1078-0432.CCR-04-1402. [DOI] [PubMed] [Google Scholar]
- 4.Kasper S, Cookson MS. Mechanisms leading to the development of hormone-resistant prostate cancer. Urol Clin North Am. 2006;33:201–210. doi: 10.1016/j.ucl.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, Chen H, Kong X, Melamed J, Tepper CG, Kung HJ, Brodie AM, et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69:2305–13. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang JC, Ok JH, Busby JE, Borowsky AD, Kung HJ, Evans CP. Aberrant activation of androgen receptor in a new neuropeptide-autocrine model of androgen-insensitive prostate cancer. Cancer Res. 2009;69:151–60. doi: 10.1158/0008-5472.CAN-08-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinoshita H, Shi Y, Sandefur C, Meisner LF, Chang C, Choon A, Reznikoff CR, Bova GS, Friedl A, Jarrard DF. Methylation of the androgen receptor minimal promoter silences transcription in human prostate cancer. Cancer Res. 2000;60:3623–30. [PubMed] [Google Scholar]
- 8.Nakayama T, Watanabe M, Suzuki H, Toyota M, Sekita N, Hirokawa Y, Mizokami A, Ito H, Yatani R, Shiraishi T. Epigenetic regulation of androgen receptor gene expression in human prostate cancers. Lab Invest. 2000;80:1789–96. doi: 10.1038/labinvest.3780190. [DOI] [PubMed] [Google Scholar]
- 9.Mosca A, Berruti A, Russo L, Torta M, Dogliotti L. The neuroendocrine phenotype in prostate cancer: basic and clinical aspects. J Endocrinol Invest. 2005;28:141–5. [PubMed] [Google Scholar]
- 10.Sharifi N, Kawasaki BT, Hurt EM, Farrar WL. Stem cells in prostate cancer: resolving the castrate-resistant conundrum and implications for hormonal therapy. Cancer Biol Ther. 2006;5:901–906. doi: 10.4161/cbt.5.8.2949. [DOI] [PubMed] [Google Scholar]
- 11.Titus MA, Schell MJ, Lih FB, Tomer KB, Mohler JL. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11:4653–7. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- 12.Jin RJ, Lho Y, Connelly L, Wang Y, Yu X, Saint Jean L, Case TC, Ellwood-Yen K, Sawyers CL, Bhowmick NA, Blackwell TS, Yull FE, et al. The nuclear factor-kappaB pathway controls the progression of prostate cancer to androgen-independent growth. Cancer Res. 2008;68:6762–9. doi: 10.1158/0008-5472.CAN-08-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta S, Afaq F, Mukhtar H. Selective growth-inhibitory, cell-cycle deregulatory and apoptotic response of apigenin in normal versus human prostate carcinoma cells. Biochem Biophys Res Commun. 2001;287:914–20. doi: 10.1006/bbrc.2001.5672. [DOI] [PubMed] [Google Scholar]
- 14.Bosland MC, Kato I, Melamed J, Taneja S, Lepor H, Torre P, Walden P, Zeleniuch-Jacquotte A, Lumey LH. Chemoprevention trials in men with prostate-specific antigen failure or at high risk for recurrence after radical prostatectomy: Application to efficacy assessment of soy protein. Urology. 2001;57(4 Suppl1):202–4. doi: 10.1016/s0090-4295(00)00975-4. [DOI] [PubMed] [Google Scholar]
- 15.Moyad MA. The use of complementary/preventive medicine to prevent prostate cancer recurrence/progression following definitive therapy. Part II--rapid review of dietary supplements. Curr Opin Urol. 2003;13:147–51. doi: 10.1097/00042307-200303000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Knowles LM, Zigrossi DA, Tauber RA, Hightower C, Milner JA. Flavonoids suppress androgen-independent human prostate tumor proliferation. Nutr Cancer. 2000;38:116–22. doi: 10.1207/S15327914NC381_16. [DOI] [PubMed] [Google Scholar]
