Abstract
The lysosomal cysteine proteases cathepsin B (Ctsb) and cathepsin Z (Ctsz, also called cathepsin X/P) have been implicated in cancer pathogenesis. Compensation of Ctsb by Ctsz in Ctsb −/− mice has been suggested. To further define the functional interplay of these proteases in the context of cancer, we generated Ctsz null mice, crossed them with Ctsb-deficient mice harboring a transgene for the mammary duct–specific expression of polyoma middle T oncogene (PymT), and analyzed the effects of single and combined Ctsb and Ctsz deficiencies on breast cancer progression. Single Ctsb deficiency resulted in delayed detection of first tumors and reduced tumor burden, whereas Ctsz-deficient mice had only a prolonged tumor-free period. However, only a trend toward reduced metastatic burden without statistical significance was detected in both single mutants. Strikingly, combined loss of Ctsb and Ctsz led to additive effects, resulting in significant and prominent delay of early and advanced tumor development, improved histopathologic tumor grading, as well as a 70% reduction in the number of lung metastases and an 80% reduction in the size of these metastases. We conclude that the double deficiency of Ctsb and Ctsz exerts significant synergistic anticancer effects, whereas the single deficiencies demonstrate at least partial reciprocal compensation.
Keywords: lysosome, mammary adenocarcinoma, mouse model, protease
Proteolysis is a hallmark of invasive tumor growth and metastasis. Proteases regulate these processes through several possible mechanisms. Processing of cell-adhesion molecules (e.g., E-cadherin) disrupts cell–cell contacts, facilitating cancer cell dissemination from the primary site (1). The breakdown of the basement membrane by degradation of ECM proteins is essential for invasion into surrounding tissue or blood vessels (2). Furthermore, proteolytic events affect tumor biological processes, such as proliferation and angiogenesis, by liberating ECM-bound growth and angiogenic factors or by processing and activating these factors (3, 4). Several protease classes, including metallo-, aspartic, serine, and cysteine proteases, are implicated in promoting tumor progression and metastasis (5 –8). The family of lysosomal cysteine proteases, the cysteine cathepsins (clan CA; C1 family), has recently attracted attention as tumor-promoting enzymes (9). Eleven cysteine cathepsins are annotated in the human genome: cathepsin B, C, F, H, K, L, L2/V, O, S, W, and X/Z/P (10). Cysteine cathepsins are implicated primarily in terminal protein degradation in the acidic environment of the lysosomes (11); however, they also exhibit specific functions in physiological (12) and pathological processes (13). Despite the cathepsins’ general localization in the endosomal/lysosomal compartment, changes in distribution during neoplasia formation result in secretion and extracellular effects (14, 15). Various human malignancies show overexpression of some cysteine cathepsins and accumulation at the invasive tumor front of solid cancers (16, 17). This increased expression and proteolytic activity correlates with poor prognosis in several human cancers, including breast cancer (18). Histological studies on human cancers and studies using ex vivo models implicate mainly cathepsin B and L, and to a lesser extent cathepsin H, S, and Z, in the proteolytic events promoting cancer progression (19).
Cancer mouse models with cathepsin deficiencies allow the elucidation of specific functions of individual cathepsins in tumor progression and metastasis (1, 20). Using the transgenic polyoma middle T oncogene (PymT)-induced mouse model for breast cancer (21), we recently showed that ablation of one cathepsin B (Ctsb) allele was sufficient to significantly reduce lung metastasis (20). However, a deficiency of both Ctsb alleles did not further reduce the metastatic burden; rather, statistical significance compared with Ctsb WT mice was lost. This effect can be explained by a compensation of Ctsb deficiency by cathepsin Z (Ctsz) in primary Ctsb-deficient tumor cells derived from breast cancers of Ctsb −/− mice (20). Ctsz (also known as cathepsin X or cathepsin P) belongs to the same Clan CA/C1 protease family as Ctsb (22 –24). Ctsz shows unique features among these proteases, that is, the presence of a RGD motif for integrin binding in its propeptide and strict exopeptidase activity (25, 26). Notably, Ctsb and Ctsz are the only carboxypeptidases in the cysteine cathepsin family, although Ctsb also exhibits endopeptidase activity (27, 28). To elucidate specific functions of Ctsz in cancer progression, we generated Ctsz-deficient mice and bred them with transgenic mice expressing PymT under control of the mammary epithelium-specific mouse mammary tumor virus (MMTV) LTR promoter (21). We demonstrate that Ctsb and Ctsz single deficiencies have only marginal effects on most parameters of breast cancer development in this model, but that combined deficiency of Ctsb and Ctsz results in a significantly reduced tumor and metastatic burden of PymT-induced breast cancer in mice.
Results
Generation and Phenotyping of Mice with Ctsb/Ctsz Double Deficiency.
To investigate the effects of single and combined deficiencies of Ctsb and Ctsz in the context of breast cancer, we generated Ctsz-deficient mice (Ctsz −/−) and backcrossed them to the FVB/N genetic background (SI Methods and Fig. S1). We then further crossed these mice with congenic Ctsb-deficient (Ctsb −/−) mice harboring a construct for expression of PymT in mammary epithelial cells under control of the MMTV LTR promoter (20, 21). Female mice hemizygous for the PymT transgene were grouped according to their Ctsb and Ctsz genotypes as PymT+/0;wt, PymT+/0;Ctsb −/−, PymT+/0;Ctsz −/−, and PymT+/0;Ctsb −/− Ctsz −/−, and the four respective cathepsin genotypes without the PymT transgene. Ctsz −/− mice show no gross phenotype for observation periods of up to 2 years and can be maintained as homozygous mutants. In addition, we investigated the postnatal development of Ctsb −/− Ctsz −/− mice with respect to weight gain and steroid hormone levels. There was no evidence of impaired quality of maternal care or overall health of the Ctsb −/− Ctsz −/− mice (Fig. S2A). The offspring of wt and Ctsb −/− Ctsz −/− weighed the same at weaning on day 21 after birth and demonstrated equal weight gain (Fig. S2B). Quantification of steroid hormone concentrations in serum of wt and Ctsb −/− Ctsz −/− mice at day 42 after birth by ELISA revealed no differences in circulating levels of estrogen and progesterone, indicating a similar onset of puberty (Fig. S2C). Most importantly, similar levels of steroid hormones suggest comparable activation of the MMTV promoter, which is costimulated by steroid hormones.
