Abstract
Glial cell line-derived trophic factor (GDNF) is a peptide with pleiotropic survival and growth promoting effects on neurons. We found that intraspinal injection of a non-replicating herpes simplex virus (HSV)-based vector coding for GDNF 2 hours after blunt trauma to the thoraco-lumbar spinal cord produced sustained improvement in motor behavioral outcomes up to 5 weeks following injury. The improvement in behavior correlated with an increase in synaptophysin and glutamic acid decarboxylase (GAD) in the spinal cord at the level of injury. Addition of recombinant GDNF protein to primary spinal cord neurons in-vitro resulted in enhanced neurite growth and a marked increase in protein levels of GAD65 and GAD67, synapsin I and synaptophysin. GDNF-mediated increases in GAD and the synaptic markers were blocked by the MEK inhibitor UO126, but not by the PI3K inhibitor LY294002. These results suggest that GDNF, acting through the MEK-ERK pathway enhances axonal sprouting, synaptic connectivity, and GABAergic neurotransmission in the spinal cord, that result in improved behavioral outcomes after spinal cord contusion injury.
Keywords: Glial cell line-derived neurotrophic factor, gamma amino butyric acid, spinal cord injury, extracellular signal related kinase, herpes simplex virus, gene therapy
Introduction
Glial-derived neurotrophic factor (GDNF) is a pleiotropic peptide that provides trophic cues to midbrain dopaminergic neurons and spinal motor neurons in the central nervous system (CNS), and sensory and autonomic neurons in the peripheral nervous system during development (Airaksinen and Saarma 2002). In addition to its effects during development, GDNF has been shown to promote the survival of neurons in the face of a variety of cellular insults (Sauer et al. 1995; Choi Lundberg et al. 1997; Ramer et al. 2000; Akerud et al. 2001; Natsume et al. 2002; Ducray et al. 2006). GDNF signals through a receptor complex consisting of glycosyl-phospatidylinositol (GPI)-anchored co-receptor (GFRα) and RET receptor tyrosine kinase to activate phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) pathways to enhance cell survival, lamelopodia formation and axonal elongation (Takahashi 2001; Airaksinen and Saarma 2002). Through additional interactions with adhesion proteins, NCAM and integrins, GDNF promotes plasticity and facilitiates synapse formation (Cao et al. 2008; Paratcha and Ledda 2008).
The potential utility of GDNF to support axonal regeneration in the CNS has been reported in a number of studies. Extension of sensory axons after dorsal root crush and sprouting of uninjured motor axons after unilateral corticospinal tract (CST) transection in the hindbrain has been reported (Ramer et al. 2000, Zhou and Shine 2003) and after spinal cord injury (SCI), GDNF delivered as either recombinant protein, as nanoconjugate, or by overexpression of genetically modified cells or viral vectors improves behavior outcome, neuronal survival and sprouting of fibers (Cheng et al. 2002; Tai et al. 2003; Cao et al. 2004; Chou et al. 2005; Wang et al. 2008; Guzen et al. 2009). However, the precise mechanisms through which GDNF treatment improves locomotor behavior after injury have not been fully established.
GDNF enhances migration and differentiation of ventral telencephalon precursors into GABAergic neurons, and promotes the growth of neurites from GABA neurons derived from the basal ganglia and cerebral cortex (Pozas and Ibanez 2005; Schaller et al. 2005). In the current study, we investigated the effects of GDNF on spinal cord neurons in vitro and in a model of spinal cord contusion injury using an HSV-based vector expressing GDNF (VG). We confirmed that vector-mediated expression of GDNF reduced tissue damage and improved locomotor function, and found that the improvement in behavioral outcome correlated with increased synaptophysin and GAD expression levels. In cultured spinal cord neurons in vitro, axonal growth and branching promoted by GDNF paralleled the increase in synaptic proteins, in GAD65 and GAD67 protein, and in GABA release induced by K+ depolarization. Taken together these results demonstrate that GDNF enhances GABA neuronal plasticity and GABAergic transmission which may contribute to reduced spasticity and improved functional outcome in SCI.
Materials and Methods
Vector Construct
The nonreplicating HSV-based vector VG contains the human GDNF gene under the control of the human cytomegalovirus immediate early promoter (HCMV IEp) in the HSV UL41 locus and green fluorescent protein (GFP) reporter gene in the UL54 locus (Natsume et al. 2002). The control vector vC is similar to vG but substitutes GDNF for the E. coli lacZ reporter gene in the UL41 locus (Supplementary Fig 1A).
Animal studies
Experiments were conducted on female Sprague-Dawley rats weighing 200-225 g and carried out in accordance with approved institutional animal care and use protocols. Rats were anesthetized with chloral hydrate (0.1 ml/kg i.p.) and a dorsal laminectomy was performed. The vertebral column was stabilized by clamping from T9 to T12 vertebral bodies and 150 Kdyne blunt force trauma was applied to the T12-T13 spinal cord using a 1.5 mm diameter probe controlled by a computerized impactor device (Precision Systems and Instrumentation, LLC). Two hours after injury, the spinal cord was stereotaxically injected with either vector vG or vC; 1 μl containing 1.2×108 plaque forming units (pfu) of vector were injected just above and below the lesion on both sides of the spinal cord at a rate of 0.5 μl/min (total injected volume 4 μl). Animals with spinal cord exposure but no injury or injection were used as control (sham). At the conclusion of the behavioral experiments (five wks after injury), animals were perfused transcardially with 15 mL of phosphate-buffered saline (PBS) followed by 500 ml of 4% paraphormaldehyde in PBS. The spinal cord was removed, postfixed at 4°C overnight, and cryoprotected with 30% sucrose in PBS. For protein analysis, 1 wk after injury animals were perfused transcardially with 15 mL of PBS and spinal cord removed and quickly frozen in dry-ice.
Behavioral studies
Gridwalk
Behavior was assessed 4 weeks after injury. The Grid walk, which tests the animal’s coordination between the front and hind limbs, was developed to measure the ability of the animal to precisely control hind paw placing (Bresnahan et al. 1987; Kunkel-Bagden et al. 1992). Our walkway consisted of a runway with regularly spaced horizontal bars (distance between bars is 1.5 cm). Errors consisting of misplacement of the hindpaw such that it falls through the grid hole were counted with a maximum of 30. Animals that did not display at least frequent stepping in the BBB score were unable to cross the walkway and were assigned the maximum score.
Basso-Beattie-Bresnahan (BBB) score
Hindlimb motor behavior was assessed once a week after injury for 4 weeks. Animals were trained to walk in an open area. The hind limb movement of each animal was assessed using a 21-point scale. A BBB score of 0 was given when no observable hind limb movement was observed, whereas a score of 21 was given when plantar stepping toe clearance, trunk stability, coordinated limb movement, and an erect tail was present (Basso et al. 1995). Behavioral analysis was performed in 22 animals: 8 in each SCI group and 6 in the sham group.
