Abstract
Trafficking of transmembrane receptors to a specific intracellular compartment is conducted by adaptor molecules that bind to target motifs within the cytoplasmic domains of cargo proteins. We generated mice containing a lymphoid-specific deficiency of AP-1 using RNAi knockdown technology. Inhibition of AP-1 expression in thymocytes blocks progression from double-positive immature thymocytes, resulting in complete absence of CD4+ single-positive thymocytes and severe reduction of CD3+CD8+ single-positive thymocytes. Analysis of the contribution of AP-1 deficiency on the interaction between mature CD4+ T cells and antigen-presenting cells revealed that AP-1 is essential to efficient immune synapse formation and associated T cell activation, suggesting a possible mechanism of AP-1 function in thymocyte development.
Keywords: adaptor protein, immunologic synapse, T cell development, T cell receptor
The development of the two major lineages of T cells that express clonally distributed T cell receptors (TCR) in the thymus represents the central event in T cell development. The process entails generation of CD4+ or CD8+ single-positive (SP) progeny from immature double-positive (DP) cell (CD4+CD8+) precursors after engagement of the TCR by self-peptide–MHC complexes. At the same time, thymocytes that bind to peptide–MHC complexes with high affinity are deleted (negative selection), whereas the remaining thymocytes that express TCR that fail to bind to MHC–self-peptides undergo “death by neglect” (1 –3). Although this core process has been studied extensively, the precise mechanism that regulates selection and maturation of DP thymocytes is not entirely understood.
An unusual aspect of the interaction between DP thymocytes and self-peptide–MHC ligands is the ability of low levels of TCR expressed by DP precursor cells to efficiently activate them in a way that promotes further differentiation. Although surface expression of the TCR on DP thymocytes is approximately 10-fold lower than on mature SP cells, these immature thymocytes respond much more readily to peptide–MHC complexes displayed on antigen-presenting cells (APC) (4 –6). Low levels of surface TCR on preselection DP thymocytes are associated with SRC-like adaptor protein (SLAP)/c-Cbl–mediated CD3 degradation in the absence of TCR engagement, whereas ligand binding that triggers CD3ζ phosphorylation and Zap70 recruitment may bypass SLAP-dependent inhibition and transduce stronger signals (7). Although these observations may help explain how a low level of surface TCR expression is maintained on DP thymocytes, they do not address the unusual sensitivity of DP thymocytes to self-peptide–MHC complexes. We addressed this question on the basis of recent analyses indicating that supramolecular TCR-associated structures formed with APC can regulate the efficiency of T cell activation.
Thymocytes form immunologic synapses that represent TCR-enriched clusters containing a panel of signaling molecules that can potentiate sustained T cell activation (8, 9). Das et al. (10) have shown that these newly formed microclusters in peripheral T cells are continuously supplied with unoccupied TCR molecules through a recycling mechanism that directs fresh TCR to surface microclusters. This process of polarized recycling of small numbers of TCR to the synapse results in repetitive TCR-based sampling of rare or low-affinity MHC–peptide ligands on APC, and thus may increase the efficiency of the thymocyte–APC interaction and selection. Although the underlying mechanism has not been defined, adaptor protein 1 (AP-1) plays a key role in this process, because it was implicated in recycling of unoccupied TCR from trans-Golgi network to the surface membranes (11 –13), whereas ligated TCR are targeted by other mechanisms to lysosomes for degradation (14).
AP-1 is a heterotetrameric complex that consists of γ1-, β1-, σ1-, and μ1-adaptins (15, 16) that belongs to a family of clathrin adaptors—AP-1 through AP-4 (reviewed in ref. 17). Each adaptor segregates to a particular cellular compartment and mediates a specific set of activities. For example, AP-2 mediates transmembrane receptor endocytosis, whereas AP-3 contributes to lysosomal trafficking (reviewed in ref. 18). AP-1, the subject of this study, transmits cargo from the trans-Golgi network to recycling endosomes but may also participate in retrograde transport (19). AP-1 expression is essential to eukaryotic cells, because knockdown of AP-1 subunits is lethal at the embryonic stage in Caenorhabditis elegans and mice (20, 21). Interestingly, AP-1γ heterozygous animals harbor diminished numbers of CD4+ T cells in peripheral lymphoid tissues (22).
Here we report that the transition from double-negative (DN) thymocytes to the DP compartment is accompanied by a substantial increase in AP-1 expression. Analysis of thymocytes and T cell development in mice that harbor a lymphoid-specific AP-1 deficiency revealed a complete block in MHC-dependent positive selection of CD4+ SP cells and a profound defect in positive selection of CD8+ SP cells. Further analysis indicated that AP-1–dependent control of T cell activation reflects its essential contribution to the formation of TCR-containing synaptic structures with APC. These findings suggest that the initial steps in positive selection of DP thymocytes depend on amplification of TCR signaling by AP-1–dependent recycling precisely at this pivotal stage of T cell development.
Results
AP-1 Is Up-Regulated at the DP Stage During Thymocyte Development.
Serial analysis of gene expression revealed that AP-1 was up-regulated in CD4+CD24low thymocytes but not in CD4+CD24hi thymocytes shortly after lineage commitment (23). Quantitative real-time PCR was used to further delineate AP-1 expression during thymocyte development. AP-1γ transcription, apparent in bone marrow progenitor cells and in the DN compartment of thymocytes (Fig. 1A), increased almost 5-fold during the transition from the DN (CD4−8−) to the DP (CD4+8+) stage, followed by a sharp decrease in gene expression in SP thymocytes and resting peripheral T cells. Western blot analysis confirmed that these developmental changes in AP-1γ mRNA expression were also apparent at the protein level (Fig. 1B).
