Abstract
Protein micropatterning techniques are increasingly applied in cell choice assays to investigate fundamental biological phenomena that contribute to the host response to implanted biomaterials, and to explore the effects of protein stability and biological activity on cell behavior for in vitro cell studies. In the area of neuronal regeneration the protein micropatterning and cell choice assays are used to improve our understanding of the mechanisms directing nervous system during development and regenerative failure in the central nervous system (CNS) wound healing environment. In these cell assays, protein micropatterns need to be characterized for protein stability, bioactivity, and spatial distribution and then correlated with observed mammalian cell behavior using appropriate model system for CNS development and repair. This review provides the background on protein micropatterning for cell choice assays and describes some novel patterns that were developed to interrogate neuronal adaptation to inhibitory signals encountered in CNS injuries.
1 Rationale for studying the role of proteoglycans in CNS injuries
Neurons from the central nervous system (CNS) possess a limited capacity to regenerate beyond scar tissue formation barriers in injuries even in the presence of minimal trauma to tissue organization [1, 2]. A major culprit in CNS neuronal regenerative failure are the inihibitory proteoglycans (PGs), which are upregulated at the site of CNS injuries. PGs are composed of a core protein with varying numbers of attached glycosaminoglycan (GAG) side chains [3, 4]. Proteoglycans are first translated intracellularly into proteins in the endoplasmic reticulum, and then GAG chains are later attached in the Golgi apparatus before secretion into the extracellular space [5–8]. The study of PGs has particularly been pronounced in the field of neurobiology and development of the nervous system. Numerous studies have concluded that PGs act as barriers that direct the migration of neural crest cells and the outgrowth direction of pathfinding neurons during development [9–17]. A number of these studies have additionally shown that cell guidance by PGs is based on an inhibitory signal located within the chondroitin sulfate GAG chains [9, 18, 19], with the result that the artificial addition of chondroitin sulfate (CS) sugars to the developing nervous system disrupts normal pathfinding [20] as well as does the removal of chondroitin sulfate chains through enzymatic treatment [9, 19]. Davies et al. found that chondroitin sulfate proteoglycans (CSPG) are a major constituent of the glial scar and that dorsal root ganglion (DRG) outgrowth termination coincided with an increase in CSPG expression density [21]. Other sources have also shown that CSPGs are upregulated after CNS injuries [22–25].
Interestingly, native adult CNS neurons actually posses the intrinsic ability to regenerate when the wound healing environment is prepared by eliminating inhibitory factors [23, 26, 27]. Stettler et al. provided evidence that matrix metalloproteinases that normally digest CSPGs in peripheral nervous system injuries are also present in the CNS, and that laminin and CSPG expression are both upregulated at the site of CNS injuries [24]. In a separate study, hyaluronidase treatment of adult spinal cord injuries (SCI) regions also helped remove CSPGs from the wound site. However, areas containing residual CSPGs caused regeneration failure [27].
In addition to CSPGs, inhibitory molecules such as myelin-associated glycoprotein (MAG) found in myelin debris have been shown to inhibit neuron regeneration and cause growth cone collapse upon contact. It has been found, however, that oligodendrocytes, which express MAG, also express CSPGs [28] and that removal of the proteoglycans permitted DRG growth onto oligodendrocyte processes [29]. It has been demonstrated that reactive astrocytes are the major contributors of CSPGs in the CNS and produce neurocan, phosphocan, and NG2 [28, 29–33].
A number of repair strategies have been devised to eliminate the inhibitory factors in the CNS wound environment, many of which are directed at lowering or removing the inhibitory effects of CSPGs. One strategy has used the enzyme chondroitinase ABC (ChABC), which digests the chondroitin sulfate chains on CSPGs. ChABC treatment of SCI or tissues explanted from spinal cord wounds has resulted in an improvement of axonal outgrowth both in vivo and in vitro [25, 26]. However, it has also been shown that even proteoglycan core proteins can still exert an inhibitory effect on axon regeneration [34]. Other recent repair strategies have focused on reducing CSPG expression in CNS injuries by decorin infusion [23] or by inhibition of proteoglycan glycosylation initiation [35]. Increasing nerve growth factor (NGF) secretion in the wound environment also appears to promote outgrowth of injured axons [36]. Bunge et al. devised a multifaceted repair strategy in which inflammation was reduced by the local administration of cyclic adenosine monophosphate (cAMP), a cAMP production stimulator, and the implantation of Schwann cells. Rats treated with cyclic AMPs, stimulators, and Schwann cell implantation showed significantly higher levels of recovered locomotion and fine motor skill than rats that only received one-part or two-part treatments [37].
In addition to in vivo studies, protein surface patterning and model surfaces have played a role in understanding the mechanisms directing the growth promoting and inhibitory signals encountered in the CNS wound healing environment. For instance, neuronal cell attachment and outgrowth on the neurite outgrowth promoting molecule laminin and on patterned laminin fields have been studied for over two decades [38, 39]. A variety of model cell types have been used in the literature including the immortalized pheochromocytoma (PC-12) rat cell line that exhibits a neuronal phenotype in the presence of NGF [39, 40]. The use of dorsal root ganglion neurons or other sensory neurons has also been widespread in the literature perhaps because of the relative ease by which these cell types can be explanted and purified. DRGs exhibit robust outgrowth, and although these cells originate in the peripheral nervous system, they extend into the CNS and generally fail to regenerate past CNS injuries [21].
DRGs and other neurons extend axons and dendrites that form connections with the ECM and other cells. In many cell culture studies, no attempt is made at distinguishing axons or dendrites and both outgrowth structures are simply designated as neurites. Still, specific expression markers in axons exist that can be immunolabeled employing the appropriate antibodies [41]. Neurites contain important cytoskeletal elements including microtubules and actin microfilaments, which provide structural support to the cell outgrowth structures and are associated with the membrane bound receptor contacts that form with surrounding cells and proteins [42, 43–46]. It has been demonstrated that microtubules, myosin, and actin are essential to proper locomotion and outgrowth behavior observed in neurites [43, 44, 47].
At the end of each neurite extension is a fan-like structure known as the growth cone (GC) in which the cellular machinery for locomotion is located [45, 46, 48]. Major components involved in growth cone locomotion are the filopodia and lamellapodia structures, which are supported by actin filaments and associated with dynamic microtubules [43–46]. Growth cones also act as the sensory unit of the neurite where signals sampled in the growth environment are integrated and cell responses are initiated [48–50]. Growth cones interact with extracellular matrix molecules such as laminin through specific integrin receptors [42, 51]. There is also some evidence that GCs express receptors for CSPGs [52–57]. Growth cone dynamics have been studied extensively in vivo and in vitro and GC morphology, trajectory, and dynamics have been used as a means to study how environmental cues affect neurite outgrowth [58–64]. In many of these studies surface patterning techniques were devised to enable the study of growth cone integration of multiple protein signals.
2 Chemical surface patterning followed by protein adsorption
Implanted biomaterials and standard tissue culture materials used in cell research present interfaces at which complex biomolecular interactions dictate the host or cellular response. The most notable interaction at the biological interface occurs between material surface and soluble proteins, which may irreversibly adsorb to the interface through nonspecific events driven by the lowering of the overall energy of the system [65, 66]. Nonspecific protein interactions with interfaces occur as spontaneous events dictated purely by the energy of the system and are not a function of the biomolecular recognition events that normally occur during protein secretion, deposition, and organization in vivo [67–70]. Conversely, in the human body, specific molecular recognition events dictate protein interactions with native interfaces such as the basal lamina, cell surfaces, and the extracellular matrix. Such specific protein-protein interfacial interactions are a necessity of normal biological function and direct extracellular organization and define homeostasis.
