Abstract
Radiation therapy is a relatively effective therapeutic method for localized prostate cancer (PCa) patients. However, radioresistance occurs in nearly 30% of patients treated with potentially curative doses. Therapeutic synergy between radiotherapy and androgen ablation treatment provides a promising strategy for improving the clinical outcome. Accordingly, the androgen deprivation-induced signaling pathway may also mediate radiosensitivity in PCa cells. The C4-2 cell line was derived from the androgen-sensitive LNCaP parent line under androgen-depleted condition and had acquired androgen-refractory characteristics. In our study, the response to radiation was evaluated in both LNCaP and C4–2. Results showed that C4-2 cells were more likely to survive from irradiation and appeared more aggressive in their resistance to radiation treatment compared with LNCaP, as measured by clonogenic assays and cell viability and cell cycle analyses. Gene expression analyses revealed that a set of genes involved in cell cycle arrest and DNA repair were differentially regulated in LNCaP and C4-2 in response to radiation, which was also consistent with the radiation-resistant property observed in C4-2 cells. These results strongly suggested that the radiation-resistant property may develop with progression of PCa to androgen-independent status. Not only can the LNCaP and C4-2 PCa progression model be applied for investigating androgen-refractory progression, but it can also be used to explore the development of radiation resistance in PCa.
Keywords: LNCaP, C4-2, prostate cancer, radiation response
Introduction
Prostate cancer (PCa) continues to be one of the most prevalent cancers in men of western countries. A total of 218 890 new PCa cases and 27 050 deaths from PCa were projected to occur in the United States in 2007 1. In the past 15 years, active screening for PCa has led to a significantly decreased proportion of patients presenting with high-risk, advanced disease 2. A majority of prostate carcinomas are now detected while the disease is still clinically localized and with intermediate or low risk-factor characteristics. Radiation is a preferred treatment option for localized PCa. However, clinical evidence reveals that conventional-dose radiation often does not provide complete tumor eradication and a small fraction of tumor cells survive the lethal effects of radiation and eventually repopulate the irradiated site, which results in radiorecurrent PCa and a 5-year distant metastasis-free survival of < 80% 3, 4, 5. The combination of radiation and androgen deprivation therapy provides an effective strategy for preventing treatment failure. Adjuvant androgen deprivation therapy has been shown to confer a survival advantage over radiation alone in high-risk localized PCa 6. Moreover, clinical observation also shows that patients with hormone-resistant (HR) PCa caused by long-term hormone treatment seem to have higher biochemical failure rates after radiation therapy. Previous studies also indicate that the response to radiation treatment is different between HR PCa cells and androgen-sensitive PCa cells 7. These observations indicate that molecular events mediated by AD may also function in radiosensitization, and that androgen-refractory development may be associated with radiation resistance in PCa.
LNCaP is an androgen-dependent, non-metastasis and marginally tumorigenic PCa cell line 8. The C4-2 subline was derived from LNCaP through interaction with stromal cells under androgen-depleted condition in castrated hosts. The C4-2 subline is tumorigenic in androgen-depleted environment, which indicates that C4-2 has the acquired characteristics of androgen independence 9, 10. LNCaP and C4-2 cells have the same genetic background and the unique advantage of remarkably mimicking the phenotypic and genotypic changes that are often observed in clinical human PCa. Even though LNCaP and C4-2 have provided a very useful model for studying the mechanism underlying the progression of PCa from androgen-dependent (AD) to androgen independent (AI) state, the model's response to radiation has not been studied systematically until now. In this study, the radiation responses of AD LNCaP and AI C4-2 were evaluated. As a result, androgen-refractory C4-2 cells were found to possess radiation-resistant properties compared with androgen-sensitive LNCaP. Taken together, our data strongly suggest that C4-2 also acquires radiation resistance during the process of transition to the androgen-refractory stage. Accordingly, LNCaP and C4-2 PCa cells may provide an ideal cell model for studying the molecular mechanism underlying PCa cells' progression from the radiation-sensitive state to resistance, and for investigating critical determinants in the efficacy of irradiation and androgen ablation therapy.
