Echolocation, or biosonar, is a sensory mechanism to locate, range, and identify objects in an environment by emitting calls to the environment and listening to the echoes of those calls that return from the objects [1]. Only microbats and toothed whales have acquired sophisticated echolocation, which is indispensable for their orientation and foraging [1]. Although the bat and whale biosonars originated independently and differ substantially in many aspects [2], we here report the surprising finding that the bottlenose dolphin, a toothed whale, is clustered with microbats in the gene tree constructed using the protein sequences of the hearing gene Prestin. The dramatic parallel evolution of prestin strongly suggests that a common molecular change of the electromotility motor of cochlear outer hair cells (OHCs) underlies the extraordinary high-frequency acoustic sensitivities and selectivities of echolocating bats and whales.
Mammalian prestin is a member of the SLC26 anion-transport family found primarily on the membrane of cochlear OHCs. It is composed of intracellular N and C termini, ~10 transmembrane domains, and multiple intra- and extracellular loops [3]. Prestin provides the electromobility of OHCs that is thought to be responsible for cochlear amplification, an active process that confers sensitivity and frequency selectivity to the mammalian auditory system [4]. To examine the potential role of prestin in echolocation, we compiled all eutherian Prestin sequences that are publicly available. The resulting sequence data of 25 species include 10 (echolocating) microbats and 3 (nonecholocating) megabats, but unfortunately only one toothed whale, for which the Prestin sequence is incomplete. We thus amplified and sequenced all 18 coding exons of Prestin from the genomic DNA of a bottlenose dolphin (Tursiops truncatus) and used this sequence in subsequent analysis.
Using maximum likelihood (ML), maximum parsimony (MP), and neighbor-joining (NJ) methods [5], we reconstructed the prestin protein tree. Surprisingly, all methods group the dolphin within bats (specifically with the green-labeled families of Rhinolophidae and Hipposideridae in Fig. 1A), rather than with cow, its true closest relative represented in our data, and this unexpected grouping