Abstract
In mature, differentiated neurons in the central nervous system (CNS), epigenetic mechanisms – including DNA methylation, histone modification, and regulatory noncoding RNAs – play critical roles in encoding experience and environmental stimuli into stable, behaviorally-meaningful changes in gene expression. For example, epigenetic changes in mature hippocampal neurons have been implicated in learning and memory and in a variety of neuropsychiatric disorders, including depression. With all the recent (and warranted) attention given to epigenetic modifications in mature neurons, it is easy to forget that epigenetic mechanisms were initially described for their ability to promote differentiation and drive cell fate in embryonic and early postnatal development, including neurogenesis. Given the discovery of ongoing neurogenesis in the adult brain and the intriguing links among adult hippocampal neurogenesis, hippocampal function, and neuropsychiatric disorders, it is timely to complement the ongoing discussions on the role of epigenetics in mature neurons with a review on what is currently known about the role of epigenetics in adult hippocampal neurogenesis. The process of adult hippocampal neurogenesis is complex, with neural stem cells (NSCs) giving rise to fate-restricted progenitors and eventually mature dentate gyrus granule cells. Notably, neurogenesis occurs within an increasingly well-defined “neurogenic niche”, where mature cellular elements like vasculature, astrocytes, and neurons release signals that can dynamically regulate neurogenesis. Here we review the evidence that key stages and aspects of adult neurogenesis are driven by epigenetic mechanisms. We discuss the intrinsic changes occurring within NSCs and their progeny that are critical for neurogenesis. We also discuss how extrinsic changes occurring in cellular components in the niche can result in altered neurogenesis. Finally we describe the potential relevance of epigenetics for understanding the relationship between hippocampal neurogenesis in neuropsychiatric disorders. We propose that a more thorough understanding of the molecular and genetic mechanisms that control the complex process of neurogenesis, including the proliferation and differentiation of NSCs, will lead to novel therapeutics for the treatment of neuropsychiatric disorders.
Adult neurogenesis and the neurogenic niche
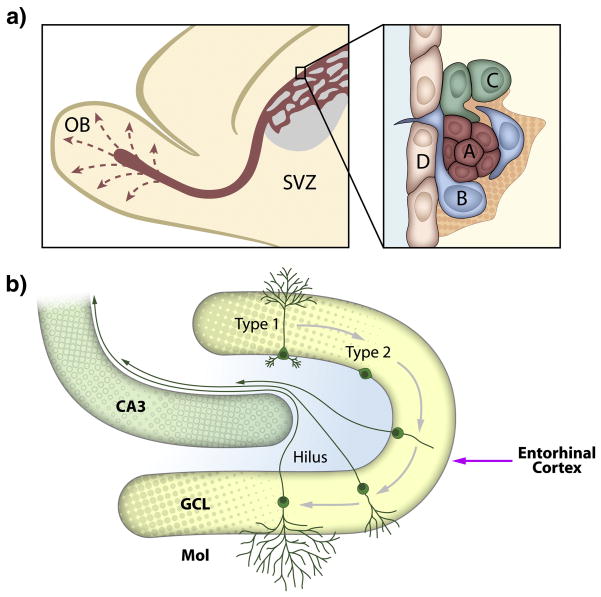
Two regions in the adult mammalian brain retain the ability to generate neurons: the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG), which is the focus of this review, and the more anterior subventricular zone (SVZ). Nestled within their discrete microenvironments or “niches”, SGZ and SVZ resident neural stem cells (NSCs) undergo self-renewal to maintain a lifelong supply of mature hippocampal DG granule neurons and olfactory bulb interneurons, respectively (Figure 1). Much is now known about the “process” of adult neurogenesis. For example, in the SGZ, Type 1 NSCs present a characteristic radial morphology and stem-like protein expression (GFAP, nestin, BLBP, Sox2), and appear to give rise to non-radial Type 2 progenitors that maintain the expression of nestin and Sox2 but downregulate Sox2 (Suh et al., 2007). We also know that different stages of neurogenesis are regulated by discrete environmental and physiological stimuli (Eisch et al., 2008; Ming and Song, 2005). For example, the number of Type 2 progenitors is increased by voluntary exercise, extended exposure to antidepressant drugs, and seizures, but is decreased with age and extended exposure to drugs of abuse like nicotine, opiates, and psychostimulants. As discussed below, this dynamic regulation of neurogenesis was among the first clue that adult-generated neurons might be relevant for neuropsychiatric disorders, like depression, addiction, and epilepsy.
Figure 1. Ongoing neurogenesis occurs in two discrete regions in the adult mammalian brain.
(a) Progenitor cells (A–C) in the anterior subventricular zone (SVZ) lie adjacent to the ependymal cell (D) layer lining the lateral ventricles and interact with basal lamina extending from the vasculature. SVZ progenitors differentiate and migrate through the rostral migratory stream before they reach the olfactory bulb (OB) and integrate as granule neurons in the granule cell layer and as periglomerular neurons (not shown). (b) Type 1 and Type 2 progenitor cells in the subgranular zone (SGZ) proliferate and go through several stages of morphological and physiological changes as they differentiate into newborn neurons in the dentate gyrus of the hippocampus. Abbreviations are as follows: GCL, granule cell layer; Mol, molecular layer.
Despite the identification of morphology and marker expression in NSCs and their progeny and increased understanding of how they are regulated by discrete stimuli, our specific understanding of the molecular and genetic basis for how NSCs self-renew and generate neurons in vivo is still very limited. This is largely due to two related factors. First, there is an inherent difficulty in unambiguously tracking adult-generated neurons in vivo and identifying and isolating NSCs in vitro (Morrison and Spradling, 2008). Recent technical advances in viral-mediated gene transfer and transgenic mouse development now allow the study and manipulation of NSCs and their progeny both in vivo and in vitro, and much of the work reviewed here relies on these new approaches (Imayoshi et al., 2009; Ming and Song, 2005).
A second factor limiting our knowledge about the genetic mechanisms that drive adult neurogenesis is the demonstrated reliance of the process of adult neurogenesis on the microenvironment, or “neurogenic niche”. Classic transplantation first demonstrated the contribution of extrinsic factors within the niche to neurogenesis (Shihabuddin et al., 2000; Suhonen et al., 1996), and many cellular components of the neurogenic niche have been identified. These include endothelial cells (Palmer et al., 2000; Shen et al., 2004; Shen et al., 2008; Tavazoie et al., 2008) which can release vascular-derived factors (Cao et al., 2004; Jin et al., 2003; Jin et al., 2002; Schanzer et al., 2004), astrocytes (Song et al., 2002a; Song et al., 2002b), which can release Wnt3, IL-1 beta and IL-6 (Barkho et al., 2006; Lie et al., 2005) and various mature neurons which via terminals and fibers of passage can release neurotransmitters, such as GABA and glutamate (Deisseroth et al., 2004; Tashiro et al., 2006) (Ben-Ari, 2002; Ge et al., 2006; Liu et al., 2005; Tozuka et al., 2005). Scientists have begun to understand how neurogenesis is regulated by the factors released by these and other cellular components of the neurogenic niche. For example, GABA-mediated depolarization of Type 2 hippocampal progenitors leads to calcium influx and increased expression of the neurogenic bHLH transcription factor NeuroD1 (Tozuka et al., 2005) and the transcription factor cAMP response element-binding protein (CREB) (Jagasia et al., 2009) to promote maturation and survival of adult-born hippocampal neurons. While important progress is being made in identifying components of the niches and understanding how signals in the niche regulate neurogenesis, the exact molecular mechanisms regarding how such diffusible factors signal to the NSC genome within the niche is unknown.
Here we review evidence that epigenetic modifications are in part responsible for maintenance and regulation of the process of adult hippocampal neurogenesis. This review is timely given several advances in the fields of epigenetics and adult neurogenesis. First, while overwhelming evidence supports the existence of adult-generated neurons and their functional integration (Zhao et al., 2008; Zhao et al., 2006), more recent evidence indicates their importance in neuropsychiatric disorders (Eisch et al., 2008; Kempermann et al., 2008). In order to harness our knowledge of adult hippocampal neurogenesis for future translational applications that target these disorders, it is imperative to understand the molecular and genetic mechanisms that control the maintenance and regulation of adult neurogenesis. Second, evidence strongly supports the importance of epigenetic mechanisms in adult neurogenesis in regards to both cell-intrinsic (Lim et al., 2006) and cell-extrinsic (Ma et al., 2009) regulation. As reviewed here, the cell-intrinsic epigenetic regulation is reminiscent of classical studies on epigenetic regulation of differentiation and cell fate during embryonic and early postnatal development, while cell-extrinsic epigenetic regulation provides insight into the intriguing phenomena of “activity-dependent neurogenesis” (Deisseroth and Malenka, 2005; Deisseroth et al., 2004). Third, as indicated above, tools have been developed that allow dissection of intrinsic versus extrinsic regulators of adult-hippocampal neurogenesis (Jessberger et al., 2009b; Johnson et al., 2009). In addition, tools to study epigenetic modifications have become more standardized and thus more reliable for use; for example, high quality antibodies needed for approaches like chromatin immunoprecipitation are readily available. Therefore, we hope this review will highlight areas of research that need greater attention, and thus encourage applications of these new tools to further advance our understanding of the links among epigenetics, adult neurogenesis, and neuropsychiatric disorders.
Overview of epigenetic mechanisms
A major advance in our understanding of gene regulation was the discovery of transcription factors. Downstream of canonical intracellular signaling pathways, transcription factors bind DNA and activate or repress gene expression, opening enormous combinatorial options upon control of gene expression in regards to environmental or physiological stimuli. Epigenetic chromatin modification has emerged as an equally important discovery that, in working together with the action of transcription factors, allows fine-tuning and coordination of gene expression, thus provides even greater number of combinations and permutations to respond to stimuli. Our working definition of the term “epigenetics” refers to “changes above the genome”: heritable changes in patterns of gene expression that are not encoded in the primary DNA sequence itself, thus leading to new cellular phenotypes without a change in genotype (Riggs and Porter, 1996). As briefly described below, epigenetic mechanisms include histone modification, DNA methylation, and noncoding RNAs.