Proteins typically interact nonspecifically with foreign interfaces through hydrophobic and protein-material charge interactions often resulting in the unfolding of the native protein structure [65, 66, 71]. Disruption of protein conformation most often causes diminished or abolished native function and can trigger undesirable side effects at the implant surface. It is well documented that the typical host response to implanted materials is a coagulation cascade triggered by nonspecific protein adsorption events [72] and complement protein reaction at the implant-host interface [73–75].
By acquiring an understanding of basic protein interactions with interfaces, it may be possible to more accurately predict how proteins and surfaces can be manipulated for the creation of cell assays or biocompatible materials that mimic native in vivo protein signals. To this end, numerous methods have been developed to produce model surfaces so that in vitro protein interactions at interfaces and resulting cellular responses can be investigated and evaluated as models of in vivo interactions and signaling. Such model surfaces, when properly validated, additionally provide a means to investigate cell behavior in a simplified format where the cell environment can be readily controlled and resulting behavior easily interrogated.
Chemical modification of surfaces has widely been used as a means to tailor the interfacial properties of materials to both investigate protein adsorption as a function of surface group characteristics and moiety coverage. Surfaces have also been modified with chemistries that allow the covalent attachment of proteins, circumventing possible denaturation events caused by adsorption processes [76]. Self-assembled mono-layers (SAMs), which are molecular films formed by the spontaneous organization of molecules at interfaces, have also been used extensively to modify surfaces [77]. SAMs may be adhered to a substrate through physisorption or chemisorption depending on the presence of functional groups on the individual SAM molecules. SAMs have been widely used because they present the flexibility of forming diffuse or compact layers or mixed films of multiple species to investigate myriad arrangements of molecular interactions with proteins [78–81].
The main strategies for the preparation of chemical patterns on a macroscopic surface include.
photochemical modification of uniform, thin organic films or polymers [82–86],
spatially controlled delivery of a surface modifying compound through a simple stamping [89] or ink-jet printing [90], followed by a second “back-filling” step in which the unmodified surface areas are reacted with a second compound [91].
With the advent of micromachining techniques, like micro-lithography, the size of surface patterns can be made quite small, typically in the μm size range. Of note are the so-called “soft lithography” techniques, which employ an elastomeric material containing a surface relief pattern as an initial adsorption substrate for transferring SAM components. After SAM deposition on the elastomeric material, the molecular film is transferred to the final substrate in a pattern, much as ink is transferred from a stamp to paper, through a process known as microcontact printing (μCP) [92–94]. Any surface areas not modified by the stamping procedure may be left untreated to conserve the original substrate molecules or further modified by allowing a second SAM to form in between the stamped areas through a solution deposition process [95]. The stamping process provides precise control over the placement of SAMs, and the level of spatial complexity and resolution in the pattern is limited only by the process used to fabricate the elastomeric stamp and the material properties of the stamp [96].
Soft lithography techniques gain their name from the process used to produce μCP stamps and microfluidic network (μFN) devices and the materials from which they are formed [97,98]. These devices are made of soft elastomers, such as silicones, and the molds are traditionally fabricated through photolithography techniques used in microelectronic fabrication. An example of such process is shown in Figure 1. In the mold production process, a photolithographic mask is first patterned through a UV exposure step, the photoresist is developed, and then a metal layer is etched from the mask uniquely where the photoresist has been removed. The mask can then be used to expose a second resist layer deposited on a smooth planar material, such as a polished silicon wafer, and then is developed to form a hard relief patterned layer on the wafer. The hardened patterned film and the silicon substrate finally serve as the mold for silicone device fabrication.
Figure 1.
Microfabrication steps for soft lithography: 1 - Exposure of 1st mask with pattern generator, 2 - Development and etch of pattern, 3 - 5x Image reduction of 1st pattern, 4 - Development and etch, 5 - UV exposure through 2nd mask onto SU-8 spin coated on silicon wafer, 6 - SU-8 development, 7 - PDMS curing step, 8 -Removal of PDMS stamp, or alternatively 5a - PDMS curing step, and 6a - Removal of PDMS stamp.
A Dow Corning silicone, Sylgard 184, a poly(dimethylsiloxane) (PDMS), has predominantly been used as an elastomeric material for soft lithography device fabrication [98,99]. The base and curing agents are commercially available and are mixed and cured with relative ease. PDMS has optical properties comparable to glass and is biocompatible [100]. Researchers have used PDMS alone to produce μCP features as small as 200 nm in width, but it has been shown that for smaller features, PDMS sags and produces artifacts in stamped patterns [101]. To remedy this problem, a formulation known as hard PDMS (hPDMS) was developed to be used as a backing to thin films of patterned PDMS [96]. In these composite devices, the hPDMS layer provides a stiff mechanical support to prevent sagging but also allows the flexibility to stamp curved surfaces [102]. Efforts have also been made to produce stiffer photocurable polysiloxanes to both improve patterning resolution and reduce fabrication time [103]. Other research efforts have resulted in the production of stamped features smaller than 50 nm in size, which is beyond the resolution limit of traditional photolithographic microfabrication processes [104].
Often it is unclear, however, what effect specific surface patterning chemistry and subsequent protein deposition by adsorption from solution have on protein conformation and bioactivity. The design of a cell choice assay must not only be based on the presumed effects of a protein on a particular cell type, as demonstrated ideally by both in vivo and in vitro evidence, but also on an understanding of how the protein interacts with the substrate material being employed in the assay and with other proteins present in the assay. To better understand how material surfaces affect protein structure, several protein characterization methods have been developed to determine adsorbed protein conformation and its effect on biological activity. Specifically, molecular recognition events through antibody binding to protein epitopes has been used as a means to confirm that the targeted peptide sequences are available and not disrupted by the adsorption process or other denaturing conditions [105]. Although conformation may be affected by adsorption, biological activity may remain intact, and thus cell based assays have also proven valuable as a means to investigate the resulting adsorbed protein activity on cell attachment and phenotypic expression [106].
3 Protein μCP stamping and μFN deposition
In addition to the chemical surface patterning and protein adsorption from solution, one can employ μCP and stamp proteins directly to the suitable substrate. It has been shown that a number of proteins can be adsorbed to elastomeric stamps and then transferred to substrates such as glass or polystyrene or even to a surface preadsorbed with another protein. In most cases, nearly 100% of the protein film transfers from the stamp onto the surface being patterned, presumably due to favorable interactions between the exposed protein film surface and the substrate to which the film is transferred. Some printed protein fields have also shown largely unaltered epitope presentation in antibody immunostaining assays [107, 108]. It has been demonstrated that stamped protein fields retain sufficient activity to promote the levels of cell adhesion and phenotypic expression found for the same cell-protein systems where the protein was deposited by adsorption. For example, proteins printed in lanes have been used as a means to direct the outgrowth of neurons in an attempt to form neural networks [109–111].
