Abstract
Dendritic cells (DCs) must achieve a critical balance between activation and tolerance, a process influenced by cytokines and growth factors. IL-10, which transduces signals through Stat3, has emerged as one important negative regulator of DC activation. To directly examine the role Stat3 plays in regulating DC activity, the Stat3 gene was targeted for deletion with a CD11c-cre transgene. Stat3 CKO mice developed cervical lymphadenopathy as well as a mild ileocolitis that persisted throughout life and was associated with impaired weight gain. Consistent with this, Stat3-deficient DCs demonstrated enhanced immune activity, including increased cytokine production, Ag-dependent T-cell activation and resistance to IL-10–mediated suppression. These results reveal a cell-intrinsic negative regulatory role of Stat3 in DCs and link increased DC activation with perturbed immune homeostasis and chronic mucosal inflammation.
Dendritic cells (DCs) play an important role in host immunity through their ability to distinguish between self-Ags and those associated with microbes (reviewed in Refs. 1–3). Microbial associated Ags potently activate DCs through engagement of innate pattern recognition receptors, including TLRs. Activation is associated with an up-regulation in the expression of MHC class II (MHC-II) and costimulatory molecules (e.g., CD80 and CD86), which in turn promote enhanced T cell activation. This process is also regulated by inflammatory cytokines, such as IFN-Is, TNF, IL-6, IL-10, IL-12, and IL-23, serving both to regulate DC activity and direct effector T cell differentiation [e.g., IFN-γ–producing Th1 cells, IL-4–producing Th2 cells, or IL-17–producing Th17 cells (4, 5)]. By contrast, self-Ags fail to activate DCs, endorsing a tolerant state. Failure of negative regulation can lead to aberrant DC activation and loss of tolerance (2, 6, 7). However, a direct causal relationship between exuberant DC activation and inflammatory disease has been more difficult to demonstrate in vivo.
Conventional DCs (cDCs), phenotypically marked by the expression of CD11c, are found throughout the host (1, 2). They can be further divided into CD8+ DCs, which cross-present endogenous Ags on MHC class I, and CD8− DCs, which prefer to present exogenous Ags on MHC-II (8). Plasmacytoid DCs (pDCs; CD11clo, mPDCA-1+, and B220+) represent a functionally distinct lineage that also arises from a Flt3+ and M-CSFR+ common DC progenitor (1, 2). In contrast to cDCs, pDCs can be found in the bone marrow BM and secrete prodigious quantities of IFN-Is. The transcription factor Stat3 has recently been ascribed an important role in Flt3-mediated DC progenitor proliferation, especially for pDCs, whereas Stat5 directs a phenotypically dominant GM-CSF–driven expansion of cDCs (9–11).
STATs play a critical role in directing the biological responses for members of the four-helix bundle family of cytokines (12). Of these, Stat3 is unique for its capacity to direct the anti-inflammatory activities of IL-10 and related cytokines (13). To overcome the embryonic lethal phenotype associated with the targeted deletion of the Stat3 gene, several conditional knockout models have been generated (14). This includes deletion in myeloid (i.e., LysM-Cre) as well as additional hematopoietic lineages (9, 15–19). Notably, each of these conditional deletions has been associated with a severe colitis, analogous to what had been observed in IL-10–deficient mice (20). In the myeloid deletion model, the inflammatory process is associated with increased IL-12 production and enhanced Th1 T cell activity, raising the possibility of aberrant DC activity (15, 21, 22). Moreover, a potential role for Stat3 as a cell intrinsic negative regulator of DC activity has been observed in several experimental systems (23–25). However, the complex phenotypes associated with hematopoietic deletion of Stat3 have precluded a more careful analysis of the specific role Stat3 plays in regulating DC activity.
To clarify the role Stat3 plays in DC function, the Stat3 gene was targeted for deletion with a DC-specific CD11c-cre transgene (26–28). Underscoring the value of this strategy, Stat3 CKO mice developed a significantly less severe colitis than observed with other targeting strategies (9, 16–19). In addition, CKO mice exhibited cervical lymphadenopathy, increased peribronchiolar inflammation, as well as impaired weight gain and fertility that were associated with ileocolitis. The constellation of intestinal, peribronchiolar, and likely nasopharyngeal inflammation suggests that DC-specific deletion of Stat3 is associated with impaired mucosal tolerance. Consistent with this, Stat3 CKO DCs, which developed normally, were resistant to IL-10 suppression and exhibited both an enhanced ability to secrete inflammatory cytokines and stimulate T cells. These observations underscore an important role for Stat3 in antagonizing DC activity and directly link DC hyperactivity with inflammatory disease.
Materials and Methods
Mice
Stat3flolx/flox mice, in a 129/C57BL/6J mixed background (16), were crossed seven generations onto C57BL/6J background, and then with CD11c-BAC-Cre transgenic mice (26). The genotype of Stat3flox/flox × CD11cCre (Stat3 CKO) mice was confirmed by PCR (16). OT-I and OT-II TCR transgenic mice obtained were from The Jackson Laboratory (Bangor, ME). The Columbia University Institutional Animal Care and Use Committee approved the murine studies.
Cell culture
DCs were either isolated from the spleen, mesenteric lymph node (MLN), or derived from BM. Purified DCs were cultured in 24-well plates at 0.5 × 106 cells/ml in RPMI 1640 medium (Life Technologies; Grand Island, NY), with 10% FCS (HyClone, Logan, UT), penicillin/streptomycin (Life Technologies), and 2-ME (Life Technologies). For BM-derived DCs, BM cells were plated in 24 well plates (0.5 × 106 cells/ml) in RPMI 1640 medium, 10% FCS, penicillin/streptomycin, 2-ME, and either 1/30 GM-CSF conditioned medium [from J558 cells as previously described (26)] or Flt3L (100 ng/ml; PeproTech, Rocky Hill, NJ). Loosely adherent clustering DCs were recovered on day 6 (~65% CD11c+) or day 10 (≥85% CD11c+). BM macrophages were prepared in 20% L929 conditioned medium as described previously (29, 30). Splenic and lymph node (LN) leukocytes were recovered by digestion with collagenase D (1 mg/ml; Roche Diagnostics; Indianapolis, IN) and DNase type IV (5 µg/ml; Sigma-Aldrich, St. Louis, MO). Single-cell suspensions were RBC depleted (Sigma-Aldrich), resuspended in RPMI 1640/10% FCS, counted, and/or stained for FACS. In one set of experiments, splenic DCs were prepared 3 h after mice were i.v. injected with LPS (250 ng in 200 µl) or PBS. Lamina propria leukocytes (LPLs) were isolated from the small intestine (minus Peyer’s patches), cecum, and colon. The epithelial layer was removed with EDTA (1.3 mM), and the remaining tissue digested in collagenase D (1 mg/ml, 37°C, 30 min) and DNase (0.1 mg/ml, 37°C, 30 min; Roche Diagnostics), followed by separation on Percoll, as reported previously (31). Cells were then analyzed by flow cytometry.
