Abstract
A hallmark of graft-versus-host-disease (GVHD), a life-threatening complication after allogeneic hematopoietic stem cell transplantation, is the cytopathic injury of host tissues mediated by persistent alloreactive effector T cells (TE). However, the mechanisms that regulate the persistence of alloreactive TE during GVHD remain largely unknown. Using mouse GVHD models, we demonstrate that alloreactive CD8+ TE rapidly diminished in vivo when adoptively transferred into irradiated secondary congenic recipient mice. In contrast, although alloreactive CD8+ TE underwent massive apoptosis upon chronic exposure to alloantigens, they proliferated in vivo in secondary allogeneic recipients, persisted and caused severe GVHD. Thus, the continuous proliferation of alloreactive CD8+ TE, which is mediated by alloantigenic stimuli rather than homeostatic factors, is critical to maintaining their persistence. Gene expression profile analysis revealed that while alloreactive CD8+ TE increased the expression of genes associated with cell death, they activated a group of stem cell genes normally expressed in embryonic and neural stem cells. Most of these stem cell genes are associated with cell cycle regulation, DNA replication, chromatin modification and transcription. One of these genes, Ezh2, which encodes a chromatin modifying enzyme, was abundantly expressed in CD8+ TE. Silencing Ezh2 significantly reduced the proliferation of alloantigen-activated CD8+ T cells. Thus, these findings identify that a group of stem cell genes could play important roles in sustaining terminally differentiated alloreactive CD8+ TE and may be therapeutic targets for controlling GVHD.
Introduction
Upon antigen-presenting cell (APC) activation, T cells are “programmed” to undergo clonal expansion, generating large numbers of effector T cells (TE) while contracting to minimize their potentially lethal activity (1-6). Consequently, the majority of CD8+ TE (∼95%) may die after clearance of the antigen, with some memory T cells surviving contraction (4, 6-8). However, chronically activated TE can be continually generated during chronic inflammatory conditions, such as responses to chronic infections, autoantigens and alloantigens. A unique clinical example is graft-versus-host disease (GVHD), a life-threatening complication after allogeneic hematopoietic stem cell transplantation (HSCT) (9-13). A hallmark of GVHD is the cytopathic injury mediated by persistent alloreactive TE, which can occur within weeks and persist for years after transplantation (10-15). GVHD therapy which typically targets TE have disappointing response rates(∼40%) (16). However, the molecular mechanisms that regulate the persistence of alloreactive T cells during GVHD remain largely unknown.
Emerging evidence indicates that a group of stem cell signals may play important roles in antigen-experienced memory T cells. CD8+ memory T cells have the ability to self-renew to survive the lifetime of an individual and can rapidly generate protective TE upon antigenic rechallenge (1-5). Gene expression profile analysis reveals that CD8+ memory T cells and long-term hematopoietic stem cells (HSCs) share a self-renewal transcriptional program (17). Furthermore, antigen-stimulated CD8+ T cells undergo an asymmetrical division to regulate the generation of long-term memory T cells (18). Thus, memory T cells are considered to be stem cell-like cells (1, 3-4, 19). Interestingly, Wnt/β-catenin signaling, which is essential for proliferation and self-renewal of adult stem cells (20), has been shown to regulate the generation of CD44loCD62LhiCD122hiBcl-2hiSca-1hi CD8+ T memory stem cells (TMSC) (21). These CD8+ TMSC have greater ability than either CD44hiCD62Lhi central memory (TCM) or CD44hiCD62Llo effector memory T cells (TEM) to proliferate and generate TE, thereby destroying tumors (21). This supports our previous observation that CD8+ TMSC are important for sustaining alloreactive TE mediating GVHD (15). However, these data do not explain why alloreactive CD8+ TE can persist and cause severe GVHD in secondary recipients (14-15). Given that TE and memory T cells are developmentally linked to each other (1-6, 22), we asked whether alloreactive TE exposure to chronic alloantigens proliferate and persist through reactivation of distinct families of stem cell genes.
Using mouse models of human GVHD directed against minor histocompatibility antigens (miHAs), we demonstrate that alloantigenic stimuli rather than homeostatic factors are critical to sustaining continuous proliferation of alloreactive CD8+ TE to counteract their massive apoptotic death. We found that a group of stem cell genes normally expressed in embryonic stem cells (ESCs) and neural stem cells (NSCs) was activated in these proliferating alloreactive CD8+ TE upon chronic exposure to alloantigens. Most of these stem cell genes are associated with DNA replication, cell cycle regulation, chromatin modification and transcription. Silencing one of these genes, Ezh2, which encodes an enzyme with methyltransferase activity, inhibited the proliferation of alloantigen-activated T cells. Thus, these stem cell genes could be important therapeutic targets for modulating allogeneic T cell responses and GVHD.
Materials and Methods
Mice
We purchased C57BL/6 (B6; H-2Db, CD45.2+), B6.SJL-Ptprca (B6/SJL, H-2Db, CD45.1+), C3H.SW (H-2Db, CD45.2+ and Ly9.1+) mice, BALB/b (H-2Db, CD45.2+), B6.β2 microglobulin gene-deficient mice (B6.B2M-/-) and BALB/c (H-2Dd, CD45.2+) from Jackson Laboratory (Maine, USA). We supplied transplant recipients with drinking water containing neomycin sulfate and polymyxin B (Sigma) as previously described (23). The Institutional Animal Care and Use Committee of the University of Michigan approved all mouse protocols.
Antibodies, cell lines, cytokines and flow cytometry analysis
All antibodies (Abs) used for immunofluorescence staining were obtained from BD Bioscience Pharmingen. Microbead-conjugated Abs and streptavidin were purchased from Miltenyi-Biotech, and all recombinant cytokines including IL-2, IL-4, IL-15, granulocyte-monocyte colony-stimulating factor (GM-CSF), stem cell factor (SCF) and tumor necrosis factor-α (TNF-α) were from R&D Systems. miHA peptide H60 / MHC-I dimmers were prepared by conjugating H60 peptide to MHC-I dimmers as instructed by the manufacturers (BD Bioscience). We performed immunofluorescence analyses of cell surface phenotypes and intracellular cytokines using FACScan and Canto cytometer (Becton Dickinson) as previously described (23).
For 5-bromo-2′-deoxyuridine (BrdU) incorporation experiments, mice were given sterile drinking water containing 0.8 mg/ml BrdU (Sigma) for 3 days. BrdU labeling was performed as previously described (24). In brief, after surface staining, cells were resuspended in cold 0.15 NaCl, fixed by addition of cold 95% ethanol, incubated for 30 minutes on ice, and washed with PBS. The cells were then fixed using fixation solution from BD Cytofix/Cytoperm™ Kit (BD Bioscience) for 30 minutes, pelleted, and then incubated at 37°C for 30 minutes with 50KU of DNase I (Sigma) in 0.15 NaCl and 4.2 mM MgCl2, pH5. After washing, cells were stained with FITC-conjugated anti-BrdU for 60 minutes at room temperature, followed by flow cytometry analysis.
Cell preparations
T cell-depleted bone marrow (TCD BM) was prepared by incubating donor BM with microbead-conjugated anti-CD4 Ab and anti-CD8 Ab as previously described (23). CD8+ T cells were magnetically isolated from spleens and lymph nodes of mice using microbead-conjugated anti-CD8 Ab (MiniMACS, Miltenyi Biotech). CD8+ T cell subsets were further separated using fluorescence activated cell sorter (MoFlo, Beckman Coulter). The purity of each sorted T cell subset was consistently more than 92%. Donor CD8+ T cells were labeled with fluorescent dye carboxyfluorescein diacetate succinimidyl ester (CFSE) as described (23). We prepared mature DCs from B6 BM as described (25).
GVHD induction
Mice underwent allogeneic BMT as previously described (23). Briefly, for the C3H.SW anti-B6 mouse GVHD model, we irradiated B6/SJL recipients using a split-dose totaling 10.0Gys from a 137Cs source. We mixed donor C3H.SW TCD BM (5 × 106) with or without C3H.SW CD44loCD62Lhi CD8+ naïve T cells (TN) (2.0 × 106) and transplanted into lethally irradiated B6/SJL recipients (4 to 8 mice per group per experiment). In some experiments, donor C3H.SW CD44loCD62Lhi CD8+TN (2.0 × 106) were transplanted together with B6/SJL TCD BM (0.5 × 106) into lethally irradiated B6/SJL recipients. In the B6/SJL anti-BALB/b mouse GVHD model, we mixed B6 TCD BM (5 × 106) with or without CFSE-labeled B6/SJL CD44loCD62Lhi CD4+ and CD8+ TN (2.5 × 106 T cells for each) and transplanted into lethally irradiated BALB/b recipients.
Array based mRNA assays
In three repeated experiments, donor alloreactive day-14 CD8+ TMSC and TE were highly purified, respectively, from 4 B6/SJL mice receiving donor CD44loCD62Lhi CD8+ TN derived from 6 C3H.SW mice. Donor CD44loCD62Lhi CD8+ TN were highly purified from pooled CD8+ T cells of 2 C3H.SW mice in three separate experiments. Total RNA was prepared from these T cell subsets using TRIzol (Invitrogen Life Technologies, Carlsbad, CA). Biotinylated cDNA was prepared for each sample from 600 ng total RNA using two rounds of reverse-transcription and T7 promoter-based in vitro transcription following hybridization to the arrays. Hybridization, scanning and image analysis of the arrays were performed according to the manufacturer's protocol (Affymetrix, Santa Clara, CA). Mouse Genome 430A 2.0 Arrays containing 22690 probe sets (Affymetrix, Santa Clara, CA) were used to broadly compare the transcription profile of CD8+ TE and TMSC to that of TN. The array data are available from Gene Expression Omnibus, accession GSE13743. Using publicly available software (26), we computed trimmed averages of PM-MM differences for each probe set and quantile-normalized these after scaling the arrays to give average probe set values of 1500 units. We then log-transformed using log (max(x+50;0) + 50). Using a 1-way ANOVA model we selected transcripts that gave p<0.01 for comparing pairs of groups that also gave at least a 1.5-fold difference from the means for the paired groups, computed based on differences in the means of log-transformed data. We collapsed probe sets to 13142 distinct genes using Entrez gene IDs. Data can be viewed from the following website: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=tjunpugmcoqomxc&acc=GSE13743.
Western blot
Samples were separated on a 12% SDS-PAGE gel and transferred onto a nitrocellulose membrane (Millipore Corporation, MA). After an overnight incubation at 4°C with primary Abs, membranes were washed five times and probed with HRP-conjugated secondary Ab (Vector Laboratories, CA) for one hour at room temperature, and immunoreactivity was detected by chemiluminescence (GE Healthcare, NJ).
Real-time reverse transcription polymerase chain reaction (RT-PCR)
The total RNA was extracted from the sorted CD8+ T cell subsets using TRIzol (Invitrogen Life Technologies, Carlsbad, CA). Real-time RT-PCR was performed using a SYBR green PCR mix (ABI Biosystem, CA) in the Realplex2 Eppendorf Real-time PCR instrument (Eppendorf AG, Westbury, NY). Gene expression levels were calculated relative to the 18S gene. The primer sequences used for real-time RT-PCR include: 18S (5′-GCTGCTGGCACCAGACTT and 3′- CGGCTACCACATCCAAGG), Ifng (5′- ATGAACGCTACACACTGCATC and 3′- CCATCCTTTTGCCAGTTCCTC), Granzyme B (5′- CCACTCTCGACCCTACATGG and 3′- GGCCCCCAAAGTGACATTTATT), Ezh2 (5′- TGCCTCCTGAATGTACTCCAA and 3′- AGGGATGTAGGAAGCAGTCATAC), Tacc3(5′- GAGATGGGGAAGTCCGTTGATG and 3′- CTCTGCTTGGGCCTTGCTGTGT), Birc5 (5′- AACTACCGCATCGCCACCTTC and 3′- TTCTTCCATCTGCTTCTTGACA), Hells (5′- GGGGAGTACCTGGACCTTTTCTTG and 3′- CTGCAGTGTCCCTTGTCTTTTGTG), Pd1(5′- ACCCTGGTCATTCACTTGGG and 3′-CATTTGCTCCCTCTGACACTG), p18 (5′- GTAAACGTCAACGCTCAAAATGG and 3′- GAACCTGGCCAAGTCGAAGG), Casp4 (5′- ACAAACACCCTGACAAACCAC and 3′-CACTGCGTTCAGCATTGTTAAA), and Bcl2 (5′- GTCGCTACCGTCGTGACTTC and 3′-CAGACATGCACCTACCCAGC).
Lentiviral vector construction and viral production
Doxyclycline (Dox) regulated lentiviral vector pLVPT-rtTRKRAB2SM2 (pLVPToff) was obtained from Addgene (Cambridge, MA)(27). We cloned short-hairpin RNA duplex that specifically targets Ezh2 (Ezh2-shRNA, 5′CGCGTCCCCAAGAGGTTCAGAAGAGCTGTTCAAGAGACAGCTCTTCTGACTGAACCTCTTTTTTTGGAAAT3′) (28) into this pLVPToff, in which Ezh2-shRNA and GFP are separately driven by H1 promoter and phosphoglycerate kinase (PGK) promoter (named Ezh2-shRNA/GFP-pLVPToff), as previously described (27). Lentiviral vector encoding scrambled shRNA and GFP was generated as control (named Con-shRNA/GFP-pLVPToff). In the absence of Dox, Ezh2-shRNA and GFP will be induced, whereas addition of Dox will repress the expression the transcription of both shEzh2 and GFP (27). Production of lentiviruses was done in 293T cells as described (27).
In vivo reconstitution of T cells with inducible knockdown of Ezh2
C-kit+ hematopoietic cells were magnetically isolated from B6 mice and infected with Ezh2-shRNA-pLVPToff in vitro as previously described (29), followed by transplantation into lethally irradiated B6 mice. To repress the expression of Ezh2-shRNA in HSCs during their hematopoietic and thymic reconstitution, all of these recipient mice were given sterile water containing Dox (2mg/ml) from day -2 to 12 weeks after transplantation. HSCs infected with Control-shRNA-pLVPToff were transplanted as control. Twelve weeks after transplantation, Dox was removed from these mice to induce the expression of Ezh2-shRNA. Seven days later, CD8+ T cells were isolated from the spleens and lymph nodes of these mice. GFP+CD8+ T cells expressing Ezh2-shRNA (named Ezh2-shRNA GFP+CD8+ T cells) or Control shRNA (named Control-shRNA GFP+CD8+ T cells) were sorted using the BD FACSAria II cell sorter (BD, Bioscience).
Ex vivo stimulation of CD8+ T cells
Sorted Ezh2-shRNA GFP+CD8+ TN and Control-shRNA GFP+CD8+ TN were stimulated with anti-CD3 Ab and anti-CD28 Ab (2.5μg/ml for each) in 96 well plate as previously described (14-15, 30). In some experiments, unfractionated splenic mononuclear cells that contained Ezh2-shRNA GFP+CD8+ T cells were cultured in the presence of allogeneic DCs or IL-7. The recovery number of GFP+CD8+ T cells was assessed by flow cytometry analysis.
Statistical analysis
Survival in different groups was compared by using the log-rank analysis. Comparison of two means was analyzed using the two-tailed unpaired Student t test. Statistical analysis from the gene array is indicated in the text.