The process of protein μCP presents some clear advantages over traditional methods to coat surfaces with protein layers. For instance, μCP enables precise spatial control over protein deposition and allows the placement of multiple protein species on a single surface in overlapping or segregated fields. μCP also allows control of the adsorption of each protein independently of other species. In a traditional adsorption scheme, two or more proteins typically compete for the same binding sites and additionally interact with each other in solution to affect adsorption kinetics, making it difficult to predict or control the final concentration of each protein species. Additionally, in μCP, undesirable substrate-protein interactions during adsorption can be minimized or eliminated due to the ability to run the adsorption step on the stamp material. This is especially beneficial when μCP can be used to deposit a greater density of protein on the stamp material than could be adsorbed directly to the substrate of interest.
A second soft lithography technique, microfluidic networks (μFN) patterning, has also been used to directly pattern proteins to surfaces. In this technique, protein solutions are flowed through microchannels formed between an elastomeric stamp and the substrate to which the proteins are to be adsorbed. Because the protein solution is in contact with the substrate only where it is exposed through the microchannels, a surface can be selectively patterned by controlling microchannel geometries. After the adsorption process, the stamp is removed and can be reused for other substrates. Microfluidic patterning provides an advantage over μCP for proteins that do not retain biological activity through the stamping process [112–114]. A few investigators have combined μCP and (μFN to simultaneously or sequentially pattern segregated and overlapping fields of different proteins to a single substrate [115, 116]. The three approaches used in stamping proteins are schematically shown in Figure 2.
Figure 2.
Protein deposition by μCP stamping on a bare substratum (panel A), on a substratum already covered with an adsorbed protein layer (panel B), and by a combination of the μCP and μFN (panel C).
In addition to providing complexity in and control over the proteins presented in an assay, soft lithography patterning provides a tool to place two otherwise disparate surfaces into adjacent locations on a single substrate. One difficulty in characterizing protein films is defining an absolute baseline or reference so that multiple samples can be characterized and compared. Additionally, depending upon the characterization method employed, experimental variations can make the comparison between two separate samples difficult. Thus, when two or more surface treatments can be combined onto a single surface, instrument and sample substrate variables are eliminated for that sample and the two treatments can be compared side by side.
4 Protein patterns and cell choice assays in studying neuronal regeneration
Protein surface patterning is the methods of choice for the cell choice assays where two separate protein signals need to be spatially segregated on a substrate. Subsequent cell attachment on either of the protein fields may then reveal how cells respond to multiple protein signals in vivo [112, 117, 118]. In vivo, cells normally form contacts with other cells or with proteins in the extracellular matrix (ECM). The extracellular matrix is largely composed of adhesion molecules that contain specific protein epitopes that cell receptors recognize and to which they attach. A large family of cell adhesion receptors known as integrin receptors attach to ubiquitous ECM adhesion molecules such as laminin, fibronectin, collagen, and vitronectin. Integrins mediate cell adhesion to the ECM but in addition affect cell phenotypic expression [42, 119]. An example of how two spatially segregated proteins, laminin and serum albumin, affect DGR adhesion and outgrowth is shown in Figure 3. However, inert protein such as albumin was not always completely effective in blocking the laminin—cell interactions and constraining the outgrowth (120).
Figure 3.
Stamped 80 μm stripes of FITC-labeled human serum albumin separated by 40 μm-wide gaps with a Alexafluor488 dye-laminin and albumin adsorbed from two protein solution mixture. The image is the combination of brightfield (DRGs) and fluorescence images. From ref. 120.
In addition to integrins, proteoglycans are a second constituent of the ECM that exert signals on cell attachment and cell phenotype. To improve the understanding of the competing signals between growth promoting molecules such as laminin and inhibitory proteoglycans we and other labs have developed in vitro assays to test how the chondroitin sulfate containing proteoglycan signals presented in a mixed CSPG-laminin surface-attached layer are affecting neuronal cell adhesion and growth cone pathfinding [120–125]. It is known that neurons possess the ability to integrate multiple signals in their environment [120, 125–128], and it has been demonstrated that the ratio of substratum bound laminin and CSPGs such as aggrecan can be varied to yield net neurite outgrowth promoting or inhibiting signals [120, 129–131]. It has also been found that aggrecan affects laminin activity on neuron cell body substratum attachment depending on the order in which the two molecules are adsorbed, providing evidence that the effect of aggrecan is not simply additive [132]. It has thus been suggested that aggrecan and other CSPGs may exert their inhibitory effect by adversely changing the activity of laminin and other growth promoting molecules through protein-protein interactions and conformational changes.
Interactions between proteoglycans and laminins have been elucidated including CSPG direct binding to and masking of laminin integrin sites [133–135]. It has also been found that certain CSPGs appear to affect only particular growth promoting molecules. For instance, neuronal adhesion to fibronectin is inhibited by versican and decorin but is not affected by the same molecules in the presence of laminin [136]. NG2 has been shown to override the growth promoting properties of laminin even after CS chain digestion but did not affect outgrowth on LI surfaces [137]. Additionally, CS sugars and keratan sulfate inhibit DRG outgrowth on laminin but not on LI [138]. It has also been found that laminin molecules bind in solution with the PGs cerebroglycan, glypican, and syndecan [139].
Although many studies have addressed the effects of CSPGs on neuronal outgrowth in vitro, it appears that there are little or no experimental data addressing neuronal interactions at laminin boundaries with pure proteoglycan fields. In addition, it appears that little information is available to accurately predict the effect of laminin deposition through soft lithography techniques on its growth promoting activity. In an effort to address these shortfalls, we have developed a set of cell choice stripe assays in order to separate CSPG-laminin interactions from possible intrinsic effects that CSPGs may exert on neuronal cell attachment and neurite outgrowth [120, 125]. Additionally, antibody attachment to a laminin epitope known to promote neuronal cell attachment and neurite outgrowth was used to relate the method of laminin deposition to resulting bioactivity [120, 125].
It was found that the activity of substrate bound laminin on glass differed whether it was adsorbed from a solution, using microfluidic networks patterning, or transferred by microcontact printing, and that laminin conformation, as measured by integrin presentation, was a strong predictor of neuronal cell adhesion and neurite outgrowth behavior [120, 125]. It was additionally observed that the order in which laminin and the CSPG aggrecan were adsorbed onto glass clearly affected laminin activity, suggesting interactions between CSPGs and laminin. An example of such differences in protein deposition is shown in Figure 4, where the upper panel shows DRGs on a pattern consisting of μCP stamped aggrecan and μFN deposited laminin and the lower panel shows the DRG on the pattern made of adsorbed laminin over which aggrecan was stamped using μCP.
Figure 4.
The method of protein deposition determines how DRGs react to the combination of permissive (laminin) and inhibitory (aggrecan) signals. The upper panel shows DRGs on the pattern consisting of μCP stamped aggrecan and μFN deposited laminin (scheme C from Fig 2) and the lower panel shows the DRG on the pattern made of μCP stamped aggrecan on the uniform adsorbed laminin (scheme B in Fig 2). The image is the combination of brightfield (DRGs) and fluorescence images. Aggrecan was stained with AlexaFluor-594 dye. From ref. 120.
5 The effects of CSPG surface concentration
The neuroscience field has borrowed or devised a number of patterning techniques to study of growth cone integration of multiple protein signals. The patterning techniques including protein film UVor laser ablation [140, 141], nitrocellulose paper strip protein transfer [142], microfluidic networks [112, 113, 143], and microcontact printing [76] have been utilized to produce such substrates. Of interest are studies in which these techniques have been used to generate concentration gradients and protein field boundaries to discover how bound factor density and spatial distrubition affect cell behavior. In particular, the inhibitory CSPGs and growth promoting laminins have been placed in patterns to discover their combined effect on neuronal cell regeneration.