has a significant bootstrap support (Fig. 1A). Furthermore, as was recently reported [6], unlike the species tree where microbats are paraphyletic [7] (Fig. 1B), the prestin tree clusters the 10 microbats in exclusion of the three megabats with a moderate bootstrap support, resulting in the misplacement of two purple-labeled microbats (Fig. 1AB). Although the prestin tree also has other differences from the mammalian species tree, none of these differences are statistically supported (Fig. 1A).
Figure 1.
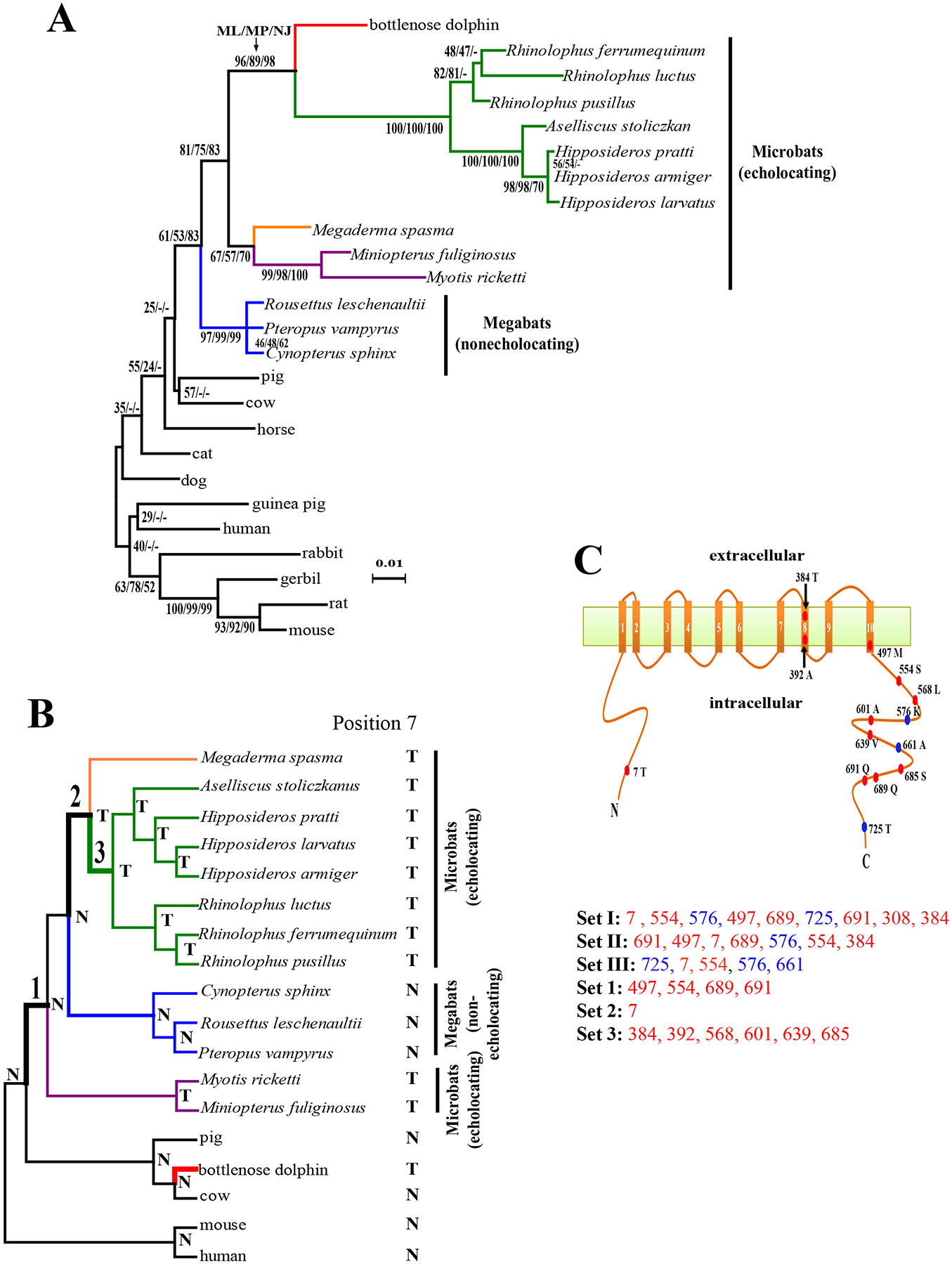
Parallel evolution of prestins of echolocating bats and bottlenose dolphin. (A) The maximum-likelihood (ML) tree reconstructed using the prestin protein sequences of 25 mammals, under the model of JTT-f with a gamma distribution of substitution rate variation among sites (shape parameter =0.14). Numbers on interior branches are bootstrap percentages from ML, maximum-parsimony (MP), and neighbor-joining (NJ) analyses. The bootstrap value from an analysis is indicted as “-” when the branch does not exist in that analysis. The scale bar shows 0.01 amino acid substitution per amino acid site. (B) The species tree of 18 mammals. Tree branches are not drawn to scale. Parallel substitutions were examined between the red branch and the branches labeled 1, 2, and 3. The amino acid (N: Asn; T: Thr) at position 7 of prestin is shown for each interior and exterior node. (C) Locations of evolutionarily interesting amino acid sites in the structural model of prestin with 10 transmembrane domains. Numbers associated with colored circles are the amino acid positions in the dolphin prestin sequence, with the residues observed in dolphin indicated. Sets I, II, and III are the most important sites responsible for the misplacement of both dolphin and the two purple-labeled microbats in panel A, misplacement of dolphin, and misplacement of the two microbats, respectively, and are listed in the order of their support of the gene tree relative to the species tree (from high to low). Sets 1, 2, and 3 are sites that have experienced parallel amino acid substitutions between the dolphin branch (red in panel B) and branches 1, 2, and 3, respectively. Parallel-evolution sites are shown in red, while the other sites are shown in blue.
