Abstract
We investigated the effects of different ischemia-mimetic factors on intracellular Ca2+ concentration ([Ca2+]i). Ventricular myocytes were isolated from adult Wistar rats, and [Ca2+]i was measured using fluorescent indicator fluo-4 AM by confocal microscopy. Intracellular pH was measured using c5-(and-6)-carboxy SNARF-1 AM, a dual emission pH-sensitive ionophore. Myocytes were exposed to hypoxia, extracellular acidosis (pH 6.8), Na-lactate (10 mM), or to combination of those factors for 25 min. Monitoring of [Ca2+]i using fluo-4 AM fluorescent indicator revealed that [Ca2+]i accumulation increased immediately after exposing the cells to Na-lactate and extracellular acidosis, but not during cell exposure to moderate ischemia. Increase in [Ca2+]i during Na-lactate exposure decreased to control levels at the end of exposure period at extracellular pH 7.4, but not at pH 6.8. When combined, Na-lactate and acidosis had an additive effect on [Ca2+]i increase. After removal of solutions, [Ca2+]i continued to rise only when acidosis, hypoxia, and Na-lactate were applied together. Analysis of intracellular pH revealed that treatment of cells by Na-lactate and acidosis caused intracellular acidification, while short ischemia did not significantly change intracellular pH. Our experiments suggest that increase in [Ca2+]i during short hypoxia does not happen if pHi does not fall, while extracellular acidosis is required for sustained rise in [Ca2+]i induced by Na-lactate. Comparing to the effect of Na-lactate, extracellular acidosis induced slower [Ca2+]i elevation, accompanied with slower decrease in intracellular pH. These multiple effects of hypoxia, extracellular acidosis, and Na-lactate are likely to cause [Ca2+]i accumulation after the hypoxic stress.
Keywords: intracellular Ca2+, intracellular acidosis, ischemia
Introduction
Elevated intracellular Ca2+ concentration ([Ca2+]i) during ischemia and reperfusion is known to induce damage to the heart. During ischemia, interruption of the oxygen supply and substrates promotes ATP hydrolysis and glycolysis, which together induce intracellular acidosis. Intracellular acidosis during ischemia may cause elevation of [Ca2+]i by stimulation Na+-H+ exchanger resulting in Na+ influx, which increases [Ca2+]i by activation of Na+-Ca+ exchanger. On the other hand, ATP depletion decreases function of Ca-ATPases of sarcoplazmic reticulum and sarcolema further leading to elevation of [Ca2+]i [1-4].
Ischemia-induced changes in intracellular environment like depletion of ATP, lactate accumulation, and intracellular acidosis are often used to simulate ischemic damage of the heart. Different cellular models, including single fresh cardiomyocyte preparations, require short-lasting ischemia to study the effects of ischemia and reperfusion on cellular function. Differences in ischemia-mimetic solutions can differently affect [Ca2+]i, and thus be crucial for the research of simulated cardiac ischemic injury. In this study, we compared the effects of 25 min-lasting simulated hypoxia with glucose deficiency, lactate accumulation, and extracellular acidosis on [Ca2+]i during hypoxia and reoxygenation in single ventricular cardiomyocytes. Additionally, we examined the role of intracellular pH (pHi) in changes of [Ca2+]i under the same conditions. The results of this study provide new insight into effects of simulated cellular ischemia that alter [Ca2+]i, which produce cellular damage.
Materials and Methods
Animals
Adult male Wistar rats (175-250 g) were used in this study. Animals were kept on 12-h cycles of light and dark, and received water and standard food ad libitum. The investigation was conducted in accordance with the Institutional Animal Use and Care Committee of the Medical College of Wisconsin (Milwaukee, Wisconsin), and in accordance with U.S. National Institutes of Health standards (NIH Publication 95-23, revised 1996).