Primary Culture
Primary spinal cord neurons from E17 rat were plated at a concentration of 2×106 cells per well (12 well plate) for Western blot or 1×104 cells/well (24 well plate with glass cover slips) for immunocytochemistry, coated with poly-d-lysine in Neurobasal media containing B27, Glutamax I, Albumax II, and penicillin/streptomycin (Invitrogen). At 3 and 10 d in vitro (DIV), 100 ng/ml recombinant human GDNF (rhGDNF, R&D) was added to the cells for 15 minutes, 30 minutes, 24 hours, and 48 hours, either alone or in the presence of 10 μM of MEK inhibitor U0126 (Promega) or 50 μM of PI3K inhibitor LY 294002 (Cell Signaling) added 40 minutes before GDNF. Each Western blot was performed using two samples per treatment condition and each experiment was repeated 3 times. Each immunocytochemical study was repeated 4 times.
Histology and Immunocytochemistry
Post-traumatic Cavity Measurements
At 5 weeks post-injury the animals were perfused with 4% paraformaldehyde in PBS and the spinal cord removed, post-fixed and cryoprotected. Twenty μm cryostat sections of spinal cord were thaw-mounted onto cold Superfrost glass slides (Fisher Scientific), heated at 37°C and stained with hematoxylineosin dye following standard protocol. Five serial sections were selected every 10 sections at the epicenter of the lesion. The area of the cavity containing tissue damage was determined from images captured using a Nikon Eclipse E1000 microscope equipped with a Plan Apo 2X/0.1 lens using Metamorph 7.0 software (Molecular Devices).
Immunohistochemestry
Control and injured spinal cords, one week after injury were fixed and cryo-sectioned as above. Twenty μm sections were obtained starting from 2 mm below the center of the lesion. Immunolocalization of synaptophysin was performed using a polyclonal antibody (1:1000, Chemicon) followed by complementary secondary tagged fluorescent antibody (Alexa Fluor 488, Invitrogen).
Neurite Growth Assay
Primary spinal cord neurons from E17 rat were plated at a concentration of 1×104 per well (24 well plate) on poly-d-lysine-coated cover slips in culture media as described above. At 3 and 10 DIV, 100 ng/ml recombinant human GDNF (R&D) was added to the cells for 24 hours after which the cells were fixed with formalin, blocked for one hour in PBS with 5% normal goat serum, 1% bovine serum albumin, and 0.2% Triton X-100, and incubated overnight at 4°C with the following antibodies [Neurofilament 1:500 (SMI 31, Covance); MAP2, 1:4000 (Sigma and Chemicon); GAD65, 1:200 (Sigma and Chemicon); GAD67, 1:200 (Chemicon); Synapsin I, 1:500 (Chemicon)], followed by complimentary secondary tagged fluorescent antibodies (Alexa Fluor 594 and Alexa Fluor 488 (1:2000; Invitrogen). The cells were then washed three times and mounted in water-based Fluoromount G (Electron Microscopy Sciences). Analysis of neurite length was performed from images acquired using a Nikon Eclipse E1000 fluorescent microscope with Plan Apo 20X/0.75 lens using a DXM1200F digital camera (Cool Snap ES; Photometrics) and Neurite Growth software from Metamorph Digital Imaging (Molecular Devices). Each experiment was repeated 3 times.
Western Blot
Homogenates were prepared from primary spinal cord neuron cultures and rat spinal cord (0.5 cm tissue block extending caudally from the impact center) in 1% SDS, 50 mM Tris-HCl buffer pH 7.0 containing glycerol and 1:100 dilution of protease inhibitor and phosphatase inhibitor cocktail (Sigma). Cell lysates were sonicated while spinal cord tissues were homogenized in glass homogenizers and centrifuged at 10,000 g for 10 minutes at 4°C and the supernatants collected. The protein concentration was estimated using a Dc protein assay kit (Bio-Rad Laboratories) and spectrophotometry (AD340; Beckman Coulter). Sample aliquots were dissolved in Lemmli buffer and boiled at 95° C for 5 min; the proteins were separated using SDS-PAGE (Bio-Rad Laboratories) and transferred to PVDF membranes, blocked and incubated with the following primary antibodies [GAD65 1:1000 (Chemicon); GAD67 1:1000 (Chemicon); synapsin I, 1:1000 (Chemicon); synaptophysin, 1:1000 (Chemicon); serotonin transporter, 1:1000 (Chemicon); pERK, 1:1000 (Santa Cruz); pCREB, 1:1000 (Cell Signaling); zif268, 1:200 (Santa Cruz)]. HRP conjugated complementary secondary antibodies (Santa Cruz Biotechnology) were used for amplification and visualized using SuperSignal West Dura Extended Duration Substrate (Pierce). Each membrane was probed with anti-β-actin, 1:2000 (Sigma) or anti-GAPDH, 1:2000 (Chemicon) as internal control. The intensity of each band was determined by quantitative chemiluminescence using image analysis software (ChemiDoc XRS System; Bio-Rad Laboratories). The values were normalized to β-actin or GAPDH used as internal controls. Six samples were analyzed in each group. GDNF release into the medium was determined by ELISA using a commercially available kit (Promega, Madison WI).
GABA Determination
The amount of GABA released from spinal cord neurons (10 DIV) was determined by gas chromatography (GC). After 48h exposure to GDNF (100 ng/ml) or control, defined culture medium was replaced by artificial cerebrospinal fluid aCSF (120 mM NaCl, 3.5 mM KCl, 2.5 mM CaCl2, 1.3 mM MgSO4, 1.25 mM NaH2PO4) prior to exposure to modified aCSF containing 40 mM KCl and 83.5 mM NaCl for 5 minutes.
Five hundred μl of aCSF was treated with an equal volume of a saturated solution of NaHCO3 and 2 μl of Norvaline (1mg/ml, internal standard) in 0.1 N HCl added. The mixture was centrifuged at 15,000 rpm for 15 min, the supernatant transferred into 1ml of CH2Cl2 (anhydrous) containing 10 ml ethyl chloroformate (ECF) and the pH adjusted to >12 with NaOH followed by addition of another 10 ml of ECF. The pH of the aqueous layer was brought to <2.0 with 50 μl of 18 N H2SO4 and the hydrophobic product (dimethyl carbonyl derivative of amino acid) extracted with 2 ml each of diethyl ether and ethyl acetate, the combined extract subjected to second derivative (tert-butyl dimethyl silyl) preparation by adding equal parts of toluene (anhydrous) and N-methyl-N-tert-butyl dimethylsilyltriflouroaceteamide (MTBSTFA). GC analyses were performed on a flame ionization detector (FID) gas chromatography (Agilent, Model 6890 N, Agilent Technologies, Atlanta, GA) equipped with an auto sampler and Chemstation software. A capillary column (Agilent Model HP-88; 30 m × 0.25 mm I. D., 0.25 mm film thickness) was used with H2 as carrier gas. A linear calibration curve was created using Norvaline after subjecting to mixed derivative preparation for GC analysis.
