Abstract
Exercise enhances branched-chain amino acid (BCAA) catabolism, and BCAA supplementation influences exercise metabolism. However, it remains controversial whether BCAA supplementation improves exercise endurance, and unknown whether the exercise endurance effect of BCAA supplementation requires catabolism of these amino acids. Therefore, we examined exercise capacity and intermediary metabolism in skeletal muscle of knockout (KO) mice of mitochondrial branched-chain aminotransferase (BCATm), which catalyzes the first step of BCAA catabolism. We found that BCATm KO mice were exercise intolerant with markedly decreased endurance to exhaustion. Their plasma lactate and lactate-to-pyruvate ratio in skeletal muscle during exercise and lactate release from hindlimb perfused with high concentrations of insulin and glucose were significantly higher in KO than wild-type (WT) mice. Plasma and muscle ammonia concentrations were also markedly higher in KO than WT mice during a brief bout of exercise. BCATm KO mice exhibited 43–79% declines in the muscle concentration of alanine, glutamine, aspartate, and glutamate at rest and during exercise. In response to exercise, the increments in muscle malate and α-ketoglutarate were greater in KO than WT mice. While muscle ATP concentration tended to be lower, muscle IMP concentration was sevenfold higher in KO compared with WT mice after a brief bout of exercise, suggesting elevated ammonia in KO is derived from the purine nucleotide cycle. These data suggest that disruption of BCAA transamination causes impaired malate/aspartate shuttle, thereby resulting in decreased alanine and glutamine formation, as well as increases in lactate-to-pyruvate ratio and ammonia in skeletal muscle. Thus BCAA metabolism may regulate exercise capacity in mice.
Keywords: mitochondrial branched-chain aminotransferase, alanine and glutamine formation, lactate, ammonia, malate/aspartate shuttle
despite extensive studies, there is still no agreement on whether branched-chain amino acid (BCAAs) supplementation improves endurance performance (8, 12, 19, 31, 44). However, it has been shown that BCAAs influence intermediary metabolism during exercise. BCAA administration was shown to increase their concentrations in plasma and subsequently their uptake by muscle during exercise (27, 29). Long-term exercise following BCAA administration results in enhanced alanine and glutamine production in muscle (5, 27, 35), consistent with the idea that BCAA metabolism is important for alanine and glutamine formation in muscle via the so-called alanine-glucose cycle during fasting and exercise (11, 15, 17, 36, 41, 42). BCAA supplementation also suppresses the increase in blood lactate concentration and lactate release from muscle during exercise in humans and rats (13, 27) and increases the lactate threshold during an incremental exercise test in humans (28). Thus it is possible that improvement of exercise performance might require enhanced BCAA metabolism.
Indeed, it has long been recognized that skeletal muscle is a major site of BCAA utilization (22). BCAAs are largely transaminated by abundantly expressed mitochondrial branched-chain aminotransferase (BCATm) in muscle, and the resulting branched-chain α-keto acids (BCKA) are usually not oxidized, because the rate-limiting enzyme, BCKA dehydrogenase complex, is inactive at rest (4, 34). However, during exercise, BCKA dehydrogenase complex is activated, and BCAA oxidative decarboxylation in skeletal muscle is elevated (39, 40). Thus increases in BCAA oxidation occur during endurance exercise, and BCAAs are used as an energy source for exercise (2, 20). In fact, BCAA are among the few amino acids that can be actively metabolized in skeletal muscle during exercise (44). Furthermore, increased proteolysis, amino acid oxidation, and elevated pyruvate from glycolysis probably generate alanine and α-ketoglutarate (α-KG), thereby feeding the TCA cycle during exercise (6, 17, 33, 45). Increased alanine production in skeletal muscle is observed during exercise, and transaminations by alanine aminotransferase (AAT) and BCATm are thought to play an important role in these processes (11, 15–17, 36, 41, 42).
In the face of dramatically increased plasma BCAA levels, BCATm knockout (KO) mice exhibit markedly altered metabolic parameters, including increased food intake, leanness, enhanced energy expenditure, and improved insulin sensitivity and glucose tolerance (38). BCATm KO mice have an increased percentage of lean mass, consistent with the use of BCAAs by body builders. In contrast, based on previous studies, one might expect that disrupting BCAA metabolism would impair the ability to exercise. It is unclear, therefore, how exercise and metabolic changes produced by exercise would be affected by disruption of the first step in BCAA metabolism that is normally so active in muscle. Thus we examined the consequences of BCATm disruption on intermediary muscle metabolism during exercise and its relationship to exercise endurance and fatigue. The data presented here confirm that BCATm-catalyzed BCAA metabolism is essential for alanine and glutamine synthesis in muscle and also suggest a possible role of BCAA metabolism in regulating lactate and ammonia production in skeletal muscle, as well as exercise endurance in mice. The biochemical basis for metabolic abnormalities caused by loss of BCAA metabolism is discussed.
MATERIALS AND METHODS
Animals.
BCATm KO and wild-type (WT) control mice were generated and maintained, as previously described (38). The mice were backcrossed to the C57/B6 background, and age-matched male mice were used for all experiments. All animal experiments were approved by the Institutional Animal Care and Use Committee at the Pennsylvania State University College of Medicine. Because the extremely high BCAA levels of BCATm KO mice are detrimental for the central nerve system, all mice were offered a choice of two diets, rodent normal chow (NC; Harland Teklad 2018, Madison, WI) that has 18% protein as a percentage of total weight, and a defined amino acid BCAA-free diet (Dyets, Bethlehem, PA) that has 17% amino acids as percentage of total weight. Subsequently, the rodent chow was replaced with a choice of a defined amino acid diet that had 17% amino acids, including BCAA and a defined amino acid BCAA-free diet (+BCAA/−BCAA). The compositions of these amino acid-purified diets and dietary selectivity in BCATm WT and KO mice were described previously (38).
Exercise protocol.
Two exercise experiments, i.e., an endurance test and a brief exercise test for metabolic profiling, were performed in randomly fed mice at 9–12 AM of the day. In the endurance test, 14 mice (7 for each genotype) of similar age were trained for 3 days in the morning by running on the treadmill (Columbus Instruments) at 11 m/min and zero incline for 5 min each day. Blood samples were collected before training to obtain baseline levels of relevant metabolites. The mice rested for 1 day before the endurance exercise test and then began running at 11 m/min and 10% incline; treadmill speed was increased 2 m/min every 5 min, up to a maximum speed of 23 m/min. Total running time was recorded, and running distance was calculated. Mice were encouraged to run as long as possible with the use of an electric grid located at the back of the treadmill (1.5 mA, 200-ms pulses, 4 Hz). Mice were defined as exhausted if they remained on the shock grid for 5 continuous seconds. For the KO mice, blood samples were collected from tail clipping at exhaustion. For control mice, the first blood samples were collected at the average time when the KO mice were exhausted (10.5 min for the first experiment) by tail clipping within 10 s after the mice were removed from the treadmill. Mice continued to run until exhaustion, at which time the second blood sample was taken. Then all of the mice were fed a choice of two defined amino acid diets, either with or without BCAAs for 6 wk, and ran again, using the same protocol.
