Abstract
Null alleles of the gene encoding NEMO (NF-κB essential modulator) are lethal in hemizygous mice and men, whereas hypomorphic alleles typically cause a syndrome of immune deficiency and ectodermal dysplasia. Here we describe an allele of Ikbkg in mice that impaired Toll-like receptor signaling, lymph node formation, development of memory and regulatory T cells, and Ig production, but did not cause ectodermal dysplasia. Degradation of IκBα, which is considered a primary requirement for NEMO-mediated immune signaling, occurred normally in response to Toll-like receptor stimulation, yet ERK phosphorylation and NF-κB p65 nuclear translocation were severely impaired. This selective loss of function highlights the immunological importance of NEMO-regulated pathways beyond IκBα degradation, and offers a biochemical explanation for rare immune deficiencies in man.
Keywords: mutagenesis, N-ethyl-nitrosourea, nuclear factor–κB essential modulator, p65, Toll-like receptor
NF-κB transcription factors orchestrate numerous immunological and developmental responses (1). Among the receptors that trigger their activity are the Toll-like receptors (TLRs), the IL-1 receptor family, B and T cell antigen receptors (BCR/TCR), and TNF receptor family members, including the lymphotoxin β receptor (LTβR), CD40, and ectodysplasin-A receptor (EDAR). NF-κB activity is restrained by members of the IκB family, which prevent nuclear translocation of NF-κB subunits. IκB proteins (IκBα, IκBβ, and IκBε) are phosphorylated and degraded in response to stimulation, allowing release and nuclear translocation of NF-κB. IκB phosphorylation is mediated by the IκB kinase (IKK) complex, which consists of IKK1 (IKKα) and IKK2 (IKKβ) catalytic subunits, and the regulatory subunit NEMO (IKKγ).
IKK activity is also required for phosphorylation of the NF-κB members p105 (NF-κB1) and p65 (RelA). Phosphorylated p105 is polyubiquitinated and degraded in a manner similar to the IκB proteins (2), promoting activation of the TPL2 (tumor progression locus 2) → ERK axis (3, 4). p65 undergoes IKK-dependent phosphorylation at serine 536 (5), although the significance of this is unclear (1).
The range of diseases caused by NEMO mutations highlights the physiological importance of NEMO and the IKK complex. Null alleles of the X-linked gene encoding NEMO, IKBKG, cause the inflammatory skin disease incontinentia pigmenti in heterozygous females (6), and are lethal in hemizygous males, as they are in mice (7–9). Milder hypomorphic alleles are compatible with viability in males, but cause severe immune deficiency and developmental abnormalities of the teeth, hair, or sweat glands (10). These abnormalities of ectodermal derivatives are thought to result from disruption of EDAR signaling, yet there are reports of IKBKG mutations in immune-deficient patients without ectodermal dysplasia (11, 12). Other mutations appear to disrupt EDAR signaling and CD40-mediated Ig class switching but not TLR signaling (13), whereas another mutation disrupts EDAR signaling, but leaves TLR and CD40 signaling largely intact (14).
Whatever the combination of pathways affected, immune deficiency is a unifying consequence of germline NEMO mutations. But of all of the targets of NEMO and the IKK complex, there is no consensus of which are most critical for immunity. A great deal has been learned from conditional mutations of IKKα, IKKβ, and NEMO, but a full understanding of their physiological function has been precluded by the embryonic lethality that occurs in their absence. The same may be true of the biochemical functions of NEMO, some of which may occur downstream of pathways arrested in the absence of NEMO (e.g., p65 translocation or TPL2 activation).
Here we describe an N-ethyl-N-nitrosourea (ENU)–induced mutation of Ikbkg in mice that disrupted TLR signaling and conferred susceptibility to viral and bacterial infection. Hemizygous males were fully viable, yet azoospermic, and most lacked inguinal lymph nodes. Serum Ig concentrations were reduced, whereas memory, regulatory, and natural killer (NK) T cells were fewer in number. In response to TLR stimulation, IκBα degradation and phosphorylation of the MAPK homologue p38 were intact, yet phosphorylation of ERK and nuclear translocation of p65 were severely diminished. These data indicate that broad suppression of NEMO-mediated immune signaling can occur despite IκBα degradation.