Results
Alloantigen-induced continuous proliferation is essential to maintaining chronically activated alloreactive CD8+ TE
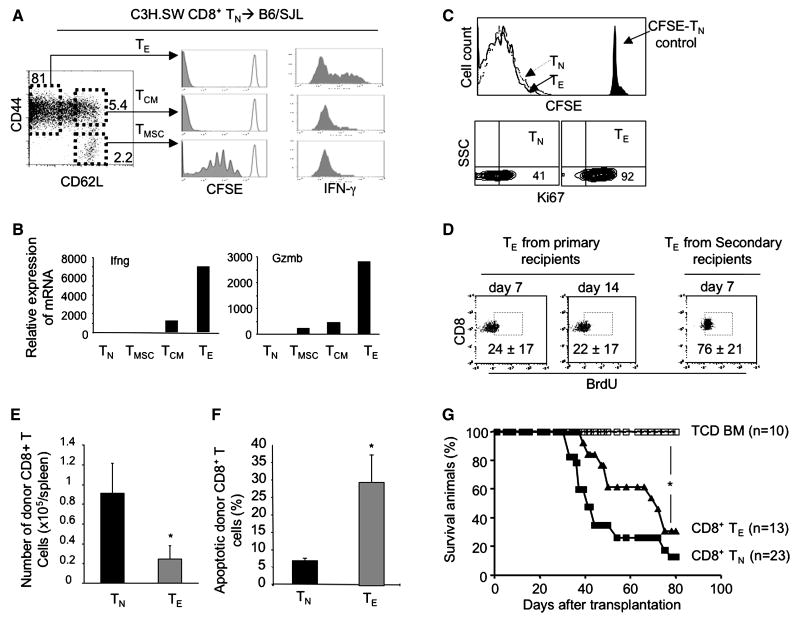
Since CD8+ TE are known to be “terminally differentiated” and short-lived cells (1-6), we first determined under what conditions alloreactive CD8+ TE were able to persist in vivo and cause GVHD. We transplanted CFSE-labeled donor CD8+ TN from normal C3H.SW mice together with B6/SJL TCD BM into lethally irradiated B6/SJL recipients. This allowed us to strictly track the fate of infused donor mature T cells while inducing GVHD (15). By day 14 after transplantation, donor alloreactive CD8+ TE became the dominant population (>80%) (named day-14 CD8+ TE, Fig.1A). They had undergone extensive division and produced significantly higher levels of IFN-γ and Granzyme B (Gzmb) (Fig.1A and B). When later adoptively transferred into lethally irradiated secondary allogeneic B6/SJL mice, all the day-14 CD8+ TE had extensively divided in vivo, expressed high levels of proliferating antigen Ki67 seven days after transplantation (Fig.1C). BrdU incorporation analysis showed that about 22% of day 14 CD8+ TE derived from primary GVHD recipient mice and 76% of alloreactive CD8+ TE recovered from secondary recipients of day 14 CD8+ TE had incorporated BrdU (Fig. 1D). These results suggest that a substantial proportion of day 14 CD8+ TE are dividing during GVH reaction.
Fig.1.
Alloreactive CD8+ TE are highly replicating cells causing GVHD. (A) CFSE-labeled donor CD8+ TN (2×106) derived from normal C3H.SW mice were transplanted with B6/SJL TCD BM (0.5×106) into lethally irradiated B6/SJL mice. Donor CD8+ T cells were recovered at day 14 after transplantation from the spleen and lymph node of 3 recipients, numerated and stained for flow cytometric analysis. Histograms show the cell division based on CFSE dilution and production of IFN-γ by CD8+ TMSC, TCM and TE. Data are representative of three independent experiments. (B) Real-time RT-PCR analysis shows the relative mRNA expression of selected genes in donor day-14 CD8+ TE, TCM and TMSC. Data are representative of three independent experiments. (C and D) Day-14 CD8+ TE were re-labeled with CFSE and adoptively transferred into lethally irradiated secondary B6/SJL mice. CFSE-labeled C3H.SW CD8+ TN were transferred as controls. Donor T cells were recovered at day 7 after adoptive transfer, numerated and analyzed for flow cytometry. Histogram shows the cell division of indicated cells. Non-stimulated CFSE-labeled CD8+ TN were used as non-dividing cell control. Contour plots show the expression of Ki67 (C). For BrdU labeling, secondary recipient mice were given drinking water containing BrdU for 3 days prior to the end of the experiments. At day 7, donor cells were recovered, stained with BrdU, and analyzed by flow cytometry. Dot plots show the labeling of BrdU in gated CD8+ TE population (Mean ± SD) (D). (E and F) As described above in (C), the number of donor T cells recovered from these secondary recipients (n=3 for each group) was calculated (E) and the percentage of apoptotic cell was shown (F). Data are representative of two independent experiments. (G) Donor C3H.SW TCD BM (5×106) were transplanted alone, or with donor day-14 CD8+ TE (0.5×106) and donor CD8+ TN (1.0×106) into lethally irradiated secondary B6/SJL mice. Survival of animals was analyzed with the Kaplan-Meier method. *, P<0.05, significant difference.
Interestingly, 4-fold fewer donor T cells were recovered from secondary recipients of day-14 CD8+ TE than that of donor CD8+ TN (Fig.1E). This was associated with significantly increased apoptotic death of donor T cells in day-14 CD8+ TE recipients as compared to CD8+ TN recipients (Fig.1F). Furthermore, adoptive transfer of these day-14 CD8+ TE caused GVHD in secondary recipients, with 70% of them dying from the disease by day 75 after transplantation (Fig.1G). Thus, upon chronic exposure to alloantigens, alloreactive CD8+ TE continuously proliferated to persist while undergoing increased apoptotic death. However, all alloreactive CD8+ TE diminished in vivo without causing GVHD when adoptively transferred into secondary B6.B2M-/- mice (data not shown). These results suggest that allogeneic stimuli could be essential to sustaining alloreactive CD8+ TE.
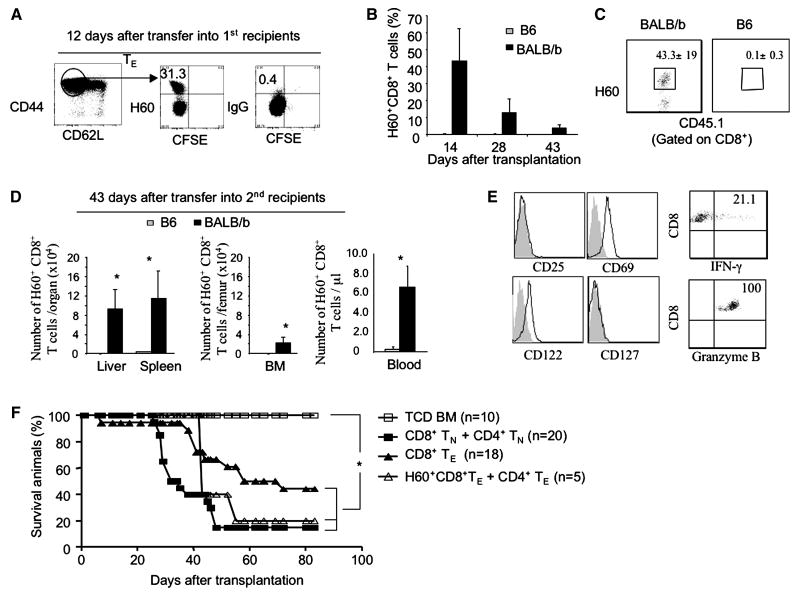
In lethally irradiated allogeneic recipient mice, both alloantigens and lymphopenia-related homeostatic factors (e.g., self-antigen peptides and cytokines IL7 and IL-15) (31) could be responsible for the proliferation of alloreactive CD8+ TE. To assess the possible impact of alloantigens versus homeostatic factors in regulating the persistence of alloreactive TE, we used the CD4+ T cell-dependent B6 anti-BALB/b GVHD model, in which miHA H60-specific (H60+) CD8+ TE could be tracked using miHA peptide/MHC class-I dimmer staining (Fig.2A)(14, 32-33). We highly purified H60+CD8+ TE at day 12 post-transplantation from GVHD BALB/b mice receiving donor B6/SJL TN (CD45.1), labeled with CFSE, and adoptively transferred together with B6/SJL CD4+ TE and donor B6 TCD BM into lethally irradiated congenic B6 (CD45.2+) mice and allogeneic BALB/b mice. Lethal irradiation of congenic B6 mice creates a lymphopenic environment that might induce homeostatic proliferation and survival of adoptively transferred day 12 B6/SJL CD8+ T cells in the absence of miHA H60, whereas lethally irradiated allogeneic BALB/b mice could provide persistent alloantigen-stimulation in addition to homeostatic factors.
Fig.2.
Proliferation and persistence of alloantigen-specific CD8+ TE depend on the presence of chronic alloantigen. (A) CFSE-labeled donor T cells (CD4+ and CD8+ T cells, 3×106 for each) from normal B6/SJL mice (CD45.1) were transplanted, together with B6 TCD BM (CD45.2, 5×106), into lethally irradiated (10Gys) primary BALB/b recipients (CD45.2). Dot plots show percent of host miHA H60-specific CD8+ TE that were recovered at day 12 after transplantation from spleens and livers of primary GVHD BALB/b recipients (n=4) of B6/SJLTCD BM and B6/SJL T cells. Data are representative of three independent experiments. (B, C and D) Donor alloreactive B6/SJL H60+CD8+ TE (5×104) and CD4+ T cells (3×105) were isolated 12 days after transplantation, respectively, from these primary GVHD BALB/b recipients (CD45.2) of B6/JL T cells, re-labeled with CFSE, and adoptively transferred together with B6 TCD BM (5×106) into lethally irradiated secondary BALB/b recipients and congenic B6 recipients (CD45.2), respectively. The percentage of donor alloreactive H60+CD8+ TE in the peripheral blood of these secondary recipients at day 14, 28 and 43 after adoptive transfer (B). Dot plots show the fraction of H60+CD8+ TE in the peripheral blood (C). Donor alloreactive H60+CD8+ TE recovered at day 43 from secondary recipients were calculated (D). Data are presented as mean ± SD. * P<0.01. (E) Histograms show the surface markers of donor H60+CD8+ TE isolated from the secondary allogeneic BALB/b mice (left), and dot plots show the production of IFN-γ and GZMB (right). Data shown in B, C, D and E are representative of two independent experiments. (F) Survival rate of animals was analyzed by the Kaplan-Meier Method. *, P<0.05, significant difference.
We found that donor H60+CD8+ TE were readily detected in the peripheral blood of secondary allogeneic BALB/b recipients (43.3±19%) by day 14 after transplantation, but not in that of secondary congenic B6 recipients (0.1±0.3%) (Fig.2B and C). Forty-three days after transfer, allogeneic BALB/b recipients showed about 80-fold more proliferating H60+CD8+ T cells than B6 congenic recipients (Fig.2D). These donor H60+CD8+ TE from secondary allogeneic BALB/b recipients expressed high levels of CD69, suggesting a recent antigenic stimulation (Fig.2E). Furthermore, they produced high levels of IFN-γ and Granzyme B (Fig.2E) and caused GVHD in these secondary allogeneic BALB/b mice (Fig.2F). In separate experiments, we observed that adoptive transfer of donor alloreactive CD8+ TE alone also caused GVHD in secondary allogeneic BALB/b mice (Fig.2F), suggesting that CD4-help is not essential to already differentiated CD8+ TE to mediate GVHD. None of these congenic B6 mice receiving donor alloreactive CD8+ TE developed clinical signs of GVHD (data not shown). Thus, alloantigenic stimuli rather than homeostatic factors are critical to the continual proliferation and persistence of alloreactive CD8+ TE during GVH reaction.
Gene expression profiles of alloreactive CD8+ TE
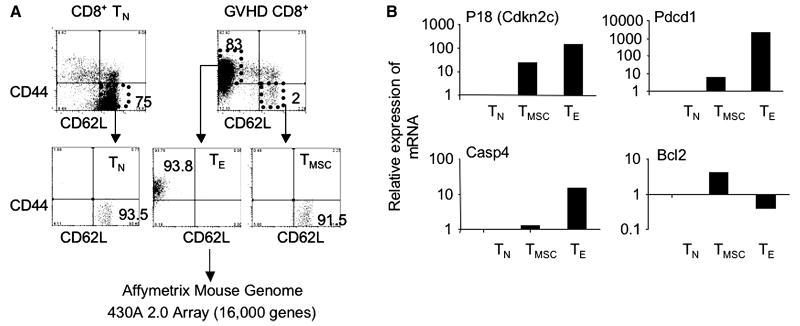
To understand the molecular mechanisms by which alloreactive CD8+ TE acquire the ability to continually proliferate upon chronic exposure to alloantigens, we used Affymetrix Mouse Genome A430A 2.0 Array to broadly compare the gene expression profiles of day 14 CD8+ TE to that of CD8+ TN and CD8+ TMSC (Fig.3A). Compared to TN, a total of 2744 distinct genes were up-regulated (1359) or down-regulated (1385) in CD8+ TE by a 1.5 fold that gave a p<0.01 value for comparing pairs of groups. As predicted by our experimental data (14-15) and others (12, 34), relative to CD8+ TN and TMSC, CD8+ TE expressed significantly higher levels of genes known to be important for effector functions, including effector molecules, chemokines and chemokine receptors (Table 1). As expected, many other genes engaged in TCR signaling pathway, glycolysis, MAPK pathway and cell adhesion were also altered in CD8+ TE (Table 1).
Fig.3.
Array based mRNA arrays of alloreactive CD8+ T cells. (A) Donor CD44loCD62Lhi CD8+ TN (2×106) from C3H.SW mice (CD45.2) were transplanted with B6/SJL TCD BM(0.5×106) into lethally irradiated B6/SJL mice (CD45.1). Donor CD8+ T cells were recovered at day 14 after transplantation from the spleens and lymph nodes of these recipients, magnetically purified and stained with indicated Abs for flow cytometry cell sorting. Affymetrix Mouse Genome 430A 2.0 Array was used to broadly compare the transcription profiles of these CD8+ TMSC and TE to that of TN cells. (B) Real-time RT-PCT analysis shows the relative expression of mRNA in each T cell subset. Data are representative of three independent preparations of alloreactive T cells.
Table 1. Altered transcripts in TMSC and TE as compared to TN.