Cell isolation
Ventricular myocytes were isolated by enzymatic dissociation [5, 6]. Animals were anesthetized with an intraperitoneal injection of thiobutabarbital (Inactin, 100-150 mg/kg, Sigma-Aldrich, St. Louis, MO) and heparin (1000 U) to prevent blood clotting. The heart was rapidly removed and mounted to Langendorff perfusion system. All perfusion solutions were continuously gassed with 95% O2 and 5% CO2, and temperature was held at 37°. Perfusion with a 0 mM Ca2+ solution for 5 min was followed by 11 min of recirculation with the same solution containing 0.5 mg/mL collagenase type II (Invitrogen, Carlsberg, CA) and 0.25 mg/mL protease XIV (Sigma-Aldrich). The left ventricle was minced and shaken for 5 min and then filtered through a nylon mesh (200 μm; Spectrum Laboratories, Inc. Rancho Dominguez, CA). After isolation, the myocytes were stored in the HEPES-buffered Tyrode solution at 20°-22°C and used for experiments within 5 h after isolation. All cells used in this study were rod shaped in appearance, had well-defined striations, and did not spontaneously contract.
Measurement of [Ca2+]i
Fluorescence measurements were made with an inverted laser-scanning confocal microscope (Nikon Eclipse TE2000-U, Nikon, Japan). [Ca2+]i was monitored with Ca2+ sensitive indicator Fluo-4 AM (Fluo-4). Cells were incubated with Fluo-4 AM (1 μM; Invitrogen) at room temperature for 20 min. Following incubation, the myocytes were superfused with dye-free Tyrode solution for additional 15 min. Fluo-4 was excited at the 488 nm with an argon laser, and emitted fluorescence was recorded at 520 nm using a 40x/1.4 oil-immersion objective (Nikon). Changes in [Ca2+]i were expressed as relative changes compared to the baseline. Relative [Ca2+]i was calculated as the ratio (F/F0 × 100), where F is the measured fluorescence and F0 is the baseline fluorescence at the beginning of experiment.
Measurement of pHi
The pHi was monitoring in single myocytes using 5-(and-6)-carboxy SNARF-1, acetoxymethiyl ester, acetate (SNARF-1) as the fluorescent indicator [7, 8]. Myocytes were loaded with 10 μM SNARF-1 (Molecular Probes, Eugene, OR) in Tyrode solution at room temperature for 15 min [9]. After dye loading, cells were superperfused with the same solution, containing no dye, for additional 20 min before recordings started. SNARF-1 was excited at 543 nm with a green HeNe laser. Emitted fluorescence was simultaneously collected at 590 and 650 nm via a 40x/1.4 oil-immersion objective (Nikon). The 640 nm/ 590 nm emission ratio from each cell was calculated and converted to pHi values using nigericin calibration technique [7, 10]. Nigericin, a H+/K+ exchanger ionophore sets [K+]o = [K+]i and thus pHo = pHi. Calibration curve was made by exposing the cells to different pH (pH 6.0, pH 7.5, and pH 9.0) buffers in a depolarizing high K+ buffer (140 mM KCl, 1.0 mM MgCl2, 5 mM D-glucose, 10 mM HEPES, in the presence of 10 μM nigericin) [11]. To reduce contamination of the superfusion system with nigericin [12], calibration was not done after every experiment. Instead, the pH calibration data were obtained separately from individual cells and were averaged for more than 15 cells.
Solutions
For baseline measurements, myocytes were superperfused with normoxic Tyrode solution containing (in mM): 132 NaCl, 10 HEPES, 5 D-glucose, 5 KCl, 1 CaCl2, and 1.2 MgCl2 equilibrated at room air; PO2 199.1±14.1 mmHg; pH 7.4 adjusted with NaOH. For simulated hypoxia, cells were placed into a closed hypoxic chamber (RC-21B, Warner Instruments, Hamden, CT) and perfused under controlled conditions. Hypoxia was simulated by perfusing the myocytes with hypoxic Tyrode solution containing (in mM): 132 NaCl, 10 HEPES, 0 D-glucose, 8 KCl, 1 CaCl2, and 1.2 MgCl2; pH 7.4; PO2 26.5±2.8 mmHg, bubbled with 100% nitric oxide for 20 min. Acidosis was simulated by perfusing the cells with Tyrode solution at pH 6.8. Lactate accumulation was induced by adding 10 mM Na-lactate to Tyrode solution without osmotic compensation. Hypoxia, acidosis, and lactate accumulation were simulated with HEPES-buffered glucose-free Tyrode solution containing (in mM): 118 NaCl, 10 HEPES, 8 KCl, 1 CaCl2, 1.2 MgCl2, 1 Na2HPO4, and 10 mM Na-L-lactate, pH 6.8; gassed with 100% N2 for 20 min; PO2 26.5±2.8 mmHg. To diminish contamination by room air, all solutions were placed in glass syringes and delivered to recording chamber using an air-tight tubing system. The PO2 of used solutions was measured with a gas analyzer (Radiometer ABL 505, Radiometer, Copenhagen, Denmark).