Statistical analysis
The statistical significance of the difference between groups for the in-vitro and in-vivo western results was determined by one-way ANOVA (SPSS 12.0) using Bonferonni’s correction for the multiple post hoc analyses. Parametric statistics using the general linear model for repeated measures were used to identify significant effects of treatment conditions on the behavioral analysis of motor function. The statistical significance of the difference between groups for the neurite length measurements was determined using the student t-test. All results were expressed as mean ± SEM with P < 0.05 considered significant.
Results
Characterization of the GDNF-expressing HSV-vector vG
In order to characterize for expression and localization of transgene from vG, in vitro and in vivo studies were performed. Cultured spinal cord neurons were infected at a multiplicity of infection (MOI) of 2 for 2 hrs and the amount of GDNF synthesized and released into the medium was measured by ELISA at 24 h. Neurons transduced with vG released substantial amounts of GDNF into the medium compared to control vector vC (untreated and vC = undetectable and vG = 1.32 ± 0.68 ng/ml). In adult spinal cord, injection of vG (1.2×108 pfu) 2 hrs after injury showed extensive GFP expression around the injection site at 7 days post vector delivery (Supplemental Fig 1B).
Intraspinal injection of vG improves hind limb locomotor function after SCI
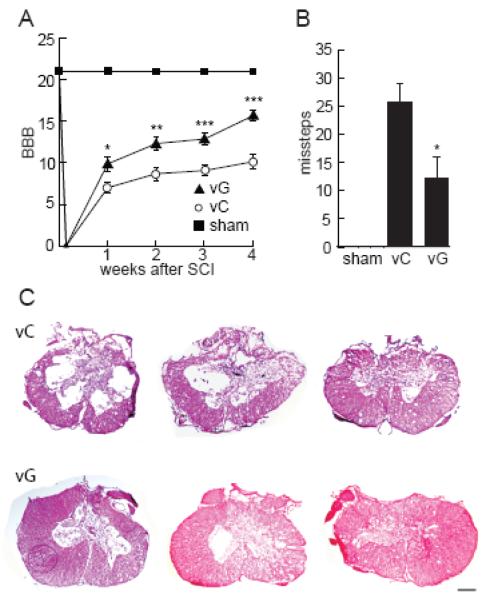
Two hours after blunt force trauma to the T12-T13 spinal cord animals injected intraspinally with the HSV-based vector expressing GDNF showed a significantly higher BBB score compared to the animals injected with control vector, which began at 1 week and persisted over the course of 4 weeks post-injury (Fig 1A). A significant improvement in hindlimb stepping accuracy in the grid walk test was determined at 4 weeks in animals injected with vG compared to animals injected with vC (Fig 1B). At the completion of the behavior analysis, the degree of tissue injury and sparing was determined by H&E staining of serial sections of spinal cord. Animals treated with vG had significantly reduced post-traumatic cavity area within the spinal cord demonstrating decreased severity of injury as compared to animals treated with vC (vG = 0.54 ± 0.085 mm2 and vC = 0.99 ± 0.22 mm2; p<0.05) (Fig 1C).
Figure 1.
vG improves locomotor functioning and tissue sparing after T12-T13 SCI compared to control vector vC. (A) BBB score measured each week for 4 weeks and (B) gridwalk measured at 4 weeks following injury. (C) Spinal cord cavity area was significantly reduced at the site of injury from 0.99 ± 0.22 mm2 in vC treated animals to 0.54 ± 0.085 mm2 in vG treated animals. Data is presented as mean ± SEM *P < 0.05, **P < 0.01, ***P < 0.005; N = 6 animals per group; scale bar = 500 μm.
Transgene mediated GDNF results in sprouting of GABAergic neurons in the spinal cord
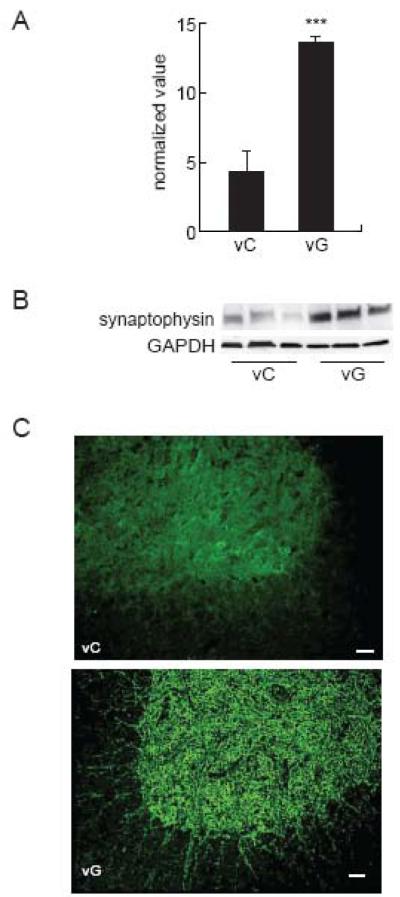
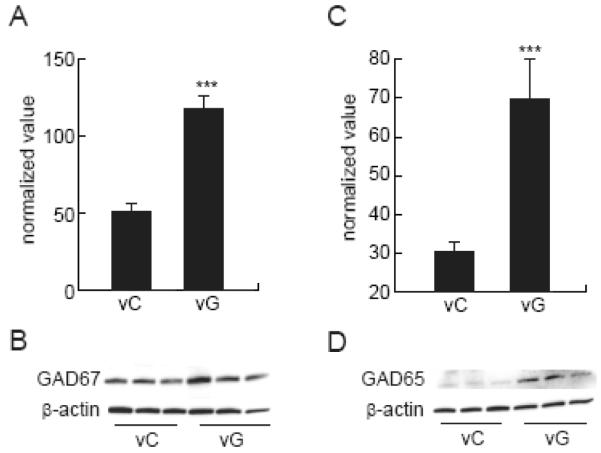
In order to determine whether GDNF might be enhancing synaptogenesis in the spinal cord we examined expression of synaptophysin at 1 week after injury. There was a significant increase in synaptophysin protein in the injured spinal cord inoculated with vG compared to animals inoculated with the vC (vC, 0.3 ± 0.1; vG, 0.87 ± 0.09; p < 0.005) (Fig 2A,B). Photomicrographs from the anterior quadrant of the spinal cord below the lesion showed the increased synaptophysin expression in boutons resembling synaptic contacts (Fig 2C). In order to establish the neurotransmitter phenotype of the neurons responsible for the sprouting we examined levels of serotonin transporter and GAD proteins. Animals injected with the GDNF-expressing vector showed increased expression of GAD65 (vC, 0.03 ± 0.002; vG, 0.06 ± 0.003; p < 0.005) (Fig 3A,B) and GAD67 (vC, 0.06 ± .005; vG, 0.14 ± .004; p < 0.005) (Fig 3C,D). We did not observe an increase in serotonin transporter protein levels in the spinal cord between vG and vC treated animals (data not shown).
