Abstract
Mutualistic interactions are taxonomically and functionally diverse. Despite their ubiquity, however, the basic ecological and evolutionary processes underlying their origin and maintenance are poorly understood. A major reason for this is the lack of an experimentally tractable model system. We examine the evolution of an experimentally imposed obligate mutualism between sulfate-reducing and methanogenic microorganisms that have no known history of previous interaction. Twenty-four independent pairings (cocultures) of the bacterium Desulfovibrio vulgaris and the archaeon Methanococcus maripaludis were established and followed for 300 community doublings in two environments, one allowing for the development of a heterogeneous distribution of resources and the other not. Evolved cocultures grew up to 80% faster and were up to 30% more productive (biomass yield per mole of substrate) than the ancestors. The evolutionary process was marked by periods of significant instability leading to extinction of two of the cocultures, but it resulted in more stable, efficient, and productive mutualisms for most replicated pairings. Comparisons of evolved cocultures with those assembled from one evolved mutualist and one ancestral mutualist showed that evolution of both species contributed to improved productivity. Surprisingly, however, overall improvements in growth rate and yield were less than the sum of the individual contributions, suggesting antagonistic interactions between mutations from the coevolved populations. Physical constraints on the transfer of metabolites in the evolution environment affected the evolution of M. maripaludis, but not of D. vulgaris. Together, these results demonstrate that challenges can imperil nascent obligate mutualisms and demonstrate the evolutionary responses that enable their persistence and future evolution.
Keywords: adaptation, experimental evolution, environmental heterogeneity, syntrophy , methanogenesis
The existence of mutually beneficial interactions between species has often puzzled evolutionary biologists because of the benefits of avoiding costly investments in genetically unrelated populations (1, 2). Such interactions are thought to originate when species begin trading byproducts or evolve from parasitic relationships (2, 3). The persistence of newly formed nascent mutualisms depends on their ability to adapt to several ecological challenges. First, the mutualists must initially use preexisting traits, so the functional basis for the mutualism likely is not optimal. Second, the mutualists’ growth may be less stable, because it depends on a resource produced by another population. This situation can lead to extinction of the mutualism if the abundance of one or both populations becomes too low or if one population stops cooperating, especially if the mutualism is obligate (1, 4, 5). Finally, adaptation to mutualism also may be affected by properties of the environment in which the mutualism is occurring. In particular, the spatial distribution of interacting populations affects the transfer of resources between them and may be key to their stability (6 –9).
Studying the effect of these challenges on the evolution of new mutualisms is difficult without the possibility to experiment with the original populations. Some adaptations arising early in mutualistic associations from preexisting traits have been identified through comparative analyses, a common approach to studying evolution (10, 11). Empirical data on the evolutionary and ecological dynamics giving rise to these adaptations are scarce, however, because the original populations and ecological conditions are unknown.
Here we use experimental evolution to address this issue while avoiding the methodological limitations of past comparative approaches. We control the selective environment and examine adaptations as they occur (12) and use microorganisms so that we can establish initially identical replicate populations and thus analyze the role of chance events in determining evolutionary outcomes (13). A similar approach has been used to rigorously examine various questions involving interactions between genotypes and species, including the evolution of predator–prey interactions (14, 15), the evolution of intraspecific cooperation (16, 17) and the ecological factors that stabilize commensal relationships against competition (7, 18, 19). The evolution of mutual cooperation between distinct species has not been addressed with experimental evolution, except to explore the relationships between mutualism and parasitism (20 –22).
We use experimental evolution to study the first steps in the evolution of a mutualism that we formed by experimentally imposing a requirement for exchange of byproducts (3). A nascent syntrophic mutualism was established by pairing the bacterium Desulfovibrio vulgaris Hildenborough with the archaeon Methanococcus maripaludis S2 (23). This mutualism is based on interspecies transfer of hydrogen, a byproduct of anaerobic metabolism that is commonly exchanged among species that inhabit anoxic environments (24). Both species can be propagated in pure culture on appropriate substrates, but under the conditions of our experiments they can grow only through syntrophic cooperation, or “feeding together.” In the absence of hydrogen and sulfate, the species feed together by cooperating to complete the following energy-yielding reaction:
D. vulgaris ferments lactate, producing acetate, carbon dioxide, and hydrogen, but this reaction does not produce sufficient energy for growth unless the concentration of one of the reaction products is kept very low. M. maripaludis ensures that this condition is met by consuming hydrogen and using it to reduce carbon dioxide to methane. Growth of M. maripaludis depends on the availability of hydrogen produced by D. vulgaris, because no other suitable substrate is available. Thus, D. vulgaris provides food for M. maripaludis as a byproduct of lactate fermentation, and M. maripaludis provides a permissive growth environment as a byproduct of feeding. Syntrophies similar to our experimental system function in the sediments of freshwater lakes, the guts of ruminants, and anaerobic digesters used to process waste (23). Desulfovibrio and related species also may function in syntrophies that degrade other complex growth substrates (24), sometimes involving obligate syntrophs (25), may use carbon monoxide or formate in lieu of hydrogen (26, 27), and may have acquired syntrophy-related genes through horizontal gene transfer (28).