Statistical analysis
Data are presented as means ± SD, with the sample size (n). To test differences between groups, we used one-way analysis of variance with Bonferroni's post-hoc test. Analysis of variance for repeated measures with Tukey’s post-hoc test was used for temporal comparisons between groups. A P value of 0.05 or less was considered significantly different.
RESULTS
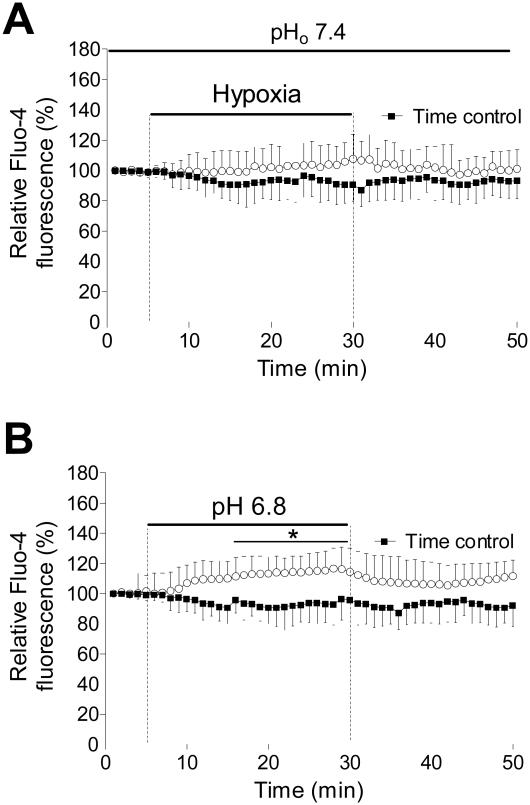
Following equilibration in normoxic HEPES-buffered Tyrode solution for 5 min, myocytes were exposed to simulated hypoxia (PO2 = 25 mmHg) at extracellular pH of 7.4. Figure 1A shows the effect of hypoxia applied for 25 min on [Ca2+]i in ventricular myocytes. During the exposure to hypoxia there was a slow increase of [Ca2+]i. At the end of this period [Ca2+]i increased to 106±5% (P>0.05). When hypoxic solution was replaced with normoxic Tyrode solution, [Ca2+]i returned to the preischemic levels.
Figure 1.
Intracellular Ca2+ changes in response to hypoxia and acidosis. A) Summarized recordings of relative fluo-4 fluorescence in cardiomyocytes exposed to glucose-free Tyrode solution with decreased O2 concentration (25 mmHg) for 25 min and in cells which were perfused with normoxic Tyrode solution (Time control). B) Time course of the relative fluo-4 fluorescence change in cells exposed to normoxic Tyrode solution with decreased pH to 6.8. *P<0.05 versus time control, n=12 from 3 rats, error bars represent SD.
Figure 1B shows the effect of acidotic Tyrode solution (pH 6.8) on [Ca2+]i. The rate of [Ca2+]i increase was faster compared to hypoxia-induced [Ca2+]i increase, and at the end of 25 min period it was 116±4% (P<0.05). Washing out acidotic Tyrode solution slowly retuned [Ca2+]i to control levels. In contrast to the effect of hypoxia, [Ca2+]i recovery was prolonged. This suggests that the [Ca2+]i regulating mechanisms are impaired by lowering extracellular pH.
