Abstract
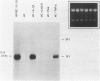
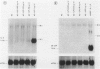
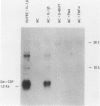
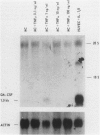
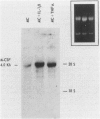
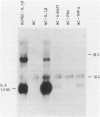
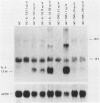
The capacity of human cultured mesangial cells to produce soluble factors potentially relevant for mechanisms of inflammation and immunity at the glomerular site was analyzed. The nature of the secreted factors initially was investigated by Northern blot analysis using total cellular RNAs isolated from resting and activated mesangial cells. On exposure of mesangial cells to human recombinant interleukin-1 β (IL-1β), high levels of interleukin-8 (IL-8) and monocyte chemotactic protein-1 (MCP-1) mRNAs were detected. Similar transcripts were found after stimulation with human recombinant tumor necrosis factor-α (TNF-α). Active secretion of IL-8 was documented by radioimmunoassay in supernatants of mesangial cells activated by either IL-1β or TNF-α. Using an in vitro migration assay, supernatants from resting mesangial cells were found to be devoid of any chemotactic activity for granulocytes or monocytes. On stimulation withIL-lβ, however, mesangial cell supernatants expressed MCP-1 biologic activity detected as induction of a strong migratory response for human monocytes but not for granulocytes. In addition, IL-1β and TNF-β induced high levels of granulocyte-macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) mRNAs. Similarly IL-1β and TNF-α induced the interleukin-6 (IL-6) gene and active secretion of its mature protein. These data strongly support an effector role for mesangial cells in modulating immune-inflammatory responses in glomeruli. Release of cytokines may activate not only infiltrating inflammatory cells through short paracrine pathways, but also mesangial cells themselves through an autocrine pathway.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins R. C., Holdsworth S. R., Hancock W. W., Thomson N. M., Glasgow E. F. Cellular immune mechanisms in human glomerulonephritis: the role of mononuclear leucocytes. Springer Semin Immunopathol. 1982;5(3):269–296. doi: 10.1007/BF01892089. [DOI] [PubMed] [Google Scholar]
- Baggiolini M., Walz A., Kunkel S. L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989 Oct;84(4):1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi A., Rossi V., Bassan R., Barbui T., Bettoni S., Sironi M., Mantovani A., Rambaldi A. Constitutive expression of the interleukin-6 gene in chronic lymphocytic leukemia. Blood. 1989 Apr;73(5):1279–1284. [PubMed] [Google Scholar]
- Bolton W. K., Innes D. J., Jr, Sturgill B. C., Kaiser D. L. T-cells and macrophages in rapidly progressive glomerulonephritis: clinicopathologic correlations. Kidney Int. 1987 Dec;32(6):869–876. doi: 10.1038/ki.1987.288. [DOI] [PubMed] [Google Scholar]
- Bottazzi B., Colotta F., Sica A., Nobili N., Mantovani A. A chemoattractant expressed in human sarcoma cells (tumor-derived chemotactic factor, TDCF) is identical to monocyte chemoattractant protein-1/monocyte chemotactic and activating factor (MCP-1/MCAF). Int J Cancer. 1990 Apr 15;45(4):795–797. doi: 10.1002/ijc.2910450436. [DOI] [PubMed] [Google Scholar]
- Bottazzi B., Polentarutti N., Acero R., Balsari A., Boraschi D., Ghezzi P., Salmona M., Mantovani A. Regulation of the macrophage content of neoplasms by chemoattractants. Science. 1983 Apr 8;220(4593):210–212. doi: 10.1126/science.6828888. [DOI] [PubMed] [Google Scholar]