Results
The panr2 Mutation Impairs TLR Signaling and Resistance to Infection.
Among more than 30,000 third generation (G3) progeny of C57BL/6J males mutagenized with ENU, we observed a heritable phenotype marked by diminished secretion of soluble, bioactive TNF by macrophages stimulated with TLR ligands. This phenotype, designated panr2 (pan-resistance 2), was characterized by an impaired response to ligands for TLR3 [poly(I:C)], TLR4 (LPS), TLR7 (R-848) and TLR9 (CpG oligodeoxynucleotides) and the heterodimers TLR1/2 (Pam3CSK4) and TLR2/6 (MALP-2, peptidoglycan; Fig. 1). Multiple cytokines were affected, including TNFα, IL-6, IL-12p40, and MCP-1, as well as the inflammatory mediator NO. Secretion of type 1 IFN in response to TLR3 and TLR4 stimulation was not affected. panr2 mice were also highly susceptible to an otherwise sublethal dose of murine cytomegalovirus (MCMV; Fig. S1A). Death within 6 d of MCMV infection was indicative of an innate immune defect, as combined deficiency of B and T cells permits survival of a similar infection for more than 2 weeks (15). Similarly, panr2 mice succumbed to infection with Listeria monocytogenes within 4 d (Fig. S1B).
Fig. 1.
The panr2 mutation impairs TLR-induced cytokine secretion. Thioglycollate-elicited peritoneal macrophages from WT (n = 12) and panr2 (n = 6) mice were stimulated with TLR ligands, and TNFα, IL-6, IL-12p40, and MCP-1 concentrations were calculated by ELISA. NO was measured by Griess assay, and type I IFN was measured by bioassay. Ligand concentrations were as follows: LPS (800 pg/mL), poly(I:C) (60 μg/mL), Pam3CSK4 (100 ng/mL), R-848 (40 ng/mL), CpG (1 μM), peptidoglycan (PGN, 2 μg/mL), MALP-2 (20 ng/mL). ND, not detected. Bars indicate mean and SE.
panr2 Mice Have a Mutation in Ikbkg.
Given the broad suppression of NF-κB–dependent cytokine secretion (TNFα, IL-6, IL-12p40, MCP-1), along with normal secretion of an IRF3-dependent cytokine (type 1 IFN), we sequenced the coding exons and splice junction of five genes known to be required exclusively for TLR-induced activation of NF-κB (Traf6, Tak1, Ikbkg, Chuk, Ikbkb). Of a total of 26,246 target nucleotides, 21,056 (80.2%) were covered by high-quality reads in both wild type and mutant templates, and a single mutation was identified in Ikbkg (Fig. 2A). The mutation was a T-to-C transition at position 473 of Ikbkg cDNA, in exon 4 of a total of 10, leading to a leucine to proline substitution at residue 153 (L153P). The affected amino acid lay within the first coiled-coil domain of NEMO (Fig. 2D), and did not alter protein expression or electrophoretic mobility in macrophage lysates (Fig. 2C). Consistent with the chromosomal location of Ikbkg, the panr2 phenotype was inherited in an X-linked recessive manner.
Fig. 2.
panr2 is a viable allele of Ikbkg. (A) Representative sequence trace of the Ikbkg gene from C57BL/6J and panr2 mice. (B) Genotype ratios of 4-week-old offspring from an Ikbkg+/Y x Ikbkg+/panr2 cross. (C) Expression of NEMO protein in macrophage lysates. (D) Domain structure and location of the panr2 mutation (L153P) in the NEMO protein. H&E staining (E) and mean weight (F) of pooled testes from Ikbkg+/Y and Ikbkgpanr2/Y littermates.
Unlike Ikbkg knockout mice, panr2 hemizygotes were viable and born at Mendelian ratios (Fig. 2B). Conditional deletion of Ikbkg is known to affect a variety of tissues, including the skin (16), liver (17), and intestinal epithelium (18), yet none of these tissues showed histological abnormalities in mutant mice. Survival of T (19) and B (20) lymphocytes is also precluded by conditional deletion of Ikbkg, yet both of these populations were present in panr2 mice (Fig. S2). panr2 hemizygous males were azoospermic, however, with histological analysis revealing cystic formations in the seminiferous tubules, as well as abnormal maturation of spermatids (Fig. 2E), and a mean testis weight approximately 35% lower than WT siblings (Fig. 2F). Inguinal lymph nodes were also absent or of diminished size in most mutant mice (Fig. S3).