DRGs in particular have exhibited the capacity to adapt to substrate bound inhibitory CSPGs in vitro providing implications for possible in vivo regeneration mechanisms. Letourneau and Snow created step gradients of CSPG on laminin fields and monitored DRG neurite outgrowth into the gradient. Although outgrowth speeds slowed with increasing CSPG concentration, neurites more readily grew into higher CSPG concentrations after having grown through intermediate densities [129]. Condic et al. later showed that DRGs upregulate integrin laminin receptor expression to overcome CSPG inhibition or low levels of substratum bound laminin, and further showed that increasing adult DRG integrin expression through viral transfection was sufficient to overcome the inhibitory effects of CSPGs [132, 144, 145]. Additional evidence exhibits that integrin receptors are also upregulated in vivo following spinal cord injuries [146].
Although DRGs and other neurons modulate integrin receptor expression in response to proteoglycans, it is still unclear how upregulation dynamics proceed in response to changing concentrations of CSPGs that occur in the injury milieu. It is proposed that valuable insights into the mechanisms directing neuronal adaptation to CSPGs remain to be discovered and that investigation of the CSPG dose and time dependent response of upregulation could provide information about the ability of neurons to adapt to varying degrees of inhibition. Such information could be useful in developing CNS repair strategies as the benefits of the degree of CSPG signal removal or suppression are assessed. Additionally, an understanding of the effects of changing CSPG signals on neuronal adaptation may provide insights into the role CSPGs play in neuronal behavior observed in vivo during development.
Significantly, a number of investigators have taken advantage of soft lithography techniques to produce patterns that test geometric effects or integrin density effects on cell behavior and that are directly applicable to signal integration by neuron growth cones [109, 110, 147–152]. Ingber et al. showed that cell bound surface area when controlled by micropatterning can have a profound effect on apoptosis [153] and provided new patterns to increase cell spread while diminishing cell substrate contacts with integrins [154]. Ingber also showed that lamellapodial extension direction and the orientation of tractional forces can be controlled through surface patterning [155, 156].
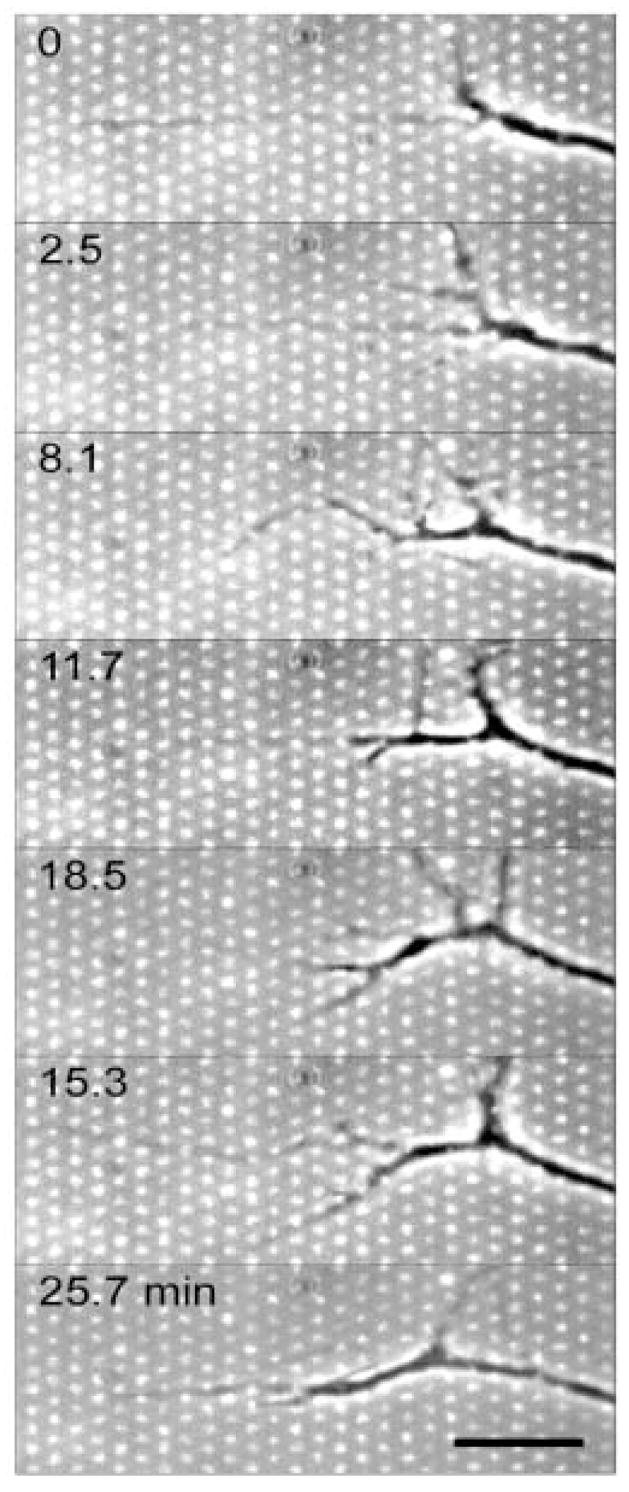
To explore the CSPG dose and time dependent integrin receptor upregulation response, we have developed a soft lithography stamp that allows the patterning of several distinct CSPG densities on a single substrate (Figure 5) [120]. These, so-called “polka dot” patterns provided very interesting insight in growth cone pathfinding dynamics. Time-lapse microscopy images showed that a narrow growth cone of a DRG chooses an outgrowth pathway predominately between aggrecan islands after the filopodia sampled the area inbetween aggrecan islands (Figure 6) [120].
Figure 5.
Fluorescence images of μCP stamped aggrecan patterns on uniform laminin adsorbed layer. The aggrecan island coverages were 2 % (left), 7 % (middle) and 31 % (right) coverage. Aggrecan was stained with the AlexaFluor-488 dye. Scale bar: 10 μm. From ref. 120.
Figure 6.
Combined phase contrast and fluorescence time-lapse microscopy images showing that a narrow growth cone of a DRG chooses an outgrowth pathway predominately between aggrecan islands (brighter dots) after the filopodia sample the area inbetween aggrecan islands. Substratum: uniform laminin field covered by μCP stamped aggrecan micrometer-sized islands (11% average aggrecan surface coverage). Scale bar −10 μm, time in minutes as indicated. [120]
A separate but similar pattern was additionally used to produce laminin containing outgrowth lanes with step function changes in CSPG density in order to explore the effects of abrupt density changes on growth cone pathfinding dynamics [120]. In general, experiments indicated that both α1β1 and α6β1 integrin receptor growth cone densities were upregulated in response to increasing levels of the CSPG aggrecan, and that increased receptor expression translated into improved cell adhesion and outgrowth in the presence of aggrecan. It was also found that short encounters with CSPG molecules triggerred inhibited outgrowth while persistent growth on a diffuse CSPG pattern promoted robust outgrowth, suggesting a CSPG induced transformation in DRGs. It was additionally demonstrated that filopodial sampling of CSPGs is sufficient to trigger long term slowing in outgrowth dynamics. Several lines of evidence are demonstrated that suggest aggrecan acts locally to inhibit integrin attachment at least in part through an anti-adhesive mechanism [120].