In the brief exercise experiment, another group of 7 KO and 10 WT mice fed NC/−BCAA choice diets were trained with exercise on a treadmill for 5 min at 12 m/min for 4 days and rested for 1 day between bouts of exercise. The mice were divided into resting and exercised groups. The exercising mice ran for 7 min at 13 m/min and 10% slope and then were quickly anesthetized with isoflurane. Gastrocnemius and quadriceps muscles were immediately collected and freeze-clamped in liquid nitrogen. The time elapsed from anesthesia and tissue freezing was within 1 min. The first piece of gastrocnemius was used for nucleotide assay, and the second piece for glycogen assay. The quadriceps was used for analyses of TCA cycle intermediates (TCAI), lactate, pyruvate, and ammonia and amino acids. Similarly, tissues were also obtained from the 7 KO and 10 WT resting mice.
HPLC amino acid analysis.
Powdered gastrocnemius was extracted with 3× 10% 5-sulfosalicylic acid by homogenizing with a Polytron. The extracts were centrifuged at 3,000 g for 15 min, and supernatant was diluted 2× with an assay buffer. Amino acid analysis was accomplished using a BioChrom 30 Amino Acid Analyzer (BioChrom, Cambridge, UK) with a cation exchange column with postcolumn ninhydrin detection at 440 and 570 nm.
Ultraperformance liquid chromatography nucleotide analysis.
Perchloric acid extracts were neutralized to pH ∼7, and the precipitated perchlorate salt was removed by centrifugation. One microliter of the supernatant was injected into a Waters AccQTag Ultra BEH column (2.1 × 100 mm, 1.7 μm), and chromatographic separation was achieved on a Waters ACQUITY ultraperformance liquid chromatography system using a binary high-pressure gradient of 0.2–5.0% acetonitrile in 0.2 M triethylamonium phosphate, pH 6.6. The flow rate was 0.60 ml/min, and column temperature was maintained at 30°C. Components were monitored by UV detection at 260 nm and quantified by comparison with standards (obtained from Sigma-Aldrich), which were run individually and in mixtures at several concentrations (1,000, 200, and 40 pmol injections).
Hindlimb perfusion.
The surgical procedure for the mouse hindlimb perfusion was performed as previously described (10). After cannulation, the mouse was placed in the Vanderbilt perfusion apparatus maintained at 37°C. The perfusion pump was immediately turned on, and the influx tube was connected to the catheter placed in the abdominal aorta. The perfusion was performed in a non-recirculating (flow-through) mode, with a flow rate of 1.0 ml/min. The arterial and venous samples were collected from a perfusion line 10–15 cm proximal to the aorta cannula and distal to the inferior vena cava cannula, respectively. The standard perfusion medium was made up of Krebs-Henseleit bicarbonate buffer containing 3% BSA and 20% saline-washed bovine erythrocytes and varied amounts of glucose. Perfusate was gassed constantly with O2/CO2 (95%/5%) and maintained at 37°C. Insulin (1 mU/ml saline) was infused with a syringe pump into a polyethylene tube connected close to the aortic cannula at the infusion rate of 20 μl/min. At the end of perfusion, leg skin was stripped, and hindlimb muscle was excised and weighed to normalize rates of glucose uptake and lactate release from hindlimb muscle.
Analytic methods.
Glucose in plasma and perfusate was measured using a commercial kit (Infinity glucose, Thermo Scientific). Muscle glycogen content was measured using the glucose kit after digestion with KOH and amyloglucosidase. Plasma BCAA concentrations were measured using an enzymatic assay, as described by Beckett (3). Plasma blood urea nitrogen, ammonia and creatine kinase were measured using a Vitros Chemistry Analyzer (Ortho-Clinical Diagnosis). Muscle ammonia concentration was measured immediately after perchloric acid extraction and neutralization with KHCO3. Lactate concentrations in plasma, perfusate, and muscle extracts and muscle phosphocreatine (PCr) were measured using enzymatic analysis spectrophotometrically (23). Muscle pyruvate, malate, citrate, and α-KG were measured using enzymatic analysis, coupled with fluorophotometry (23). Protein concentrations in the pellets of muscle extracts were measured to normalize concentrations of amino acids, nucleotides, and other metabolites.
Electron microscope.
Tissue was dissected for electron microscopy and immersion fixed for 24 h in 5% glutaraldehyde and 4% paraformaldehyde, buffered with 0.1 M sodium cacodylate, pH 7.3. Following fixation, the tissue was washed in 0.1 M sodium cacodylate buffer, postfixed overnight in buffered 1% osmium tetroxide, dehydrated in a graded series of ethanol, transferred to propylene oxide, and embedded in EMbed 812 (Electron Microscopy Sciences). Then 70- to 90-nm sections were cut with a diamond knife, were mounted on 200-mesh copper grids, and stained with 2% aqueous uranyl acetate and lead citrate. The sections were examined in a Philips 400 transmission electron microscope.
Statistical analysis.
Two tailed nonpaired t-test was used to assess the difference between two groups. One-way ANOVA or repeated-measures ANOVA with Newman-Keuls posttests was used to analyze data obtained from more than two groups or multiple time points during exercise. Values are means ± SE, and P < 0.05 was considered significantly different.
RESULTS
Exercise intolerance in BCATm KO mice.
It became apparent that, during initial training, the KO mice did not run well. During the treadmill endurance exercise test, the total running time and running distance to exhaustion in BCATm KO mice at the age of ∼15 wk was only 32 and 24%, respectively, of those in WT group (Fig. 1, A and B, respectively), indicating that BCATm KO had an impaired exercise endurance. To eliminate the possibility that differences in diet composition and amino acid source (protein vs. free amino acids) might influence exercise, the same mice were switched from feeding the NC/−BCAA diet choice to the +BCAA/−BCAA diets. The endurance exercise experiment was performed again after a 6-wk diet switch. The running time (12.1 ± 1.4 min in KO vs. 27.9 ± 2.5 min in WT) and running distance (151.5 ± 19.4 m in KO vs. 418.6 ± 43.8 m in WT) to exhaustion in BCATm KO mice was 43 and 36%, respectively, of those of WT mice (P < 0.01, n = 7). The plasma BCAA concentration was elevated 22- and 4.6-fold in BCATm KO compared with WT mice fed NC/−BCAA and +BCAA/−BCAA choice diets, respectively (38) (Fig. 2D). To further examine whether plasma BCAA concentrations affect endurance, we fed another cohort of BCATm KO mice with fixed percentages of +BCAA/−BCAA diets to make their plasma BCAA levels similar to that of WT mice (unpublished protocol). We then performed an endurance test of these KO mice. The running time (16.7 ± 2.1 min in KO vs. 23.9 ± 2.4 min in WT) and running distance to exhaustion (263.3 ± 39.2 min in KO vs. 409.4 ± 52.0 min in WT) in BCATm KO mice was 67 and 64%, respectively, of those of WT mice (P < 0.05, n = 7). Thus it appears that decreasing high-plasma BCAA levels in BCATm KO mice improves their endurance. However, lower exercise capacity in BCATm KO mice, even when plasma BCAA levels are similar to those of WT mice, suggest that loss of BCAA metabolism may also cause endurance impairment in mice.