| Gene symbols | Gene ID# | Fold Change | Gene symbols | Gene ID# | Fold Change | Gene symbols | Gene ID# | Fold Change | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TMSC/TN | TE/TN | TMSC/TN | TE/TN | TMSC/TN | TE/TN | ||||||
| Effector function | Glycolysis / Gluconeogenesis | mTOR pathway | |||||||||
| Gzmb | 14939 | 38.3 | 306.4 | Hk2 | 15277 | 2 | 7.1 | Rps6kb2 | 58988 | 2.7 | |
| Ifng | 15978 | 4.2 | 211 | Tpi1 | 21991 | 1.9 | 4.1 | Akt1 | 11651 | 2.1 | |
| Fasl | 14103 | 66.3 | Eno3 | 13808 | 4 | Eif4e2 | 26987 | 2 | |||
| Klrk1 | 27007 | 2.9 | 3.3 | Gapdh | 14433 | 1.5 | 3.8 | Rps6ka1 | 20111 | 1.7 | |
| Prf1 | 18646 | 3 | Acyp2 | 75572 | 1.8 | 2.4 | Eif4b | 75705 | -1.9 | ||
| Tnfsf10 | 22035 | 2.7 | Pfkp | 56421 | 1.5 | 1.9 | Tsc1 | 64930 | -2 | ||
| Klra3 | 16634 | 2.3 | Bpgm | 12183 | 1.6 | Rps6ka2 | 20112 | -2.9 | |||
| Chemokine-chemokine receptor interactions | MAPK signaling pathway | Adhesion and trans-endothelial migration | |||||||||
| Ccl5 | 20304 | 15.9 | 453.5 | Stmn1 | 16765 | 5 | 19.3 | Cd44 | 12505 | 14.2 | |
| Ccl4 | 20303 | 69.6 | Dusp1 | 19252 | 1.8 | 4.9 | Hmmr | 15366 | 7.2 | ||
| Ccl3 | 20302 | 42 | Map3k8 | 26410 | 7.6 | 3.4 | Itgb1 | 16412 | 5.6 | ||
| Ccr5 | 12774 | 4.7 | 32.8 | Gadd45b | 17873 | 3.2 | Spp1 | 20750 | 4.5 | ||
| Ccr2 | 12772 | 10.8 | Gadd45g | 23882 | 15.3 | 2.7 | Itga4 | 16401 | 2.7 | ||
| Cxcr3 | 12766 | 4.1 | 8.3 | Dusp3 | 72349 | 1.8 | 2.5 | Sdc4 | 20971 | 2.5 | |
| Cx3cr1 | 13051 | 4.8 | Mapkapk2 | 17164 | 1.8 | Itgav | 16410 | 2.4 | |||
| Xcl1 | 16963 | 4.7 | Mapk3 | 26417 | 1.8 | Lamc1 | 226519 | 2 | |||
| Cxcl10 | 15945 | 3.1 | Map2k3 | 26397 | 1.7 | Itga3 | 16400 | 2 | |||
| Cxcl10 | 15945 | 3.1 | Mapk8 | 26419 | -1.6 | Npnt | 114249 | 1.9 | |||
| Ccr6 | 12458 | 2.9 | Mapk14 | 26416 | -1.6 | Itgb2 | 16414 | 1.9 | |||
| Ccl6 | 20305 | 2.7 | Map2k6 | 26399 | -1.6 | Vcl | 22330 | 1.7 | |||
| Ccr7 | 12775 | -29.1 | Dusp2 | 13537 | -1.6 | Tnc | 21923 | 1.6 | |||
| Cytokine-cytokine receptor interactions | Jak-STAT signaling pathway | Pxn | 19303 | 1.5 | |||||||
| Il10ra | 16154 | 1.7 | 9.9 | Pim1 | 18712 | 2.3 | 2.3 | Apoptosis | |||
| Csf1 | 12977 | -2 | 6.4 | Jak3 | 16453 | 1.8 | 1.9 | Casp3 | 12367 | 5.4 | |
| Il2rb | 16185 | 1.7 | 6.3 | Socs5 | 56468 | -1.7 | -1.7 | Capn2 | 12334 | 2.3 | |
| Il10 | 16153 | 6.1 | Stat6 | 20852 | -1.7 | Bid | 12122 | 2.2 | |||
| Csf2 | 12981 | 4.7 | Stat1 | 20846 | 2 | -1.8 | Apaf1 | 11783 | 2.2 | ||
| Il15ra | 16169 | 2 | 2.5 | Jak1 | 16451 | -1.5 | -1.8 | Casp7 | 12369 | 2.1 | |
| Il12rb1 | 16161 | 1.9 | 2.5 | Socs3 | 12702 | 2.6 | -2.4 | Bax | 12028 | 1.8 | |
| Il4ra | 16190 | -2.8 | Socs1 | 12703 | 1.9 | Bcl2l1 | 12048 | 1.6 | |||
| Il6st | 16195 | -1.6 | -4.3 | Myc | 17869 | 1.5 | Bad | 12015 | 1.5 | ||
| Il7r | 16197 | -1.9 | -7.3 | Toll-like receptor signaling pathway | Bcl2 | 12043 | 2 | -1.5 | |||
| Il6ra | 16194 | -18.6 | Tlr7 | 170743 | 2.6 | Trp53 | 22059 | 1.9 | |||
| Il10rb | 16155 | 1.6 | Tlr6 | 21899 | 1.5 | ||||||
| TCR signaling | TCR signaling | TCR signaling | |||||||||
| Tbx21 | 57765 | 8.8 | 21.5 | Cdc42 | 12540 | 1.6 | Ikbkb | 16150 | -2.5 | ||
| Map3k8 | 26410 | 7.6 | 3.4 | Hras1 | 15461 | 1.5 | 1.5 | Pak1 | 18479 | -5.8 | |
| Nfatc1 | 18018 | 2.6 | Ikbkg | 16151 | -1.5 | Pdk1 | 228026 | -6.3 | |||
| Fyn | 14360 | 2.1 | Itk | 16428 | -1.5 | -2 | Jun | 16476 | 6.1 | ||
| Ppp3cc | 19057 | 1.6 | 1.7 | Map3k14 | 53859 | -1.5 | -2.2 | Fos | 14281 | 4 | |
| Zap70 | 22637 | 1.7 | Tec | 21682 | -2.5 | Lcp2 | 16822 | -1.6 | |||
To better define a specific gene signature of alloreactive T cells, we further tested each list of distinct genes for over-representation in 507 lists of Gene Ontology (GO) terms for which there were at least 10 genes present on the array (Affymetrix web site), as well as for 190 lists of pathways from the Kyoto Encyclopedia of Genes and Genomes (KEGG, http://www.genome.jp/kegg/). Compared to both CD8+ TN and TMSC, CD8+ TE demonstrated a signature expression of genes related to cell cycle, mitosis, apoptosis, and transcription and translation (Table 2).
Table 2. Most significantly enriched GO terms and KEGG pathways.
| A) Tests of 507 Geneontology (GO) biological process terms with at least 10 genes present on the arrays. | |||||||
|---|---|---|---|---|---|---|---|
| Comparison | direction | number of probe sets selected (of 22690) | number of distinct genes selected (of 13142) | GO term title | number of genes with GO term on the array | number of those genes selected | p-value |
| Tmsc vs Tn | up in Tmsc | 672 | 543 | cell cycle | 335 | 41 | 4.1E-10 |
| immune response | 263 | 29 | 1.4E-06 | ||||
| DNA replication | 96 | 16 | 1.8E-06 | ||||
| mitosis | 109 | 17 | 2.2E-06 | ||||
| cell division | 173 | 22 | 2.6E-06 | ||||
| regulation of progression through cell cycle | 185 | 20 | 8.1E-05 | ||||
| Tmsc vs Tn | up in Tn | 545 | 448 | transcription | 1125 | 72 | 1.1E-07 |
| regulation of transcription, DNA-dependent | 1486 | 86 | 4.6E-07 | ||||
| Te vs Tmsc | up in Te | 1486 | 1098 | cell cycle | 335 | 102 | 1.5E-32 |
| mitosis | 109 | 53 | 1.1E-28 | ||||
| cell division | 173 | 66 | 1.1E-27 | ||||
| DNA replication | 96 | 40 | 6.7E-19 | ||||
| apoptosis | 296 | 54 | 3.1E-08 | ||||
| DNA replication initiation | 13 | 9 | 1.0E-07 | ||||
| Te vs Tmsc | up in Tmsc | 1372 | 1036 | ribosome biogenesis and assembly | 47 | 21 | 9.6E-12 |
| translation | 259 | 51 | 7.5E-10 | ||||
| Te vs Tn | up in Te | 1817 | 1359 | cell cycle | 335 | 109 | 2.6E-29 |
| mitosis | 109 | 52 | 2.9E-23 | ||||
| cell division | 173 | 67 | 3.5E-23 | ||||
| DNA replication | 96 | 41 | 1.8E-16 | ||||
| DNA replication initiation | 13 | 10 | 2.9E-08 | ||||
| apoptosis | 296 | 59 | 5.1E-07 | ||||
| DNA recombination | 35 | 13 | 2.4E-05 | ||||
| regulation of progression through cell cycle | 185 | 38 | 2.6E-05 | ||||
| Te vs Tn | up in Tn | 1837 | 1359 | ribosome biogenesis and assembly | 47 | 18 | 5.3E-07 |
| translation | 259 | 49 | 3.3E-05 | ||||
| transcription | 1125 | 158 | 6.9E-05 | ||||
| B) Tests of 190 KEGG pathways | |||||||
|---|---|---|---|---|---|---|---|
| Comparison | direction | number of probe sets selected (of 22690) | number of distinct genes selected (of 13142) | Pathway Title | number of genes in pathway on the array | number of those genes selected | p-value |
| Tmsc vs Tn | up in Tmsc | 672 | 543 | Cell cycle | 102 | 16 | 4.1E-06 |
| Pyrimidine metabolism | 80 | 12 | 1.0E-04 | ||||
| Jak-STAT signaling pathway | 122 | 14 | 5.0E-04 | ||||
| Tmsc vs Tn | up in Tn | 545 | 448 | Wnt signaling pathway | 133 | 11 | 5.8E-03 |
We fit a 1-way ANOVA model to the data for each probe set and used it to compare each pair of groups. We selected those probe sets that gave p-values of p<.01 and average fold differences of at least 1.5-fold as being differentially expressed. We estimated the false discovery rate (FDR) by performing an identical analysis of all data sets with permuted sample labels that did not recapitulate the actual groupings of the 3 groups of samples, and dividing the average number of probe sets selected in these permuted data sets by the actual number of probe sets selected for the original data, and obtained estimated FDRs of 7.5% for TMSC vs. TN, 3.1% for TE vs. TMSC, and 2.5% for TE vs. TN. The number of probe sets selected as being increased or decreased is shown, as well as the number of distinct genes these represented, determined using Entrez Gene identifiers. The mapping of probe sets to genes, as well as the Gene Ontology terms for each gene, were obtained from Affymetrix web site, and were dated July 11, 2007. We tested each list of distinct genes for over-representation in 507 lists of Gene Ontology terms for which there were at least 10 genes present on the array, as well as for 190 lists of pathways from the Kyoto Encyclopedia of Genes and Genomes, obtained Jun 12, 2007 (http://www.genome.jp/kegg/). We present only the most significant lists obtained in each category, and of the most significant lists present all of them (that is, none are hidden). The total number of genes in each list that were on the array is given as well as the number of those that were selected as altered in each pair-wise comparison of groups of samples. The p-values shown are from using these counts to perform one-sided Fisher's Exact tests, however, since many lists were tested, we also give the average number of lists that obtained a p-value this smaller or smaller in 100 analyses of the same data in which the gene labels were randomly permuted.
Detailed analysis revealed that alloreactive CD8+ TE showed decreased expression of anti-apoptotic gene Bcl2, but had increased genes related to apoptosis, including Pdcd1 (also named Pd1), Klrg1, Casp1, Casp3, Caps4, Casp7, Bax, and Bad (Table 1). In contrast, CD8+ TMSC expressed higher levels of Bcl2 than both TN and TE, while only minimally changing in the expression of other pro-apoptotic genes (Table 1 and Fig.3B). When compared to CD8+ TN, CD8+ TE show significantly decreased expression of genes related to ribosome biogenesis and assembly, ribosome, translation and transcription (Table 2). Real time RT-PCR validated the expression some of these genes (Fig.3B).
Notably, alloreactive CD8+ TE also had 150-fold more expression of p18Ink4c (encoded by Cdkn2c) than CD8+ TN and 15-fold than CD8+ TMSC (Fig.3B). Previous studies have shown that p18Ink4c plays a critical role in negatively regulating cell proliferation and survival (35-38). The loss of p18Ink4c in T cells causes their hyperproliferation response and proliferation disorder (38-39).
Altogether, this transcriptional signature confirmed that alloreactive CD8+ TE are replicating cells, but are more likely susceptible than CD8+ TMSC to apoptotic death and senescence, consistent with our previous observations (15).
Alloreactive CD8+ TE activate stem cell transcriptional programs
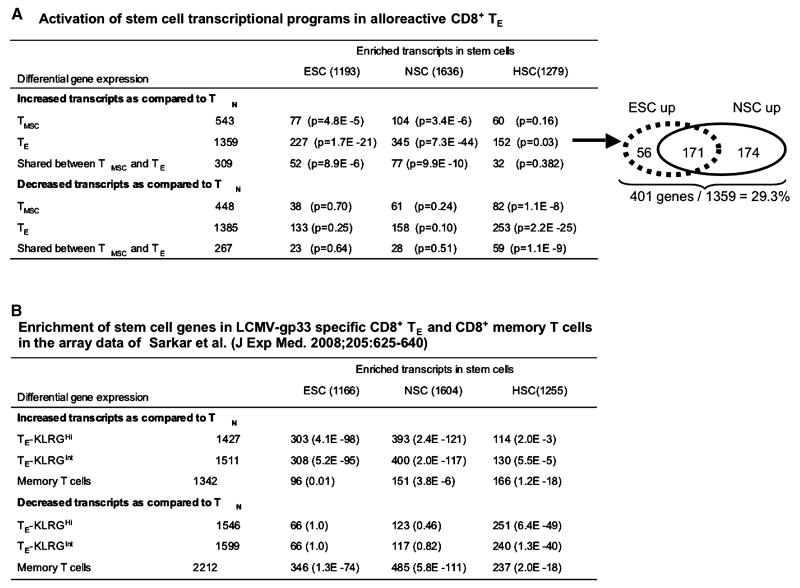
Next we tested 1687 curated gene lists from Molecular Signature Database v2 (MSigDB v2)(40) to inquire which transcriptional program(s) could be associated with the continuous proliferation property of alloreactive CD8+ TE. Based on one-sided Fisher's exact test, we identified that transcripts increased in CD8+ TE were significantly enriched in lists of genes increased in NSCs (345 of 1636 genes, p=7.3 × 10-44) and ESCs (227 of 1193 genes, p=1.7 × 10-21) (Fig.4A), which were identified by Ramalho-Santos et al. (41). These alloreactive CD8+ TE-related ESC genes and NSC genes are listed in Table 3 and Table 4. Among them, 171 genes were shared by ESCs and NSCs, 56 appeared in ESCs, and 174 were found only in NSCs. Thus, 401 out of 1369 (29%) of transcripts that were increased in alloreactive CD8+ TE were enriched for ESCs and/or NSCs (Fig.4A). In contrast, transcripts decreased in CD8+ TE were over-represented among HSC-related genes (253 of 1279, 19.8%, p=2.2×10-25) (Fig.4A).
Fig.4.
Acquisition of stem cell transcriptional programs in alloreactive CD8+ T cells. (A) Genes distinctively expressed by CD8+ TMSC and TE relative to TN were analyzed for functional set enrichment analysis using curated gene lists from the Ramalho-Santos's array data. (B) Acquisition of stem cell transcriptional programs in P14 LCMV-gp33-specific CD8+ T cells. Genes differentially expressed by CD8+ TE-KLRG1Hi, CD8+ TE-KLRG1Hint and CD8+ memory T cells relative to TN were analyzed for enrichment for the same stem cell gene lists. Sizes of stem cell gene lists differ slightly from that in Fig.4A since the Sarkar's study used Affymetrix Mouse_430_2, which also had slightly different probe-set annotation than our arrays.
Table 3.