Figure 2.
Delivery of vG 2 hrs after injury resulted in a substantial increase in synaptophysin at 1 week compared to animals injected with the control vector (vC; P < 0.005). (A) Amount of synaptophysin protein expressed as normalized value using GAPDH as a loading control. (B) Representative Western blot. Data is presented as mean ± SEM. N = 3 animals per group. (C) Photomicrograph from the anterior horn of the spinal cord below the center of the lesion showing the distribution of synaptophysin immunoreactivity as boutons 1 week after vG treatment compared to vC-treated animals; scale bar = 70 μm.
Figure 3.
Injection of vG 2 hrs after injury showed a substantial increase in GAD65 and GAD67 protein levels at 1 week compared to animals injected with the control vector (vC; P < 0.005). (A) Amount of GAD65 protein expressed as normalized value using β-actin as a loading control. (B) Representative Western blot. (C) Amount of GAD67 determined by Western blot expressed as normalized value using β-actin as a loading control. (D) Representative Western blot. Data is presented as mean ± SEM. N = 3 animals per group.
GDNF promotes axonal elongation, neurite growth and synapse formation of spinal GABAergic neurons
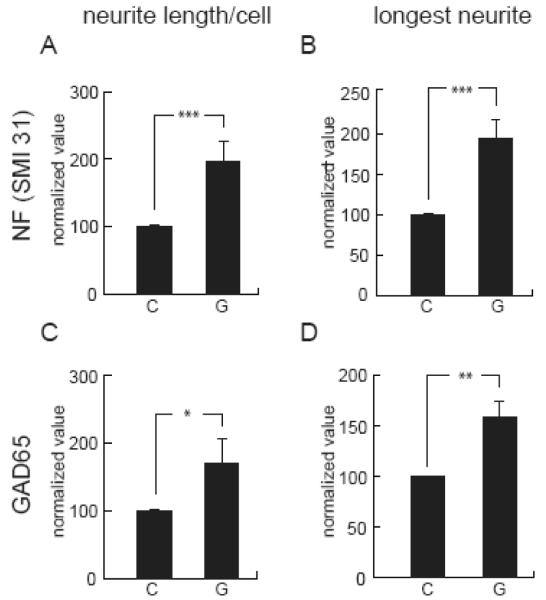
The effects of GDNF on the initial formation of neurite processes were studied in primary spinal cord neurons at 3 DIV using axonal and dendritic markers. We found a significant increase in neurite length and in the length of the longest neurite determined by SMI31 immunoreactivity in neurons treated with 100 ng/ml of GDNF for 24 hours compared to control (Fig 4A,B). We did not observe an increase in the length of the neurites immunostained with anti-MAP2 suggesting that GDNF preferentially enhanced early axonal elongation over dendritic arborization. Immunostaining with an antibody against GAD65 confirmed that GDNF increased sprouting of GABAergic neurons. (Fig 4C,D). At 10 DIV, we found a significant increase in neurite length, in the length of the longest neurite, and branch number in cells treated with GDNF immunostained against GAD65 and GAD67 (Table 1).
Figure 4.
GDNF increases neurite length in spinal cord neurons in vitro. Treatment of spinal cord neurons at 3 DIV with GDNF resulted in an increase in the total neurite length (A,C) and in the length of the longest neurites (B,D). These neurites were stained with SMI31 antibody against phosphorylated neurofilament and tau epitopes, indicating axonal processes, and with the anti GAD65 antibody, confirming the GABAergic phenotype. There was no change in the length of neurites measured by MAP2 immunostaining. C = control untreated and G = GDNF (100 ng/ml) for 24 h. Data is expressed as normalized value and presented as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.005.
Table 1.
GDNF promotes neurite growth of GABAergic spinal cord neurons. GDNF (100 ng/ml) added to 10 DIV neurons for 24 hrs resulted in significant increases of total neurite growth, longest neurite length, and branching number by GAD65 and GAD67 immunoreactivity.
| % increase GDNF/control |
P | ||
|---|---|---|---|
| GAD65 | Total neurite length | 188.5 ± 7.0 | < 0.01 |
| Longest neurite | 199.5 ± 6.1 | < 0.005 | |
| Branch number | 186.3 ± 13.7 | < 0.05 | |
| GAD67 | Total neurite length | 144.8 ± 5.1 | < 0.005 |
| Longest neurite | 144.5 ± 6.0 | < 0.005 | |
| Branch number | 145.9 ± 4.5 | < 0.005 |
Data presented as the mean percentage ± SEM increase with GDNF-treatment over control. Data represents four separate experiments;
P < 0.05,
P < 0.01,
P < 0.005.
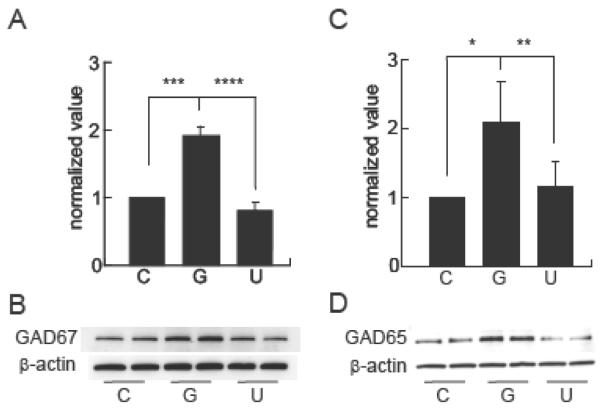
GDNF enhances GABAergic phenotype and synapse formation through the MEK-ERK pathway
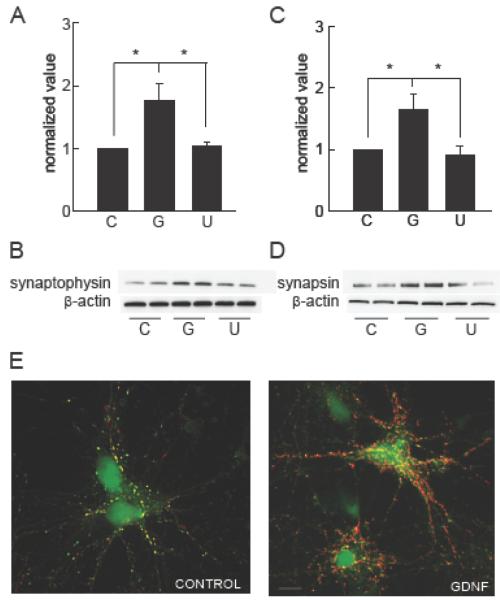
The morphological changes observed at 10 DIV correlated with a substantial increase in GAD65 and GAD67 protein in spinal cord neurons treated with GDNF compared to control untreated neurons by Western blot (Fig 5A-D). In addition, GDNF promoted synapse formation and induced expression of synaptophysin protein (Fig 6A,B), and synapsin I (Fig 6C,D) which was confirmed by colocalization of synapsin I and GAD65 immunoreactivity on GABAergic neurons (Fig 6E).