The most classically studied epigenetic modification that is also important for the present review is DNA methylation. Mammalian DNA can be covalently modified through methylation of the carbon at the fifth position on the pyrimidine ring of the cytosine residue, which is usually found at symmetrical CpG dinucleotides. DNA methylation is a major epigenetic modification for the establishment of parental-specific imprints during gametogenesis and gene silencing of the inactivated X-chromosome (Jaenisch and Bird, 2003). Cellular methyltransferases like Dnmt3a and Dnmt3b add methyl groups de novo to unmethylated DNA. Upon cell division, Dnmt1 preferentially recognizes hemimethylated DNA and methylates the unmethylated strand, thus serving as a maintenance methyltransferase. Interestingly, both classes of methyltransferases been shown to participate in various stages of neural fate and neurogenesis. During the initial specification of neurons and glia (Feng et al., 2005), as well as during later stages of neuronal maturation and function (Levenson et al., 2006), Dnmt3a and Dnmt3b are important. Dnmt1 is also very important in the brain and involved in JAK-STAT signaling to control the timing of when precursor cells switch from neurogenesis to gliogenesis during development (Fan et al., 2001; Fan et al., 2005; Namihira et al., 2009). With each round of cell division following DNA replication, passive demethylation of DNA occurs when maintenance methylases become inactivated. But what is known regarding active DNA demethylation? Plants use 5-methylcytosine glycosylases and the base excision repair pathway to remove excess cytosine methylation (Zhu, 2009). However, active DNA demethylation in mammals - though still controversial - is proposed to operate via several very different mechanisms from plants, but initiated by the same enzymes that are important in DNA methylation (Dnmt3a and Dnmt3b) (Gehring et al., 2009; Metivier et al., 2008; Ooi and Bestor, 2008). Clearly, more work is needed to elucidate the exact players and detailed mechanism of active DNA demethylation in mammals.
A second epigenetic modification of note is chromatin remodeling, which includes changes in histone modification. Chromatin is comprised of nucleosome repeats of 147 base pairs of DNA sequence physically wrapped around two copies of four distinct histone proteins: H2A, H2B, H3 and H4 (Luger and Richmond, 1998). One of the most exciting breakthroughs in chromatin biology this last decade is the discovery that the amino (N)-terminal tails of core histones are subject to a variety of covalent modifications or ‘marks’ such as acetylation, methylation, phosphorylation, and ubiquitylation. The DNA site- or domain-specific histone modifications (histone “code”) appear to exert powerful control over the activation or repression of the associated genes (Jenuwein and Allis, 2001). Some histone marks, like acetylation of lysine 9 and 14, di- or tri-methylation of lysine 4, and phosphorylation of serine 10 on histone H3, are signatures of actively expressed chromatin. Other marks, such as di- or tri-methylation of lysine 9 or 27 on histone H3, are associated with silent chromatin domains. The histone code is established and maintained by dozens of chromatin-modifying enzymes such as histone acetyltransferases and deacetylases (HDACs) and histone methyltransferases and demethylases, which are targeted to specific chromatin loci, possibly through direct association with sequence-specific DNA binding proteins in large, multi-component complexes. Many of the components of these complexes – even the chromatin-modifying enzymes themselves – are signal responsive, resulting in a complex regulatory hierarchy for control of the genome. Notably, recent research suggests well-known neuronal transcription factors like ATF2 and CLOCK and CNS signaling molecules like nitric oxide have chromatin-modifying properties (Doi et al., 2006; Kawasaki et al., 2000; Nott et al., 2008), and likely more of such multi-purpose molecules will be identified in the future.
The complexity of this system raises the obvious question: why are there so many modifications? One hypothesis is that specific modifications link with individual biological processes and read out as simple binary “on” or “off” states (Strahl and Allis, 2000; Turner, 2000). An alternate, more complicated, but perhaps more realistic scenario is that histone modifications set up a platform for recognition and binding of many other proteins to carry out a multitude of cellular functions (Schreiber and Bernstein, 2002).
Yet another layer of complexity on top of DNA methylation, histone modification, and transcription factor expression is the presence of regulatory noncoding RNAs. Transcribed from non-protein-coding regions, several classes of noncoding RNAs are expressed in a regulated manner and serve to “fine-tune” gene expression networks. For example, microRNAs (miRNAs) are 21–23 bp noncoding RNAs that bind 3 untranslated regions of target mRNAs resulting in translational repression or mRNA destabilization (Kosik, 2006). Other small noncoding RNAs include small-interfering RNAs (siRNAs), small nucleolar RNAs (snoRNAs), and piwi protein-interacting RNAs (piRNAs) (Mattick and Makunin, 2005). Recently, many small noncoding RNAs have been discovered to function in development and disease, including those of the mammalian brain (Cao et al., 2006; Mehler and Mattick, 2007).
Below we expand on current aspects of the epigenetic regulation of adult neurogenesis. We begin with a review of classic and recent studies on epigenetic regulation of nervous system development, and then provide an overview of the progress made in understanding epigenetic mechanisms in regards to adult neurogenesis (Figure 2). We conclude with a summary of the progress made in exploring the links among epigenetics, hippocampal neurogenesis, and neuropsychiatric disorders (Figure 3).
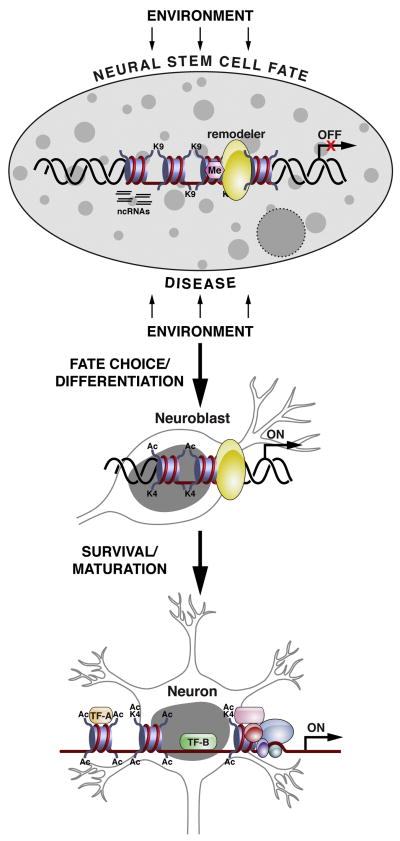
Figure 2. Environmental factors signal to the neural stem cell genome to regulate cell fate decisions and neurogenesis.
Sequence-specific transcription factors work in concert with the chromatin machinery to direct the neuronal lineage program within neural stem cells. In the stem cell state, repressive chromatin remodeling machinery maintains neuronal gene repression (OFF) through one set of histone modifications, such as histone H3 lysine K9 methylation and DNA cytosine methylation. Stimulation by environmental factors and/or stress signals (during disease) can induce adult neurogenesis and survival/maturation of newborn neurons by de-repressing or activating neuronal gene expression (ON) through the hyperacetylation and/or switch in histone modification to histone H3 lysine K4 methylation. The emergence of noncoding RNAs adds another layer of regulatory complexity to help fine-tune gene expression. Abbreviations are as follows: Me: methylation, Ac: acetylation, K: lysine, ncRNAs: noncoding RNAs, TF: transcription factor.
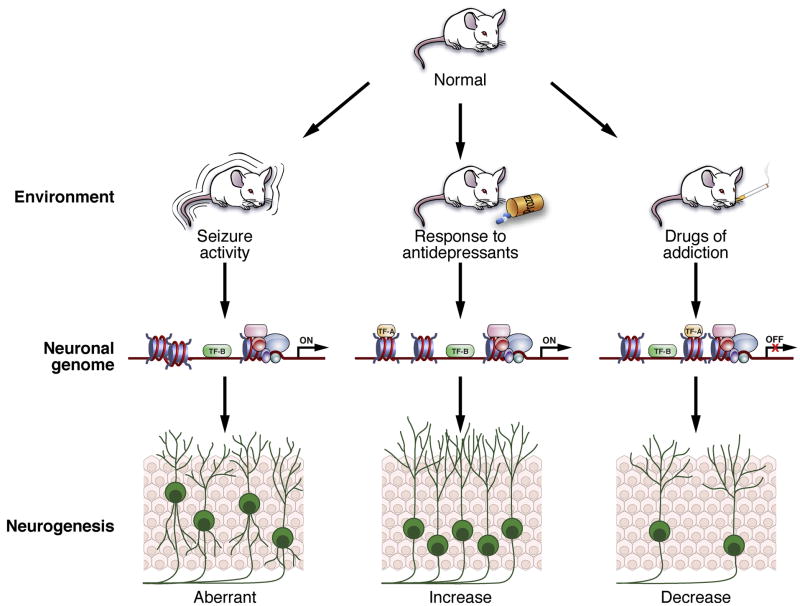
Figure 3. Hypothetical relationship among animal models of psychiatric disorders, epigenetic regulation in SGZ stem cells, and altered adult hippocampal neurogenesis.
Environmental/physiological stimuli, such as drug-induced seizure activity or chronic exposure to antidepressant agents or drugs of abuse, lead to complex neuroadaptations in discrete brain regions including altered neurogenesis. We hypothesize that these stimuli may also produce cell-intrinsic changes in chromatin remodeling that contributes to altered neurogenesis and ultimately altered neuronal genome structure. Recent advances in understanding epigenetic regulation and technical advances in determining and independently regulating chromatin modifications now make it possible to test these hypothetical relationships.
Epigenetic regulation of nervous system development from mouse and in vitro models
The understanding of postnatal and adult neurogenesis to date has greatly benefited from studies of embryonic nervous system development. During CNS development, a diverse spectrum of neuronal and glial cell-types originate from multipotent neuroepithelial precursor cells lining the ventricles of the brain and spinal cord (Guillemot, 2007; Temple, 2001). Neuroepithelial cells differentiate into radial glia progenitors cells which further divide in a temporal fashion (‘first neuron and then glia’) to generate transit-amplifying progenitors to expand the population of neurons and glia that constitute the developing cortex (Okano and Temple, 2009). A number of cell-intrinsic factors have been reported to play a role in the switch from neurogenesis to gliogenesis, including proneural bHLH genes, Smad/CBP-p300 proteins, and nuclear hormone receptors (Schuurmans and Guillemot, 2002; Sun et al., 2001; Tomita et al., 2000) as well as chromatin remodeling and DNA methylation of glia-specific genes (Nakashima et al., 1999; Namihira et al., 2004; Takizawa et al., 2001). However, these studies still leave open the exact role of chromatin-based epigenetic mechanisms in early neural development.
