Abstract
There is controversy about whether pathologic abnormalities are associated with pregnancies complicated by factor V Leiden (FVL) mutation. The purpose of this study was to evaluate 105 placentas delivered to mothers heterozygous for FVL mutation to determine if there are pathologic changes suggestive of hypoxia or thrombosis, which correlate with mutation status. We examined placentas obtained as part of a prospective study of 5188 pregnancies analyzed for the presence of FVL mutation in either the mother or the infant. One hundred five placentas from mothers heterozygous for the mutation were compared with 225 controls matched for maternal age, race, and geographic site. Of the 330 pregnancies, 50 infants were FVL mutation heterozygotes. Maternal FVL heterozygote status was associated with more frequent increased numbers of syncytial knots (13% vs 4%); the difference remained significant after controlling for hypertension, preeclampsia, small-for-gestational-age infants, and delivery prior to 35 weeks of gestation (odds ratio 3.6, 95% confidence interval 1.5–8.7, P = 0.004). Maternal FVL heterozygotes had more hypervascular villi (10% vs 3%), with significance retained controlling for delivery route (odds ratio 3.4, 95% confidence ratio 1.2–9.4, P = 0.018). Placentas from infants heterozygous for FVL mutation had more avascular villi than controls (odds ratio 2.9, 95% confidence interval 1.5–5.6, P = 0.001). Fetal or maternal FVL heterozygosity was not associated with infarcts, small-for-gestational-age placentas, or fetal thrombotic vasculopathy. This analysis demonstrates that pathologic findings associated with placental hypoxia, specifically focal avascular villi, increased numbers of syncytial knots, and hypervascular villi, also correlate with FVL heterozygosity in infants or mothers.
Keywords: avascular, chorangiosis, Leiden, pathology, placenta, thrombophilia
INTRODUCTION
Factor V Leiden (FVL) mutation is the most common inherited mutation associated with thrombophilia, present in as much as 5% of the general population [1]. Individuals affected in the homozygous or heterozygous state have an increased risk of thrombotic events.
It is known that thrombotic tendencies in the mother associated with other thrombophilias, such as the antiphospholipid antibody syndrome, may result in placental pathologic findings of decreased placental weight, infarcts, increased numbers of syncytial knots, “accelerated villous maturation” and atherosis, identical to the findings associated with severe pregnancy-induced hypertension [2,3]. Because the presumed pathogenesis of the placental pathology is thrombosis of decidual arterioles, it is a logical assumption that similar changes may be seen in the presence of maternal FVL mutation in the mother. In addition, inheritance of the mutation by the fetus could be hypothesized to result in thrombosis of the fetal vascular tree, resulting in avascular villi or fetal thrombotic vasculopathy.
The medical literature is contradictory regarding whether the presence of FVL mutation in the mother and/or fetus is associated with placental pathologic changes [4–14]. Vern and colleagues retrospectively evaluated extracted DNA for FVL mutation from placentas with fetal thrombotic vasculopathy and found heterozygous mutations in 5 of 27 placentas (18.5%) [5]. In their study, fetal thrombotic vasculopathy was defined as either vascular thrombi in the umbilical cord, chorionic vessels, or stem villous vessels or avascular villi involving at least one 20× field. In contrast, Mousa and Alfirevic reported no specific pathologic alterations associated with the FVL mutation [10]. These authors reviewed reports of placentas from 79 women with severe preeclampsia, abruption, fetal growth restriction, or unexplained stillbirth, of which 4 (5%) were positive for the FVL mutation. Additional thrombophilias were identified by functional or genetic assays. They reviewed placental pathology reports assessing for thrombotic lesions of the placenta and found no difference in incidence of women with or without thrombophilic mutation. Khong and colleagues suggested in an editorial that the study was “…seriously flawed,” citing a lack of understanding of placental pathology [12]. Sebire had similar comments [11].