The strains of D. vulgaris and M. maripaludis used in this work have been propagated in pure culture in the laboratory for years and were isolated from very different environments (29, 30); thus, the selective environment during adaptation to syntrophy is expected to be similar to that of a nascent mutualism. Both strains must rely on traits that have been adapted to pure culture growth. Their continued association also is dependent on the individual success of both syntrophic partners. Finally, because efficient interspecies transfer of hydrogen (and possibly other materials) depends on the spatial distribution of each species and the resources that they produce, our experimental design also incorporates spatial heterogeneity as an environmental factor, allowing us to test how the efficiency of byproduct transfer affects mutualist evolution.
To explore the evolution of this model mutualism, 24 nearly identical cocultures were evolved independently for 300 coculture doublings in two environments that promoted different distributions of populations, substrates, and metabolites. Throughout the experiment, nearly all of the cells were free-living and not aggregated. Twelve cultures were evolved in an environment in which cells and substrates were uniformly distributed and metabolic byproducts were transferred by rapid shaking of the culture. The remaining 12 replicates were evolved in cultures that remained static during incubation (but were mixed weekly for propagation), creating an environment in which substrates and metabolites could be transferred only by diffusion. To test how the heterogeneity of the environment affected the evolution of each species in mutualism, we isolated the species populations from each coculture and used them to produce cocultures of mixed ancestry. We compared these cocultures with cocultures that had only ancestral or only evolved populations.
Results
Evolutionary Changes in Stability of Syntrophic Communities.
The early stage of mutualist evolution was characterized by erratic growth (Fig. 1). Cocultures typically consumed all of the resources within 1 week and achieved stationary-phase densities between 0.25 and 0.35 OD600 nm. However, after the first four transfers, a few cocultures did not show appreciable growth (0.0–0.06 OD600); these were not transferred until they reached stationary-phase densities 1 week or more later. Every coculture entered one of these slow-growth phases at least once during the first 6 months of propagation. The final densities achieved by slow-growing cocultures varied from 0.15 to 0.35 OD600. The timing, frequency, and duration of slow growth varied considerably among the cocultures, suggesting the involvement of a stochastic process that resulted in the extinction of two cocultures in the heterogeneous evolution environment. After about 30 transfers, erratic growth became infrequent, and coculture growth cycles stabilized (Fig. 1).
Fig. 1.
Coculture density at each transfer during the evolution experiment.
Increases in Growth Rate and Yield of Evolved Communities.
As the cocultures became stable, they consistently achieved higher densities in stationary phase compared with those at the beginning of the evolution experiment (Fig. 1). To test whether each coculture was also growing faster, we used freezer stocks of each evolved and ancestral coculture to inoculate media selective for D. vulgaris and M. maripaludis, thereby separating the populations in each coculture. We acclimated these populations in pure culture conditions before using them to inoculate new cocultures, to minimize differences in previous acclimation to syntrophic growth. This allowed us to more accurately measure differences caused by genetic changes accumulated in evolved cocultures. We tested only 20 cocultures in these experiments, because 2 cocultures went extinct and 2 others did not reach their 45th transfer for several weeks after these assays were completed.
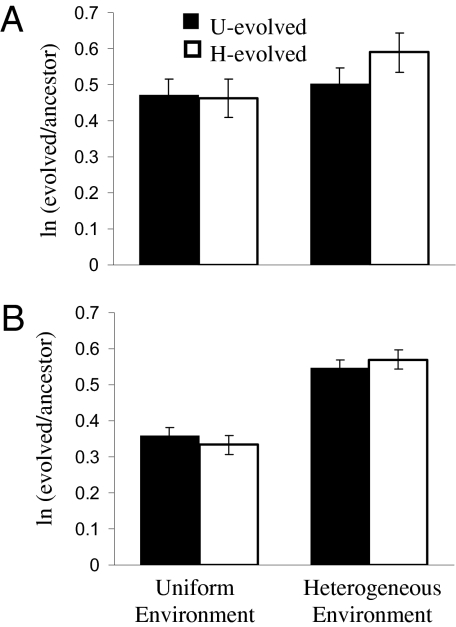
Nineteen of the 20 cocultures tested exhibited a significant improvement in growth rate relative to the ancestor, indicating that one or both species had adapted to some aspect of the syntrophic environment. The average doubling time of the ancestral cocultures was 20 h (± 1.3, 95% confidence interval) in the uniform (U) environment and 23 h (± 1.3) in the heterogeneous (H) environment. In contrast, evolved cocultures doubled every 13 h on average when grown in the treatment in which they evolved (± 1.5, H-evolved; ± 1.7, U-evolved; when the U-evolved coculture that did not improve was removed, the average for U-evolved cocultures was 12 ± 0.2 h). Thus, on average U-evolved cocultures improved by roughly 1.6-fold, whereas H-evolved cocultures improved by 1.8-fold (Fig. 2A).