Figure 5.
GDNF increases GAD65 and GAD67 in spinal cord neurons. (A) The amount of GAD67 protein expressed as normalized value using β-actin as a loading control (P < 0.005). (B) Representative Western blot. (C) Amount of GAD65 protein expressed as normalized value using β-actin as a loading control (P < 0.005). (D) Representative Western blot. C = untreated control and G= GDNF (100 ng/ml) for 48 h. Treatment with the MEK inhibitor U0126 (U; 10 μM) prevented the increased expression of GAD67 (P < 0.001) and GAD65 (P < 0.01). Data is presented as mean ± SEM.
Figure 6.
GDNF increases synaptophysin and synapsin I in primary spinal cord neurons. (A) Amount of synaptophysin protein expressed as normalized value using β-actin as a loading control (P < 0.05). (B) Representative Western blot. (C) Amount of synapsin I protein expressed as normalized value using β-actin as a loading control (P < 0.05). (D) Representative Western blot. C= untreated control and G= GDNF (100 ng/ml) for 48 h. Treatment with the MEK inhibitor U0126 (U; 10 μM) prevented the increased expression of synaptophysin (P < 0.05) and synapsin I (P < 0.05). Data is presented as mean ± SEM. (E) Photomicrograph of primary spinal cord neurons treated with GDNF showing colocalization of synapsin 1 (red) and GAD65 (green) immunoreactivity; scale bar = 70 μm.
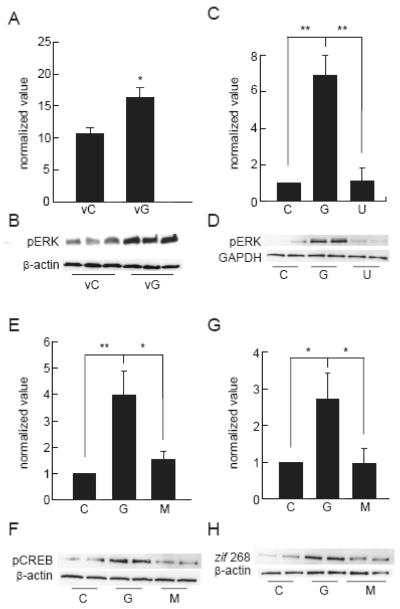
GDNF has been shown to exert effects on primary striatal GABAergic neurons through MEK/ERK and PI3K/AKT pathways downstream of the GDNF receptor (Garcia-Martinez et al. 2006). In the SCI model, we found an increase in pERK in the injured spinal cord of animals injected with the GDNF expressing vector 2 hrs after blunt trauma compared to animals injected with the control vector (Fig 7A,B). In vitro, inhibition of ERK activation by addition of 10 μM of the MEK inhibitor U0126 to cells treated with GDNF for 48 hrs (Fig 7C,D) blocked the increase in GAD protein otherwise produced by exposure to GDNF (Fig 5). Inhibition of the ERK pathway also prevented the increase of synaptophysin (Fig 6A,B) and synapsin I proteins induced by GDNF (Fig 6C,D). In contrast, application at the PI3K inhibitor LY294002 (50 μM) did not block the increase in GAD and synaptic related proteins (data not shown). GDNF treatment induced within 30 min an increase in pERK (Fig 7C,D), phosphorylation of downstream transcriptional activator CREB (Fig 7E,F), and expression of the downstream immediate early gene zif268 (Fig 7G,H). All of these effects were blocked by the MEK inhibitor U0126.
Figure 7.
vG in vivo and recombinant GDNF in vitro activate the ERK pathway. Animals injected with vG 2 hrs after injury had increased pERK in the spinal cord compared to control vector (vC) at 1 week after vector delivery. Western blot normalized to internal control. (A,B; P < 0.05). Spinal cord neurons at 10 DIV treated with GDNF show increases in pERK (C,D; P < 0.01), pCREB (E,F; P < 0.01), and zif 268 (G,H; P < 0.05). All values are expressed as mean ± SEM and normalized to internal control. C= control untreated and G = GDNF (100 ng/ml) for 48 h. Treatment with the MEK inhibitor U0126 (U; 10 μM) prevented the increase in pERK (C,D; P < 0.01), pCREB (E,F; P < 0.05) and zif 268 (G,H; P < 0.05).
GDNF treatment results in increased release of GABA from primary spinal cord neurons
In order to evaluate the functional significance of increased expression of GAD and synaptic related proteins we examined the release of GABA from primary spinal cord neurons. Primary cultures at 10 DIV were treated with GDNF (100 ng/ml) for 48 h. Membrane depolarization was induced with 40 μM potassium chloride for 5 min and the amount of GABA released into the medium was determined by HPLC. GABA release increased from 1.37 ± 0.035 nmol/ ml/ min/ 106 neurons in untreated cultures to 1.49 ± 0.018 nmol/ ml/ min/ 106 neurons (P < 0.05) in cultures treated with GDNF.
Discussion
In our study, we report several findings with important implications: 1) HSV-mediated release of GDNF in spinal cord improves locomotor function and reduces tissue damage after SCI; 2) improved function correlates with evidence of neurite sprouting and increases in synaptic marker proteins in the spinal cord; 3) coincident increases in spinal GAD suggests sprouting of GABAergic interneurons; 4) exposure of primary spinal cord neurons in vitro to GDNF results in GABAergic neurite growth, enhanced synaptic bouton formation, and GABA release that correlates with the increased expression of GAD and synaptic proteins mimicking the results of the in vivo studies and, 5) these effects are mediated through activation of the MEK/ERK pathway possibly through phosphorylation of CREB and increased expression of the immediate early gene zif 268. Taken together, these results suggest that HSV-mediated gene transfer of GDNF may improve functional recovery after spinal cord injury in part by enhancing GABAergic neurotransmission.