ESC-related genes that are activated in CD8+ TE
| Gene symbol | Gene ID# | Fold Change | Gene symbol | Gene ID# | Fold Change | Gene symbol | Gene ID# | Fold Change | Gene symbol | Gene ID# | Fold Change | Gene symbol | Gene ID# | Fold Change | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TMSC/TN | TE/TN | TMSC/TN | TE/TN | TMSC/TN | TE/TN | TMSC/TN | TE/TN | TMSC/TN | TE/TN | ||||||||||
| Cell cycle | Ubiquitin/Proteolysis/Proteasome | Transcription/Transcription regulation | Receptor/Signal transduction | Microtubule-based process | |||||||||||||||
| Ccnb1 | 268697 | 2.28 | 16.85 | Cdc20 | 107995 | 5.27 | Mdfic | 16543 | 2.77 | 18.58 | Spp1 | 20750 | 4.52 | Prc1 | 233406 | 1.86 | 12.05 | ||
| Sgol1 | 72415 | 11.04 | Capn2 | 12334 | 2.34 | Prdm1 | 12142 | 10.62 | Aurka | 20878 | 4.47 | Cks2 | 66197 | 4.85 | 11.65 | ||||
| Chek1 | 12649 | 2.6 | 10.34 | Xpnpep1 | 170750 | 2.32 | Tacc3 | 21335 | 6.06 | Exo1 | 26909 | 4.02 | Anln | 68743 | 4.82 | ||||
| Cks1b | 54124 | 2.58 | 9.51 | Ide | 15925 | 2.28 | Csda | 56449 | 5.52 | Hif1a | 15251 | 3.71 | Dstn | 56431 | 1.74 | ||||
| Cdkn1a | 12575 | 9.17 | Coq3 | 230027 | 1.67 | 2.12 | Cdkn2b | 12579 | 4.89 | Csrp2 | 13008 | 3.01 | Cytokinesis | ||||||
| Plk1 | 18817 | 7.54 | Psma1 | 26440 | 1.99 | Plagl1 | 22634 | 3.25 | Sap30 | 60406 | 2.5 | Igfbp7 | 29817 | 2.68 | |||||
| Aspm | 12316 | 7.53 | Psmb2 | 26445 | 1.55 | 1.78 | Sub1 | 20024 | 2.62 | Dusp16 | 70686 | 2.3 | Wisp1 | 22402 | 2.26 | ||||
| Ect2 | 13605 | 6.86 | Psmc2 | 19181 | 1.63 | Myef2 | 17876 | 2.09 | Rap2a | 76108 | 2.27 | Miscellaneous | |||||||
| Itgb1 | 16412 | 5.58 | Psmb5 | 19173 | 1.62 | Gtf2e2 | 68153 | 1.99 | Bax | 12028 | 1.84 | Fignl1 | 60530 | 3.2 | 15.73 | ||||
| Ccne1 | 12447 | 5.31 | Psmd6 | 66413 | 1.57 | Tceb1 | 67923 | 1.95 | Pex7 | 18634 | 1.6 | 1.74 | Trip13 | 69716 | 1.88 | 8.69 | |||
| Syce2 | 71846 | 5.16 | Psma3 | 19167 | 1.55 | Polr3k | 67005 | 1.77 | Bdnf | 12064 | 1.72 | Pcbp4 | 59092 | 6.39 | |||||
| Cdc25c | 12532 | 4.72 | Psma5 | 26442 | 1.55 | Bzw1 | 66882 | 1.68 | Gnb1 | 14688 | 1.69 | Tmem49 | 75909 | 2.26 | 6.27 | ||||
| Ccnf | 12449 | 3.96 | Metabolism | Cnot7 | 18983 | 1.63 | Hnrpab | 15384 | 1.65 | Tacstd1 | 17075 | 5.17 | |||||||
| Dlg7 | 218977 | 2.72 | Gldc | 104174 | 23.94 | Klf9 | 16601 | 1.6 | Ddx1 | 104721 | 1.65 | Smoc2 | 64074 | 4.05 | |||||
| Cdkn2a | 12578 | 2.55 | Gsto1 | 14873 | 2.29 | 7.34 | Mtdh | 67154 | 1.57 | Inhbb | 16324 | 1.64 | Synpo | 104027 | 1.75 | 3.68 | |||
| Mybl2 | 17865 | 2.43 | Bcat1 | 12035 | 5.54 | Nfyb | 18045 | 1.52 | Nasp | 50927 | 1.61 | Tipin | 66131 | 3.5 | |||||
| Ran | 19384 | 1.89 | Myo5a | 17918 | 1.55 | 4.68 | RNA processing | Sfrs1 | 110809 | 1.61 | Ccdc99 | 70385 | 2.81 | ||||||
| Ccng1 | 12450 | 1.84 | Asns | 27053 | 2.95 | 3.87 | Syncrip | 56403 | 1.79 | 2.88 | Snrpd1 | 20641 | 1.55 | Lactb2 | 212442 | 1.64 | 2.68 | ||
| Erh | 13877 | 1.67 | 1.74 | Gcat | 26912 | 2.71 | Hnrpll | 72692 | 2.73 | Hrb | 15463 | 1.55 | Gmfb | 63985 | 1.67 | 2.64 | |||
| Psmd1 | 70247 | 1.69 | Shmt1 | 20425 | 1.53 | 2.55 | Ell2 | 192657 | 2.42 | Rab1 | 19324 | 1.53 | Wbp5 | 22381 | 2.42 | ||||
| Cops5 | 26754 | 1.64 | Uxs1 | 67883 | 2.48 | Ncbp2 | 68092 | 1.9 | 2.01 | Vdac3 | 22335 | 1.52 | Errfi1 | 74155 | 1.54 | 2.4 | |||
| DNA replication/Repair | Hmgcr | 15357 | 1.96 | Snrpa1 | 68981 | 1.91 | Transport (Protein/Ion) | Ccdc80 | 67896 | 2.34 | |||||||||
| Rrm2 | 20135 | 9.19 | 60.07 | Pfkfb1 | 18639 | 1.8 | Cstf3 | 228410 | 1.84 | Kif20a | 19348 | 4.26 | Nucks1 | 98415 | 2.3 | ||||
| Cdc6 | 23834 | 5.09 | 21.54 | Lypla1 | 18777 | 1.79 | Sfrs3 | 20383 | 1.71 | Arl5a | 75423 | 3.8 | Mpdu1 | 24070 | 1.85 | 2.29 | |||
| Il1rl1 | 17082 | 19.15 | Ywhah | 22629 | 1.74 | Cops2 | 12848 | 1.7 | Snx10 | 71982 | 1.97 | 2.74 | Ipp | 16351 | 2.25 | ||||
| Dtl | 76843 | 4.19 | 18.32 | Acot9 | 56360 | 1.66 | Lsm5 | 66373 | 1.7 | Clic4 | 29876 | 2.31 | 2.56 | Pls3 | 102866 | 2.16 | |||
| Rad51 | 19361 | 2.92 | 15.71 | Soat1 | 20652 | 1.65 | Sfrs2 | 20382 | 1.65 | Slc35b1 | 110172 | 2.15 | Phlda3 | 27280 | 2.1 | ||||
| Pttg1 | 30939 | 8.1 | Nup93 | 71805 | 1.62 | Translation | Arl1 | 104303 | 2.07 | Dsp | 109620 | 1.96 | |||||||
| Dna2l | 327762 | 1.68 | 7.48 | Kpna3 | 16648 | 1.56 | Farsb | 23874 | 4.68 | Lman1 | 70361 | 1.98 | Prdx1 | 18477 | 1.91 | ||||
| Brca1 | 12189 | 2.04 | 5.87 | Electron transport | Mrpl27 | 94064 | 1.84 | 2.41 | Vps54 | 245944 | 1.94 | Tmem41b | 233724 | 1.83 | |||||
| Smc2 | 14211 | 3.55 | Nqo2 | 18105 | 3.3 | Eif4e2 | 26987 | 1.52 | 2.04 | Mtch2 | 56428 | 1.86 | Rcn1 | 19672 | 1.6 | 1.77 | |||
| Mcm2 | 17216 | 1.56 | 3.4 | Etfb | 110826 | 3.11 | Eif1ay | 66235 | 1.86 | Snx5 | 69178 | 1.77 | 1.85 | Esd | 13885 | 1.73 | |||
| Rfc5 | 72151 | 2.7 | Pdia6 | 71853 | 1.6 | 2.42 | Mrpl18 | 67681 | 1.69 | Clcn5 | 12728 | 1.85 | Psmc3ip | 19183 | 1.71 | ||||
| Mcm3 | 17215 | 2.7 | Gsr | 14782 | 2.06 | Eif3s1 | 78655 | 1.63 | Slc2a3 | 20527 | 1.83 | Ranbp1 | 19385 | 1.67 | |||||
| Mcm4 | 17217 | 2.59 | Cyp51 | 13121 | 1.94 | Mrpl16 | 94063 | 1.52 | Nup62 | 18226 | 1.78 | Nup85 | 445007 | 1.62 | |||||
| Nudt1 | 17766 | 1.66 | 2.53 | Uqcrb | 67530 | 1.92 | Protein phosphorylation | Slc33a1 | 11416 | 1.72 | Ppp1r7 | 66385 | 1.62 | ||||||
| Orc6l | 56452 | 2.44 | Txnl1 | 53382 | 1.81 | Melk | 17279 | 4.05 | 17.84 | Golt1b | 66964 | 1.7 | Psmd7 | 17463 | 1.84 | 1.54 | |||
| Rfc4 | 106344 | 2.4 | Sqle | 20775 | 1.69 | Bub1 | 12235 | 7.69 | Slc4a7 | 218756 | 1.69 | Cox4nb | 18117 | 1.54 | |||||
| Rpa3 | 68240 | 2.22 | Txndc4 | 76299 | 1.6 | Wee1 | 22390 | 2.07 | 6.06 | Bcap29 | 12033 | 1.61 | 1.65 | Asah1 | 11886 | 1.53 | |||
| Dtymk | 21915 | 1.65 | 2.21 | Txnl5 | 52700 | 1.6 | Ryk | 20187 | 5.21 | Slc12a2 | 20496 | 1.57 | Uck2 | 80914 | 1.74 | 1.52 | |||
| Hat1 | 107435 | 2.15 | Chromatin modification/Assembly | Plk4 | 20873 | 1.54 | 4.75 | Inhibitorysignal/Apoptosis | Tiprl | 226591 | 1.51 | ||||||||
| Brca2 | 12190 | 2.01 | Nusap1 | 108907 | 1.86 | 9.22 | Ttk | 22137 | 3.62 | Ckap2 | 80986 | 5.44 | |||||||
| Blm | 12144 | 1.97 | Mad2l1 | 56150 | 1.61 | 6.8 | Mapk6 | 50772 | 2.07 | Gas2 | 14453 | 4.73 | |||||||
| Topbp1 | 235559 | 1.96 | Tmem38b | 52076 | 2.7 | Chek2 | 50883 | 2.07 | Cell adhesion/Communication | ||||||||||
| Ranbp5 | 70572 | 1.72 | Nek2 | 18005 | 2.45 | Ppap2a | 19012 | 2.05 | Adam19 | 11492 | 4.89 | ||||||||
| Dbf4 | 27214 | 1.69 | Suv39h2 | 64707 | 2.04 | Pigf | 18701 | 1.61 | Adam9 | 11502 | 2.82 | ||||||||
| Ube2n | 93765 | 1.64 | Cbx1 | 12412 | 1.94 | Acp1 | 11431 | 1.56 | Tnc | 21923 | 1.94 | 1.56 | |||||||
| Nudt5 | 53893 | 1.52 | Nap1l1 | 53605 | 1.65 | Ptprk | 19272 | 1.54 | Vcl | 22330 | 1.66 | ||||||||
| Smarcad1 | 13990 | 1.56 | |||||||||||||||||
Table 4. NSC-related genes that are activated in CD8+ TE.
| Gene symbol | Gene ID# | Fold Change | Gene symbol | Gene ID# | Fold Change | Gene symbol | Gene ID# | Fold Change | Gene symbol | Gene ID# | Fold Change | Gene symbol | Gene ID# | Fold Change | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TMSC/TN | TE/TN | TMSC/TN | TE/TN | TMSC/TN | TE/TN | TMSC/TN | TE/TN | TMSC/TN | TE/TN | ||||||||||
| Cell cycle | DNA replication/Repair | Receptor/Signal transduction | Transport (Protein/Ion) | Transcription/Transcription regulation | |||||||||||||||
| Ccnb2 | 12442 | 3.64 | 29.94 | Rrm2 | 20135 | 9.19 | 60.07 | Epas1 | 13819 | 7.54 | Sypl | 19027 | 4.33 | Mdfic | 16543 | 2.77 | 18.58 | ||
| Ccna2 | 12428 | 3.31 | 21.13 | Cdc6 | 23834 | 5.09 | 21.54 | Rhoc | 11853 | 6.85 | Kif20a | 19348 | 4.26 | Uhrf1 | 18140 | 2.89 | 9.26 | ||
| Cdca5 | 67849 | 4.46 | 19.44 | Dtl | 76843 | 4.19 | 18.32 | Rhoq | 104215 | 1.69 | 5.32 | Syt11 | 229521 | 2.9 | 3.88 | Hells | 15201 | 1.91 | 7 |
| Ccnb1 | 268697 | 2.28 | 16.85 | Uhrf1 | 18140 | 2.89 | 9.26 | Arl5a | 75423 | 3.8 | Snx10 | 71982 | 1.97 | 2.74 | Ezh2 | 14056 | 6.95 | ||
| Sgol1 | 72415 | 11.04 | Dna2l | 327762 | 1.68 | 7.48 | Hif1a | 15251 | 3.71 | Slc35b2 | 73836 | 2.61 | Tacc3 | 21335 | 6.06 | ||||
| E2f8 | 108961 | 10.39 | Lig1 | 16881 | 5.73 | Nudt1 | 17766 | 1.66 | 2.53 | Clic4 | 29876 | 2.31 | 2.56 | Eomes | 13813 | 1.61 | 5.7 | ||
| Chek1 | 12649 | 2.6 | 10.34 | Rpa2 | 19891 | 4.52 | Sap30 | 60406 | 2.5 | Pdzd11 | 72621 | 1.66 | 2.25 | Csda | 56449 | 5.52 | |||
| Cks1b | 54124 | 2.58 | 9.51 | Rfc3 | 69263 | 1.6 | 4.27 | Dusp16 | 70686 | 2.3 | Slc35b1 | 110172 | 2.15 | Cenpk | 60411 | 4.51 | |||
| Cdkn1a | 12575 | 9.17 | Rrm1 | 20133 | 4.18 | Dtymk | 21915 | 1.65 | 2.21 | Dbi | 13167 | 2.11 | Nsbp1 | 50887 | 3.85 | ||||
| Plk1 | 18817 | 7.54 | Mcm5 | 17218 | 4.07 | Gpr56 | 14766 | 2.08 | Lman1 | 70361 | 1.98 | Klf10 | 21847 | 2.98 | |||||
| Aspm | 12316 | 7.53 | Smc2 | 14211 | 3.55 | Akt1 | 11651 | 2.07 | Vps54 | 245944 | 1.94 | Wdhd1 | 218973 | 2.85 | |||||
| Ect2 | 13605 | 6.86 | Fen1 | 14156 | 3.43 | Arl1 | 104303 | 2.07 | Gipc1 | 67903 | 1.55 | 1.93 | Sub1 | 20024 | 2.62 | ||||
| Cdkn2c | 12580 | 2.13 | 6.27 | Prim1 | 19075 | 3.42 | Arhgap21 | 71435 | 2 | Arf4 | 11843 | 1.87 | Pmf1 | 67037 | 2.45 | ||||
| Itgb1 | 16412 | 5.58 | Mcm2 | 17216 | 1.56 | 3.4 | Tank | 21353 | 1.95 | Mtch2 | 56428 | 1.86 | Myef2 | 17876 | 2.09 | ||||
| Gmnn | 57441 | 2.1 | 5.33 | Hmgn2 | 15331 | 3.37 | Fut8 | 53618 | 1.91 | Snx5 | 69178 | 1.77 | 1.85 | Rab8b | 235442 | 2.09 | |||
| Cdc45l | 12544 | 1.68 | 5.13 | Mcm7 | 17220 | 2.94 | Rbl1 | 19650 | 1.83 | Clcn5 | 12728 | 1.85 | Gtf2e2 | 68153 | 1.99 | ||||
| Cdkn2b | 12579 | 4.89 | Prim2 | 19076 | 2.76 | Tmpo | 21917 | 1.82 | Vps29 | 56433 | 1.83 | Inppl1 | 16332 | 1.98 | |||||
| Cdc25c | 12532 | 4.72 | Rfc5 | 72151 | 2.7 | Ptprj | 19271 | 1.74 | Rrbp1 | 81910 | 1.8 | Cdca7l | 217946 | 1.8 | 1.97 | ||||
| Pola1 | 18968 | 1.59 | 4.55 | Mcm3 | 17215 | 2.7 | Csnk2b | 13001 | 1.73 | Trappc1 | 245828 | 1.79 | Tceb1 | 67923 | 1.95 | ||||
| Aurka | 20878 | 4.47 | Mcm4 | 17217 | 2.59 | Ranbp5 | 70572 | 1.72 | Ssr2 | 66256 | 1.73 | Hnrpdl | 50926 | 1.72 | 1.78 | ||||
| Ccnf | 12449 | 3.96 | Orc6l | 56452 | 2.44 | Gnb1 | 14688 | 1.69 | Slc33a1 | 11416 | 1.72 | Polr3k | 67005 | 1.77 | |||||
| Gsg2 | 14841 | 1.75 | 3.11 | Rfc4 | 106344 | 2.4 | Dck | 13178 | 1.66 | Vps45 | 22365 | 1.72 | Bzw1 | 66882 | 1.68 | ||||
| Dlg7 | 218977 | 2.72 | Recql | 19691 | 1.71 | 2.28 | Ddx1 | 104721 | 1.65 | Atp6v0a2 | 21871 | 1.71 | Cnot7 | 18983 | 1.63 | ||||
| Nfatc1 | 18018 | 2.51 | Rpa3 | 68240 | 2.22 | Inhbb | 16324 | 1.64 | Slc4a7 | 218756 | 1.69 | Sin3b | 20467 | 1.63 | |||||
| Ran | 19384 | 1.89 | Smc6 | 67241 | 2.15 | Tyms | 22171 | 1.63 | Slc39a6 | 106957 | 1.65 | Mtdh | 67154 | 1.57 | |||||
| Ccng1 | 12450 | 1.84 | Hat1 | 107435 | 2.15 | Aaas | 223921 | 1.59 | Bcap29 | 12033 | 1.61 | 1.65 | Th1l | 57314 | 1.53 | ||||
| Erh | 13877 | 1.67 | 1.74 | Blm | 12144 | 1.97 | Smap1 | 98366 | 1.58 | Nup93 | 71805 | 1.62 | Microtubule-based process | ||||||
| Rb1 | 19645 | 1.72 | Topbp1 | 235559 | 1.96 | Rab1 | 19324 | 1.53 | Sfxn1 | 14057 | 1.7 | 1.57 | Kif2c | 73804 | 3.23 | 20.24 | |||
| Psmd1 | 70247 | 1.69 | Hmgb1 | 15289 | 1.82 | Asna1 | 56495 | 1.53 | Timm17b | 21855 | 1.57 | Kif11 | 16551 | 1.82 | 7.9 | ||||
| Cops5 | 26754 | 1.64 | Ube2n | 93765 | 1.64 | Nudt5 | 53893 | 1.52 | Kpna3 | 16648 | 1.56 | Kif22 | 110033 | 2.33 | 7.45 | ||||
| Mapre1 | 13589 | 1.6 | Smarcad1 | 13990 | 1.57 | Cytokinesis | Slc25a10 | 27376 | 1.56 | Kif4 | 16571 | 3.58 | |||||||
| Rap1a | 109905 | 1.53 | Prc1 | 233406 | 1.86 | 12.05 | Kdelr2 | 66913 | 1.56 | Mid1ip1 | 68041 | 2.07 | |||||||
| Cks2 | 66197 | 4.85 | 11.65 | Sec14l1 | 74136 | 1.54 | Nde1 | 67203 | 2.04 | ||||||||||
| Incenp | 16319 | 4.68 | Kif23 | 71819 | 1.68 | ||||||||||||||
| Metabolism | Ubiquitin/Proteolysis/Proteasome | Skap2 | 54353 | 1.76 | 2.09 | Chromatin modification/Assembly | Pnkd | 56695 | 2.29 | ||||||||||