Fig. 2.
Improvements in growth rate and yield of cocultures after 300 generations of evolution. The growth rate (A) and yield (B) of uniform (solid bars) or heterogeneous (open bars) evolved cocultures and their ancestors was measured in both evolution environments. Bars indicate least squares means from the ANOVA results reported in Table S1, and error bars indicate SE.
These evolutionary improvements in coculture growth rate could represent general adaptations to conditions that are the same in both treatments, such as the challenge of growing on lactate without an electron acceptor. In that case, evolved cocultures would perform similarly whether they were examined in the heterogeneous environment or in the uniform environment. Alternatively, the populations might have adapted to different ecological conditions in the two environments. In that case, cocultures that evolved in the uniform environment would perform poorly in the heterogeneous environment and vice versa. We investigated this possibility by measuring the growth rate of all of the evolved cocultures in their alternate evolution environment (Fig. 2A and Table S1). The magnitude of improvement in coculture growth rate was not affected by environment (F1,18 = 0.41; P = .531) or by the interaction between the evolution and assay environment (F1,136 = 2.03; P = .157). Thus, it appears that the populations have mostly adapted to general aspects of syntrophic growth, not specifically to the heterogeneity of the environment in which they evolved. Regardless of how the populations evolved, the relative improvement was greater when examined in the heterogeneous environment (F1,136 = 5.35; P = .022), slightly more so if they also evolved in the heterogeneous environment (t 136 = −2.42; P = .0169, t test) (Fig. 2A). This latter difference was due to poorer growth of U-evolved lines in the heterogeneous environment (15 ± 1.0 h average doubling time) compared with H-evolved lines.
All evolved cocultures exhibited significant increases in yield. The magnitude of the increase was independent of the evolution environment (F1,18 =0.0; P = .969) but was relatively higher when yield was measured in the heterogeneous environment compared to the uniform environment (F1,136 =228.9; P < .0001) (Fig. 2B). This difference in relative magnitude can be attributed to the performance of the ancestor. The average yield was similar for U-evolved cocultures (OD600 nm = 0.488 ± 0.01, 95% confidence interval) and H-evolved cocultures (OD600 nm = 0.492 ± 0.03), but the ancestor reached yields of 0.339 ± 0.01 when growing in the uniform environment and 0.278 ± 0.01 when growing in the heterogeneous environment. Increases in optical density corresponded with increases in average stationary-phase cell densities of both species. An average 3.9-fold increase in D. vulgaris cell density (to 4 × 108 cells/mL) and a 1.6-fold increase in M. maripaludis cell density (to 3 × 108 cells/mL) were determined in evolved cocultures. Although M. maripaludis predominated in ancestral cocultures, after 300 generations D. vulgaris became the predominant species in almost all U-evolved cocultures and half of the H-evolved cocultures (Fig. S1).
Contributions of D. vulgaris and M. maripaludis Populations to Improved Coculture Growth.
The evolutionary improvements in coculture growth rate and yield could be caused by adaptations in D. vulgaris, M. maripaludis, or both species. To identify the population responsible for these community-level changes, we compared the growth rate and yield improvements in cocultures with only one evolved species (DEMA, evolved D. vulgaris in coculture with ancestral M. maripaludis, and DAME, ancestral D. vulgaris and evolved M. maripaludis) and fully evolved cocultures (DEME) in their evolution treatment only. For these comparisons, cocultures were formed from potentially genetically diverse populations of D. vulgaris and M. maripaludis that had been isolated by enrichment with pure culture growth conditions and antibiotics. Each of the evolved D. vulgaris and M. maripaludis populations could improve syntrophic growth by themselves (Figs. 3 and 4). Independently evolved D. vulgaris populations were able to improve coculture growth rate and yield by similar magnitudes (except in coculture U12), but the capacity of M. maripaludis populations to affect syntrophy was more variable.
Fig. 3.