- 17.Dall’Era MA, Cooperberg MR, Chan JM, Davies BJ, Albertsen PC, Klotz LH, Warlick CA, Holmberg L, Bailey DE, Jr, Wallace ME, Kantoff PW, Carroll PR. Active surveillance for early-stage prostate cancer: review of the current literature. Cancer. 2008;112:1650–9. doi: 10.1002/cncr.23373. [DOI] [PubMed] [Google Scholar]
- 18.Singh YN. Kava: an overview. J Ethnopharmacol. 1992;37:13–45. doi: 10.1016/0378-8741(92)90003-a. [DOI] [PubMed] [Google Scholar]
- 19.Steiner GG. The correlation between cancer incidence and kava consumption. Hawaii Med J. 2000;59:420–2. [PubMed] [Google Scholar]
- 20.Zi X, Simoneau AR. Flavokawain A, a novel chalcone from kava extract, induces apoptosis in bladder cancer cells by involvement of Bax protein-dependent and mitochondria-dependent apoptotic pathway and suppresses tumor growth in mice. Cancer Res. 2005;65:3479–86. doi: 10.1158/0008-5472.CAN-04-3803. [DOI] [PubMed] [Google Scholar]
- 21.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 22.Tang Y, Simoneau AR, Xie J, Shahandeh B, Zi X. Effects of the kava chalcone flavokawain A differ in bladder cancer cells with wild-type versus mutant p53. Cancer Prev Res. 2008;1:439–51. doi: 10.1158/1940-6207.CAPR-08-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dharmaratne HR, Nanayakkara NP, Khan IA. Kavalactones from Piper methysticum, and their 13C NMR spectroscopic analyses. Phytochemistry. 2002;59:429–33. doi: 10.1016/s0031-9422(01)00443-5. [DOI] [PubMed] [Google Scholar]
- 24.Ho PK, Hawkins CJ. Mammalian initiator apoptotic caspases. FEBS J. 2005;272:5436–53. doi: 10.1111/j.1742-4658.2005.04966.x. [DOI] [PubMed] [Google Scholar]
- 25.Finnberg N, El-Deiry WS. TRAIL death receptors as tumor suppressors and drug targets. Cell Cycle. 2008;7:1525–8. doi: 10.4161/cc.7.11.5975. [DOI] [PubMed] [Google Scholar]
- 26.Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. Hierarchical regulation of mitochondrial-dependent apoptosis by BCL-2 subfamilies. Nat Cell Biol. 2006;8:1348–58. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- 27.Peehl DM. Primary cell cultures as models of prostate cancer development. Endocr Relat Cancer. 2005;12:19–47. doi: 10.1677/erc.1.00795. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Nan B, Yu J, Snabboon T, Andriani F, Marcelli M. From castration-induced apoptosis of prostatic epithelium to the use of apoptotic genes in the treatment of prostate cancer. Ann N Y Acad Sci. 2002;963:191–203. doi: 10.1111/j.1749-6632.2002.tb04110.x. [DOI] [PubMed] [Google Scholar]
- 29.Catz SD, Johnson JL. BCL-2 in prostate cancer: a mini-review. Apoptosis. 2003;8:29–37. doi: 10.1023/a:1021692801278. [DOI] [PubMed] [Google Scholar]
- 30.Gao S, Lee P, Wang H, Gerald W, Adler M, Zhang L, Wang YF, Wang Z. The androgen receptor directly targets the cellular Fas/FasL-associated death domain protein-like inhibitory protein gene to promote the androgen-independent growth of prostate cancer cells. Mol Endocrinol. 2005;19:1792–802. doi: 10.1210/me.2004-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gleave ME, Zellweger T, Chi K, Miyake H, Kiyama S, July L, Leung S. Targeting anti-apoptotic genes upregulated by androgen withdrawal using antisense oligonucleotides to enhance androgen- and chemo-sensitivity in prostate cancer. Invest New Drugs. 2002;20:145–58. doi: 10.1023/a:1015694802521. [DOI] [PubMed] [Google Scholar]
