Summary
Ano-genital distance (AGD) is a sexually dimorphic trait that is a well established reproductive toxicity endpoint in animals. In male animals, a shortened AGD is associated with a variety of genital abnormalities including hypospadias and cryptorchidism. Consensus on the anatomical definition of AGD in humans remains to be established and few data exist on the determinants and normal variance in the general population. We implemented a standardized anthropometric protocol to measure AGD, ano-scrotal distance (ASD), and ano-fourchette distance (AFD) in 169 (82 male, 87 female) infants in the University of Washington newborn nursery in 2008. We collected data on the following characteristics: weight, length, and occipital head circumference, race and relevant gestational complications. Using linear regression modelling, we examined AGD⁄ASD⁄AFD for sexual dimorphism, normal population variance and predictors of the measurement in infants. The mean male and female AGD measurements were 52.0 mm (SD ± 5.5) and 37.2 mm (SD ± 3.7). The mean ASD and AFD were 23.0 mm (SD ± 3.8) and 15.1 mm (SD ± 2.9). Weight, length, occipital head circumference and gestational age were associated with AGD (p < 0.05). Weight and length were the most important correlates to AGD. We confirmed previous findings that AGD is a sexually dimorphic measurement that is most strongly predicted by infant weight. The application of this measurement to clinically relevant outcomes remains to be explored in further depth.
Keywords: androgen, ano-genital distance, male reproductive development
Introduction
Ano-genital distance (AGD) is a sexually dimorphic anatomic landmark that develops in response to hormone signalling and end-organ response during foetal life (Bowman et al., 2003; Marty et al., 2003). In animal studies, it is associated with male reproductive tract abnormalities and is an important reproductive toxicity endpoint (Gray et al., 1999). While AGD measurements have been preliminarily examined and applied to clinical outcomes in humans, studies have been limited in number and comprised of racially homogenous populations (Salazar-Martinez et al., 2004; Thankamony et al., 2009). In addition, the definition of AGD differs depending on the study. A recent international panel of experts stated that AGD is an important clinical measure to address endocrine-sensitive endpoints in the first year of life and recommended more in depth examination of the measurement in humans (Arbuckle et al., 2008).
In animal studies, changes in androgen action during the foetal period can lead to genital defects and shortened AGD in offspring (Mylchreest et al., 1998; Gray et al., 2001). A shortened AGD in rats is associated with various reproductive tract abnormalities including hypospadias, abnormal testicular descent and decreased testicular volume (Gray et al., 2001). Exposure to endocrine disruptors, synthetic and natural chemicals known to interfere with hormone action, can impact AGD development, which may be a predictor of other adverse reproductive outcomes (Gray et al., 2001). The Environmental Protection Agency has designated AGD as a reproductive toxicity endpoint in animal studies, from which data are used for human health risk assessments of endocrine disrupting chemicals.
Though AGD measurements are routine and established biomarkers in animals, few studies have evaluated these measurements in male and female newborn human infants (Bongiovanni & Root, 1963; Callegari et al., 1987; Salazar-Martinez et al., 2004; Longnecker et al., 2007; Romano-Riquer et al., 2007; Thankamony et al., 2009). Some researchers define AGD as the distance from the anus to the base of the penis in males or clitoral hood in females, while others measure the distance from the mid-anus to the base of the scrotum in males and from the mid-anus to the base of the posterior fourchette in females. As a potentially valuable biomarker of foetal androgen exposure and action, AGD could be a useful clinical measurement if the definition is standardized, distribution in the general population is established and the determinants are identified. We report AGD measurements and correlates in a racially and ethnically diverse population of male and female newborn infants in Washington state.
Methods
Subjects
We recruited 169 healthy infants from the University of Washington (UW) Medical Center newborn nursery in the first 1–3 days of the post-partum period from May through August of 2008. Any healthy newborn infant whose mother was English speaking was eligible for the study. Infants who were admitted to the neonatal intensive care unit were eligible if they transitioned into the newborn nursery before being discharged from the hospital. Exclusion criteria included a documented positive exam finding of hypospadias, cryptorchidism, chordee, hydrocele, imperforate anus or any known syndrome or genital anomaly. Of the families approached for the study, 95% agreed to participate.
Standard anthropometric measurements were recorded from the patient’s chart including birth weight, occipitofrontal head circumference (OFC) and length. Infant race was documented based on parental response to a standardized question administered by the trained study provider. All study methods, procedures and modifications were approved by the UW Human Subjects Board.