To further address PymT oncogene expression, we measured the PymT mRNA levels in tumors of 14-week-old PymT+/0;wt, PymT+/0;Ctsb −/−, PymT+/0;Ctsz −/−, and PymT+/0;Ctsb −/− Ctsz −/− mice by quantitative RT-PCR (Fig. S2D). These experiments revealed no cathepsin-genotype dependent differences in PymT expression. Thus, any alteration of tumor progression in the cathepsin-deficient MMTV-PymT mice is not caused by impaired postnatal development or reduced oncogene levels, but rather is most likely a consequence of altered cathepsin expression in the various knockout mice.
Expression Pattern, Activity Profile, and Histological Localization of Ctsb and Ctsz.
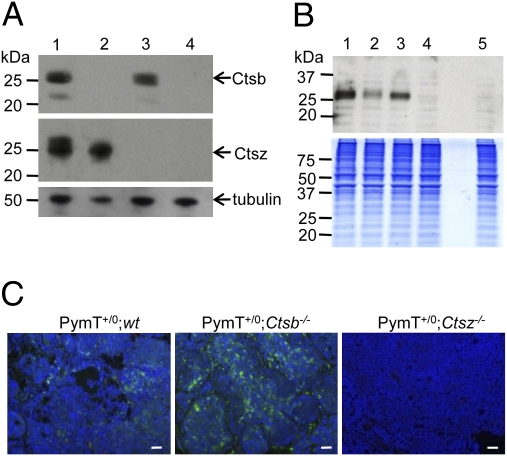
Western blot analyses of Ctsb and Ctsz (Fig. 1A) revealed the expected expression pattern in the corresponding groups, with PymT+/0;wt expressing both Ctsb and Ctsz, PymT+/0;Ctsb −/− lacking expression of Ctsb, and PymT+/0;Ctsz−/− lacking expression of Ctsz. PymT+/0;Ctsb −/− Ctsz −/− showed no expression of Ctsb and Ctsz. Having recently reported the extracellular expression of Ctsz as a second cysteine cathepsin on PymT tumor cells (20), we analyzed activity profiles of cysteine cathepsins on the surface of PymT tumor cells with different Ctsb and Ctsz genotypes (Fig. 1B). Cell surface labeling of cysteine cathepsins with the membrane-impermeable active site probe DCG-04 (29) revealed the presence of Ctsb and Ctsz on PymT+/0;wt cells. PymT+/0;Ctsb −/− and PymT+/0;Ctsz −/− showed distinct cysteine cathepsin bands considered to be Ctsz on Ctsb-deficient tumor cells and Ctsb on Ctsz-deficient tumor cells. Notably, no active cysteine cathepsins were detectable on the surface of PymT+/0;Ctsb −/− Ctsz −/− tumor cells. Because Ctsz has not yet been investigated in the context of PymT-induced mammary cancer, we analyzed the histological localization of Ctsz in primary PymT tumors of PymT+/0;wt, PymT+/0;Ctsb −/− , and PymT+/0;Ctsz −/− as control (Fig. 1C). Ctsz expression was detected in tumor cells and cells of the tumor stroma. Interestingly, immunofluorescence staining of Ctsz revealed particularly high Ctsz expression in PymT+/0;Ctsb −/− tumors. Moreover, Ctsb and Ctsz showed the same expression pattern in primary PymT tumors and lung metastases (SI Methods and Fig S3).
Fig. 1.
Expression pattern of Ctsb and Ctsz in PymT-induced mammary carcinomas. (A and B) Detection of Ctsb and Ctsz expression by Western blot analyses in primary tumor lysats (A) and cell surface labeling of active cysteine proteases by the biotinylated activity–based probe DCG-04 (B) on primary tumor cells of PymT+/0;wt [1], PymT+/0;Ctsb −/− [2], PymT+/0;Ctsz −/− [3], and PymT+/0;Ctsb −/− Ctsz −/− [4] mice. Unlabeled PymT+/0;wt control [5] was analyzed to detect endogenously biotinylated proteins. α-tubulin served and colloidal Coomassie blue–stained gel served as loading controls. (C) Detection of Ctsz (green staining) by immunofluorescence staining of PymT+/0;wt, PymT+/0;Ctsb −/−, and PymT+/0;Ctsz −/− tumor sections. (Scale bar: 50 μm.)
Progression and Histopathology of PymT-Induced Mammary Carcinomas.