- Broudy V. C., Kaushansky K., Segal G. M., Harlan J. M., Adamson J. W. Tumor necrosis factor type alpha stimulates human endothelial cells to produce granulocyte/macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7467–7471. doi: 10.1073/pnas.83.19.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolino F., Wang J. M., Defilippi P., Turrini F., Sanavio F., Edgell C. J., Aglietta M., Arese P., Mantovani A. Granulocyte- and granulocyte-macrophage-colony stimulating factors induce human endothelial cells to migrate and proliferate. Nature. 1989 Feb 2;337(6206):471–473. doi: 10.1038/337471a0. [DOI] [PubMed] [Google Scholar]
- Cannistra S. A., Griffin J. D. Regulation of the production and function of granulocytes and monocytes. Semin Hematol. 1988 Jul;25(3):173–188. [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Cotran R. S. American Association of Pathologists president's address. New roles for the endothelium in inflammation and immunity. Am J Pathol. 1987 Dec;129(3):407–413. [PMC free article] [PubMed] [Google Scholar]
- Dahinden C. A., Kurimoto Y., De Weck A. L., Lindley I., Dewald B., Baggiolini M. The neutrophil-activating peptide NAF/NAP-1 induces histamine and leukotriene release by interleukin 3-primed basophils. J Exp Med. 1989 Nov 1;170(5):1787–1792. doi: 10.1084/jem.170.5.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Biology of interleukin 1. FASEB J. 1988 Feb;2(2):108–115. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fillit H. M., Zabriskie J. B. Cellular immunity in glomerulonephritis. Am J Pathol. 1982 Nov;109(2):227–243. [PMC free article] [PubMed] [Google Scholar]
- Hooke D. H., Hancock W. W., Gee D. C., Kraft N., Atkins R. C. Monoclonal antibody analysis of glomerular hypercellularity in human glomerulonephritis. Clin Nephrol. 1984 Oct;22(4):163–168. [PubMed] [Google Scholar]
- Kawasaki E. S., Ladner M. B., Wang A. M., Van Arsdell J., Warren M. K., Coyne M. Y., Schweickart V. L., Lee M. T., Wilson K. J., Boosman A. Molecular cloning of a complementary DNA encoding human macrophage-specific colony-stimulating factor (CSF-1). Science. 1985 Oct 18;230(4723):291–296. doi: 10.1126/science.2996129. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. The biology of interleukin-6. Blood. 1989 Jul;74(1):1–10. [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Appella E., Oppenheim J. J., Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989 Mar 17;243(4897):1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- Le J., Vilcek J. Tumor necrosis factor and interleukin 1: cytokines with multiple overlapping biological activities. Lab Invest. 1987 Mar;56(3):234–248. [PubMed] [Google Scholar]
- Leonard E. J., Yoshimura T. Human monocyte chemoattractant protein-1 (MCP-1). Immunol Today. 1990 Mar;11(3):97–101. doi: 10.1016/0167-5699(90)90035-8. [DOI] [PubMed] [Google Scholar]
- Lovett D. H., Sterzel R. B. Cell culture approaches to the analysis of glomerular inflammation. Kidney Int. 1986 Aug;30(2):246–254. doi: 10.1038/ki.1986.176. [DOI] [PubMed] [Google Scholar]
- Lovett D. H., Szamel M., Ryan J. L., Sterzel R. B., Gemsa D., Resch K. Interleukin 1 and the glomerular mesangium. I. Purification and characterization of a mesangial cell-derived autogrowth factor. J Immunol. 1986 May 15;136(10):3700–3705. [PubMed] [Google Scholar]
- Mantovani A., Dejana E. Cytokines as communication signals between leukocytes and endothelial cells. Immunol Today. 1989 Nov;10(11):370–375. doi: 10.1016/0167-5699(89)90270-3. [DOI] [PubMed] [Google Scholar]
- Mantovani A. Tumor-associated macrophages. Curr Opin Immunol. 1989;2(5):689–692. doi: 10.1016/0952-7915(90)90031-b. [DOI] [PubMed] [Google Scholar]