GDNF was originally purified as a neurotrophic factor for embryonic midbrain dopamine neurons, but was later found to be trophic for neurons with diverse neurotransmitter phenotype. GDNF promotes neurite outgrowth and survival of developing dopaminergic neurons of the substantia nigra (Akerud et al. 1999) and vector-mediated release of GDNF protects dopaminergic neurons from 6-OHDA-induced degeneration in-vivo (Choi-Lundberg et al. 1997; Natsume et al. 2001). GDNF administered subcutaneously to mice at birth increases the number of axons converging at the neuromuscular junction and increases localized arborization in motor neurons (Keller-Peck et al. 2001). HSV-mediated delivery of GDNF after proximal spinal nerve injury results in an increased number of surviving motor neurons in the ventral horn (Natsume et al. 2002) and exogenously administered GDNF can support long-term motor neuron survival and axon regeneration after peripheral nerve injury in newborn and adult animals (Hottinger et al. 2000).
Several studies have reported that GDNF either delivered by gene transfer, biodegradable nanoparticles, or infusion improves locomotor function following spinal cord injury (Cheng et al. 2002; Tai et al. 2003; Guzen et al. 2009). In our study, inoculation of the HSV-vector expressing GDNF in animals that received a blunt force contusion injury to the lower thoracic spinal cord also led to an improvement in locomotor function over four weeks. At 1 week post-injury, we observed an increase in synaptophysin as previously reported (Chou et al. 2005) and synapsin I in the anterior horn of the spinal cord below the level of the lesion suggesting that vector-mediated expression of GDNF induced sprouting and the formation of new synapses. The fact that we observed increases in GAD65 and GAD67 but did not observe an increase in serotonin transporter protein suggests that the effect of vector-mediated expression of GDNF was on spinal cord GABAergic interneurons, rather than an effect on descending projection fibers. These results are not unexpected as GDNF treatment has been reported to stimulate neuritic outgrowth from GABAergic, cortical, ventral mesencephalic and striatal neurons in culture (Pozas and Ibanez 2005).
GDNF is known to activate both src-PI3K-AKT and raf-MEK–ERK pathways. Activation of the MEK-ERK cascade appears to be essential for axon extension and synaptic connectivity (Soler et al. 1999; Markus et al. 2002; Hollis et al. 2009) in agreement with our results. GDNF promoted an early response to axonal differentiation, seen by neurofilament immunostaining, over dendrites in our in vitro studies. In addition glutamic acid decarboxylase, synapsin I and II, and neurofilament 68 genes have known zif 268 transcriptional regulatory sites on their promoter regions suggesting possible regulation of protein expression by MEK-ERK-CREB-zif 268 pathway (Knapska and Kaczmarek 2004).
While our findings would suggest that HSV-mediated expression of GDNF improves motor functional recovery through increased expression of GAD and synaptic related proteins resulting in enhanced release of GABA and consequent reduction in spasticity, GDNF may also contribute to enhance synaptic function through mechanisms that are independent of gene transcription. For example, GDNF has been shown to promote synapse formation by inducing ligand–dependent cell adhesion between GFRα1 expressing neurons (Ledda 2007). GDNF also increases neurotransmitter release through direct potentiation of N-type Ca2+ channel currents (Wang et al. 2001) and causes ERK-dependent enhancement of voltage-gated Ca2+ current density by altering channel gating (Fitzgerald 2000; Woodall et al. 2008). In addition, Ca2+ entry through presynaptic N-type Ca2+ channels is important for synaptic growth (Rieckhof et al. 2003). These GDNF mediated effects that result in improved synaptic connectivity and neurotransmitter release could also influence functional recovery after SCI by further enhancing GABAergic transmission.
In addition, increased production and release of GABA may also influence the tissue response to injury by preventing macrophage activation, inhibiting pro-inflammatory cytokine release by glial cells (Kuhn et al. 2004; Lubick et al. 2007; Seidel et al. 2007; Roach et al. 2008; Cheung et al. 2009), or by reducing oxygen consumption and blood flow (Caesar et al. 2008). GDNF acting either directly, or through increased GABA release may also alter cell migration (Bolteus and Bordey 2004; Seidel et al. 2007; Su et al. 2009).
There have been previous studies demonstrating that gene delivery through viral vectors may be applied to models of spinal cord injury (Tai et al. 2003; Zhou and Shine 2003; Chou et al. 2005), and the salutary effects of GDNF have been previously reported (Cheng et al. 2002; Sharma 2007; Oh et al. 2009). The new studies reported herein enhance our understanding of the many effects that GDNF exerts after spinal cord injury and suggests that the behavioral improvement observed in SCI after treatment with a GDNF expressing vector may result in part from enhanced GABAergic transmission resulting in a possible reduction in posttraumatic spasticity, thus pointing towards a novel indication of GDNF for spinal cord trauma.
Supplementary Material
Figure 8.
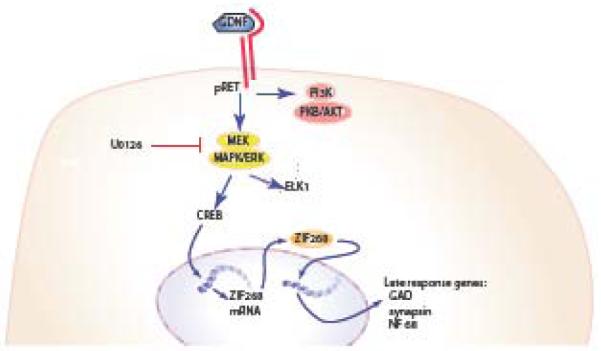
Schematic representation of the zif 268 pathway in spinal cord neurons. Activation of receptor tyrosine kinase by GDNF activates the MEK/ERK pathway leading to phosphorylation of the transcription factor CREB, which translocates to the nucleus and promotes the expression of zif 268. Acting as a transcription factor, zif 268 regulates the expression of late response genes, such as glutamic acid decarboxylase (GAD), synapsins and neurofilament 68.
Acknowledgements
This work was supported by grants from the Department of Veterans Affairs and the National Institutes of Health to Drs Mata and Fink. We gratefully acknowledge the excellent technical assistance of Mr. Vikram Thakur Singh in vector propagation, and Ms. Shue Liu in tissue culture.