AGD measurement technique
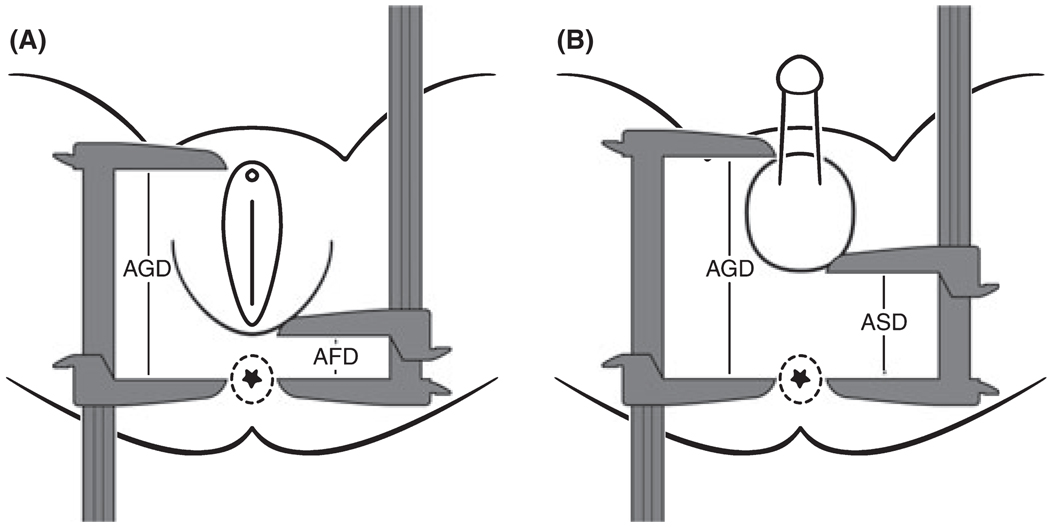
We defined AGD as the distance between the anus to the anterior base of the penis (males) or the clitoral hood (females). This definition is similar to that used by Swan (2008) in the Study for Future Families, a study that examined environmental exposures and health outcomes, as well as Hsieh et al. (2008) in a case–control study of ano-scrotal distance (ASD) in males with genital abnormalities compared to controls (Swan et al., 2005; Hsieh et al., 2008). In this study, we also report other sexually dimorphic measures: (i) distance from the anus to the base of the scrotum (ASD) in males and (ii) distance from the anus to the posterior fourchette (AFD) in females (see Fig. 1). ASD and AFD measures are reported by Romano-Riquer and Salazar-Martinez in studies of infants in Mexico (Romano-Riquer et al., 2007; Salazar-Martinez et al., 2004).
Figure 1.
An-ogenital distance⁄ano-scrotal distance⁄ano-fourchette distance diagrams. (A) Center of the anus to the anterior clitoral surface (AGD) and the center of the anus to the posterior fourchette (AFD) measurements made in female subjects. (B) Center on the anus to the anterior base of the penis (AGD) and the center of the anus to the junction of the perineum with the rugated scrotal skin (ASD) measurements made in male subjects.
Prior to recruiting subjects, we conducted a standardized training session in which five health-care providers learnt how to conduct AGD measurements. For this session, two males and three females aged 2 weeks–7 months were recruited for the training session from the UW medical center. Providers standardized both technique of infant positioning and AGD measurement landmarks by reviewing a presentation on measurement techniques together. The providers measured each child separately and were blinded as to the previously recorded measurements of the other providers. The results were then evaluated for inter-rater and intra-rater reliability. Inter-rater reliability for AGD measurement in infants was 54% for females and 89% for males and intra-rater reliabilities were 81% for females and 96% males. Inter-rater reliabilities for AFD and ASD were 18% for females and 82% for males respectively. Intra-rater reliabilities for AFD and ASD measurements were 76% for females and 91% for males respectively. Given that this was a standardized training, we expected poor-to-fair reliability values because observers were learning how to position infants and conduct measurements. In addition, we had two female infants for the training, and one was very fussy and therefore difficult to measure. Conducting a post-study session to re-examine reliability values would likely show that reliabilities improve after training and conducting many measurements, but was not feasible in this study.