Flow cytometry
Cells were stained with different fluorochrome-coupled Abs. Anti-CD11c (HL3), anti-CD3e (145-2C11), anti-CD86 (GL1), anti-CD4 (L3T4), anti-CD40, anti-CD80 (16-10A1), anti-CD8 (53-6.7), streptavidin-PerCP, and streptavidin-allophycocyanin were from BD Pharmingen (San Diego, CA). Anti-CD11b (M1/70), anti-B220 (RA3-6B2), and anti-CD19 (MB19-1) were from eBioscience (San Diego, CA). Anti-murine PDCA-1 was from Miltenyi Biotec (Auburn, CA). Propidium iodide-negative (live) cells were gated on scatter, and at least 1 × 106 cells were acquired per mouse on an LSRII (BD Biosciences, Franklin Lakes, NJ). Data were analyzed with FlowJo software (Tree Star, Ashland, OR). Cell sorting was carried out on a FACSAria (BD Biosciences).
Biochemical studies
Whole-cell extracts were prepared from IL-10 (100 ng/ml; PeproTech)– or IFN-α (1000 U/ml; PBL, Piscataway, NJ)–treated cells and evaluated by immunoblotting, as reported previously (29, 32). Abs included those directed against phospho-Stat3 (Cell Signaling Technology, Beverly, MA), Stat3 (Cell Signaling Technology), and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA).
Quantitative-PCR
DNA was recovered from FACS-purified leukocyte populations and amplified with specific primers by quantitative-PCR (Q-PCR) (ABI Prism 7700 with SYBR green master mix; Applied Biosystems, Foster City, CA). Primers were annealed at 60°C and run 40 cycles. Stat3 primers included 5′-CCA AGT TCA TCT GTG TGA CAC C-3′ (common); 5′-ATC GGC AGG TCA ATG GTA TTG C-3′ (wild-type [WT]); and 5′-GAG TCA GGG ATC CAT AAC TTC-3′ (flox). Control cyclophillin A primers included 5′-CTG AGC ACT GGA GAG AAA GG-3′ and 5′-CTT GCT GGT CTT GCC ATT CC-3′). Cre primers included 5′-GGA CAT GTT CAG GGA TCG CCA GGC G-3′ and 5′-GCA TAA CCA GTG AAA CAG CAT TGC TG-3′ (26). Ct values and a standard curve were generated by plotting log of DNA concentration versus Ct values from 1:5 serial dilutions of DNA for each primer set SDS1.9.1 software).
Cytokine profiling
Cultured DCs were stimulated with LPS (1 µg/ml, 12 h; from Escherichia coli serotype 055:B5; Sigma-Aldrich), IL-10 (10 ng/ml), or CpG-oligodeoxynucleotides (1 µM, 12 h; Invitrogen, Frederick, MD), including D19 (A/D-type), 1668 (K-type), and D (negative control). Cytokines levels were measured by Bead Array (IL-6, TNF, and IL-12; BioSource International [Camarillo, CA] on Luminex [Austin, TX]), ELISA (IFN-γ, IL-17, IL-4, IL-13, IL-10, and IL-23 [eBioscience, San Diego, CA] and IFN-α [PBL]), or intracytoplasmic staining (i.e., anti–CD4-FITC, anti–IFN-γ–allophycocyanin, anti–IL-4-PE, or IL-12–allo-phycocyanin; BD Pharmingen), after treating cells with GolgiStop and Cytofix/Cytoperm (BD Pharmingen).
T cells analysis
A total of 3 × 105 OVA-specific CD4+ T cells (anti-CD4 MACS beads; Miltenyi Biotec) from OT-II TCR transgenic spleens were cultured with 3 × 104 WT CD11c+ DCs that had been loaded with increasing concentrations of OVA (Worthington Biochemical, Lakewood, NJ) or OVA323–339 peptide, as indicated. T cell proliferation was measured by [3H]thymidine incorporation (16 h, 37°C; PerkinElmer Life Sciences, Shelton, CT). In some studies, cytokine production was measured by intracellular staining after 7 d of OVA-DC stimulation, followed by restimulation with plate bound anti-mouse CD3 (5 µg/ml, 6 h; 145-2C11; BD Pharmingen). For Ag presentation by purified MLN-DCs, 0.25–1 × 104 OVA323–339-loaded (100 and 1000 ng/ml) primary DCs were incubated with 105 OT-II CD4+ splenic T cells. Cytokine accumulation (72 h) was evaluated by ELISA (eBioscience) 3 d after restimulation with 3 µg/ml anti-CD3 and 3 µg/ml anti-CD28 (eBioscience). Alternatively, 0.25–1 × 104 SIINFKL (100 and 1000 ng/ml)-loaded, CD11c+ MLN-DCs were incubated with 105 OT-I CD8+ splenic T cells.
Histopathology
Tissues were fixed in 10% neutral buffered formalin and embedded in paraffin, and sections (3 µm) were stained with H&E. The severity of inflammation was scored using a four-tiered scale (see Fig. 3).
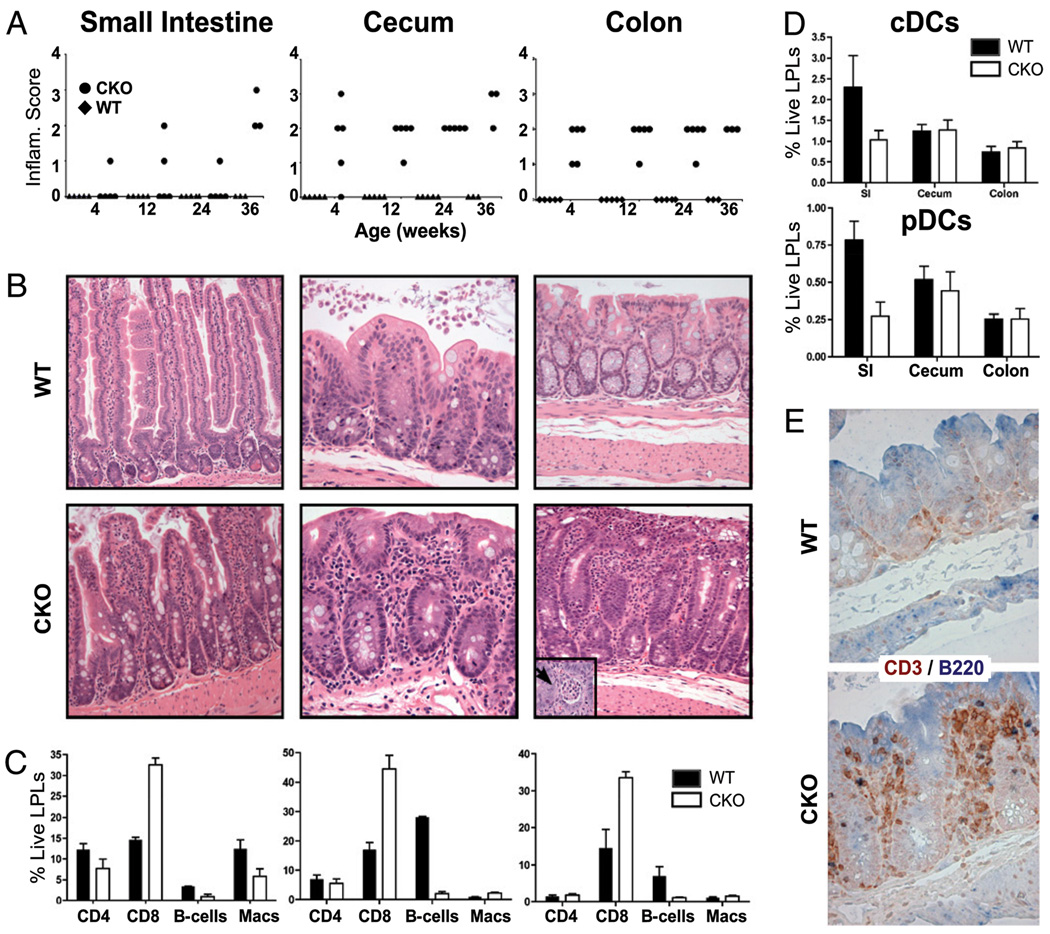
Figure 3.