Fig. 1.
Running time and distance of mitochondrial branched-chain aminotransferase (BCATm) knockout (KO) and wild-type (WT) mice during endurance exercise on treadmill. Mice fed normal chow (NC)/branched-chain amino acid-free (−BCAA) choice diets were trained for 3 days to run and then run on treadmill to exhaustion, according to the exercise protocol described in materials and methods. Total running time was recorded (A), and running distance was calculated (B). Values are means ± SE; n = 7 for each group. *P < 0.05.
Fig. 2.
Plasma concentrations of lactate, ammonia, glucose, and BCAA before and during exercise and at exhaustion. In both BCATm KO (open bars) and WT (solid bars) mice, blood samples were collected from tail clippings immediately before and during exercise when BCATm KO mice were exhausted. Blood samples were also collected from WT mice when they were exhausted. Plasma lactate (A) and glucose (C) were measured in samples collected from mice fed NC/−BCAA choice diets. Plasma ammonia (B) and BCAA (D) were measured in sample collected from mice fed +BCAA/−BCAA choice diets. When fed these different diets, the time for BCATm KO and WT mice to reach exhaustion was not the same, but did not differ statistically. Plasma lactate when BCATm KO was exhausted was also higher in BCATm KO (14.5 ± 2.0 mM) than WT (6.5 ± 1.3 mM, P < 0.01, n = 7) mice fed +BCAA/−BCAA choice diets. Values are means ± SE; n = 7 for each group. *P < 0.05.
Elevated plasma lactate and ammonia concentrations in response to exercise in BCATm KO mice.
We measured plasma concentrations of lactate, ammonia, glucose, and BCAA in mice before and during exercise and at exhaustion. Basal plasma lactate level in BCATm KO mice did not differ from that in WT mice (Fig. 2A). In response to exercise, plasma lactate concentration in BCATm KO at exhaustion was increased 2.4-fold from the baseline level (Fig. 2A). In contrast, in WT mice, plasma lactate levels during exercise (10.5 min, at which time the KO mice were exhausted) and at the time of exhaustion (average 33 min) were not different from their resting levels.
Plasma ammonia levels of the two groups of mice at rest did not differ and rose in response to exercise in both genotypes (Fig. 2B). However, the rate of increase of plasma ammonia in BCATm KO mice was much faster than that in WT mice, leading to a 62% higher level of plasma ammonia in the KO than in the WT at 12 min of exercise, when BCATm KO mice became exhausted (Fig. 2B). In WT mice at exhaustion (average 27.9 min), plasma ammonia was increased 280% compared with their resting levels.
While plasma glucose concentration did not differ between the two groups of mice at rest, it was 21% lower in BCATm KO than that in WT mice at 10.5 min of exercise when the KO mice became exhausted (Fig. 2C). Plasma glucose in WT mice at exhaustion was significantly decreased compared with that after 10.5 min of exercise, but did not differ from the resting level. We also measured muscle glycogen contents before and after exercise, as well as during fasted-refeeding. While fed muscle glycogen content did not differ between the two groups of mice at rest, it was decreased by 34 and 46% by a brief bout of exercise in BCATm WT and KO mice, respectively (Fig. 3A). In contrast, muscle glycogen content in mice refed for 3 h after a 21-h fast was 37% higher in BCATm KO than in WT, suggesting elevated glycogen synthesis during refeeding in the KO muscle (Fig. 3B).
Fig. 3.
Muscle glycogen content during exercise and fasted-refeeding. A: gastrocnemius glycogen content in mice at rest and with a brief exercise, as described in materials and methods. Values are means ± SE; n = 10 for WT at rest and exercise, and n = 7 for BCATm KO at rest and exercise. aP < 0.05 compared with respective resting level. B: muscle glycogen content in mixed hindlimb muscle of mice during fasting and refeeding. Male mice were fasted for 21 h and then refed with NC/−BCAA choice diets for 3 h and killed to collect mixed hind muscle for glycogen measurement. Values are means ± SE; n = 8 for BCATm WT and KO during refeeding, and n = 3 for BCATm WT and KO after a 21-h fast. *P < 0.05.
Basal plasma BCAA concentration was 4.6-fold higher in BCATm KO than in WT mice when fed a choice of +BCAA/−BCAA purified amino acid diets (Fig. 2D). Plasma BCAA in BCATm KO mice at exhaustion (12 min of exercise) was further elevated from their baseline level. In contrast, plasma BCAA level in WT mice during exercise and at exhaustion did not differ from their resting level (Fig. 2D).
Plasma blood urea nitrogen concentrations at 12 min of exercise, when the KO mice reached exhaustion, were not different between BCATm KO (20 ± 3 mg/dl) and WT mice (19 ± 2 mg/dl).
Alterations in muscle nucleotide concentrations.
To explore the mechanisms underlying impaired exercise in BCATm KO mice, we measured muscle concentrations of relevant metabolites in mice at rest and after a brief exercise. The brief period of exercise was chosen to detect early metabolic alterations before the mice became exhausted. Table 1 shows the nucleotide profiling measured using ultraperformance liquid chromatography. The major change was a 12-fold increase of muscle IMP from resting level in BCATm KO mice after 7 min of exercise, which was not observed in WT mice. Muscle ATP concentrations after exercise tended to be lower in the KO than in WT mice (P < 0.08); however, they did not differ significantly from their resting levels. There were also some small differences in muscle CMP and UMP levels between the two groups of mice after exercise.
Table 1.