| Gldc | 104174 | 23.94 | Cdc20 | 107995 | 5.27 | Protein phosphorylation | Nusap1 | 108907 | 1.86 | Mpdu1 | 24070 | 1.85 | 2.29 | ||||||
| Brca1 | 12189 | 2.04 | 5.87 | Capn2 | 12334 | 2.34 | Melk | 17279 | 4.05 | 17.84 | Mad2l1 | 56150 | 1.61 | Ipp | 16351 | 2.25 | |||
| Gpd2 | 14571 | 4.17 | Xpnpep1 | 170750 | 2.32 | Bub1 | 12235 | 7.69 | Asf1b | 66929 | Tmem97 | 69071 | 2.24 | ||||||
| Lycat | 225010 | 3.06 | Ide | 15925 | 2.28 | Wee1 | 22390 | 2.07 | 6.06 | Nek2 | 18005 | Pls3 | 102866 | 2.16 | |||||
| Gcat | 26912 | 2.71 | Psma1 | 26440 | 1.99 | Ddr1 | 12305 | 2.26 | 5.82 | Cbx5 | 12419 | Dek | 110052 | 2.16 | |||||
| Azin1 | 54375 | 2.29 | Psmb1 | 19170 | 1.88 | Stk39 | 53416 | 1.51 | 5.32 | Pvt1 | 19296 | 1.57 | Lancl2 | 71835 | 2.15 | ||||
| Pkm2 | 18746 | 2.19 | Ube2l3 | 22195 | 1.82 | Ryk | 20187 | 5.21 | Suv39h2 | 64707 | Pqlc3 | 217430 | 2.14 | ||||||
| Idh3a | 67834 | 2 | Blmh | 104184 | 1.71 | Plk4 | 20873 | 1.54 | 4.75 | Cbx1 | 12412 | Phlda3 | 27280 | 2.1 | |||||
| Gyg | 27357 | 1.98 | Ube2t | 67196 | 1.64 | Ttk | 22137 | 3.62 | Nap1l1 | 53605 | Pih1d1 | 68845 | 1.59 | 2.03 | |||||
| Hmgcr | 15357 | 1.96 | Psmb4 | 19172 | 1.64 | Mapk6 | 50772 | 2.07 | Translation | Anp32e | 66471 | 1.96 | |||||||
| Rpe | 66646 | 1.86 | Psmc2 | 19181 | 1.63 | Cdk2 | 12566 | 1.87 | Farsb | 23874 | 4.68 | Tmem30a | 69981 | 1.92 | |||||
| Sdhb | 67680 | 1.8 | Ube2e3 | 22193 | 1.63 | Commd1 | 17846 | 1.81 | Lamp2 | 16784 | 2.63 | 3.48 | Prdx1 | 18477 | 1.91 | ||||
| Acsl5 | 433256 | 1.79 | Psmb5 | 19173 | 1.62 | Ptp4a2 | 19244 | 1.74 | Mrpl27 | 94064 | 1.84 | 2.41 | Mlf2 | 30853 | 1.88 | ||||
| Lypla1 | 18777 | 1.79 | Psmd6 | 66413 | 1.57 | Rps6ka1 | 20111 | 1.73 | Eif4e2 | 26987 | 1.52 | 2.04 | Hccs | 15159 | 1.85 | ||||
| Decr1 | 67460 | 1.79 | Psma3 | 19167 | 1.55 | Ptpn9 | 56294 | 1.59 | Eif1ay | 66235 | 1.86 | Actb | 11461 | 1.84 | |||||
| Gstt1 | 14871 | 1.78 | Psma5 | 26442 | 1.55 | Ppp5c | 19060 | 1.53 | 1.58 | Mrpl13 | 68537 | 1.71 | Tex9 | 21778 | 1.8 | ||||
| Ywhah | 22629 | 1.74 | RNA processing | Acp1 | 11431 | 1.56 | Mrpl18 | 67681 | 1.69 | Rcn1 | 19672 | 1.6 | 1.77 | ||||||
| Gusb | 110006 | 1.72 | Syncrip | 56403 | 1.79 | 2.88 | Inhibitory signal/Apoptosis | Eif3s1 | 78655 | 1.63 | Nubp1 | 26425 | 1.59 | 1.74 | |||||
| Soat1 | 20652 | 1.65 | Slbp | 20492 | 2.76 | Lgals1 | 16852 | 3.91 | 27.4 | Mrpl16 | 94063 | 1.52 | Psmc3ip | 19183 | 1.71 | ||||
| Aytl2 | 210992 | 1.65 | Hnrpll | 72692 | 2.73 | Birc5 | 11799 | 5.33 | 21.6 | Miscellaneous | Pdap1 | 231887 | 1.69 | ||||||
| Aco1 | 11428 | 1.58 | Thoc4 | 21681 | 2.21 | Casp3 | 12367 | 5.37 | Fignl1 | 60530 | 3.2 | 15.73 | Arpc5 | 67771 | 1.67 | ||||
| Pcyt1a | 13026 | 1.57 | Cpsf2 | 51786 | 2.02 | Gas2 | 14453 | 4.73 | Trip13 | 69716 | 1.88 | 8.69 | Ranbp1 | 19385 | 1.67 | ||||
| Electron transport | Ncbp2 | 68092 | 1.9 | 2.01 | Dap | 223453 | 2.37 | Pcbp4 | 59092 | 6.39 | Tmem77 | 67171 | 1.65 | ||||||
| Acadl | 11363 | 5.27 | Snrpa1 | 68981 | 1.91 | Bcl7c | 12055 | 2.23 | Sh3bgrl | 56726 | 4.77 | Pon2 | 330260 | 1.65 | |||||
| Pdia6 | 71853 | 1.6 | 2.42 | Cstf3 | 228410 | 1.84 | Api5 | 11800 | 2.16 | Lxn | 17035 | 4.32 | Nup85 | 445007 | 1.62 | ||||
| Gsr | 14782 | 2.06 | Rod1 | 230257 | 1.79 | Casp7 | 12369 | 2.14 | Capg | 12332 | 1.95 | 4.17 | Ppp1r7 | 66385 | 1.62 | ||||
| Cyp51 | 13121 | 1.94 | Sfrs3 | 20383 | 1.71 | Phlda1 | 21664 | 1.94 | Ltb4dh | 67103 | 4.01 | Sephs2 | 20768 | 1.61 | |||||
| Uqcrb | 67530 | 1.92 | Cops2 | 12848 | 1.7 | Bax | 12028 | 1.84 | Gtse1 | 29870 | 3.59 | Zdhhc6 | 66980 | 1.59 | |||||
| Glrx | 93692 | 1.87 | Lsm5 | 66373 | 1.7 | Bnip2 | 12175 | 1.51 | Tipin | 66131 | 3.5 | Pdia3 | 14827 | 1.58 | |||||
| Txnl1 | 53382 | 1.81 | Sfrs2 | 20382 | 1.65 | Cell adhesion/Communication | Ccdc99 | 70385 | 2.81 | Mgat2 | 217664 | 1.56 | |||||||
| Cyb5b | 66427 | 1.7 | Sfrs1 | 110809 | 1.61 | Itgb1 | 16412 | 5.58 | Mtm1 | 17772 | 2.71 | Psmd7 | 17463 | 1.84 | 1.54 | ||||
| Sqle | 20775 | 1.69 | Regulation of cell growth/Proliferation | Adam19 | 11492 | 4.89 | Wbp5 | 22381 | 2.42 | Psmc1 | 19179 | 1.54 | |||||||
| Txndc4 | 76299 | 1.6 | Scin | 20259 | 3.06 | Adam9 | 11502 | 2.82 | Acyp2 | 75572 | 1.75 | 2.41 | Asah1 | 11886 | 1.53 | ||||
| Cyb5r3 | 109754 | 1.57 | Chpt1 | 212862 | 2.36 | Tnc | 21923 | 1.94 | 1.56 | Nucks1 | 98415 | 2.3 | Peci | 23986 | 1.53 | ||||
Like TE, the 543 transcripts increased in TMSC versus TN showed significant over-representation of ESC- and NSC-related genes (p=4.8 × 10-5 and 3.4 × 10-6, respectively) (Fig.4A). Notably, 72% of ESC- and NSC-related genes selected as increased in TMSC were also increased in TE (87 of 121 genes, Tables 3 and 4). Thus, despite their different proliferation capability, both alloreactive CD8+ TE and TMSC share some common stem cell transcriptional programs.
To validate our observations using an unsupervised approach, we obtained the Sarkar's dataset (GSE10239) (42), fit one-way ANOVA models, and performed probe-set selections identical to those of our array data. In their experiments, both KLRG1Int and KLRG1High P14 CD8+ TE specific to lymphocytic choriomenigitis (LCMV)-gp33 showed probe set differences compared to TN that had significantly overlaps with probe sets that we found to differ between alloreactive CD8+ TE and TN in our GVHD model (p<10-200, by Fisher's Exact test in both cases). Most importantly, as with our alloreactive CD8+ TE, P14 CD8+ TE highly enriched for ESC- and NSC-related genes (Fig.4B). In contrast, P14 CD8+ memory cells decreased the expression of ESC- and NSC-related genes while activating genes enriched for HSCs (Fig.4B). These data independently confirmed our gene microarray data.
Characterization of stem cell genes activated in alloreactive CD8+ TE
These observations were surprising to us because CD8+ TE have been believed to be terminally differentiated cells (1, 43). To understand what these CD8+ TE-related stem cell genes were, we assigned these genes to several functional categories by filtering for keywords in the GO terms as previously described by Ramalho-Santos et al. (41). As shown in Table 5, we found that CD8+ TE shared the similarity with ESCs and NSCs in the expression of genes associated with: 1) regulation of cell cycle, 2) resistance to stress, 3) chromatin modification and transcription / translation regulation, 4) cell survival and death, 5) signaling, and 6) others. In cell cycle category, CD8+ TE increased both negative (e.g., p18Ink4c and p21Cip1) and positive cell cycle regulators (e.g., Ccna and Ccnb). Interestingly, after removing all these cell cycle genes from our array data and Ramalho-Santos's stem cell data set (41), we found that the rest of transcripts increased in alloreactive CD8+ TE remained significant over-representation of ESC- and NSC-related genes (p<5.0-15, by Fisher's Exact test in both cases). Our further evaluation of Sarkar's (42) and Ramalho-Santos's (41) data sets without these cell cycle genes also revealed significant enrichments in P14 CD8+ TE for ESC- and NSC-related transcripts (p<4.2-82 in both cases). Thus, cell cycle genes are not the only attributes to the similarity of gene expression between CD8+ TE and embryonic and neural stem cells.
Table 5. Functional categories of 401 CD8+ TE-related stem cell genes (See gene symbols and identification# in Table 3 and Table 4).
| Category | Gene Symbols | |
|---|---|---|
| 1. Cell cycle regulation | ||
| Cell cycle | (35) | Ccna2, Cdca5, Ccnb1, Sgol1, E2f8, Chek1, Cks1b, Cdkn1a, Ncaph, Plk1, Aspm, Ect2, Cdkn2c, Gmnn, Ccne1, Syce2, Cdc45l, Cdkn2b, Cdc25c, Pola1, Aurka, Ccnf, Gsg2, Dlg7, Cdkn2a, Nfatc1, Mybl2, Ran, Ccng1, Erh, Rb1, Psmd1, Cops5, Mapre1, Rap1a |
| 2. Resistance to stress | ||
| DNA replication / repair | (34) | Rrm2, Cdc6, Il1rl1, Dtl, Rad51, Uhrf1, Pttg1, Dna2l, Lig1, Rpa2, Rfc3, Rrm1, Mcm5, Smc2, Fen1, Prim1, Mcm2, Hmgn2, Mcm7, Prim2, Mcm3, Rfc5, Mcm4, Orc6l, Rfc4, Recql, Rpa3, Smc6, Brca2, Blm, Topbp1, Hmgb1, Dbf4, Ube2n |
| Ubiquitin / Proteolysis / Proteasome | (20) | Cdc20, Coq7, Nedd4, Capn2, Xpnpep1, Ide, Coq3, Psma1, Psmb1, Ube2l3, Psmb2, Blmh, Ube2t, Psmb4, Psmc2, Psmb5, Psmd6, Psma3, Psma5, Ube2e3 |
| Metabolism | (27) | Gldc, Gsto1, Brca1, Bcat1, Myo5a, Gpd2, Lycat, Gcat, Shmt1, Uxs1, Azin1, Pkm2, Idh3a, Gyg, Hmgcr, Rpe, Pfkfb1, Sdhb, Decr1, Acsl5, Lypla1, Gstt1, Gusb, Acot9, Aytl2, Soat1, Aco1 |
| Electron transport | (14) | Acadl, Nqo2, Etfb, Pdia6, Gsr, Cyp51, Uqcrb, Glrx, Txnl1, Cyb5b, Sqle, Txndc4, Txnl5, Cyb5r3 |
| 3. Chromatin modification, and transcription / translation regulation | ||
| Chromatin modification/Assembly | (12) | Nusap1, Mad2l1, Asf1b, Tmem38b, Nek2, Cbx5, Pvt1, Hat1, Suv39h2, Cbx1, Nap1l1, Smarcad1 |
| Transcription/Transcription regulation | (33) | Mdfic, Prdm1, Uhrf1, Hells, Ezh2, Tacc3, Eomes, Csda, Cenpk, Nsbp1, Klf10, Wdhd1, Sub1, Sap30, Pmf1, Myef2, Rab8b, Gtf2e2, Inppl1, Cdca7l, Tceb1, Rbl1, Tmpo, Hnrpdl, Polr3k, Cops2, Bzw1, Sin3b, Cnot7, Klf9, Mtdh, Th1l, Nfyb |
| RNA processing | (16) | Syncrip, Slbp, Hnrpll, Ell2, Thoc4, Cpsf2, Ncbp2, Snrpa1, Cstf3, Rod1, Sfrs3, Lsm5, Sfrs2, Ddx1, Sfrs1, Snrpd1 |
| Translation | (11) | Farsb, Lamp2, Mrpl27, Eif4e2, Lamc1, Ap3s1, Mrpl13, Mrpl18, Eif3s1, Dnajc9, Mrpl16 |
| 4. Survival / apoptosis | ||
| Survival/Apoptosis | (16) | Lgals1, Birc5, Ckap2, Casp3, Gas2, Scin, Grn, Dap, Bcl7c, Api5, Casp7, Skap2, Phlda1, Bax, Nasp, Bnip2 |
| 5. Signaling | ||
| Protein phosphorylation | (20) | Melk, Bub1, Wee1, Ddr1, Stk39, Ryk, Plk4, Ttk, Mapk6, Chek2, Ppap2a, Cdk2, Commd1, Ptp4a2, Rps6ka1, Pigf, Ptpn9, Ppp5c, Acp1, Ptprk |
| Receptor/Signal transduction | (24) | Epas1, Rhoc, Rhoq, Hif1a, Grn, Dusp16, Rap2a, Gpr56, Akt1, Arhgap21, Tank, Gipc1, Fut8, Rrbp1, Csnk2b, Ssr2, Ranbp5, Gnb1, Nup93, Nasp, Smap1, Timm17b, Kpna3, Rab1 |
| 6. Others | ||
| Transport (Protein/Ion) | (33) | Sypl, Kif20a, Syt11, Arl5a, Snx10, Slc35b2, Clic4, Slc35b1, Dbi, Arl1, Lman1, Vps54, Arf4, Mtch2, Clcn5, Snx5, Vps29, Slc2a3, Trappc1, Nup62, Slc33a1, Vps45, Atp6v0a2, Golt1b, Slc4a7, Bcap29, Slc39a6, Aaas, Sfxn1, Slc12a2, Slc25a10, Sec14l1, Asna1 |
| Cell adhesion/Communication | (5) | Itgb1, Adam19, Adam9, Vcl, Tnc |
| Microtubule-based process / cytokinesis | (12) | Kif2c, Kif11, Kif22, Kif4, Mid1ip1, Nde1, Kif23, Prc1, Cks2, Anln, Incenp, Dstn |
| Miscellaneous | (89) | Wisp1, Igfbp7, Chpt1, Fignl1, Trip13, Pcbp4, Tmem49, Tacstd1, Sh3bgrl, Spp1, Lxn, Capg, Smoc2, Exo1, Ltb4dh, Asns, Synpo, Gtse1, Tipin, Plagl1, Ccdc99, Mtm1, Lactb2, Gmfb, Nudt1, Wbp5, Acyp2, Errfi1, Htatip2, Ccdc80, Nucks1, Pnkd, Mpdu1, Ipp, Tmem97, Dtymk, Apaf1, Pls3, Dek, Lancl2, Pqlc3, Fyn, Phlda3, Pih1d1, Anp32e, Dsp, Tmem30a, Prdx1, Mlf2, Hccs, Actb, Tmem41b, Tex9, Rcn1, Ptprj, Nubp1, Pex7, Ywhah, Esd, Bdnf, Psmc3ip, Nab1, Pdap1, Arpc5, Ranbp1, Dck, Hnrpab, Tmem77, Pon2, Inhbb, Tyms, Nup85, Ppp1r7, Sephs2, Zdhhc6, Pdia3, Pcyt1a, Mgat2, Kdelr2, Hrb, Psmd7, Psmc1, Cox4nb, Asah1, Peci, Uck2, Nudt5, Vdac3, Tiprl |
Genes engaged in resistance to stress represented were a large group of stem cell transcripts activated in CD8+ TE. This group of genes included transcripts of DNA replication and repair, ubiquitin/proteasome, metabolism and electron transport (Table 5). These genes are considered to be related to the stress condition of stem cells (41). For example, genes including Coq7, Ube2l3, Nedd4 and Psma play important role in modifying abnormal or short-lived proteins (44-45). DNA repair gene Rad51 is critical to maintaining chromosomal stability and preventing genetic mutation potentially occurring during cell division (41, 46). Genes associated with metabolisms, such as Gldc and Gsto1, are critical to amino acid and antioxidant metabolism (47-48). The functions of these genes in T cells and stem cells remain largely unknown.