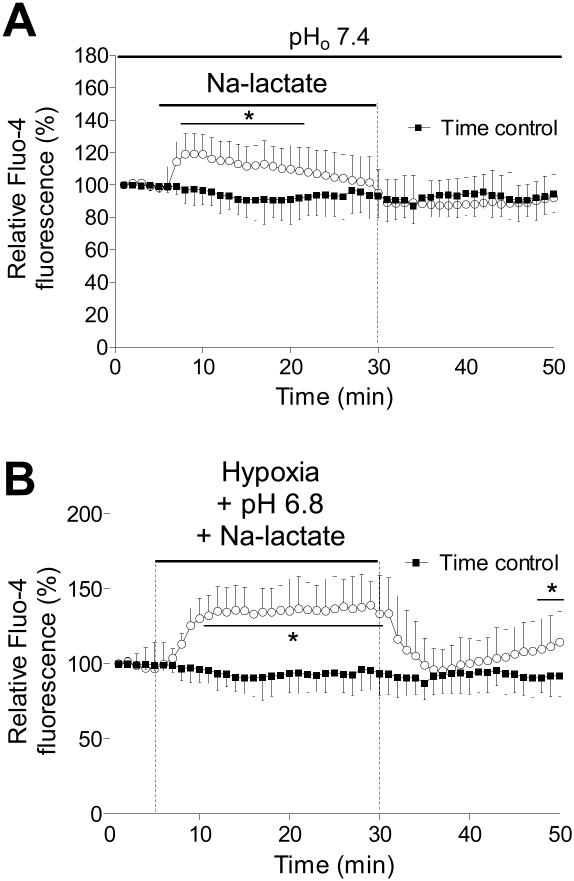
Na-lactate (10 mM) was used to induce intracellular lactate accumulation and intracellular acidosis. Figure 2A shows the changes in [Ca2+]i during the application of lactate at extracellular pH 7.4. Initially, there was rapid increase in [Ca2+]i, which was similar to that induced by acidotic Tyrode solution, 118±10% (P<0.05). After the initial rise in [Ca2+]i, there was a slow partial recovery of [Ca2+]I, which reached control levels after 25 min. This result suggests that [Ca2+]i regulating process is active at normal extracellular pH.
Figure 2.
The effect of Na-lactate and hypoxia with Na-lactate and extracellular acidosis on intracellular Ca2+. A) Fluorescence measurements of intracellular pH in cardiomyocytes exposed to 10 mM Na-lactate (pH 7.4) for 25 min. B) Relative fluo-4 fluorescence intensity during hypoxia together with Na-lactate (10 mM) and extracellular acidosis (pH 6.8). *P<0.05 versus time control, n=12 from 3 rats, error bars represent SD.
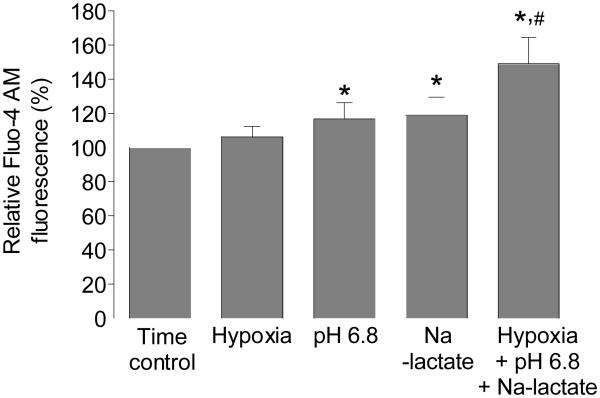
When cells were exposed to hypoxia together with acidosis (pH 6.8) and Na-lactate, there was rapid increase in [Ca2+]i (149±15%), which did not recover during the hypoxic period. This implies that Na-lactate induces fast increase in [Ca2+]I, which persist when extracellular pH is decreased, indicating that regulating processes are less active at decreased extracellular pH. During early reperfusion, [Ca2+]i decreased to the preischemic levels, but then significantly increased through reoxygenation, indicating that cells were damaged during simulated hypoxia (Figure 2B). Figure 3 summarized the change in [Ca2+]i induced by hypoxia, Na-lactate, and extracellular acidosis or with combination of these factors.
Figure 3.
Changes in intracellular Ca2+ in response to hypoxia, Na-lactate, extracellular acidosis, and combination of those factors. Graph shows summarized data for relative fluo-4 fluorescence of cardiomyocytes exposed to different ischemia-mimetic factors. *P<0.05 versus time control, #P<0.05 versus pH 6.8 or Na-lactate, n=10 from 3 rats, error bars represent SD.
The changes in pHi resulting from exposure to hypoxia, Na-lactate, extracellular acidosis, and combination of these factors are shown in Figure 4. Hypoxia induced small decrease in pHi (0.07±0.02 pH units, P>0.05). Na-lactate induced faster pHi decrease (0.15±0.03 pH units, P<0.05), which remained constant. Exposure of the cells to extracellular acidosis caused the decrease in pHi that was slower than pHi decrease during the Na-lactate exposure, but it was more profound (0.22±0.05 pH units, P<0.05). This corresponds to an increase in [Ca2+]i as seen when cells were exposed to acidotic Tyrode solution. Under the conditions of hypoxia with decreased extracellular pH and Na-lactate, the decrease in pHi was the largest (0.42±0.08 pH units, P<0.05).