- 32.Wang W, Kim SH, El-Deiry WS. Small-molecule modulators of p53 family signaling and antitumor effects in p53-deficient human colon tumor xenografts. Proc Natl Acad Sci U S A. 2006;103:11003–8. doi: 10.1073/pnas.0604507103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Z, Li B, Li X, Simoneau A, Zi X. The Kava chalcone Flavokawain A potentiates the inhibitory effect of Nutilin-3a on cancer cell growth via enhancement of p53 activity. Proceedings of the 100th Annual Meeting of the American Association for Cancer Research; 2009 Apr 18–22; Denver, CO. Philadelphia (PA): AACR; 2009. Abstract nr (4728) [Google Scholar]
- 34.Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, Tu HC, Kim H, Cheng EH, Tjandra N, Walensky LD. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–81. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erlacher M, Labi V, Manzl C, Böck G, Tzankov A, Häcker G, Michalak E, Strasser A, Villunger A. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J Exp Med. 2006;203:2939–51. doi: 10.1084/jem.20061552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Egle A, Harris AW, Bouillet P, Cory S. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc Natl Acad Sci U S A. 2004;101:6164–9. doi: 10.1073/pnas.0401471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zantl N, Weirich G, Zall H, Seiffert BM, Fischer SF, Kirschnek S, Hartmann C, Fritsch RM, Gillissen B, Daniel PT, Häcker G. Frequent loss of expression of the pro-apoptotic protein Bim in renal cell carcinoma: evidence for contribution to apoptosis resistance. Oncogene. 2007;2:7038–48. doi: 10.1038/sj.onc.1210510. [DOI] [PubMed] [Google Scholar]
- 38.Tagawa H, Karnan S, Suzuki R, Matsuo K, Zhang X, Ota A, Morishima Y, Nakamura S, Seto M. Genome-wide array-based CGH for mantle cell lymphoma: identification of homozygous deletions of the proapoptotic gene BIM. Oncogene. 2005;24:1348–58. doi: 10.1038/sj.onc.1208300. [DOI] [PubMed] [Google Scholar]
- 39.Ley R, Ewings KE, Hadfield K, Howes E, Balmanno K, Cook SJ. Extracellular signal-regulated kinases 1/2 are serum-stimulated “Bim(EL) kinases” that bind to the BH3-only protein Bim(EL) causing its phosphorylation and turnover. J Biol Chem. 2004;279:8837–47. doi: 10.1074/jbc.M311578200. [DOI] [PubMed] [Google Scholar]
- 40.Guo Y, Schoell MC, Freeman RS. The von Hippel-Lindau protein sensitizes renal carcinoma cells to apoptotic stimuli through stabilization of BIM(EL) Oncogene. 2009;28:1864–74. doi: 10.1038/onc.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qi XJ, Wildey GM, Howe PH. Evidence that Ser87 of BimEL is phosphorylated by Akt and regulates BimEL apoptotic function. J Biol Chem. 2006;281:813–23. doi: 10.1074/jbc.M505546200. [DOI] [PubMed] [Google Scholar]
- 42.Sunters A, Fernández de Mattos S, Stahl M, Brosens JJ, Zoumpoulidou G, Saunders CA, Coffer PJ, Medema RH, Coombes RC, Lam EW. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem. 2003;278:49795–805. doi: 10.1074/jbc.M309523200. [DOI] [PubMed] [Google Scholar]
- 43.Kinkade CW, Castillo-Martin M, Puzio-Kuter A, Yan J, Foster TH, Gao H, Sun Y, Ouyang X, Gerald WL, Cordon-Cardo C, Abate-Shen C. Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J Clin Invest. 2008;118:3051–64. doi: 10.1172/JCI34764. [DOI] [PMC free article] [PubMed] [Google Scholar]