Stat3 CKO mice exhibit a mild ileocolitis. A, Intestinal inflammation was scored on H&E-stained sections (see B) with a four-tiered scale (0, normal; 1, minimal; 2, mild; 3, moderate; and 4, severe) by a pathologist (G.B.) blinded for age (4, 8, 12, 24, and 36 wk; n = 5 for each group except 36 wk, where n = 3) and genotype. Mice were independently housed. B, Representative H&E-stained sections of the small intestine (original magnification ×40), cecum (original magnification ×100), and colon (original magnification ×40) from WT and CKO mice. A crypt abscess (original magnification ×200) is shown in the inset. C, Enterocolitis correlates with changes in LPLs. Leukocytes were harvested from 9- to 12-mo-old WT (n = 3) and CKO (n = 5) small intestine, cecum, and colon. Cells were stained and evaluated by FACS for their distribution of CD4+ and CD8+ T cells, B cells (B220+/CD11c−), and macrophages (Macs; CD11b+/CD11c−). Data are presented as percentage of total live LPLs. The data are mean ± SEM of tissues from three WT and CKO mice. D, DC populations in cecum and colon are unchanged in CKO mice. cDCs (CD11chi/MHC-IIhi) and pDCs (B220+/CD11cint) were detected using cells isolated as in C and are presented as percentage of total live LPLs. The data are mean ± SEM of three WT and CKO mice. E, Enterocolitis is associated with T cell infiltration into lamina propria. Epitopes were retrieved from paraffin sections of WT and CKO cecum as previously reported (36) and stained with B220 (biotinylated rat anti-CD45R; clone RA3-6B2 [eBioscience, San Diego, CA]; 10 mg/ml), polyclonal rabbit anti-CD3 (BioExpress), or control Abs and detected with streptavidin-HRP (Jackson ImmunoResearch Laboratories, West Grove, PA) plus 3-amino-9-ethylcarbazole (NovaRed; Vector Laboratories, Burlingame, CA) or with goat anti-rabbit alkaline phosphatase and NBT/5-bromo-4-chloro-3-indolyl phosphate (dark blue; Sigma-Aldrich). Representative photographs, taken at magnification ×200, as presented. SI, small intestine.
Statistics
Statistical significance was estimated by calculating two-tailed p values in unpaired Student t test from independent studies (n = 3; unless otherwise stated).
Results
CD11c-Cre directs efficient and DC-specific Stat3 deletion
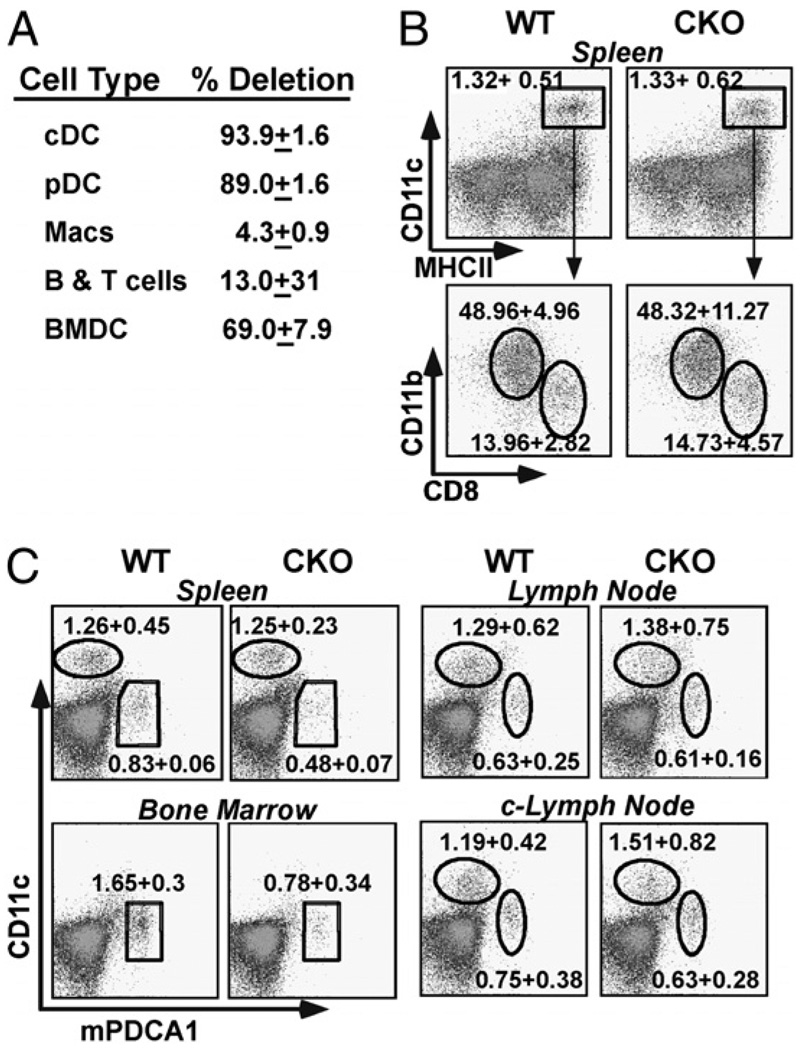
To test the hypothesis that Stat3 is an important regulator of DC function, mice carrying a loxP-flanked Stat3 allele (Stat3flox [16]) were crossed with CD11c-Cre transgenic mice (26). To evaluate Stat3 deletion specificity and efficiency in Stat3flox/flox × CD11c-Cre mice, genomic DNA from purified leukocyte populations (i.e., cDCs, pDCs, macrophages, T cells, and B cells) was analyzed by Q-PCR (Fig. 1A). Consistent with previous studies with the CD11c-Cre transgene, the Stat3flox allele was deleted ~94% of cDCs and 89% of pDCs from CKO mice (26–28). This contrasted a relatively low deletion efficiency in CKO monocytes and lymphocytes (4 and 13%, respectively; Fig. 1A), as well as DX5+ NK cells (~10% [26]). Q-PCR analysis of day 8 BM-derived DCs (BMDCs) (~75% pure; Supplemental Fig. 1) revealed ~70% deletion efficiency in CKO cells. Consistent with this, the level of Stat3 protein expression was also dramatically reduced in CKO BMDCs, but not BM-derived macrophages (Supplemental Fig. 2) (30). No compensatory increase in Stat1 was observed (data not shown [16]). Likewise, little if any activated (i.e., phosphorylated) Stat3 could be detected in IL-10–stimulated Stat3 CKO BMDCs (Supplemental Fig. 2). These results correlated well with Southern blotting studies (data not shown) and demonstrate that the CD11c-Cre transgene directs both the efficient and specific deletion of Stat3 in DCs, consistent with studies on other LoxP flanked loci (26–28).
Figure 1.
