Abstract
Complement factor I (CFI) mutations are implicated in the pathogenesis of atypical hemolytic uremic syndrome (aHUS). Nevertheless, there is evidence that CFI deficiency is a weak effector of aHUS. Bienaime et al. report that homozygous deletion of CFHR-1 in the RCA gene cluster of chromosome 1q is a major risk factor for poor outcome for patients with CFI mutations. The basic and clinical implications of the findings are further elaborated here.
Remarkable advances have been made in understanding the pathophysiology of thrombotic microangiopathy. As exemplified by thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS), thrombotic microangiopathy is characterized by thrombotic occlusion of the arterioles and capillaries. The thrombosis may involve the brain, kidney, and other vital organs and is associated with serious morbidity and high mortality. With the exception of Streptococcus pneumoniae neuraminidase and the shiga toxins of Escherichia coli or related microorganisms, the pathogenesis of thrombotic microangiopathy remained a mystery until recent years.
It is now widely recognized that defective proteolysis of von Willebrand factor, due to genetic mutations of the ADAMTS13 gene or autoantibodies of the ADAMTS13 metalloprotease, causes the microvascular thrombosis of TTP.1 Among patients with atypical hemolytic uremic syndrome (aHUS), that is, those without evidence of infectious or other etiologies, three categories of abnormality have been detected in up to 50% of the cases: inactivating mutations involving the complement regulators, such as factor H (CFH), factor I (CFI), membrane cofactor protein (MCP, or CD46), C4b-binding protein, and thrombomodulin; autoantibodies of CFH; and gain-of-function mutations involving C3 convertase components, including C3 and complement factor B (CFB).2,3 All of these abnormalities involve the complement system. It is assumed that uncontrolled activation of the complement cascade causes endothelial injury and microvascular thrombosis of aHUS. Nevertheless, analysis of kindred suggests that the correlation between genetic mutations of the complement components and aHUS is anything but straightforward.
An obvious example of this complexity is the relatively low penetrance of the aHUS phenotype among patients with CFI mutations. Fremeaux-Bacchi et al. first described heterozygous mutations of CFI in three patients with aHUS.4 Yet neither of the two parents carrying the implicated mutations ever developed aHUS. Such low penetrance has been repeatedly observed in subsequently reported pedigrees of CFI mutations.
CFI is a plasma two-chain serine esterase that controls the amplification loop of the alternative complement pathway. CFI cleaves the α-chains of C3b and C4b in the presence of CFH or MCP as a cofactor. Inactivation of C3b and C4b prevents the formation of C3/C5 convertases. When CFI is deficient, relentless activation of the alternative pathway lowers the C3, factor B, CFH, and properdin levels. Consequently, deficiency of CFI is expected to predispose patients to bacterial infections.
Hereditary deficiency of CFI, previously known as C3b inactivator, was first described nearly four decades ago in a patient with a lifelong history of recurrent bacterial infection. 5 Since then, more than 24 pedigrees with homozygous CFI deficiency patients have been described. Recurrent bacterial infections have been the hallmark. Yet none of the patients with heterozygous or homozygous CFI deficiency was noted to develop HUS.
To reconcile this apparent discordance between CFI deficiency and aHUS, one may postulate that although it may be a risk factor, CFI deficiency per se is insufficient to cause HUS. What then are the other factors that are necessary to cause HUS in patients with CFI deficiency?
Bienaime and colleagues6 (this issue) identified exonic mutations of CFI in 23 of a cohort of 202 aHUS patients. Interestingly, 30% of the patients with CFI mutations carried at least one additional known genetic risk factor for aHUS, such as mutations in CFH, MCP, C3, or CFB. Additionally, 21% of the patients with CFI mutations carried a homozygous deletion of CFH-related protein 1 (CFHR-1). In contrast, homozygous deletion of CFHR-1 is detected in 2.9% of the control population.7
The conspicuous overrepresentation of homozygous CFHR-1 deletion or other defects again strongly suggests that CFHR-1 protects patients with CFI deficiency from developing aHUS. This interpretation is further supported by the patients’ outcomes. In the survival analysis, Bienaime et al. report that more than 95% of the patients with concurrent homozygous CFHR-1 gene deletion died or developed end-stage renal failure within 3 years.6 In contrast, after 10 years such events had occurred in only 40% of the patients with ‘exclusive’ CFI mutations. Although the series of Bienaime et al.6 includes small numbers of cases, the difference in the outcome between the groups with and without other mutations is striking. Further examination of the survival curves reveals that in the group with ‘exclusive’ CFI mutations, the events of death or end-stage renal failure occurred within the first 2 years of the disease onset, raising the possibility that those patients might have additional yet-unidentified abnormalities.