Fig. 1.
AP-1 is up-regulated in DP thymocytes. (A) Quantitative real-time PCR analysis of AP-1γ subunit. Cells isolated from 4- to 5-week-old C57BL/6 mice were sorted to >98% purity. AP-1 expression in different subsets was normalized to β-actin, and AP-1 expression was determined relative to peripheral CD4 (pCD4) cells, which was set as 1. Data shown represent mean ± SD of three independent experiments. BM, bone marrow; DN1, CD44+CD25−; DN2, CD44+CD25+; DN3, CD44−CD25+; DN4, CD44−CD25−; DN, CD4−CD8−; DP, CD4+CD8+; thSP, thymic single-positive cells; pSP, peripheral single-positive cells. (B) AP-1γ protein expression was analyzed by immunoblotting with anti-γ1-adaptin. Membrane was stripped and reprobed with anti-β-actin mAb. (C) Quantitative real-time PCR of AP-1 σ, μ and β subunits, as performed as in A.
Because the AP-1 complex is a heterotetramer consisting of γ1, β1, μ1, and σ1 subunits, we analyzed expression of the other AP-1 subunits by quantitative real-time PCR. The expression pattern of μ1 subunit during thymocyte development closely resembled γ1-adaptin, whereas β1 subunit expression was relatively modest at all stages and increased only slightly during the DN→DP transition. Finally, the σ1 subunit was expressed at relatively high levels during the early stages (DN→DP) of thymocyte development before decreasing at the SP stage (Fig. 1C). In sum, expression of the AP-1 complex is up-regulated at the DP stage, consistent with our previous analysis of AP-1 gene components in developing thymocytes (23).
AP-1 Knockdown Blocks T Cell Development in the Thymus.
Up-regulation of AP-1 gene expression in DP thymocytes immediately before selection opened the possibility that this gene might contribute to thymocyte selection. To investigate this hypothesis, we generated bone marrow chimeric mice that harbored a lymphoid-specific deficiency of the AP-1γ1 gene in thymocytes and T cells, according to RNAi-dependent AP-1 knockdown (KD), as described in Materials and Methods. Analysis of AP-1 expression in WT or AP-1 KD thymocytes 8–12 weeks after hematopoietic stem cell (HSC) injection showed that AP-1 expression was reduced substantially both in thymus and peripheral lymphoid organs relative to GFP controls (Fig. S1A).
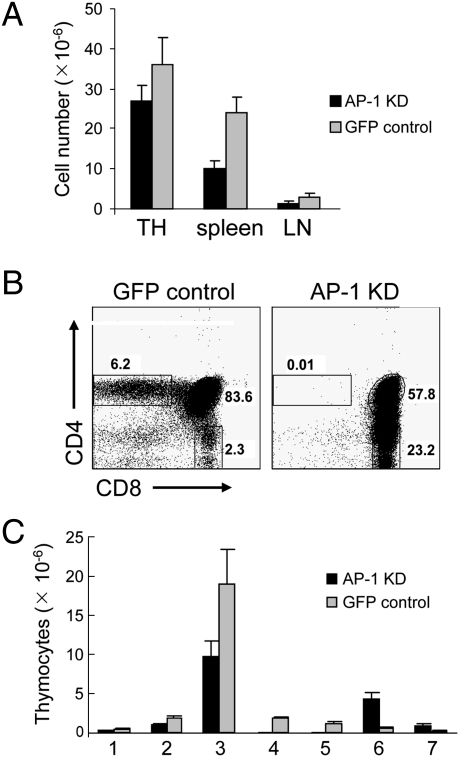
AP-1–deficient mice displayed a 30–50% decrease in lymphoid cell numbers that was apparent in spleen, lymph nodes, and thymus (Fig. 2A). Analysis of the thymocyte subpopulations revealed that generation of CD4+ SP thymocytes was essentially abolished in AP-1 KD mice (WT: 3 ×106 cells vs. KD: 1.6 ×104 cells), consistent with the absence of detectable GFP+CD4+ SP thymocytes (Fig. 2 B and C and Fig. S1B). Although total numbers of CD8+ thymocytes were increased, the number of peripheral CD8+ T cells was not, and the phenotype of CD8+ thymocytes was abnormal. Expression of CD69 and CD5, which are up-regulated on positively selected thymocytes (24, 25), was decreased substantially on DP and CD8+ thymocytes from AP-1–deficient mice compared with control mice (Fig. 3A). In addition, AP-1–deficient DP and CD8+ thymocytes expressed high levels of CD24 (HSA), consistent with an immature preselection phenotype (ISP). A decisive finding was that AP-1–deficient DP and CD8+ thymocytes expressed almost no detectable levels of either TCRβ or CD3ε at their surface (Fig. 3B). Moreover, peripheral lymphocytes were essentially absent, and those few that could be detected had GFP+CD8+TCRlo phenotype (Fig. S2). Taken together, these findings indicate that AP-1 deficiency completely blocks development of the CD4+ SP lineage and severely impairs development of the CD8+ SP thymocyte lineage.
Fig. 2.
Analysis of lymphocyte subsets in AP-1 KD mice. (A) Comparison of cell numbers between AP-1 KD and control mice in different organs. TH, thymus; LN, lymph nodes. (B) Flow cytometry of thymic subpopulations in AP-1–deficient mice (AP-1 KD) vs. GFP control mice (plasmid only). The percentage of different subpopulations is shown. (C) Comparison of cell numbers between AP-1 KD and GFP control mice in different thymocyte subsets: 1, DN cells; 2, CD4loCD8lo (DP low); 3, CD4hiCD8hi (DP high); 4, CD4+CD8lo; 5, mature CD4 SP; 6, CD4lo CD8hi; 7, mature CD8 SP. Only GFP+ cells carrying lentivirus construct with GFP reporter were included in analysis.