A set of recent papers sheds light on this issue, implicating mammalian SWI/SNF-like ATP-dependent chromatin-remodeling complexes and demonstrating that a subunit composition change of the Brg- and Brahma- (Brm) associated factor-complexes (BAFs or mSWI/SNF) is critical for neuronal differentiation and dendritic development during early embryonic and postnatal development (Lessard et al., 2007; Wu et al., 2007). The transition of multipotent progenitors to postmitotic neurons involves an essential switch in ATP-dependent complexes: the BAF45a and BAF53a subunits are exchanged for the homologous BAF45b and BAF53b subunits. Knockdown of individual BAF subunits (BAF45a and/or 53a) affected the proliferation of E14.5 cortical progenitors in vitro and exogenous BAF45a was sufficient to drive progenitor proliferation in vivo (Lessard et al., 2007). Consistent with these findings, stem cell deletion of the core subunit Brg, which is associated with BAF45a and BAF53a, resulted in reduced cortical thickness and decreased proliferation of progenitors. Perhaps the most interesting observation was that the neuron-specific subunit BAF45b, a closely related subunit, could not substitute for BAF45a, suggesting a level of specificity that is necessary for the transition from proliferation of progenitors to the differentiation of neuronal subtypes.
Recently, an intriguing study came out regarding a microRNA-based mechanism that controls the essential BAF subunit composition switch. Specifically miR-9* and miR-124 appear necessary to repress BAF53a as neural progenitors differentiate into neurons (Yoo et al., 2009). Moreover, expression of neuron-restrictive silencing factor (NRSF, also known as repressor element-1 silencing transcription factor or REST; a known transcriptional repressor of miR-9* and miR-124 as well as many other neuronal genes) leads to de-repression of BAF53a in postmitotic neurons. These results highlight the intricate interplay between transcriptional regulators and regulatory noncoding RNAs, which will be discussed in a subsequent section.
In contrast to the downregulation of BAF subunits, CNS deletion of HDACs 1 and 2 during embryonic development resulted in increased proliferation of ventricular zone progenitors that similarly resulted in abrogated neuronal differentiation in vivo and in vitro (Montgomery et al., 2009). However it remains to be determined whether BAF chromatin remodeling complexes and HDACs interact to control neuronal and glial lineage specific differentiation during CNS development.
In addition to traditional mouse models of developmental neurogenesis, studies utilizing in vitro stem cell systems - such as embryonic stem (ES) and induced pluripotent stem cells (iPSCs) - have emerged to address the question: to what extent do epigenetic mechanisms maintain cells in an undifferentiated or differentiated state? A major breakthrough came in 2006 when it was discovered that terminally differentiated somatic cells can be coaxed to adopt a pluripotent state via introduction of four transcription factors Oct4, Sox2, Klf4, and c-Myc (Takahashi and Yamanaka, 2006). These “reprogrammed” cells represent an immensely powerful tool for biomedical research and potentially allow every man, woman, and child to have his or her own matched stem cells for therapeutic use (Park et al., 2008; Takahashi et al., 2007). Moreover, the possibility of modeling neurological disorders like Parkinson’s disease in vitro where primary neuronal tissue is not available (Soldner et al.; Wernig et al., 2008) make patient-specific iPSCs immediately valuable. A major obstacle to widespread use of iPSCs for neuroregenerative medicine is the knowledge gap regarding the basic mechanisms underlying direct reprogramming to pluripotency and subsequent neurogenesis from iPSCs. A slew of exciting recent papers (Hester et al., 2009; Kim et al., 2009a; Kim et al., 2009b; Kim et al., 2008; Marchetto et al., 2009) showed that NSCs from human and mice could be reprogrammed to pluripotency with the expression of a single transcription factor Oct4 and/or a combination of two factors in defined culture conditions.
These studies are consistent with the previous work of global chromatin modification in ES cells and support the hypothesis that NSCs represent an intermediate state between differentiated and pluripotent ES cells. Of great interest in this regard is a study by Bernstein and colleagues (Bernstein et al., 2006). Using a combination of chromatin immunoprecipitation and tiling oligonucleotide arrays, they examined histone H3 lysine 4 and lysine 27 methylation patterns in ES cells across a subset of highly conserved noncoding elements (~2.5% of the genome), which are enriched for the 4 Hox clusters as well as transcription factor genes. Their study revealed a novel histone modification pattern termed “bivalent domains” consisting of smaller regions of lysine 4 methylation within larger regions of lysine 27 methylation within ES cells. Moreover, upon differentiation into neural lineages, bivalent domains appeared to resolve into regions selectively enriched for either lysine 27 or lysine 4 methylation. These resolved regions may provide an “epigenetic memory” for the maintenance of lineage-specific gene activation or repression. These studies also suggest that bivalent domains silence differentiation-specific genes in ES cells, but keep them poised for activation. Thus, uncovering the mechanisms that initiate and maintain bivalent domains in ES cells may shed light on the underlying mechanisms involved in reprogramming somatic cells, such as neurons, to a pluripotent state.
DNA methylation/demethylation and chromatin remodeling during adult neurogenesis
Compared to studies of embryonic and early postnatal neurogenesis, relatively little is known about cell-intrinsic epigenetic mechanisms that control adult neurogenesis. In fact, most of what is known about epigenetic modifications and adult neurogenesis comes from mouse models in which epigenetic mediators, like methyl-CpG binding protein-1 (MBD1), are constitutively deleted.
For example, one of the first studies of epigenetic regulation in adult hippocampal NSCs showed that MBD1-deficient mice had reduced neurogenesis and deficits in spatial learning and DG-specific LTP (Zhao et al., 2003), in addition to exhibiting enhanced susceptibility to depressive behaviors (Allan et al., 2008). Although there was no change in global methylation levels in hippocampal NSCs in MBD1 deficient mice, there was increased expression of the endogenous virus IAP and increased aneuploidy, supporting the important role that DNA methylation may play in maintaining genomic stability. MBDs can bind directly to methylated gene promoters and silence gene expression by blocking transcription factor binding and/or recruit HDACs to promote transcriptional repression (Klose and Bird, 2006). In a follow-up paper, MBD-1 was shown to directly regulate the methylation status of the FGF-2 promoter in adult NSCs, consistent with its intrinsic epigenetic effects (Li et al., 2008b). Future work is needed to determine whether MBD-1 actually plays a cell-intrinsic role in vivo or whether MBD-1 in other cells in the niche regulates neurogenesis (e.g. via cell-extrinsic mechanisms).
Another MBD, MeCP2, is highly expressed in mature neurons in the adult CNS. More recently, MeCP2 appears to be involved in suppressing the expression of glia-specific genes (like GFAP) in neurons (Kohyama et al., 2008). This finding is consistent with the importance of DNA methylation in the maintenance of neuronal identity and function which is critical for the maturation of new neurons in adult brain (Smrt et al., 2007). Interestingly, acute overexpression of MeCP2 in NSCs in vitro leads to enhanced neuronal differentiation (Tsujimura et al., 2009), suggesting that MeCP2 regulates NSC function. In related work, the maintenance of hyperacetylation with HDAC inhibitors in adult hippocampal NSCs resulted in neuronal differentiation, and dominantly blocked glial differentiation (Hsieh et al., 2004). These studies lay the groundwork for additional in vivo experiments to determine whether MeCP2 has a cell-intrinsic vs. cell-extrinsic regulation of neurogenesis in vivo, what role specific HDACs play in neurogenesis in vivo, and what the precise relationship is between HDACs and MBDs during adult neurogenesis.
As mentioned above, electrochemical activity is emerging to be a potent trigger of adult neurogenesis. While the detailed molecular mechanisms underlying this ‘activity-dependent neurogenesis’ are still unknown, an exciting recent report attempted to address the question of how transient activation of mature neurons modulates adult neurogenesis. The authors focused on the functional role of the activity-induced gene Gadd45b (DNA-damage-inducible protein 45 beta) (Ma et al., 2009), which was previously implicated in 5-methylcytosine excision (Barreto et al., 2007; Jung et al., 2007; Tran et al., 2002). Mice deficient for Gadd45b display decreased proliferation after electroconvulsive treatment (ECT) or voluntary exercise. Moreover, Gadd45b is required for ECT-induced dendritic development of newborn neurons in DG. Interestingly, the mechanism by which Gadd45b regulates activity-dependent neurogenesis appears to be epigenetic in nature. While Gadd45b did not appear to affect global DNA demethylation after ECT, DNA from micro-dissected DG from knockout mice apparently lacked demethylation at the regulatory regions of two ECT-induced genes, FGF-1B and BDNF IX, suggesting that the role of Gadd45b is to promote epigenetic DNA demethylation at specific activity-induced target genes to control adult neurogenesis and dendritic development in a non-cell autonomous manner. The precise nature of how, for example, BDNF from mature neurons signals to dividing cells in the neurogenic niche remains unclear. However, these studies importantly highlight that epigenetic mechanisms like DNA methylation and demethylation may be broadly employed for long-lasting modulation of plasticity after neuronal activity. In addition, recent studies of active DNA demethylation activity mediated by Gadd45a were reported to occur in mammalian cell lines as well as in Xenopus oocytes (Barreto et al., 2007), and in zebrafish embryos (Rai et al., 2008). However, in a further study, the functional role of Gadd45a in DNA demethylation was unsubstantiated (Jin et al., 2008), possibly due to species differences. This and work reviewed elsewhere (Wu and Sun, 2009)thus leave open the controversial role for Gadd45a as a DNA demethylase. In sum, this groundbreaking work by Ma and colleagues provides a tantalizing example of how cell-extrinsic epigenetic modifications (likely in mature neurons, and thus cell-extrinsic relative to NSCs) can lead to potent alterations in adult neurogenesis.