There are many variables among the studies that attempt to determine whether there is an association between FVL mutation and placental pathology. Studies differ in many respects, including whether the mutation status of the mother or fetus is assessed, the type of placental lesions evaluated, and the ascertainment of subjects [5,6,10,13,14]. The 2 largest studies to date that assessed the effect of thrombophilic mutations carried by either the mother or the fetus are those of Ariel and colleagues [6] and Gogia and Machin [14]. Ariel and colleagues evaluated a pregnancy population selected for preeclampsia, abruption, and fetal growth restriction. Fetal thrombophilic mutations were identified in 19 of 64 pregnancies, with 9 carrying a FVL mutation. There was no association of fetal vascular lesions of the placenta with thrombophilic mutations. Gogia and Machin reported an association between maternal thrombophilias and maternal floor infarct, massive perivillous fibrin deposition, and fetal thrombotic vasculopathy. Of 138 pregnancies with placental abnormalities and thrombophilic testing, FVL mutation was present in 10 mothers [14].
Vern and colleagues assessed the presence of thromboses of the fetal placental vascular tree in the setting of FVL mutation [5]. They found no association of placental lesions with the infant’s FVL mutation status from a prospective study of 169 consecutive placentas in which only 5 contained a mutation. Alternatively, when these same authors looked retrospectively, they noted a significant association of fetal thrombotic vasculopathy with FVL mutation of the fetus. Compounding the heterogeneity of the studies reported thus far is the fact that many of the sample sizes were small, limiting the power of the studies and the conclusions presented.
The purpose of our study was to identify if there were placental pathologic changes associated with FVL mutation in a large cohort of pregnancies, assessed for the presence of FVL mutation in the mother or infant.
MATERIALS AND METHODS
The placentas were selected from a prospective study done by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network, with the purpose of assessing the “frequency of pregnancy-related thromboembolic events among carriers of the factor V Leiden mutation and evaluating the impact of maternal and fetal FVL mutation carriage or other thromophilias on the risk of adverse outcomes [15].” Informed consent was obtained for 5188 pregnant women at gestational age less than 14 weeks from 13 clinical centers. Each of the identified 140 FVL mutation carriers was matched to 2 noncarriers by clinical center, maternal age (±years), and race/ethnicity. These women were asked to provide additional samples for analysis of other inherited thrombophilias, including the prothrombin G20210A mutation and the methylenetetrahydrofolate reductase mutation C677T. The placentas from these deliveries were used in this study, and maternal demographics were similar to those from pregnancies in which the placentas were not used for this study. A total of 105 placentas from pregnancies in which mothers were heterozygous for the FVL mutation and 225 placentas from the matched pregnancies without the FVL mutation were collected. Placentas were weighed after removal of the umbilical cord and membranes. Gross examination was performed at a central laboratory, and slides were reviewed at a separate location. The central laboratory used for gross examination was initially Magee Women’s Hospital and later the University of Utah School of Medicine. Histologic slides were taken to include 2 sections of umbilical cord, a section of placental membranes, and at least 2 sections of placental parenchyma. Gross evaluation included the weight of the placenta, with cord and membranes removed, and evaluation for infarction, intervillous thrombi, calcification, amnion nodosum, meconium staining, marginal hemorrhage, abruption, circumvallate membrane insertion, and velamentous insertion of the umbilical cord. Because the gross examination was performed by personnel distant from where histologic evaluation was performed, only certain grossly identified abnormalities, felt to be most reliably identified on gross examination, were abstracted for the final analysis. These include placental weight and the presence or absence of infarction, which was also confirmed histologically.