DC populations in Stat3 CKO null mice. A, Stat3 is efficiently deleted in CKO DCs. WT and Stat3 CKO splenocytes were FACS sorted into the following: cDCs (CD11c+ and CD11b+); pDCs (CD11clo and mPDCA1+); macrophages (CD11c− and CD11b+); T cells (CD3+); and B cells (CD19+). DNA was evaluated for presence of WT or deleted Stat3 gene by Q-PCR. Results are representative of two independent studies and were confirmed by Southern blotting. B, Circulating cDC populations are normal in CKO mice. WT and CKO splenic DCs (CD11c+ and MHC-II+) were evaluated for mDCs (CD11c+, MHCII+, and CD11b+) and lymphoid DCs (CD11c+, MHC-II+, and CD8+) populations. Analysis is representative of more than three independent studies. C, Circulating pDCs are reduced in CKO mice. Analysis of WT and CKO cDCd (Cd11chi and mPDCA1−) and pDCs (Cd11clo and mPDCA1hi) populations from spleen, BM, normal-sized LNs (see text), and enlarged CKO c-LNs (see Supplemental Fig. 3, Table I). Analysis is representative of more than three independent studies. The ~2-fold decrease in CKO pDCs was significant in spleen (p < 0.004) and BM (p < 0.03).
cDC and pDC populations in Stat3 CKO mice
To examine the effect of Stat3 deletion on DC homeostasis, cells from the spleen, BM, and LNs of CKO mice were evaluated by immunophenotyping (Fig. 1, Table I). No significant differences were observed in the splenic cDCs (CD11chi and MHC-II+), including CD8− and CD8+ populations (Fig. 1B). Likewise, there were no significant differences in the expression of activation markers (e.g., CD80, CD86, CD40, and MHC-II; data not shown). Moreover, circulating lymphocyte populations were not affected by DC-specific Stat3 deletion (data not shown) (Table I). There was, however, a consistent ~2-fold reduction in the number of pDCs (CD11clo, mPDCA+, and B220+), in the spleen (0.48 ± 0.07 versus 0.83 ± 0.06%), and in the BM(0.78 ± 0.34 versus 1.65 ± 0.30%) of CKO mice (Fig. 1C), likely reflecting the ~50% deletion efficiency directed by the CD11c-Cre transgene in developing pDCs (M. Caton and B. Reizis, unpublished observation) (26). Yet, the number of pDCs found in “normal” LNs and the lower gastrointestinal tract was similar, likely reflecting a compensatory recruitment (Figs. 1C, 3D) (33). In addition, CKO mice were noted to develop persistently enlarged cervical LNs (c-LNs). This was associated with a 2-fold increase in pDCs but did not represent an absolute increase in the tissue concentration of these cells (see Fig. 1C, Table I). In addition, the enlarged c-LNs featured more lymphoid follicles, with a corresponding increase in lymphocytes (B cells > T cells) (Supplemental Fig. 4, Table I). Stat3 deletion was also not associated with a significant change in the capacity of splenic or BM pDCs to secrete IFN-α (see Supplemental Fig. 3).
Table I.
Comparison of WT and CKO leukocyte counts
| pDCs | cDCs | T Cell | B Cell | |
|---|---|---|---|---|
| Spleen | ||||
| WT | 8.22 × 105 ± 6.49 × 104 | 1.25 × 106 ± 4.49 × 105 | 2.78 × 107 ± 3.47 × 106 | 5.79 × 107 ± 6.09 × 106 |
| CKO | 4.80 × 105 ± 7.55 × 104 | 1.25 × 106 ± 2.31 × 105 | 2.70 × 107 ± 3.50 × 106 | 6.19 × 107 ± 7.29 × 106 |
| BM | ||||
| WT | 9.90 × 105 ± 1.81 × 105 | NA | NA | Not counted |
| CKO | 3.91 × 105 ± 1.70 × 105 | NA | NA | Not counted |
| LN | ||||
| WT | 2.48 × 104 ± 8.50 × 103 | 3.56 × 104 ± 6.64 × 103 | 1.67 × 106 ± 1.57 × 105 | 9.60 × 105 ± 1.60 × 105 |
| CKO | 2.42 × 104 ± 5.69 × 103 | 3.04 × 104 ± 5.15 × 103 | 1.63 × 106 ± 1.87 × 105 | 9.89 × 105 ± 1.54 × 105 |
| c-LN | ||||
| WT | 2.72 × 104 ± 1.37 × 104 | 4.28 × 104 ± 1.52 × 104 | 2.13 × 106 ± 1.06 × 105 | 1.22 × 106 ± 1.40 × 105 |
| CKO | 6.37 × 104 ± 2.83 × 104 | 1.51 × 105 ± 8.26 × 104 | 4.39 × 106 ± 1.03 × 105 | 4.98 × 106 ± 1.15 × 105 |
Total leukocytes populations from spleen, BM, and LNs were calculated based on cell counts and FACS analysis (n = 5 for WT and CKO spleens; n = 5 for BM; n = 8 for LNs and c-LNs) for cDCs (CD11chi, CD11b+, and mPDCA1−), pDCs (CD11clo and mPDCA1hi), macrophages (CD11c−, CD11bhi), T cells 3+), and B cells (B220+).
NA, not available.
To determine whether the absence of Stat3 affected DC development in vitro, WT and CKO BM cells were cultured in media supplemented with GM-CSF or Flt3 ligand to generate cDCs and pDCs, respectively (Supplemental Fig. 1) (26, 34). Consistent with the relatively late onset of Cre recombination associated with in vitro DC development (26), no differences were observed in the ratio of these two populations (data not shown). Overall, these studies reveal a relatively normal DC compartment in CKO mice, as well as cultured DCs, affording the opportunity to carry out functional studies on Stat3-deficient DCs in the absence of any confounding developmental or systemic defects.
Stat3 CKO mice exhibit a mild inflammatory phenotype
Although Stat3 CKO mice were born at the expected Mendelian ratio, they exhibited isolated cervical lymphadenopathy (see above; see also Supplemental Fig. 4), developed infertility and failed to accumulate body fat with increasing age (Supplemental Fig. 5). To determine whether these latter two phenotypes could be attributed to a chronic inflammatory state, serum cytokine levels were evaluated at 24 wk of age. Stat3 CKO mice revealed a significant and consistent 1.5- to 2-fold increase in levels of circulating TNF (i.e., cachexin [35]) and IFN-γ, as well as less significant increases in IL-12 and IL-6 (see Fig. 2). No significant differences in circulating levels of IL-1β, IL-10, or Th2-type cytokines (i.e., IL-4 and IL-5) (see Fig. 2; data not shown) were noted. These data highlight a mild proinflammatory state in CKO mice, which may account for their reduced fertility and body fat.
Figure 2.
Inflammatory cytokines are increased in serum of Stat3 CKO mice. Circulating cytokines levels (TNF, IL-12, IL-6, IFN-γ, IL-10, and IL-4) were measured in the serum of five independently housed WT and CKO mice by bead array. Differences between WT and CKO were significant for TNF (p < 0.04) and IFN-γ (p < 0.008) but not for IL-12, IL-10, IL-6, and IL-4 (p values of 0.07, 0.19, 0.06, and 0.05, respectively).
To determine the etiology of chronic inflammation in Stat3 CKO mice, a histopathologic survey was carried out. Specific pathology was not evident in most CKO organs systems including, heart, pancreas, liver, kidneys, thymus, non–c-LNs, and spleen. However, four WT and eight CKO mice (n = 16 for each group) exhibited peribronchial thickening and inflammation. The inflammatory infiltrate consisted largely of lymphocytes as well as some monocytes (data not shown).