Materials and methods
Cell culture and reagents
LNCaP and C4-2 cell lines were cultured in RPMI–1640 (Invitrogen, Carlsbad, CA, USA) with 8% fetal bovine serum (FBS; Hyclone, Logan, UT, USA), 10 mmol L−1 HEPES and 1.0 mmol L−1 sodium bicarbonate. All cells were cultured at 37°C with 5% CO2 in a humidified incubator. To study the cell growth in androgen-depleted condition, LNCaP and C4-2 cells were cultured in fresh phenol red-free RPMI–1640 with 5%–10% dextran/charcoal absorbed fetal bovine serum (cFBS; Hyclone). G418 was obtained from GIBCO (Invitrogen), and Casodex was supplied by Sigma (Woodlands, TX, USA).
3-(4,5-Dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay
Cell growth rate was measured using an MTT proliferation assay. Briefly, 4 000–5 000 cells were seeded in 96-well plates. Cell growth was examined at the indicated time points. Before testing, 20 μL of MTT reagent (2.5 mg mL−1 MTT in PBS, Amresco Inc., Solon, OH, USA) was added and the cells were incubated for a further 4-h at 37°C. Then 150 μL of dissolving reagent DMSO (Amresco Inc., ) was added to dissolve the formazan crystals. The optical density (OD) was measured at wavelength of 490 nm on a microplate reader. Pilot experiments were conducted to determine the optimal cell concentration for the experiments.
Colony formation in soft agar
A total of 400–10 000 cells were suspended in 0.17% low melting agarose (Difco Laboratories, Detroit, MI, USA) dissolved in 2 mL of RPMI 1640 with 10% FBS or with 10% androgen-free cFBS medium and placed on top of a 2-mL underlayer of 0.5% agarose in the same medium in six-well culture plates. After 3 weeks of incubation at 37°C in a 5% CO2 incubator in a humidified atmosphere, the colonies with more than 15 cells were counted.
Cell cycle analysis
LNCaP and C4-2 cells were harvested at several different time points after radiation. Following treatment, the cells were fixed, washed with phosphate-buffered saline, incubated with 5 μg mL−1 DNase-free RNase for 30 min at 37°C, and stained with 40 μg mL−1 propidium iodide (Sigma-Aldrich, St. Louis, MO, USA). The propidium iodide-stained cells were detected in a FACScan flow cytometer and analyzed with CellQuest software (Becton Dickinson, Franklin Lakes, NJ, USA).
Real-time reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from LNCaP and C4-2 cells and the control cells using the RNeasy Minikit (Qiagen, Hilden, Germany). Double-stranded cDNA was synthesized from 1 μg of total RNA with random primer (Promega, San Luis Obispo, CA, USA). Real-time PCR was performed using an ABI Prism 7000 Sequence Detection System (ABI Applied Biosystems, Foster City, CA, USA) according to the manufacturer's instructions. Reactions were performed in 50 μL volume with 0.5 μmol L−1 primers and MgCl2 concentration optimized between 2 and 5 μmol L−1. Nucleotides, Taq DNA polymerase and buffer were included in the SYBR Green PCR Master Mix (ABI Applied Biosystems). A typical protocol included a 5-min denaturation step followed by 40 cycles involving 95°C denaturation for 30 s, 55°C annealing for 30 s and 72°C extension for 30 s. Extension periods varied with specific primers depending on the length of the product (∼1 s/25 bp). Detection of the fluorescent product was carried out after an additional 2-s step at 2°C below the product melting temperature. To confirm the amplification specificity of the PCR, products from each primer pair were subjected to a melting curve analysis.