References
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Akerud P, Canals JM, Snyder EY, Arenas E. Neuroprotection through delivery of glial cell line-derived neurotrophic factor by neural stem cells in a mouse model of Parkinson’s disease. J Neurosci. 2001;21:8108–8118. doi: 10.1523/JNEUROSCI.21-20-08108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerud P, Alberch J, Eketjall S, Wagner J, Arenas E. Differential effects of glial cell line-derived neurotrophic factor and neurturin on developing and adult substantia nigra dopaminergic neurons. J Neurochem. 1999;73:70–78. doi: 10.1046/j.1471-4159.1999.0730070.x. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Bolteus AJ, Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J Neurosci. 2004;24:7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnahan JC, Beattie MS, Todd FD, 3rd, Noyes DH. A behavioral and anatomical analysis of spinal cord injury produced by a feedback-controlled impaction device. Exp Neurol. 1987;95:548–570. doi: 10.1016/0014-4886(87)90299-8. [DOI] [PubMed] [Google Scholar]
- Caesar K, Offenhauser N, Lauritzen M. Gamma-aminobutyric acid modulates local brain oxygen consumption and blood flow in rat cerebellar cortex. J Cereb Blood Flow Metab. 2008;28:906–915. doi: 10.1038/sj.jcbfm.9600581. [DOI] [PubMed] [Google Scholar]
- Cao JP, Yu JK, Li C, Sun Y, Yuan HH, Wang HJ, Gao DS. Integrin beta1 is involved in the signaling of glial cell line-derived neurotrophic factor. J Comp Neurol. 2008;509:203–210. doi: 10.1002/cne.21739. [DOI] [PubMed] [Google Scholar]
- Cao L, Liu L, Chen ZY, Wang LM, Ye JL, Qiu HY, Lu CL, He C. Olfactory ensheathing cells genetically modified to secrete GDNF to promote spinal cord repair. Brain. 2004;127:535–549. doi: 10.1093/brain/awh072. [DOI] [PubMed] [Google Scholar]
- Cheng H, Wu JP, Tzeng SF. Neuroprotection of glial cell line-derived neurotrophic factor in damaged spinal cords following contusive injury. J Neurosci Res. 2002;69:397–405. doi: 10.1002/jnr.10303. [DOI] [PubMed] [Google Scholar]
- Cheung G, Kann O, Kohsaka S, Faerber K, Kettenmann H. GABAergic activities enhance macrophage inflammatory protein-1(alpha) release from microglia (brain macrophages) in postnatal mouse brain. J Physiol. 2009;587:753–768. doi: 10.1113/jphysiol.2008.163923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi-Lundberg DL, Lin Q, Chang Y-N, Chiang YL, Hay CM, Mohajeri H, Davidson BL, Bohn MC. Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science. 1997;275:838–841. doi: 10.1126/science.275.5301.838. [DOI] [PubMed] [Google Scholar]
- Choi Lundberg DL, Lin Q, Chang YN, Chiang YL, Hay CM, Mohajeri H, Davidson BL, Bohn MC. Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science. 1997;275:838–841. doi: 10.1126/science.275.5301.838. [DOI] [PubMed] [Google Scholar]
- Chou AK, Yang LC, Wu PC, Wong WT, Liu GS, Chen JT, Howng SL, Tai MH. Intrathecal gene delivery of glial cell line-derived neurotrophic factor ameliorated paraplegia in rats after spinal ischemia. Brain Res Mol Brain Res. 2005;133:198–207. doi: 10.1016/j.molbrainres.2004.10.036. [DOI] [PubMed] [Google Scholar]
- Ducray A, Krebs SH, Schaller B, Seiler RW, Meyer M, Widmer HR. GDNF family ligands display distinct action profiles on cultured GABAergic and serotonergic neurons of rat ventral mesencephalon. Brain Res. 2006;1069:104–112. doi: 10.1016/j.brainres.2005.11.056. [DOI] [PubMed] [Google Scholar]
- Fitzgerald EM. Regulation of voltage-dependent calcium channels in rat sensory neurones involves a Ras-mitogen-activated protein kinase pathway. J Physiol. 2000;527(Pt 3):433–444. doi: 10.1111/j.1469-7793.2000.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Martinez JM, Perez-Navarro E, Gavalda N, Alberch J. Glial cell line-derived neurotrophic factor promotes the arborization of cultured striatal neurons through the p42/p44 mitogen-activated protein kinase pathway. J Neurosci Res. 2006;83:68–79. doi: 10.1002/jnr.20713. [DOI] [PubMed] [Google Scholar]
- Guzen FP, de Almeida Leme RJ, de Andrade MS, de Luca BA, Chadi G. Glial cell line-derived neurotrophic factor added to a sciatic nerve fragment grafted in a spinal cord gap ameliorates motor impairments in rats and increases local axonal growth. Restor Neurol Neurosci. 2009;27:1–16. doi: 10.3233/RNN-2009-0454. [DOI] [PubMed] [Google Scholar]
- Hollis ER, 2nd, Jamshidi P, Low K, Blesch A, Tuszynski MH. Induction of corticospinal regeneration by lentiviral trkB-induced Erk activation. Proc Natl Acad Sci U S A. 2009;106:7215–7220. doi: 10.1073/pnas.0810624106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hottinger AF, Azzouz M, Deglon N, Aebischer P, Zurn AD. Complete and long-term rescue of lesioned adult motoneurons by lentiviral-mediated expression of glial cell line-derived neurotrophic factor in the facial nucleus. J Neurosci. 2000;20:5587–5593. doi: 10.1523/JNEUROSCI.20-15-05587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller-Peck CR, Feng G, Sanes JR, Yan Q, Lichtman JW, Snider WD. Glial cell line-derived neurotrophic factor administration in postnatal life results in motor unit enlargement and continuous synaptic remodeling at the neuromuscular junction. J Neurosci. 2001;21:6136–6146. doi: 10.1523/JNEUROSCI.21-16-06136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, Kaczmarek L. A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Prog Neurobiol. 2004;74:183–211. doi: 10.1016/j.pneurobio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Kuhn SA, van Landeghem FK, Zacharias R, Farber K, Rappert A, Pavlovic S, Hoffmann A, Nolte C, Kettenmann H. Microglia express GABA(B) receptors to modulate interleukin release. Mol Cell Neurosci. 2004;25:312–322. doi: 10.1016/j.mcn.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Kunkel-Bagden E, Dai HN, Bregman BS. Recovery of function after spinal cord hemisection in newborn and adult rats: differential effects on reflex and locomotor function. Exp Neurol. 1992;116:40–51. doi: 10.1016/0014-4886(92)90174-o. [DOI] [PubMed] [Google Scholar]