We used the same measurement protocol for the training and subsequent study. One trained medical provider, LB, consented parents inside their post-partum hospital room. Every effort was made to conduct measurements at the mother’s bedside. If this was not possible, measurements were conducted in the progressive care nursery (an area where infants are watched by nurses while parents attend to other activities). Infants were positioned in the dorsal decubitus position on a flat surface. Infant engagement and distraction by the assistant were important to create the opportunity for consistent measurement. The provider positioned the infants’ legs at a 90 degree angle at the hip with the infant’s hips relaxed out laterally and an assistant was then instructed to maintain this position. The assistant held the infant firmly ensuring minimal movement and all infants were measured while still and relatively calm. The provider then positioned herself in front of the infant. Measurements were made with the provider holding the calliper in a sagittal plane with her right hand and the handle of the calliper pointing up and tilted towards the infant’s head. The measuring surfaces were immediately adjacent to the landmarks being measured and the fixed measuring edge of the calliper was placed at the centre of the anus while the movable end was slid to the appropriate anatomical landmark. Very minimal pressure was applied to the landmarks in an attempt to reduce variation and measurement error. The first measurement was made from the centre of the anus to the anterior base of the penis in males (Fig. 1B) and from the centre of the anus to the anterior surface of the clitoris in females (Fig. 1A). The second measurement was made from the centre of the anus to the posterior scrotum (ASD) in males (Fig. 1B), using the junction of the perineum with the rugated scrotal skin as a marker, and from the centre of the anus to the posterior fourchette (AFD) in females (Fig. 1A). This was done three times consecutively with the digital face of the calliper facing away from the provider during the measurement to avoid measurement bias. With the exception of two infants, all subjects were measured three times for AGD⁄ASD⁄AFD. Measurements were made using Dura-tool Digital vernier callipers (2008).
Statistical analysis
We calculated the intra-rater reliability and inter-rater reliability, as estimated by the proportion of contributing variance components to the total variance, for AGD measurements in the standardized training and intra-rater reliability for the primary study, using mixed effects models. We examined summary statistics for genital distance measures as well as gestational age, OFC, length, weight, and race. We conducted univariate and multivariate regression analysis of the correlates of AGD⁄ASD⁄AFD. Because weight, length, and gestational age were all highly correlated with one another and could not be placed into one model together, we created three different multivariate models to assess the most important correlate of AGD: (i) weight as a predictor of AGD adjusted for gestational age, (ii) length as a predictor of AGD adjusted for gestational age and (iii) gestational age as a predictor of AGD adjusted for weight. We used the statistical software sas proc 9.1.3 (Cary, NC, USA) mixed for inter and intra-rater reliabilities and stata version 9.0 (College Station, TX, USA) for all other statistical analyses.
Results
Subjects recruited into the study were mostly singleton pregnancies with the exception of five infants. Table 1 shows the racial and anthropometric characteristics of the 169 infants measured for the study. There were an approximately equal numbers of male and female subjects recruited, boys (n = 82) and girls (n = 87). Half of the subjects were Caucasian (n = 86). About 15% (n = 26) of mothers identified their infants as African-American, while an additional 15% (n = 26) of infants were classified as mixed race. All other racial groups combined accounted for less than 20% of the study population.
Table 1.
Characteristics of 169 healthy newborn infants, University of Washington Medical Center, 2008*
| Infant | N | % |
|---|---|---|
| Gender | ||
| Male | 82 | 49 |
| Female | 87 | 51 |
| Race | ||
| Caucasian | 86 | 51 |
| African-American | 26 | 15 |
| Native American | 5 | 3 |
| Asian⁄Pacific Islander | 15 | 9 |
| Hispanic | 11 | 6 |
| Mixed race | 25 | 15 |
| Length | ||
| 41 – <48 cm | 23 | 14 |
| 48 – <53 cm | 102 | 60 |
| 53–56 cm | 44 | 26 |
| Weight | ||
| 1500 – <2500 g | 19 | 11 |
| 2500 – <4000 g | 129 | 76 |
| 4000 – <4800 g | 21 | 12 |
| Occipito-frontal head circumference | ||
| 30 – <34 cm | 76 | 45 |
| 34 – <36 cm | 71 | 42 |
| 36–39 cm | 22 | 13 |
| Gestational age | ||
| 34 – <38 weeks | 41 | 24 |
| 38–42 weeks | 128 | 76 |
Missing data not shown.