Figure 4.
Changes in pHi in response to hypoxia, Na-lactate, extracellular acidosis, and combination of those factors. Figure shows representative examples of pHi changes of cardiomyocytes following exposure to different ischemia-mimetic factors. *P<0.05 versus time control, #P<0.05 versus pH 6.8 or Na-lactate, n=12 from 5 rats, error bars represent SD.
Discussion
In this study, we examined the effects of different ischemia-mimicking factors on [Ca2+]i and pHi in isolated ventricular myocytes. The main objective of this study was to compare the measurements with different types of hypoxia-mimicking solutions and to determine temporal relationship between the rise in [Ca2+]i and pHi. Our results showed that moderate ischemia (PO2 = 25 mmHg) had no effect on the rise in [Ca2+]i and decrease in pHi during the tested period. Na-lactate at 10 mM concentration rapidly increased [Ca2+]i, which subsequently recovered at extracellular pH 7.4. When Na-lactate was combined with extracellular acidosis (pH 6.8) this effect was prevented. Extracellular acidosis induced slower rise in [Ca2+]i that was accompanied with slow intracellular acidification. Combination of those factors also induced the rise in [Ca2+]i after the removal of solutions.
Accumulation of [Ca2+]i is one of the main factors causing cardiac damage during ischemia, and rise in [Ca2+]i causes irreversible myocardial damage [4]. In myocardial ischemia pHi decreases due to ATP hydrolysis, increased glycolysis, and increased lactate production. In addition, protons can not be removed due to impaired circulation. The lack of ATP impairs the function of Ca2+ pumps, while acidosis induces an additional rise in [Ca2+]i by activation of Na+-Ca2+ exchanger [1, 2]. When myocytes are exposed longer to an acidic solution, Na+-H+ exchanger activity is stimulated resulting in a Na+ influx, which subsequently induces Na+-Ca2+ exchanger to operate in reverse mode resulting in rise in [Ca2+]i. Studies performed on animal models showed that Na+-H+ exchanger inhibitors, such as amiloride derivatives, can reduce intracellular Na+ accumulation and subsequent rise in [Ca2+]i during ischemia [2]. These inhibitors were also beneficial in reducing myocardial ischemia and reperfusion injury [13].
To study the effects of ischemia and reoxygenation on cardiac myocytes, different ischemia-mimicking solutions are used. Those solutions can induce hypoxia, glucose deficiency, hyperkalemia, hypercapnia, acidosis, lactate accumulation, or substrate deprivation [3, 14]. For ischemia and reperfusion experiments, extended cellular viability is essential to study different cellular processes at reoxygenation. The long duration of hypoxia is limiting factor to study ischemia and reperfusion injury in fresh cellular preparations, like cardiac myocytes, which have to be used within several hours of isolation. On the other hand, to induce ischemic damage of resting cardiomyocytes by sole hypoxia, usually several hours of hypoxia are necessary. Additionally, cardioprotective strategies like ischemic and anesthetic preconditioning are maximally potent when fresh cells are used [3]. For that reason the use of the short ischemia and reoxygenation-mimicking factors is necessary in many experimental models.