Fig. 3.
AP-1 KD impairs preselection thymocyte maturation. Flow cytometric analysis of positive selection markers expressed on thymocyte subsets from AP-1 KD (gray) or GFP control mice (solid line). Thymocyte populations were gated as in Fig. 2B. (A) Histograms of CD69, CD5, and CD24 surface expression. (B) Histograms of TCRβ and CD3ε surface expression.
Transition from DN to DP stage is accompanied by tremendous changes in thymocyte transcriptional signature (23). It is important to distinguish whether AP-1 expression is up-regulated as a part of this program or whether it is due to TCR signaling. To test these possibilities we assessed AP-1 expression in MHC-deficient DP thymocytes. AP-1 expression was similar in C57BL/6 and MHC−/− DP (Fig. S3A). We also found that AP-1 expression was not regulated through TCR signaling in mature CD4 cells, which was determined by monitoring AP-1γ mRNA levels in response to anti-CD3 TCR cross-linking (Fig. S3B).
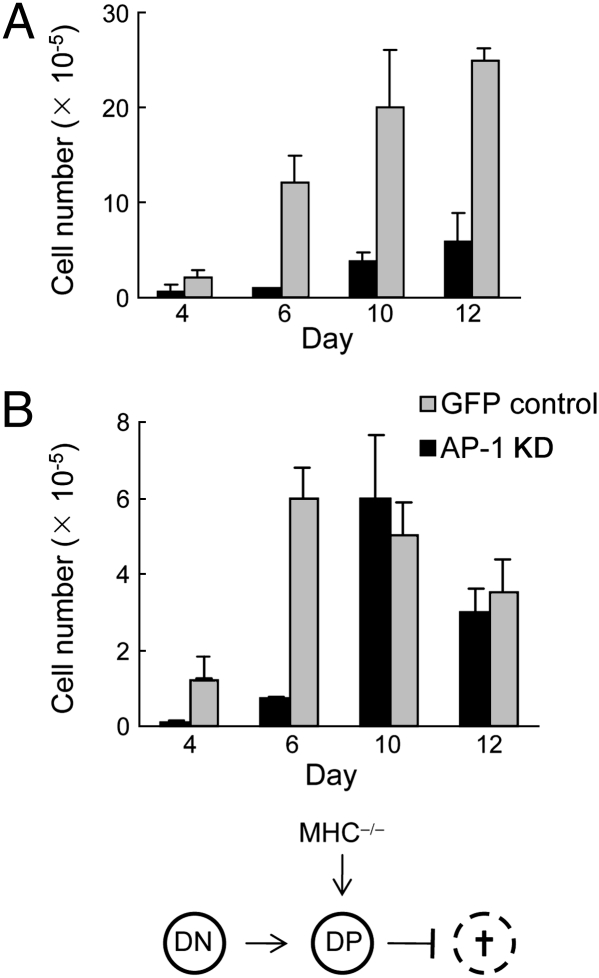
Another critical issue is whether AP-1 deficiency impairs MHC-dependent selection of DP thymocytes or whether reduced levels of AP-1 as a housekeeping gene might compromise survival and/or expansion of DP thymocytes, independent of MHC selection. To distinguish between these alternative hypotheses, we transferred WT or AP-1 KD DN thymocytes into MHC+/+ or MHC−/− thymi of (syngeneic) C57BL/6 Thy1.1 hosts. Transfer of AP-1–deficient DN cells in MHC+/+ thymi resulted in markedly reduced numbers of DP thymocytes that expressed almost undetectable levels of surface CD3 by day 12, consistent with our previous results shown in Figs. 2 and 3. In contrast, AP-1 WT DN cells rapidly proliferated and expressed CD3 levels comparable to C57BL/6 hosts (Fig. 4A; day 6: AP-1wt, 12 × 105 vs. AP-1KD, 8 × 104). Transition from DN to DP stage seems to be largely independent from MHC signals as thymocytes progress normally to DP stage in MHC−/− mice (26). Thus, failure of AP-1–deficient DN cells to expand in MHC−/− mice would reflect defective intracellular housekeeping, resulting in increased thymocyte necrosis or apoptosis rather than defective MHC-dependent positive selection. To test this hypothesis, we injected AP-1 WT and AP-1 KD DN cells into MHC−/− thymi. Although with some delay AP-1–deficient DN cells populated the host thymus to the same extent as AP-1 WT thymocytes (i.e., 104 DN cells expanded into ≈5 × 105 DP thymocytes by day 10–12. Virtually all of the cells in both groups were DP thymocytes that expressed low but detectable TCR levels. These data strongly suggest that AP-1 is up-regulated during DN→DP transition through yet-unknown mechanisms and is required for positive selection.
Fig. 4.
Intrathymic transfer of cells from GFP control or AP-1 KD mice. Approximately 104 thymocytes at the DN stage were isolated from both strains of mice. Cells were transferred to (A) irradiated C57BL/6 mice or (B) MHC−/− hosts. The number of GFP+TCRβ+ cells was calculated at the indicated time points.
AP-1 Regulates Peripheral T Cell Function.