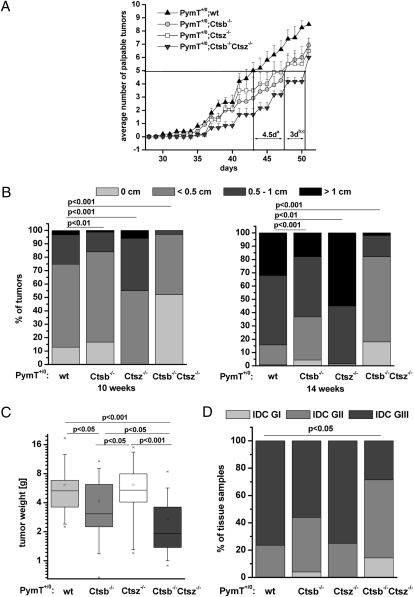
To detect first palpable tumors, we palpated 10 mammary glands per mouse every second day in a genotype-blinded fashion starting on day 28 after birth. To assess genotype-dependent differences in the occurrence of first palpable tumors, we determined the time point at which each genotype developed 50% of the tumors (Fig. 2A). The PymT+/0;Ctsb −/− and PymT+/0;Ctsz −/− mice had an average of five palpable tumors 4.5 days later than the PymT+/0;wt mice (P <.05). Remarkably, the PymT+/0;Ctsb −/− Ctsz −/− mice exhibited a further delay, with five palpable tumors arising 3 days later compared with the PymT+/0;Ctsb −/− and PymT+/0;Ctsz −/− mice (P <.05). To analyze the effects of Ctsb and Ctsz on progression of established tumors, mice were palpated at week 10 and 14, and tumors were grouped by size rulers into the following categories: no tumor, tumor diameter <0.5 cm, tumor diameter 0.5–1 cm, and tumor diameter >1 cm (Fig. 2B). The PymT+/0;Ctsz −/− mice showed enlarged tumors in this assessment, whereas the PymT+/0;Ctsb −/− mice developed large tumors significantly later than the PymT+/0;wt mice. A further significant decrease in tumor burden was detected in the PymT+/0;Ctsb −/− Ctsz −/− mice with nonpalpable and small tumors even at 14 weeks of age. These findings were evaluated by measuring the tumor weight of all 10 mammary tumors per animal at age 14 weeks (Fig. 2C). Compared with the PymT+/0;wt mice, tumor weight was reduced to 65% in the PymT+/0;Ctsb −/− mice (P <.05) and to 45% in the PymT+/0;Ctsb −/− Ctsz −/− mice (P <.001). In contrast, the PymT+/0;Ctsz −/− mice showed no difference in tumor weight compared with the PymT+/0;wt mice.
Fig. 2.
Tumor progression, tumor burden, and histopathology of mammary tumors. (A) Detection of first palpable tumors of PymT+/0;wt (n = 5), PymT+/0;Ctsb −/− (n = 12), PymT+/0;Ctsz −/− (n = 8), and PymT+/0;Ctsb −/− Ctsz −/− mice (n = 6) of all 10 mammary glands from day 28 to day 51. The time point at which each genotype developed 50% of tumors is indicated. Student´s t test was used for statistical analysis with (a) P <.01 for PymT+/0;wt compared with PymT+/0;Ctsb −/− and PymT+/0;Ctsz −/−, (b) P <.01 for PymT+/0;Ctsb−/−Ctsz −/− compared with PymT+/0;wt, and (c) P <.05 for PymT+/0;Ctsb −/− Ctsz −/− compared with PymT+/0;Ctsb −/− and PymT+/0;Ctsz −/−. (B) Estimation of the tumor size by palpation. Distribution of tumor size (0 cm, <0.5 cm, 0.5–1 cm, and >1 cm) per genotype is shown for the time points 10 and 14 weeks with PymT+/0;wt (n = 30), PymT+/0;Ctsb −/− (n = 30), PymT+/0;Ctsz −/− (n = 10), and PymT+/0;Ctsb −/− Ctsz −/− mice (n = 10). The χ2 test was used for statistical analysis. (C) The tumor weight of all 10 tumors per mouse was measured for PymT+/0;wt (n = 30), PymT+/0;Ctsb −/− (n = 40), PymT+/0;Ctsz −/− (n = 24), and PymT+/0;Ctsb −/− Ctsz −/− (n = 23). Data are presented as statistical boxplots; boxes include data between the 25th and 75th percentiles. Statistics wre analyzed using Student´s t test. (D) Histopathologic grading of H&E-stained tumor sections of 14-week-old PymT+/0;wt (n = 17), PymT+/0;Ctsb −/− (n = 25), PymT+/0;Ctsz −/− (n = 8), and PymT+/0;Ctsb −/− Ctsz −/− mice (n = 14). The χ2 test was used for statistical analysis.
Tumorigenesis of PymT-induced mammary cancers is characterized by stepwise development of distinct histopathologic stages (30). To investigate the impact of different Ctsb and Ctsz genotypes on tumor grade, we classified premalignant and malignant lesions in a genotype-blinded fashion into the following defined histopathologic stages: atypical ductal hyperplasia, ductal carcinoma in situ, and invasive ductal carcinoma, further subdivided into grades GI–GIII with regard to mitotic activity and cellular differentiation (31, 32) (Fig. 2D). Although all tumors were graded as invasive carcinoma at 14 weeks of age, only 56% of PymT+/0;Ctsb −/− tumors and 28% of PymT+/0;Ctsb −/− Ctsz −/− tumors were poorly differentiated (GIII), compared with 75% of both PymT+/0;wt and PymT+/0;Ctsz −/− tumors. Consequently, more of the PymT+/0;Ctsb −/− and PymT+/0;Ctsb −/− Ctsz −/− tumors were well differentiated (GI) or moderately differentiated (GII). Notably, Ctsb/Ctsz double-deficient tumors demonstrated a further reduction in tumor grade compared with tumors from Ctsb −/− mice (14% GI and 57% GII vs. 4% GI and 40% GII invasive ductal carcinomas).
Formation of Lung Metastases.