Histologic examination was performed over a 2-month period by one observer (BBR), who was blinded to clinical outcomes and mutation status. Pathologic findings were defined based on descriptions in Placental Pathology [16] and selected articles [17,18]. Pathologic alterations suggestive of hypoxia or thrombosis were assessed, and histologic characteristics are described in Table 1 and shown in Figure 1. Lesions hypothesized to reflect maternal thrombotic disease included placentas with weight small for gestational age (<10th percentile [19]), infarcts, and increased numbers of syncytial knots (Tenney-Parker change). Increased syncytial knots, although most commonly seen with maternal vascular underperfusion, was also assessed in the face of fetal heterozygosity for the FVL mutation. Changes hypothesized to reflect fetal thrombotic disease included fetal thrombotic vasculopathy, avascular villi, or partially avascular villi. The presence of hypervascular villi (chorangiosis) was hypothesized to be suggestive of either maternal or fetal thrombophilia. Uncommon lesions looked for but rarely, if ever, found were atherosis and increased fetal nucleated blood cells. Increased syncytial knots was scored histologically instead of distal villous hypoplasia to reflect maternal vascular underperfusion, because distal villous hypoplasia was also seen only rarely.
Table 1.
Gross and histologic pathology defined
| Pathologic finding | Definition |
|---|---|
| Small-for-gestational-age placenta | Placenta below the 10th percentile for gestation |
| Infarct | Localized area of coagulative necrosis in the parenchyma, confirmed histologically |
| Increased number of syncytial knots | Excessive number of villi with prominent syncytial knots; also known as Tenney-Parker change |
| Hypervascular villi | More than 10 terminal villi with more than 10 capillaries in several areas, with at least 15 vessels in occasional villi; also known as chorangiosis |
| Fetal thrombotic vasculopathy | Vascular occlusion of a large placental vessel with associated avascular villi |
| Avascular villi | One or more foci of avascular villi without an identified large-vessel thrombus |
| Partially avascular villi | Focal intermediate or tertiary villi with an area suggestive of vascular occlusion accompanied by small capillaries peripherally |
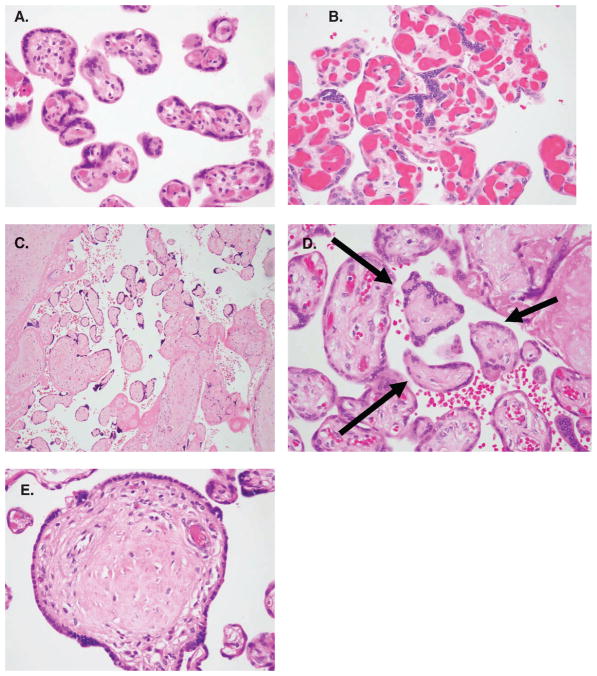
Figure 1.
Placental pathologic findings include (A) prominent syncytial knots, (B) hypervascular villi, (C) fetal thrombotic vasculopathy, (D) focal avascular villi, and (E) partial avascular villi.
Fisher exact or Pearson chi-square statistics and odds ratios (OR) with 95th percent confidence intervals (CI) were used to compare categoric variables. Associations were explored using stratified analyses or logistic regression, as appropriate. The covariates’ associations with outcomes were investigated with multivariable analyses. Nominal statistical significance was set at a P value less than 0.05.