Nucleotide concentrations in gastrocnemius of mitochondrial branched-chain aminotransferase wild-type and knockout mice during rest and brief exercise
| Rest |
Brief Exercise |
|||
|---|---|---|---|---|
| WT | KO | WT | KO | |
| CMP | 0.02 ± 0.00 | 0.02 ± 0.01 | 0.03 ± 0.00* | 0.02 ± 0.00‡ |
| UMP | 0.88 ± 0.13 | 0.45 ± 0.12 | 0.32 ± 0.03* | 0.29 ± 0.04 |
| IMP | 0.06 ± 0.01 | 0.05 ± 0.02 | 0.09 ± 0.03 | 0.60 ± 0.19†‡ |
| CTP | 0.14 ± 0.01 | 0.13 ± 0.01 | 0.15 ± 0.01 | 0.12 ± 0.01 |
| GDP | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.00 |
| UTP | 3.81 ± 0.22 | 3.80 ± 0.43 | 4.04 ± 0.28 | 3.50 ± 0.41 |
| AMP | 0.14 ± 0.03 | 0.17 ± 0.04 | 0.17 ± 0.04 | 0.20 ± 0.02 |
| GTP | 0.37 ± 0.02 | 0.40 ± 0.04 | 0.42 ± 0.03 | 0.35 ± 0.04 |
| ADP | 1.45 ± 0.23 | 1.61 ± 0.28 | 1.64 ± 0.38 | 1.76 ± 0.19 |
| ATP | 19.03 ± 1.22 | 17.16 ± 1.57 | 19.46 ± 1.32 | 15.21 ± 1.71§ |
| ATP+ADP+AMP | 20.62 ± 1.10 | 18.94 ± 1.76 | 19.46 ± 1.32 | 17.17 ± 1.89 |
Values are means ± SE in nmol/mg protein; n = 7 for each group at rest and exercise. WT, wild type; KO, knockout.
P <0.05, WT exercise vs. WT rest;
P <0.05, KO exercise vs. KO rest;
P <0.05, KO exercise vs. WT exercise;
P ≤ 0.077, KO exercise vs. WT exercise.
Alterations in muscle amino acid concentrations.
Table 2 shows muscle concentrations of amino acids and related amines. Consistent with previously reported plasma data (38), muscle Val, Ile, and Leu concentrations in resting BCATm KO mice fed a choice of NC/−BCAA amino acid diet was increased 23-, 28-, and 12-fold, respectively, compared with WT mice; and the increases were 31-, 39-, and 21-fold, respectively, after exercise. Interestingly, muscle concentrations of alanine and glutamine and related amino acids, glutamate and aspartate, were decreased by ∼70, 60, 75, and 45%, respectively, in the KO mice, both at rest and after exercise. The decreases in these amino acids were also found in plasma (38) or other tissues such as liver and heart (data not shown). Resting muscle concentrations of o-phosphoserine, tyrosine, histidine, carnosine, and anserine in the KO mice were lowered by 30–55%, compared with that in WT mice. Resting arginine levels were elevated by 85% in the KO mice, while their muscle urea concentrations were 35% less than in WT mice. Brief exercise significantly elevated only Leu concentration in BCATm KO mice and decreased tyrosine concentration in WT mice from resting levels.
Table 2.
Concentrations of amino acids and related metabolites in quadriceps muscle of mitochondrial branched-chain aminotransferase wild-type and knockout mice during rest and brief exercise
| Rest |
Brief Exercise |
|||
|---|---|---|---|---|
| WT | KO | WT | KO | |
| Valine | 1.16 ± 0.13 | 26.5 ± 4.5* | 0.92 ± 0.06 | 28.9 ± 4.3† |
| Isoleucine | 0.33 ± 0.05 | 9.2 ± 0.9* | 0.28 ± 0.02 | 10.9 ± 0.6† |
| Leucine | 0.83 ± 0.10 | 10.1 ± 1.2* | 0.67 ± 0.03 | 14.1 ± 1.1†§ |
| Aspartate | 4.99 ± 0.73 | 2.68 ± 0.44* | 4.66 ± 0.24 | 2.64 ± 0.17† |
| Glutamate | 6.37 ± 0.74 | 1.33 ± 0.18* | 5.55 ± 0.36 | 1.55 ± 0.21† |
| Glutamine | 8.20 ± 0.63 | 3.27 ± 0.49* | 7.78 ± 0.37 | 3.41 ± 0.37† |
| Alanine | 12.9 ± 1.3 | 3.7 ± 0.7* | 12.0 ± 0.78 | 3.9 ± 0.6† |
| Urea | 58.6 ± 3.8 | 38.0 ± 5.8* | 46.9 ± 3.2 | 39.2 ± 1.8 |
| Ornithine | 0.28 ± 0.07 | 0.42 ± 0.10 | 0.20 ± 0.02 | 0.42 ± 0.08 |
| Arginine | 1.26 ± 0.13 | 2.27 ± 0.16* | 1.38 ± 0.0.16 | 1.86 ± 0.19 |
| Citrulline | 0.48 ± 0.03 | 0.40 ± 0.06 | 0.50 ± 0.02 | 0.40 ± 0.03 |
| Threonine | 2.85 ± 0.28 | 2.34 ± 0.53 | 2.29 ± 0.12 | 2.26 ± 0.41 |
| Serine | 2.47 ± 0.27 | 1.88 ± 0.32 | 2.28 ± 0.11 | 2.01 ± 0.21 |
| Glycine | 23.1 ± 2.5 | 22.8 ± 3.5 | 21.2 ± 2.2 | 21.5 ± 1.6 |
| Sarcosine | 4.15 ± 1.54 | 1.21 ± 0.40 | 1.11 ± 0.07 | 0.65 ± 0.08 |
| O-phosphoserine | 0.28 ± 0.03 | 0.14 ± 0.02* | 0.20 ± 0.01 | 0.18 ± 0.01 |
| Asparagine | 0.37 ± 0.06 | 0.23 ± 0.05 | 0.28 ± 0.02 | 0.23 ± 0.03 |
| Methionine | 4.18 ± 0.84 | 3.67 ± 0.86 | 4.19 ± 0.50 | 4.93 ± 0.72 |
| Tyrosine | 0.86 ± 0.06 | 0.38 ± 0.08* | 0.58 ± 0.03‡ | 0.38 ± 0.06 |
| β-Alanine | 0.39 ± 0.04 | 0.40 ± 0.05 | 0.49 ± 0.06 | 0.42 ± 0.03 |
| Phenylalanine | 0.44 ± 0.04 | 0.33 ± 0.06 | 0.30 ± 0.01 | 0.31 ± 0.03 |
| Lysine | 4.55 ± 0.50 | 3.99 ± 0.73 | 4.51 ± 0.55 | 4.10 ± 0.83 |
| Histidine | 0.85 ± 0.08 | 0.54 ± 0.09* | 0.82 ± 0.05 | 0.56 ± 0.03 |
| Carnosine | 25.4 ± 2.3 | 17.0 ± 1.6* | 22.7 ± 2.4 | 17.3 ± 2.0 |
| Anserine | 38.9 ± 3.7 | 25.9 ± 2.9* | 30.0 ± 2.7 | 24.8 ± 1.3 |
| Taurine | 159.2 ± 13.0 | 139.0 ± 10.5 | 146.8 ± 3.6 | 150.0 ± 5.7 |
| Proline | 1.40 ± 0.40 | 1.05 ± 0.18 | 1.09 ± 0.12 | 1.55 ± 0.26 |
| Hydroxyproline | 1.10 ± 0.17 | 1.01 ± 0.15 | 1.30 ± 0.15 | 0.97 ± 15 |
Values are means ± SE in nmol/mg protein; n = 10 for WT at rest and exercise, and n = 7 for mitochondrial branched-chain aminotransferase KO at rest and exercise for each group.