The most striking pathology in Stat3 CKO mice was an inflammatory process that extended throughout the lower intestinal tract. To evaluate this more carefully, a blinded histopathologic survey was carried out in WT and CKO GI tracts (n = 5 for both genotypes at 4, 12, and 24 wk and n = 3 at 36 wk; see Fig. 3A, 3B). This revealed mild to moderate inflammation that was most evident in the lamina propria of the large intestine and cecum, an area with a large Ag load and abundant DCs (21). A more detailed analysis of the leukocytes accumulating in the lamina propria (i.e., LPLs) revealed a significant increase in CD8+ cells in CKO mice (p < 0.05, 0.01, and 0.01 for small intestine, cecum, and colon, respectively), but no differences in the limited number of recovered CD4+ cells, especially in regions where inflammation was prevalent (i.e., cecum and colon) (Fig. 3C, 3E). In addition, no significant differences were observed in the intracytoplasmic accumulation of IFN-γ or IL-17 in CD4+ cells (data not shown). Likewise, there were no significant differences in the DCs recovered from the cecum and colon (Fig. 3D, 3E). Intriguingly, there was a decrease in B cells isolated from cecum and colon of CKO mice (Fig. 3C), which may reflect a decrease in the number of observed Peyer’s patches (data not shown). Finally, there was a trend toward increasing inflammation as mice aged, which did not achieve significance in our study.
A more careful analysis of the inflammatory infiltrate revealed a variable lymphocytic cryptitis, expansion of intercryptal spaces secondary to inflammatory cells and occasional crypt abscesses, which were composed of clusters of neutrophils within crypt lumens (see inset in Fig. 3B, CKO large intestine). Although some mucin depletion (decreased goblet cell mass) was evident in areas of inflammation, granulomas were not observed. Disease was most apparent in the cecum (four of five at 4 wk and all older CKO mice) and the large intestine (all CKO mice), where inflammation was patchy, with intervening normal mucosa. The small intestine, especially in the distal ileum, was largely spared of disease until 36 wk of age. Overall, the pattern of inflammation bore similarities to Crohn’s disease (37). Not only was the extent of colonic inflammation considerably less severe than that observed in other Stat3 CKO models, but it also extended into the distal small intestine (16–18, 38, 39).
Functional characterization of Stat3 CKO DCs
To determine whether the increased inflammation observed in CKO mice could be attributed to augmented DC activity, the capacity of WT and Stat3 CKO BMDCs to be activated by TLR agonists was evaluated. Although CKO BMDCs developed normally (Supplemental Fig. 1), they appeared to mature a little more rapidly in several independent experiments when interrogated for CD40, CD80, CD86, and MHC-II expression (data not shown). As anticipated, stimulation of WT BMDCs with synthetic TLR9 agonists (i.e., D19 and 1668 CpG oligonucleotides) or the TLR4 agonist LPS stimulated robust secretion of IL-6, TNF and IL-12, as well as more modest increases in IL-10, IL-23, and IFN-α (see Fig. 4A, Supplemental Fig. 3) (26, 40). Other TLR ligands (e.g., poly[I]•poly[C] and imiquimod) were considerably less effective in inducing cytokine expression. Intriguingly, Stat3 CKO DCs produced significantly higher levels of IL-12 and IL-23 after stimulation with the two most potent agents, 1668 and LPS (Fig. 4A). There was also a trend toward higher TNF and IL-6 but not IFN-α secretion in CpG-treated CKO DCs (Fig. 4A, Supplemental Fig. 3) (data not shown).
Figure 4.
Functional characterization of Stat3 CKO DCs. A, Stimulated CKO DCs exhibit enhanced capacity to secrete cytokines in vitro. WT and CKO BMDCs (day 6 GM-CSF; see Supplemental Fig. 1) were evaluated for cytokine expression after stimulation with TLR agonists, including D (negative control CpG; 1 µM), D19 (A/D-type CpG; 1 µM), 1668 (K-type CpG; 1 µM), and LPS (1000 ng/ml). Twelve-hour supernatants were evaluated for cytokine production by ELISA (IL-23 and IL-10) or bead array (TNF, IL-12, and IL-6). Values of p for WT versus CKO were as follows: for IL-6, D19 < 0.01, 1668 < 0.007; for TNF, D19 < 0.0001, 1668 < 0.032; for IL-12, 1668 < 0.04, LPS < 0.003; for IL-10, LPS < 0.19; and for IL-23, LPS < 0.03). Results are representative of more than three independent experiments. B, Stimulated CKO DCs exhibit enhanced capacity to secrete IL-12 in vivo. WT (n = 5) and CKO (n = 8) mice were injected with LPS (WT, n = 3; CKO, n = 5) or PBS. Three hours later, splenocytes were harvested, stained (anti–CD11c-PE and anti–IL-12-allophycocyanin), and analyzed by FACS (see also Supplemental Fig. 6A). The increase in IL-12 production was quantified by mean fluorescence intensity in table at right. C, Increased Th1 polarization by CKO BMDCs. Day 6 WT and CKO GM-CSF DCs were evaluated for their ability to present Ag to purified CD4+ OT-II splenocytes after loading (16) with indicated concentrations of OVA. OT-II T cells were then evaluated for their capacity to proliferate (Supplemental Fig. 6B) or produce IFN-γ and IL-4 (data not shown) by intracytoplasmic staining. Graph shows percentage of IFN-γ+,CD4+ T cells. Data are representative of three independent experiments and were confirmed by ELISA. Values of p of WT versus CKO were significant for IFN-γ production at 0.5 mg/ml OVA (p < 0.0004) and 1 mg/ml OVA (p < 0.001). D, Increased Th1 polarization by CKO MLN DCs. OT-II CD4+ T cells (105 cells/well) were stimulated with CD11c+ cells isolated from the MLNs of WT (n = 3) and CKO (n = 5) mice in the presence or absence of the peptide OVA323–339 (OVAp) at DC: T cell ratio of 1:10. After 72 h, T cells were restimulated and evaluated for IFN-γ production by ELISA (eBioscience). Results are expressed as means (± SEM) of six independent experiments (*p < 0.05).
To determine whether endogenous CKO DCs exhibited the same pattern of enhanced cytokine secretion, splenic DCs were examined after the mice had been injected with LPS (9, 26). Consistent with in vitro observations, splenic CD11c+ DCs from both WT and CKO mice exhibited comparable levels of CD86 and MHC-II (Supplemental Fig. 6A). More importantly, Stat3-deficient splenic DCs produced significantly more IL-12 in response to LPS than WT DCs, paralleling results with cultured DCs (Fig. 4B).
Next, the ability of CKO DCs to present Ag to T cells was evaluated. At lower doses, WT and CKO OVA-loaded BMDCs were equivalent in their capacity to stimulate proliferation and IFN-γ secretion in naive OT-II transgenic CD4+ T cells (Fig. 4C, Supplemental Fig. 6B). Consistent with elevated levels of IL-12 secreted by CKO DCs (Fig. 4A, 4B), these cells exhibited a significant increase in their capacity to stimulate T cell proliferation and IFN-γ secretion when loaded with higher doses of OVA (i.e.,>0.1 mg/ml) (see Fig. 4C, Supplemental Fig. 6B). In contrast, however, OVA-dependent induction of IL-4 and IL-17 secretion was considerably more modest, suggesting that corresponding effector populations may not contribute to the inflammatory response in Stat3 CKO mice (Supplemental Fig. 6) (data not shown). A similar increased capacity to promote OT-II cell polarization toward IFN-γ–producing Th1, but not other effectors, was observed when DCs were harvested from the MLNs of CKO mice and loaded with OVA peptide (OVAp) (Fig. 4D, Supplemental Fig. 6D). Likewise, OVAp-loaded MLN CKO DCs exhibited an enhanced trend toward promoting IFN-γ production in OT-I T cells (see Supplemental Fig. 6E). Collectively, these data reveal that Stat3-deficient DCs are hyperresponsive to TLR stimulation, thereby directing a more exuberant activation of proinflammatory effector T cells.