Improvements in growth rate of each coculture caused by both evolved species together (open triangles), evolved D. vulgaris only (open circles), or evolved M. maripaludis only (white stars on solid squares). The average of four replicate measures for cocultures from the uniform environment and the heterogeneous environment are plotted separately in A and B, respectively. Error bars indicate 95% CIs.
Fig. 4.
Improvements in yield of each coculture caused by both evolved species together. Symbols and error bars are the same as in Fig. 3. The average of four replicate measures for cocultures from the uniform environment and the heterogeneous environment are plotted separately in A and B, respectively.
M. maripaludis populations that evolved in the heterogeneous environment had a greater and more consistent effect on coculture growth compared with those evolved in the uniform environment (Fig. 3 and Table S2). The interaction between coculture composition (DEME vs. DEMA vs. DAME) and evolution environment had a significant effect on coculture growth rate (F2, 214 =15.2; P < .0001). The mean growth rate improvement was lower in U-evolved DAME cocultures than in H-evolved DAME cocultures, because only some U-evolved M. maripaludis populations could improve coculture growth rates, whereas every H-evolved M. maripaludis population caused faster growth (Fig. 3 A and B). When evolved in a uniform environment, almost all DEME and DEMA cocultures had similar growth rate improvements, suggesting that D. vulgaris might be the primary determinant of this feature (Fig. 3A). In contrast, H-evolved DEMA and DAME cocultures both yielded similar improvements as DEME cocultures (Fig. 3B). Thus, either or both species in combination may have contributed to the growth rate improvements of H-evolved cocultures.
The effects of D. vulgaris and M. maripaludis on coculture yield were similar and appear to be unaffected by the heterogeneity of their evolution environment (F2, 36 = 0.2; P = .819). Fully evolved cocultures (DEME) tended to obtain higher yields than cocultures in which only one of the species had evolved in syntrophy (DEMA or DAME) (F2,36 = 19.8; P <.0001), suggesting that both species contributed to the yield improvements of the fully evolved cocultures (Fig. 4 A and B).
Interactions Between Evolved D. vulgaris and Evolved M. maripaludis Populations.
All of the evolved D. vulgaris and M. maripaludis populations were able to enhance coculture growth, indicating that one or more new mutations became prevalent in each of these populations during evolution. In some cocultures, both species could cause improvements similar to those of the fully evolved cocultures; thus, the relative contributions of mutations in each species to the growth improvements of DEME cocultures is unclear. Consequently, we tested for interactions between the mutations that could cause improvements in D. vulgaris and M. maripaludis beyond what might be predicted from their independent effects, using methods developed for detecting epistatic interactions (31). We used the growth improvements of DEMA and DAME (relative to DAMA) cocultures to calculate multiplicative (DEMA/DAMA × DAME/DAMA) and additive (DEMA/DAMA + DAME/DAMA − 1) null models for combinations of mutational effects.
The observed improvements in growth rate and yield of all H-evolved cocultures were lower than could be predicted from additive and multiplicative models (Table S3). Compared with both null models, growth rate improvements were significantly lower in cocultures H3, H5, and H6 (P <.05, two-tailed, two-sample t test; n = 4), and yield improvements were significantly lower in cocutures H1, H2, and H6. These results indicate that in the heterogeneous environment there is a tendency toward antagonistic interactions between mutations affecting syntrophic growth efficiency in the coevolving populations. In U-evolved cocultures, antagonistic interactions between mutational effects were not universal. Growth rate was lower than predicted by both null models for eight U-evolved cocultures and yield was lower in nine, but these differences were not statistically significant, except for coculture U12, which had a significantly lower growth rate than predicted by both models. In five U-evolved cocultures, the observed improvements in growth were either the same as or slightly higher than predicted.
Discussion
Few empirical examples of the initial stages of adaptation to mutualism are available. By experimentally imposing a mutualism and then monitoring its evolution, we were able to demonstrate rapid improvement in productivity and stability in response to the challenges of a new interdependent relationship. The evolved mutualism grew up to 80% faster and produced up to 30% more biomass than the ancestral pairings. Although evolutionary changes in both species contributed to improvements, the contribution of each population varied with the environment in which the mutualism evolved. Most significantly, all M. maripaludis populations that evolved in a heterogeneous environment contributed to a faster growth rate, whereas the contribution of those populations that evolved in a uniform environment was highly variable, with some not contributing to improvement. Our findings also suggest that substantial challenges are associated with the early stages of evolution of this mutualism. This characteristic was demonstrated by initially erratic growth that led to extinction in 2 of the 24 cocultures.