Western blot assay
Cells were treated with lysis buffer containing 150 mmol L−1 Tris base (pH 7.5), 50 mmol L−1 NaCl, 1 mmol L−1 EDTA (pH 8.0), 1% NP40, 1 mg mL−1 leupeptin and 1 mmol L−1 PMSF. Protein concentrations were determined by BCA protein assay (Pierce Chemical Co., Rockford, IL, USA). Equal amounts of protein denatured in 2 × sodium dodecyl sulfate (SDS) sample buffer (100 mmol L−1 Tris base [pH 6.8], 200 mmol L−1 DTT, 4% SDS, 20% glycerol and 0.005% bromphenol blue) were loaded into 15% SDS-polyacrylamide gel electrophoresis, and gels were transferred onto nitrocellulose membranes (Amersham Biosciences, Uppsala, Sweden). The membranes were blocked overnight in Tris-buffered saline containing 5% (w/v) skimmed milk powder, and then were stained by appropriate dilution of primary antibodies against p53, p21, Bcl-2, CHK2 and β-actin (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) respectively. After a series of washes the blots were further incubated with goat anti-mouse or anti-rabbit IgG antibody conjugated to horseradish peroxidase (Zhongshan Golden Bridge Biotechnology Co. Ltd., Beijing, China) and detected using the ECL kit (Pierce, Rockford, IL, USA).
Statistical analysis
Analyses were performed using the statistical software SAS/STAT (SAS Institute Inc., Cary, NC, USA). P < 0.01 was considered the threshold value for statistical significance.
Results
Cell growth in vitro in response to radiation treatment
The effects of radiation treatment on cell growth in vitro were determined by MTT assay. Androgen-sensitive LNCaP and androgen-refractory C4-2 cells were exposed to radiation doses of 0, 2.5, 5 and 10 Gy and the values of OD490 were measured at 0, 6, 12, 24, 48 and 72 h post-radiation. The survival curve showed that the growth rates of LNCaP and C4-2 were inhibited by irradiation in dose-dependent manner, whereas C4-2 acquired greater radioresistance compared with LNCaP control (Ρ < 0.01, Figure 1). The growth ratio of LNCaP exposed to 5-Gy radiation was 1.098 at 72 h post-radiation, while it was 5.886 in the non-treatment control LNCaP at the same time point. Hence, the growth of LNCaP was inhibited by 81.34% as a result of the 5-Gy radiation. The growth ratio of C4-2 exposed to 5-Gy radiation was 4.224 at 72 h post-radiation, while it was 6.379 in the control group. Therefore, the growth inhibition mediated by 5-Gy radiation was 33.79% in C4–2. When exposed to 10-Gy radiation, the growth ratio was reduced by 88.32% in LNCaP at 72 h post-radiation, while it decreased by 35.88% in C4-2 in the same condition. These results indicate that the growth-inhibitory effect caused by radiation is obviously reduced in C4-2, and that the growth ability of C4-2 exhibited increased radioresistance compared with LNCaP.
Figure 1.
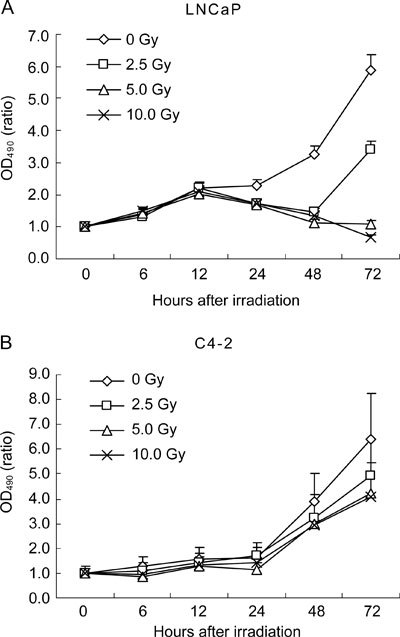
Growth curve of LNCaP and C4-2 cells after irradiation. 5 000 LNCaP (A) and 4000 C4-2 (B) cells were irradiated with 0, 2.5, 5 and 10 Gy and the cell viability was determined by MTT assay at 0, 6, 12, 24, 48 and 72 h post-radiation. Each point is an average of three experiments. Each data point is presented as mean ± SD.