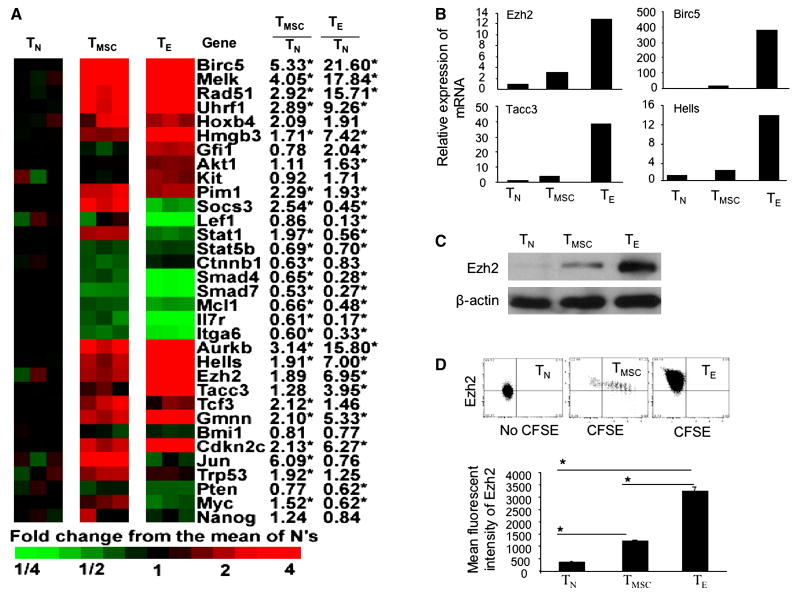
Another important finding was that CD8+ TE activated many genes engaged in DNA methylation, chromatin modification, transcription and survival in ESCs and NSCs. For example, Uhrf1 protein forms complexes with DNA methyltransferase Dnmt1, which may result in an inheritable DNA methylation (49). Hells (also known as Lsh) protein associates with Dnmt3a and Dnmt3b in embryonic cells for DNA methylation and transcription (50). Tacc3 protein can activate gene transcription even prior to demethylation (51). Birc5 (also known as Survivin), is an apoptosis inhibitor in both normal and malignant cells (52). Ezh2, which encodes a chromatin modifying enzyme with methyltransferase activity, orchestrates gene expression in both embryonic and adult stem cells (53-55). The representative gene expression of these chromatin modifiers and transcriptional regulators were shown in Fig.5A, and validated by real-time RT-PCR (Fig.5B). Thus, genes in this category have multiple roles in controlling cell fate, self-renewal, differentiation, survival and memory function.
Fig.5.
Characterization of stem cell genes in alloreactive CD8+ T cells. (A) The relative expression of stem cell genes in alloreactive CD8+ TE and CD8+ TMSC are shown with genes identified by functional set enrichment analysis using curated gene lists from MSigDBv2 (Fig.4). (B) Real-time RT-PCR analysis shows the relative mRNA expression of selected genes in each T cell subset. Data are representative of three independent preparations of alloreactive T cells. (C) Western blot analysis shows the expression of EZH2 protein in purified day-14 CD8+ TE, TMSC and control TN. (D) donor CD8+ TN and day-14 CD8+ T cells were stained with anti-Ezh2 Ab using intracellular staining and analyzed by flow cytometry. Dot plots show the expression of Ezh2 in cell subset of donor T cells. Mean fluorescent intensity shows the amount of testing antigen.
Finally, we noted that CD8+ TE did not activate those genes associated with pluripotency of ESCs, such as Oct4, Sox2, Klf4, Nanog, and c-Myc(56) (Fig. 5A, other data not shown). Furthermore, genes associated with HSC self-renewal were decreased in CD8+ TE. For example, CD8+ TE markedly down-regulated the expression of many genes related to receptor, signaling and transcription that are normally expressed in HSCs, such Il6ra, Il6st, Smad4, Smad7, and c-Myc (Table 6, Fig.5A). Among them, Smad4 and Smad7 have been shown to be required for self-renewal and quiescence of HSCs (57).
Table 6. HSC-enriched genes that are decreased in CD8+ TE.
| Gene symbol | Gene ID# | Fold Change | Gene symbol | Gene ID# | Fold Change | Gene symbol | Gene ID# | Fold Change | Gene symbol | Gene ID# | Fold Change | Gene symbol | Gene ID# | Fold Change | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TMSC/TN | TE/TN | TMSC/TN | TE/TN | TMSC/TN | TE/TN | TMSC/TN | TE/TN | TMSC/TN | TE/TN | ||||||||||
| Cell cycle | Translation | Receptor/Signal transduction | Regulation of cell growth/Proliferation | Camsap1 | 227634 | -1.75 | |||||||||||||
| Lats2 | 50523 | -1.6 | Mknk2 | 17347 | -1.62 | Aplp2 | 11804 | -1.75 | -1.5 | Ppan | 235036 | -1.6 | Las1l | 76130 | -1.76 | ||||
| Rassf2 | 215653 | -1.64 | Mrpl24 | 67707 | -1.75 | Prpf6 | 68879 | -1.5 | Creg1 | 433375 | -2.03 | Pcf11 | 74737 | -1.78 | |||||
| Flcn | 216805 | -2.1 | Eif4g2 | 13690 | -1.55 | -1.8 | Mettl3 | 56335 | -1.51 | Pctk1 | 18555 | -2.09 | Serpini1 | 20713 | -1.79 | ||||
| Sesn1 | 140742 | -2.71 | -5.69 | Rpl37a | 19981 | -2.18 | Notch1 | 18128 | -1.51 | Epc1 | 13831 | -2.03 | -2.28 | Fnbp4 | 55935 | -1.81 | |||
| DNA replication/Repair | Rpl13 | 270106 | -2.57 | Ikbkg | 16151 | -1.52 | Socs3 | 12702 | -2.41 | Ccdc53 | 67282 | -1.85 | |||||||
| Polg | 18975 | -1.55 | Rpl22 | 19934 | -2.59 | Chd8 | 67772 | -1.52 | Negative regulation of transcription | Phf1 | 21652 | -1.86 | |||||||
| E4f1 | 13560 | -1.63 | Protein phosphorylation | Rasa3 | 19414 | -1.54 | from RNA polymerase II promoter | Gtf2ird2 | 114674 | -1.87 | |||||||||
| Sfpq | 71514 | -1.54 | -1.75 | Tesk1 | 21754 | -1.59 | Mapk14 | 26416 | -1.55 | -1.54 | Cutl1 | 13047 | -1.52 | Numa1 | 101706 | -1.57 | -1.88 | ||
| Xpc | 22591 | -2.52 | Mast3 | 546071 | -1.59 | Mfng | 17305 | -1.56 | Id3 | 15903 | -1.77 | Brd1 | 223770 | -1.9 | |||||
| Nfix | 18032 | -6.12 | Prkcq | 18761 | -1.64 | Frap1 | 56717 | -1.57 | Bcl6 | 12053 | -1.85 | Plekha1 | 101476 | -1.91 | |||||
| Ubiquitin/Proteolysis/Proteasome | Jak1 | 16451 | -1.52 | -1.75 | Itpkb | 320404 | -1.81 | -1.59 | Tcf25 | 66855 | -2.02 | Frmd6 | 319710 | -1.93 | -1.98 | ||||
| Usp3 | 235441 | -1.54 | Csnk1g2 | 103236 | -1.75 | Xab2 | 67439 | -1.59 | Tle1 | 21885 | -3.63 | Akap8l | 54194 | -1.98 | |||||
| Fbxo38 | 107035 | -1.57 | Ptpn21 | 24000 | -1.68 | -1.87 | Dtx2 | 74198 | -1.77 | -1.6 | Foxp1 | 108655 | -1.67 | -4.93 | Grwd1 | 101612 | -1.99 | ||
| Ubl4 | 27643 | -1.62 | Prkce | 18754 | -1.96 | Dicer1 | 192119 | -1.77 | -1.63 | Transport (Protein/Ion) | Otud5 | 54644 | -1.99 | ||||||
| Usp9x | 22284 | -1.64 | Map4k4 | 26921 | -2.44 | Srpk2 | 20817 | -1.53 | -1.68 | Mcfd2 | 193813 | -1.52 | Etnk1 | 75320 | -2.02 | ||||
| Fbxo9 | 71538 | -1.72 | Tec | 21682 | -2.47 | Mapk8 | 26419 | -1.58 | -1.73 | Bet1l | 54399 | -1.59 | Abcf3 | 27406 | -2.03 | ||||
| Usp19 | 71472 | -1.66 | -1.85 | Ikbke | 56489 | -2.52 | Stat6 | 20852 | -1.74 | Abcb8 | 74610 | -1.61 | Acyp1 | 66204 | -2.04 | ||||
| Rnpepl1 | 108657 | -1.56 | -2.04 | Ptpn23 | 104831 | -2.87 | Ppp2r5e | 26932 | -1.76 | Acox1 | 11430 | -1.65 | Nsun4 | 72181 | -2.06 | ||||
| Ube2d2 | 56550 | -1.77 | -2.06 | Prkd2 | 101540 | -2.98 | Phc2 | 54383 | -1.78 | Ecgf1 | 72962 | -1.65 | Tmem141 | 51875 | -2.07 | ||||
| Rbbp6 | 19647 | -1.5 | -2.1 | Transcription/Transcription regulation | Rrad | 56437 | -1.84 | Pitpnm1 | 18739 | -1.76 | Snord22 | 83673 | -2.07 | ||||||
| Zfp292 | 30046 | -2.31 | Zzz3 | 108946 | -1.51 | Stub1 | 56424 | -1.84 | Acadm | 11364 | -1.82 | Lrrc8a | 241296 | -1.6 | -2.1 | ||||
| Rffl | 67338 | -2.35 | Zmynd11 | 66505 | -1.51 | Arrb1 | 109689 | -1.87 | Mybbp1a | 18432 | -1.89 | Paqr7 | 71904 | -1.68 | -2.1 | ||||
| Klk8 | 259277 | -4.21 | Zkscan6 | 52712 | -1.53 | Azi2 | 27215 | -1.74 | -1.91 | Pitpnc1 | 71795 | -1.53 | -1.97 | Cdc42se2 | 72729 | -1.56 | -2.12 | ||
| Metabolism | Nfe2l1 | 18023 | -1.53 | Rai1 | 19377 | -1.61 | -1.91 | Scamp1 | 107767 | -1.59 | -1.99 | Bola2 | 66162 | -2.16 | |||||
| Tk2 | 57813 | -1.5 | Ezh1 | 14055 | -1.55 | Mfhas1 | 52065 | -1.98 | Hcn3 | 15168 | -2.12 | Crlf3 | 54394 | -1.55 | -2.21 | ||||
| Glb1l | 74577 | -1.51 | Scmh1 | 29871 | -1.56 | Foxo3a | 56484 | -1.99 | P2rx4 | 18438 | -2.44 | Zdhhc9 | 208884 | -2.26 | |||||
| Idua | 15932 | -1.52 | Zkscan3 | 72739 | -1.6 | Spred2 | 114716 | -2.07 | Ramp3 | 56089 | -2.54 | Gas5 | 14455 | -2.3 | |||||
| Glul | 14645 | -1.54 | Zkscan1 | 74570 | -1.69 | Gaa | 14387 | -2.13 | Laptm4b | 114128 | -1.88 | -2.88 | Tmem66 | 67887 | -2.33 | ||||
| Elovl6 | 170439 | -1.55 | Rarg | 19411 | -2.56 | -1.73 | Dvl1 | 13542 | -2.15 | Kcnn4 | 16534 | -3.1 | Rbm38 | 56190 | -2.39 | ||||
| Supt6h | 20926 | -1.61 | Zfx | 22764 | -1.73 | Il16 | 16170 | -1.64 | -2.26 | Rab3ip | 216363 | -3.12 | Jarid1b | 75605 | -2.4 | ||||
| Pcyt2 | 68671 | -1.69 | Mef2d | 17261 | -1.77 | Spsb1 | 74646 | -2.26 | Slco3a1 | 108116 | -3.18 | Marveld1 | 277010 | -2.47 | |||||
| Mthfr | 17769 | -1.71 | Ncor2 | 20602 | -1.6 | -1.81 | Gbp2 | 14469 | -2.29 | Slc12a7 | 20499 | -4.35 | Ctdsp2 | 52468 | -1.94 | -2.56 | |||
| Ndufa6 | 67130 | -1.85 | Zfml | 18139 | -1.79 | -2.05 | Crebbp | 12914 | -1.64 | -2.41 | Abca1 | 11303 | -4.42 | Armcx2 | 67416 | -2.68 | |||
| Pgs1 | 74451 | -2.1 | Zfp96 | 22758 | -2.06 | Macf1 | 11426 | -1.61 | -2.57 | Ramp1 | 51801 | -1.68 | -17.13 | Ipo4 | 75751 | -2.82 | |||
| Ihpk1 | 27399 | -2.26 | Zeb1 | 21417 | -2.11 | Il4ra | 16190 | -2.8 | Miscellaneous | Rabac1 | 14470 | -3.3 | |||||||
| Cbr1 | 12408 | -2.55 | Taf1a | 21339 | -2.28 | Ltb | 16994 | -2.81 | Tnip1 | 57783 | -1.69 | -1.51 | Plekho1 | 67220 | -3.54 | ||||
| Lpin1 | 14245 | -2.57 | Bach1 | 12013 | -1.55 | -2.34 | Eng | 13805 | -3.43 | Nphp1 | 53885 | -1.52 | Peli1 | 67245 | -1.94 | -4.03 | |||
| Man2c1 | 73744 | -2.88 | Klf3 | 16599 | -2.52 | Smad4 | 17128 | -1.53 | -3.53 | Isca1 | 69046 | -1.53 | Btbd14a | 67991 | -4.63 | ||||
| Gstk1 | 76263 | -2.99 | Jmjd1a | 104263 | -2.54 | Il6st | 16195 | -1.61 | -4.31 | Msl2l1 | 77853 | -1.54 | Rreb1 | 68750 | -7.28 | ||||
| Adcy6 | 11512 | -3.08 | Basp1 | 70350 | -2.71 | -2.64 | Smad1 | 17125 | -5.01 | Nol1 | 110109 | -1.56 | Cxxc5 | 67393 | -2.79 | -7.61 | |||
| Dph5 | 69740 | -3.81 | Ash1l | 192195 | -1.83 | -2.84 | Mettl4 | 76781 | -1.58 | -5.5 | Dctn1 | 13191 | -1.56 | ||||||
| Ldhb | 16832 | -1.97 | -4.83 | Zfp1 | 22640 | -2.91 | Notch2 | 18129 | -1.58 | -5.62 | Klhl7 | 52323 | -1.62 | ||||||
| Pdk1 | 228026 | -6.29 | Zbtb20 | 56490 | -2.61 | -3.08 | Thra | 21833 | -5.8 | Sh3gl1 | 20405 | -1.63 | |||||||
| RNA processing | Dbp | 13170 | -1.96 | -3.63 | Inadl | 12695 | -6.08 | Mgea6 | 217615 | -1.65 | |||||||||
| Rpusd4 | 71989 | -1.51 | Smad7 | 17131 | -1.87 | -3.68 | Il6ra | 16194 | -18.59 | Bcl7b | 12054 | -1.66 | |||||||
| Imp3 | 102462 | -1.54 | Irf6 | 54139 | -2.29 | -4.63 | Cell adhesion/Communication | Gltscr2 | 68077 | -1.7 | |||||||||
| Mphosph10 | 67973 | -1.62 | Ssbp2 | 66970 | -6.88 | Nisch | 64652 | -1.74 | -2.16 | Serf2 | 378702 | -1.7 | |||||||
| Prpf38b | 66921 | -1.65 | Chromatin modification/Assembly | Itga6 | 16403 | -1.67 | -3.24 | Dhx30 | 72831 | -1.71 | |||||||||
| Nola3 | 66181 | -1.8 | Chd1 | 12648 | -1.59 | Cep350 | 74081 | -1.57 | -1.74 | ||||||||||
| Utp20 | 70683 | -2.32 | Smarca2 | 67155 | -2.44 | Cramp1l | 57354 | -1.78 | -1.74 | ||||||||||
Role of chromatin modifying enzyme Ezh2 in CD8+ T cells
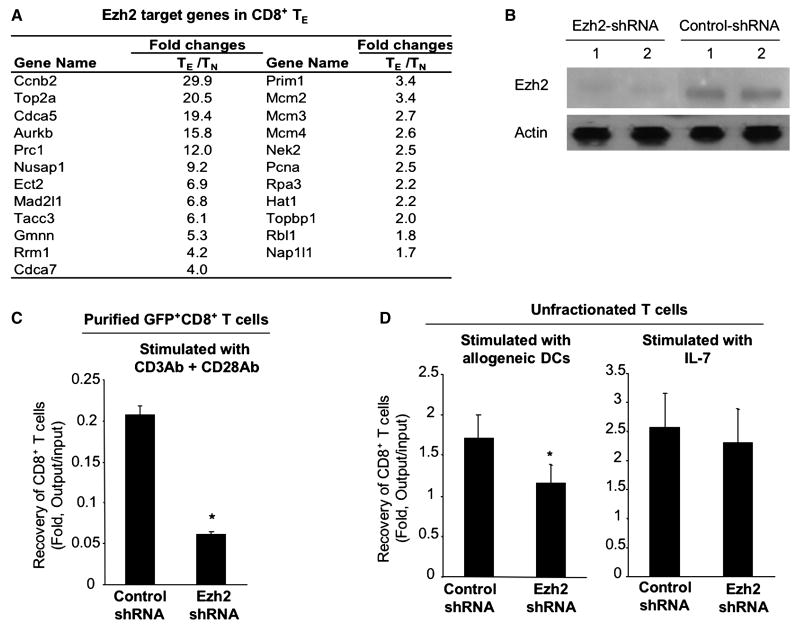
Data from previous studies demonstrate that the loss of Ezh2 in mature T cells impairs their proliferative response to anti-CD3 Ab (58). We observed that Ezh2 mRNA and protein were significantly increased in alloreactive CD8+ TE (Fig.5 B and C). Flow cytometry analysis showed at the single cell level that all day 14 CD8+ TE expressed higher levels of Ezh2 protein than TN (Fig.5D). Further tests using MSigDBv2 demonstrated that alloreactive CD8+ TE activated 23 of 30 Ezh2 target or partner genes previously identified by others(54) (Fig.6A). Ex vivo culture confirmed that purified Ezh2-shRNA GFP+CD8+ TN had reduced expression of Ezh2 protein (Fig.6B) and decreased their expansion by approximate 4-folds in response to anti-CD3 and anti-CD28 Abs as compared to Control-shRNA GFP+CD8+ T cells (Fig.6C). Thus, Ezh2 may play important roles in antigen-activated CD8+ T cells.
Fig.6.
Identification of stem cell gene Ezh2 in alloreactive CD8+ T cells. (A) The relative expression of 23 Ezh2 target or partner genes that were also selected as increased in alloreactive CD8+ TE relative to TN. (B) Western blot shows Ezh2 protein in CD8+ TN expressing with Ezh2-shRNA or Control-shRNA. These Ezh2-shRNA GFP+CD8+ TN were derived from B6 mice reconstituted with HSCs infected by Ezh2-shRNA/GFP-pLVPToff. GFP+CD8+ TN from B6 mice reconstituted with Con-shRNA/GFP-pLVPToff were isolated as controls. (C) Sorted Ezh2-shRNA GFP+CD8+ TN and Control-shRNA GFP+CD8+ TN (1×105 / well, 96 well plate) were stimulated with anti-CD3Ab and anti-CD28 (2.5 μg/ml for each). Five days later, cells were recovered and analyzed with flow cyotmetry for measuring GFP+CD8+ T cells. (D) Unfractionated Ezh2-shRNA GFP+CD8+ TN were stimulated with BABL/c mouse-derived DCs, or with IL-7 alone. Five days later, cells were recovered and analyzed using flow cytometry for measuring GFP+CD8+ T cells. Fold change of GFP+CD8+ T cells was calculated based on the output number of GFP+CD8+ T cells after culture divided by the input number of GFP+CD8+ T cells before culture. Data shown in (B, C and D) are representative of two independent experiments.