It has been reported that rat cardiomyocyte superfused with an anoxic medium lacking glucose does not increase [Ca2+]i during prolonged hypoxia (more than 1 hour) [15]. This is in agreement with our experiments where 25 min of hypoxia without glucose did not significantly increase [Ca2+]i. Our experiments suggest that increase in [Ca2+]i during short hypoxia does not happen if pHi does not fall. In an earlier study, it was found that 20 mM Na-lactate induces intracellular acidosis and rise in [Ca2+]i. But when it was accompanied with extracellular acidosis (pH 6.4) at used concentration, Na-lactate induced severe twitch potentiations and myocytes went into an irreversible contracture at reoxygenation [16]. We observed similar effect when 20 mM Na-lactate was used. The high lactate concentrations can damage the myocytes through increased osmolarity, intracellular acidosis, and increase in [Ca2+]i. Myocyte hypercontracture leads to rupture of plasma membrane and rapid cell killing (within 10 min), which is not suitable for studying the effect of reoxygenation on cellular function. Contrary, others have reported that Na-lactate is not responsible for anoxia and reoxygenation damage in cardiac myocytes [17]. Intracellular lactate increases up to 5-10 mM during anoxia in isolated ferret heart. In the present study, we used 10 mM Na-lactate to mimic conditions present during ischemia in isolated hearts. Cells exposed to Na-lactate and acidosis had more severe increase in [Ca2+]i, and decrease in pHi. This is in agreement with the finding that intracellular acidosis induced by CO2 and Na-lactate can increase Ca2+ transients [1]. Furthermore, there was a faster increase in [Ca2+]i during Na-lactate exposure, than during extracellular acidosis. Increase in [Ca2+]i has an early rapid phase and slower delayed phase dependent on Na+-H+ exchanger activity. There are several mechanisms responsible for the early increase in [Ca2+]i during Na-lactate exposure. One of the factors that could induce rapid rise in [Ca2+]i upon exposure to Na-lactate is acute intracellular acidosis, when intracellular buffering mechanisms become hypersaturated. Under the conditions of intracellular acidosis, binding capacity of intracellular proteins for Ca2+ is reduced, and acidosis reduces Ca2+ uptake by sarcoplasmic reticulum. In addition to this, protons directly inhibit Na+-Ca2+ exchanger, which normally extrudes Ca2+ in resting myocytes [2, 3].
The decrease in [Ca2+]i observed under prolonged exposure to Na-lactate was prevented with extracellular acidosis. During continued exposure to lactate, another acidosis buffering mechanisms like Na+-H+ exchanger might be activated and thus attenuate decrease in pHi and, therefore, rise in [Ca2+]i. Indeed, it has been shown that pHi recovery of cardiac myocytes exposed to ischemia-mimetic solution containing Na-lactate can be prevented with Na+-H+ exchange inhibitors. Part of this increase results from uncharged form of lactate under conditions of extracellular acidosis, which is more membrane permeable, and lactate can induce greater intracellular acidosis. In addition, it is known that reducing extracellular pH leads to an intracellular acidosis in cardiac myocytes, which promotes [Ca2+]i accumulation [1]. Low extracellular pH inhibits Na+-H+ exchanger so that H+ efflux is inhibited, and cellular ability to regulate pHi is impaired [1].
Increase in [Ca2+]i during reoxygenation of an ischemic myocardium correlates with the degree of cellular damage [1]. Cells damaged by hypoxia are not able to maintain [Ca2+]i within physiological values. Monitoring changes in [Ca2+]i and pHi after cells were exposed to hypoxia-mimicking solutions reveled that pHi recovered with the similar kinetics in all tested solutions. Faster pHi recovery in solutions containing Na-lactate is due to activation of lactate-H+ symport, which has been shown to be the muscle membrane transport systems with the highest capacity for H+ removal in rat [18, 19]. However, [Ca2+]i increased only when cells were exposed to hypoxia, acidosis, and Na-lactate together suggesting that some other mechanisms including hypoxia, together with decreased pHi, are responsible for the myocyte damage with hypoxia-mimicking solutions.
In summary, the results show that intracellular acidosis is the main trigger for acute rise in [Ca2+]i during acute exposure to ischemia-mimicking solutions in cardiomyocytes. The magnitude and duration of increase in [Ca2+]i produced by superfusing the myocytes with lactate containing solution is increased at reduced extracellular pH. The inability of cells to prevent [Ca2+]i rise at reduced extracellular pH showed that other processes dependent on extracellular pH contribute to this process. Continued [Ca2+]i elevation after hypoxia suggests that other mechanisms, independently from intracellular acidosis and lactate accumulation, are involved in ischemic damage of the heart.
Acknowledgements
This work was supported in part by the National Institutes of Health Grants P01GM066730 and R01HL034708 (to ZJB), Bethesda, Maryland.