Clonogenicity in response to radiation treatment
Colony formation assays were performed in plates and soft agar to examine the anchorage-dependent and anchorage-independent clonogenicity of LNCaP and C4-2 in response to radiation treatment (5 Gy). Clonogenic assays in plates revealed that C4-2 retained a low level of clonogenicity after 5-Gy radiation treatment, whereas LNCaP cells almost lost their clonogenicity under the same condition (Figure 2). Moreover, anchorage-independent clonogenic assays in soft agar showed that the clonogenicities of LNCaP and C4-2 were obviously inhibited by the treatment with 5-Gy radiation. When seeded at 4 000 cells/well and exposed to 5-Gy radiation, the colony formation ratio of LNCaP was 0.325%, while the ratio was 2.275% in the non-treatment control. Hence, the colony formation ratio of LNCaP was reduced by 85.71% when exposed to 5-Gy radiation. Under the same condition, the colony formation ratio of C4-2 was 2.60% when exposed to 5-Gy radiation, whereas it was 5.40% in the non-treatment control. The colony formation ratio was lowered by 51.82% as a result of radiation exposure. Similarly, when seeded at 6 000 cells per well and exposed to 5-Gy radiation, the colony formation ratio of LNCaP was decreased by 86.70% compared with the control, while the ratio of C4-2 was reduced by 47.52% in the same environment (P < 0.01, Figure 3). From the results described above, it can be seen that the radiation-mediated inhibitory effect on clonogenicity is obviously decreased in C4-2, and that C4-2 possesses evidently increased radioresistance compared with radiosensitive LNCaP as measured by clonogenic assays.
Figure 2.

Colony formation of LNCaP (A) and C4-2 (B) cells after irradiation. Cells were seeded into six-well plates at 2 000–8 000 cells per well and were then irradiated with 5 Gy. Cell survival was determined by colony formation assay.
Figure 3.
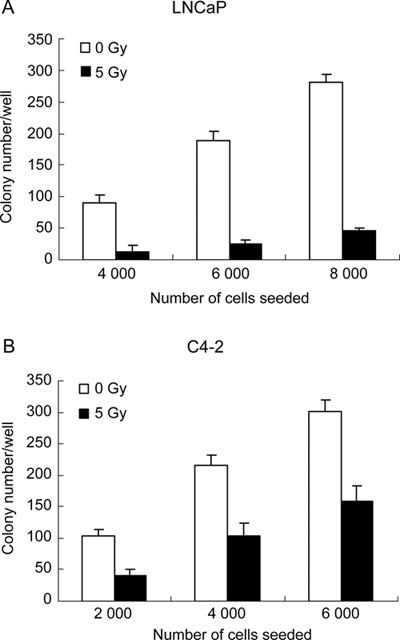
Soft agar assay. A total of 2 000–8 000 cells were suspended in 2 mL of 0.34% low-melting-point agarose and irradiated with 5-Gy. After 20 days of anchorage-independent growth, colonies with at least 15 cells were counted. Each data point is presented as mean ± SD of triplicate independent experiments.
G2/M arrest of LNCaP and C4-2 induced by radiation treatment
Under normal condition, the percentage of C4-2 cells in S phase is higher than that of LNCaP (33.28% vs. 20.25% on average; Figure 4). LNCaP and C4-2 were exposed to 5-Gy radiation and the cell cycle distribution was detected by flow cytometry at 6, 12, 24, 48 and 72 h after treatment. Cell cycle analysis showed that the proportion of LNCaP in S phase was significantly decreased at 12 h post-radiation (3.49% vs. 22.14%), and partially restored at 48 h post-radiation (10.00% vs. 19.12%). Moreover, obvious G2/M arrest was observed at 12 h post-radiation (37.01% vs. 14.42%) and persistent at 72 h post-radiation (40.37% vs. 16.84%). In contrast, the proportion of C4-2 in S phase was significantly increased at 6 h post-radiation (48.98% vs. 35.85%), decreased dramatically at 12 h post-radiation (5.77% vs. 31.71%) and was partially restored at 48 h post-radiation (11.45% vs. 32.65%). Evident G2/M arrest was observed at 12 h post-radiation (56.08% vs. 15.73%) and the arrest was retained at 24 h post-radiation (47.52% vs. 15.24%), but almost abrogated at 48 h post-radiation (23.27% vs. 10.46%) (Figure 4). The cell cycle results showed that the G2 checkpoint was more quickly (6 h in C4-2 vs. 12 h in LNCaP) and intensively (56.08% in C4-2 vs. 37.01% in LNCaP at 12 h) activated in C4-2, but the G2/M arrest of C4-2 was more easily abrogated compared with LNCaP.