In view of the essential contribution of AP-1 to MHC class II–dependent selection of CD4+TCR+ thymocytes, we focused on the contribution of AP-1 to the activities of this subset in peripheral tissues. Because disruption of either AP-1γ or AP-1β subunits leads to degradation of the remaining subunits and aborts assembly of the functional complex, we analyzed the impact of AP-1β shRNA on the OT-II CD4 T cell response. Lentiviral infection with AP-1β shRNA or vector alone with GFP reporter resulted in a >50% reduction in AP-1 complex expression, as judged by the anti-AP-1γ Ab response (Fig. 5A). Analysis of the proliferative response to graded doses of ovalbumin (OVA) indicated an approximately 10-fold reduction in potency (Fig. 5B). Similar results were obtained by monitoring the in vitro recall of OVA-immune CD4+ T cells (Fig. S4), suggesting that AP-1 is essential for lymphocyte function in this more physiologic setting.
Fig. 5.
AP-1 KD impairs peripheral CD4+ T cell function. (A) Representative AP-1 KD in peripheral CD4+ T cells. Cells were isolated from 5- to 7-week-old OT-II mice and lentivirally infected with vector only (GFP) or shRNA against AP-1β subunit for 4 to 5 h. Cells were cultured for 48 h in the presence of APC and 1 μg/mL anti-CD3ε. AP-1 complex expression was monitored by immunoblotting with anti-γ1-adaptin. Membrane was stripped and reprobed with anti-β-actin mAb. (B). Purified OT-II CD4+ T cells were infected as in A. T cells (1 × 105) were cocultured with irradiated splenocytes (4 × 105) from C57BL/6 in the presence of incremental concentrations of OVA peptide for 72 h. [3H] thymidine was included in cultures during the last 16–18 h. (C) Mouse CD4+ T cells were enriched by negative selection and infected with either GFP control or shRNA against AP-1γ. Cells (105) were cultured for 72 h with 2 μg/mL anti-CD28 and increasing concentrations of anti-CD3ε Ab in the presence of [3H] thymidine during the last 18 h of culture. Data represent triplicates of three independent experiments.
We then investigated the mechanism accountable for AP-1–dependent responses (i.e., interactions between T cells and APC). To avoid a potential APC influence on conjugation and signal transduction, we engaged TCR directly by plate-bound anti-CD3/CD28 Ab in GFP control-infected and AP-1 KD cells (Fig. 5C). The response of AP-1 KD cells was similar to AP-1 WT (GFP+) control cells, again indicating that antibody-dependent ligation of surface CD3 can bypass AP-1 deficiency, pointing to a contribution of AP-1 to regulating the physiologic interaction between the TCR and antigen presented by APC.
AP-1 Deficiency Modulates Formation of Immune Synapse Between T and B Cells.
One consequence of the interaction between T cells and APC is TCR polarization toward the immune synapse (IS) (27). Because AP-1 contributes to TCR recycling, AP-1 deficiency and associated reduction in targeted delivery of TCR to the synapse may blunt the T cell response to antigen. Indeed, activated OT-II cells that are deficient in AP-1 display diminished surface TCR expression, suggesting defective spontaneous TCR recycling to the cell surface (Fig. S5). To define the contribution of AP-1 deficiency on synapse formation, we analyzed the interaction between OT-II cells and OVA-pulsed B cells (Fig. 6A and Fig. S6A). AP-1 deficiency diminished T–B conjugate formation by ≈60%; the ability of T–B conjugates to form TCR-containing synapses was also reduced by approximately two thirds, whereas the degree of endosomal compartment polarization was reduced by half and the intensity of TCR accumulation was also reduced by ≈40% (Fig. 6B and Table 1). In experiments using human Jurkat T cells conjugated with superantigen-pulsed Raji B cells, conjugate formation was reduced by ≈80%, and both polarization and maximal TCR accumulation were substantially reduced (Fig. S6 B and C). However, the degree of synapse formation was less affected, suggesting that strong antigens may overcome the defect imposed by AP-1 deficiency. Thus, AP-1 regulates T cell response through enhancing IS by targeted TCR delivery to the synapse, suggesting a mechanism that thymocytes may use during positive selection.
Fig. 6.
AP-1 regulates immune synapse formation. (A) Primary mouse OT-II cells were lentivirally infected with vector only (GFP) or shRNA against AP-1γ subunit. Cells were cultured for 72 h and conjugated with OVA-pulsed B cells for 20–30 min at 37 °C on 0.01% poly-L-lysine–coated slides. Conjugates were fixed/permeabilized with methanol and stained with anti-CD3ε and anti-I-Ab. (B) Percentage of T cells that formed conjugates are mean ± SD from three independent experiments. N conjugates were analyzed for each group. Percentage synapse was calculated as TCR enrichment at the cell interface relative to the T cell periphery. Percentage IC polarization is percentage of T cells in conjugates that reposition TCR in intracellular compartment toward synapse.
Table 1.
TCR accumulation at the T-B cell interface
| OT-II | Jurkat T | |||
| Parameter | GFP | AP-1 KD | GFP | AP-1 KD |
| Accumulation ± SD | 1.2 ± 0.5 | 1.0 ± 0.3 | 3.8 ± 3.1 | 1.9 ± 0.9 |
| Maximum accumulation | 3.1 | 1.9 | 10.6 | 3 |
| Minimum accumulation | 0.5 | 0.6 | 1 | 0.4 |
| No. of conjugates | 40 | 39 | 21 | 24 |
Discussion
AP-1 is an essential component of transmembrane protein trafficking. Here we have identified a function of AP-1 in thymocyte development and mature T cell signaling. Up-regulation of AP-1 during transition from the DN compartment to preselection of DP thymocytes is critical for MHC-dependent positive selection, and lymphoid-specific deficiency of AP-1 results in a complete block of positive selection. Analysis of thymocyte differentiation has defined a transition from DP thymocytes to CD4+CD8lo pivotal subpopulation as the earliest step in MHC-dependent selection, upon which the thymus engraves lineage choice (28). This essential CD4+CD8lo cell and its positively selected CD4+ SP progeny are not detectable in the AP-1–deficent thymus. In addition, CD8+ SP progeny is also absent from thymus. The block in CD8+ SP development is partially obscured by the increased numbers of immature CD8+ thymocytes (TCR−/loCD5loCD69−CD24hi) that resemble ISP intermediaries. Because ISP are normally generated from DN4 cells by signals from the pre-TCR, AP-1 deficiency may also affect the pre-TCR compartment of thymocyte differentiation that precedes the DP stage. In this case, AP-1 deficiency may decrease pre-TCR degradation, allowing more efficient generation of ISP.