A final example of recent work on chromatin remodeling and adult neurogenesis focuses on proteins in the polycomb (PcG) and trithorax (TrxG) groups, which are required for establishing and maintaining cellular states during development and been the subject of intense study for decades (Gould, 1997). PcG and TrxG genes are organized into large multimeric complexes that regulate their target genes through a “ying-yang” fashion, most notably Hox (and other) promoters that control fate of individual body segments in Drosophila, by counterbalancing each other functionally and modulating chromatin structure. During SVZ neurogenesis, Bmi-1, a member of the Polycomb group of chromatin remodeling factors, is important for the self-renewal of embryonic and postnatal NSCs (Fasano et al., 2009; Molofsky et al., 2003). In the adult SVZ, Lim and colleagues (Lim et al., 2009; Lim et al., 2006) showed that the histone methyltransferase (HMT) Mll1 is expressed in the SVZ and olfactory bulb (OB) and is required for proliferation and neurogenesis of SVZ/OB NSCs. One downstream gene that failed to up-regulate in Mll1-deficient SVZ neurospheres was Dlx2, a homeodomain-containing transcription factor. Interestingly, although Mll1 is known to be a HMT for histone H3 lysine 4, the level of methylated lysine 4 did not change between wild type and Mll1 KO cells. However, the level of tri-methylated lysine 27 on histone H3, usually associated with silent chromatin, was apparently higher in the absence of Mll1. Thus, the main function of Mll1 in the regulation of Dlx2 appears to be to recruit a histone H3 lysine 27 demethylase. Mll1 is a member of the Trithorax family of proteins, which are known to antagonize Polycomb-mediated silencing (Ringrose and Paro, 2004). Future work will likely be aimed at uncovering the detailed mechanisms by which these chromatin-remodeling factors control adult neurogenesis, and dissecting whether the modifications are cell-intrinsic to NSCs and their immediate progeny or - as shown in the first part of this section - are rather cell-extrinsic and occur in postmitotic components in the neurogenic niche.
Noncoding RNAs and control of NSC fate
In addition to chromatin remodeling and neurogenesis, recent progress has been made in investigating the links between noncoding RNAs and neurogenesis. Noncoding RNAs play key roles in the modulation of transcriptional networks and appear to have important functions in CNS development and neurological disease as well (reviewed in Cao et al., 2006; Kosik and Krichevsky, 2005; Mehler, 2008; Mehler and Mattick, 2007). An interesting recent study profiled microRNA expression in developing and adult olfactory epithelium and found a dynamic expression of microRNAs, particularly miR-200 family members (Choi et al., 2008). Interestingly, inducible conditional deletion of Dicer, an enzyme required for the production of functional miRNAs (Bernstein et al., 2001), in olfactory progenitors during development resulted in defects in terminal differentiation of olfactory neurons as well as the maintenance of olfactory progenitor cells. Moreover, a forebrain-specific deletion of Dicer displays smaller brains, abnormally large ventricles, increased cortical apoptosis and defects in hippocampal development (Davis et al., 2008) and conditional deletion of Dicer also results in defects in cerebellar Purkinje neurons (Schaefer et al., 2007), striatal neurons (Kim et al., 2007) and retinal degeneration (Damiani et al., 2008). Although these studies point to the global role of microRNAs in brain development, there are ~500 microRNA genes (and many mRNA targets per individual microRNA) identified in human and mice to date(Saini et al., 2008; Saini et al., 2007). Therefore, detailed studies of individual microRNAs are warranted to further establish the link between small noncoding RNAs and their contribution to neurological disorders. However, it is important to note that the work by Berstein and colleagues is one of the few to identify a causal role for epigenetic modification intrinsic to neural progenitors in modification of neurogenesis in vivo.
One of the most fascinating stories regarding noncoding RNAs and neuronal differentiation is that of miR-124, the most abundant microRNA in the adult brain (Lagos-Quintana et al., 2002). During neurogenesis, miR-124 expression is undetectable or expressed at low levels in progenitor cells and is upregulated in differentiating and mature neurons (Deo et al., 2006). Initial studies demonstrated that overexpression of miR-124 in HeLa cells led to decreased expression of many non-neuronal genes to reflect a gene expression program more similar to that of neuronal cells (Lim et al., 2005). Another set of studies showed that the transcriptional repressor NRSF/REST can silence the expression of miRNA-124 and the down-regulation of NRSF and increased levels of miR-124 and neuronal gene expression is permissive for neuronal differentiation in mouse embryonal carcinoma cells (Conaco et al., 2006). However, inhibiting miR-124 does not affect neuronal differentiation, at least in chick neural tube (Cao et al., 2007). A regulatory noncoding RNA/NRSF transcriptional circuit has been described previously, with the discovery of a double-stranded small RNA (smRNA) that acts to convert NRSF from a repressor to an activator in adult hippocampal NSCs during neurogenesis (Kuwabara et al., 2004).
To add to the controversial role of miR-124 in the brain, Makeyev and colleagues (Makeyev et al., 2007) found that miR-124 targets PTBP1, a polypyrimidine tract binding protein and a repressor of neuronal splicing. Thus as neurons differentiate, miR-124 reduces PTBP1 mRNA levels, which results in a switch from general to neuron-specific alternative splicing. MiR-124 is also critical during adult neurogenesis, in SVZ stem cells (Cheng et al., 2009). Blocking miR-124 maintains SVZ stem cells as dividing precursors at the transient amplifying stage and ectopic expression of miR-124 in earlier stages results in precocious neuronal differentiation in vitro and in vivo. Interestingly, miR-124 targets the SRY-box transcription factor Sox9, as well as the Dlx2 transcription factor and the Notch ligand Jag1, and functional experiments indicate that Sox9 protein levels need to be tightly regulated by miR-124 for the transition of SVZ progenitors to neurons.
In addition to microRNAs, another subset of noncoding RNAs is long, polyadenylated noncoding RNAs (lpncRNAs). LpncRNAs may act cooperatively and recruit protein partners to regulate gene expression (Shamovsky and Nudler, 2006). The discovery of an embryonic brain noncoding RNA called Evf2, a lpncRNA target of sonic hedgehog signaling and cooperates with Dlx homeodomain proteins in NSCs (Feng et al., 2006) is consistent with noncoding RNAs having transcription-regulating activity. Recently, it was shown that Evf2 controls the balance of positive (Dlx) and negative (Mecp2) transcriptional factor recruitment to regulate Gad1 and early GABAergic interneuron development reinforcing the notion that epigenetic regulation is critical in the developing embryo which may impact mental disorders (Bond et al., 2009).
These above studies highlight the fundamental role that epigenetic mechanisms have in the regulation of adult neurogenesis under basal conditions and suggest that they may also have important effects in the context of neurological disease. In the next section, we present the current knowledge regarding the links between hippocampal neurogenesis and the pathophysiology of neuropsychiatric disorders and examine the question of whether epigenetic mechanisms underlying hippocampal neurogenesis might contribute to neuropsychiatric diseases.
Adult hippocampal neurogenesis and neuropsychiatric diseases
Strong evidence shows that adult-generated neurons are incorporated into hippocampal circuitry (Figure 1b) and the hippocampus itself is clearly involved in myriad neuropsychiatric disorders (Kobayashi, 2009; Sapolsky, 2000). Thus it is perhaps not surprising that adult-generated hippocampal neurons themselves have been implicated in the pathophysiology of disorders as diverse as depression, addiction, schizophrenia, epilepsy, Alzheimer’s disease and even autism. As several excellent recent reviews have highlighted the links between adult neurogenesis and neuropsychiatric disorders (e.g. Danzer, 2008; Eisch et al., 2008; Kuruba et al., 2009; Parent et al., 2007; Perera et al., 2008; Scharfman and Hen, 2007), only a few critical points will be reiterated here.
First, animal models of neuropsychiatric disorders are in general marked by decreased or abnormal hippocampal neurogenesis. For example, animals chronically exposed to stress (a predisposing event in depression) or to drugs like nicotine, opiates, ethanol, or psychostimulants have fewer Type 2 hippocampal progenitors, which in general leads to decreased hippocampal neurogenesis (Figure 3). Interestingly, while pharmacologically-induced seizure activity leads to increased neurogenesis, the resulting neurons are abnormal and have dramatically aberrant migration and dendritic processes (Figure 3). Such work with animal models suggests that similar changes would be seen in the brains of humans diagnosed with neuropsychiatric disorders. Indeed, tissue from epileptic patients reveals both increases and abnormalities in neurogenesis (Geha et al., 2009; Liu et al., 2008b; Liu et al., 2007) and tissue from schizophrenics reveals decreased neurogenesis (Reif et al., 2006), in accordance with what might be expected based on work in animal models of these disorders. While tissue from depressed patients does not have increased indices of neurogenesis relative to tissue from control patients (Boldrini et al., 2009; Reif et al., 2006), antidepressants appear to enhance proliferation in humans and non-human primates (Boldrini et al., 2009; Perera et al., 2007), which is consistent with laboratory animal work. Data from patients diagnosed with Alzheimer’s disease are more challenging to interpret, as both animal models and human studies show both increased and decreased indices of neurogenesis (Donovan et al., 2006; He and Shen, 2009; Jin et al., 2004y; Jin et al., 2004b; Li et al., 2008a; Yu et al., 2009b; Zhang et al., 2007). Other disorders, like addiction, have yet to be thoroughly assessed for their impact on human neurogenesis. Clearly, more work is needed on human neurogenesis in relation to neuropsychiatric disorders. However, major challenges exist to performing human studies in a meaningful manner, such as the limited availability of proven markers appropriate for human neurogenesis and for consideration of the many stages of neurogenesis, and other general complexities that accompany human post-mortem studies (as reviewed in DeCarolis and Eisch, in press). Thus more studies – and more technical advances, such as imaging neurogenesis in the human brain (Manganas et al., 2007) – are needed to clarify whether indeed neuropsychiatric disorders are linked to decreased or abnormal neurogenesis as is the case in most animal models of these disorders.