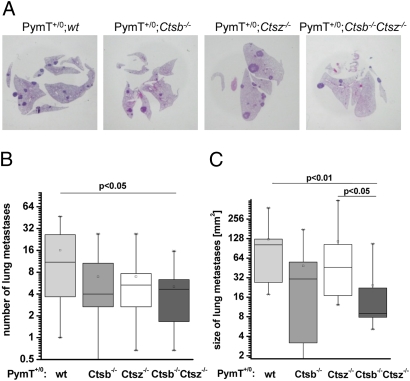
Formation of lung metastases is characteristic in female mice expressing the PymT transgene (21). H&E-stained lung sections of three independent sectional planes were examined for the degree of metastatic burden (Fig. 3A). At 14 weeks of age, 100% of the mice examined exhibited lung metastases. The number and size of lung metastases was about 50% less in PymT+/0;Ctsb −/− mice compared with PymT+/0;wt mice, but comparisons revealed no statistically significant differences (Fig. 3 B and C), confirming previous results (20). PymT+/0;Ctsz −/− mice exhibited a similarly reduced number, but no decrease in the average size, of lung metastases. Again, the combined deficiency of Ctsb and Ctsz had the most prominent and significant effect, with PymT+/0;Ctsb −/− Ctsz −/− mice exhibiting 70% fewer lung metastases (Fig. 3B) and an 80% smaller average metastasis size compared with PymT+/0;wt mice (Fig. 3C).
Fig. 3.
Formation of lung metastases. (A) Representative images of H&E-stained lung sections. (B and C) Metastatic burden in lungs of 14-week-old PymT mice was assessed for number (B) and size (C) of metastases in three independent sectional planes per lung; n = 10 per genotype. Statistics were analyzed using Student´s t test.
Tumor Cell Proliferation, Cell Death, and Angiogenesis.
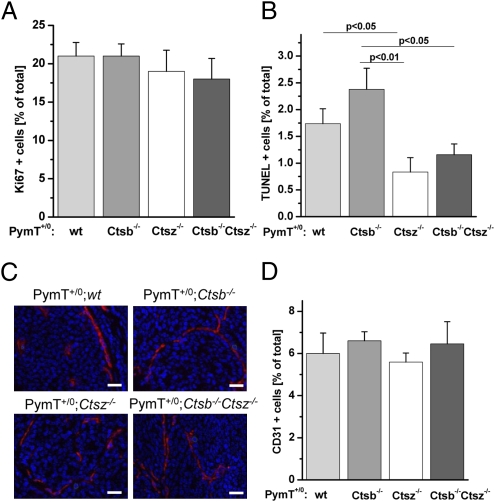
To address tumor biological processes affected by Ctsb and Ctsz, we investigated proliferation, cell death, and angiogenesis in late-stage primary PymT tumors (Fig. 4). We quantified cell proliferation by immunohistochemical detection of the proliferation marker Ki67. Although tumor weight was significantly lower in the PymT+/0;Ctsb −/− and PymT+/0;Ctsb −/− Ctsz −/− mice, no genotype-dependent difference in proliferation rate was seen (Fig. 4A). Increased rates of cell death may contribute to smaller cancers in PymT+/0;Ctsb −/− and PymT+/0;Ctsb −/− Ctsz −/− mice. To quantify dead cells in primary PymT tumors, TUNEL was performed to measure DNA fragmentation due to cell death. The fraction of dead cells in primary tumors generally is very low, ranging from 1% to 2.5% TUNEL-positive cells. Interestingly, PymT+/0;Ctsz −/− mice showed the lowest number of TUNEL-positive cells (P <.05 compared with PymT+/0;wt mice and P <.01 compared with PymT+/0;Ctsb −/− mice) (Fig. 4B). Over time, the significantly reduced cell death in cancers of PymT+/0;Ctsz −/− mice might result in the enlarged tumors seen in these mice (Fig. 2B). PymT+/0;Ctsb −/− Ctsz −/− mice also had less cell death compared with PymT+/0;Ctsb −/− mice (P <.05); however, the tumor burden was even smaller in PymT+/0;Ctsb −/− Ctsz −/− mice compared with PymT+/0;Ctsb −/− mice (Fig. 2C).
Fig. 4.
Proliferation, cell death and mean vessel density in late-stage mammary carcinomas. (A and B) Quantification of Ki67+ cells (A) and TUNEL-positive cells (B) as percentage of total cells. The percentage of Ki67+ and TUNEL-positive cells was calculated from 60 high-power fields (HPF) per animal by computer-assisted data analyses using the histoquest software (TissueGnostics); n = 6 per genotype. (C) Representative images of immunofluoresence staining of the endothelial cell specific marker CD31 (red staining). (D) Quantification of CD31+ cells as percentage of total cells. The percentage of CD31+ cells was calculated from 300 HPF per animal; n = 5 per genotype. Data acquisition and analyses were performed with the microscope-based imaging platform ScanR (Olympus). Data are presented as mean ± SE. Student´s t test was used for statistical analysis. (Scale bar: 50 μm.)
Because sufficient supplies of oxygen and nutrients are critical for tumor growth, the connection of growing tumors to the vasculature of the host and the formation of a tumor vasculature by angiogenesis is essential. To investigate the effects of Ctsb and Ctsz on tumor angiogenesis, we evaluated endothelial cells by immnunofluorescence staining of the marker CD31 (Fig. 4C). Quantification of CD31+ cells revealed no genotype-dependent differences in mean vessel density (Fig. 4D).
Tumor Cell Migration and Invasive Strand Formation.