P <0.05, KO rest vs. WT rest;
P <0.05, KO exercise vs. WT exercise;
P <0.05, WT exercise vs. WT rest;
P <0.05, KO exercise vs. KO rest.
Alterations in muscle concentrations of lactate, TCAI, ammonia and PCr.
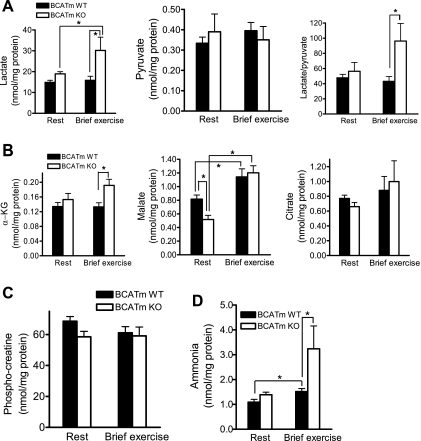
As in plasma (Fig. 2A), muscle lactate levels in BCATm KO at rest did not differ from that in WT mice, but were markedly elevated after exercise, compared with WT mice (Fig. 4A). Muscle pyruvate concentrations did not differ between the KO and WT mice and was unaffected by a brief exercise. The lactate-to-pyruvate ratio was significantly higher in the KO than in WT after exercise. Muscle α-KG concentration did not differ between the two groups at rest; however, it was higher in the KO mice with exercise than in WT mice (Fig. 4B). Muscle malate concentration was 37% lower in the KO than WT mice at rest and rose with exercise in both groups, so that no difference was found between them after exercise. Muscle citrate concentrations did not differ between the KO and WT mice and was unaffected by brief exercise. Resting muscle PCr level did not differ between the KO and WT mice and was barely influenced by brief exercise (Fig. 4C). Muscle ammonia level (Fig. 4D) did not differ between the two groups at rest and rose with exercise in both groups. However, the increment in ammonia was greater in the KO mice than in WT mice after exercise, consistent with the alterations in plasma ammonia (Fig. 4B).
Fig. 4.
Concentrations of lactate and pyruvate (A), TCA cycle intermediates (B), phosphocreatine (C), and ammonia (D) in quadriceps of mice at rest and with a brief exercise. All mice fed NC/−BCAA choice diets were trained for 4 days to run and rest for 1 day before death. Mice were killed either at rest or after running for 7 min at 13 m/min and 10% slope, and skeletal muscles were collected for analyses of metabolites. Values are means ± SE; n = 10 for WT at rest and exercise, and n = 7 for BCATm KO at rest and exercise. α-KG, α-ketoglutarate. *P < 0.05.
Increased lactate production from glucose during hindlimb perfusion.
To confirm that elevated muscle and plasma lactate levels were indeed derived from muscle glucose metabolism, we performed in situ hindlimb perfusion to measure lactate release (Fig. 5). While insulin-stimulated glucose uptake in hindlimb perfused with either low or high glucose did not differ between the WT and KO mice, high-glucose perfusion led to an approximately threefold increase of glucose uptake compared with low glucose. The very high dose of insulin (1 mU/ml) used in this experiment might have eliminated any difference between muscle glucose uptake between the KO and WT mice. Lactate release during low glucose/insulin perfusion did not differ between the two groups of mice; however, it was 47% higher during high glucose/insulin perfusion in the KO than in WT mice, suggesting enhanced lactate production from skeletal muscle of BCATm KO mice when glycolysis is stimulated, a situation that also occurs during exercise.
Fig. 5.
Glucose uptake (A) and lactate release (B) during hindlimb perfusion. The perfusion protocol was described in materials and methods. Overnight fasted mice were perfused with 6.1 mM glucose/insulin at 1 mU/ml for 40 min and then 19.1 mM glucose/insulin at 1 mU/ml for another 40 min. Arterial and venous samples were collected every 10 min. Glucose uptake was calculated by the following formula: (arterial glucose − venous glucose) × flow rate/weight of tissue perfused. Lactate release was calculated as (venous lactate − arterial lactate) × flow rate/weight of tissue perfused. Values are means ± SE; n = 5 for WT and 4 for BCATm KO mice. *P < 0.05.
Apparently normal muscle ultrastructure and plasma creatine kinase activity.
We performed transmission electron microscopy in gastrocnemius muscle sections. A representative image is shown in Fig. 6. Inspection of the photo electron micrographs revealed no systematic differences in the sarcomere length, numbers of subsarcolemmal mitiochondria, cristae pattern of the mitochondria, mitochondria associated with sarcomeres, or myofibril structure and organization. We also measured basal plasma creatine kinase activity and found no difference between the KO (240 ± 28 U/l) and WT (207 ± 29 U/l) mice. These results suggest that the exercise intolerance in BCATm KO mice is not a result of structural alterations or damage to skeletal muscle.
Fig. 6.
Transmission electron image of gastrocnemius from BCATm WT (+/+) and KO (−/−) mice. Electron photomicroscope images were developed from gastrocnemius of 3 WT and 3 KO mice. A representative image is shown.
DISCUSSION
The data reported here show that BCATm KO mice are exercise intolerant, and this impairment is strongly associated with markedly increased lactate release and ammonia concentration from skeletal muscle during exercise. During brief exercise, lactate and ammonia concentrations and the lactate-to-pyruvate ratio in skeletal muscle were greatly elevated in BCATm KO compared with WT mice. Meanwhile, BCATm KO mice exhibited 43–79% decreases in muscle concentrations of alanine, glutamine, aspartate, and glutamate during rest and exercise. Basal muscle malate level was 37% lower in the KO than WT mice, and, in response to a brief exercise, the malate level was increased 40 and 132% from their resting levels in the WT and KO mice, respectively. Basal muscle α-KG level did not differ between the KO and WT mice, but was 44% higher in the KO during exercise. While muscle [ATP] tended to be lower, muscle [IMP] (where brackets denote concentration) was sevenfold higher in the KO compared with WT mice with a brief exercise.