The intra-rater reliability for AGD measurements in all 169 infants were 96% for males and 92% for females. The intra-rater reliabilities for ASD and AFD in all 169 infants were 94% and 91% respectively. The mean male AGD was 52.0 mm (SD ± 5.5) and mean female AGD was 37.2 mm (SD ± 3.7). The mean male ASD was 23.0 (SD ± 3.8), and mean female AFD was 15.1 (SD ± 2.9) (Table 2). Infant weight, length, head circumference and gestational age were statistically significant correlates of one or more of AGD⁄AFD⁄ASD in univariate analyses (Table 3). Asians and Native Americans had consistently smaller AGD⁄ASD⁄AFD measurements as compared to Caucasians, but the number of subjects was small and confidence intervals were wide.
Table 2.
Distribution of AGD⁄ASD⁄AFD for 169 healthy newborn infants, University of Washington Medical Center, 2008
| Male AGD (mm) |
Female AGD (mm) |
Male ASD (mm) |
Female AFD (mm) |
|
|---|---|---|---|---|
| 10% | 45 | 33 | 19 | 12 |
| 25% | 48 | 34 | 20 | 13 |
| 50% (median) | 51 | 37 | 23 | 15 |
| 75% | 56 | 39 | 26 | 17 |
| 90% | 59 | 43 | 28 | 19 |
| Mean | 52 | 37 | 23 | 15 |
| SD (mean) | 6 | 4 | 4 | 3 |
AGD, ano-genital distance; ASD, ano-scrotal distance; AFD, ano-four-chette distance.
Table 3.
Regression coefficients for associations between individual demographic and anthropometric factors and ano-genital distance among 169 healthy newborn infants, University of Washington Medical Center, 2008
| AGD (mm) | ASD (mm) | AFD (mm) | ||||
|---|---|---|---|---|---|---|
| Characteristics | Coefficients | 95% CI | Coefficients | 95% CI | Coefficients | 95% CI |
| Infant Gender | ||||||
| Male | Referent | N⁄A | N⁄A | |||
| Female | −14.7 | −16.1, −13.3 | N⁄A | N⁄A | ||
| Gestational age (weeks) | 0.8 | 0.1, 1.5 | 0.5 | 0.0, 0.9 | 0.4 | 0.1, 0.7 |
| Weight (kg) | 5.0 | 3.0, 7.1 | 2.3 | 1.0, 3.5 | 2.2 | 1.2, 3.2 |
| Length (cm) | 1.1 | 0.7, 1.5 | 0.4 | 0.1, 0.7 | 0.3 | 0.1, 0.5 |
| Occipito-frontal head circumference (cm) | 1.3 | 0.6, 2.0 | 0.4 | −0.05, 0.9 | 0.5 | 0.1, 0.8 |
| Race | ||||||
| Caucasian | Referent | Referent | Referent | |||
| African-American | 0.4 | −3.5, 4.3 | 1.0 | −1.4, 3.3 | 1.5 | −0.3, 3.3 |
| Latino | 0.4 | −5.2, 5.9 | 1.6 | −2.0, 5.2 | −1.1 | −4.2, 1.3 |
| Asian | −3.1 | −8.0, 1.7 | −3.0 | −6.6, 0.6 | −1.5 | −3.2, 0.9 |
| Native American | −4.7 | −13.6, 4.2 | −0.2 | −5.8, 5.3 | −1.8 | −6.0, 2.3 |
| Other | −0.8 | −4.7, 3.2 | −0.7 | −3.2, 1.9 | −0.3 | −2.0, 1.5 |
In multivariate analyses, weight and length were the strongest correlates of male AGD, female AGD, ASD and AFD (Table 4). Model fit, as determine by R2, values were similar for all models within a specific measurement category. Confidence intervals were narrow for both weight and length.
Table 4.
Multivariate regression coefficients for correlates of AGD⁄ASD⁄AFD among 169 healthy newborn infants, University of Washington Medical Center, 2008
| Male AGD (mm) | Female AGD (mm) | ASD (mm) | AFD (mm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Coefficients | 95% CI | R2 | Coefficients | 95% CI | R2 | Coefficients | 95% CI | R2 | Coefficients | 95% CI | R2 |
| Gestational age (weeks) |
0.5a | −0.2, 1.2 | 0.4 | 0.03a | −0.4, 0.5 | 0.3 | −0.1a | −0.7, 0.5 | 0.1 | −0.02a | −0.4, 0.4 | 0.2 |
| Weight (kg) | 4.7b | 2.8, 6.6 | 0.4 | 3.7b | 2.1, 5.2 | 0.3 | 2.5b | 0.9, 4.1 | 0.1 | 2.2b | 0.9, 3.5 | 0.2 |
| Length (cm) | 0.8b | 0.4, 1.3 | 0.4 | 0.6b | 0.3, 0.9 | 0.3 | 0.4b | 0.04, 0.8 | 0.1 | 0.2b | −0.03, 0.5 | 0.1 |
Multivariate adjusted regression coefficient adjusted for infant weight.