IL-10, which transduces critical signals through Stat3, is an important negative regulator of DC activity (5, 41). To determine whether Stat3 mediates the inhibitory effect of IL-10 on these cells, WT and CKO DCs were incubated with IL-10 prior to LPS stimulation. As anticipated, IL-10 pretreatment blocked the robust LPS-dependent upregulation of MHC-II and CD86 (see Fig. 5A). In contrast, MHC-II and CD86 expression was insensitive to IL-10 pretreatment in Stat3 CKO DCs (Fig. 5A). Similarly, IL-10 failed to block LPS-dependent cytokine secretion in Stat3 CKO DCs (see Fig. 5B). A similar autocrine phenomenon may also account for the enhanced levels of cytokines observed in CKO serum and in CpG-stimulated DCs (Figs. 2, 4A). These observations are consistent with previous studies on Stat3-deficient macrophages (16), underscoring an important role for Stat3 in directing the anti-inflammatory activity of IL-10 on DCs. These studies raise the possibility that a loss in autocrine IL-10 response, or another analogous Stat3-dependent negative regulator, may account for the hyperactivity observed in Stat3 CKO DCs.
Figure 5.
Inhibitory activity of IL-10 on Stat3 CKO DCs. A, CKO DC activation is not sensitive to IL-10 suppression. Day 9 WT and CKO GM-CSF DCs were stimulated with LPS (100 ng/ml, 12 h) either before or after a 12-h pretreatment with IL-10 (10 ng/ml). Bar graph quantifies significant decreases (graphed as percent change) in the LPS-stimulated CD86 and MHC-II expression in WT (versus CKO) CD11c+, IL-10–pretreated DCs (p values of 0.00001 and 0.008, respectively). Results are representative of greater than three independent experiments. B, CKO DC cytokine secretion is not suppressed by IL-10. Twelve-hour LPS culture supernatants were collected from DCs treated as outlined in A and evaluated for cytokine production by bead array. Each data point is representative of four independent samples.
Discussion
The development of a more specific CD11c-Cre transgene has provided an opportunity to explore the role Stat3 plays in regulating DC activity in vivo. Previous efforts, exploiting panhematopoietic or inducible Stat3 deletion, yielded complex phenotypes. Specifically, deletion of Stat3 in hematopoietic progenitors led to a severely contracted cDC compartment and a substantial block in pDC development, with the rapid emergence of a lethal ileocolitis (9, 39). Defects in DC development were attributed to impaired Flt3-Stat3–dependent signaling (9, 10). Inducible Stat3 deletion has been associated with systemic granulocytosis and a comparable aggressive colitis marked by a neutrophil predominant infiltrate (L. Song and C. Schindler, unpublished observation) (17, 18). This inflammatory process featured a disrupted epithelial barrier, reminiscent of the pathophysiology reported for T-bet/RAG2 double-knockout mice (42). Analogously, deletion of Stat3 in regulatory T cells was associated with an aggressive, albeit Th17-dependent destructive colitis (19). In contrast, the colitis that developed in mice with a myeloid-specific (i.e., LysM-Cre transgene targeting polymorphonuclear neutrophils > macrophages ≈ DCs), albeit incomplete, Stat3 deletion (L. Song and C. Schindler, unpublished observation) (43) was associated with life spans that approached 24 wk and an infiltrate featuring neutrophils, lymphocytes, macrophages, and DCs (15, 16). Although colitis was ascribed to an enhanced TLR4-dependent IL-12 secretion and lymphocyte activation, the considerably more abundant macrophages appeared to direct this response (15). This contrasted the chronic (i.e., nonlethal), neutrophil poor, inflammatory process found in CD11c-Cre Stat3 CKO mice, suggesting that either the macrophage predominant IL-12 secretion in LysM-Cre mice was more proinflammatory or that activation of the IL-23-Th17 axis may have accounted for at least some of IL-20p40–dependent inflammatory response in these mice (15). This axis does, however, not appear to play a significant role in the CD11c-Cre Stat3 CKO mice.
Studies with the CD11c-Cre transgene have determined that it directs the efficient deletion in target genes, including Stat3, in cDCs and pDCs but not in other myeloid or lymphoid lineages (26–28). Consistent with evidence that this deletion largely occurs after cDC lineage commitment, cDCs developed normally in Stat3 CKO mice (26). In contrast, splenic and BM pDC populations were reduced by 50%. These observations underscore the earlier expression of the CD11c-Cre transgene during pDC development, as well as a role for the Flt3-Stat3 axis in pDC expansion (9–11). Defective Stat3 signaling may, however, also perturb hematopoiesis through a pathway that is independent of Flt3 (44, 45). Despite this 2-fold reduction, the number of pDCs recovered from LNs, cecum, and colon was equivalent in WT and Stat3 CKO mice. This observation highlights a compensatory recruitment of CKO pDCs to lymphoid tissues, further underscoring the use of a model where potentially confounding developmental defects are avoided.
Mice with steady-state, DC-specific Stat3 deletion exhibited several proinflammatory features in addition to their ileocolitis, including isolated cervical lymphadenopathy (c-LNs drain into the nasopharyngeal cavity) and increased peribronchiolar infiltration. This constellation suggested that a DC-specific deletion of Stat3 was associated with an increased proclivity toward mucosal inflammation, likely reflecting a loss in mucosal tolerance. Consistent with this, the lower gastrointestinal tract, with its relatively large burden of microbes, exhibited the highest level of inflammation. Indeed, previous studies have highlighted an important role for lamina propria DCs in sampling commensal bacterial and food Ags to promote tolerance (21, 22). Similarly, deletion of the transcription factor T-bet in the DCs of RAG2−/− mice was associated with enhanced DC-dependent TNF secretion (42). Although these mice developed an aggressive, granulocyte predominant colitis that was quite distinct from the Crohn’s-like disease found in Stat3 CKO mice; both studies underscore an important role for DCs in promoting mucosal tolerance. Intriguingly, recent evidence that Stat3 may control DC plasticity (i.e., inflammatory versus tolerogenic states) through a capacity to regulate the expression of IDO has provided additional insight into how Stat3 may control mucosal tolerance (46, 47). In addition, our data support a role for Stat3 as a negative transcriptional regulator, serving to antagonize aberrant or exuberant DC activity through ligand(s) from the IL-10 family of cytokines (see below) (48). This process may also be associated with changes in regulatory T cell activity (7). Hence, loss of Stat3 is associated with an increase in inflammatory cytokines, including TNF (i.e., cachexin), which we speculate is responsible for the cachexia and infertility observed in CKO mice (21, 35). Although the number of infiltrating CD8+ and not CD4+ T cells correlated most closely with disease, analysis of these CD4+ T cells failed to detect significant differences in their cytokine signatures (e.g., IFN-γ versus, IL-4 versus IL-17; data not shown). The observed lymphocytic infiltration may reflect changes in the CKO DC-dependent CD4+ T cell activation, or these CD8+ T cells may directly be modulated by altered DC function. Consistent with the latter possibility, there was a trend toward higher OT-I–dependent IFN-γ secretion when these cells were stimulated with SIINFEKL-loaded CKO MLN DCs (Supplemental Fig. 6E). The current data did not evaluate the potentially pathogenic, compensatory, or regulatory roles changing B cell and/or CD8+ T cell populations may have played in the development of enterocolitis. Finally, it seems likely that malabsorption did not contribute significantly to weight loss in Stat3 CKO mice, because the onset of small intestinal inflammation was both relatively modest and post adolescence.