Ikbkgpanr2 Impairs Adaptive Immunity.
Although NEMO is essential for survival of all T cells, IKKβ is not, and is known to be required specifically for the development of memory and regulatory T cells (19). Similarly, mice with an engineered mutation of p105 that cannot be phosphorylated by IKK (Nfkb1SSAA/SSAA) also have fewer memory and regulatory T cells, and additionally have a reduction of NKT cells (21). These populations were also reduced in panr2 mutants (Fig. 3), despite normal development of naive T cells (Fig. S2).
Fig. 3.
Impaired development of memory, regulatory and NKT cells. Representative flow cytometry plots and relative frequencies of CD44lo (naive) and CD44hi (memory) T cells in spleen (A), Foxp3+ regulatory T cells in thymus and spleen (B), and CD3ε+NK1.1+ NKT cells in thymus and spleen (C). Absolute numbers are represented in panels D–F. Numbers in each flow cytometry plot represents percentages of total lymphocytes, and bars represent mean and SE. (*P < 0.05; **P < 0.01; ***P < 0.001; Student’s t test in D–F.)
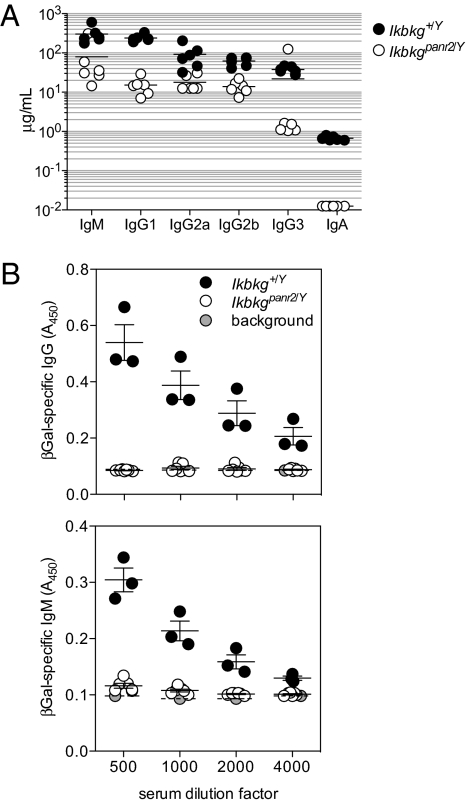
IKK activity is further required for signals transduced from the BCR, and for CD40-mediated recombination of Ig heavy chains. Serum of unimmunized panr2 mice was deficient for all Ig isotypes tested, including IgM, indicating a broad impairment of B cell activation (Fig. 4A). After immunization with a recombinant Semliki Forest virus (rSFV) expressing β-gal vector, a strong inducer of antibody responses in WT mice, panr2 mice produced no specific IgG or IgM antibody (Fig. 4B).
Fig. 4.
Low serum Ig levels and impairment of antibody responses in panr2 mice. (A) Total Ig isotypes in the serum of naive mice as measured by ELISA. Sample concentrations below the detection thresholds for IgG2a and IgA were given values of 12.5 μg/mL and 12.5 ng/mL, respectively. (B) Mice were immunized with a recombinant rSFV-β-gal, and β-gal–specific IgG and IgM at d 14 postimmunization was measured by ELISA.
Impairment of MAPK Phosphorylation and p65 Translocation, but Not IκBα Degradation.
To characterize the biochemical consequences of the panr2 mutation, NF-κB and MAPK signaling events were examined in TLR-stimulated macrophages. In response to both LPS (Fig. 5A) and MALP-2 (Fig. S4A), p38 MAPK phosphorylation occurred normally in panr2 cells, yet phosphorylation of p105, MEK (MAPK or ERK kinase), and ERK was severely impaired. Despite a mild impairment of IκBα phosphorylation, IκBα degradation remained intact. Nonetheless, nuclear translocation of p65 was suppressed in panr2 cells (Fig. 5C), with accumulation occurring instead in the cytoplasmic fraction. Degradation of two other classical IκB proteins, IκBβ and IκBε, was not detected in cells of either genotype, although phosphorylation of IκBε was impaired to a similar degree as IκBα (Fig. S4B). And combined with its reduced phosphorylation, p105 was stabilized in panr2 cells (Fig. S4B).