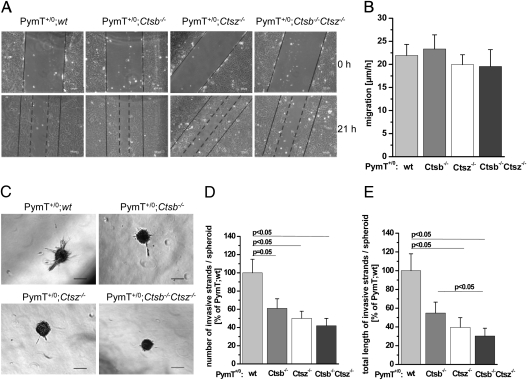
The migratory and invasive properties of tumor cells are crucial to tumor growth and metastasis. To quantify the migratory potential of primary PymT tumor cells with different Ctsb and Ctsz genotypes, we performed in vitro scratch assays. Migration was estimated as the distance of scratch closure per hour within the first 21 h (Fig. 5A). Quantification of cell migration revealed no genotype-dependent differences (Fig. 5B). Operating under the assumption that extracellularly expressed cathepsins are critical for invasion of PymT tumor cells, we embedded tumor spheroids with different Ctsb and Ctsz genotypes into a collagen I matrix. Within 24 h, the spheroids formed invasive strands (Fig. 5C). We estimated the number and average length of invasive strands per spheroid as a measure of the invasive potential of the tumor cells (Fig. 5 D and E). Notably, Ctsb −/− and Ctsz −/− tumor spheroids formed significantly less and shorter invasive strands compared with the wt controls. In line, Ctsb/Ctsz double-deficient spheroids had the most impaired invasive strand formation, suggesting a synergistic effect of Ctsb and Ctsz on cancer cell invasion by proteolytic matrix remodeling.
Fig. 5.
Cell migration and invasive strand formation of primary PymT tumor spheroids. (A) Representative images of scratch closure in in vitro scratch assay. (B) Quantification of cell migration as the distance of scratch closure per hour within the first 21 h; n = 3–7 per genotype. (C) Representative images of primary PymT tumor cell spheroids in a collagen I matrix. (D and E) Formation of invasive strands of primary PymT tumor cell spheroids was assessed for number (D) and average strand length (E) per spheroid; n = 6–8 per genotype, with 40–50 spheroids analyzed. Data are presented as mean ± SE. Student´s t test was used for statistical analysis. (Scale bar: 100 μm.)
Discussion
Here we show synergistic effects of a combined deletion of two cysteine cathepsins, Ctsb and Ctsz, on cancer development. Only the combined loss of both proteases led to significant reductions in tumor and metastatic burden; single deficiencies resulted in reciprocal compensation. Ctsb, one of the most potent tumor-promoting cysteine cathepsins, is overexpressed in various human cancers and is secreted by malignant cells and cells of the tumor microenvironment (20, 33). Although Ctsb is most stable and active at pH 5.5, the enzyme also exerts proteolytic activity at neutral or slightly acidic pH (34) and is capable of degrading the ECM proteins laminin, fibronectin, and collagen (35). Ctsb also is known to initiate proteolytic cascades through activation of other tumor-promoting proteases, including matrix metalloproteinases and urokinase–type plasminogen activator (35). Accordingly, inhibition of Ctsb by inhibitors or neutralizing antibodies or knock-down by antisense RNA results in reduced invasion through ECM in vitro (36, 37), whereas overexpression of Ctsb facilitates invasion and metastasis (38, 39).
Compared with Ctsb, relatively little is known about Ctsz’s involvement in cancer. Increased levels of Ctsz have been reported in several cancers, however, including gastric and breast cancers (40, 41). In addition, our previous work suggested that Ctsz (then called cathepsin X) compensates for the loss of Ctsb in primary cancer cells (20).
To address single and double deficiencies of Ctsb and Ctsz in vivo, we analyzed the development of PymT-induced breast cancer with regard to early tumor formation, progression of advanced tumors, lung metastasis, and tumor biological processes including proliferation, cell death, angiogenesis, and invasion in relation to the Ctsb and Ctsz genotypes. Proliferation, cell death, and the density of blood/lymphatic vessels in primary tumors were not altered in any way that could provide an explanation for the antitumor effect of combined Ctsb/Ctsz deficiency; however, we recently reported that inhibition of Ctsz by neutralizing antibodies reduced invasion of Ctsb-deficient PymT tumor cells through extracellular matrix in vitro (20). Here we provide genetic evidence suggesting that ablation of Ctsb and Ctsz significantly impaired the formation of invasive strands of PymT tumor cell spheroids embedded in collagen I. Most importantly, Ctsb and Ctsz double-deficient tumor cells that expressed no active cysteine cathepsins on their cell surfaces exhibited strongly reduced formation of invasive strands. Because Ctsb and Ctsz are the only enzymes with carboxypeptidase activity among the cysteine cathepsins (27), these data suggest that cathepsin carboxypeptidase activity significantly contributes to cancer cell invasion and thus to metastasis. The spontaneously occurring metastatic process in PymT mice depends largely on the rate of primary breast cancer development. Consistently, a Ctsz deficiency that did not ameliorate the tumor burden caused only a trend toward a decreased number of metastatic foci, but no alteration in the size of metastases. The significantly reduced primary tumor burden in the PymT+/0;Ctsb −/− mice led to a trend toward a reduced number and size of lung metastases. Consequently, Ctsb/Ctsz double-deficient mice showed prominent and significant disturbances of all parameters of primary cancer development together with strongly impaired lung metastasis.