In the past decades, it has been established that, by acting as a nitrogen donor, BCAA metabolism is important for alanine and glutamine formation in muscle in the so-called alanine-glucose cycle during fasting and exercise (11, 15, 17, 36, 41, 42). Our finding that loss of BCATm function in BCATm KO mice is associated with a decrease in skeletal muscle alanine and glutamine concentrations confirms the belief that BCAA catabolism is essential for alanine and glutamine production. These in vivo data also support and extend an in vitro study of rat muscle conducted by Snell at al. 25 years ago (42). Using enzymatic inhibitors and biochemical approaches, they provided evidence that mitochondrial BCAA metabolism and cytosolic alanine and glutamine formation are strongly coupled, most likely through aspartate aminotransferase (42). The decreased alanine and glutamine production and decreased transport of these amino acids to liver from skeletal muscle may contribute to hypoglycemia during prolonged fasting observed in BCATm KO mice, probably due to diminished hepatic gluconeogenesis (38).
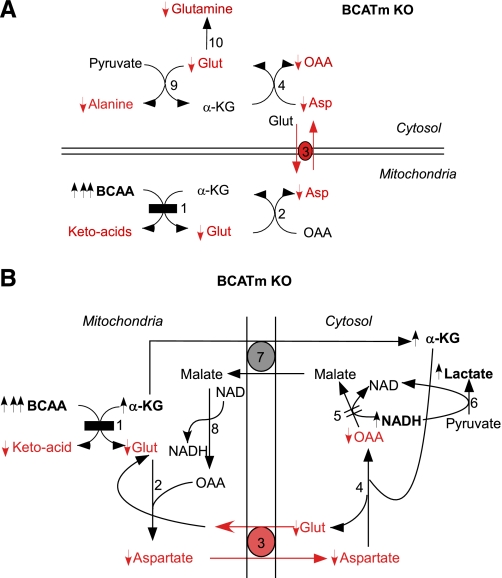
We hypothesize that the metabolic imbalance caused by BCATm disruption slows the malate/aspartate shuttle in skeletal muscle, leading to other metabolic perturbations and an exercise defect (Fig. 7). The lack of BCATm activity may deplete the mitochondrial glutamate and aspartate pools. The rate-limiting step in the malate/aspartate shuttle is the exchange of intramitochondrial aspartate for cytosolic glutamate. This exchange is energy dependent and electrogenic, since a proton is transported with glutamate but not aspartate. A deficit in cytosolic aspartate slows transamination of aspartate with α-KG and the formation of oxaloacetate and glutamate in cytosol, even though α-KG is present in excess. Depleted glutamate prevents alanine and glutamine formation by AAT and glutamine synthetase, respectively (Fig. 7A). Cytosolic oxaloacetate deficit slows formation of malate from a depleted oxaloacetate pool (Fig. 7B). This is the key step that converts cytosolic NADH to NAD+. A high ratio of NADH to NAD+ in cytosol increases the lactate-to-pyruvate ratio, independent of O2 supply, and limits pyruvate to supply the TCA cycle. Lactate dehydrogenase likely catalyzes a near-equilibrated reaction of lactate + NAD+ ↔ pyruvate + NADH, so that the lactate-to-pyruvate ratio provides an estimate of cytosolic, but not mitochondrial, NADH-to-NAD+ ratio (47).
Fig. 7.
Metabolic impairments in skeletal muscle caused by disruption of the first step of BCAA metabolism. A: blockage of BCAA transamination leads to decreased alanine glutamine synthesis from pyruvate and glutamate. The scheme emphasizes the importance of translocation of glutamate from mitochondria to cytosol via aspartate aminotransferanses and glutamate-aspartate antiporter. B: model of perturbed malate/aspartate shuttle in mice lacking BCAA metabolism. The diagram emphasizes the importance of BCATm-catalyzed transamination to provide glutamate and aspartate for the malate/aspartate shuttle and the role of this shuttle to transfer electrons from cytosol to mitochondria to avoid lactate overproduction during exercise. BCATm disruption (shown as black rectangle) leads to decreases in metabolites shown in red and as “↓”, and increases in metabolites are shown in bold characters and as “↑”. The changes in branched-chain α-ketoacids, OAA (oxaloacetate), and NADH concentrations were not measured and are deduced. The enzymes or proteins involved are as follows: 1, BCATm; 2, mitochondrial aspartate aminotransferanse; 3, glutamate-aspartate antiporter; 4, cytosolic aspartate aminotransferanse; 5, cytosolic malate dehydrogenase (the “=” sign donates a diminished flux in BCATm KO mice); 6, lactate dehydrogenase; 7, malate-α-ketoglutarate antiporter; 8, mitochondrial malate dehydrogenase; 9, alanine aminotransferanse; and 10, glutamine synthetase.
On the other hand, we cannot rule out the effect of possible existence of O2 deficit on lactate overproduction and other cellular stress in BCATm KO mice. We previously found that O2 uptake (V̇o2) in BCATm KO mice at rest was significantly elevated (38). Exercise significantly increases V̇o2 in correlation with exercise intensity. Given exercise intolerance in BCATm KO mice, the maximum running speed in BCATm KO mice would be lower compared with that in WT mice. Thus, although we compared metabolic responses at the same absolute speed, the mice were running at different relative exercise intensities (different percentage of maximum V̇o2). This could enhance the cellular stress in BCATm KO mice, as recently reported in a kinase-dead AMP-activated protein kinase-α2 mice (24). BCATm is also abundantly expressed in heart, and the amino acid profile in heart of BCATm KO mice is similar to that in skeletal muscle (data not shown). Thus the cardiac function of these animals could be compromised during exercise, leading to decreased O2 delivery to peripheral tissues and lowered anaerobic threshold. Although we did not measure glucose uptake and glycolysis in vivo during exercise in this study, plasma glucose level was lower in the KO during exercise at the time of exhaustion compared with WT mice, and glycogenolysis during exercise appeared to be greater in the KO (Fig. 3A). Moreover, markedly improved glucose tolerance and insulin sensitivity, as well as increased basal respiratory quotient (38) and muscle glycogen synthesis during fasted-refeeding conditions (Fig. 3B) in these mice, suggest that they exhibit faster glucose utilization, which would lead to greater cytosolic NADH production from glycolysis and a greater reliance on the malate/aspartate shuttle. However, BCATm KO mice might be unable to meet demands during exercise because this process is impaired, as discussed.