Figure 4.

G2 checkpoint activation and G2/M arrest induced by irradiation treatment in LNCaP and C4–2. LNCaP and C4-2 were exposed to 5-Gy radiation. Cell cycle distribution was detected by flow cytometry at 6, 12, 24, 48 and 72 h post-treatment.
DNA repair and cell cycle-associated genes expression
Real-time PCR was performed to examine the expressions of p53, p21, ATR, CHK1 and CHK2 under normal condition. Results indicated that the expressions of the respective genes were down-regulated in C4-2 compared with LNCaP. Besides p21, which was observed to be down-regulated only by 1.28-fold, the other genes were found to be down-regulated more evidently in C4–2. For example, ATR, CHK1 and CHK2 were down-regulated in C4-2 by 5.21-, 6.02- and 1.93-fold, respectively (Figure 5). Then we examined the changes induced by radiation in the respective gene expressions. Results showed that the pattern of gene expression changes mediated by radiation were completely different in LNCaP and C4–2. As shown in Figures 5B–G, the expressions of p53, ATR, MRE11, CHK1 and CHK2 were obviously increased after radiation in C4–2. In contrast, almost all of the genes described above were down-regulated in LNCaP. For instance, the expression of ATR was reduced by 2.03-fold in LNCaP after 5-Gy radiation treatment, whereas it increased by 31.56-fold in C4-2 in response to 5-Gy radiation. The expression of CHK1 was decreased by 6.92-fold in LNCaP after treatment with 5-Gy radiation, while it increased by 6.28-fold in C4-2 under the same condition. The mRNA expression of p21 was found to increase in response to 5- and 10-Gy radiation both in LNCaP and in C4–2. We also evaluated the protein expression changes mediated by radiation in p53, p21, Bcl-2 and Chk2. As a result, we found that the p53 protein expression was slightly decreased in response to 5-Gy radiation in LNCaP, whereas it was obviously up-regulated in C4-2 after 5- and 10-Gy radiation. The expression of Bcl-2 before and after 5-Gy radiation was increased in C4-2 compared with LNCaP (data not shown). The immunoblotting results also showed that Chk2 protein expression was down-regulated in response to 5- and 10-Gy radiation in LNCaP (Figure 6), while it slightly increased in response to 5-Gy radiation in C4–2. The protein expression of p21 was revealed to be evidently elevated after radiation in LNCaP and C4–2. The Western blot results were consistent with the quantitative RT-PCR results.
Figure 5.

Real-time PCR analysis of gene expression associated with DNA repair and cell cycle arrest in LNCaP and C4–2. (A): P53, p21, ATR, CHK1 and CHK2 mRNA expression were examined using real-time PCR assays in LNCaP and C4–2. (B)–(G): Gene expression changes induced by 5- or 10-Gy radiation in LNCaP and C4-2 were examined in p53, p21, ATR, MRE11, CHK1 and CHK2 at 24h post-radiation. Data shown are mean ± SD of triplicate independent measurements. The relative amounts of mRNA of target genes to β-actin were calculated using the equation 2−(C(t)target−C(t)β−actin).
Figure 6.

Immunoblotting analysis of gene expression changes in response to radiation in LNCaP and C4–2. p53, p21 and Chk2 protein expression changes in response to radiation were evaluated in LNCaP and C4-2 at 24h post-radiation using Western blot assays, with β-actin used as normalizing control.
Discussion
Besides radical prostatectomy, radiation therapy has been proven to be a relatively effective treatment for localized PCa, with local tumor control and improvement of patient survival rates. However, PCa cells vary in their sensitivity to ionizing radiation and nearly 30% of patients treated with potentially curative doses relapsed at the sites of irradiated tumors 11, 12, 13. AD through surgery or chemical methods is the most effective therapeutic approach for advanced PCa. Unfortunately, despite the effectivity of such procedures during the initial period, PCa eventually progresses to the androgen-independent stage and resists additional androgen withdrawal 14. It is known that hormone-independent PCa cells are only modestly sensitive to chemotherapy and to radiation therapy 15. No curative and durable therapeutic methods are available for AI and metastatic PCa.