6 Conclusions
Protein micropatterning can be used as a valuable tool to research cell-protein interactions as well as a means to better control protein signaling at interfaces. However, in using protein micropatterns all three different sets of interactions, namely, protein-substrate, protein-protein, and protein-cell, must be considered for vitro cell assays and tissue engineering purposes. Evidence exists that protein-material interactions can affect cell behavior. In addition, the method of protein deposition can affect adsorbed amount and bioactivity. The nature of protein-protein and protein-material interactions must be considered when predicting protein behavior and resulting effects on cell attachment and outgrowth on experimental interfaces. Overall, there exists a wealth of possibilities still to be explored through micropatterning and through studying the effects of protein-protein and protein-material interactions on cell behavior for in vitro and in vivo applications. Novel patterning techniques, such as the “polka dot” patterns, can be further evolved from the existing methods and used to better control protein conformation and bioactivity as well as more precisely control spatial placement and integration of various protein signals.
Acknowledgments
The authors acknowledge the discussions with Drs. D.W. Britt, C.-B. Chien, W.M. Reichert and PA. Tresco. This work has been supported by the NIH grants EB00278 and HL084586.
References
- 1.Davies SJ, Field PM, Raisman G. Exp Neurol. 1996;142:203. doi: 10.1006/exnr.1996.0192. [DOI] [PubMed] [Google Scholar]
- 2.Li Y, Raisman G. J Neurosci. 1994;14:4050. doi: 10.1523/JNEUROSCI.14-07-04050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardingham TE, Fosang AJ. J FASEB. 1992;6:861. [PubMed] [Google Scholar]
- 4.Shibata S, Midura RJ, Hascall VC. J Biol Chem. 1992;267:6548. [PubMed] [Google Scholar]
- 5.Silbert JE, Sugumaran G. IUBMB Life. 2002;54:177. doi: 10.1080/15216540214923. [DOI] [PubMed] [Google Scholar]
- 6.Deutsch AJ, Midura RJ, Plaas AH. J Orthop Res. 1995;13:230. doi: 10.1002/jor.1100130211. [DOI] [PubMed] [Google Scholar]
- 7.Luo W, Guo C, Zheng J, Chen TL, Wang PY, Vertel BM, Tanzer ML. J Bone Miner Metab. 2000;18:51. doi: 10.1007/s007740050011. [DOI] [PubMed] [Google Scholar]
- 8.Sugumaran G, Silbert JE. J Biol Chem. 1989;264:3864. [PubMed] [Google Scholar]
- 9.Brittis PA, Canning DR, Silver J. Science. 1992;255:733. doi: 10.1126/science.1738848. [DOI] [PubMed] [Google Scholar]
- 10.Landolt RM, Vaughan L, Winterhalter KH, Zimmermann DR. Development. 1995;121:2303. doi: 10.1242/dev.121.8.2303. [DOI] [PubMed] [Google Scholar]
- 11.Hoke A, Silver J. Prog Brain Res. 1996;108:149. doi: 10.1016/s0079-6123(08)62538-8. [DOI] [PubMed] [Google Scholar]
- 12.Pearlman AL, Sheppard AM. Prog Brain Res. 1996;108:117. [PubMed] [Google Scholar]
- 13.Rhodes KB, Fawcett JW. J Anat. 2004;204:33. doi: 10.1111/j.1469-7580.2004.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snow DM, Steindler DA, Silver J. Dev Biol. 1990;138:359. doi: 10.1016/0012-1606(90)90203-u. [DOI] [PubMed] [Google Scholar]
- 15.Perissinotto D, Iacopetti P, Bellina I, Doliana R, Colombatti A, Pettway Z, Bronner-Fraser M, Shinomura T, Kimata K, Morgelin M, Lofberg J, Perris R. Development. 2000;127:2823. doi: 10.1242/dev.127.13.2823. [DOI] [PubMed] [Google Scholar]
- 16.Faissner A, Steindler D. Glia. 1995;13:233. doi: 10.1002/glia.440130402. [DOI] [PubMed] [Google Scholar]
- 17.Perris R, Johansson S. Dev Biol. 1990;137:1. doi: 10.1016/0012-1606(90)90002-z. [DOI] [PubMed] [Google Scholar]
- 18.Oakley RA, Tosney KW. Dev Biol. 1991;147:187. doi: 10.1016/s0012-1606(05)80017-x. [DOI] [PubMed] [Google Scholar]
- 19.Silver J. J Neurol. 1994;242:S22. doi: 10.1007/BF00939236. [DOI] [PubMed] [Google Scholar]
- 20.Walz A, Anderson RB, Irie A, Chien CB, Holt CE. J Neurobiol. 2002;53:330. doi: 10.1002/neu.10113. [DOI] [PubMed] [Google Scholar]
- 21.Davies SJ, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J. Nature. 1997;390:680. doi: 10.1038/37776. [DOI] [PubMed] [Google Scholar]
- 22.Levine JM. J Neurosci. 1994;14:4716. doi: 10.1523/JNEUROSCI.14-08-04716.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies JE, Tang X, Denning JW, Archibald SJ, Davies SJ. Eur J Neurosci. 2004;19:1226. doi: 10.1111/j.1460-9568.2004.03184.x. [DOI] [PubMed] [Google Scholar]
- 24.Duchossoy Y, Horvat JC, Stettler O. Mol Cell Neurosci. 2001;17:945. doi: 10.1006/mcne.2001.0986. [DOI] [PubMed] [Google Scholar]
- 25.McKeon RJ, Hoke A, Silver J. Exp Neurol. 1995;136:32. doi: 10.1006/exnr.1995.1081. [DOI] [PubMed] [Google Scholar]
- 26.Chau CH, Shum DK, Li H, Pei J, Lui YY, Wirthlin L, Chan YS, Xu XM. FASEB J. 2004;1:194. doi: 10.1096/fj.03-0196fje. [DOI] [PubMed] [Google Scholar]
- 27.Moon LD, Asher RA, Fawcett JW. J Neurosci Res. 2003;71:23. doi: 10.1002/jnr.10449. [DOI] [PubMed] [Google Scholar]
- 28.Schmalfeldt M, Bandtlow CE, Dours-Zimmermann MT, Winterhalter KH, Zimmermann DR. J Cell Sci. 2000;113:807. doi: 10.1242/jcs.113.5.807. [DOI] [PubMed] [Google Scholar]
- 29.McKeon RJ, Jurynec MJ, Buck CR. J Neurosci. 1999;19:10778. doi: 10.1523/JNEUROSCI.19-24-10778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fidler PS, Schuette K, Asher RA, Dobbertin A, Thornton SR, Calle-Patino Y, Muir E, Levine JM, Geller HM, Rogers JH, Faissner A, Fawcett JW. J Neurosci. 1999;19:8778. doi: 10.1523/JNEUROSCI.19-20-08778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudge JS, Silver J. J Neurosci. 1990;10:3594. doi: 10.1523/JNEUROSCI.10-11-03594.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snow DM, Lemmon V, Carrino DA, Caplan AI, Silver J. Exp Neurol. 1990;109:111. doi: 10.1016/s0014-4886(05)80013-5. [DOI] [PubMed] [Google Scholar]