What may account for the difference in the outcome? Homozygous deletion of an 84-kb genomic segment in the RCA (regulators of complement activation) gene cluster of chromosome 1q, which encompasses CFHR-1 and CFHR-3, is a risk factor for aHUS, particularly in patients with autoantibodies of CFH or CFI mutations.7 Nevertheless, the deletion is quite common among normal subjects, indicating that deletion of CFHR-1 (and CFHR-3) is insufficient to cause aHUS or renal failure. Could the absence of the CFHR-1 protein aggravate renal injury of excessive complement activation caused by CFI deficiency? This scenario is certainly a possibility and is supported by a recent report that CFHR-1 blocks C5 convertase activity and the formation of the terminal complement complex (Figure 1).8 Nevertheless, one also needs to consider the possibility that the CFHR-1 deletion allele may be linked to other as-yet unidentified genetic variations that play a direct role in causing renal failure.
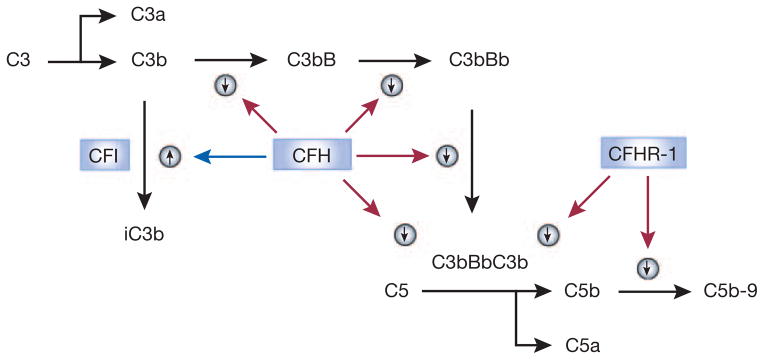
Figure 1. Deficiency of complement factor H-related protein 1 aggravates the atypical hemolytic uremic syndrome in patients with complement factor I (CFI) mutations.
This scheme depicts the roles of complement factor H (CFH), CFI, and CFH-related protein 1 (CFHR-1) in regulating the activation of the alternative complement pathway and the formation of the terminal complement complex C5b-9. Because activation of the alternative pathway is spontaneous, regulators are particularly critical for preventing its excessive activation. CFH, by binding to C3b, promotes the cleavage of the latter by CFI to iC3b. In addition, CFH also prevents the association of C3b with factor B, thereby preventing the formation of C3 convertase (C3bBb) and C5 convertase (C3bBbC3b). CFHR-1 binds to C5, inhibiting its activation by C5 convertase and the formation of the terminal complement complex (C5b-9). This scheme explains why, compared with CFH deficiency, CFI deficiency is a weak cause of atypical hemolytic uremic syndrome (aHUS). It also explains why CFHR-1 deficiency does not cause aHUS but markedly worsens aHUS in association with CFI deficiency. Other complement pathways, regulators, and receptors are not shown in this simplified scheme.
Another enigma is that different disease phenotypes, including infections, mem-branoproliferative glomerulonephritis type II (dense-deposit disease (DDD), OMIM #609814), and age-related macular degeneration (ARMD, OMIM # 603075) as well as aHUS, have been independently associated with mutations of CFH.9 In contrast, DDD has not been associated with CFI mutations. In fact, in mouse models of CFH deficiency, the development of DDD requires the presence of CFI.10 Furthermore, while the data of Bienaime et al.6 suggest that homozygous deletion of CFHR-1 is an important risk factor for poor outcome in patients with heterozygous CFI mutations, this same deletion genotype is reportedly protective against the development of ARMD.11 It is apparent that further mechanistic investigations will be needed to elucidate the pathophysiology of aHUS, ARMD, and DDD.
Current evidence suggests that in many cases aHUS is the consequence of epistatic interactions of two or more genetic mutations. Therefore, identification of one genetic mutation may not offer an adequate guide of therapeutic decisions in individual cases. It has been proposed that patients with mutations involving circulating proteins such as CFH and CFI respond well to plasma replacement therapy but have a very high risk of relapse after renal transplantation. In contrast, patients with mutations involving membrane-anchored proteins such as MCP do not respond well to plasma therapy but may be essentially cured with renal transplantation. Although this distinction has a rational basis, exceptions exist in published case reports. The distinction may be of little use for aHUS patients whose molecular defects are not completely defined.