Therapeutic synergy between radiotherapy and androgen ablation in patients with advanced metastatic PCa has been documented with promising clinical outcome. Neoadjuvant AD improves intro prostatic local control and reduces distant metastasis. Neoadjuvant androgen withdrawal achieves this endpoint through sensitization of the tumor to radiation and through improved oxygenation 16. In addition, HR PCa cells appeared to be more aggressive in their resistance to radiation treatment in comparison with androgen-sensitive PCa cells 7. Accordingly, androgen ablation-mediated molecular events may have important roles in the radiosensitization of PCa cells. Conversely, gene expression alterations mediating androgen-refractory progression of PCa may also have an impact on the development of radiation resistance.
LNCaP is an androgen-responsive, non-metastatic and marginally tumorigenic PCa cell. The C4-2 subline was derived from LNCaP through interaction with stromal cells under androgen-depleted conditions in vivo and has acquired the phenotypes of androgen independence and osseous metastases. In our study, C4-2 was found to possess increased radioresistance and decreased radiosensitivity compared with the parent cell line LNCaP. C4-2 sustained the ability of clonogenicity after radiation, whereas LNCaP lost the ability. The survival and proliferation rates of C4-2 after exposure to radiation are increased compared with LNCaP. The increased expression of Bcl-2 both in non-irradiated and in irradiated C4-2 indicates the enhanced anti-apoptosis characteristics in C4-2, because Bcl-2 expression is required for tumor maintenance and prevents cells from apoptosis 17, 18. The basical expression levels of p53 and p21 were found to be suppressed in C4-2 in comparison with LNCaP. The expressions of ATR, Chk1 and Chk2, which have been implicated to have important roles in cell cycle arrest, were also shown to exist in lower levels in C4–2. Decreased basical expressions of the genes described above indicate that C4-2 is able to pass the cell cycle checkpoints more easily under normal conditions, which is consistent with the increased percentage of S phase C4-2 cells observed in cell cycle analysis. We also found that the G2/M checkpoint of C4-2 was activated more quickly and intensively than that of LNCaP after radiation exposure, which can be explained by the increased expression of ATR, MRE11, CHK1 and CHK2 as well as p53 in C4-2 after radiation treatment. The increased expression of these genes may be transient, but they still have important roles in initiating the G2/M checkpoints and in responding to DNA damage. ATR is one of the major regulators of DNA damage response (DDR) and targets a set of substrates that promote cell cycle arrest and DNA repair. As a downstream substrate of ATR, Chk1 functions to signal DNA damage to the rest of the nucleus. Chk1 phosphorylation of the CDC25 proteins inhibits their activity and prevents CDK activations, which is a major checkpoint mechanism that prevents entry into mitosis. The ATR/Chk1/CDC25 signaling pathway has an essential role in the checkpoint response to radiation. Even the initiation of the G2/M checkpoint is impaired in the absence of ATR. ATR signaling also promotes the repairing of a variety of DNA lesions by regulating recombination at stalled and collapsed replication forks. Moreover, the ATM/Chk2 signaling pathway has an essential role in G2 checkpoint activation and the ATM-dependent repair pathway (presumably homologous recombination) functions to restore chromosomal integrity after radiation exposure 19, 20, 21. As for p53, it has been well established that the p53 protein contributes to both G1 and G2 arrest through transcriptional regulation, which includes the induction of CDKN1/p21 22, 23. The cell cycle arrest maintenance role of p53 in response to radiation-induced damage may be protective and allows sufficient time for DNA damage repair. Transient increase in p53 expression in response to radiation contributes to cell cycle checkpoint activation and increases cell survival. Functional p53 expression significantly increased PC-3 