References
- 1.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88(2):581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy E, Steenbergen C. Ion transport and energetics during cell death and protection. Physiology (Bethesda) 2008;23:115–23. doi: 10.1152/physiol.00044.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Brien JD, Ferguson JH, Howlett SE. Effects of ischemia and reperfusion on isolated ventricular myocytes from young adult and aged Fischer 344 rat hearts. Am J Physiol Heart Circ Physiol. 2008;294(5):H2174–83. doi: 10.1152/ajpheart.00058.2008. [DOI] [PubMed] [Google Scholar]
- 4.O'Rourke B. The ins and outs of calcium in heart failure. Circ Res. 2008;102(11):1301–3. doi: 10.1161/CIRCRESAHA.108.178095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aizawa K, et al. Protein kinase C-epsilon primes the cardiac sarcolemmal adenosine triphosphate-sensitive potassium channel to modulation by isoflurane. Anesthesiology. 2004;101(2):381–9. doi: 10.1097/00000542-200408000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Marinovic J, Bosnjak ZJ, Stadnicka A. Preconditioning by isoflurane induces lasting sensitization of the cardiac sarcolemmal adenosine triphosphate-sensitive potassium channel by a protein kinase C-delta-mediated mechanism. Anesthesiology. 2005;103(3):540–7. doi: 10.1097/00000542-200509000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Buckler KJ, Vaughan-Jones RD. Application of a new pH-sensitive fluoroprobe (carboxy-SNARF-1) for intracellular pH measurement in small, isolated cells. Pflugers Arch. 1990;417(2):234–9. doi: 10.1007/BF00370705. [DOI] [PubMed] [Google Scholar]
- 8.Hoshino K, Avkiran M. Effects of moderate hypothermia on sarcolemmal Na(+)/H(+) exchanger activity and its inhibition by cariporide in cardiac ventricular myocytes. Br J Pharmacol. 2001;134(7):1587–95. doi: 10.1038/sj.bjp.0704405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaughan-Jones RD, et al. Intrinsic H(+) ion mobility in the rabbit ventricular myocyte. J Physiol. 2002;541:139–58. doi: 10.1113/jphysiol.2001.013267. Pt 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spitzer KW, Bridge JH. Relationship between intracellular pH and tension development in resting ventricular muscle and myocytes. Am J Physiol. 1992;262:C316–27. doi: 10.1152/ajpcell.1992.262.2.C316. 2 Pt 1. [DOI] [PubMed] [Google Scholar]
- 11.Salvi A, Quillan JM, Sadee W. Monitoring intracellular pH changes in response to osmotic stress and membrane transport activity using 5-chloromethylfluorescein. AAPS PharmSci. 2002;4(4):E21. doi: 10.1208/ps040421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richmond PH, Vaughan-Jones RD. Assessment of evidence for K+-H+ exchange in isolated type-1 cells of neonatal rat carotid body. Pflugers Arch. 1997;434(4):429–37. doi: 10.1007/s004240050417. [DOI] [PubMed] [Google Scholar]
- 13.Namekata I, et al. Reduction by SEA0400 of myocardial ischemia-induced cytoplasmic and mitochondrial Ca2+ overload. Eur J Pharmacol. 2006;543(1-3):108–15. doi: 10.1016/j.ejphar.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Lu J, et al. Effects of ischaemia-mimetic factors on isolated rat ventricular myocytes. Exp Physiol. 2005;90(4):497–505. doi: 10.1113/expphysiol.2004.029421. [DOI] [PubMed] [Google Scholar]
- 15.Allshire A, et al. Cytosolic free Ca2+ in single rat heart cells during anoxia and reoxygenation. Biochem J. 1987;244(2):381–5. doi: 10.1042/bj2440381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cairns SP, Westerblad H, Allen DG. Changes in myoplasmic pH and calcium concentration during exposure to lactate in isolated rat ventricular myocytes. J Physiol. 1993;464:561–74. doi: 10.1113/jphysiol.1993.sp019651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geisbuhler TP, Rovetto MJ. Lactate does not enhance anoxia/reoxygenation damage in adult rat cardiac myocytes. J Mol Cell Cardiol. 1990;22(11):1325–35. doi: 10.1016/0022-2828(90)90068-d. [DOI] [PubMed] [Google Scholar]
- 18.Coady MJ, et al. Establishing a definitive stoichiometry for the Na+/monocarboxylate cotransporter SMCT1. Biophys J. 2007;93(7):2325–31. doi: 10.1529/biophysj.107.108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pilegaard H, et al. Effect of high-intensity exercise training on lactate/H+ transport capacity in human skeletal muscle. Am J Physiol. 1999;276:E255–61. doi: 10.1152/ajpendo.1999.276.2.E255. 2 Pt 1. [DOI] [PubMed] [Google Scholar]