TCA cycle flux in normal exercising skeletal muscle is dramatically increased from the resting level, thereby predominantly providing ATP from mitochondrial oxidative phosphorylation during prolonged exercise. Meanwhile within the first 5–10 min of exercise, TCAI, especially malate, are increased approximately four- to fivefold in human muscles (6). The AAT-catalyzed reaction (glutamate + pyruvate ↔ alanine + α-KG) is probably the main anaplerotic mechanism responsible for TCAI expansion during exercise (6). In WT mice, we saw a 40% increase in malate level, but no alterations in α-KG or citrate concentrations after 7 min of treadmill exercise (Fig. 3). The much lower TCAI response to exercise in this study compared with others may be due to low level of exercise intensity and species (mice vs. humans). Because the AAT-catalyzed reaction could be diminished, as suggested by a large decrease in alanine concentration in BCATm KO mice, it is possible that the much enhanced purine nucleotide cycle (PNC) is responsible for the elevated TCAI, malate, and α-KG, as well as for the decreased aspartate in BCATm KO animals during exercise. The second half of PNC includes these reactions: IMP + aspartate + GTP → adenylosuccinate + GDP + Pi by adenylosuccinate synthase, and adenylosuccinate → AMP + fumarate by adenylosuccinate lyase. At the expense of aspartate, the end product fumarate enters TCA cycle; thus it has been proposed that, at least in rodents, the PNC is a major anaplerotic process (1, 9). Also, the totally blocked BCATm-catalyzed transamination (α-KG + BCAA ↔ BCKA + glutamate) within skeletal muscle mitochondria of the KO mice should contribute to the elevated α-KG and decreased glutamate, in agreement with the observation that BCAA metabolism provides a drain on TCAI during exercise (44, 45). Thus it appears that BCATm KO mice use an alternative way to provide TCAI so that anaplerosis is unimpaired in muscle during exercise. However, since TCAI pool size can be disassociated with oxidative phosphorylation (6), further studies are warranted to determine whether mitochondrial function and TCA cycle activity during exercise are impaired in these animals.
Decreases in [ATP]-to-[ADP] ratio and increases in [H+] (14, 37), especially increased muscle lactate in BCATm KO, can cause a much greater PNC in muscle and, therefore, dramatically higher muscle [IMP] and ammonia. Enhanced PNC could, therefore, help maintain the energy state of the cell, preventing a further decrease in ATP-to-ADP ratio (21, 26). It was reported that transamination of BCAAs in muscle may be a source of ammonia production (27). However, BCAA transamination in tissues except neurons is blocked in BCATm KO mice, and the glutamate dehydrogenase-catalyzed reaction (glutamate + NADP+ → α-KG + NH3 + NADPH) in skeletal muscle of BCATm KO mice could be slowed due to deceased glutamate formation. Moreover, GDH activity in skeletal muscle is normally low (1). Thus, as it is thought that a major source of ammonia in skeletal muscle during exercise is the PNC (25, 26), the elevated ammonia production during exercise in BCATm KO mice seems primarily to be derived from PNC.
Finally, it should be pointed out that, although the metabolic defects in BCATm KO mice lead to greater lactate and ammonia production, further studies are warranted to determine whether lactate and ammonia overproduction directly link to endurance impairment in these mice. It has been debated whether lactate is a direct fatigue factor of exercise (32, 43, 46); however, lactate threshold and onset of blood lactate accumulation are often good predicators of endurance performance, muscle oxidative capacity, and the rate of carbohydrate utilization during exercise (7, 18). The concentrations of ammonia often rise in blood during both high-intensity and prolonged exercises (30, 37). In conclusion, the data obtained from this study suggest that BCAA metabolism is required for normal exercise metabolism and endurance.
GRANTS
The research is supported from an Ajinomoto Amino Acid Research grant (to P. She) and National Institute of Diabetes and Digestive and Kidney Diseases Grants RO1 DK053843 and DK062880 (to C. J. Lynch).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Beth Halle, Vance Albaugh, and Stephanie Goshorn for technical assistance and Kathryn LaNoue and Charles Lang for reading the manuscript. We also thank Patrick Donahue and Ken Grimes from the Vanderbilt DRTC for amino acid analysis.
REFERENCES
- 1. Aragon JJ, Lowenstein JM. The purine-nucleotide cycle. Comparison of the levels of citric acid cycle intermediates with the operation of the purine nucleotide cycle in rat skeletal muscle during exercise and recovery from exercise. Eur J Biochem 110: 371–377, 1980 [DOI] [PubMed] [Google Scholar]
- 2. Babij P, Matthews SM, Rennie MJ. Changes in blood ammonia, lactate and amino acids in relation to workload during bicycle ergometer exercise in man. Eur J Appl Physiol Occup Physiol 50: 405–411, 1983 [DOI] [PubMed] [Google Scholar]
- 3. Beckett PR. Spectrophotometric assay for measuring branched-chain amino acids. In: Methods in Enzymology Branched-chain amino acids, Part B, edited by Harris RA, Sokatch JR. New York: Academic, 2000, p. 40–47 [DOI] [PubMed] [Google Scholar]
- 4. Block KP, Richmond WB, Mehard WB, Buse MG. Glucocorticoid-mediated activation of muscle branched-chain α-keto acid dehydrogenase in vivo. Am J Physiol Endocrinol Metab 252: E396–E407, 1987 [DOI] [PubMed] [Google Scholar]
- 5. Blomstrand E, Ek S, Newsholme EA. Influence of ingesting a solution of branched-chain amino acids on plasma and muscle concentrations of amino acids during prolonged submaximal exercise. Nutrition 12: 485–490, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Bowtell JL, Marwood S, Bruce M, Constantin-Teodosiu D, Greenhaff PL. Tricarboxylic acid cycle intermediate pool size: functional importance for oxidative metabolism in exercising human skeletal muscle. Sports Med 37: 1071–1088, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Cairns SP. Lactic acid and exercise performance: culprit or friend? Sports Med 36: 279–291, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Calders P, Matthys D, Derave W, Pannier JL. Effect of branched-chain amino acids (BCAA), glucose, and glucose plus BCAA on endurance performance in rats. Med Sci Sports Exerc 31: 583–587, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Canela EI, Ginesta I, Franco R. Simulation of the purine nucleotide cycle as an anaplerotic process in skeletal muscle. Arch Biochem Biophys 254: 142–155, 1987 [DOI] [PubMed] [Google Scholar]
- 10. Chan TM, Dehaye JP. Hormone regulation of glucose metabolism in the genetically obese-diabetic mouse. Diabetes 30: 211–218, 1981 [DOI] [PubMed] [Google Scholar]
- 11. Chang TW, Goldberg AL. The origin of alanine produced in skeletal muscle. J Biol Chem 253: 3677–3684, 1978 [PubMed] [Google Scholar]
- 12. Crowe MJ, Weatherson JN, Bowden BF. Effects of dietary leucine supplementation on exercise performance. Eur J Appl Physiol 97: 664–672, 2006 [DOI] [PubMed] [Google Scholar]