cell clonogenic survival after exposure to daily doses of 2-Gy of IR 24. This result is consistent with our data showing that p53 gene expression is up-regulated in C4-2 after 5- and 10-Gy radiation. A normal function of cell cycle checkpoints is to protect cells from the deleterious consequences of replicating or segregating damaged chromosomes. Abrogation of the G2 checkpoint often leads to a marked increase in the sensitivity to ionizing radiation. The sensitizing actions of caffeine and UCN-01 have raised the probability that adjunctive therapy with G2 checkpoint inhibitors will increase the therapeutic efficacies of radiation 25, 26. Cell cycle analysis and molecular examination after radiation revealed that C4-2 had acquired an enhanced G2 checkpoint in comparison with LNCaP, which is consistent with the decreased radiation sensitivity in C4–2. More interestingly, we found that the cell cycle checkpoint-associated gene expressions maintained a relatively low level in C4-2 under normal condition, but were elevated by radiation. Conversely, in LNCaP, these gene expressions were at a higher level under normal condition, but were inhibited or unchanged by radiation. It is intriguing to propose that C4-2 is able to pass the checkpoints more easily under normal conditions, while acquiring more effective cell cycle checkpoints to allow sufficient time for DNA repair when DNA damage occurs. Furthermore, as observed in cell cycle analysis, C4-2 recovered from G2/M phase arrest caused by radiation more easily than LNCaP. The MRE11/RAD50/NBS complex is a central player in most aspects of cellular response to DNA double-strand breaks, including homologous recombination, non-homologous end joining, telomere maintenance and DNA damage checkpoint activation 27, 28. The increased ATR and MRE11 expressions in C4-2 in response to radiation indicated that C4-2 had acquired increased potential of DNA repair after IR exposure, which may contribute to the ability of C4-2 to recover from G2/M arrest more easily and quickly. The molecular examinations are consistent with results observed in the cell viability and cell cycle analysis described above. All of these results revealed that the radiation-resistant property may correlate with the androgen-independent status in PCa development. Accordingly, LNCaP and C4-2, which, respectively, represent the androgen-dependent and independent status of PCa, can also be applied to explore new therapeutic strategies for increasing the radiosensitivity of PCa cells. The gene expression differentiation between LNCaP and C4-2 may also shed novel insights into the molecular determinants in the efficacy of androgen depletion and radiation therapeutic strategies.
Acknowledgments
This work was supported by grants from National “863” Research Program Foundation (No. 2008AA02Z123), Key Project for drug discovery and development in China (No. 2009ZX09501-027), and National Natural Science Foundation of China (No. 30770834 and 30870961).
References
- Jemal A, Siegel R, Ward E, Murray T, Xu J, et al. Cancer Statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Cooperberg MR, Broering JM, Litwin MS, Lubeck DP, Mehta SS, et al. The contemporary management of prostate cancer in the United States: lessons from the cancer of the prostate urologic research endeavor (CapSURE), a national disease registry. J Urol. 2004;171:1393–401. doi: 10.1097/01.ju.0000107247.81471.06. [DOI] [PubMed] [Google Scholar]
- Zelefsky MJ, Ben-Porat L, Chan HM, Fearn PA, Venkatraman ES. Evaluation of postradiotherapy PSA patterns and correlation with 10-year disease free survival outcomes for prostate cancer. Int J Radiat Oncol Biol Phys. 2006;66:382–8. doi: 10.1016/j.ijrobp.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Pollack A, Zagars GK, Starkschall G, Antolak JA, Lee JJ, et al. Prostate cancer radiation dose response: results of the M.D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53:1097–105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
- Rosen EM, Fan SJ, Rockwell S, Goldberg ID. The molecular and cellular basis of radiosensitivity: implications for understanding how normal tissues and tumors respond to therapeutic radiation. Cancer Invest. 1999;17:56–72. [PubMed] [Google Scholar]