A second notable link is that decreased or abnormal hippocampal neurogenesis is strongly correlated to deficits in hippocampal structure and function in animal models of these disorders. This is reviewed extensively elsewhere (Abrous et al., 2005; Leuner and Gould; Ming and Song, 2005; Zhao et al., 2008), but the overview is worth mentioning. In general, stimuli or manipulations that improve performance on cognitive tasks (like exercise or the opportunity to learn a spatial task) increase neurogenesis, while stimuli or manipulations that diminish performance on behavioral testing on cognitive tasks (like stress, age, drugs of abuse, or as is relevant for this review, HDAC inhibitors (Umka et al., 2009)) decrease neurogenesis. These correlative links suggest a functional importance for hippocampal neurogenesis in key aspects of cognition, and this is supportive by numerous publications in which neurogenesis is inducibly ablated (e.g. Clelland et al., 2009; Deng et al., 2009; Dupret et al., 2008; Garthe et al., 2009; Hernandez-Rabaza et al., 2009; Imayoshi et al., 2008; Jessberger et al., 2009a; Ko et al., 2009). Of great interest for this review, suppression of hippocampal neurogenesis in mice blocks behavioral responses in antidepressant-sensitive tests (Santarelli et al., 2003), is anxiogenic (Revest et al., 2009), and confers vulnerability in an animal model of cocaine addiction (Noonan et al., in press). However, a role for new neurons in cognition and mental health remains controversial (e.g. DeCarolis and Eisch, in press; Leuner and Gould; Sapolsky, 2004), as ablation of neurogenesis does not always diminish cognition, result in anxiety or lead to a depressive phenotype, and new neurons are not always needed for antidepressant efficacy (e.g. David et al., 2009; Hernandez-Rabaza et al., 2009; Holick et al., 2008; Jaholkowski et al., 2009; Ko et al., 2009; Singer et al., 2009; Surget et al., 2008). These discrepancies emphasize the need for more studies, which are further prompted by the intriguing support from human imaging studies (Manganas et al., 2007) that NSCs may be important for cognitive function. Thus more work is needed to fully clarify the relationship between the regulation of adult neurogenesis and the pathophysiology or treatment of many neuropsychiatric disorders.
A third and final link between neurogenesis and neuropsychiatric disorders worth mentioning is that treatments that ameliorate the alterations in behavior in animal models or in humans typically enhance or normalize neurogenesis. The first example of this was with antidepressants, where Duman and colleagues showed that chronic, but not acute, exposure to several different classes of antidepressants enhanced hippocampal neurogenesis (Malberg et al., 2000). In fact, all major pharmacological and non-pharmacological treatments for depression enhance proliferation and/or neurogenesis in laboratory animals, including electroconvulsive shock (ECS), monoamine oxidase inhibitors (MAOIs) like tranylcypromine, serotonin selective reuptake inhibitors (SSRIs) like fluoxetine, norepinephrine (NE) selective reuptake inhibitors like reboxetine, (all shown in Malberg et al., 2000), NMDA antagonists like memantine (Jin et al., 2006; Namba et al., 2009), tricyclic antidepressants like imipramine (Sairanen et al., 2005), transcranial magnetic stimulation (Arias-Carrion et al., 2004), and exercise (e.g. van Praag et al., 1999). Intriguingly, some antidepressants themselves do not statistically enhance neurogenesis but rather block stress-induced decreases in proliferation or neurogenesis (Czeh et al., 2001; Liu et al., 2008a). After this surge in research on antidepressants, medications for other neuropsychiatric disorders have similarly been found to normalize neurogenesis. For example, some antiepileptic drugs block seizure-induced changes in neurogenesis in animal models (e.g. Chen et al., 2009a).
Epigenetics, adult neurogenesis and neuropsychiatric diseases
As stated above, there are notable links between neuropsychiatric disorders and hippocampal neurogenesis. This leads us to the main point of this review: might epigenetics play a role in mediating hippocampal neurogenesis and perhaps contribute to neuropsychiatric disorders? Certainly epigenetic modifications in non-hippocampal brain regions are linked to neuropsychiatric disorders, including depression (Castren et al., 2007; Renthal et al., 2007; Renthal and Nestler, 2008) and schizophrenia (Sharma, 2005). In addition, a wealth of information is known now about epigenetic modifications with regards to hippocampal function and dysfunction (Alarcon et al., 2004; Crepaldi and Riccio, 2009; Sweatt, 2009; Tsankova et al., 2006). Thus it is timely to turn the spotlight on what is known about the relationship among epigenetics, neurogenesis and neuropsychiatric disorders which has also been the focus of a recent review (also see reviews Newton and Duman, 2006).
Prior to reviewing what is specifically known about the links among epigenetics, neurogenesis, and neuropsychiatric disorders, it is important to state that there is still great complexity – and some understandable confusion – around the progress in understanding these links and advancing the promise of utilizing epigenetic regulation to modify neurogenesis and perhaps treat or prevent neuropsychiatric disorders. To help guide future research on these important topics, here we suggest two ways of thinking about the relationship among epigenetics, adult neurogenesis, and neuropsychiatric disorders. The first is that perhaps there are cell-intrinsic epigenetic mechanisms that underlie altered neurogenesis in animal models of these disorders or even in human disease. An alternative – and complementary way – of thinking about the relationship among epigenetics, adult neurogenesis, and neuropsychiatric disorders is that perhaps epigenetic mechanisms in mature cellular components in the neurogenic niche – or cell-extrinsic mechanisms – contribute to altered signaling in the niche, which then modulates adult hippocampal neurogenesis indirectly. Distinguishing between these two possibilities is important since it clarifies the challenges ahead.
However, distinguishing between these two possibilities is also extremely challenging. As mentioned throughout this review, constitutive knockout mice are frequently the focus of epigenetic studies, and the pharmaceutical agents typically used to manipulate epigenetic mechanisms are given systemically (e.g. intraperitoneal sodium butyrate) or at best intra-hippocampally, thus influencing all cells in the hippocampus. For example, mice with constitutive mutations in or deletions of epigenetic-relevant genes, like CBP, MBD, Mecp2, HDAC1/2, or NRSF, present robust behavioral and cognitive deficits that are associated with numerous neuropsychiatric disorders (Adachi et al., 2009; Allan et al., 2008; Amir et al., 1999; Guan et al., 2009; Lepagnol-Bestel et al., 2007; Zhao et al., 2003). Intriguingly, mice deficient in these and other relevant genes, such as NRSF/REST, MeCP2, Gadd45b or MRG15 (a component of HAT and HDAC complexes) also have abnormal neurogenesis under basal or stimulated conditions (e.g. Ballas et al., 2005; Chen et al., 2009b; Ma et al., 2009; Smrt et al., 2007; Zhao et al., 2003), However, it is not clear if these results are a function of mutations in mature neurons leading to altered neurogenesis or to the mutations also causing cell-intrinsic changes in neurogenesis. As another example, exposure to antidepressants or drugs of abuse alter epigenetic-relevant molecules in the hippocampus (e.g. Cassel et al., 2006; Tsankova et al., 2006; Tsankova et al., 2004) similar to actions in other brain regions (e.g. Renthal et al., 2007; Renthal and Nestler, 2008), perhaps driving the changes in neurogenesis seen after antidepressants and cocaine (Malberg et al., 2000; Yamaguchi et al., 2004). However, it is unclear whether epigenetic changes occur within NSCs and their progeny to mediate changes in neurogenesis, or whether the changes occur within nonneurogenic components of the niche. Essentially, these published studies are elegant and excellent for what they reveal about epigenetic mechanisms in hippocampal neurons and thus the relationship between the hippocampus and neuropsychiatric disorders. However, few mechanistic conclusions can be drawn from these about how cell-intrinsic or cell-extrinsic epigenetic modifications regulate neurogenesis and adult-generated neuron involvement in neuropsychiatric disorders.
There are a few lines of research, however, that have utilized a clever combination of in vivo cell-specific targeting and in vitro approaches, and whose results support our hypothesis that epigenetic changes in mature neurons or cell-intrinsic epigenetic changes in NSCs and their progeny contribute to altered neurogenesis and are linked to neuropsychiatric disorders. For example, valproic acid (VPA) is a clinically effective mood stabilizer that drives hippocampal neurogenesis (Manji et al., 2000) partially via its positive influence on ERK signaling (Hao et al., 2004). However, VPA also is an HDAC inhibitor, and it drives neuronal differentiation in vitro in part through upregulation of the proneural transcription factor NeuroD1 (Hsieh et al., 2004). Intriguingly, VPA’s HDAC inhibition blocks kainic acid-induced seizures and prevents the resulting abnormal neurogenesis and cognitive deficits (Jessberger et al., 2007), strongly suggesting a role for HDACs in seizure-induced behavioral problems. More recent work has emphasized a role for VPA in induction of proneural factors (Yu et al., 2009a) and the importance of these factors, like NeuroD1, in driving neurogenesis (Gao et al., 2009). As VPA also can decrease memory function and neurogenesis in vivo (Umka et al., 2009), the question of whether and how HDAC inhibition alters neurogenesis will await the utilization of modern transgenic mice and viral-mediated gene transfer to answer these more mechanistic questions (Johnson et al., 2009).
Another example of a line of research that support our hypothesis that epigenetic changes in mature neurons or cell-intrinsic epigenetic changes in NSCs and their progeny contribute to altered neurogenesis and are linked to neuropsychiatric disorders involves the gene Disc1 (disrupted-inschizophrenia-1 gene). Disc1 is notable since the clinical link was discovered prior to its epigenetic involvement. Initially linked to schizophrenia (Millar et al., 2000), Disc1 was subsequently found to be important in neurite outgrowth and other fundamental neuronal functions (Miyoshi et al., 2003; Morris et al., 2003). Seminal work showing the importance of Disc1 in adult neurogenesis (Duan et al., 2007) was followed by recent work that Disc1 interacts with the GSK3β/β-catenin pathway to regulate adult neurogenesis (Mao et al., 2009). This is exceptionally intriguing since the GSK3β/β-catenin pathway is considered a common target for many neuropsychiatric disorders (Wada, 2009), including its involvement in the regulation of histone modifications that are hallmarks of epigenetic mechanisms (Mosimann et al., 2009).
Several other papers that were discussed above (Allan et al., 2008; Ma et al., 2009; Zhao et al., 2003) are other excellent examples of how altered epigenetic signaling in mature neurons might be linked to adult neurogenesis and altered behavior relevant to neuropsychiatric disorders. Taken together, these studies point to a set of hypothetical epigenetic changes that may occur within NSCs in the adult SGZ after seizure activity, antidepressant administration, or exposure to drugs of abuse (Figure 3), and that are the focus of current studies within many laboratories. Clearly, more work is needed utilizing cell-specific transgenic and viral-mediated protein expression in order to fully understand the relationship among epigenetics, neurogenesis, and neuropsychiatric disorders.