When populations first engage in a mutualistic relationship, they must adapt to new growth conditions, and they most likely use preexisting traits for new functions. Thus, one of the first adaptations for mutualism may be optimization of these traits for mutualistic performance. In support of this hypothesis, both species in nearly every coculture appear to have substituted mutations that improved the overall productivity of syntrophy. Cocultures could grow faster and produce more cells even though the resources remained constant throughout the experiment. Each species contributed to one or both of these community-level changes, presumably because they were able to more efficiently use the available resources and hence acquire more energy for growth.
In an obligate mutualism, growth may not occur if both interacting populations are not at a minimum density (4, 5). The positive feedback between populations can lead to unsustainable levels of growth (4), and evolution may cause substantial fluctuations in the population densities of commensals (32). We found that the growth dynamics of communities were erratic during the early evolution of an experimentally imposed obligate syntrophy. The cause of this erratic growth is unclear, but the extinction of two cocultures demonstrated significant ecological consequences. The surviving mutualisms eventually evolved stable, predictable responses to batch culture growth.
As populations evolve in mutualisms or other interactions, they acquire mutations that may affect not only their own fitness, but also the environment for their coevolving partner. The coevolving partner may acquire mutations that mitigate or enhance these changes, depending on how they affect its fitness. This process underlies interactions between genotypes, such as those described by Heath and Tiffin (33) between Sinorhizobium medicae and Medicago truncatulata genotypes. A surprising result from our study was the tendency toward antagonistic interactions between coculture growth-enhancing mutations in some D. vulgaris and M. maripaludis populations. These interactions between mutational effects could indicate an ecological constraint on growth of the syntrophy that limits the combined effects of two efficient syntrophs, each of which is capable of improving the growth of both species, bringing syntrophy to near-maximal levels. The species also could be actively competing for a limiting resource. For example, evolved D. vulgaris might obtain more resources than the ancestor (e.g., incorporating more lactate into cellular carbon), thereby limiting growth opportunities for evolved M. maripaludis when they are together. In this scenario, M. maripaludis would have higher fitness without its evolved partner.
The efficiency of a mutualism based on byproduct exchange is affected by how easily goods can be transferred between interacting populations. In our experiments, one species maintained thermodynamically permissible conditions as a byproduct of feeding while the other produced a metabolic byproduct, hydrogen. The transfer of this metabolite to M. maripaludis probably was most efficient in the uniform environment, where it was vigorously dispersed by mixing. In contrast, if hydrogen were not efficiently transferred between species in the heterogeneous environment (which might require their close proximity), its accumulation in the headspace during growth would reduce its availability in liquid (23). Inefficient hydrogen transfer is consistent with the observation that ancestral cocultures were slower and less productive in the heterogeneous environment.
Cocultures that evolved in the heterogeneous environment overcame this obstacle. They could grow as fast in the heterogeneous environment as all evolved cocultures could grow in the uniform environment. This evolved capacity required a special adaptation that evidently was not acquired by the uniform-evolved cocultures, which could not grow at maximal rates in the heterogeneous environment. Evolutionary responses to this challenge were confined to M. maripaludis, the species that used hydrogen for growth. All of the M. maripaludis populations from the heterogeneous evolution environment improved the coculture growth rate, but few of the uniform-evolved M. maripaludis had this capacity. In contrast, this variable had a more subtle effect (if any) on adaptation in D. vulgaris.
Other research with microbial systems has shown that the diversification of populations into new niches (8, 34), the evolution of exploitative relationships (7), and community diversity (6, 9) are affected by heterogeneous distributions of resources and populations that limit the diffusion of metabolites in communities. Our results confirm the importance of metabolite transfer rates on evolution of microorganisms. M. maripaludis relied on a diffusible metabolic byproduct for growth and had a different evolutionary response in the heterogeneous environment, where this resource must be transferred through diffusion or enhanced in some way by interspecies contact. In contrast, D. vulgaris relied on lactate, a soluble growth substrate that would be evenly dispersed in either heterogeneity treatment, and it showed a similar evolutionary response in both environments.
In conclusion, using experimental evolution of a model microbial mutualism, we were able to demonstrate several evolutionary responses of nascent mutualisms that may be predicted intuitively but have rarely been examined empirically. This model system for studying mutualistic interactions is now poised to address a variety of issues relating to the evolution of interacting populations, including how quickly coevolving populations become specialized to one another, the effects of adaptation to mutualism on solitary fitness, and the genetic and physiological basis of adaptations to mutualism.
Methods
Strains and Culture Conditions.