RESULTS
The gestational age at delivery was 25.6–42.6 weeks (mean gestational age of 38.9 ± 1.9 weeks), with only 16 deliveries occurring at less than 36 weeks’ gestation (Table 2). Of the 330 pregnancies, DNA testing for the FVL mutation was performed on 315 infants, and 50 infants were FVL mutation heterozygotes (mean gestational age of 39.1 ± 1.6 weeks). Forty infants inherited the FVL mutation from the mother and 10 from the father (Table 3). Maternal FVL heterozygosity was associated with the presence of increased numbers of syncytial knots compared with controls (13% vs 4%); the difference remained significant after controlling for hypertension, preeclampsia, small-for-gestational-age infants [20], and delivery at less than 35 weeks (OR 3.6, 95% CI 1.5–8.7, P = 0.004) (Table 4). Increased numbers of syncytial knots were also associated independently with maternal hypertension (P = 0.01). Placentas delivered to mothers heterozygous for FVL were also more likely to have hypervascular villi than placentas from mothers without FVL mutation (10% vs 3%) (Table 4). Significance was retained after controlling for delivery by Cesarean section, which might produce congestion, giving the appearance of hypervascular villi (OR 3.4, 95% CI 1.2–9.4, P = 0.018).
Table 2.
Clinical data
| FVL mutation
|
||
|---|---|---|
| Heterozygous positive, n (%) | Heterozygous negative, n (%) | |
| Gestational age at delivery | ||
| <32 weeks | 1/105 (1.0) | 3/225 (1.3) |
| 32–36 weeks | 10/105 (9.5) | 20/225 (8.9) |
| 37–40 weeks | 81/105 (77.1) | 176/225 (78.2) |
| 41+ weeks | 13/105 (12.4) | 26/225 (11.6) |
| Hypertension treated with medication | 0 | 3/225 (1.3) |
| Pregestational diabetes | 4/105 (3.8) | 4/225 (1.8) |
| Gestational diabetes | 3/105 (2.9) | 9/225 (4.0) |
| Body mass index >30 | 15 (14.3) | 47 (21.2) |
Table 3.
Factor V Leiden (FVL) status of mothers and infants
| Infant FVL negative | Infant FVL heterozygous | Infant FVL not determined | Total | |
|---|---|---|---|---|
| Mother FVL negative | 204 | 10 | 11 | 225 |
| Mother FVL heterozygous | 61 | 40 | 4 | 105 |
| Total | 265 | 50 | 15 | 330 |
Table 4.
Pathology correlated with maternal factor V Leiden (FVL) mutation status
| Maternal FVL mutation
|
P value | Odds ratio (95% confidence interval) | ||
|---|---|---|---|---|
| Heterozygous positive (N = 105), n (%) | Heterozygous negative (N = 225), n (%) | |||
| Placenta small for gestational age | 13 (12) | 42 (19) | 0.15 | 0.6 (0.3, 1.2) |
| Infarcts | 16 (15) | 39 (17) | 0.63 | 0.9 (0.5, 1.6) |
| Increased number of syncytial knots | 14 (13) | 10 (4) | 0.004 | 3.3 (1.4, 7.7) |
| Hypervascular villi | 10 (10) | 7 (3) | 0.01 | 3.3 (1.2, 8.9) |
Thirty-six percent of placentas from infants heterozygous for FVL had focal or multifocal avascular villi vs 16% of controls (OR 2.9, 95% CI 1.5–5.6, P = 0.001). These foci of avascular villi characteristically consisted of rare villi, between 1 and 3, without stromal capillaries. Fetal FVL mutation was not associated with fetal thrombotic vasculopathy, partial avascular villi, or hypervascular villi. Neither fetal nor maternal FVL heterozygosity was associated with infarcts or small-for-gestational-age placentas. Placentas from pregnancies in which the infant was heterozygous for FVL mutation had a significant increase in avascular villi, controlling for maternal FVL mutation status (OR 2.9, 95% CI 1.3–6.8), and placentas from pregnancies in which the mother was heterozygous for FVL mutation had a significant increase in the number of syncytial knots and hypervascular villi, controlling for fetal FVL mutation status (OR 3.6, 95% CI 1.3–10.2 and OR 4.4, 95% CI 1.3–16.3, respectively).