- Lee AK. Radiation therapy combined with hormone therapy for prostate cancer. Semin Radiat Oncol. 2006;16:20–8. doi: 10.1016/j.semradonc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Wu CT, Chen WC, Liao SK, Hsu CL, Lee KD, et al. The radiation response of hormone resistant prostate cancer induced by long-term hormone therapy. Endocr Relat Cancer. 2007;14:633–43. doi: 10.1677/ERC-07-0073. [DOI] [PubMed] [Google Scholar]
- Horoszewicz JS, Leong SS, Kawinski E, Karr JP, Rosenthal H, et al. LNCaP model of human prostatic carcinoma. Cancer Res. 1983;43:1809–18. [PubMed] [Google Scholar]
- Thalmann GN, Anezinis PE, Chang SM, Zhau HE, Kim EE, et al. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994;54:2577–81. [PubMed] [Google Scholar]
- Wu HC, Hsieh JT, Gleave ME, Brown NM, Pathak S, et al. Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: role of bone stromal cells. Int J Cancer. 1994;57:406–12. doi: 10.1002/ijc.2910570319. [DOI] [PubMed] [Google Scholar]
- Scardino PT, Wheeler TM. Local control of prostate cancer with radiotherapy: frequency and prognostic significance of positive results of postirradiation prostate biopsy. NCI Monogr. 1988;7:95–103. [PubMed] [Google Scholar]
- Crook JM, Perry GA, Robertson S, Esche BA. Routine prostate biopsies following radiotherapy for prostate cancer: results for 226 patients. Urology. 1995;45:624–31. doi: 10.1016/S0090-4295(99)80054-5. [DOI] [PubMed] [Google Scholar]
- Zelefsky MJ, Leibel SA, Gaudin PB, Kutcher GJ, Fleshner NE, et al. Dose escalation with three-dimensional conformal radiation therapy affects the outcome in prostate cancer. Int J Radiat Oncol Biol Phys. 1998;41:491–500. doi: 10.1016/s0360-3016(98)00091-1. [DOI] [PubMed] [Google Scholar]
- Isaacs JT. The biology of hormone refractory prostate cancer. Uro Clin North Am. 1999;26:263–73. doi: 10.1016/s0094-0143(05)70066-5. [DOI] [PubMed] [Google Scholar]
- Kaliks RA, Giglio AD. Management of advanced prostate cancer. Rev Assoc Med Bras. 2008;54:178–82. doi: 10.1590/s0104-42302008000200025. [DOI] [PubMed] [Google Scholar]
- Wo JY, Zietman AL. Why does androgen deprivation enhance the results of radiation therapy. Urol Oncol. 2008;26:522–9. doi: 10.1016/j.urolonc.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–37. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letai A, Sorcinelli MD, Beard C, Korsmeyer SJ. Antiapoptotic BCL-2 is required for maintenance of a model leukemia. Cancer Cell. 2004;6:241–9. doi: 10.1016/j.ccr.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol. 2008;9:616–27. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez D, Guntuku S, Qin J, Elledge SJ. ATR and ATRIP: partners in checkpoint signaling. Science. 2001;294:1713–6. doi: 10.1126/science.1065521. [DOI] [PubMed] [Google Scholar]
- Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15:2177–96. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- Chan TA, Hwang PM, Hermeking H, Kinzler KW, Vogelstein B. Cooperative effects of genes controlling the G2/M checkpoint. Genes Dev. 2000;14:1584–8. [PMC free article] [PubMed] [Google Scholar]
- Scott SL, Earle JD, Gumerlock PH. Functional p53 increases prostate cancer cell survival after exposure to fractionated doses of ionizing radiation. Cancer Res. 2003;63:7190–6. [PubMed] [Google Scholar]
- Sarkaria JN, Busby EC, Tibbetts RS, Roos P, Taya Y, et al. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 1999;59:4375–82. [PubMed] [Google Scholar]
- Tenzer A, Pruschy M. Potentiation of DNA-damage-induced cytotoxicity by G2 checkpoint abrogators. Curr Med Chem Anticancer Agents. 2003;3:35–46. doi: 10.2174/1568011033353533. [DOI] [PubMed] [Google Scholar]
- Paull TT. New glimpses of an old machine. Cell. 2001;107:563–5. doi: 10.1016/s0092-8674(01)00591-8. [DOI] [PubMed] [Google Scholar]
- Paull TT, Gellert M. Nbs l potentiates ATP-driven DNA unwinding and endonuclease cleavage by the Mre11/Rad50 complex. Genes Dev. 1999;13:1276–88. doi: 10.1101/gad.13.10.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]