All cultures were grown at 37 °C. D. vulgaris Hildenborough (ATCC 29579) was obtained from Dr. T.C. Hazen (Lawrence Berkeley National Laboratory). We isolated a clone of this strain, D1, and a spontaneous nalidixic acid–resistant derivative of that clone, D2, to use as ancestors in the evolution experiment. D. vulgaris was grown on plates with LS4D media (30) or in 10 mL of coculture medium A (CCMA) (23) with 4.3 g/L of NaSO4 in a Balch tube (18 × 150-mm glass tube with a narrow opening to hold a 1-inch-thick rubber septum) with a 80% N2 and 20% CO2 headspace. See SI Methods for detailed recipes. All media were buffered with bicarbonate to maintain a pH of 7.2.
We isolated clone M1 of M. maripaludis S2 (29) and a neomycin-resistant clone (M2) from this population to use as ancestors in the evolution experiments. Clone M2 lost its neomycin resistance during culturing. M. maripaludis was cultured in 5 mL of CCMA without lactate and supplemented with 0.82 g/L of acetate, 1 g/L of casamino acids, and a higher sulfide concentration (0.5 g/L NaS • 9H2O) in Balch tubes pressurized to 30 psi with 20% CO2 and 80% H2, and then incubated in a horizontal position with shaking (300 rpm). Syntrophic cocultures were initiated by adding 0.1 mL (>1 × 107 cells) each of stationary-phase cultures of D. vulgaris and M. maripaludis to 20 mL of CCMA (no sulfate) in a 30-mL Balch tube under 20% CO2 and 80% N2 atmosphere.
Evolution Experiment.
Four cocultures (consisting of D1 and M1, D1 and M2, D2 and M1, and D2 and M2) were used as inocula (0.2 mL) for six independent cultures. Three cocultures were incubated without shaking (heterogeneous environment), and three were incubated in a horizontal position with shaking at 300 rpm (uniform environment). Thus, the evolution experiment was started with 12 independently evolving cocultures in each heterogeneity treatment. Every 7 days, coculture density was measured in a spectrophotometer at OD600 nm, and the coculture was transferred to fresh media (1% inoculum) if it had achieved its “maximum density” of 0.25–0.35 OD, or left to incubate until the next weekly transfer otherwise. If the density declined from one week to the next, the coculture was transferred even if the maximum density was low (0.1–0.16 OD600nm). At the 45th 100-fold dilution (300 doublings of the syntrophic community, 6.6 per transfer) and several previous intervals, samples of each evolved coculture were stored in 10%–20% glycerol at −80 °C. In the heterogeneous lines, a significant number of cells were concentrated at the bottom of the culture tubes at each transfer interval; thus, these tubes were shaken vigorously to redistribute cells before transfer. Purity of the cocultures was checked periodically by plating on R2A and by microscopic examination.
Assay of Coculture Growth Rate and Yield.
Coculture growth rate and yield was assayed for all 12 cocultures from the uniform environment but for only 8 cocultures from the heterogeneous environment, because two cocultures in the latter environment went extinct and two others had not reached their 45th transfer until after the assays were completed. Freezer stocks of the evolved cocultures at the 45th transfer and all four ancestral cocultures were each used to inoculate a Balch tube containing media typically used to propagate D. vulgaris and antibiotic against M. maripaludis (5 μg/mL of puromycin), and another tube containing media for M. maripaludis and antibiotic against D. vulgaris (1 mg/mL of spectinomycin). The resulting separated D. vulgaris and M. maripaludis populations were used to inoculate two each of the following combinations per evolved coculture: DEME, DEMA, DAME, and DAMA. All of these cocultures were incubated in their evolution environment. Two extra DEME replicates were made for each evolved coculture and incubated in the alternate environment in which they did not evolve, resulting in a total of 200 cocultures per temporal block (400 total for the entire assay). Once a coculture maintained its maximum density for 3 days, it was transferred (1%) into fresh CCMA, and its density was recorded periodically until it once again maintained a maximum density for 3 days. Coculture yield (maximum OD600 nm reached by the coculture) and growth rate were estimated independently for each replicate growth curve. The coculture growth rate was the slope (obtained from several data points) of the linear portion of the curve obtained by plotting ln(OD600 nm) = time.
Statistical Analyses.
Evolutionary changes in coculture growth rate and yield were obtained by dividing each measurement of a coculture containing one or more evolved population (DEME, DEMA, or DAME) by a randomly chosen measure from the same temporal block of that coculture’s direct ancestor (DAMA). The natural log of this ratio was used to complete the mixed-model ANOVAs using the Mixed procedure and the Satterthwaite approximation of degrees of freedom in SAS version 9.1 (SAS Institute). Formal ANOVA tables and detailed descriptions of the statistical models are provided in Tables S1 and S2 and SI Methods.