Thirty-seven mothers were heterozygous for the methylenetetrahydrofolate reductase mutation, and 14 mothers were heterozygous for the prothrombin mutation. There was an insufficient number of study subjects to assess whether an association existed between these mutations and placental pathology or to determine if there was an additive or synergistic effect of double heterozygosity for FVL and one of these other mutations on placental pathology. Similarly, there were no cases of massive intervillous fibrin deposition or maternal floor infarct and insufficient numbers of fetal thrombotic vasculopathy to assess whether an association with FVL mutation existed.
DISCUSSION
If one ponders the state of pregnancy, 3 distinct individuals are involved in the process: the fetus, the mother, and the father. Each has its own unique genetic material, and each has its own physiologic relationship to the others in the triad. In addition to the genetic heterogeneity, there is also physiologic complexity between the mother and fetus, through the shared circulation of the placenta. Both maternal and fetal health affect the placenta, and health is a combination of genetics and environment, adding further complexity to the situation.
It is no wonder, then, that many articles investigating the effect of thrombophilic mutations on pregnancy have come to the conclusion that there is no clear relationship between thrombophilic mutation with placental abnormalities and/or adverse pregnancy outcomes [4,6,8,9]. This is also confounded by the fact that these mutations are infrequent and many studies had small sample sizes. The suspicion still exists that there may be additional factors that, when combined with inherited thrombophilias, predispose to fetal vascular thrombosis [4,14]. Studies have supported an association between the most common inherited thrombophilic mutation, FVL, and placental vascular lesions [5,14]; have not found an association [6,10]; and have called the methodology of previous reports into question [11,12]. Vern and colleagues indicated that if cases are evaluated prospectively, there are no placental alterations associated with FVL mutation, and if evaluated retrospectively, such an association exists [5].
The purpose of this study was single-fold: to ask if FVL heterozygosity in the mother and/or fetus is associated with placental pathologic changes hypothesized to reflect either fetal or maternal hypoxia or vascular thrombosis. Ours is the only study that has had sufficient statistical power to ask the question in a prospective manner. In addition, a single pathologist, blinded to the clinical data, reviewed the slides to maintain consistency of observation.
A hypothesis-driven approach was used to evaluate lesions in the placenta that are, or might be, associated with hypoxia of either the fetus or mother. The placenta is a complex organ and is sustained by blood from both the mother and the fetus. Therefore, thrombophilias in the mother, which might result in thrombosis of the spiral arterioles, could be hypothesized to have one effect on the placenta, whereas a thrombophilia in the fetus would be expected to occlude the fetal vasculature with a different pattern of pathology.
We divided the lesions into those that have been described in fetal hypoxia or vascular disease, those that are described in maternal states, and those that might represent hypoxia or thrombosis in either system [16].
Placental lesions hypothesized to be associated with FVL mutation in only the mother were small for gestation placenta, infarcts, and increased syncytial knotting. Each of these pathologic changes is characteristic of the findings seen in placentas from mothers with pregnancy-induced hypertension. Those findings hypothesized to be characteristic of FVL mutation only in the fetus were fetal thrombotic vasculopathy, focal avascular villi, and partially avascular villi. Both fetal thrombotic vasculopathy and avascular villi reflect thrombosis of fetal vessels. Villi that are partially avascular are not described as a pathologic entity, but we hypothesized that these may be associated with fetal vascular thrombosis affecting only part of a villus. The last lesion recorded, hypervascular villi, has been described in infants of diabetic mothers, in association with fetal thrombotic vasculopathy, and mild maternal vascular underperfusion, and it is found more often in placentas delivered at higher elevations [16]. It was hypothesized to be present in either mothers or fetuses with FVL heterozygosity.