- 33.Johnson-Green PC, Dow KB, Riopelle RJ. Glia. 1991;4:314. doi: 10.1002/glia.440040309. [DOI] [PubMed] [Google Scholar]
- 34.Lemons ML, Sandy JD, Anderson DK, Howland DR. Exp Neurol. 2003;184:981. doi: 10.1016/S0014-4886(03)00383-2. [DOI] [PubMed] [Google Scholar]
- 35.Grimpe B, Silver J. J Neurosci. 2004;24:1393. doi: 10.1523/JNEUROSCI.4986-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones LL, Sajed D, Tuszynski MH. J Neurosci. 2003;23:9276. doi: 10.1523/JNEUROSCI.23-28-09276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, Bunge MB. Nature Med. 2004;10:610. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- 38.Baron-Van Evercooren A, Kleinman HK, Ohno S, Marangos P, Schwartz JP, Dubois-Dalcq ME. J Neurosci Res. 1982;8:179. doi: 10.1002/jnr.490080208. [DOI] [PubMed] [Google Scholar]
- 39.Darmon MY. In Vitro. 1982;18:997. doi: 10.1007/BF02796374. [DOI] [PubMed] [Google Scholar]
- 40.Rossino P, Gavazzi I, Timpl R, Aumailley M, Abbadini M, Giancotti F, Silengo L, Marchisio PC, Tarone G. Exp Cell Res. 1990;189:100. doi: 10.1016/0014-4827(90)90262-9. [DOI] [PubMed] [Google Scholar]
- 41.Ruthel G, Hollenbeck PJ. J Neurosci. 2000;20:2266. doi: 10.1523/JNEUROSCI.20-06-02266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Letourneau PC, Condic ML, Snow DM. J Neurosci. 1994;14:915. doi: 10.1523/JNEUROSCI.14-03-00915.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin CH, Espreafico EM, Mooseker MS, Forscher P. Neuron. 1996;16:769. doi: 10.1016/s0896-6273(00)80097-5. [DOI] [PubMed] [Google Scholar]
- 44.Rochlin MW, Wickline KM, Bridgman PC. J Neurosci. 1996;16:3236. doi: 10.1523/JNEUROSCI.16-10-03236.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldberg DJ, Burmeister DW. Trends Neurosci. 1989;12:503. doi: 10.1016/0166-2236(89)90110-0. [DOI] [PubMed] [Google Scholar]
- 46.Suter DM, Forscher P. J Neurobiol. 2000;44:97. [PubMed] [Google Scholar]
- 47.Forscher P, Smith SJ. J Cell Biol. 1988;107:1505. doi: 10.1083/jcb.107.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dickson BJ. Science. 2002;298:1959. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka E, Sabry J. Cell. 1995;83:171. doi: 10.1016/0092-8674(95)90158-2. [DOI] [PubMed] [Google Scholar]
- 50.Kuhn TB, Schmidt MF, Kater SB. Neuron. 1995;14:275. doi: 10.1016/0896-6273(95)90285-6. [DOI] [PubMed] [Google Scholar]
- 51.Ivins JK, Yurchenco PD, Lander AD. J Neurosci. 2000;20:6551. doi: 10.1523/JNEUROSCI.20-17-06551.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyasaka M. Int Immunol. 2001;13:359. doi: 10.1093/intimm/13.3.359. [DOI] [PubMed] [Google Scholar]
- 53.Sivasankaran R, Pei J, Wang KC, Zhang YP, Shields CB, Xu XM, He Z. Nature Neurosci. 2004;7:261. doi: 10.1038/nn1193. [DOI] [PubMed] [Google Scholar]
- 54.Ernst H, Zanin MK, Everman D, Hoffman S. J Cell Sci. 1995;108:3807. doi: 10.1242/jcs.108.12.3807. [DOI] [PubMed] [Google Scholar]
- 55.Snow DM, Atkinson PB, Hassinger TD, Letourneau PC, Kater SB. Dev Biol. 1994;166:87. doi: 10.1006/dbio.1994.1298. [DOI] [PubMed] [Google Scholar]
- 56.Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, Poo M. Science. 1998;281:1515. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- 57.Aricescu AR, McKinnell IW, Halfter W, Stoker AW. Mol Cell Biol. 2002;22:1881. doi: 10.1128/MCB.22.6.1881-1892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin CH, Forscher P. J Cell Biol. 1993;121:1369. doi: 10.1083/jcb.121.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sabry JH, O’Connor TP, Evans L, Toroian-Raymond A, Kirschner M, Bentley D. J Cell Biol. 1991;115:381. doi: 10.1083/jcb.115.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bovolenta P, Mason C. J Neurosci. 1987;7:1447. doi: 10.1523/JNEUROSCI.07-05-01447.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Caudy M, Bentley D. J Neurosci. 1986;6:1781. doi: 10.1523/JNEUROSCI.06-06-01781.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim GJ, Shatz CJ, McConnell SK. J Neurobiol. 1991;22:629. doi: 10.1002/neu.480220608. [DOI] [PubMed] [Google Scholar]
- 63.Mason CA, Wang LC. J Neurosci. 1997;17:1086. doi: 10.1523/JNEUROSCI.17-03-01086.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gundersen RW. J Neurosci Res. 1988;21:298. doi: 10.1002/jnr.490210222. [DOI] [PubMed] [Google Scholar]
- 65.Andrade JD. Protein Adsorption. Vol. 2. New York: Plenum Press; 1985. Surface and Interfacial Aspects of Biomaterials. [Google Scholar]
- 66.Fang F, Szleifer I. Biophys J. 2001;80:2568. doi: 10.1016/S0006-3495(01)76228-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rogers SL, Edson KJ, Letourneau PC, McLoon SC. Dev Biol. 1986;113:429. doi: 10.1016/0012-1606(86)90177-6. [DOI] [PubMed] [Google Scholar]
- 68.Li S, Harrison D, Carbonetto S, Fassler R, Smyth N, Edgar D, Yurchenco PD. J Cell Biol. 2002;157:1279. doi: 10.1083/jcb.200203073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Friedman R, Gelfand T, Weiss DW, Doljanski F. Int J Tissue React. 1984;6:291. [PubMed] [Google Scholar]
- 70.McDonald JA, Kelley DG, Broekelmann TJ. J Cell Biol. 1982;92:485. doi: 10.1083/jcb.92.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Elwing H, Nilsson B, Svensson KE, Askendahl A, Nilsson UR, Lundström I. J Coll Interface Sci. 1988;125:139. [Google Scholar]
- 72.Leonard EF, Turrito VT, Vroman L. Blood in Contact with Natural and Artificial Surfaces. New York: Ann NY Acad Sci; 1987. [PubMed] [Google Scholar]
- 73.Andersson J, Larsson R, Richter R, Ekdahl KN, Nilsson B. Biomaterials. 2001;22:2435. doi: 10.1016/s0142-9612(00)00431-2. [DOI] [PubMed] [Google Scholar]