We further asked whether Ezh2 inhibition had differential effects on alloantigen stimulated versus homeostatic cytokine IL-7 mediated CD8+ T cell proliferation. Since our previous studies suggest that purified mouse T cells rapidly diminish in cultures in the absence of DCs (30), we stimulated unfractionated Ezh2-shRNA GFP+CD8+ TN with allogeneic DCs or with IL-7. Five days later, cells were recovered from the culture and analyzed for the expansion GFP+CD8+ T cells using flow cytometry. Interestingly, as compared to Control-shRNA GFP+CD8+ T cells, the expansion of Ezh2-shRNA GFP+CD8+ T cells was decreased in the culture supplemented with allogeneic DCs but not with IL-7 (Fig.6D). This suggests that Ezh2 may be required for antigen-driven T cell responses rather than homeostatic T cell proliferation.
Discussion
These studies identify a group of stem cell genes that are normally expressed in ESCs and NSCs in alloreactive CD8+ T cells. Most of these stem cell genes are found to be essential to cell cycle regulation, DNA replication and repair, stress resistance, chromatin modification and transcription regulation. One of these genes, Ezh2, emerges as an important regulator for the proliferation of antigen-activated CD8+ T cells. On the other hand, alloreactive CD8+ TE increase the expression of many genes that mediate cell apoptosis and growth arrest. We demonstrate that these alloreactive CD8+ TE were rapidly diminished in vivo in lethally irradiated secondary congenic recipients, suggesting that homeostatic factors alone are not sufficient to sustaining alloreactive CD8+ TE. However, upon chronic exposure to alloantigens alloreactive CD8+ TE proliferate to persist in vivo despite their massive apoptotic death. These data indicated that although alloreactive CD8+ TE were “terminally differentiated” with drastically increased susceptibility to apoptotic death, they had the ability to survive and persist via the mechanisms of continuous replication in the presence of alloantigens. Thus, these newly identified stem cell genes could be important targets for understanding and modulating allogeneic T cell responses and GVHD.
T cells are known to be stem cell-like cells (1, 3-4). Gene expression profile analysis reveals that memory T cells may trigger HSC-related transcriptional programs to regulate their self-renewal in the absence of antigens (17). We found that alloreactive CD8+ TE activated stem cell transcriptional programs which are operational in both ESCs and NSCs, while genes decreased in CD8+ TE were over-represented in HSC-enriched genes. Our observations were further validated by our re-analyzing the gene expression profile of Sarkar et al.(42), which revealed that ESC- and NSC-related genes were activated in LCMV gp33-specific CD8+ TE, but decreased in CD8+ memory T cells. It appears that functional T cell subsets can be defined by different stem cell transcriptional programs which regulate their unique properties of TE versus memory T cells. It is likely that alloreactive CD8+ TE require ESC- and NSC-related transcriptional programs to generate a large number of functionally active effectors sufficient to eliminate the target antigen.
Interestingly, reactivation of stem cell transcriptional programs occurs in both alloantigen-specific CD8+ TE generated during GVHD and viral antigen-reactive CD8+ TE derived from acute infection. We identified these CD8+ TE-related stem cell transcriptional programs using curated gene lists from MSigDBv2 (40). Although it has been reported that stem cell transcriptional profiles identified by different groups do not correlate well to each other (59), we found that approximately 30% of the genes increased in our alloreactive CD8+ TE are present on ESC and/or NSC gene lists of Ramalho-Santos et al.(41). Other studies also clearly showed a significant correlation of stem cell gene expression profiles identified by different groups (41, 60). Furthermore, we independently validated that the gene expression profiles of our alloreactive CD8+ TE were correlated significantly with those P14 CD8+ TE of Sarkar et al.(42). Thus, these CD8+ TE-related stem cell transcriptional programs may have significant implications in regulating T cells across various types of immune responses.
Overlapped gene expression profiles between CD8+ TE and embryonic and neural stem cells suggest that these cells could share some common properties. ESCs and NSCs characterized in Rammalho-Santos' study are highly proliferating stem cells, whereas HSCs are quiescent cells (41). In their study, ESCs were derived from the inner cell mass of the blastocyst stage of embryos, whereas NSCs were isolated from brain-derived neurospheres. Both ESCs and NSCs were highly purified after ex vivo cultures for gene expression profile analyses. In contrast, HSCs were freshly isolated from BM of normal B6 mice based on dual-dye efflux and HSC markers (41). We found that many of these CD8+ TE-related stem cell genes were associated with cell cycle regulation, DNA replication and repair, and stress resistance. This was in agreement with our findings that a proportion of alloreactive CD8+ TE continually proliferated upon chronic exposure to alloantigens. Thus, genes controlling proliferation of ESCs and NSCs are an important component of the similarity between CD8+ TE and embryonic and neural stem cells.
However, CD8+ TE did not increase the expression of genes associated with pluripotency of ESCs. In contrast, they activated many other stem cell genes that are found to be important for controlling cell fate, differentiation, survival, self-renewal and memory function in ESCs and NSCs, such as Uhrf1,Tacc3, Hells, Birc5, and Ezh2 (37, 49-54, 61). For example, Ezh2 binds to chromatin and DNA during cell dividing, thereby preserving transcriptional programs and cell identity established during earlier response phase (62-63). We found that CD8+ TE had the ability to sustain their effector functions over numerous rounds of cell dividing upon chronic exposure to allogeneic stimuli. Previous reports have also suggested that CD8+ TE can become self-renewing memory T cells upon clearance of the target antigen (4-6, 8, 22). These observations suggest that CD8+ TE share some common properties with ESCs and NSCs in the expression of stem cell transcriptional programs that are engaged in cell fate decision, self-renewal, survival, differentiation and memory function.
Several lines of evidence suggest that Ezh2 may be important for antigen-driven T cell responses. We found that Ezh2 was abundantly expressed in antigen-activated CD8+ T cells but not in CD8+ TN. Silencing Ezh2 inhibited CD8+ T cell proliferation activated by TCD/CD28 costimulation and allogeneic DCs, which is consistent with a previous report of others (58). Interestingly, knockdown of Ezh2 did not affect mature T cells to proliferate in response to homeostatic cytokine IL-7 alone. Thus, it is unlikely that inhibition of Ezh2 in alloreactive TE can globally affect donor T cell immunity after allogeneic HSCT. However, further studies are necessary to investigate the impact of Ezh2 inhibition in antigen-activated T cell responses and GVHD.
Our results suggest that APCs may play an important role in regulating stem cell transcriptional programs in CD8+ T cells. We found that alloreactive CD8+ TE continuously replicated in secondary allogeneic recipients and caused severe GVHD, but rapidly diminished in congenic recipients where alloantigens were absent. Thus, allogeneic stimuli rather than homeostatic factors are critical to the continuous replication in vivo of alloreactive CD8+ TE. This may explain why APCs are important for alloreactive T cell-mediated GVHD at both the induction and effector phase (13, 23, 64-66). Other studies suggest that antigenic stimulation is also necessary for protective immunity during chronic infection (67-69). It is likely that antigen stimulation sustains the replication of TE through the activation of stem cell transcriptional programs. However, other non-antigenic stimuli, such as inflammatory cytokines and co-stimulatory signals, could also be important for regulating stem cell transcriptional programs in CD8+ TE. For example, CD4+ T cells are found to be important for in vivo development of long- lasting CD8+ memory T cells and are required for mediating chronic GVHD (70-72). It is possible that signals derived from CD4+ help T cells might affect the expression of these stem cell genes in antigen-activated CD8+ T cells.
Stem cell transcriptional programs may also play an important role in alloreactive CD8+ TMSC. Gene expression profile analysis showed that these CD8+ TMSC were less differentiated as they did not produce cytotoxic molecules and inflammatory cytokines. A recent study suggests that activation of Wnt-signaling arrests the effector differentiation while enhancing the generation of CD8+ TMSC with greater ability than mature memory T cells to proliferate and generate tumor-reactive TE in vivo (21). This further supports that CD8+ TMSC themselves do not mediate tissue injury, but can further differentiate into functional CD8+ TE (15). Notably, 70% of stem cell genes activated in alloreactive CD8+ TMSC remained increased in CD8+ TE. It will be interesting to determine how these ESC- and NSC-related genes affect the proliferation and differentiation of CD8+ TMSC.
In summary, we have identified that “terminally differentiated” alloreactive CD8+ T cells activate stem cell transcriptional programs that are normally expressed in ESCs and NSCs. This group of stem cell genes may play important roles in regulating the proliferation and persistence of alloreactive T cells upon chronic exposure to alloantigens in GVHD. Further exploring the specific roles of ESC- and NSC-related stem cell genes in chronically activated T cells could have significant impact on understanding and modulating pathogenic T cell responses in many other inflammatory conditions, such as chronic infections, autoimmune diseases and rejection of grafted solid organs.
Acknowledgments
This work is supported by grant of R01 CA102464 from National Institutes of Health, Damon-Runyon Rachleff Innovation Award, Start-up funding from Department of Internal Medicine and Medical School, University of Michigan, and developmental funds from the Cancer Center Core Grant, University of Michigan. We are grateful for the technical support of Elizabeth Bacon (Department of Medicine, University of Michigan), thoughtful comments by Ivan Maillard of Departments of Medicine & Cell and Developmental Biology, University of Michigan) and edits by Evelyn Nieves (Department of Internal Medicine, University of Michigan).