Fig. 5.
The panr2 mutation impairs MAPK and p105 phosphorylation, p65 translocation, and TNF production, but not IκBα degradation. (A) Thioglycollate-elicited macrophages were stimulated with LPS (1 μg/mL) for the indicated times, and cell lysates analyzed by Western blotting. (B) Macrophages were stimulated with LPS, and intracellular (Upper) or surface (Lower) TNF was measured by flow cytometry. (C) Cytoplasmic and nuclear fractions of LPS-stimulated macrophages stimulated with 1 μg/mL LPS were probed with antibodies against NF-κB p65 and β-tubulin.
With respect to TNFα, the predominant role of the TPL2/MEK/ERK cascade is to promote its secretion, rather than accumulation of the 26-kDa membrane-associated precursor protein inside the cell (22). To examine the accumulation of TNFα inside panr2 cells, macrophages were stimulated with LPS in the presence or absence of the secretory pathway inhibitor brefeldin A. Brefeldin A–treated panr2 cells accumulated approximately twofold less intracellular TNFα, in contrast to a three- to fourfold reduction of surface TNFα (Fig. 5B) and a fivefold reduction of TNFα in the supernatant (Fig. 1). This disparity between intracellular and secreted TNF is consistent with observations in TPL2-deficient (22) and mutant (23) cells, but also imply that synthesis of TNFα precursor protein is affected independently of TPL2.
Discussion
By separating developmental and immunological functions of NEMO, the panr2 mutant models a rare immune deficiency of man (11, 12). This allele is likely to be hypomorphic (rather than null or hypermorphic) for a number of reasons, viability being chief among them. TLR signaling is reduced, but not abolished as it is in NEMO-deficient fibroblasts (24), and survival of naive lymphocytes is also intact, in contrast to conditional knockouts of Ikbkg in B and T cells (19, 20). An absence of ectodermal dysplasia, which develops in mice with mutations of Eda (25), Edar (26), and Edaradd (27), suggests that the panr2 mutation is permissive to signals transduced from EDAR, but not from the TLRs, CD40, LTβR, BCR, and/or TCR.
Our data reveal that NEMO is important for the development of lymph nodes. IKK-mediated phosphorylation of the p50 precursor p105 is required for complete lymph node development (21), as are the NF-κB subunits p50 and p52 (28). Activation of both subunits via signaling from LTβR is required for secondary lymphoid organogenesis, with combined p50/p52 deficiency preventing lymph node development altogether (28). Together with our observations in panr2 mice, these data suggest that canonical NF-κB activation through the IKK complex is important for the development of lymphoid architecture. Conversely, azoospermia has never, to our knowledge, been associated with mutations of the IKK complex, and may indicate a new developmental role for NEMO. Yet although the phenotypes of immune deficiency and sterility are linked, we have not excluded the possibility that an additional X-linked mutation is responsible for azoospermia.
An L153R mutation (as opposed to L153P in panr2) has also been described in a patient with hypohidrotic ectodermal dysplasia and immune deficiency (29). Unlike panr2, L153R mutant cells do not degrade IκBα in response to TLR stimulation (29), potentially accounting for a disruption of EDAR signaling and the development of ectodermal dysplasia in this patient. Mice expressing a “superrepressive” form of IκBα also develop ectodermal dysplasia (30). These results may be consistent with the largely intact nuclear translocation of p65 in an IKBKG mutant patient without ectodermal dysplasia (12).