What could have caused the misplacement of dolphin to the bat clade in the prestin tree? Horizontal gene transfer, DNA contamination, gene paralogy, long-branch attraction, and biased amino acid frequencies are all unlikely (see Supplemental Experimental Procedures and Fig. S1A). The only remaining reason is the convergence of the prestin sequences of echolocating bats and whales, likely resulting from a common selection for amino-acid-altering mutations that are beneficial to echolocation. Indeed, the same misplacement of dolphin is observed in the Prestin tree reconstructed with only nonsynonymous nucleotide substitutions (Fig. S1B); but, when only synonymous substitutions are used, dolphin and cow are correctly grouped with 100% bootstrap support (Fig. S1C).
We used two approaches to identify the amino acid sites causing the clustering of dolphin and bat prestins. To avoid confounding factors, we analyzed a subset of 18 species that includes only cetartiodactyls and bats, with human and mouse as outgroups (Fig. 1B). The prestin protein tree again places dolphin within bats and groups all echolocating bats (Fig. S2). We found that a minimum of 9 amino acid sites (referred to as set I in Fig. 1C) need to be removed to make the likelihood of the species tree higher than that of the prestin tree (see Supplementary Experimental Procedures). Because the prestin tree misplaces both dolphin and two purple-labeled microbats (Fig. 1A), we further examined whether the two problems are caused by the same sites. By comparing the prestin tree without the two misplaced microbats and the corresponding species tree, we found that a minimum of 7 sites (referred to as set II in Fig. 1C) need to be removed to correct the phylogenetic position of dolphin. By comparing the prestin tree without dolphin and the corresponding species tree, we identified a minimum of 5 sites (referred to as set III in Fig. 1C) that need to be removed to rectify the positions of the two microbats. Sets II and III share 3 sites, significantly exceeding the random expectation (P<0.002, binomial test under the consideration that only variable sites may be included in any above set), suggesting that the misplacements of the dolphin and the two microbats are in large part due to the same sites.
The second approach we used is to identify convergent or parallel amino acid substitutions [8] that occurred between the dolphin branch (red in Fig. 1B) and any of the three bat branches marked 1, 2, and 3 in the established species tree in Fig. 1B, by comparing the inferred ancestral prestin sequences in all interior nodes of the species tree and the extant sequences. We considered these three bat branches because prestin function associated with echolocation in bats (especially Rhinolophidae and Hipposideridae) likely have emerged in one or more of these branches. We identified no convergent site, but 4, 1, and 6 parallel sites between the red branch and branches 1, 2, and 3, respectively, and named these three sets of sites as set 1, 2, and 3, respectively (Fig. 1C). A statistical test [8] shows that sets 1, 2, and 3 contain significantly more parallel sites than their respective random expectations (P <10−8, <0.0045, and <10−8, respectively), suggesting that a common selection, rather than chance, underlies the observed parallel substitutions. The total number of parallel substitutions observed here is the largest in all proteins reported to have undergone parallel sequence evolution [8-10]. Six of the 7 sites in set II, which cause the misplacement of dolphin, experienced parallel changes (Fig. 1C), supporting our hypothesis that parallel amino acid substitutions is the reason for the grouping of dolphin and bats in the prestin tree.