Multivariate adjusted regression coefficient adjusted for gestational age.
Discussion
We found that AGD⁄ASD⁄AFD can be consistently learnt and performed measurements with good reproducibility within provider. AGD was sexually dimorphic in newborn infants, and intra-rater reliability of the measurement was very good for both males and females. Anthropometric factors were strongly associated with genital distances, with weight and length having the strongest associations.
Ano-genital distance is an important reproductive toxicity endpoint that reflects the degree of in utero androgenization in newborns in animal studies (Gray et al., 1999). It is associated with a variety of male reproductive disorders and predictive of future fertility outcomes in animals (Foster, 2006). The clinical significance of AGD as a biomarker of reproductive disease and potential target for endocrine disrupting action in humans remains to be elucidated. Studies published by Bongiovanni & Root (1963) document ano-genital measurements in humans with endocrine disorders such as adrenal cortical hyperplasia and congenital adrenal hyperplasia (Bongiovanni & Root, 1963). In cases of female infants exposed to increased androgens in utero, they found masculinization of the female foetus including clitoral hypertrophy and increased labial fusion (which is similar to AGD) (Goldman & Bongiovanni, 1967). For males, decreased exposure to in utero androgens resulted in decreased AGD and severe hypospadias (Goldman & Bongiovanni, 1967).
Recent human studies examining the impact of endocrine disrupting chemicals on foetal AGD development are conflicting. Swan et al. (2005) reported that increased third trimester pre-natal phthalate (known anti-androgenic chemicals) concentrations were associated with a shorter AGD in male infants (Swan, 2008). Longnecker et al. (2007) reported no association between prenatal dichlorodiphenyldichloroethylene (DDE – chemical that is known to have estrogenic and anti-androgenic properties) concentrations and AGD (Longnecker et al., 2007). Hsieh et al. (2008) found that AGD was shorter in older, anaesthetized children with male reproductive tract disorders as compared to healthy controls (Hsieh et al., 2008). These are the first data to support that AGD may be a clinical marker of reproductive disease in humans as it is in rodents; more data are needed to support these hypotheses in humans.
Initial studies not only indicate AGD may be a more reliable measurement than previously used markers such as penile dimension but also note the lack of standardization and need for further evaluation of measurement technique and variability (Romano-Riquer et al., 2007; Thankamony et al., 2009). Three studies including this one have measured ASD⁄AFD in newborn infants with a mean ASD range of 20–23 mm and mean AFD range of 9–15 mm (Table 5). The Mexican and European Caucasian cohorts only conducted ASD⁄AFD measures and not AGD as we define it in this publication. Their measurements were made using a similar protocol for ASD⁄AFD as described in the methods section. The variation of ASD⁄AFD between studies may be attributable to racial⁄ethnic variation or measurement error. There may also be regional⁄geographical variation because of differing environmental factors that could be contributing to genital development. Methods of measurement should be clearly defined for all measurements and standardized between research centres to compare data accurately. In addition, inter-rater and intra-rater reliabilities should be examined during the pre-study training sessions and post-study assessments to determine if they improve over time.
Table 5.
Ano-scrotal distance (ASD) (males) and ano-fourchette distance (AFD) (females) measurements in three different newborn studies
| ASD (mm) | AFD (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| Study population | N | Mean | SD | Median | N | Mean | SD | Median |
| Morelos, Mexico (Salazar-Martinez et al., 2004) | 45 | 21 | 3 | 22 | 42 | 11 | 2 | 11 |
| Washington state, USA | 82 | 23 | 4 | 23 | 87 | 15 | 3 | 15 |
| Cambridge, England (Thankamony et al., 2009) | 285 | 20 | 6 | – | 279 | 9 | 3 | – |
Birth weight and length were the strongest correlates of AGD⁄ASD⁄AFD in our population, based on the width of the confidence intervals and the magnitude of the coefficient estimates. These results are similar to those found in Mexican and European Caucasian populations (Salazar-Martinez et al., 2004; Thankamony et al., 2009). We found that AGD was smaller in Asian, Native American and mixed race infants as compared to Caucasian infants, but the size of several racial groups was very limited and therefore results were not statistically significant. This may be partially explained by the increased incidence of pre-term birth and small for gestational age in certain minority groups. We could not control for these factors in analysis given that we had small sample numbers of Native American and mixed race groups. These observations should be confirmed by future studies with a larger sample size. Additionally, as only English speaking mothers were recruited into the study, there may have been selection bias that contributed to the racial characteristics of the study population. For example, English speaking Asian mothers might have infants with longer⁄shorter AGD as compared with non-English speaking Asian mothers.