The statistically significant association of increased syncytial knotting and hypervascular villi with maternal FVL mutation suggests that hypoxia of the placental vascular bed occurs more frequently in mothers with FVL mutation than those without. The absence of an association of maternal FVL mutation with changes typical of severe pregnancy-induced hypertension, including small-for-gestational-age placentas, infarcts, and distal villous hypoplasia, may indicate that there is obstruction of decidual vessels but to a lesser extent than in cases of pregnancy-induced hypertension that affect of the placenta.
The only lesion associated with the FVL mutation in the fetus was avascular villi, which were present very focally. There was only 1 placenta with fetal thrombotic vasculopathy in a fetus heterozygous for FVL mutation and 10 placentas with fetal thrombotic vasculopathy in fetuses without a FVL mutation. This is the classic lesion associated with pregnancies complicated by FVL mutation, whereas focal avascular villi have not been evaluated. In the current study, this suggests that there may be more microvascular involvement at the level of the intermediate or tertiary villus than involvement at the stem villous level. This results in less severe pathologic changes.
There are several limitations to our study, primarily related to the lack of sufficient number of cases to address all of the possible clinical questions. First, our study was not intended to address the effect of the placental findings on the fetus, and there is insufficient power in the analysis to assess for growth restriction, fetal demise, or neonatal stroke. Ours was an ancillary study derived from a cohort of more than 5000 women who were followed for evidence of thrombotic events related to large-vessel thrombosis [15]. We found no association between the large-vessel thromboses and FVL heterozygosity in the mother. Therefore, it is not surprising that such lesions thought to derive from large-vessel thrombosis, such as infarcts and small-for-gestational-age placentas, may not be present in the placenta from mothers heterozygous for the mutation. Another limitation was the fact that there was an insufficient number of cases to assess for the additive effect of other inherited or acquired thrombophilias. It is possible that mothers who are compound heterozygous for genetic alterations producing thrombophilias or who carry both genetic and acquired thrombophilias may have more extensive placental pathology, as is suggested by Gogia and Machin [14]. Because of the relative rarity of these inherited mutations, very few individuals would be carriers of more than 1 mutation. Clearly, our study was not powered to answer this question, because the required sample size would be enormous. Third, the gross examination of the placentas occurred at a location separate from the microscopic examination. This resulted in final statistical evaluation for only 2 gross features, the placental size and the presence of infarcts, to ensure the validity of results.
The clinical question is one that cannot be answered by our data. That is, “What is the long-term effect of the placental findings seen in this study on the fetus?” This would likely require following the infants from this study for years to assess cognitive and behavioral function, as well as growth and health issues. This is out of the scope of the study. Various studies that have attempted to assess the effect of FVL mutation, typically of the mother, on pregnancy outcome correlated the mutation status with findings at delivery, such as stroke, hypoxic/ischemic encephalopathy, and stillbirth [21,22].
In summary, there are alterations of the placenta indicative of the presence of FVL mutation in either the mother or the fetus.
Table 5.
Pathology correlated with fetal factor V Leiden (FVL) mutation status
| Fetal FVL mutation
|
P value | Odds ratio (95% confidence interval) | ||
|---|---|---|---|---|
| Heterozygous positive (N = 50), n (%) | Heterozygous negative (N = 265), n (%) | |||
| Fetal thrombotic vasculopathy | 1 (2) | 10 (4) | 1.0 | 0.5 (0.0, 3.8) |
| Focal avascular villi | 18 (36) | 43 (16) | 0.001 | 2.9 (1.5, 5.6) |
| Partially avascular villi | 8 (16) | 48 (18) | 0.72 | 0.9 (0.4, 2.0) |
| Hypervascular villi | 2 (4) | 13 (5) | 1.0 | 0.8 (0.1, 3.7) |
Acknowledgments
This work was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD27860, HD36801, HD27917, HD21414, HD27861, HD27869, HD27905, HD34208, HD34116, HD21410, HD27915, HD34136, HD34122, HD34210).