- Ledda F. Ligand-induced cell adhesion as a new mechanism to promote synapse formation. Cell Adh Migr. 2007;1:137–139. doi: 10.4161/cam.1.3.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubick K, Radke M, Jutila M. Securinine, a GABAA receptor antagonist, enhances macrophage clearance of phase II C. burnetii: comparison with TLR agonists. J Leukoc Biol. 2007;82:1062–1069. doi: 10.1189/jlb.0407255. [DOI] [PubMed] [Google Scholar]
- Markus A, Patel TD, Snider WD. Neurotrophic factors and axonal growth. Curr Opin Neurobiol. 2002;12:523–531. doi: 10.1016/s0959-4388(02)00372-0. [DOI] [PubMed] [Google Scholar]
- Natsume A, Mata M, Goss JR, Huang S, Wolfe D, Oligino T, Glorioso JC, Fink DJ. Bcl-2 and GDNF delivered by HSV-mediated gene transfer act additively to protect dopaminergic neurons from 6-OHDA induced degeneration. Exp Neurol. 2001;169:231–238. doi: 10.1006/exnr.2001.7671. [DOI] [PubMed] [Google Scholar]
- Natsume A, Mata M, Wolfe D, Oligino T, Goss J, Huang S, Glorioso J, Fink DJ. Bcl-2 and GDNF delivered by HSV-mediated gene transfer after spinal root avulsion provide a synergistic effect. J. Neurotrauma. 2002;19:61–68. doi: 10.1089/089771502753460240. [DOI] [PubMed] [Google Scholar]
- Oh MJ, Seo TB, Kwon KB, Yoon SJ, Elzi DJ, Kim BG, Namgung U. Axonal outgrowth and Erk1/2 activation by training after spinal cord injury in rats. J Neurotrauma. 2009 doi: 10.1089/neu.2008.0800. [DOI] [PubMed] [Google Scholar]
- Paratcha G, Ledda F. GDNF and GFRalpha: a versatile molecular complex for developing neurons. Trends Neurosci. 2008;31:384–391. doi: 10.1016/j.tins.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Pozas E, Ibanez CF. GDNF and GFRalpha1 promote differentiation and tangential migration of cortical GABAergic neurons. Neuron. 2005;45:701–713. doi: 10.1016/j.neuron.2005.01.043. [DOI] [PubMed] [Google Scholar]
- Ramer MS, Priestley JV, McMahon SB. Functional regeneration of sensory axons into the adult spinal cord. Nature. 2000;403:312–316. doi: 10.1038/35002084. [DOI] [PubMed] [Google Scholar]
- Rieckhof GE, Yoshihara M, Guan Z, Littleton JT. Presynaptic N-type calcium channels regulate synaptic growth. J Biol Chem. 2003;278:41099–41108. doi: 10.1074/jbc.M306417200. [DOI] [PubMed] [Google Scholar]
- Roach JD, Jr., Aguinaldo GT, Jonnalagadda K, Hughes FM, Jr., Spangelo BL. Gamma-aminobutyric acid inhibits synergistic interleukin-6 release but not transcriptional activation in astrocytoma cells. Neuroimmunomodulation. 2008;15:117–124. doi: 10.1159/000148194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer H, Rosenblad C, Bjorklund A. Glial cell line-derived neurotrophic factor but not transforming growth factor beta 3 prevents delayed degeneration of nigral dopaminergic neurons following striatal 6-hydroxydopamine lesion. Proc Natl Acad Sci U S A. 1995;92:8935–8939. doi: 10.1073/pnas.92.19.8935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller B, Andres RH, Huber AW, Meyer M, Perez-Bouza A, Ducray AD, Seiler RW, Widmer HR. Effect of GDNF on differentiation of cultured ventral mesencephalic dopaminergic and non-dopaminergic calretinin-expressing neurons. Brain Res. 2005;1036:163–172. doi: 10.1016/j.brainres.2004.12.054. [DOI] [PubMed] [Google Scholar]
- Seidel J, Niggemann B, Punzel M, Fischer J, Zanker KS, Dittmar T. The neurotransmitter GABA is a potent inhibitor of the stromal cell-derived factor-1alpha induced migration of adult CD133+ hematopoietic stem and progenitor cells. Stem Cells Dev. 2007;16:827–836. doi: 10.1089/scd.2007.0004. [DOI] [PubMed] [Google Scholar]
- Sharma HS. A select combination of neurotrophins enhances neuroprotection and functional recovery following spinal cord injury. Ann N Y Acad Sci. 2007;1122:95–111. doi: 10.1196/annals.1403.007. [DOI] [PubMed] [Google Scholar]
- Soler RM, Dolcet X, Encinas M, Egea J, Bayascas JR, Comella JX. Receptors of the glial cell line-derived neurotrophic factor family of neurotrophic factors signal cell survival through the phosphatidylinositol 3-kinase pathway in spinal cord motoneurons. J Neurosci. 1999;19:9160–9169. doi: 10.1523/JNEUROSCI.19-21-09160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su CM, Lu DY, Hsu CJ, Chen HT, Huang CY, Yang WH, Su YC, Yang SN, Fong YC, Tseng WP, Tang CH. Glial cell-derived neurotrophic factor increases migration of human chondrosarcoma cells via ERK and NF-kappaB pathways. J Cell Physiol. 2009;220:499–507. doi: 10.1002/jcp.21802. [DOI] [PubMed] [Google Scholar]
- Tai MH, Cheng H, Wu JP, Liu YL, Lin PR, Kuo JS, Tseng CJ, Tzeng SF. Gene transfer of glial cell line-derived neurotrophic factor promotes functional recovery following spinal cord contusion. Exp Neurol. 2003;183:508–515. doi: 10.1016/s0014-4886(03)00130-4. [DOI] [PubMed] [Google Scholar]
- Takahashi M. The GDNF/RET signaling pathway and human diseases. Cytokine Growth Factor Rev. 2001;12:361–373. doi: 10.1016/s1359-6101(01)00012-0. [DOI] [PubMed] [Google Scholar]
- Wang CY, Yang F, He X, Chow A, Du J, Russell JT, Lu B. Ca(2+) binding protein frequenin mediates GDNF-induced potentiation of Ca(2+) channels and transmitter release. Neuron. 2001;32:99–112. doi: 10.1016/s0896-6273(01)00434-2. [DOI] [PubMed] [Google Scholar]
- Wang YC, Wu YT, Huang HY, Lin HI, Lo LW, Tzeng SF, Yang CS. Sustained intraspinal delivery of neurotrophic factor encapsulated in biodegradable nanoparticles following contusive spinal cord injury. Biomaterials. 2008;29:4546–4553. doi: 10.1016/j.biomaterials.2008.07.050. [DOI] [PubMed] [Google Scholar]
- Woodall AJ, Richards MA, Turner DJ, Fitzgerald EM. Growth factors differentially regulate neuronal Cav channels via ERK-dependent signalling. Cell Calcium. 2008;43:562–575. doi: 10.1016/j.ceca.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Zhou L, Shine HD. Neurotrophic factors expressed in both cortex and spinal cord induce axonal plasticity after spinal cord injury. J Neurosci Res. 2003;74:221–226. doi: 10.1002/jnr.10718. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.