Collectively, our results demonstrate that both Ctsb and Ctsz are critical for early tumor formation (i.e., development of first palpable tumors) and affect this process independent of each other. Nevertheless, loss of Ctsz function in promotion of established tumors is completely compensated for by Ctsb alone or in combination with proteases other than cysteine cathepsins, whereas Ctsz only partially compensates for a lack of Ctsb. Given the identical cell- and tissue-specific expression patterns of these two ubiquitous proteases, differences in the effects of Ctsb and Ctsz deletion might highlight the importance of their distinctive biochemical features (42). The carboxypeptidase activity of Ctsz likely is fully compensated for by the corresponding Ctsb activity, whereas the accessory endoproteolytic activity of Ctsb appears to result in additional cancer-promoting functions of this protease, and thus a significantly reduced primary tumor burden in the Ctsb knockout. On the other hand, the unique presence of the RGD motif in the propeptide of Ctsz might contribute to formation of early cancers by binding to integrins (26). These functional aspects of the proteases remain to be elucidated, however, because our genetic approach does not allow us to distinguish between the proteolytic and nonproteolytic features of Ctsz or the endopeptidase and exopeptidase activities of Ctsb. Cysteine cathepsins have been the focus of clinical investigations as potential targets for cancer therapy (43). In this regard, our data on reciprocal functional compensation of Ctsb and Ctsz single deficiencies in tumor promotion and the efficacy of their combined knockout suggest to test a therapeutic strategy using a combination of selective inhibitors for both proteases.
Materials and Methods
Generation of Mice with Targeted Disruption of Ctsz.
Part of exon 2 (which contains the active site cysteine critical for enzyme activity), and part of intron 3 of the murine Ctsz gene were deleted by homologous recombination in HM-1 mouse ES cells and substituted by a cassette comprising an independent ribosomal entry sequence (IRES), followed by a lacZ reporter gene including an SV40 polyadenylation signal, and a neomycin resistance [from MC1Neo poly(A)] (Fig. S1A). G418-resistant HM-1 ES cell clones were screened by Southern blot analysis on genomic DNA, which was digested with Hind III or Hinc II and hybridized with the 5′ or 3′ external probes, respectively. Mutant ES cells were used to generate chimeric mice by blastocyst injection. Germline transmission of the modified Ctsz allele was confirmed in offspring from male chimeras bred to WT C57BL/6N mice. These mice were backcrossed for eight generations to the FVB/N background required in the present study. Mice were genotyped for the introduced Ctsz gene mutation by Southern blot and PCR analysis of tail genomic DNA. Disruption of the Ctsz gene was confirmed by RT-PCR (primers: forward, 5′-TAT GCC AGC GTC ACC AGG AAC-3′; reverse, 5′-CCT CTT GAT GTT GAT TCG GTC TGC-3′) and Western blot analyses.
Mouse Model for Breast Cancer.
Ctsb−/− mice (44) were previously backcrossed to the transgenic mouse strain FVB/N-TgN(MMTVPyVT)634-Mul/J (here abbreviated PymT+/0), which develops invasive and metastatic mammary cancers (21). Ctsz heterozygous (Ctsz+/−) mice were backcrossed for eight generations to the FVB/N background and then crossed with the congenic PymT+/0;Ctsb−/− mouse strain (20). The resulting PymT+/ 0;Ctsb+/−Ctsz+/− males and Ctsb+/−Ctsz+/− females were further bred to obtain PymT+/ 0;Ctsb+/+Ctsz+/+ (abbreviated as PymT+/ 0;wt), PymT+ /0;Ctsb−/−, PymT+ /0;Ctsz−/−, and PymT+/ 0;Ctsb−/−Ctsz−/− mice and the corresponding genotypes without the PymT transgene. The experimental cohorts were obtained by mating PymT+ /0 males with PymT-negative females of the same cathepsin genotype, for example, PymT+/ 0;Ctsb−/−Ctsz−/− males with Ctsb−/−Ctsz−/− females. The maintenance and breeding of the animals used in this study were performed in accordance with the German law for animal protection (Tierschutzgesetz) as published on May 25, 1998.
Isolation of Primary PymT Tumor Cells.
Primary tumor cells were obtained by mechanical and enzymatic dissociation of solid PymT-induced carcinomas as described previously (20).
Ctsb and Ctsz Immunoblotting.
Frozen tumor tissue was disrupted in lysis buffer (200 mM sodium acetate, 1 mM EDTA, 0.05% Brij; pH 5.5) using an IKA ULTRA-TURRAX disperser, followed by Dounce homogenization (Wheaton) and centrifugation at 1,500 × g for 10 min. Then 10 μg of protein from the postnuclear supernatant was resolved by 15% SDS-PAGE and transferred onto PVDF membranes. For immunodetection of Ctsb and Ctsz, membranes were probed with biotinylated goat anti-Ctsb or anti-Ctsz antibodies (R&D Systems; 1:500 dilution), followed by streptavidin peroxidase binding and detection by enhanced chemiluminescence reaction (Thermo Scientific).
Surface Labeling of Cysteine Proteases by Active Site Probes.
Surface labeling of cysteine proteases using the biotinylated active site probe DCG-04 (29) (10 μM, 1 h at 4°C) was performed as described previously (20).
Histological Analyses.
Scratch Assay and Three-Dimensional Culture of Tumor Cell Spheroids.
Cryopreserved PymT tumor cells were recultivated for 24 h, harvested by trypsinization, and plated on 6-well plates with 3 × 105 cells per well. Upon confluency, the cell monolayer was scraped using a 1,000-μL tip. Images were obtained at 0, 15, and 21 h at 10× magnification. Scratch closure distances were measured using Axiovision LE 4.4 software (Zeiss). Primary PymT tumor cell spheroids were generated as described previously (45).
Data Presentation and Statistical Analyses.
The quantitative data are presented as mean ± SE or as statistical boxplots. Multiple-group comparisons were performed by ANOVA. Post hoc range tests and pairwise multiple comparisons were conducted using Student´s t test (two-sided). Proportions were compared using the χ2 test. P ≤ 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Dr. M. Bogyo (Department of Pathology, Stanford University) for kindly providing the cysteine cathepsin active site probe DCG-04. This work was supported by European Commission FP7 Grant 201279 (Microenvimet), Deutsche Forschungsgemeinschaft SFB 850 Project B7, and by the Excellence Initiative of the German Federal and State Governments (EXC 294 and GSC-4, Spemann Graduate School).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907240107/DCSupplemental.