Ano-genital distance landmarks on infants rely on soft tissue points of measurement, thus making measurement errors an important concern. One advantage to this study is that the same examiner measured all infants thereby eliminating inter-observer variation. Positioning and pressure variation while making AGD measurements may distort the results and affect both inter-rater and intra-rater reliability. Additionally, landmarks in newborn infants are not always clearly demarcated, particularly in female subjects. The clitoral hood can be difficult to identify precisely and the entire genital region may be significantly swollen following delivery. Using the centre of the anus as a landmark also requires visual estimation, which is another source of potential error. Finally, while most infants were intentionally measured while calm and held in a still position by an assistant for the duration of the measurement, the effect of infant resistance and movement may also provide a source of error.
Our study indicates that AGD is a non-invasive measurement that can be performed in newborn infants with proper training and reliability assessment. It is a sexually dimorphic marker probably reflecting the differences in hormone concentrations in male and female infants during foetal life. Further research on population distribution, especially examining large and diverse racial groups, is needed to determine more fully the precise nature and normal variation of AGD in humans. The National Children’s Study presents an opportunity to understand AGD among a large representative sample of births in the United States. If measurements can be standardized and normative values established, AGD has the potential to be a clinical marker of reduced androgenization in foetal life, abnormal development of reproductive organs and future fertility.
Acknowledgements
We thank the UW Medical Student Research Training Programme, UW K-12 Male Reproductive Health Research Training Grant and the UW Pediatric Environmental Health Specialty Unit which supported Lauren Beard to participate in this project. We also thank all of the study participants and newborn nursery staff at the UW Medical Center.
Panel discussion
Christine Wohlfahrt Veje (Copenhagen, Denmark)
Have you any data from older children?
Sheela Sathyanarayana
We have a little experience with older children but they are difficult to measure. Newborn babies are the easiest population to measure because they eat and sleep, and they have an easily manoevered pliable anatomy after feeding. Older children are very difficult to control because they are not easily distracted in order to get them into the right position to measure. Over the age of 15 months there is usually a period of distraction no longer than 6 seconds in which to make the measurements. We also did measurements in some teenagers which was much easier because they could understand what we were doing.
Ieuan Hughes (Cambridge, UK)
I must voice concern about the methodology you use to measure the AGD, and we must be clear if we are comparing like with like from different institutions. It is difficult to define the base of the penis for measurement of AGD. Your clinical picture showing the calipers in position during measurement is confusing and suggests that it is difficult to be accurate and consistent. There is a suprapubic fat pad in some infants masking the dorsal base of the penis, and in other infants the scrotal skin has a shawl effect extending to, and masking, the ventral base of the penis. In our studies, we measure from mid anus to mid scrotum – the anoscrotal distance (ASD). This gives a well defined measurement and a consistent ratio of 2:1 between ASD in boys and anofourchette distance (AFD) in girls. This is in agreement with the Mexican study which described the ASD. This is a more consistent and reliable measurement, and is probably a better measurement for comparative studies where consistency is required amongst different studies from different centres. Uniform methodology must be agreed before embarking on large scale clinical studies.
Sheela Sathyanarayana
I agreed that there are inconsistencies in AGD measurements, but there is only one paper using ASD and AFD measurements while all other papers use AGD. We have tried measuring ASD and AFD in comparison to AGD but there was no greater reliability. As a collaboration, we should all probably do all of these measurements. I am presently collaborating with other institutions to produce a paper outlining standardization of AGD measurements.