Differential expression of particular intracellular kinases has been associated with thymocyte selection. Sustained low levels of ERK activity may contribute to positive selection, whereas high but transient ERK activity (associated with JNK/p38 activation) may contribute to negative selection (reviewed in ref. 29). Despite these observations, the ability of immature DP thymocytes but not mature thymocytes or T cells responding to weak self-antigens has remained unresolved. The unusual sensitivity of DP thymocytes to self-peptide MHC ligands, despite low TCR surface expression, was reported almost a decade ago (4 –6). Only recently has it become apparent that thymocytes may change their intrinsic properties to undergo successful positive selection. For example, differential expression of microRNA miR-181a in DN thymocytes and mature T cells modulates TCR sensitivity to ligand and governs positive and negative selection of thymocytes (30).
We find that thymocytes may use an additional internal mechanism to modulate the developing cells’ TCR signaling threshold: utilization of increased TCR trafficking to the IS through up-regulation of AP-1. Previous studies have suggested that the TCR may recycle more rapidly in DP thymocytes than in SP thymocytes (31). Additional studies revealed that a dominant negative Rab35 mutation decreasing TCR transfer from recycling endosomes to the IS impairs development of conjugates (32, 33). We find that AP-1 knockdown in OT-II T cells reduced T–B conjugation, synapse formation, and diminished T cell activation (Figs. 5 and 6); that is, increased AP-1 expression in DP thymocytes results in increased TCR recycling to the IS and an increase in the number of surface TCR molecules on a developing DP thymocyte that can engage a limiting number of ligands, thus amplifying TCR signaling in the DP population. Interestingly, weak ligands that fail to induce robust and long-lived TCR down-regulation (34) allow for continuous entry of internalized TCR molecules into the recycling pool for rerouting to the IS. In contrast, interactions with strong ligands result in rapid TCR internalization and degradation (35, 36). Thus, AP-1–dependent amplification of TCR recycling after engagement of low-affinity ligand would enhance positive selection, whereas high-affinity ligand would enhance TCR degradation, resulting in apoptosis and negative selection. The effect of AP-1 deficiency on prolonged TCR signaling might result in a more pronounced effect on CD4 development than on CD8 development (Fig. 2). In addition, CD8+ ISP may reflect the impact of AP-1 deficiency on the pre-TCR, a hypothesis that remains to be tested. Interestingly, thymocyte development was not impaired in mice harboring a mutated di-leucine motif in CD3γ (37). Possibly, the effect was masked by the normal di-leucine motif of CD3δ. Because the di-leucine motif interacts with both AP-1 and AP-2, disruption of AP-2 binding may dominate TCR internalization and down-modulation. Indeed, a slight increase of surface TCR expression was noted.
The contribution of AP-1 to TCR-based engagement of antigen on APC extends to mature T cells. Down-regulation of AP-1 expression in peripheral CD4 cells was associated with the failure of AP-1–dependent T cells to respond to low-affinity self-peptides, possibly reflecting loss of the AP-1 signal amplification mechanism. It should be noted that the impact of AP-1 may not be limited to its effect on TCR recycling but may also reflect modulation of costimulatory activity in both thymocytes and mature T cells. Indeed, both CD28 and CTLA-4 play a role in the organization of the IS (38, 39), and AP-1 has been implicated in the regulation of the latter protein at the cell surface (40). In sum, these findings delineate a unique interaction between the clathrin adaptor AP-1 and the CD3 complex that regulates both early positive selection and, later, efficient T cell function.
Materials and Methods
Mice.
All age- and sex-matched mice were from Taconic Farms: C57BL/6, Rag2 −/− (H-2b; B6.129S6-Rag2tm1Fwa N12), MHC class I and class II-deficient (DKO; B6.129-H2-Ab1tm1Gru B2mtm1Jae N17), and Rag2 −/− OT-II TCR-transgenic [C57BL/6-Rag2tm1Fwa Tg(TcraTcrb)425Cbn]. Animal experimentation conducted in compliance with federal laws and institutional guidelines was approved by the Dana-Farber Cancer Institute Animal Care and Use Committee.
Reagents and Antibodies.
All reagents were from Sigma unless otherwise noted. Anti-CD8α (Ly-2), anti-CD4 (L3T4), anti-CD24 (HSA, M1/69), anti-TCRβ (H57-597), anti-CD3ε (145-2C11), anti-CD11c, anti-CD117 (c-Kit, 2B8), anti-Ly-6A/E (Sca-1, D7), anti-Gr-1 (RB6-8c5), and anti-CD11b (Mac-1, M1/70); anti-CD19 (1D3), anti-Ly-76 (TER-119), anti-CD49b/pan NK (DX5), anti-NK1.1 (PK136), anti-B220 (RA3-6B2), anti-CD25 and anti-CD44 (BD Biosciences); biotin conjugated anti-CD127 (IL-7Ra; A7R34) (eBioscience); hamster anti-mouse IgG-Alexa555 (Invitrogen); and anti-human CD3-Alexa647 (AbD Serotec).