But of all of the biochemical pathways disrupted by mutations in NEMO, which are the most important for immunity? The data presented here would imply that IκBα degradation alone is insufficient for immune competence, at least in macrophages, as TLR-induced IκBα degradation proceeds normally in panr2 cells. Nuclear translocation of p65 is severely affected, however, implying a requirement for NEMO beyond IκBα degradation. In agreement with this finding, cytoplasmic retention of most p65 can occur in the combined absence of IκBα, IκBβ, and IκBε (31). The molecule(s) that retain p65 in the cytoplasm in this context are not known, although greater quantities of p100 and p105 can be recovered by p65 immunoprecipitation. As p105 phosphorylation and degradation are impaired in TLR-stimulated panr2 cells, this may account for p65 cytoplasmic retention in the presence of IκBα degradation.
Impairment of the TPL2/MEK/ERK pathway could also account for immune deficiency, although this cannot be entirely responsible for the phenotypes observed. TLP2 mutant mice do not exhibit reduced numbers of memory or regulatory T cells (21), suggesting that development of these subsets depends on a TPL2-independent pathway. This pathway nevertheless requires IKK-mediated phosphorylation of p105, as Ikbkgpanr2/Y, p105 (Nfkb1SSAA/SSAA), and IKKβ (IkbkbFL/Y;CD4-cre) mutant mice all show reductions in these populations. And with respect to the role of p105 phosphorylation in innate immunity, examination of the susceptibility of Nfkb1SSAA/SSAA mice to infection, as well as p65 nuclear translocation, will be of great interest.
Finally, TRAF6-mediated ubiquitination of NEMO is also necessary for complete activation of TLR-induced cytokine secretion. As revealed by mice lacking the target lysine residue (32), TLR-induced IκBα degradation, NF-κB activation and ERK phosphorylation are all intact in the absence of NEMO ubiquitination, whereas the cytokine response is not, suggesting that NEMO ubiquitination may regulate processes beyond IκBα degradation and ERK activation. The panr2 mutation, in contrast, uncouples ERK phosphorylation and nuclear translocation of p65 without impairing IκBα degradation. We suggest that this is caused by alteration of the scaffolding function of NEMO, disrupting some but not all functions of NEMO and the IKK complex. This separation of function may offer a mechanistic explanation for rare immune deficiencies in man, and provides an animal model for their investigation.
Materials and Methods
Mice.
Ikbkgpanr2 (MGI:3808877, MMRRC:030659-UCD) was generated on a pure C57BL/6J background by ENU mutagenesis as previously described (33). The panr2 strain was maintained by breeding heterozygous females with C57BL/6J males. All experiments were performed using age-matched male littermates between 5 and 10 weeks of age, and were in accordance with institutional animal care guidelines.
Macrophage Isolation and TLR Stimulation.
Mice were injected with 1.5 mL of a 4% solution of Brewer’s thioglycollate (wt/vol in distilled H2O, autoclaved and aged for at least 1 month; BBL Microbiology Systems). Four days later, peritoneal cells were recovered from isofluorane-anesthetized mice by peritoneal lavage with 5 mL PBS solution. Peritoneal macrophages were plated in 96-well tissue culture treated plates (Costar) at a density of 1 × 105 cells per well. Following overnight incubation (37 °C in 5% CO2), supernatant was discarded and replaced with 100 μL/well of TLR ligand solutions [in DMEM (Cellgro) plus 5% FBS (Atlanta Biosciences) and 2% penicillin/streptomycin (Gibco)], and incubated for 4 h. Supernatant was harvested to assay for the presence of cytokines, and replaced with a 2 mg/mL solution of MTT (M2128; Sigma) in DMEM plus 5% FBS and 2% penicillin/streptomycin to confirm macrophage viability. For the induction of NO, macrophages were preactivated with 20 ng/mL IFN-γ (R&D Systems) for 24 h, and stimulated with TLR ligands for an additional 24 h. LPS (from Salmonella minnesota R595), poly(I:C), MALP-2, and R-848 were all obtained from Enzo Life Sciences. Pam3CSK4 was from EMC Microcollections, CpG ODN from IDT (sequence TCCATGACGTTCCTGATGCT with phosphorothioate bonds), and peptidoglycan from Sigma. Purity of all ligands was validated using cells deficient for their corresponding TLR.
Detection of Cytokines and NO.