Consistent with a direct effect of a loss in Stat3, CKO DCs exhibited an augmented proinflammatory capacity. There were significant increases in TLR dependent IL-12 and IL-23 secretion in these DCs, supporting recent evidence that Stat3 negatively regulates the IL-12-p40 promoter (49). Similar results were observed when Stat3 was deleted through a tamoxifen-inducible Cre-ERT2 transgene in BM-derived DCs (Ref. 50; data not shown). Functionally, the increased IL-12 secretion correlated with a more robust Th1 polarization in vitro and ex vivo. Likewise, the more modest TLR-dependent IL-23 secretion in CKO DCs was not associated with a significant increase in T cell-dependent IL-17 expression, either in vitro or in lamina propria lymphocytes (Supplemental Fig. 6C; data not shown) (51). Notably, there was only a modest difference between WT and CKO DCs in TLR-stimulated secretion of IL-6 and IL-10, two Stat3-dependent negative regulators of DC activity (25, 52, 53). Likewise, although there was a trend toward enhanced, TLR-dependent upregulation of DC activation markers in Stat3 CKO mice, this also failed to reach statistical significance (Figs. 1, 5, Supplemental Fig. 6; data not shown). Stat3 CKO DCs were, however, resistant to the potent inhibitory activity of IL-10 in vitro, raising the possibility that a more modest defective response to IL-10, or a related cytokine family member, may contribute to the enhanced inflammatory activity of CKO DCs.
Supplementary Material
Acknowledgments
We thank Michele Caton for help with DC analysis.
This work was supported by National Institutes of Health Grants AI 058211 (to C.S.), HL 55413 (to C.S.), AI067804 (to B.R.), 5T32 AI07525 (to J.A.M. and C.R.P.), and AI044236 (to C.B.), Burroughs Wellcome Fund Grant APP#2010 (to C.S.), and the Sandler Program for Asthma Research (to B.R.).
Abbreviations used in this paper
- BM
bone marrow
- BMDC
bone marrow dendritic cell
- c-LN
cervical lymph node
- cDC
conventional dendritic cell
- DC
dendritic cell
- LN
lymph node
- LPL
lamina propria leukocyte
- MHC-II
MHC class II
- MLN
mesenteric lymph node
- OVAp
OVA peptide
- pDC
plasmacytoid dendritic cell
- Q-PCR
quantitative-PCR
- SI
small intestine
- WT
wild-type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
The online version of this article contains supplemental material.
References
- 1.Reis e Sousa C. Dendritic cells in a mature age. Nat. Rev. Immunol. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 2.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat. Rev. Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 3.Colonna M, Pulendran B, Iwasaki A. Dendritic cells at the host-pathogen interface. Nat. Immunol. 2006;7:117–120. doi: 10.1038/ni0206-117. [DOI] [PubMed] [Google Scholar]
- 4.Reiner SL. Development in motion: helper T cells at work. Cell. 2007;129:33–36. doi: 10.1016/j.cell.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu. Rev. Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 7.Darrasse-Jèze G, Deroubaix S, Mouquet H, Victora GD, Eisenreich T, Yao KH, Masilamani RF, Dustin ML, Rudensky A, Liu K, Nussenzweig MC. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J. Exp. Med. 2009;206:1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 9.Laouar Y, Welte T, Fu XY, Flavell RA. STAT3 is required for Flt3L-dependent dendritic cell differentiation. Immunity. 2003;19:903–912. doi: 10.1016/s1074-7613(03)00332-7. [DOI] [PubMed] [Google Scholar]
- 10.Onai N, Obata-Onai A, Tussiwand R, Lanzavecchia A, Manz MG. Activation of the Flt3 signal transduction cascade rescues and enhances type I interferon-producing and dendritic cell development. J. Exp. Med. 2006;203:227–238. doi: 10.1084/jem.20051645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esashi E, Wang YH, Perng O, Qin XF, Liu YJ, Watowich SS. The signal transducer STAT5 inhibits plasmacytoid dendritic cell development by suppressing transcription factor IRF8. Immunity. 2008;28:509–520. doi: 10.1016/j.immuni.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schindler C, Plumlee C. Inteferons pen the JAK-STAT pathway. Semin. Cell Dev. Biol. 2008;19:311–318. doi: 10.1016/j.semcdb.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D, Hanada T, Takeda K, Akira S, Hoshijima M, et al. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat. Immunol. 2003;4:551–556. doi: 10.1038/ni938. [DOI] [PubMed] [Google Scholar]
- 14.Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, Kishimoto T, Akira S. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc. Natl. Acad. Sci. USA. 1997;94:3801–3804. doi: 10.1073/pnas.94.8.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi M, Kweon MN, Kuwata H, Schreiber RD, Kiyono H, Takeda K, Akira S. Toll-like receptor-dependent production of IL-12p40 causes chronic enterocolitis in myeloid cell-specific Stat3-deficient mice. J. Clin. Invest. 2003;111:1297–1308. doi: 10.1172/JCI17085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Förster I, Akira S. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 17.Alonzi T, Newton IP, Bryce PJ, Di Carlo E, Lattanzio G, Tripodi M, Musiani P, Poli V. Induced somatic inactivation of STAT3 in mice triggers the development of a fulminant form of enterocolitis. Cytokine. 2004;26:45–56. doi: 10.1016/j.cyto.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Lee CK, Raz R, Gimeno R, Gertner R, Wistinghausen B, Takeshita K, DePinho RA, Levy DE. STAT3 is a negative regulator of granulopoiesis but is not required for G-CSF-dependent differentiation. Immunity. 2002;17:63–72. doi: 10.1016/s1074-7613(02)00336-9. [DOI] [PubMed] [Google Scholar]
- 19.Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 21.Niess JH, Reinecker HC. Dendritic cells: the commanders-in-chief of mucosal immune defenses. Curr. Opin. Gastroenterol. 2006;22:354–360. doi: 10.1097/01.mog.0000231807.03149.54. [DOI] [PubMed] [Google Scholar]
- 22.Hapfelmeier S, Müller AJ, Stecher B, Kaiser P, Barthel M, Endt K, Eberhard M, Robbiani R, Jacobi CA, Heikenwalder M, et al. Microbe sampling by mucosal dendritic cells is a discrete, MyD88-independent step in DeltainvG S. Typhimurium colitis. J. Exp. Med. 2008;205:437–450. doi: 10.1084/jem.20070633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kortylewski M, Kujawski M, Wang T, Wei S, Zhang S, Pilon-Thomas S, Niu G, Kay H, Mulé J, Kerr WG, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat. Med. 2005;11:1314–1321. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 24.Nefedova Y, Cheng P, Gilkes D, Blaskovich M, Beg AA, Sebti SM, Gabrilovich DI. Activation of dendritic cells via inhibition of Jak2/STAT3 signaling. J. Immunol. 2005;175:4338–4346. doi: 10.4049/jimmunol.175.7.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park SJ, Nakagawa T, Kitamura H, Atsumi T, Kamon H, Sawa S, Kamimura D, Ueda N, Iwakura Y, Ishihara K, et al. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J. Immunol. 2004;173:3844–3854. doi: 10.4049/jimmunol.173.6.3844. [DOI] [PubMed] [Google Scholar]