Conclusions and Future Directions
In this review, we hope to convey that many types of epigenetic mechanisms are interrelated – DNA methylation and demethylation, histone modifications, noncoding RNAs – to regulate gene expression in adult neural stem/progenitor cells which crossover to diverse neuropsychiatric conditions. While these epigenetic approaches to explore the links between adult neurogenesis and neurological disease remain vastly promising, there are still unanswered questions that need to be addressed in future research towards the development of potential therapeutics. Do the recently identified epigenetic mechanisms that function to regulate embryonic neurogenesis also function in adult neurogenesis? What other CNS signaling molecules have chromatin-modifying properties? As human studies with in vivo imaging of NSCs become more commonplace, and as our ability to study neurogenesis in human tissues improve, it will be important to aggressively test whether, as in animal models of these disorders, strong links exist between hippocampal function and neurogenesis. Importantly for this review, it will be critical to assess how epigenetic mechanisms in human tissue contribute to regulation of neurogenesis and whether, as we hypothesize, chromatin remodeling is in fact a promising target for future treatment avenues. Finally, it will further be critical to establish whether treatments that target the epigenetic state of mature hippocampal neurons have untoward or additional unappreciated effect on the adult-generated neurons that reside nearby.
Acknowledgments
We apologize to the many researchers whose work was not cited in this review due to space limitations. We thank Jiang Wu for critical reading of the manuscript and Jose Cabrera for graphics. Work in the laboratory of JH is supported by grants from the NIH, the Ellison Medical Foundation, the Welch Foundation, and the Citizens United for Research in Epilepsy. Work in the laboratory of AJE is supported by grants from the NIH, the National Institute on Drug Abuse, and NASA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrous DN, et al. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–69. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- Adachi M, et al. MeCP2-mediated transcription repression in the basolateral amygdala may underlie heightened anxiety in a mouse model of Rett syndrome. J Neurosci. 2009;29:4218–27. doi: 10.1523/JNEUROSCI.4225-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon JM, et al. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42:947–59. doi: 10.1016/j.neuron.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Allan AM, et al. The loss of methyl-CpG binding protein 1 leads to autism-like behavioral deficits. Hum Mol Genet. 2008;17:2047–57. doi: 10.1093/hmg/ddn102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir RE, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–8. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Arias-Carrion O, et al. Neurogenesis in the subventricular zone following transcranial magnetic field stimulation and nigrostriatal lesions. J Neurosci Res. 2004;78:16–28. doi: 10.1002/jnr.20235. [DOI] [PubMed] [Google Scholar]
- Ballas N, et al. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell. 2005;121:645–57. doi: 10.1016/j.cell.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Barkho BZ, et al. Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cells Dev. 2006;15:407–21. doi: 10.1089/scd.2006.15.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto G, et al. Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature. 2007;445:671–5. doi: 10.1038/nature05515. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–39. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Bernstein BE, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–26. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- Bernstein E, et al. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–6. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- Boldrini M, et al. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology. 2009;34:2376–89. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond AM, et al. Balanced gene regulation by an embryonic brain ncRNA is critical for adult hippocampal GABA circuitry. Nat Neurosci. 2009;12:1020–7. doi: 10.1038/nn.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, et al. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–35. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- Cao X, et al. A functional study of miR-124 in the developing neural tube. Genes Dev. 2007;21:531–6. doi: 10.1101/gad.1519207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, et al. Noncoding RNAs in the Mammalian Central Nervous System. Annu Rev Neurosci. 2006 doi: 10.1146/annurev.neuro.29.051605.112839. [DOI] [PubMed] [Google Scholar]
- Cassel S, et al. Fluoxetine and cocaine induce the epigenetic factors MeCP2 and MBD1 in adult rat brain. Mol Pharmacol. 2006;70:487–92. doi: 10.1124/mol.106.022301. [DOI] [PubMed] [Google Scholar]
- Castren E, et al. Role of neurotrophic factors in depression. Curr Opin Pharmacol. 2007;7:18–21. doi: 10.1016/j.coph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Chen J, et al. Effects of lamotrigine and topiramate on hippocampal neurogenesis in experimental temporal-lobe epilepsy. Brain Res. 2009a doi: 10.1016/j.brainres.2009.12.024. [DOI] [PubMed] [Google Scholar]
- Chen M, et al. MRG15, a component of HAT and HDAC complexes, is essential for proliferation and differentiation of neural precursor cells. J Neurosci Res. 2009b;87:1522–31. doi: 10.1002/jnr.21976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng LC, et al. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci. 2009;12:399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi PS, et al. Members of the miRNA-200 family regulate olfactory neurogenesis. Neuron. 2008;57:41–55. doi: 10.1016/j.neuron.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–3. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conaco C, et al. Reciprocal actions of REST and a microRNA promote neuronal identity. Proc Natl Acad Sci U S A. 2006;103:2422–7. doi: 10.1073/pnas.0511041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepaldi L, Riccio A. Chromatin learns to behave. Epigenetics. 2009;4:23–6. doi: 10.4161/epi.4.1.7604. [DOI] [PubMed] [Google Scholar]
- Czeh B, et al. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci U S A. 2001;98:12796–801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani D, et al. Dicer inactivation leads to progressive functional and structural degeneration of the mouse retina. J Neurosci. 2008;28:4878–87. doi: 10.1523/JNEUROSCI.0828-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer SC. Postnatal and adult neurogenesis in the development of human disease. Neuroscientist. 2008;14:446–58. doi: 10.1177/1073858408317008. [DOI] [PubMed] [Google Scholar]
- David DJ, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–93. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TH, et al. Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. J Neurosci. 2008;28:4322–30. doi: 10.1523/JNEUROSCI.4815-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarolis NA, Eisch AJ. Hippocampal neurogenesis as a target for the treatment of mental illness: A critical evaluation. Neuropharmacology. doi: 10.1016/j.neuropharm.2009.12.013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K, Malenka RC. GABA excitation in the adult brain: a mechanism for excitation- neurogenesis coupling. Neuron. 2005;47:775–7. doi: 10.1016/j.neuron.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, et al. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–52. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- Deng W, et al. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009;29:13532–42. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deo M, et al. Detection of mammalian microRNA expression by in situ hybridization with RNA oligonucleotides. Dev Dyn. 2006;235:2538–48. doi: 10.1002/dvdy.20847. [DOI] [PubMed] [Google Scholar]
- Doi M, et al. Circadian regulator CLOCK is a histone acetyltransferase. Cell. 2006;125:497–508. doi: 10.1016/j.cell.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Donovan MH, et al. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer’s disease. J Comp Neurol. 2006;495:70–83. doi: 10.1002/cne.20840. [DOI] [PubMed] [Google Scholar]
- Duan X, et al. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–58. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, et al. Spatial relational memory requires hippocampal adult neurogenesis. PLoS ONE. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, et al. Adult neurogenesis, mental health, and mental illness: hope or hype? J Neurosci. 2008;28:11785–91. doi: 10.1523/JNEUROSCI.3798-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan G, et al. DNA hypomethylation perturbs the function and survival of CNS neurons in postnatal animals. J Neurosci. 2001;21:788–97. doi: 10.1523/JNEUROSCI.21-03-00788.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan G, et al. DNA methylation controls the timing of astrogliogenesis through regulation of JAK-STAT signaling. Development. 2005;132:3345–56. doi: 10.1242/dev.01912. [DOI] [PubMed] [Google Scholar]
- Fasano CA, et al. Bmi-1 cooperates with Foxg1 to maintain neural stem cell self-renewal in the forebrain. Genes Dev. 2009;23:561–74. doi: 10.1101/gad.1743709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, et al. The Evf-2 noncoding RNA is transcribed from the Dlx-5/6 ultraconserved region and functions as a Dlx-2 transcriptional coactivator. Genes Dev. 2006;20:1470–84. doi: 10.1101/gad.1416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, et al. Dynamic expression of de novo DNA methyltransferases Dnmt3a and Dnmt3b in the central nervous system. J Neurosci Res. 2005;79:734–46. doi: 10.1002/jnr.20404. [DOI] [PubMed] [Google Scholar]
- Gao Z, et al. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat Neurosci. 2009;12:1090–2. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthe A, et al. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS ONE. 2009;4:e5464. doi: 10.1371/journal.pone.0005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–93. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geha S, et al. NG2+/Olig2+ Cells Are the Major Cycle-Related Cell Population of the Adult Human Normal Brain. Brain Pathol. 2009 doi: 10.1111/j.1750-3639.2009.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring M, et al. DNA demethylation by DNA repair. Trends Genet. 2009;25:82–90. doi: 10.1016/j.tig.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Gould A. Functions of mammalian Polycomb group and trithorax group related genes. Curr Opin Genet Dev. 1997;7:488–94. doi: 10.1016/s0959-437x(97)80075-5. [DOI] [PubMed] [Google Scholar]
- Guan JS, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot F. Cell fate specification in the mammalian telencephalon. Prog Neurobiol. 2007;83:37–52. doi: 10.1016/j.pneurobio.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Hao Y, et al. Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J Neurosci. 2004;24:6590–6599. doi: 10.1523/JNEUROSCI.5747-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, Shen Y. Interruption of beta-catenin signaling reduces neurogenesis in Alzheimer’s disease. J Neurosci. 2009;29:6545–57. doi: 10.1523/JNEUROSCI.0421-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Rabaza V, et al. Inhibition of adult hippocampal neurogenesis disrupts contextual learning but spares spatial working memory, long-term conditional rule retention and spatial reversal. Neuroscience. 2009;159:59–68. doi: 10.1016/j.neuroscience.2008.11.054. [DOI] [PubMed] [Google Scholar]