D. vulgaris and M. maripaludis Density Estimation.
Using the same methods in assays of coculture growth rate and yield, we separated species populations and used them to prepare DEME, and DAMA cocultures for all 20 evolved lines and 4 ancestral pairings. We determined the density of D. vulgaris and M. maripaludis in cocultures that had been at stationary-phase densities for 3 days using a Petroff–Hauser counting chamber and a light microscope at 400× magnification. D. vulgaris (vibriod) and M. maripaludis (cocci) were identified by cellular morphology. We repeated this experiment three times.
Supplementary Material
Acknowledgments
We thank Lara Rajeev, Sergey Stolyar, Paul Berube, Willm Martens-Habbena, Elizabeth Ostrowski, Tim Cooper, and Vaughn Cooper for their comments on the manuscript. We also thank Charles Atkinson for his valuable technical assistance and Siobhan Everson-Stewart for advice on statistical models. This work was part of the Virtual Institute for Microbial Stress and Survival (http://VIMSS.lbl.gov), supported by the US Department of Energy, Office of Science, Office of Biological and Environmental Research, Genomics Program:GTL through contract DE-AC02-05CH11231 between the Lawrence Berkeley National Laboratory and the US Department of Energy.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908456107/DCSupplemental.
References
- 1.Sachs JL, Simms EL. Pathways to mutualism breakdown. Trends Ecol Evol. 2006;21:585–592. doi: 10.1016/j.tree.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 2.Bergstrom CT, et al. In: Genetic and Cultural Evolution of Cooperation. Hammerstein P, editor. Cambridge, MA: MIT Press; 2003. pp. 241–256. [Google Scholar]
- 3.Sachs JL, Mueller UG, Wilcox TP, Bull JJ. The evolution of cooperation. Q Rev Biol. 2004;79:135–160. doi: 10.1086/383541. [DOI] [PubMed] [Google Scholar]
- 4.May RM. In: Theoretical Ecology: Principles and Applications. May RM, editor. Philadelphia: WB Saunders; 1976. pp. 49–71. [Google Scholar]
- 5.Shou WY, Ram S, Vilar JMG. Synthetic cooperation in engineered yeast populations. Proc Natl Acad Sci USA. 2007;104:1877–1882. doi: 10.1073/pnas.0610575104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HJ, Boedicker JQ, Choi JW, Ismagilov RF. Defined spatial structure stabilizes a synthetic multispecies bacterial community. Proc Natl Acad Sci USA. 2008;105:18188–18193. doi: 10.1073/pnas.0807935105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansen SK, Rainey PB, Haagensen JA, Molin S. Evolution of species interactions in a biofilm community. Nature. 2007;445:533–536. doi: 10.1038/nature05514. [DOI] [PubMed] [Google Scholar]
- 8.Habets MG, Rozen DE, Hoekstra RF, de Visser JA. The effect of population structure on the adaptive radiation of microbial populations evolving in spatially structured environments. Ecol Lett. 2006;9:1041–1048. doi: 10.1111/j.1461-0248.2006.00955.x. [DOI] [PubMed] [Google Scholar]
- 9.Saxer G, Doebeli M, Travisano M. Spatial structure leads to ecological breakdown and loss of diversity. Proc R Soc Lond B Biol Sci. 2009;276:2065–2070. doi: 10.1098/rspb.2008.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pellmyr O, Thompson JN, Brown JM, Harrison RG. Evolution of pollination and mutualism in the yucca moth lineage. Am Nat. 1996;148:827–847. [Google Scholar]
- 11.Shingleton AW, Stern DL, Foster WA. The origin of a mutualism: A morphological trait promoting the evolution of ant-aphid mutualisms. Evolution. 2005;59:921–926. [PMC free article] [PubMed] [Google Scholar]
- 12.Elena SF, Lenski RE. Evolution experiments with microorganisms: The dynamics and genetic bases of adaptation. Nat Rev Genet. 2003;4:457–469. doi: 10.1038/nrg1088. [DOI] [PubMed] [Google Scholar]
- 13.Travisano M, Mongold JA, Bennett AF, Lenski RE. Experimental tests of the roles of adaptation, chance, and history in evolution. Science. 1995;267:87–90. doi: 10.1126/science.7809610. [DOI] [PubMed] [Google Scholar]
- 14.Hillesland KL, Lenski RE, Velicer GJ. Experimental evolution of a microbial predator’s ability to find prey. Proc R Soc Lond B Biol Sci. 2009;276:459–467. doi: 10.1098/rspb.2008.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poullain V, Gandon S, Brockhurst MA, Buckling A, Hochberg ME. The evolution of specificity in evolving and coevolving antagonistic interactions between a bacteria and its phage. Evolution. 2008;62:1–11. doi: 10.1111/j.1558-5646.2007.00260.x. [DOI] [PubMed] [Google Scholar]