As researchers continue the endeavor to attack the mysteries of aHUS, what can clinicians do to translate the recent advances into improved care for patients with aHUS? First, it is important to recognize that, although focal neurological deficits or mental changes are much more frequent in TTP and renal failure is much more prominent in aHUS, it is impossible to distinguish all cases of TTP, aHUS, and other types of thrombotic microangiopathy solely on a clinical basis. When a patient presents with thrombocytopenia and microangiopathic hemolysis, infectious etiologies, systemic autoimmune diseases such as lupus erythematosus, metastatic neoplasms, HELLP syndrome (hemolysis with elevated liver enzymes and low platelet count) during pregnancy, and potential medication culprits should be considered or excluded. For the diagnosis of TTP, measurements of ADAMTS13 activity and inhibitors are becoming more readily available, although appropriate interpretation of the test results requires attention to certain potential laboratory pitfalls. Global complement assess such as CH50 and AH50 and individual complement component measurements may be used to assess the integrity of the complement system. Nevertheless, these tests do not invariably detect abnormalities of the complement system and do not provide a specific molecular diagnosis. Most of the genetic and molecular tests are not yet available for disease characterization in clinical laboratories. Referral to research laboratories is encouraged. However, regulatory concern, access, cost, and lengthy turnaround times hamper the role that research laboratories will play in clinical practice.
With the defects of aHUS being characterized in an increasing number of cases at the genetic and molecular levels, clinicians face a mounting challenge of deciding how to apply the vast albeit incomplete knowledge to the management of individual patients. In this regard, measures to block complement activation with inhibitors such as eculizumab seem attractive for the treatment of aHUS.12,13 Nevertheless, clinical experience with this therapeutic modality is quite limited for aHUS, and serious questions remain unanswered. It is hoped that with further elucidation of the molecular mechanisms, better measures will become available in the near future to tackle the serious business of aHUS.
Acknowledgments
This work was supported in part by grant R01HL62136 from the National Heart, Lung, and Blood Institute of the US National Institutes of Health.
Footnotes
DISCLOSURE
The author declared no competing interests.
References
- 1.Tsai HM. Mechanisms of microvascular thrombosis in thrombotic thrombocytopenic purpura. Kidney Int Suppl. 2009;75 (Suppl 112):S11–S14. doi: 10.1038/ki.2008.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skerka C, Jozsi M, Zipfel PF, et al. Autoantibodies in haemolytic uraemic syndrome (HUS) Thromb Haemost. 2009;101:227–232. [PubMed] [Google Scholar]
- 3.Delvaeye M, Noris M, De VA, et al. Thrombomodulin mutations in atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:345–357. doi: 10.1056/NEJMoa0810739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fremeaux-Bacchi V, Dragon-Durey MA, Blouin J, et al. Complement factor I: a susceptibility gene for atypical haemolytic uraemic syndrome. J Med Genet [online. 2004;41:e84. doi: 10.1136/jmg.2004.019083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abramson N, Alper CA, Lachmann PJ, et al. Deficiency of C3 inactivator in man. J Immunol. 1971;107:19–27. [PubMed] [Google Scholar]
- 6.Bienaime F, Dragon-Durey M-A, Regnier CH, et al. Mutations in components of complement influence the outcome of Factor I-associated atypical hemolytic uremic syndrome. Kidney Int. 2010;77:339–349. doi: 10.1038/ki.2009.472. [DOI] [PubMed] [Google Scholar]
- 7.Dragon-Durey MA, Blanc C, Marliot F, et al. The high frequency of complement factor H related CFHR1 gene deletion is restricted to specific subgroups of patients with atypical haemolytic uraemic syndrome. J Med Genet. 2009;46:447–450. doi: 10.1136/jmg.2008.064766. [DOI] [PubMed] [Google Scholar]
- 8.Heinen S, Hartmann A, Lauer N, et al. Factor H-related protein 1 (CFHR-1) inhibits complement C5 convertase activity and terminal complex formation. Blood. 2009;114:2439–2447. doi: 10.1182/blood-2009-02-205641. [DOI] [PubMed] [Google Scholar]
- 9.Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9:729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 10.Rose KL, Paixao-Cavalcante D, Fish J, et al. Factor I is required for the development of membranoproliferative glomerulonephritis in factor H-deficient mice. J Clin Invest. 2008;118:608–618. doi: 10.1172/JCI32525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spencer KL, Hauser MA, Olson LM, et al. Deletion of CFHR3 and CFHR1 genes in age-related macular degeneration. Hum Mol Genet. 2008;17:971–977. doi: 10.1093/hmg/ddm369. [DOI] [PubMed] [Google Scholar]
- 12.Nurnberger J, Philipp T, Witzke O, et al. Eculizumab for atypical hemolytic-uremic syndrome. N Engl J Med. 2009;360:542–544. doi: 10.1056/NEJMc0808527. [DOI] [PubMed] [Google Scholar]
- 13.Gruppo RA, Rother RP. Eculizumab for congenital atypical hemolytic-uremic syndrome. N Engl J Med. 2009;360:544–546. doi: 10.1056/NEJMc0809959. [DOI] [PubMed] [Google Scholar]