Quantitative Real-Time PCR.
Freshly isolated lymphocyte populations from 4- to 5-week-old C57BL/6 mice were sorted by MoFlo (Dako) to 98% purity as determined by FACS analysis. Results were analyzed using Flow Jo software (Tree Star). Total RNA was isolated with RNeasy Mini Kit (Qiagen) and cDNA synthesized using oligo dT primers and the ThermoScript RT-PCR system (Invitrogen), according to the manufacturer’s instructions. Quantitative real-time PCR was performed on ABI 7300 (Applied Biosystems) using the QuantiTec SYBR Green PCR kit (Qiagen). Primer sequences were obtained from Primer Bank (41).
Immunoblotting Analysis.
Cell lysates were prepared using 1% TX-100, 20 mM Tris (pH 7.5), 150 mM NaCl, and 2 mM EDTA supplemented with protease inhibitor mixture. Proteins separated by 10% SDS/PAGE were transferred to PVDF (Invitrogen) and immunoblotted with anti-AP-1γ Ab (clone 88; BD Biosciences) or anti-β-Actin-HRP (Sigma).
Generation of AP-1 KD Mice.
Bone marrow cells were isolated from healthy mice (female, aged 6–7 weeks), and lineage-positive cells were depleted with anti-TCRβ, anti-CD3ε, anti-Gr1, anti-CD11b, anti-CD19, and anti-DX5 followed by anti-PE MicroBeads (Miltenyi Biotech). Remaining cells were stained and sorted for Sca-1+ c-Kit+ cells with MoFlo to enrich for HSC. HSC were rested overnight and infected with lentivirus shRNA against mouse adaptin-β1 target sequence (5′-GAC TGT GAG GAC CCC AAT-3′), mouse adaptin-γ1 (5′-CTG CTG GTT TCC TTC GAG GTT-3′), and human adaptin-γ1 (5′-AGC TGC TGG TTT CAT TCG AGT-3′) by centrifugation at 1,200 × g, 1.5 h, 25 °C with 8 μg/mL polybrene. Virus was prepared as described previously (42) with multiplicity of infection ≈10–20. Cells were incubated for 4 to 5 h at 37 °C in 5% CO2, then 75% of supernatant was replaced with culture medium. GFP reporter was used to distinguish lentivirus-infected cells that were enriched by sorting 24–36 h after infection. Cells were incubated in Iscove’s modified Dulbecco’s medium, 20% FCS, L-glutamine, Pen/Strep, sodium pyruvate, and nonessential amino acids supplemented with cytokines (50 ng/mL stem cell factor, 50 ng/mL Flt3L, 20 ng/mL IL-7, and 20 ng/mL IL-6). GFP+ cells (5–10 × 104) were injected into irradiated Rag2−/− mice (400 cGy), and reconstituted mice were analyzed 8 weeks later.
Intrathymic Injection.
DN cells were isolated from GFP control and AP-1 KD mice by negative selection. C57BL/6 or MHC-deficient mice were irradiated (300 cGy) and intrathymically injected with ≈104 DN cells. Numbers of GFP+ cells were analyzed at various time points.
Immunization and in Vitro Recall Response.
C57BL/6 female mice were immunized with 50 μg OVA323–339 peptide (New England Peptide) and Complete Freund’s adjuvant. CD4+ cells were isolated from draining lymph nodes 10 days after immunization and enriched by negative selection. Alternatively, CD4+ cells were isolated from spleen and lymph nodes of naïve OT-II transgenic mice. CD4+ cells were lentivirally infected with either shRNA against AP-1γ or GFP control for 4 to 5 h as above. Then 105 CD4 cells were cocultured with 4 × 105 irradiated APC in the presence or absence of increasing concentrations of OVA peptide for 72 h. Supernatants were collected at 48–60 h and analyzed for IL-2 secretion by ELISA (BD Pharmingen) (43). Cells were then pulsed with 3H-thymidine for 16–18 h and proliferation measured as thymidine incorporation using a PerkinElmer scintillation counter.
Conjugation Assay and Immunofluorescence Microscopy.
OT-II blasts were prepared by stimulation of splenocytes with 0.5 μg/mL OVA peptide × 3 days and expansion in 40 U/mL mrIL-2 (PeproTech) for 2 additional days. OT-II blasts were lentivirally infected with GFP control or shRNA against AP-1γ as above. GFP+ cells were sorted with MoFlo sorter 48 h after infection and conjugated 24 h later. Primary B cells were isolated from C57BL/6 mice and loaded with 100 μg/mL OVA protein and 2 μM OVA peptide for 10 h. OT-II and B cells were mixed at a 1:2 ratio and allowed to conjugate for 20–30 min at 37 °C in 5% CO2. Conjugates were fixed/permeabilized by methanol and stained with anti-CD3ε M20 (Santa Cruz Biotechnology) and anti-I-Ab (KH74; BD Biosciences) followed by staining with donkey anti-goat Alexa555 and donkey anti-mouse Alexa647 Ab. Jurkat T cells (clone E6-1; ATCC) were treated as above and conjugated with Raji B cells loaded with 2 μg/mL Staphylococcus enterotoxin E (Toxin Technology) at 1:1 for 15 min at 37 °C in 5% CO2. Conjugates were fixed and stained as above.