TNFα, IL-6, IL-12p40 and MCP-1 were measured by ELISA according to the manufacturer’s instructions (eBioscience). For type 1 IFN, L929 mouse fibroblasts stably transfected with an IFN-sensitive luciferase element (L929-ISRE) were incubated with supernatant for 5 h, washed once with PBS solution, lysed with reporter lysis buffer (Promega), and incubated overnight at −80°C. After thawing, 35 μL luciferase reagent (Promega) was added to each well, and luminescence read immediately on an Lmax plate reader (Molecular Devices). NO was measured by Griess assay. Concentrations of all cytokines and NO were interpolated from a standard curve.
Histology.
Organs were fixed in 10% buffered formalin (Sigma), embedded in paraffin, sectioned, and stained with H&E.
Flow Cytometry.
Lymphoid organ suspensions or macrophages were stained with antibodies against CD4, CD8, CD44, NK1.1, CD3ε, and B220 (eBioscience). Intracellular staining of Foxp3 was performed according to the manufacturer’s protocol (eBioscience). For staining of TNF on macrophages, 2 × 105 cells were stimulated with the indicated dose of LPS for 2 h in the presence or absence of 5 μg/mL brefeldin A (Sigma), and stained with PE-conjugated anti-TNF (BD Biosciences). Intracellular staining of brefeldin A–treated cells was performed using the Cytofix/Cytoperm Fixation/Permeabilization solution kit (BD Biosciences).
Western Blotting.
Peritoneal macrophages (2 × 106) were stimulated in 24-well plates with LPS (1 μg/mL) or MALP-2 (100 ng/mL) for the indicated lengths of time, separated into cytoplasmic and nuclear fractions (Nuclear Extract Kit; Active Motif) or lysed directly in sample buffer (Invitrogen), separated by SDS/PAGE, and transferred to nitrocellulose membranes. Membranes were probed with antibodies to NEMO (Imgenex), phospho-IκBα (Ser32/36), phospho-IκBε (Ser18/22), IκBα, IκBβ, IκBε, phospho-p38 (Thr180/Tyr182), phospho-p105 (Ser933), p105, phospho-MEK1/2 (Ser217/221), ERK1/2, phospho-ERK1/2 (Thr202/Tyr204), p65, and β-tubulin (Cell Signaling Technology).
Immunizations and Serum Antibodies.
Single-round infectious rSFV encoding β-gal was generated and titered as previously described (34). Mice were injected i.p. with 200 μL of 0.9% sterile saline solution containing 2 × 106 infectious units of rSFV-β-gal on d 0. On d 14, blood was collected from the retro-orbital cavities of anesthetized mice into serum separator tubes and stored at −80°C. For antibody ELISA, polyvinyl chloride microtiter 96-well round-bottom plates (Fisher Scientific) were coated overnight at 4°C with 2 μg/mL β-gal (Roche). The plates were blocked with 5% milk, and serum samples were serially diluted in 1% milk. The plates were incubated with HRP-conjugated goat anti-mouse IgM or IgG (Southern Biotechnology), developed with SureBlue TMB Microwell Peroxidase Substrate and TMB Stop Solution (KPL), and read at 450 nm on a MAXline Emax Microplate Reader (Molecular Devices). Background for the assay was determined by incubating sera pooled from immunized WT mice on uncoated wells. Total serum immunoglobulins in preimmune sera were measured as previously described (35).
Viral and Bacterial Infections.
Mice were infected with an i.p. dose of 2 × 105 PFU MCMV (Smith strain) prepared from BALB/c salivary glands. L. monocytogenes strain 10403S (Xenogen) was cultured in brain–heart infusion broth at 37°C, resuspended in PBS solution, and 105 CFU were administered via the tail vein. Infected mice were monitored daily for signs of illness.
Supplementary Material
Acknowledgments
We thank Jordan Orange for valuable discussions; Liz Hanley, Christine Domingo, and Mercedes Gutierrez for mutagenesis and animal care; and the University of California San Diego histology core facility. This work was supported by National Institutes of Health Grants AI070167 (to B.B.) and RO1GM44809 (to D.N.), National Institute of Allergy and Infectious Diseases Broad Agency Announcement Contract HHSN272200700038C (to B.B.),the Bill and Melissa Gates Foundation (to B.B.), and a fellowship from the Swiss National Science Foundation (P.K.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0915098107/DCSupplemental.