- Matsushima K., Larsen C. G., DuBois G. C., Oppenheim J. J. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J Exp Med. 1989 Apr 1;169(4):1485–1490. doi: 10.1084/jem.169.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima K., Morishita K., Yoshimura T., Lavu S., Kobayashi Y., Lew W., Appella E., Kung H. F., Leonard E. J., Oppenheim J. J. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988 Jun 1;167(6):1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mené P., Dunn M. J. Contractile effects of TxA2 and endoperoxide analogues on cultured rat glomerular mesangial cells. Am J Physiol. 1986 Dec;251(6 Pt 2):F1029–F1035. doi: 10.1152/ajprenal.1986.251.6.F1029. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989 May 4;339(6219):27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- Ming W. J., Bersani L., Mantovani A. Tumor necrosis factor is chemotactic for monocytes and polymorphonuclear leukocytes. J Immunol. 1987 Mar 1;138(5):1469–1474. [PubMed] [Google Scholar]
- Minty A. J., Caravatti M., Robert B., Cohen A., Daubas P., Weydert A., Gros F., Buckingham M. E. Mouse actin messenger RNAs. Construction and characterization of a recombinant plasmid molecule containing a complementary DNA transcript of mouse alpha-actin mRNA. J Biol Chem. 1981 Jan 25;256(2):1008–1014. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Munro J. M., Cotran R. S. The pathogenesis of atherosclerosis: atherogenesis and inflammation. Lab Invest. 1988 Mar;58(3):249–261. [PubMed] [Google Scholar]
- Rambaldi A., Wakamiya N., Vellenga E., Horiguchi J., Warren M. K., Kufe D., Griffin J. D. Expression of the macrophage colony-stimulating factor and c-fms genes in human acute myeloblastic leukemia cells. J Clin Invest. 1988 Apr;81(4):1030–1035. doi: 10.1172/JCI113413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaldi A., Young D. C., Griffin J. D. Expression of the M-CSF (CSF-1) gene by human monocytes. Blood. 1987 May;69(5):1409–1413. [PubMed] [Google Scholar]
- Schreiner G. F., Cotran R. S., Unanue E. R. Macrophages and cellular immunity in experimental glomerulonephritis. Springer Semin Immunopathol. 1982;5(3):251–267. doi: 10.1007/BF01892088. [DOI] [PubMed] [Google Scholar]
- Shultz P. J., DiCorleto P. E., Silver B. J., Abboud H. E. Mesangial cells express PDGF mRNAs and proliferate in response to PDGF. Am J Physiol. 1988 Oct;255(4 Pt 2):F674–F684. doi: 10.1152/ajprenal.1988.255.4.F674. [DOI] [PubMed] [Google Scholar]
- Sica A., Matsushima K., Van Damme J., Wang J. M., Polentarutti N., Dejana E., Colotta F., Mantovani A. IL-1 transcriptionally activates the neutrophil chemotactic factor/IL-8 gene in endothelial cells. Immunology. 1990 Apr;69(4):548–553. [PMC free article] [PubMed] [Google Scholar]
- Sica A., Wang J. M., Colotta F., Dejana E., Mantovani A., Oppenheim J. J., Larsen C. G., Zachariae C. O., Matsushima K. Monocyte chemotactic and activating factor gene expression induced in endothelial cells by IL-1 and tumor necrosis factor. J Immunol. 1990 Apr 15;144(8):3034–3038. [PubMed] [Google Scholar]
- Sironi M., Breviario F., Proserpio P., Biondi A., Vecchi A., Van Damme J., Dejana E., Mantovani A. IL-1 stimulates IL-6 production in endothelial cells. J Immunol. 1989 Jan 15;142(2):549–553. [PubMed] [Google Scholar]
- Strieter R. M., Kunkel S. L., Showell H. J., Marks R. M. Monokine-induced gene expression of a human endothelial cell-derived neutrophil chemotactic factor. Biochem Biophys Res Commun. 1988 Nov 15;156(3):1340–1345. doi: 10.1016/s0006-291x(88)80779-4. [DOI] [PubMed] [Google Scholar]
- Striker G. E., Striker L. J. Glomerular cell culture. Lab Invest. 1985 Aug;53(2):122–131. [PubMed] [Google Scholar]