Confocal microscopy was performed using a Zeiss LSM510 META with 63× oil immersion objective, N.A. = 1.4. Number of conjugates was determined per imaging field and scored relative to total number of T cells per field. Nonspecific conjugation of T cells with B cells in the absence of antigen was subtracted from each T cell subset. Percentage intracellular compartment (IC) polarization was calculated as number of T cells in conjugates that reposition endosomal TCR toward cell–cell interface relative to total number of conjugates. Z series were performed at 0.5-μm increments. TCR enrichment at the synapse was evaluated as the total gray level of pixels at the cell–cell interface relative to the same area elsewhere on the plasma membrane on a midsection using Image J (National Institutes of Health).
Supplementary Material
Acknowledgments
We thank the Dana-Farber Cancer Institute (DFCI) Flow Cytometry Facility, the NeuroDiscovery Imaging Center at Harvard University, and Alison Angel and Dr. Hye-Jung Kim for help with manuscript preparation. This work was supported in part by National Institutes of Health Research Grants AI 37562 and 48125 and The Claudia Adams Barr Program (DFCI) (to H.C.); T32 AI007386 (to N.M. and L.L.); and T32 CA070083 (to M.L.S.) from DFCI/NIH.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0913671107/DCSupplemental.
References
- 1.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 2.Werlen G, Hausmann B, Naeher D, Palmer E. Signaling life and death in the thymus: Timing is everything. Science. 2003;299:1859–1863. doi: 10.1126/science.1067833. [DOI] [PubMed] [Google Scholar]
- 3.Aliahmad P, Kaye J. Commitment issues: Linking positive selection signals and lineage diversification in the thymus. Immunol Rev. 2006;209:253–273. doi: 10.1111/j.0105-2896.2006.00345.x. [DOI] [PubMed] [Google Scholar]
- 4.Davey GM, et al. Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. J Exp Med. 1998;188:1867–1874. doi: 10.1084/jem.188.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peterson DA, DiPaolo RJ, Kanagawa O, Unanue ER. Cutting edge: Negative selection of immature thymocytes by a few peptide-MHC complexes: Differential sensitivity of immature and mature T cells. J Immunol. 1999;162:3117–3120. [PubMed] [Google Scholar]
- 6.Peterson DA, DiPaolo RJ, Kanagawa O, Unanue ER. Quantitative analysis of the T cell repertoire that escapes negative selection. Immunity. 1999;11:453–462. doi: 10.1016/s1074-7613(00)80120-x. [DOI] [PubMed] [Google Scholar]
- 7.Mingueneau M, et al. The proline-rich sequence of CD3epsilon controls T cell antigen receptor expression on and signaling potency in preselection CD4+CD8+ thymocytes. Nat Immunol. 2008;9:522–532. doi: 10.1038/ni.1608. [DOI] [PubMed] [Google Scholar]
- 8.Hailman E, Burack WR, Shaw AS, Dustin ML, Allen PM. Immature CD4(+)CD8(+) thymocytes form a multifocal immunological synapse with sustained tyrosine phosphorylation. Immunity. 2002;16:839–848. doi: 10.1016/s1074-7613(02)00326-6. [DOI] [PubMed] [Google Scholar]
- 9.Richie LI, et al. Imaging synapse formation during thymocyte selection: Inability of CD3zeta to form a stable central accumulation during negative selection. Immunity. 2002;16:595–606. doi: 10.1016/s1074-7613(02)00299-6. [DOI] [PubMed] [Google Scholar]
- 10.Das V, et al. Activation-induced polarized recycling targets T cell antigen receptors to the immunological synapse: Involvement of SNARE complexes. Immunity. 2004;20:577–588. doi: 10.1016/s1074-7613(04)00106-2. [DOI] [PubMed] [Google Scholar]
- 11.Dietrich J, Kastrup J, Nielsen BL, Odum N, Geisler C. Regulation and function of the CD3gamma DxxxLL motif: A binding site for adaptor protein-1 and adaptor protein-2 in vitro. J Cell Biol. 1997;138:271–281. doi: 10.1083/jcb.138.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rapoport I, Chen YC, Cupers P, Shoelson SE, Kirchhausen T. Dileucine-based sorting signals bind to the beta chain of AP-1 at a site distinct and regulated differently from the tyrosine-based motif-binding site. EMBO J. 1998;17:2148–2155. doi: 10.1093/emboj/17.8.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu H, Rhodes M, Wiest DL, Vignali DA. On the dynamics of TCR:CD3 complex cell surface expression and downmodulation. Immunity. 2000;13:665–675. doi: 10.1016/s1074-7613(00)00066-2. [DOI] [PubMed] [Google Scholar]
- 14.Valitutti S, Müller S, Salio M, Lanzavecchia A. Degradation of T cell receptor (TCR)-CD3-zeta complexes after antigenic stimulation. J Exp Med. 1997;185:1859–1864. doi: 10.1084/jem.185.10.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glickman JN, Conibear E, Pearse BM. Specificity of binding of clathrin adaptors to signals on the mannose-6-phosphate/insulin-like growth factor II receptor. EMBO J. 1989;8:1041–1047. doi: 10.1002/j.1460-2075.1989.tb03471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson MS. Cloning of cDNAs encoding two related 100-kD coated vesicle proteins (alpha-adaptins) J Cell Biol. 1989;108:833–842. doi: 10.1083/jcb.108.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Traub LM. Common principles in clathrin-mediated sorting at the Golgi and the plasma membrane. Biochim Biophys Acta. 2005;1744:415–437. doi: 10.1016/j.bbamcr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Bonifacino JS, Rojas R. Retrograde transport from endosomes to the trans-Golgi network. Nat Rev Mol Cell Biol. 2006;7:568–579. doi: 10.1038/nrm1985. [DOI] [PubMed] [Google Scholar]