After mapping the sites of sets I-III and sets 1-3 onto the structural model of prestin [3] (Fig. 1C), we observed that all except three sites fall in the intracellular terminal regions, including one site in the N-terminus and 10 in the C-terminus, a pattern that is highly nonrandom (P<0.005, Fisher’s exact test). Previous mutagenesis studies demonstrated that both N- and C-termini are used for voltage sensing [3] and the N-terminus is also critical for homo-oligomerization of prestin [3], which may influence the speed of conformational changes of prestin that is likely crucial for high-frequency acoustic sensitivities of echolocation. Although not all parallel changes identified may be necessary for echolocation, as some have reverted in some microbats or occurred also in some nonecholocating mammals, they are strong candidates for future experimental investigation. Of particular interest is position 7, which appears in sets I-III and experienced a parallel change from Asn to Thr in three branches (Fig. 1B). This site has a Thr in all echolocating mammals but an Asn in all non-echolocating mammals examined so far. We were able to amplify exon 1 of Prestin from the bowhead whale Balaena mysticetus, a nonecholocating whale, and determined that it has an Asn at position 7. Thus, the multiple Asn to Thr changes at position 7 were likely important for the multiple origins of echolocation. Sequencing Prestin from additional echolocating and nonecholocating cetaceans will further help identify the amino acid changes critical for echolocation.
Bats and whales vary greatly in echolocation [2]. For example, bats use echolocation for ranges up to 3-4 meters, whereas whales use for ranges up to >100 meters [2]. More importantly, the speed of sound in air is about one fifth that in water, making the information transfer during sonar transmission much slower for bats than for whales [2]. Despite these gross differences, our findings suggest that the high-frequency acoustic sensitivities and selectivities of bat and whale echolocation appear to rely on a common molecular design of prestin. Because prestin function can be studied in knock-in mice and in cell lines [4], a functional analysis of the parallel amino acid substitutions identified here could shed light on the structure-function relationship of prestin and the molecular underpinnings of the acoustic adaptations in echolocation. It could also help answer why the prestin of bottlenose dolphin is particularly similar to that of Rhinolophidae and Hipposideridae bats (Fig. 1A).
Supplementary Material
Acknowledgements
We thank Meg Bakewell for valuable comments. This work was supported by research grants from US National Institutes of Health to J.Z. and a start-up fund of the Hundreds-Talent Program from Chinese Academy of Sciences to P.S. Y.L. was supported by the visiting scholarship of the Chinese Academy of Sciences and the National Natural Science Foundation of China (grant No. 30600066).
References
- 1.Jones G. (2005). Echolocation. Curr Biol 15, R484–488. [DOI] [PubMed] [Google Scholar]
- 2.Au WWL (2004). A comparison of the sonar capabilities of bats and dolphins. In Echolocation in Bats and Dolphins, Thomas JA, Moss CF and Vater M, eds. (Chicago: The University of Chicago Press; ), pp. xiii–xxvii. [Google Scholar]
- 3.Navaratnam D, Bai JP, Samaranayake H, and Santos-Sacchi J (2005). N-terminal-mediated homomultimerization of prestin, the outer hair cell motor protein. Biophys J 89, 3345–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dallos P. (2008). Cochlear amplification, outer hair cells and prestin. Curr Opin Neurobiol 18, 370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nei M, and Kumar S (2000). Molecular Evolution and Phylogenetics, (New York: Oxford University Press; ). [Google Scholar]
- 6.Li G, Wang J, Rossiter SJ, Jones G, Cotton JA, and Zhang S (2008). The hearing gene Prestin reunites echolocating bats. Proc Natl Acad Sci USA 105, 13959–13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones G, and Teeling EC (2006). The evolution of echolocation in bats. Trends Ecol Evol 21, 149–156. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, and Kumar S (1997). Detection of convergent and parallel evolution at the amino acid sequence level. Mol Biol Evol 14, 527–536. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J. (2006). Parallel adaptive origins of digestive RNases in Asian and African leaf monkeys. Nat Genet 38, 819–823. [DOI] [PubMed] [Google Scholar]
- 10.Castoe TA, de Koning AP, Kim HM, Gu W, Noonan BP, Naylor G, Jiang ZJ, Parkinson CL, and Pollock DD (2009). Evidence for an ancient adaptive episode of convergent molecular evolution. Proc Natl Acad Sci USA 106, 8986–8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