References
- 1.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 2.Heissmeyer V, Krappmann D, Hatada EN, Scheidereit C. Shared pathways of IkappaB kinase-induced SCF(betaTrCP)-mediated ubiquitination and degradation for the NF-kappaB precursor p105 and IkappaBalpha. Mol Cell Biol. 2001;21:1024–1035. doi: 10.1128/MCB.21.4.1024-1035.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beinke S, Robinson MJ, Hugunin M, Ley SC. Lipopolysaccharide activation of the TPL-2/MEK/extracellular signal-regulated kinase mitogen-activated protein kinase cascade is regulated by IkappaB kinase-induced proteolysis of NF-kappaB1 p105. Mol Cell Biol. 2004;24:9658–9667. doi: 10.1128/MCB.24.21.9658-9667.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waterfield M, Jin W, Reiley W, Zhang M, Sun SC. IkappaB kinase is an essential component of the Tpl2 signaling pathway. Mol Cell Biol. 2004;24:6040–6048. doi: 10.1128/MCB.24.13.6040-6048.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. IkappaB kinases phosphorylate NF-kappaB p65 subunit on serine 536 in the transactivation domain. J Biol Chem. 1999;274:30353–30356. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- 6.Smahi A, et al. The International Incontinentia Pigmenti (IP) Consortium. Genomic rearrangement in NEMO impairs NF-kappaB activation and is a cause of incontinentia pigmenti. Nature. 2000;405:466–472. doi: 10.1038/35013114. [DOI] [PubMed] [Google Scholar]
- 7.Makris C, et al. Female mice heterozygous for IKK gamma/NEMO deficiencies develop a dermatopathy similar to the human X-linked disorder incontinentia pigmenti. Mol Cell. 2000;5:969–979. doi: 10.1016/s1097-2765(00)80262-2. [DOI] [PubMed] [Google Scholar]
- 8.Rudolph D, et al. Severe liver degeneration and lack of NF-kappaB activation in NEMO/IKKgamma-deficient mice. Genes Dev. 2000;14:854–862. [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt-Supprian M, et al. NEMO/IKK gamma-deficient mice model incontinentia pigmenti. Mol Cell. 2000;5:981–992. doi: 10.1016/s1097-2765(00)80263-4. [DOI] [PubMed] [Google Scholar]
- 10.Zonana J, et al. A novel X-linked disorder of immune deficiency and hypohidrotic ectodermal dysplasia is allelic to incontinentia pigmenti and due to mutations in IKK-gamma (NEMO) Am J Hum Genet. 2000;67:1555–1562. doi: 10.1086/316914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niehues T, et al. Nuclear factor kappaB essential modulator-deficient child with immunodeficiency yet without anhidrotic ectodermal dysplasia. J Allergy Clin Immunol. 2004;114:1456–1462. doi: 10.1016/j.jaci.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 12.Orange JS, et al. Human nuclear factor kappa B essential modulator mutation can result in immunodeficiency without ectodermal dysplasia. J Allergy Clin Immunol. 2004;114:650–656. doi: 10.1016/j.jaci.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 13.Jain A, et al. Specific missense mutations in NEMO result in hyper-IgM syndrome with hypohydrotic ectodermal dysplasia. Nat Immunol. 2001;2:223–228. doi: 10.1038/85277. [DOI] [PubMed] [Google Scholar]
- 14.Salt BH, et al. IKBKG (nuclear factor-kappaB essential modulator) mutation can be associated with opportunistic infection without impairing Toll-like receptor function. J Allergy Clin Immunol. 2008;121:976–982. doi: 10.1016/j.jaci.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welsh RM, Brubaker JO, Vargas-Cortes M, O’Donnell CL. Natural killer (NK) cell response to virus infections in mice with severe combined immunodeficiency. The stimulation of NK cells and the NK cell-dependent control of virus infections occur independently of T and B cell function. J Exp Med. 1991;173:1053–1063. doi: 10.1084/jem.173.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nenci A, et al. Skin lesion development in a mouse model of incontinentia pigmenti is triggered by NEMO deficiency in epidermal keratinocytes and requires TNF signaling. Hum Mol Genet. 2006;15:531–542. doi: 10.1093/hmg/ddi470. [DOI] [PubMed] [Google Scholar]