References
- 1.Gocheva V, et al. Distinct roles for cysteine cathepsin genes in multistage tumorigenesis. Genes Dev. 2006;20:543–556. doi: 10.1101/gad.1407406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Werb Z, Vu TH, Rinkenberger JL, Coussens LM. Matrix-degrading proteases and angiogenesis during development and tumor formation. APMIS. 1999;107:11–18. doi: 10.1111/j.1699-0463.1999.tb01521.x. [DOI] [PubMed] [Google Scholar]
- 3.Du R, et al. HIF1alpha induces the recruitment of bone marrow–derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Damme J, Struyf S, Opdenakker G. Chemokine–protease interactions in cancer. Semin Cancer Biol. 2004;14:201–208. doi: 10.1016/j.semcancer.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Bachmeier BE, Nerlich AG, Lichtinghagen R, Sommerhoff CP. Matrix metalloproteinases (MMPs) in breast cancer cell lines of different tumorigenicity. Anticancer Res. 2001;21(6A):3821–3828. [PubMed] [Google Scholar]
- 6.Shin M, et al. Association of cathepsin E with tumor growth arrest through angiogenesis inhibition and enhanced immune responses. Biol Chem. 2007;388:1173–1181. doi: 10.1515/BC.2007.154. [DOI] [PubMed] [Google Scholar]
- 7.Del Rosso M, et al. Multiple pathways of cell invasion are regulated by multiple families of serine proteases. Clin Exp Metastasis. 2002;19:193–207. doi: 10.1023/a:1015531321445. [DOI] [PubMed] [Google Scholar]
- 8.Turk V, Kos J, Turk B. Cysteine cathepsins (proteases)—on the main stage of cancer? Cancer Cell. 2004;5:409–410. doi: 10.1016/s1535-6108(04)00117-5. [DOI] [PubMed] [Google Scholar]
- 9.Mohamed MM, Sloane BF. Cysteine cathepsins: Multifunctional enzymes in cancer. Nat Rev Cancer. 2006;6:764–775. doi: 10.1038/nrc1949. [DOI] [PubMed] [Google Scholar]
- 10.Rawlings ND, Morton FR, Kok CY, Kong J, Barrett AJ. MEROPS: The peptidase database. Nucleic Acids Res. 2008;36(Database Issue):D320–D325. doi: 10.1093/nar/gkm954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrett AJ. Cellular proteolysis: An overview. Ann N Y Acad Sci. 1992;674:1–15. doi: 10.1111/j.1749-6632.1992.tb27472.x. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa T, et al. Cathepsin L: Critical role in Ii degradation and CD4 T cell selection in the thymus. Science. 1998;280:450–453. doi: 10.1126/science.280.5362.450. [DOI] [PubMed] [Google Scholar]
- 13.Hook V, et al. Inhibition of cathepsin B reduces beta-amyloid production in regulated secretory vesicles of neuronal chromaffin cells: Evidence for cathepsin B as a candidate beta-secretase of Alzheimer’s disease. Biol Chem. 2005;386:931–940. doi: 10.1515/BC.2005.108. [DOI] [PubMed] [Google Scholar]
- 14.Mai J, Finley RL, Jr, Waisman DM, Sloane BF. Human procathepsin B interacts with the annexin II tetramer on the surface of tumor cells. J Biol Chem. 2000;275:12806–12812. doi: 10.1074/jbc.275.17.12806. [DOI] [PubMed] [Google Scholar]
- 15.Rozhin J, Sameni M, Ziegler G, Sloane BF. Pericellular pH affects distribution and secretion of cathepsin B in malignant cells. Cancer Res. 1994;54:6517–6525. [PubMed] [Google Scholar]
- 16.Berdowska I. Cysteine proteases as disease markers. Clin Chim Acta. 2004;342:41–69. doi: 10.1016/j.cccn.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Fernández PL, et al. Expression of cathepsins B and S in the progression of prostate carcinoma. Int J Cancer. 2001;95:51–55. doi: 10.1002/1097-0215(20010120)95:1<51::aid-ijc1009>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 18.Castiglioni T, et al. Immunohistochemical analysis of cathepsins D, B, and L in human breast cancer. Hum Pathol. 1994;25:857–862. doi: 10.1016/0046-8177(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 19.Jedeszko C, Sloane BF. Cysteine cathepsins in human cancer. Biol Chem. 2004;385:1017–1027. doi: 10.1515/BC.2004.132. [DOI] [PubMed] [Google Scholar]
- 20.Vasiljeva O, et al. Tumor cell–derived and macrophage-derived cathepsin B promotes progression and lung metastasis of mammary cancer. Cancer Res. 2006;66:5242–5250. doi: 10.1158/0008-5472.CAN-05-4463. [DOI] [PubMed] [Google Scholar]
- 21.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: A transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santamaría I, Velasco G, Pendás AM, Fueyo A, López-Otín C. Cathepsin Z, a novel human cysteine proteinase with a short propeptide domain and a unique chromosomal location. J Biol Chem. 1998;273:16816–16823. doi: 10.1074/jbc.273.27.16816. [DOI] [PubMed] [Google Scholar]
- 23.Nägler DK, Ménard R. Human cathepsin X: A novel cysteine protease of the papain family with a very short proregion and unique insertions. FEBS Lett. 1998;434:135–139. doi: 10.1016/s0014-5793(98)00964-8. [DOI] [PubMed] [Google Scholar]