- Hester ME, et al. Two factor reprogramming of human neural stem cells into pluripotency. PLoS One. 2009;4:e7044. doi: 10.1371/journal.pone.0007044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick KA, et al. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2008;33:406–17. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- Hsieh J, et al. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc Natl Acad Sci U S A. 2004;101:16659–64. doi: 10.1073/pnas.0407643101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I, et al. Continuous neurogenesis in the adult brain. Dev Growth Differ. 2009;51:379–86. doi: 10.1111/j.1440-169X.2009.01094.x. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–61. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–54. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Jagasia R, et al. GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J Neurosci. 2009;29:7966–77. doi: 10.1523/JNEUROSCI.1054-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaholkowski P, et al. New hippocampal neurons are not obligatory for memory formation; cyclin D2 knockout mice with no adult brain neurogenesis show learning. Learn Mem. 2009;16:439–51. doi: 10.1101/lm.1459709. [DOI] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Jessberger S, et al. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009a;16:147–54. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, et al. Making a neuron: Cdk5 in embryonic and adult neurogenesis. Trends Neurosci. 2009b;32:575–82. doi: 10.1016/j.tins.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, et al. Epigenetic modulation of seizure-induced neurogenesis and cognitive decline. J Neurosci. 2007;27:5967–75. doi: 10.1523/JNEUROSCI.0110-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, et al. Enhanced neurogenesis in Alzheimer’s disease transgenic (PDGF-APPSw, Ind) mice. Proc Natl Acad Sci U S A. 2004a;101:13363–7. doi: 10.1073/pnas.0403678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, et al. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2004b;101:343–7. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, et al. Cerebral neurogenesis is induced by intranasal administration of growth factors. Ann Neurol. 2003;53:405–9. doi: 10.1002/ana.10506. [DOI] [PubMed] [Google Scholar]
- Jin K, et al. Alzheimer’s disease drugs promote neurogenesis. Brain Res. 2006;1085:183–8. doi: 10.1016/j.brainres.2006.02.081. [DOI] [PubMed] [Google Scholar]
- Jin K, et al. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–50. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SG, et al. GADD45A does not promote DNA demethylation. PLoS Genet. 2008;4:e1000013. doi: 10.1371/journal.pgen.1000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MA, et al. Cell-intrinsic signals that regulate adult neurogenesis in vivo: insights from inducible approaches. BMB Rep. 2009;42:245–59. doi: 10.5483/bmbrep.2009.42.5.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HJ, et al. Base excision DNA repair defect in Gadd45a-deficient cells. Oncogene. 2007;26:7517–25. doi: 10.1038/sj.onc.1210557. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, et al. ATF-2 has intrinsic histone acetyltransferase activity which is modulated by phosphorylation. Nature. 2000;405:195–200. doi: 10.1038/35012097. [DOI] [PubMed] [Google Scholar]
- Kempermann G, et al. The contribution of failing adult hippocampal neurogenesis to psychiatric disorders. Curr Opin Psychiatry. 2008;21:290–5. doi: 10.1097/YCO.0b013e3282fad375. [DOI] [PubMed] [Google Scholar]
- Kim J, et al. A MicroRNA feedback circuit in midbrain dopamine neurons. Science. 2007;317:1220–4. doi: 10.1126/science.1140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JB, et al. Direct reprogramming of human neural stem cells by OCT4. Nature. 2009a doi: 10.1038/nature08436. [DOI] [PubMed] [Google Scholar]
- Kim JB, et al. Oct4-induced pluripotency in adult neural stem cells. Cell. 2009b;136:411–9. doi: 10.1016/j.cell.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Kim JB, et al. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature. 2008;454:646–50. doi: 10.1038/nature07061. [DOI] [PubMed] [Google Scholar]
- Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;31:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Ko HG, et al. Effect of ablated hippocampal neurogenesis on the formation and extinction of contextual fear memory. Mol Brain. 2009;2:1. doi: 10.1186/1756-6606-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K. Targeting the hippocampal mossy fiber synapse for the treatment of psychiatric disorders. Mol Neurobiol. 2009;39:24–36. doi: 10.1007/s12035-008-8049-5. [DOI] [PubMed] [Google Scholar]
- Kohyama J, et al. Epigenetic regulation of neural cell differentiation plasticity in the adult mammalian brain. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0808417105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik KS. The neuronal microRNA system. Nat Rev Neurosci. 2006;7:911–20. doi: 10.1038/nrn2037. [DOI] [PubMed] [Google Scholar]
- Kosik KS, Krichevsky AM. The Elegance of the MicroRNAs: A Neuronal Perspective. Neuron. 2005;47:779–82. doi: 10.1016/j.neuron.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Kuruba R, et al. Hippocampal neurogenesis and neural stem cells in temporal lobe epilepsy. Epilepsy Behav. 2009;14(Suppl 1):65–73. doi: 10.1016/j.yebeh.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara T, et al. A small modulatory dsRNA specifies the fate of adult neural stem cells. Cell. 2004;116:779–93. doi: 10.1016/s0092-8674(04)00248-x. [DOI] [PubMed] [Google Scholar]
- Lagos-Quintana M, et al. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12:735–9. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- Lepagnol-Bestel AM, et al. Nrsf silencing induces molecular and subcellular changes linked to neuronal plasticity. Neuroreport. 2007;18:441–6. doi: 10.1097/WNR.0b013e328011dc81. [DOI] [PubMed] [Google Scholar]
- Lessard J, et al. An essential switch in subunit composition of a chromatin remodeling complex during neural development. Neuron. 2007;55:201–15. doi: 10.1016/j.neuron.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Gould E. Structural plasticity and hippocampal function. Annu Rev Psychol. 61:111–40. C1–3. doi: 10.1146/annurev.psych.093008.100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JM, et al. Evidence that DNA (cytosine-5) methyltransferase regulates synaptic plasticity in the hippocampus. J Biol Chem. 2006;281:15763–73. doi: 10.1074/jbc.M511767200. [DOI] [PubMed] [Google Scholar]
- Li B, et al. Failure of neuronal maturation in Alzheimer disease dentate gyrus. J Neuropathol Exp Neurol. 2008a;67:78–84. doi: 10.1097/nen.0b013e318160c5db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, et al. Epigenetic regulation of the stem cell mitogen Fgf-2 by Mbd1 in adult neural stem/progenitor cells. J Biol Chem. 2008b;283:27644–52. doi: 10.1074/jbc.M804899200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie DC, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–5. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- Lim DA, et al. Chromatin remodelling factor Mll1 is essential for neurogenesis from postnatal neural stem cells. Nature. 2009;458:529–33. doi: 10.1038/nature07726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DA, et al. In vivo transcriptional profile analysis reveals RNA splicing and chromatin remodeling as prominent processes for adult neurogenesis. Mol Cell Neurosci. 2006;31:131–48. doi: 10.1016/j.mcn.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Lim LP, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–73. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- Liu Q, et al. Repeated clomipramine treatment reversed the inhibition of cell proliferation in adult hippocampus induced by chronic unpredictable stress. Pharmacogenomics J. 2008a;8:375–83. doi: 10.1038/sj.tpj.6500485. [DOI] [PubMed] [Google Scholar]
- Liu X, et al. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci. 2005;8:1179–87. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YW, et al. Doublecortin expression in the normal and epileptic adult human brain. Eur J Neurosci. 2008b;28:2254–65. doi: 10.1111/j.1460-9568.2008.06518.x. [DOI] [PubMed] [Google Scholar]
- Liu YW, et al. Adult neurogenesis in mesial temporal lobe epilepsy: a review of recent animal and human studies. Curr Pharm Biotechnol. 2007;8:187–94. doi: 10.2174/138920107780906504. [DOI] [PubMed] [Google Scholar]
- Luger K, Richmond TJ. The histone tails of the nucleosome. Curr Opin Genet Dev. 1998;8:140–146. doi: 10.1016/s0959-437x(98)80134-2. [DOI] [PubMed] [Google Scholar]
- Ma DK, et al. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323:1074–7. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeyev EV, et al. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell. 2007;27:435–48. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, et al. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–10. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganas LN, et al. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 2007;318:980–5. doi: 10.1126/science.1147851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji HK, et al. Clinical and preclinical evidence for the neurotrophic effects of mood stabilizers: implications for the pathophysiology and treatment of manic-depressive illness. Biol Psychiatry. 2000;48:740–54. doi: 10.1016/s0006-3223(00)00979-3. [DOI] [PubMed] [Google Scholar]
- Mao Y, et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136:1017–31. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetto MC, et al. Transcriptional signature and memory retention of human-induced pluripotent stem cells. PLoS One. 2009;4:e7076. doi: 10.1371/journal.pone.0007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick JS, Makunin IV. Small regulatory RNAs in mammals. Hum Mol Genet. 2005;14(Spec No 1):R121–32. doi: 10.1093/hmg/ddi101. [DOI] [PubMed] [Google Scholar]
- Mehler MF. Epigenetic principles and mechanisms underlying nervous system functions in health and disease. Prog Neurobiol. 2008;86:305–41. doi: 10.1016/j.pneurobio.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler MF, Mattick JS. Noncoding RNAs and RNA editing in brain development, functional diversification, and neurological disease. Physiol Rev. 2007;87:799–823. doi: 10.1152/physrev.00036.2006. [DOI] [PubMed] [Google Scholar]
- Metivier R, et al. Cyclical DNA methylation of a transcriptionally active promoter. Nature. 2008;452:45–50. doi: 10.1038/nature06544. [DOI] [PubMed] [Google Scholar]
- Millar JK, et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet. 2000;9:1415–23. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–50. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Miyoshi K, et al. Disrupted-In-Schizophrenia 1, a candidate gene for schizophrenia, participates in neurite outgrowth. Mol Psychiatry. 2003;8:685–94. doi: 10.1038/sj.mp.4001352. [DOI] [PubMed] [Google Scholar]
- Molofsky AV, et al. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–7. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery RL, et al. Histone deacetylases 1 and 2 control the progression of neural precursors to neurons during brain development. Proc Natl Acad Sci U S A. 2009;106:7876–81. doi: 10.1073/pnas.0902750106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JA, et al. DISC1 (Disrupted-In-Schizophrenia 1) is a centrosome-associated protein that interacts with MAP1A, MIPT3, ATF4/5 and NUDEL: regulation and loss of interaction with mutation. Hum Mol Genet. 2003;12:1591–608. doi: 10.1093/hmg/ddg162. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosimann C, et al. Beta-catenin hits chromatin: regulation of Wnt target gene activation. Nat Rev Mol Cell Biol. 2009;10:276–86. doi: 10.1038/nrm2654. [DOI] [PubMed] [Google Scholar]
- Nakashima K, et al. Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science. 1999;284:479–482. doi: 10.1126/science.284.5413.479. [DOI] [PubMed] [Google Scholar]
- Namba T, et al. The Alzheimer’s disease drug memantine increases the number of radial glia-like progenitor cells in adult hippocampus. Glia. 2009;57:1082–90. doi: 10.1002/glia.20831. [DOI] [PubMed] [Google Scholar]