- 74.Lundberg F, Lea T, Ljungh A. Infect Immun. 1997;65:897. doi: 10.1128/iai.65.3.897-902.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.DeHeer DH, Engels JA, DeVries AS, Knapp RH, Beebe JD. J Biomed Mater Res. 2001;54:12. doi: 10.1002/1097-4636(200101)54:1<12::aid-jbm2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 76.Zhang S, Yan L, Altman M, Lassle M, Nugent H, Frankel F, Lauffenburger DA, Whitesides GM, Rich A. Biomaterials. 1999;20:1213. doi: 10.1016/s0142-9612(99)00014-9. [DOI] [PubMed] [Google Scholar]
- 77.Whitesides GM, Grzybowski B. Science. 2002;295:2418. doi: 10.1126/science.1070821. [DOI] [PubMed] [Google Scholar]
- 78.Ulman A. J Materials Ed. 1991;11:207. [Google Scholar]
- 79.Sigal GB, Mrksich M, Whitesides GM. J Am Chem Soc. 1998;120:3464. [Google Scholar]
- 80.Prime KL, Whitesides GM. Science. 1991;252:1164. doi: 10.1126/science.252.5009.1164. [DOI] [PubMed] [Google Scholar]
- 81.Lin YS, Hlady V. Coll & Surfaces B: Biointerfaces. 1994;2:481. doi: 10.1016/0927-7765(94)80056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dulcey CS, Georger JH, Jr, Krauthamer V, Stenger DA, Fare TL, Calvert JM. Science. 1991;252:551. doi: 10.1126/science.2020853. [DOI] [PubMed] [Google Scholar]
- 83.Calvert JM, Georger JH, Jr, Peckerar MC, Pehrsson PE, Schnur JM, Schoen PE. Thin Solid Films. 1992;210/211:359. [Google Scholar]
- 84.Bhatia SK, Hickman JJ, Ligler FS. J Am Chem Soc. 1992;114:4432. [Google Scholar]
- 85.Liu J, Hlady V. Coll Surfaces B: Biointerfaces. 1996;8:25. doi: 10.1016/S0927-7765(96)01298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dupont-Gillain CC, Nysten B, Hlady V, Rouxhet PG. J Coll Interface Sci. 1999;220:163. doi: 10.1006/jcis.1999.6524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fodor SPA, Read JL, Pirrung MC, Stryer L, Lu AT, Solas D. Science. 1991;251:767. doi: 10.1126/science.1990438. [DOI] [PubMed] [Google Scholar]
- 88.Rozsnyai LF, Benson DR, Fodor SPA, Schultz PG. Angew Chem Int Ed. 1992;31:759. [Google Scholar]
- 89.Kumar A, Whitesides GM. Science. 1994;263:60. doi: 10.1126/science.263.5143.60. [DOI] [PubMed] [Google Scholar]
- 90.Kimura J, Kawana Y, Kuriyama T. Biosensors. 1998;14:41. [Google Scholar]
- 91.Wilbur JL, Biebuyck HA, MacDonald JC, Whitesides GM. Langmuir. 1995;11:825. [Google Scholar]
- 92.Kane RS, Takayama S, Ostuni E, Ingber DE, Whitesides GM. Biomaterials. 1999;20:2363. doi: 10.1016/s0142-9612(99)00165-9. [DOI] [PubMed] [Google Scholar]
- 93.Singhvi R, Kumar A, Lopez GP, Stephanopoulos GN, Wang DI, Whitesides GM, Ingber DE. Science. 1994;264:696. doi: 10.1126/science.8171320. [DOI] [PubMed] [Google Scholar]
- 94.Mrksich M, Whitesides GM. Annu Rev Biophys Biomol Struct. 1996;25:55. doi: 10.1146/annurev.bb.25.060196.000415. [DOI] [PubMed] [Google Scholar]
- 95.Mrksich M, Dike LE, Tien J, Ingber DE, Whitesides GM. Exp Cell Res. 1997;235:305. doi: 10.1006/excr.1997.3668. [DOI] [PubMed] [Google Scholar]
- 96.Schmid H, Michel B. Macromol. 2000;33:3042. [Google Scholar]
- 97.Whitesides GM, Ostuni E, Takayama S, Jiang X, Ingber DE. Annu Rev Biomed Eng. 2001;3:335. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 98.Kane RS, Takayama S, Ostuni E, Ingber DE, Whitesides GM. Biomaterials. 1999;20:2363. doi: 10.1016/s0142-9612(99)00165-9. [DOI] [PubMed] [Google Scholar]
- 99.Jackman RJ, Wilbur JL, Whitesides GM. Science. 1995;269:664. doi: 10.1126/science.7624795. [DOI] [PubMed] [Google Scholar]
- 100.Clarson SJ, Mark JE. In: Siloxane Polymers. Clarson SJ, Semlyen JA, editors. Prentice Hall; Englewood Cliffs NJ: 1993. [Google Scholar]
- 101.Delamarche E, Geissler M, Wolf H, Michel B. J Am Chem Soc. 2002;124:3834. doi: 10.1021/ja017854j. [DOI] [PubMed] [Google Scholar]
- 102.Choi D–G, Kyun H, Yang SM. Mater Sci Eng C. 2004;24:213. [Google Scholar]
- 103.Yoo PJ, Choi S–J, Kim JH, Suh D, Baek SJ, Kim TW, Lee HH. Chem Mater. 2004;16:5000. [Google Scholar]
- 104.Odom TW, Thalladi VR, Love JC, Whitesides GM. J Am Chem Soc. 2002;124:12112. doi: 10.1021/ja0209464. [DOI] [PubMed] [Google Scholar]
- 105.Speed MA, Morshead T, Wang DI, King J. Protein Sci. 1997;6:99. doi: 10.1002/pro.5560060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Webb K, Hlady V, Tresco PA. J Biomed Mater Res. 2000;49:362. doi: 10.1002/(sici)1097-4636(20000305)49:3<362::aid-jbm9>3.0.co;2-s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.James CD, Davis RC, Kam L, Craighead HG, Isaacson M, Turner JN, Shain W. Langmuir. 1998;14:741. [Google Scholar]
- 108.Bernard A, Delamarche E, Heinz S, Michel B, Bosshard HR, Biebuyck H. Langmuir. 1998;14:2225. [Google Scholar]
- 109.Kam L, Shain W, Turner JN, Bizios R. Biomaterials. 2001;22:1049. doi: 10.1016/s0142-9612(00)00352-5. [DOI] [PubMed] [Google Scholar]
- 110.Lauer L, Klein C, Offenhausser A. Biomaterials. 2001;22:1925. doi: 10.1016/s0142-9612(00)00379-3. [DOI] [PubMed] [Google Scholar]
- 111.James CD, Davis R, Meyer M, Turner A, Turner S, Withers G, Kam L, Banker G, Craighead H, Isaacson M, Turner J, Shain W. IEEE Trans Biomed Eng. 2000;47:17. doi: 10.1109/10.817614. [DOI] [PubMed] [Google Scholar]
- 112.Esch T, Lemmon V, Banker G. J Neurosci. 1999;19:6417. doi: 10.1523/JNEUROSCI.19-15-06417.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Papra A, Bernard A, Juncker D, Larsen N, Michel B, Delamarche E. Langmuir. 2001;17:4090. [Google Scholar]
- 114.Patel N, Padera R, Sanders GH, Cannizzaro SM, Davies MC, Langer R, Roberts CJ, Tendler SJ, Williams PM, Shakesheff KM. FASEB J. 1998;12:1447. doi: 10.1096/fasebj.12.14.1447. [DOI] [PubMed] [Google Scholar]
- 115.Cuvelier D, Rossier O, Bassereau P, Nassoy P. Eur Biophys J. 2003;32:342. doi: 10.1007/s00249-003-0282-2. [DOI] [PubMed] [Google Scholar]