References
- Arbuckle TE, Hauser R, Swan SH, Mao CS, Longnecker MP, Main KM, et al. Meeting report: measuring endocrine-sensitive endpoints within the first years of life. Environ Health Perspect. 2008;116:948–951. doi: 10.1289/ehp.11226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongiovanni AM, Root AW. The adrenogenital syndrome. N Engl J Med. 1963;268:1391–1399. doi: 10.1056/NEJM196306202682505. [DOI] [PubMed] [Google Scholar]
- Bowman CJ, Barlow NJ, Turner KJ, Wallace DG, Foster PM. Effects of in utero exposure to finasteride on androgen-dependent reproductive development in the male rat. Toxicol Sci. 2003;74:393–406. doi: 10.1093/toxsci/kfg128. [DOI] [PubMed] [Google Scholar]
- Callegari C, Everett S, Ross M, Brasel JA. Anogenital ratio: measure of fetal virilization in premature and full-term newborn infants. J Pediatr. 1987;111:240–243. doi: 10.1016/s0022-3476(87)80075-6. [DOI] [PubMed] [Google Scholar]
- Foster PM. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int J Androl. 2006;29:140–147. doi: 10.1111/j.1365-2605.2005.00563.x. [DOI] [PubMed] [Google Scholar]
- Goldman AS, Bongiovanni AM. Induced genital anomalies. Ann N Y Acad Sci. 1967;142:755–767. doi: 10.1111/j.1749-6632.1967.tb14686.x. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Wolf C, Lambright C, Mann P, Price M, Cooper RL, Ostby J. Administration of potentially antiandrogenic pesticides (procymidone, linuron, iprodione, chlozolinate, p,p’-dde, and ketoconazole) and toxic substances (dibutyl- and diethylhexyl phthalate, pcb 169, and ethane dimethane sulphonate) during sexual differentiation produces diverse profiles of reproductive malformations in the male rat. Toxicol Ind Health. 1999;15:94–118. doi: 10.1177/074823379901500109. [DOI] [PubMed] [Google Scholar]
- Gray LE, Ostby J, Furr J, Wolf CJ, Lambright C, Parks L, et al. Effects of environmental antiandrogens on reproductive development in experimental animals. Hum Reprod Update. 2001;7:248–264. doi: 10.1093/humupd/7.3.248. [DOI] [PubMed] [Google Scholar]
- Hsieh MH, Breyer BN, Eisenberg ML, Baskin LS. Associations among hypospadias, cryptorchidism, anogenital distance, and endocrine disruption. Curr Urol Rep. 2008;9:137–142. doi: 10.1007/s11934-008-0025-0. [DOI] [PubMed] [Google Scholar]
- Longnecker MP, Gladen BC, Cupul-Uicab LA, Romano-Riquer SP, Weber JP, Chapin RE, Hernandez-Avila M. In utero exposure to the antiandrogen 1,1-dichloro-2,2-bis(p-chlorophe-nyl)ethylene (dde) in relation to anogenital distance in male newborns from chiapas, mexico. Am J Epidemiol. 2007;165:1015–1022. doi: 10.1093/aje/kwk109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty MS, Chapin RE, Parks LG, Thorsrud BA. Development and maturation of the male reproductive system. Birth Defects Res B Dev Reprod Toxicol. 2003;68:125–136. doi: 10.1002/bdrb.10015. [DOI] [PubMed] [Google Scholar]
- Mylchreest E, Cattley RC, Foster PM. Male reproductive tract malformations in rats following gestational and lactational exposure to di(n-butyl) phthalate: an antiandrogenic mechanism? Toxicol Sci. 1998;43:47–60. doi: 10.1006/toxs.1998.2436. [DOI] [PubMed] [Google Scholar]
- Romano-Riquer SP, Hernandez-Avila M, Gladen BC, Cupul-Uicab LA, Longnecker MP. Reliability and determinants of anogenital distance and penis dimensions in male new-borns from chiapas, mexico. Paediatr Perinat Epidemiol. 2007;21:219–228. doi: 10.1111/j.1365-3016.2007.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Martinez E, Romano-Riquer P, Yanez-Marquez E, Longnecker MP, Hernandez-Avila M. Anogenital distance in human male and female newborns: a descriptive, cross-sectional study. Environ Health. 2004;3:8. doi: 10.1186/1476-069X-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res. 2008;108:177–184. doi: 10.1016/j.envres.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113:1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thankamony A, Ong K, Dunger DB, Acerini CL, Hughes IA. Anogenital distance from birth to two years: a population study. Environ Health Perspect. 2009;117:1786–1790. doi: 10.1289/ehp.0900881. [DOI] [PMC free article] [PubMed] [Google Scholar]