Thom participated in protocol/data management and statistical analysis and G. Wendel in protocol development and oversight. In addition to the authors, other members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network are as follows: University of Texas Southwestern Medical Center, Dallas—J. Gold, K. Leveno, and S. Williams; University of Alabama at Birmingham—D. Rouse, A. Northen, and K. Bailey; University of Chicago—A. Moawad, P. Jones, and G. Mallett; University of Cincinnati—T. Siddiqi, H. How, N. Elder, and W. Knox; University of Pittsburgh—M. Cotroneo, K. Lain, and T. Kamon; University of Miami—F. Doyle; Ohio State University—J. Iams, F. Johnson, and C. Latimer; University of Tennessee—W. Mabie and R. Ramsey; University of Texas at San Antonio—O. Langer and D. Dudley; University of Texas Health Science Center at Houston—M.C. Day and L. Gilstrap; Thomas Jefferson University—A. Sciscione, M. Talucci, and M. DiVito; University of Utah—M. Varner, K. Anderson, K. Jolley, A. Guzman, and J. Parsons; Wake Forest University Health Sciences—P. Meis, M. Harper, M. Swain, and K. Lanier; Wayne State University—M. Dombrowski, G. Norman, P. Lockhart, and C. Sudz; MFMU Network Steering Committee Chair (Vanderbilt University Medical Center)—S. Gabbe; George Washington University Biostatistics Center—E. Thom, A. Arrieta, and L. Leuchtenburg; Eunice Kennedy Shriver National Institute of Child Health and Human Development—M. Klebanoff, S. Pagliaro, and D. McNellis.
References
- 1.Dahlbäck B. Advances in understanding pathogenic mechanisms of thrombophilic disorders. Blood. 2008;112:19–27. doi: 10.1182/blood-2008-01-077909. [DOI] [PubMed] [Google Scholar]
- 2.Levy RA, Avvad E, Oliveira J, Porto LC. Placental pathology in antiphospholipid syndrome. Lupus. 1998;7(suppl 2):S81–S85. doi: 10.1177/096120339800700218. [DOI] [PubMed] [Google Scholar]
- 3.Abramowsky CR, Vegas ME, Swinehart G, Gyves MT. Decidual vasculopathy of the placenta in lupus erythematosus. NEJM. 1980;303:668–672. doi: 10.1056/NEJM198009183031204. [DOI] [PubMed] [Google Scholar]
- 4.Redline RW. Thrombophilia and placental pathology. Clin Obstet Gynecol. 2006;49:885–894. doi: 10.1097/01.grf.0000211957.68745.6b. [DOI] [PubMed] [Google Scholar]
- 5.Vern TZ, Alles AJ, Kowal-Vern A, Longtine J, Roberts DJ. Frequency of factor V Leiden and prothrombin G20210A in placentas and their relationship with placental lesions. Hum Pathol. 2000;31:1036–1043. doi: 10.1053/hupa.2000.16281. [DOI] [PubMed] [Google Scholar]
- 6.Ariel I, Anteby E, Hamani Y, Redline RW. Placental pathology in fetal thrombophilia. Hum Pathol. 2004;35:729–733. doi: 10.1016/j.humpath.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Katz VL, DiTomasso J, Farmer R, Carpenter M. Activated protein C resistance associated with maternal floor infarction treated with low–molecular-weight heparin. Am J Perinatol. 2002;19:273–277. doi: 10.1055/s-2002-33085. [DOI] [PubMed] [Google Scholar]
- 8.Rodger MA, Paidas M, Claire M, et al. Inherited thrombophilia and pregnancy complications revisited. Obstet Gynecol. 2008;112:320–324. doi: 10.1097/AOG.0b013e31817e8acc. [DOI] [PubMed] [Google Scholar]