- 26.Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8− dendritic cells in the spleen. J. Exp. Med. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou B, Reizis B, DeFranco AL. Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity. 2008;29:272–282. doi: 10.1016/j.immuni.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, Wang Y, Bernstein X, Huang X, Reichardt LF, et al. Loss of integrin α(v)β8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song L, Bhattacharya S, Yunus AA, Lima CD, Schindler C. Stat1 and SUMO modification. Blood. 2006;108:3237–3244. doi: 10.1182/blood-2006-04-020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao W, Cha EN, Lee C, Park CY, Schindler C. Stat2-dependent regulation of MHC class II expression. J. Immunol. 2007;179:463–471. doi: 10.4049/jimmunol.179.1.463. [DOI] [PubMed] [Google Scholar]
- 31.Yang PC, Xing Z, Berin CM, Soderholm JD, Feng BS, Wu L, Yeh C. TIM-4 expressed by mucosal dendritic cells plays a critical role in food antigen-specific Th2 differentiation and intestinal allergy. Gastroenterology. 2007;133:1522–1533. doi: 10.1053/j.gastro.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Park C, Li S, Cha E, Schindler C. Immune response in Stat2 knockout mice. Immunity. 2000;13:795–804. doi: 10.1016/s1074-7613(00)00077-7. [DOI] [PubMed] [Google Scholar]
- 33.Nakano H, Yanagita M, Gunn MD. CD11c+B220+Gr-1+ cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J. Exp. Med. 2001;194:1171–1178. doi: 10.1084/jem.194.8.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilliet M, Boonstra A, Paturel C, Antonenko S, Xu XL, Trinchieri G, O’Garra A, Liu YJ. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 2002;195:953–958. doi: 10.1084/jem.20020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gordon JN, Green SR, Goggin PM. Cancer cachexia. QJM. 2005;98:779–788. doi: 10.1093/qjmed/hci127. [DOI] [PubMed] [Google Scholar]
- 36.Fanzo JC, Yang W, Jang SY, Gupta S, Chen Q, Siddiq A, Greenberg S, Pernis AB. Loss of IRF-4-binding protein leads to the spontaneous development of systemic autoimmunity. J. Clin. Invest. 2006;116:703–714. doi: 10.1172/JCI24096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J. Clin. Invest. 2007;117:514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamezaki K, Shimoda K, Numata A, Haro T, Kakumitsu H, Yoshie M, Yamamoto M, Takeda K, Matsuda T, Akira S, et al. Roles of Stat3 and ERK in G-CSF signaling. Stem Cells. 2005;23:252–263. doi: 10.1634/stemcells.2004-0173a. [DOI] [PubMed] [Google Scholar]
- 39.Welte T, Zhang SS, Wang T, Zhang Z, Hesslein DG, Yin Z, Kano A, Iwamoto Y, Li E, Craft JE, et al. STAT3 deletion during hematopoiesis causes Crohn’s disease-like pathogenesis and lethality: a critical role of STAT3 in innate immunity. Proc. Natl. Acad. Sci. USA. 2003;100:1879–1884. doi: 10.1073/pnas.0237137100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kadowaki N, Antonenko S, Liu YJ. Distinct CpG DNA and polyinosinic-polycytidylic acid double-stranded RNA, respectively, stimulate CD11c− type 2 dendritic cell precursors and CD11c+ dendritic cells to produce type I IFN. J. Immunol. 2001;166:2291–2295. doi: 10.4049/jimmunol.166.4.2291. [DOI] [PubMed] [Google Scholar]
- 41.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 42.Garrett WS, Lord GM, Punit S, Lugo-Villarino G, Mazmanian SK, Ito S, Glickman JN, Glimcher LH. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131:33–45. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park JM, Greten FR, Li ZW, Karin M. Macrophage apoptosis by anthrax lethal factor through p38 MAP kinase inhibition. Science. 2002;297:2048–2051. doi: 10.1126/science.1073163. [DOI] [PubMed] [Google Scholar]
- 44.Chung YJ, Park BB, Kang YJ, Kim TM, Eaves CJ, Oh IH. Unique effects of Stat3 on the early phase of hematopoietic stem cell regeneration. Blood. 2006;108:1208–1215. doi: 10.1182/blood-2006-01-010199. [DOI] [PubMed] [Google Scholar]
- 45.Götze KS, Keller U, Rose-John S, Peschel C. gp130-stimulating designer cytokine Hyper-interleukin-6 synergizes with murine stroma for long-term survival of primitive human hematopoietic progenitor cells. Exp. Hematol. 2001;29:822–832. doi: 10.1016/s0301-472x(01)00652-x. [DOI] [PubMed] [Google Scholar]
- 46.Bonifazi P, Zelante T, D’Angelo C, De Luca A, Moretti S, Bozza S, Perruccio K, Iannitti RG, Giovannini G, Volpi C, et al. Balancing inflammation and tolerance in vivo through dendritic cells by the commensal Candida albicans. Mucosal Immunol. 2009;2:362–374. doi: 10.1038/mi.2009.17. [DOI] [PubMed] [Google Scholar]
- 47.Sun Y, Chin YE, Weisiger E, Malter C, Tawara I, Toubai T, Gatza E, Mascagni P, Dinarello CA, Reddy P. Cutting edge: Negative regulation of dendritic cells through acetylation of the nonhistone protein STAT-3. J. Immunol. 2009;182:5899–5903. doi: 10.4049/jimmunol.0804388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, Orenstein JM, Smith PD. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J. Clin. Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoentjen F, Sartor RB, Ozaki M, Jobin C. STAT3 regulates NF-κB recruitment to the IL-12p40 promoter in dendritic cells. Blood. 2005;105:689–696. doi: 10.1182/blood-2004-04-1309. [DOI] [PubMed] [Google Scholar]
- 50.Guo K, McMinn JE, Ludwig T, Yu YH, Yang G, Chen L, Loh D, Chua CS, Jr, Zhang Y. Disruption of peripheral leptin signaling mice results in hyperleptinemia without associated metabolic abnormalities. Endocrinology. 2007;148:3987–3997. doi: 10.1210/en.2007-0261. [DOI] [PubMed] [Google Scholar]
- 51.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat. Rev. Immunol. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 52.Corinti S, Albanesi C, la Sala A, Pastore S, Girolomoni G. Regulatory activity of autocrine IL-10 on dendritic cell functions. J. Immunol. 2001;166:4312–4318. doi: 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- 53.Kitamura H, Kamon H, Sawa S, Park SJ, Katunuma N, Ishihara K, Murakami M, Hirano T. IL-6-STAT3 controls intracellular MHC class II αβ dimer level through cathepsin S activity in dendritic cells. Immunity. 2005;23:491–502. doi: 10.1016/j.immuni.2005.09.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.