- 19.Hinners I, Tooze SA. Changing directions: Clathrin-mediated transport between the Golgi and endosomes. J Cell Sci. 2003;116:763–771. doi: 10.1242/jcs.00270. [DOI] [PubMed] [Google Scholar]
- 20.Shim J, Sternberg PW, Lee J. Distinct and redundant functions of mu1 medium chains of the AP-1 clathrin-associated protein complex in the nematode Caenorhabditis elegans . Mol Biol Cell. 2000;11:2743–2756. doi: 10.1091/mbc.11.8.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer C, et al. mu1A-adaptin-deficient mice: Lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J. 2000;19:2193–2203. doi: 10.1093/emboj/19.10.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zizioli D, et al. Early embryonic death of mice deficient in gamma-adaptin. J Biol Chem. 1999;274:5385–5390. doi: 10.1074/jbc.274.9.5385. [DOI] [PubMed] [Google Scholar]
- 23.McCarty N, Shinohara ML, Lu L, Cantor H. Detailed analysis of gene expression during development of T cell lineages in the thymus. Proc Natl Acad Sci USA. 2004;101:9339–9344. doi: 10.1073/pnas.0402654101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swat W, Dessing M, von Boehmer H, Kisielow P. CD69 expression during selection and maturation of CD4+8+ thymocytes. Eur J Immunol. 1993;23:739–746. doi: 10.1002/eji.1830230326. [DOI] [PubMed] [Google Scholar]
- 25.Dutz JP, Ong CJ, Marth J, Teh HS. Distinct differentiative stages of CD4+CD8+ thymocyte development defined by the lack of coreceptor binding in positive selection. J Immunol. 1995;154:2588–2599. [PubMed] [Google Scholar]
- 26.Grusby MJ, et al. Mice lacking major histocompatibility complex class I and class II molecules. Proc Natl Acad Sci USA. 1993;90:3913–3917. doi: 10.1073/pnas.90.9.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grakoui A, et al. The immunological synapse: A molecular machine controlling T cell activation. Science. 1999;285:221–227. [PubMed] [Google Scholar]
- 28.Singer A, Adoro S, Park JH. Lineage fate and intense debate: Myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat Rev Immunol. 2008;8:788–801. doi: 10.1038/nri2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alberola-Ila J, Hernández-Hoyos G. The Ras/MAPK cascade and the control of positive selection. Immunol Rev. 2003;191:79–96. doi: 10.1034/j.1600-065x.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 30.Li QJ, et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129:147–161. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Myers MD, et al. Src-like adaptor protein regulates TCR expression on thymocytes by linking the ubiquitin ligase c-Cbl to the TCR complex. Nat Immunol. 2006;7:57–66. doi: 10.1038/ni1291. [DOI] [PubMed] [Google Scholar]
- 32.Kouranti I, Sachse M, Arouche N, Goud B, Echard A. Rab35 regulates an endocytic recycling pathway essential for the terminal steps of cytokinesis. Curr Biol. 2006;16:1719–1725. doi: 10.1016/j.cub.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 33.Patino-Lopez G, et al. Rab35 and its GAP EPI64C in T cells regulate receptor recycling and immunological synapse formation. J Biol Chem. 2008;283:18323–18330. doi: 10.1074/jbc.M800056200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cemerski S, et al. The stimulatory potency of T cell antigens is influenced by the formation of the immunological synapse. Immunity. 2007;26:345–355. doi: 10.1016/j.immuni.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D’Oro U, Vacchio MS, Weissman AM, Ashwell JD. Activation of the Lck tyrosine kinase targets cell surface T cell antigen receptors for lysosomal degradation. Immunity. 1997;7:619–628. doi: 10.1016/s1074-7613(00)80383-0. [DOI] [PubMed] [Google Scholar]
- 36.Lauritsen JP, et al. Two distinct pathways exist for down-regulation of the TCR. J Immunol. 1998;161:260–267. [PubMed] [Google Scholar]
- 37.Bonefeld CM, et al. TCR down-regulation controls virus-specific CD8+ T cell responses. J Immunol. 2008;181:7786–7799. doi: 10.4049/jimmunol.181.11.7786. [DOI] [PubMed] [Google Scholar]
- 38.Pentcheva-Hoang T, Egen JG, Wojnoonski K, Allison JP. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004;21:401–413. doi: 10.1016/j.immuni.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 39.Jackman RP, Balamuth F, Bottomly K. CTLA-4 differentially regulates the immunological synapse in CD4 T cell subsets. J Immunol. 2007;178:5543–5551. doi: 10.4049/jimmunol.178.9.5543. [DOI] [PubMed] [Google Scholar]
- 40.Schneider H, et al. Cytolytic T lymphocyte-associated antigen-4 and the TCR zeta/CD3 complex, but not CD28, interact with clathrin adaptor complexes AP-1 and AP-2. J Immunol. 1999;163:1868–1879. [PubMed] [Google Scholar]
- 41.Wang X, Seed B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res. 2003;31:e154. doi: 10.1093/nar/gng154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCarty N, et al. Signaling by the kinase MINK is essential in the negative selection of autoreactive thymocytes. Nat Immunol. 2005;6:65–72. doi: 10.1038/ni1145. [DOI] [PubMed] [Google Scholar]
- 43.Lu L, et al. Regulation of activated CD4+ T cells by NK cells via the Qa-1-NKG2A inhibitory pathway. Immunity. 2007;26:593–604. doi: 10.1016/j.immuni.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.