- 116.Tien J, Nelson CM, Chen CS. Proc Natl Acad Sci USA. 2002;99:1758. doi: 10.1073/pnas.042493399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gundersen RW. Dev Biol. 1987;121:423. doi: 10.1016/0012-1606(87)90179-5. [DOI] [PubMed] [Google Scholar]
- 118.Gomez TM, Letourneau PC. J Neurosci. 1994;14:5959. doi: 10.1523/JNEUROSCI.14-10-05959.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hubbell JA. Matrix Effects. In: Lanza RP, Langer R, Vacanti J, editors. Principles of Tissue Engineering. 2. Academic Press; 2000. [Google Scholar]
- 120.Hodgkinson GN. PhD Dissertation. University of Utah; 2005. Interfacial biomolecular engineering: controlling material - protein - cell interactions through micro-patterning. [Google Scholar]
- 121.Yu X, Bellamkonda RV. J Neurosci Res. 2001;66:303. doi: 10.1002/jnr.1225. [DOI] [PubMed] [Google Scholar]
- 122.Challacombe JF, Snow DM, Letourneau PC. J Cell Sci. 1996;109:2031. doi: 10.1242/jcs.109.8.2031. [DOI] [PubMed] [Google Scholar]
- 123.Hynds DL, Snow DM. Exp Neurol. 1999;160:244. doi: 10.1006/exnr.1999.7212. [DOI] [PubMed] [Google Scholar]
- 124.Hynds DL, Snow DM. J Neurosci Res. 2001;66:630. doi: 10.1002/jnr.10020. [DOI] [PubMed] [Google Scholar]
- 125.Hodgkinson GN, Tresco PA, Hlady V. Biomaterials. 2007;28:2590. doi: 10.1016/j.biomaterials.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tanaka E, Sabry J. Cell. 1995;83:171. doi: 10.1016/0092-8674(95)90158-2. [DOI] [PubMed] [Google Scholar]
- 127.Kuhn TB, Schmidt MF, Kater SB. Neuron. 1995;14:275. doi: 10.1016/0896-6273(95)90285-6. [DOI] [PubMed] [Google Scholar]
- 128.Dickson BJ. Science. 2002;298:1959. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- 129.Snow DM, Letourneau PC. J Neurobiol. 1992;23:322. doi: 10.1002/neu.480230311. [DOI] [PubMed] [Google Scholar]
- 130.Snow DM, Mullins N, Hynds DL. Microsc Res Tech. 2001;54:273. doi: 10.1002/jemt.1140. [DOI] [PubMed] [Google Scholar]
- 131.Snow DM, Smith JD, Gurwell JA. J Neurobiol. 2002;51:285. doi: 10.1002/neu.10060. [DOI] [PubMed] [Google Scholar]
- 132.Condic ML, Snow DM, Letourneau PC. J Neurosci. 1999;19:10036. doi: 10.1523/JNEUROSCI.19-22-10036.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Friedlander DR, Milev P, Karthikeyan L, Margolis RK, Margolis RU, Grumet M. J Cell Biol. 1994;125:669. doi: 10.1083/jcb.125.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Milev P, Friedlander DR, Sakurai T, Karthikeyan L, Flad M, Margolis RK, Grumet M, Margolis RU. J Cell Biol. 1994;127:1703. doi: 10.1083/jcb.127.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Skubitz AP, McCarthy JB, Charonis AS, Furcht LT. J Biol Chem. 1988;263:4861. [PubMed] [Google Scholar]
- 136.Braunewell KH, Martini R, LeBaron R, Kresse H, Faissner A, Schmitz B, Schachner M. Eur J Neurosci. 1995;7:792. doi: 10.1111/j.1460-9568.1995.tb00682.x. [DOI] [PubMed] [Google Scholar]
- 137.Dou CL, Levine JM. J Neurosci. 1994;14:7616. doi: 10.1523/JNEUROSCI.14-12-07616.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dou CL, Levine JM. J Neurosci. 1995;15:8053. doi: 10.1523/JNEUROSCI.15-12-08053.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Herndon ME, Stipp CS, Lander AD. Glycobiology. 1999;9:143. doi: 10.1093/glycob/9.2.143. [DOI] [PubMed] [Google Scholar]
- 140.Hammarback JA, Palm SL, Furcht LT, Letourneau PC. J Neurosci Res. 1985;13:213. doi: 10.1002/jnr.490130115. [DOI] [PubMed] [Google Scholar]
- 141.Corey JM, Wheeler BC, Brewer GJ. J Neurosci Res. 1991;30:300. doi: 10.1002/jnr.490300204. [DOI] [PubMed] [Google Scholar]
- 142.Hynds DL, Snow DM. J Neurosci Res. 2001;66:630. doi: 10.1002/jnr.10020. [DOI] [PubMed] [Google Scholar]
- 143.Gundersen RW. J Neurosci Res. 1985;13:199. doi: 10.1002/jnr.490130114. [DOI] [PubMed] [Google Scholar]
- 144.Condic ML, Letourneau PC. Nature. 1997;389:852. doi: 10.1038/39878. [DOI] [PubMed] [Google Scholar]
- 145.Condic ML. J Neurosci. 2001;21:4782. doi: 10.1523/JNEUROSCI.21-13-04782.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wallquist W, Zelano J, Plantman S, Kaufman SJ, Cullheim S, Hammarberg H. J Comp Neurol. 2004;480:162. doi: 10.1002/cne.20345. [DOI] [PubMed] [Google Scholar]
- 147.Ito Y. Biomaterials. 1999;20:2333. doi: 10.1016/s0142-9612(99)00162-3. [DOI] [PubMed] [Google Scholar]
- 148.Kam L, Shain W, Turner JN, Bizios R. Biomaterials. 1999;20:2343. doi: 10.1016/s0142-9612(99)00163-5. [DOI] [PubMed] [Google Scholar]
- 149.Lu L, Kam L, Hasenbein M, Nyalakonda K, Bizios R, Gopferich A, Young JF, Mikos AG. Biomaterials. 1999;20:2351. doi: 10.1016/s0142-9612(99)00164-7. [DOI] [PubMed] [Google Scholar]
- 150.Stenger DA, Hickman JJ, Bateman KE, Ravenscroft MS, Ma W, Pancrazio JJ, Shaffer K, Schaffner AE, Cribbs DH, Cotman CW. J Neurosci Methods. 1998;82:167–73. doi: 10.1016/s0165-0270(98)00047-8. [DOI] [PubMed] [Google Scholar]
- 151.Wang N, Ostuni E, Whitesides GM, Ingber DE. Cell Motil Cytoskeleton. 2002;52:97. doi: 10.1002/cm.10037. [DOI] [PubMed] [Google Scholar]
- 152.Matsuzawa M, Tokumitsu S, Knoll W, Liesi P. J Neurosci Res. 1998;53:114. doi: 10.1002/(SICI)1097-4547(19980701)53:1<114::AID-JNR12>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 153.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Biotechnol Prog. 1998;14:356. doi: 10.1021/bp980031m. [DOI] [PubMed] [Google Scholar]
- 154.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Science. 1997;276:1425. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 155.Parker KK, Brock AL, Brangwynne C, Mannix RJ, Wang N, Ostuni E, Geisse NA, Adams JC, Whitesides GM, Ingber DE. FASEB J. 2002;16:1195. doi: 10.1096/fj.02-0038com. [DOI] [PubMed] [Google Scholar]
- 156.Brock A, Chang E, Ho CC, LeDuc P, Jiang X, Whitesides GM, Ingber DE. Langmuir. 2003;19:1611. doi: 10.1021/la026394k. [DOI] [PubMed] [Google Scholar]