- 24.Deussing J, von Olshausen I, Peters C. Murine and human cathepsin Z: cDNA cloning, characterization of the genes and chromosomal localization. Biochim Biophys Acta. 2000;1491:93–106. doi: 10.1016/s0167-4781(00)00021-x. [DOI] [PubMed] [Google Scholar]
- 25.Nägler DK, et al. Human cathepsin X: A cysteine protease with unique carboxypeptidase activity. Biochemistry. 1999;38:12648–12654. doi: 10.1021/bi991371z. [DOI] [PubMed] [Google Scholar]
- 26.Lechner AM, et al. RGD-dependent binding of procathepsin X to integrin alphavbeta3 mediates cell-adhesive properties. J Biol Chem. 2006;281:39588–39597. doi: 10.1074/jbc.M513439200. [DOI] [PubMed] [Google Scholar]
- 27.Klemencic I, et al. Biochemical characterization of human cathepsin X revealed that the enzyme is an exopeptidase, acting as carboxymonopeptidase or carboxydipeptidase. Eur J Biochem. 2000;267:5404–5412. doi: 10.1046/j.1432-1327.2000.01592.x. [DOI] [PubMed] [Google Scholar]
- 28.Nägler DK, et al. Major increase in endopeptidase activity of human cathepsin B upon removal of occluding loop contacts. Biochemistry. 1997;36:12608–12615. doi: 10.1021/bi971264+. [DOI] [PubMed] [Google Scholar]
- 29.Greenbaum D, Medzihradszky KF, Burlingame A, Bogyo M. Epoxide electrophiles as activity-dependent cysteine protease profiling and discovery tools. Chem Biol. 2000;7:569–581. doi: 10.1016/s1074-5521(00)00014-4. [DOI] [PubMed] [Google Scholar]
- 30.Lin EY, et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 2003;163:2113–2126. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vasiljeva O, et al. Reduced tumour cell proliferation and delayed development of high-grade mammary carcinomas in cathepsin B–deficient mice. Oncogene. 2008;27:4191–4199. doi: 10.1038/onc.2008.59. [DOI] [PubMed] [Google Scholar]
- 32.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 33.Podgorski I, Sloane BF. Cathepsin B and its role(s) in cancer progression. Biochem Soc Symp. 2003;70:263–276. doi: 10.1042/bss0700263. [DOI] [PubMed] [Google Scholar]
- 34.Linebaugh BE, Sameni M, Day NA, Sloane BF, Keppler D. Exocytosis of active cathepsin B enzyme activity at pH 7.0, inhibition and molecular mass. Eur J Biochem. 1999;264:100–109. doi: 10.1046/j.1432-1327.1999.00582.x. [DOI] [PubMed] [Google Scholar]
- 35.Skrzydlewska E, Sulkowska M, Koda M, Sulkowski S. Proteolytic–antiproteolytic balance and its regulation in carcinogenesis. World J Gastroenterol. 2005;11:1251–1266. doi: 10.3748/wjg.v11.i9.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Premzl A, Zavasnik-Bergant V, Turk V, Kos J. Intracellular and extracellular cathepsin B facilitate invasion of MCF-10A neoT cells through reconstituted extracellular matrix in vitro. Exp Cell Res. 2003;283:206–214. doi: 10.1016/s0014-4827(02)00055-1. [DOI] [PubMed] [Google Scholar]
- 37.Gondi CS, et al. RNAi-mediated inhibition of cathepsin B and uPAR leads to decreased cell invasion, angiogenesis and tumor growth in gliomas. Oncogene. 2004;23:8486–8496. doi: 10.1038/sj.onc.1207879. [DOI] [PubMed] [Google Scholar]
- 38.Coulibaly S, et al. Modulation of invasive properties of murine squamous carcinoma cells by heterologous expression of cathepsin B and cystatin C. Int J Cancer. 1999;83:526–531. doi: 10.1002/(sici)1097-0215(19991112)83:4<526::aid-ijc15>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 39.Tzanakakis GN, Margioris AN, Tsatsakis AM, Vezeridis MP. The metastatic potential of human pancreatic cell lines in the liver of nude mice correlates well with cathepsin B activity. Int J Gastrointest Cancer. 2003;34:27–38. doi: 10.1385/IJGC:34:1:27. [DOI] [PubMed] [Google Scholar]
- 40.Krueger S, et al. Up-regulation of cathepsin X in Helicobacter pylori gastritis and gastric cancer. J Pathol. 2005;207:32–42. doi: 10.1002/path.1820. [DOI] [PubMed] [Google Scholar]
- 41.Decock J, et al. Cathepsin B, cathepsin H, cathepsin X and cystatin C in sera of patients with early-stage and inflammatory breast cancer. Int J Biol Markers. 2008;23:161–168. doi: 10.1177/172460080802300305. [DOI] [PubMed] [Google Scholar]
- 42.Ménard R, et al. Cathepsins X and B display distinct activity profiles that can be exploited for inhibitor design. Biol Chem. 2001;382:839–845. doi: 10.1515/BC.2001.102. [DOI] [PubMed] [Google Scholar]
- 43.Palermo C, Joyce JA. Cysteine cathepsin proteases as pharmacological targets in cancer. Trends Pharmacol Sci. 2008;29:22–28. doi: 10.1016/j.tips.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Halangk W, et al. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J Clin Invest. 2000;106:773–781. doi: 10.1172/JCI9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schurigt U, et al. Trial of the cysteine cathepsin inhibitor JPM-OEt on early and advanced mammary cancer stages in the MMTV-PyMT-transgenic mouse model. Biol Chem. 2008;389:1067–1074. doi: 10.1515/BC.2008.115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.