Footnotes
Financial disclosure: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fearon DT, Manders P, Wagner SD. Arrested differentiation, the self-renewing memory lymphocyte, and vaccination. Science. 2001;293:248–250. doi: 10.1126/science.1062589. [DOI] [PubMed] [Google Scholar]
- 2.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 3.Hinrichs CS, Gattinoni L, Restifo NP. Programming CD8+ T cells for effective immunotherapy. Curr Opin Immunol. 2006;18:363–370. doi: 10.1016/j.coi.2006.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joshi NS, Kaech SM. Effector CD8 T cell development: a balancing act between memory cell potential and terminal differentiation. J Immunol. 2008;180:1309–1315. doi: 10.4049/jimmunol.180.3.1309. [DOI] [PubMed] [Google Scholar]
- 5.Bannard O, Kraman M, Fearon DT. Secondary replicative function of CD8+ T cells that had developed an effector phenotype. Science. 2009;323:505–509. doi: 10.1126/science.1166831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prlic M, Bevan MJ. Exploring regulatory mechanisms of CD8+ T cell contraction. Proc Natl Acad Sci U S A. 2008;105:16689–16694. doi: 10.1073/pnas.0808997105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 8.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 9.Goker H, Haznedaroglu IC, Chao NJ. Acute graft-vs-host disease: pathobiology and management. Exp Hematol. 2001;29:259–277. doi: 10.1016/s0301-472x(00)00677-9. [DOI] [PubMed] [Google Scholar]
- 10.Ho VT, Soiffer RJ. The history and future of T-cell depletion as graft-versus-host disease prophylaxis for allogeneic hematopoietic stem cell transplantation. Blood. 2001;98:3192–3204. doi: 10.1182/blood.v98.12.3192. [DOI] [PubMed] [Google Scholar]
- 11.Higman MA, Vogelsang GB. Chronic graft versus host disease. Br J Haematol. 2004;125:435–454. doi: 10.1111/j.1365-2141.2004.04945.x. [DOI] [PubMed] [Google Scholar]
- 12.Blazar BR, Murphy WJ. Bone marrow transplantation and approaches to avoid graft-versus-host disease (GVHD) Philos Trans R Soc Lond B Biol Sci. 2005;360:1747–1767. doi: 10.1098/rstb.2005.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7:340–352. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Alloreactive Memory T Cells Are Responsible for the Persistence of Graft-versus-Host Disease. J Immunol. 2005;174:3051–3058. doi: 10.4049/jimmunol.174.5.3051. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat Med. 2005;11:1299–1305. doi: 10.1038/nm1326. [DOI] [PubMed] [Google Scholar]
- 16.Ferrara JL, Reddy P. Pathophysiology of graft-versus-host disease. Semin Hematol. 2006;43:3–10. doi: 10.1053/j.seminhematol.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Luckey CJ, Bhattacharya D, Goldrath AW, Weissman IL, Benoist C, Mathis D. Memory T and memory B cells share a transcriptional program of self-renewal with long-term hematopoietic stem cells. Proc Natl Acad Sci U S A. 2006;103:3304–3309. doi: 10.1073/pnas.0511137103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, Killeen N, Orange JS, Russell SM, Weninger W, Reiner SL. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 19.Reiner SL, Sallusto F, Lanzavecchia A. Division of labor with a workforce of one: challenges in specifying effector and memory T cell fate. Science. 2007;317:622–625. doi: 10.1126/science.1143775. [DOI] [PubMed] [Google Scholar]
- 20.Staal FJ, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol. 2008;8:581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- 21.Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, Paulos CM, Muranski P, Restifo NP. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Louboutin JP, Zhu J, Rivera AJ, Emerson SG. Preterminal host dendritic cells in irradiated mice prime CD8+ T cell-mediated acute graft-versus-host disease. J Clin Invest. 2002;109:1335–1344. doi: 10.1172/JCI14989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dulude G, Roy DC, Perreault C. The effect of graft-versus-host disease on T cell production and homeostasis. J Exp Med. 1999;189:1329–1342. doi: 10.1084/jem.189.8.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Harada A, Wang JB, Zhang YY, Hashimoto S, Naito M, Matsushima K. Bifurcated dendritic cell differentiation in vitro from murine lineage phenotype-negative c-kit+ bone marrow hematopoietic progenitor cells. Blood. 1998;92:118–128. [PubMed] [Google Scholar]
- 26.Shedden K, Chen W, Kuick R, Ghosh D, Macdonald J, Cho KR, Giordano TJ, Gruber SB, Fearon ER, Taylor JM, Hanash S. Comparison of seven methods for producing Affymetrix expression scores based on False Discovery Rates in disease profiling data. BMC Bioinformatics. 2005;6:26. doi: 10.1186/1471-2105-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szulc J, Wiznerowicz M, Sauvain MO, Trono D, Aebischer P. A versatile tool for conditional gene expression and knockdown. Nat Methods. 2006;3:109–116. doi: 10.1038/nmeth846. [DOI] [PubMed] [Google Scholar]
- 28.Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt RG, Otte AP, Rubin MA, Chinnaiyan AM. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–629. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- 29.Zhu J, Zhang Y, Joe GJ, Pompetti R, Emerson SG. NF-Ya activates multiple hematopoietic stem cell (HSC) regulatory genes and promotes HSC self-renewal. Proc Natl Acad Sci U S A. 2005;102:11728–11733. doi: 10.1073/pnas.0503405102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Joe G, Zhu J, Carroll R, Levine B, Hexner E, June C, Emerson SG. Dendritic cell-activated CD44hiCD8+ T cells are defective in mediating acute graft-versus-host disease but retain graft-versus-leukemia activity. Blood. 2004;103:3970–3978. doi: 10.1182/blood-2003-09-3135. [DOI] [PubMed] [Google Scholar]
- 31.Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002;2:547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 32.Korngold R, Wettstein PJ. Immunodominance in the graft-vs-host disease T cell response to minor histocompatibility antigens. J Immunol. 1990;145:4079–4088. [PubMed] [Google Scholar]
- 33.Ryu SJ, Jung KM, Yoo HS, Kim TW, Kim S, Chang J, Choi EY. Cognate CD4 help is essential for the reactivation and expansion of CD8 memory T cells directed against the hematopoietic cell-specific dominant minor histocompatibility antigen, H60. Blood. 2009;113:4273–4280. doi: 10.1182/blood-2008-09-181263. [DOI] [PubMed] [Google Scholar]
- 34.Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, Serody JS. Leukocyte migration and graft-versus-host disease. Blood. 2005;105:4191–4199. doi: 10.1182/blood-2004-12-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bracken AP, Kleine-Kohlbrecher D, Dietrich N, Pasini D, Gargiulo G, Beekman C, Theilgaard-Monch K, Minucci S, Porse BT, Marine JC, Hansen KH, Helin K. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev. 2007;21:525–530. doi: 10.1101/gad.415507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gil J, Peters G. Regulation of the INK4b-ARF-INK4a tumour suppressor locus: all for one or one for all. Nat Rev Mol Cell Biol. 2006;7:667–677. doi: 10.1038/nrm1987. [DOI] [PubMed] [Google Scholar]
- 37.Sparmann A, van Lohuizen M. Polycomb silencers control cell fate, development and cancer. Nat Rev Cancer. 2006;6:846–856. doi: 10.1038/nrc1991. [DOI] [PubMed] [Google Scholar]
- 38.Yuan Y, Yu H, Boyer MJ, Song X, Cao S, Shen H, Cheng T. Hematopoietic stem cells are not the direct target of spontaneous leukemic transformation in p18(INK4C)-null reconstituted mice. Cancer Res. 2006;66:343–351. doi: 10.1158/0008-5472.CAN-05-2945. [DOI] [PubMed] [Google Scholar]
- 39.Kovalev GI, Franklin DS, Coffield VM, Xiong Y, Su L. An important role of CDK inhibitor p18(INK4c) in modulating antigen receptor-mediated T cell proliferation. J Immunol. 2001;167:3285–3292. doi: 10.4049/jimmunol.167.6.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. “Stemness”: transcriptional profiling of embryonic and adult stem cells. Science. 2002;298:597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- 42.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol. 2002;2:982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- 44.Stepanyan Z, Hughes B, Cliche DO, Camp D, Hekimi S. Genetic and molecular characterization of CLK-1/mCLK1, a conserved determinant of the rate of aging. Exp Gerontol. 2006;41:940–951. doi: 10.1016/j.exger.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 45.Weissman AM. Themes and variations on ubiquitylation. Nat Rev Mol Cell Biol. 2001;2:169–178. doi: 10.1038/35056563. [DOI] [PubMed] [Google Scholar]
- 46.Hilario J, Amitani I, Baskin RJ, Kowalczykowski SC. Direct imaging of human Rad51 nucleoprotein dynamics on individual DNA molecules. Proc Natl Acad Sci U S A. 2009;106:361–368. doi: 10.1073/pnas.0811965106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kume A, Koyata H, Sakakibara T, Ishiguro Y, Kure S, Hiraga K. The glycine cleavage system. Molecular cloning of the chicken and human glycine decarboxylase cDNAs and some characteristics involved in the deduced protein structures. J Biol Chem. 1991;266:3323–3329. [PubMed] [Google Scholar]
- 48.Board PG, Coggan M, Chelvanayagam G, Easteal S, Jermiin LS, Schulte GK, Danley DE, Hoth LR, Griffor MC, Kamath AV, Rosner MH, Chrunyk BA, Perregaux DE, Gabel CA, Geoghegan KF, Pandit J. Identification, characterization, and crystal structure of the Omega class glutathione transferases. J Biol Chem. 2000;275:24798–24806. doi: 10.1074/jbc.M001706200. [DOI] [PubMed] [Google Scholar]
- 49.Hashimoto H, Horton JR, Zhang X, Bostick M, Jacobsen SE, Cheng X. The SRA domain of UHRF1 flips 5-methylcytosine out of the DNA helix. Nature. 2008;455:826–829. doi: 10.1038/nature07280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xi S, Zhu H, Xu H, Schmidtmann A, Geiman TM, Muegge K. Lsh controls Hox gene silencing during development. Proc Natl Acad Sci U S A. 2007;104:14366–14371. doi: 10.1073/pnas.0703669104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piekorz RP, Hoffmeyer A, Duntsch CD, McKay C, Nakajima H, Sexl V, Snyder L, Rehg J, Ihle JN. The centrosomal protein TACC3 is essential for hematopoietic stem cell function and genetically interfaces with p53-regulated apoptosis. Embo J. 2002;21:653–664. doi: 10.1093/emboj/21.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fukuda S, Pelus LM. Survivin, a cancer target with an emerging role in normal adult tissues. Mol Cancer Ther. 2006;5:1087–1098. doi: 10.1158/1535-7163.MCT-05-0375. [DOI] [PubMed] [Google Scholar]
- 53.O'Carroll D, Erhardt S, Pagani M, Barton SC, Surani MA, Jenuwein T. The polycomb-group gene Ezh2 is required for early mouse development. Mol Cell Biol. 2001;21:4330–4336. doi: 10.1128/MCB.21.13.4330-4336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kamminga LM, Bystrykh LV, de Boer A, Houwer S, Douma J, Weersing E, Dontje B, de Haan G. The Polycomb group gene Ezh2 prevents hematopoietic stem cell exhaustion. Blood. 2006;107:2170–2179. doi: 10.1182/blood-2005-09-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ezhkova E, Pasolli HA, Parker JS, Stokes N, Su IH, Hannon G, Tarakhovsky A, Fuchs E. Ezh2 orchestrates gene expression for the stepwise differentiation of tissue-specific stem cells. Cell. 2009;136:1122–1135. doi: 10.1016/j.cell.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 57.Zon LI. Intrinsic and extrinsic control of haematopoietic stem-cell self-renewal. Nature. 2008;453:306–313. doi: 10.1038/nature07038. [DOI] [PubMed] [Google Scholar]
- 58.Su IH, Dobenecker MW, Dickinson E, Oser M, Basavaraj A, Marqueron R, Viale A, Reinberg D, Wulfing C, Tarakhovsky A. Polycomb group protein ezh2 controls actin polymerization and cell signaling. Cell. 2005;121:425–436. doi: 10.1016/j.cell.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 59.Fortunel NO, Otu HH, Ng HH, Chen J, Mu X, Chevassut T, Li X, Joseph M, Bailey C, Hatzfeld JA, Hatzfeld A, Usta F, Vega VB, Long PM, Libermann TA, Lim B. Comment on “‘Stemness’: transcriptional profiling of embryonic and adult stem cells” and “a stem cell molecular signature”. Science. 2003;302:393. doi: 10.1126/science.1086384. author reply 393. [DOI] [PubMed] [Google Scholar]
- 60.Wong DJ, Liu H, Ridky TW, Cassarino D, Segal E, Chang HY. Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell. 2008;2:333–344. doi: 10.1016/j.stem.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ringrose L, Paro R. Epigenetic regulation of cellular memory by the Polycomb and Trithorax group proteins. Annu Rev Genet. 2004;38:413–443. doi: 10.1146/annurev.genet.38.072902.091907. [DOI] [PubMed] [Google Scholar]
- 62.Francis NJ, Follmer NE, Simon MD, Aghia G, Butler JD. Polycomb proteins remain bound to chromatin and DNA during DNA replication in vitro. Cell. 2009;137:110–122. doi: 10.1016/j.cell.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hansen KH, Bracken AP, Pasini D, Dietrich N, Gehani SS, Monrad A, Rappsilber J, Lerdrup M, Helin K. A model for transmission of the H3K27me3 epigenetic mark. Nat Cell Biol. 2008;10:1291–1300. doi: 10.1038/ncb1787. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y, Shlomchik WD, Joe G, Louboutin JP, Zhu J, Rivera A, Giannola D, Emerson SG. APCs in the liver and spleen recruit activated allogeneic CD8+ T cells to elicit hepatic graft-versus-host disease. J Immunol. 2002;169:7111–7118. doi: 10.4049/jimmunol.169.12.7111. [DOI] [PubMed] [Google Scholar]
- 65.Merad M, Hoffmann P, Ranheim E, Slaymaker S, Manz MG, Lira SA, Charo I, Cook DN, Weissman IL, Strober S, Engleman EG. Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease. Nat Med. 2004;10:510–517. doi: 10.1038/nm1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haniffa M, Ginhoux F, Wang XN, Bigley V, Abel M, Dimmick I, Bullock S, Grisotto M, Booth T, Taub P, Hilkens C, Merad M, Collin M. Differential rates of replacement of human dermal dendritic cells and macrophages during hematopoietic stem cell transplantation. J Exp Med. 2009;206:371–385. doi: 10.1084/jem.20081633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci U S A. 2004;101:16004–16009. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dudani R, Chapdelaine Y, Faassen Hv H, Smith DK, Shen H, Krishnan L, Sad S. Multiple mechanisms compensate to enhance tumor-protective CD8(+) T cell response in the long-term despite poor CD8(+) T cell priming initially: comparison between an acute versus a chronic intracellular bacterium expressing a model antigen. J Immunol. 2002;168:5737–5745. doi: 10.4049/jimmunol.168.11.5737. [DOI] [PubMed] [Google Scholar]
- 69.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 70.Zhang C, Todorov I, Zhang Z, Liu Y, Kandeel F, Forman S, Strober S, Zeng D. Donor CD4+ T and B cells in transplants induce chronic graft-versus-host disease with autoimmune manifestations. Blood. 2006;107:2993–3001. doi: 10.1182/blood-2005-09-3623. [DOI] [PubMed] [Google Scholar]
- 71.Chu YW, Gress RE. Murine models of chronic graft-versus-host disease: insights and unresolved issues. Biol Blood Marrow Transplant. 2008;14:365–378. doi: 10.1016/j.bbmt.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y, Hexner E, Frank D, Emerson SG. CD4+ T cells generated de novo from donor hemopoietic stem cells mediate the evolution from acute to chronic graft-versus-host disease. J Immunol. 2007;179:3305–3314. doi: 10.4049/jimmunol.179.5.3305. [DOI] [PubMed] [Google Scholar]