- 17.Luedde T, et al. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007;11:119–132. doi: 10.1016/j.ccr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 18.Nenci A, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt-Supprian M, et al. Mature T cells depend on signaling through the IKK complex. Immunity. 2003;19:377–389. doi: 10.1016/s1074-7613(03)00237-1. [DOI] [PubMed] [Google Scholar]
- 20.Pasparakis M, Schmidt-Supprian M, Rajewsky K. IkappaB kinase signaling is essential for maintenance of mature B cells. J Exp Med. 2002;196:743–752. doi: 10.1084/jem.20020907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sriskantharajah S, et al. Proteolysis of NF-kappaB1 p105 is essential for T cell antigen receptor-induced proliferation. Nat Immunol. 2009;10:38–47. doi: 10.1038/ni.1685. [DOI] [PubMed] [Google Scholar]
- 22.Rousseau S, et al. TPL2-mediated activation of ERK1 and ERK2 regulates the processing of pre-TNF alpha in LPS-stimulated macrophages. J Cell Sci. 2008;121:149–154. doi: 10.1242/jcs.018671. [DOI] [PubMed] [Google Scholar]
- 23.Xiao N, et al. The Tpl2 mutation sluggish impairs type I IFN production and increases susceptibility to group B Streptococcal disease. J Immunol. 2009;183:7975–7983. doi: 10.4049/jimmunol.0902718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamaoka S, et al. Complementation cloning of NEMO, a component of the IkappaB kinase complex essential for NF-kappaB activation. Cell. 1998;93:1231–1240. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]
- 25.Srivastava AK, et al. The Tabby phenotype is caused by mutation in a mouse homologue of the EDA gene that reveals novel mouse and human exons and encodes a protein (ectodysplasin-A) with collagenous domains. Proc Natl Acad Sci USA. 1997;94:13069–13074. doi: 10.1073/pnas.94.24.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Headon DJ, Overbeek PA. Involvement of a novel Tnf receptor homologue in hair follicle induction. Nat Genet. 1999;22:370–374. doi: 10.1038/11943. [DOI] [PubMed] [Google Scholar]
- 27.Headon DJ, et al. Gene defect in ectodermal dysplasia implicates a death domain adapter in development. Nature. 2001;414:913–916. doi: 10.1038/414913a. [DOI] [PubMed] [Google Scholar]
- 28.Lo JC, et al. Coordination between NF-kappaB family members p50 and p52 is essential for mediating LTbetaR signals in the development and organization of secondary lymphoid tissues. Blood. 2006;107:1048–1055. doi: 10.1182/blood-2005-06-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orange JS, et al. Deficient natural killer cell cytotoxicity in patients with IKK-gamma/NEMO mutations. J Clin Invest. 2002;109:1501–1509. doi: 10.1172/JCI14858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt-Ullrich R, et al. Requirement of NF-kappaB/Rel for the development of hair follicles and other epidermal appendices. Development. 2001;128:3843–3853. doi: 10.1242/dev.128.19.3843. [DOI] [PubMed] [Google Scholar]
- 31.Tergaonkar V, Correa RG, Ikawa M, Verma IM. Distinct roles of IkappaB proteins in regulating constitutive NF-kappaB activity. Nat Cell Biol. 2005;7:921–923. doi: 10.1038/ncb1296. [DOI] [PubMed] [Google Scholar]
- 32.Ni CY, et al. Cutting edge: K63-linked polyubiquitination of NEMO modulates TLR signaling and inflammation in vivo. J Immunol. 2008;180:7107–7111. doi: 10.4049/jimmunol.180.11.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Georgel P, Du X, Hoebe K, Beutler BA. ENU mutagenesis in mice. Methods Mol Biol. 2008;415:1–16. doi: 10.1007/978-1-59745-570-1_1. [DOI] [PubMed] [Google Scholar]
- 34.Smerdou C, Liljeström P. Two-helper RNA system for production of recombinant Semliki forest virus particles. J Virol. 1999;73:1092–1098. doi: 10.1128/jvi.73.2.1092-1098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gavin AL, et al. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.