- 13. De Palo EF, Gatti R, Cappellin E, Schiraldi C, De Palo CB, Spinella P. Plasma lactate, GH and GH-binding protein levels in exercise following BCAA. Amino Acids 20: 1–11, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Dudley GA, Terjung RL. Influence of acidosis on AMP deaminase activity in contracting fast-twitch muscle. Am J Physiol Cell Physiol 248: C43–C50, 1985 [DOI] [PubMed] [Google Scholar]
- 15. Felig P, Wahren J. Amino acid metabolism in exercising man. J Clin Invest 50: 2703–2714, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Galim EB, Hruska K, Bier DM, Matthews DE, Haymond MW. Branched-chain amino acid nitrogen transfer to alamine in vivo in dogs. Direct isotopic determination with [15N]leucine. J Clin Invest 66: 1295–1304, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garber AJ, Karl IE, Kipnis DM. Alanine and glutamine synthesis and release from skeletal muscle. II. The precursor role of amino acids in alanine and glutamine synthesis. J Biol Chem 251: 836–843, 1976 [PubMed] [Google Scholar]
- 18. Gladden LB. Lactate metabolism: a new paradigm for the third millennium. J Physiol 558: 5–30, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gleeson M. Interrelationship between physical activity and branched-chain amino acids. J Nutr 135: 1591S–1595S, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Hagg SA, Morse EL, Adibi SA. Effect of exercise on rates of oxidation, turnover, and plasma clearance of leucine in human subjects. Am J Physiol Endocrinol Metab 242: E407–E410, 1982 [DOI] [PubMed] [Google Scholar]
- 21. Hancock CR, Brault JJ, Terjung RL. Protecting the cellular energy state during contractions: role of AMP deaminase. J Physiol Pharmacol 57, Suppl 10: 17–29, 2006 [PubMed] [Google Scholar]
- 22. Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu Rev Nutr 4: 409–454, 1984 [DOI] [PubMed] [Google Scholar]
- 23. Janet V, Passonneau OHL. Enzymatic Analysis: A Practical Guide. Totowa, NJ: Humana, 1993 [Google Scholar]
- 24. Lee-Young RS, Griffee SR, Lynes SE, Bracy DP, Ayala JE, McGuinness OP, Wasserman DH. Skeletal muscle AMP-activated protein kinase is essential for the metabolic response to exercise in vivo. J Biol Chem 284: 23925–23934, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lowenstein J, Tornheim K. Ammonia production in muscle: the purine nucleotide cycle. Science 171: 397–400, 1971 [DOI] [PubMed] [Google Scholar]
- 26. Lowenstein JM. The purine nucleotide cycle revisited [corrected]. Int J Sports Med 11, Suppl 2: S37–S46, 1990 [DOI] [PubMed] [Google Scholar]
- 27. MacLean DA, Graham TE, Saltin B. Stimulation of muscle ammonia production during exercise following branched-chain amino acid supplementation in humans. J Physiol 493: 909–922, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matsumoto K, Koba T, Hamada K, Tsujimoto H, Mitsuzono R. Branched-chain amino acid supplementation increases the lactate threshold during an incremental exercise test in trained individuals. J Nutr Sci Vitaminol (Tokyo) 55: 52–58, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Matsumoto K, Mizuno M, Mizuno T, Dilling-Hansen B, Lahoz A, Bertelsen V, Munster H, Jordening H, Hamada K, Doi T. Branched-chain amino acids and arginine supplementation attenuates skeletal muscle proteolysis induced by moderate exercise in young individuals. Int J Sports Med 28: 531–538, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Mutch BJ, Banister EW. Ammonia metabolism in exercise and fatigue: a review. Med Sci Sports Exerc 15: 41–50, 1983 [PubMed] [Google Scholar]
- 31. Negro M, Giardina S, Marzani B, Marzatico F. Branched-chain amino acid supplementation does not enhance athletic performance but affects muscle recovery and the immune system. J Sports Med Phys Fitness 48: 347–351, 2008 [PubMed] [Google Scholar]
- 32. Nielsen OB, de Paoli F, Overgaard K. Protective effects of lactic acid on force production in rat skeletal muscle. J Physiol 536: 161–166, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Palmer TN, Caldecourt MA, Snell K, Sugden MC. Alanine and inter-organ relationships in branched-chain amino and 2-oxo acid metabolism. Biosci Rep 5: 1015–1033, 1985 [DOI] [PubMed] [Google Scholar]
- 34. Paul HS, Adibi SA. Mechanism of increased conversion of branched chain keto acid dehydrogenase from inactive to active form by a medium chain fatty acid (octanoate) in skeletal muscle. J Biol Chem 267: 11208–11214, 1992 [PubMed] [Google Scholar]
- 35. Ren JM, Broberg S, Sahlin K, Hultman E. Influence of reduced glycogen level on glycogenolysis during short-term stimulation in man. Acta Physiol Scand 139: 467–474, 1990 [DOI] [PubMed] [Google Scholar]
- 36. Ruderman NB, Berger M. The formation of glutamine and alanine in skeletal muscle. J Biol Chem 249: 5500–5506, 1974 [PubMed] [Google Scholar]
- 37. Sahlin K, Broberg S. Adenine nucleotide depletion in human muscle during exercise: causality and significance of AMP deamination. Int J Sports Med 11, Suppl 2: S62–S67, 1990 [DOI] [PubMed] [Google Scholar]
- 38. She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ, Hutson SM. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab 6: 181–194, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shimomura Y, Honda T, Shiraki M, Murakami T, Sato J, Kobayashi H, Mawatari K, Obayashi M, Harris RA. Branched-chain amino acid catabolism in exercise and liver disease. J Nutr 136: 250S–253S, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Shimomura Y, Murakami T, Nakai N, Nagasaki M, Harris RA. Exercise promotes BCAA catabolism: effects of BCAA supplementation on skeletal muscle during exercise. J Nutr 134: 1583S–1587S, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Snell K, Duff DA. Branched-chain amino acid metabolism and alanine formation in rat diaphragm muscle in vitro. Effects of dichloroacetate. Biochem J 223: 831–835, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Snell K, Duff DA. Branched-chain amino acid metabolism and alanine formation in rat muscles in vitro. Mitochondrial-cytosolic interrelationships. Biochem J 225: 737–743, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vissing J, Gansted U, Quistorff B. Exercise intolerance in mitochondrial myopathy is not related to lactic acidosis. Ann Neurol 49: 672–676, 2001 [PubMed] [Google Scholar]
- 44. Wagenmakers AJ. Muscle amino acid metabolism at rest and during exercise: role in human physiology and metabolism. Exerc Sport Sci Rev 26: 287–314, 1998 [PubMed] [Google Scholar]
- 45. Wagenmakers AJM.(Editor). Amino Acid Metabolism in Exercise. Oxford, UK: Blackwell Science, 2000, p. 119–132 [Google Scholar]
- 46. Wilson JR, Mancini DM, Ferraro N, Egler J. Effect of dichloroacetate on the exercise performance of patients with heart failure. J Am Coll Cardiol 12: 1464–1469, 1988 [DOI] [PubMed] [Google Scholar]
- 47. Zhou L, Stanley WC, Saidel GM, Yu X, Cabrera ME. Regulation of lactate production at the onset of ischaemia is independent of mitochondrial NADH/NAD+: insights from in silico studies. J Physiol 569: 925–937, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]