- Namihira M, et al. Committed neuronal precursors confer astrocytic potential on residual neural precursor cells. Dev Cell. 2009;16:245–55. doi: 10.1016/j.devcel.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Namihira M, et al. Developmental stage dependent regulation of DNA methylation and chromatin modification in a immature astrocyte specific gene promoter. FEBS Lett. 2004;572:184–8. doi: 10.1016/j.febslet.2004.07.029. [DOI] [PubMed] [Google Scholar]
- Newton SS, Duman RS. Chromatin remodeling: a novel mechanism of psychotropic drug action. Mol Pharmacol. 2006;70:440–3. doi: 10.1124/mol.106.027078. [DOI] [PubMed] [Google Scholar]
- Noonan MA, et al. Reduction of Adult Hippocampal Neurogenesis Confers Vulnerability in an Animal Model of Cocaine Addiction. J Neurosci. doi: 10.1523/JNEUROSCI.4256-09.2010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nott A, et al. S-Nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature. 2008;455:411–5. doi: 10.1038/nature07238. [DOI] [PubMed] [Google Scholar]
- Okano H, Temple S. Cell types to order: temporal specification of CNS stem cells. Curr Opin Neurobiol. 2009;19:112–9. doi: 10.1016/j.conb.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Ooi SK, Bestor TH. The colorful history of active DNA demethylation. Cell. 2008;133:1145–8. doi: 10.1016/j.cell.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Palmer TD, et al. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–94. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Parent JM, et al. Is neurogenesis reparative after status epilepticus? Epilepsia. 2007;48(Suppl 8):69–71. doi: 10.1111/j.1528-1167.2007.01355.x. [DOI] [PubMed] [Google Scholar]
- Park IH, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–6. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- Perera TD, et al. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci. 2007;27:4894–901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera TD, et al. Cognitive role of neurogenesis in depression and antidepressant treatment. Neuroscientist. 2008;14:326–38. doi: 10.1177/1073858408317242. [DOI] [PubMed] [Google Scholar]
- Rai K, et al. DNA demethylation in zebrafish involves the coupling of a deaminase, a glycosylase, and gadd45. Cell. 2008;135:1201–12. doi: 10.1016/j.cell.2008.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif A, et al. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11:514–22. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- Renthal W, et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56:517–29. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends Mol Med. 2008;14:341–50. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revest JM, et al. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry. 2009 doi: 10.1038/mp.2009.15. [DOI] [PubMed] [Google Scholar]
- Riggs AD, Porter TN. Overview of epigenetic mechanisms. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 1996. [Google Scholar]
- Ringrose L, Paro R. Epigenetic regulation of cellular memory by the Polycomb and Trithorax group proteins. Annu Rev Genet. 2004;38:413–43. doi: 10.1146/annurev.genet.38.072902.091907. [DOI] [PubMed] [Google Scholar]
- Saini HK, et al. Annotation of mammalian primary microRNAs. BMC Genomics. 2008;9:564. doi: 10.1186/1471-2164-9-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini HK, et al. Genomic analysis of human microRNA transcripts. Proc Natl Acad Sci U S A. 2007;104:17719–24. doi: 10.1073/pnas.0703890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairanen M, et al. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25:1089–94. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–9. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–35. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Is impaired neurogenesis relevant to the affective symptoms of depression? Biol Psychiatry. 2004;56:137–9. doi: 10.1016/j.biopsych.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Schaefer A, et al. Cerebellar neurodegeneration in the absence of microRNAs. J Exp Med. 2007;204:1553–8. doi: 10.1084/jem.20070823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanzer A, et al. Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol. 2004;14:237–48. doi: 10.1111/j.1750-3639.2004.tb00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Hen R. Neuroscience. Is more neurogenesis always better? Science. 2007;315:336–8. doi: 10.1126/science.1138711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber SL, Bernstein BE. Signaling network model of chromatin. Cell. 2002;111:771–8. doi: 10.1016/s0092-8674(02)01196-0. [DOI] [PubMed] [Google Scholar]
- Schuurmans C, Guillemot F. Molecular mechanisms underlying cell fate specification in the developing telencephalon. Curr Opin Neurobiol. 2002;12:26–34. doi: 10.1016/s0959-4388(02)00286-6. [DOI] [PubMed] [Google Scholar]
- Shamovsky I, Nudler E. Gene control by large noncoding RNAs. Sci STKE 2006. 2006:pe40. doi: 10.1126/stke.3552006pe40. [DOI] [PubMed] [Google Scholar]
- Sharma RP. Schizophrenia, epigenetics and ligand-activated nuclear receptors: a framework for chromatin therapeutics. Schizophr Res. 2005;72:79–90. doi: 10.1016/j.schres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Shen Q, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–40. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Shen Q, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shihabuddin LS, et al. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J Neurosci. 2000;20:8727–35. doi: 10.1523/JNEUROSCI.20-23-08727.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer BH, et al. Conditional ablation and recovery of forebrain neurogenesis in the mouse. J Comp Neurol. 2009;514:567–82. doi: 10.1002/cne.22052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smrt RD, et al. Mecp2 deficiency leads to delayed maturation and altered gene expression in hippocampal neurons. Neurobiol Dis. 2007;27:77–89. doi: 10.1016/j.nbd.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldner F, et al. Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136:964–77. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, et al. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002a;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Song HJ, et al. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat Neurosci. 2002b;5:438–45. doi: 10.1038/nn844. [DOI] [PubMed] [Google Scholar]
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- Suh H, et al. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell. 2007;1:515–28. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhonen JO, et al. Differentiation of adult hippocampus-derived progenitors into olfactory neurons in vivo. Nature. 1996;383:624–7. doi: 10.1038/383624a0. [DOI] [PubMed] [Google Scholar]
- Sun Y, et al. Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell. 2001;104:365–376. doi: 10.1016/s0092-8674(01)00224-0. [DOI] [PubMed] [Google Scholar]
- Surget A, et al. Drug-Dependent Requirement of Hippocampal Neurogenesis in a Model of Depression and of Antidepressant Reversal. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Experience-dependent epigenetic modifications in the central nervous system. Biol Psychiatry. 2009;65:191–7. doi: 10.1016/j.biopsych.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takizawa T, et al. DNA methylation is a critical cell-intrinsic determinant of astrocyte differentiation in the fetal brain. Dev Cell. 2001;1:749–758. doi: 10.1016/s1534-5807(01)00101-0. [DOI] [PubMed] [Google Scholar]
- Tashiro A, et al. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442:929–33. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- Tavazoie M, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–88. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple S. The development of neural stem cells. Nature. 2001;414:112–7. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- Tomita K, et al. Mammalian achaete-scute and atonal homologs regulate neuronal versus glial fate determination in the central nervous system. Embo J. 2000;19:5460–72. doi: 10.1093/emboj/19.20.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozuka Y, et al. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–15. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Tran H, et al. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science. 2002;296:530–4. doi: 10.1126/science.1068712. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, et al. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–25. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, et al. Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J Neurosci. 2004;24:5603–10. doi: 10.1523/JNEUROSCI.0589-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimura K, et al. Neuronal differentiation of neural precursor cells is promoted by the methyl-CpG-binding protein MeCP2. Exp Neurol. 2009;219:104–11. doi: 10.1016/j.expneurol.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Turner BM. Histone acetylation and an epigenetic code. Bioessays. 2000;22:836–45. doi: 10.1002/1521-1878(200009)22:9<836::AID-BIES9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Umka J, et al. Valproic acid reduces spatial working memory and cell proliferation in the hippocampus. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.11.073. [DOI] [PubMed] [Google Scholar]
- van Praag H, et al. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada A. Lithium and neuropsychiatric therapeutics: neuroplasticity via glycogen synthase kinase-3beta, beta-catenin, and neurotrophin cascades. J Pharmacol Sci. 2009;110:14–28. doi: 10.1254/jphs.09r02cr. [DOI] [PubMed] [Google Scholar]
- Wernig M, et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proc Natl Acad Sci U S A. 2008;105:5856–61. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Sun YE. Reversing DNA methylation: new insights from neuronal activity-induced Gadd45b in adult neurogenesis. Sci Signal. 2009;2:pe17. doi: 10.1126/scisignal.264pe17. [DOI] [PubMed] [Google Scholar]
- Wu JI, et al. Regulation of dendritic development by neuron-specific chromatin remodeling complexes. Neuron. 2007;56:94–108. doi: 10.1016/j.neuron.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, et al. Repetitive cocaine administration decreases neurogenesis in adult rat hippocampus. Ann N Y Acad Sci. 2004;1025:351–62. doi: 10.1196/annals.1316.043. [DOI] [PubMed] [Google Scholar]
- Yoo AS, et al. MicroRNA-mediated switching of chromatin-remodelling complexes in neural development. Nature. 2009;460:642–6. doi: 10.1038/nature08139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu IT, et al. Valproic acid promotes neuronal differentiation by induction of proneural factors in association with H4 acetylation. Neuropharmacology. 2009a;56:473–80. doi: 10.1016/j.neuropharm.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Yu Y, et al. Increased hippocampal neurogenesis in the progressive stage of Alzheimer’s disease phenotype in an APP/PS1 double transgenic mouse model. Hippocampus. 2009b doi: 10.1002/hipo.20587. [DOI] [PubMed] [Google Scholar]
- Zhang C, et al. Long-lasting impairment in hippocampal neurogenesis associated with amyloid deposition in a knock-in mouse model of familial Alzheimer’s disease. Exp Neurol. 2007;204:77–87. doi: 10.1016/j.expneurol.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, et al. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–60. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhao C, et al. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, et al. Mice lacking methyl-CpG binding protein 1 have deficits in adult neurogenesis and hippocampal function. Proc Natl Acad Sci U S A. 2003;100:6777–82. doi: 10.1073/pnas.1131928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK. Active DNA demethylation mediated by DNA glycosylases. Annu Rev Genet. 2009;43:143–66. doi: 10.1146/annurev-genet-102108-134205. [DOI] [PMC free article] [PubMed] [Google Scholar]