- 9.Alfirevic A, Roberts D, Martlew V. How strong is the association between maternal thrombophilia and adverse pregnancy outcome? A systematic review. Eur J Obstet Gynecol Reprod Biol. 2002;101:6–14. doi: 10.1016/s0301-2115(01)00496-1. [DOI] [PubMed] [Google Scholar]
- 10.Mousa HA, Alfirevic A. Do placental lesions reflect thrombophilia state in women with adverse pregnancy outcome? Hum Reprod. 2000;15:1830–1833. doi: 10.1093/humrep/15.8.1830. [DOI] [PubMed] [Google Scholar]
- 11.Sebire NJ. Thrombophilias and adverse pregnancy outcome (letter to the editor) Hum Reprod. 2001;16:395. doi: 10.1093/humrep/16.2.395. [DOI] [PubMed] [Google Scholar]
- 12.Khong TY, Moore L, Hague WM. Thrombophilias and adverse pregnancy outcome (letter to the editor) Hum Reprod. 2001;16:395–396. doi: 10.1093/humrep/16.2.395-a. [DOI] [PubMed] [Google Scholar]
- 13.Many A, Schreiber L, Rosner S, Lessing JB, Eldor A, Kupferminc MJ. Pathologic features of the placenta in women with severe pregnancy complications and thrombophilia. Obstet Gynecol. 2001;98:1041–1044. doi: 10.1016/s0029-7844(01)01621-0. [DOI] [PubMed] [Google Scholar]
- 14.Gogia N, Machin GA. Maternal thrombophilias are associated with specific placental lesions. Pediatr Dev Pathol. 2008;11:424–429. doi: 10.2350/07-09-0345.1. [DOI] [PubMed] [Google Scholar]
- 15.Dizon-Townson D, Miller C, Sibai B, et al. The relationship of the factor V Leiden mutation and pregnancy outcomes for mother and fetus. Obstet Gynecol. 2005;106:517–524. doi: 10.1097/01.AOG.0000173986.32528.ca. [DOI] [PubMed] [Google Scholar]
- 16.Kraus FT, Redline RW, Gersell DJ, Nelson DM, Dicke JM, editors. Volume 3 of the Atlas of Nontumor Pathology. 2. Armed Forces Institute of Pathology; Washington, DC: American Registry of Pathology; 2004. Placental Pathology. [Google Scholar]
- 17.Redline RW, Boyd T, Campbell V, et al. Maternal vascular underperfusion: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2004;7:237–249. doi: 10.1007/s10024-003-8083-2. [DOI] [PubMed] [Google Scholar]
- 18.Redline RW, Ariel I, Baergen RN, et al. Fetal vascular obstructive lsions: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2004;7:443–452. doi: 10.1007/s10024-004-2020-x. [DOI] [PubMed] [Google Scholar]
- 19.Pinar H, Sung CJ, Oyer CE, Singer DB. Reference values for singleton and twin placenta weights. Pediatr Dev Pathol. 1996;16:901–907. doi: 10.1080/15513819609168713. [DOI] [PubMed] [Google Scholar]
- 20.Alexander GR, Kogan MD, Himes JH. 1994–1996 U.S. singleton birth weight percentiles for gestational age by race, Hispanic origin, and gender. Matern Child Health J. 1999;3:225–231. doi: 10.1023/a:1022381506823. [DOI] [PubMed] [Google Scholar]
- 21.Nelson KB. Thrombophilias, perinatal stroke, and cerebral palsy. Clin Obstet Gynecol. 2006;49:875–884. doi: 10.1097/01.grf.0000211956.61121.e0. [DOI] [PubMed] [Google Scholar]
- 22.Stella CL, Sibai BM. Thrombophilia and adverse maternal-perinatal outcome. Clin Obstet Gynecol. 2006;49:850–860. doi: 10.1097/01.grf.0000211954.66959.e1. [DOI] [PubMed] [Google Scholar]