- 16.Velicer GJ, Yu YT. Evolution of novel cooperative swarming in the bacterium Myxococcus xanthus . Nature. 2003;425:75–78. doi: 10.1038/nature01908. [DOI] [PubMed] [Google Scholar]
- 17.Rainey PB, Rainey K. Evolution of cooperation and conflict in experimental bacterial populations. Nature. 2003;425:72–74. doi: 10.1038/nature01906. [DOI] [PubMed] [Google Scholar]
- 18.Rosenzweig RF, Sharp RR, Treves DS, Adams J. Microbial evolution in a simple unstructured environment: Genetic differentiation in Escherichia coli . Genetics. 1994;137:903–917. doi: 10.1093/genetics/137.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rozen DE, Philippe N, Arjan de Visser J, Lenski RE, Schneider D. Death and cannibalism in a seasonal environment facilitate bacterial coexistence. Ecol Lett. 2009;12:34–44. doi: 10.1111/j.1461-0248.2008.01257.x. [DOI] [PubMed] [Google Scholar]
- 20.Sachs JL, Wilcox TP. A shift to parasitism in the jellyfish symbiont Symbiodinium microadriaticum . Proc R Soc Lond B Biol Sci. 2006;273:425–429. doi: 10.1098/rspb.2005.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sachs JL, Bull JJ. Experimental evolution of conflict mediation between genomes. Proc Natl Acad Sci USA. 2005;102:390–395. doi: 10.1073/pnas.0405738102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouma JE, Lenski RE. Evolution of a bacteria/plasmid association. Nature. 1988;335:351–352. doi: 10.1038/335351a0. [DOI] [PubMed] [Google Scholar]
- 23.Stolyar S, et al. Metabolic modeling of a mutualistic microbial community. Mol Syst Biol. 2007;3 doi: 10.1038/msb4100131. article 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schink B. In: Molecular Basis of Symbiosis. Overmann J, editor. Vol. 41. Berlin: Springer; 2006. pp. 1–19. [Google Scholar]
- 25.McInerney MJ, et al. The genome of Syntrophus aciditrophicus: Life at the thermodynamic limit of microbial growth. Proc Natl Acad Sci USA. 2007;104:7600–7605. doi: 10.1073/pnas.0610456104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parshina SN, et al. Carbon monoxide conversion by thermophilic sulfate-reducing bacteria in pure culture and in co-culture with Carboxydothermus hydrogenoformans . Appl Microbiol Biotechnol. 2005;68:390–396. doi: 10.1007/s00253-004-1878-x. [DOI] [PubMed] [Google Scholar]
- 27.Boone DR, Johnson RL, Liu Y. Diffusion of the interspecies electron carriers H2 and formate in methanogenic ecosystems and its implications in the measurement of Km for H2 or formate uptake. Appl Environ Microbiol. 1989;55:1735–1741. doi: 10.1128/aem.55.7.1735-1741.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scholten JC, Culley DE, Brockman FJ, Wu G, Zhang W. Evolution of the syntrophic interaction between Desulfovibrio vulgaris and Methanosarcina barkeri: Involvement of an ancient horizontal gene transfer. Biochem Biophys Res Commun. 2007;352:48–54. doi: 10.1016/j.bbrc.2006.10.164. [DOI] [PubMed] [Google Scholar]
- 29.Whitman WB, et al. Isolation and characterization of 22 mesophilic Methanococci . Syst Appl Microbiol. 1986;7:235–240. [Google Scholar]
- 30.Postgate JR. The Sulphate Reducing Bacteria. 2nd Ed. Cambridge, UK: Cambridge University Press; 1984. [Google Scholar]
- 31.Elena SF, Lenski RE. Test of synergistic interactions among deleterious mutations in bacteria. Nature. 1997;390:395–398. doi: 10.1038/37108. [DOI] [PubMed] [Google Scholar]
- 32.Rozen DE, Lenski RE. Long-term experimental evolution in Escherichia coli. VIII: Dynamics of a balanced polymorphism. Am Nat. 2000;155:24–35. doi: 10.1086/303299. [DOI] [PubMed] [Google Scholar]
- 33.Heath KD, Tiffin P. Context dependence in the coevolution of plant and rhizobial mutualists. Proc R Soc Lond B Biol Sci. 2007;274:1905–1912. doi: 10.1098/rspb.2007.0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rainey PB, Travisano M. Adaptive radiation in a heterogeneous environment. Nature. 1998;394:69–72. doi: 10.1038/27900. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.