- Suematsu S., Matsuda T., Aozasa K., Akira S., Nakano N., Ohno S., Miyazaki J., Yamamura K., Hirano T., Kishimoto T. IgG1 plasmacytosis in interleukin 6 transgenic mice. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7547–7551. doi: 10.1073/pnas.86.19.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme J., Decock B., Lenaerts J. P., Conings R., Bertini R., Mantovani A., Billiau A. Identification by sequence analysis of chemotactic factors for monocytes produced by normal and transformed cells stimulated with virus, double-stranded RNA or cytokine. Eur J Immunol. 1989 Dec;19(12):2367–2373. doi: 10.1002/eji.1830191228. [DOI] [PubMed] [Google Scholar]
- Van Damme J., Van Beeumen J., Opdenakker G., Billiau A. A novel, NH2-terminal sequence-characterized human monokine possessing neutrophil chemotactic, skin-reactive, and granulocytosis-promoting activity. J Exp Med. 1988 Apr 1;167(4):1364–1376. doi: 10.1084/jem.167.4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snick J., Cayphas S., Vink A., Uyttenhove C., Coulie P. G., Rubira M. R., Simpson R. J. Purification and NH2-terminal amino acid sequence of a T-cell-derived lymphokine with growth factor activity for B-cell hybridomas. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9679–9683. doi: 10.1073/pnas.83.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snick J., Vink A., Cayphas S., Uyttenhove C. Interleukin-HP1, a T cell-derived hybridoma growth factor that supports the in vitro growth of murine plasmacytomas. J Exp Med. 1987 Mar 1;165(3):641–649. doi: 10.1084/jem.165.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. M., Creasey A. A., Ladner M. B., Lin L. S., Strickler J., Van Arsdell J. N., Yamamoto R., Mark D. F. Molecular cloning of the complementary DNA for human tumor necrosis factor. Science. 1985 Apr 12;228(4696):149–154. doi: 10.1126/science.3856324. [DOI] [PubMed] [Google Scholar]
- Wang J. M., Colella S., Allavena P., Mantovani A. Chemotactic activity of human recombinant granulocyte-macrophage colony-stimulating factor. Immunology. 1987 Mar;60(3):439–444. [PMC free article] [PubMed] [Google Scholar]
- Wang J. M., Taraboletti G., Matsushima K., Van Damme J., Mantovani A. Induction of haptotactic migration of melanoma cells by neutrophil activating protein/interleukin-8. Biochem Biophys Res Commun. 1990 May 31;169(1):165–170. doi: 10.1016/0006-291x(90)91449-3. [DOI] [PubMed] [Google Scholar]
- Wolpe S. D., Cerami A. Macrophage inflammatory proteins 1 and 2: members of a novel superfamily of cytokines. FASEB J. 1989 Dec;3(14):2565–2573. doi: 10.1096/fasebj.3.14.2687068. [DOI] [PubMed] [Google Scholar]
- Wong G. G., Clark S. C. Multiple actions of interleukin 6 within a cytokine network. Immunol Today. 1988 May;9(5):137–139. doi: 10.1016/0167-5699(88)91200-5. [DOI] [PubMed] [Google Scholar]
- Wong G. G., Temple P. A., Leary A. C., Witek-Giannotti J. S., Yang Y. C., Ciarletta A. B., Chung M., Murtha P., Kriz R., Kaufman R. J. Human CSF-1: molecular cloning and expression of 4-kb cDNA encoding the human urinary protein. Science. 1987 Mar 20;235(4795):1504–1508. doi: 10.1126/science.3493529. [DOI] [PubMed] [Google Scholar]
- Yoshimura T., Matsushima K., Oppenheim J. J., Leonard E. J. Neutrophil chemotactic factor produced by lipopolysaccharide (LPS)-stimulated human blood mononuclear leukocytes: partial characterization and separation from interleukin 1 (IL 1). J Immunol. 1987 Aug 1;139(3):788–793. [PubMed] [Google Scholar]
- Yoshimura T., Matsushima K., Tanaka S., Robinson E. A., Appella E., Oppenheim J. J., Leonard E. J. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T., Robinson E. A., Tanaka S., Appella E., Kuratsu J., Leonard E. J. Purification and amino acid analysis of two human glioma-derived monocyte chemoattractants. J Exp Med. 1989 Apr 1;169(4):1449–1459. doi: 10.1084/jem.169.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]