More than one-third of cases of dilated cardiomyopathy (DCM) are caused by inherited mutations, with 5-10% of these mutations linked to the LMNA gene encoding the nuclear envelope proteins lamin A and C.1-3 Whereas geneticists have mostly looked for mutations in genes encoding sarcomeric and cytoskeletal proteins in the past, genes for nuclear envelope associated proteins are proving to be equally important candidate genes for DCM. The subsequent discoveries that mutations in other nuclear envelope proteins that directly bind to lamins A/C, for example, nesprin4 and emerin5, can also cause DCM has stimulated the interest in exploring the role of lamin and its binding partners in DCM. New insights into the functional mechanisms by which nuclear proteins can cause DCM have come from the identification of a mutation in another lamin A/C binding partner, the nuclear protein Lamina Associated Polypeptide 2α (LAP2 α), as cause for dilated cardiomyopathy in a large kindred6 and subsequent studies in LAP2α-deficient (LAP2α–/–) mice7. In this issue of Circulation Research, Gotic et al report that young, male, LAP2α–/– mice develop systolic dysfunction and show deregulation of the major cardiac transcription factors GATA4 and MEF2c8. These findings, taken together with previous reports showing an important role of lamin A/C in regulating gene expression by sequestering or altering the activity of transcription factors9-11, suggest that (nucleoplasmic) lamins may act as a scaffold for transcription factors and other DNA binding proteins such as LAP2α and, through this complex, modulate gene expression and cell function. This function could be distinct or overlapping with their role in providing nuclear structure and support.12
The nuclear envelope has gained particularly great interest since mutations in its components have been associated with a spectrum of diseases ranging from muscular dystrophy, dilated cardiomyopathy, familial partial lipodystrophy, peripheral neuropathy, and bone defects to premature aging (reviewed in 13). Lamins are the major building blocks of the nuclear lamina, a dense protein network underlying the inner nuclear membrane, and of a more diffuse network in the nucleoplasm.14, 15 The detailed higher order structure of lamins, their various cellular functions, and the molecular mechanisms underlying nuclear envelope associated diseases such as DCM remain unclear and are the focus of intense research. Lamins were originally regarded as mere structural proteins providing stability and structural support to the nuclear envelope but are now known to be also involved in chromatin organization, gene regulation, DNA transcription and replication, DNA repair and overall cellular integrity (reviewed in 16). Importantly, it is the interaction of lamins with a multitude of integral nuclear membrane proteins such as emerin, MAN1, Lamina Associated Polypeptide (LAP) 2 proteins, lamin B receptor, SUN-proteins and transcription factors such as SREBP-1c, c-Fos and retinoblastoma protein (pRb) that give lamins a pivotal role in the nucleus. LAP2α is the sole member of the LAP2 family that lacks a transmembrane domain and is restricted to the nucleoplasm. Here, LAP2α binds the nucleoplasmic pool of lamins A and C and forms a complex with the cell-cycle regulator and tumor suppressor pRb (Figure 1). Through the LAP2α-lamin A/C-pRb complex, LAP2α modulates pRb and thus controls cell cycle exit and thereby, the maintenance of progenitor pools.7
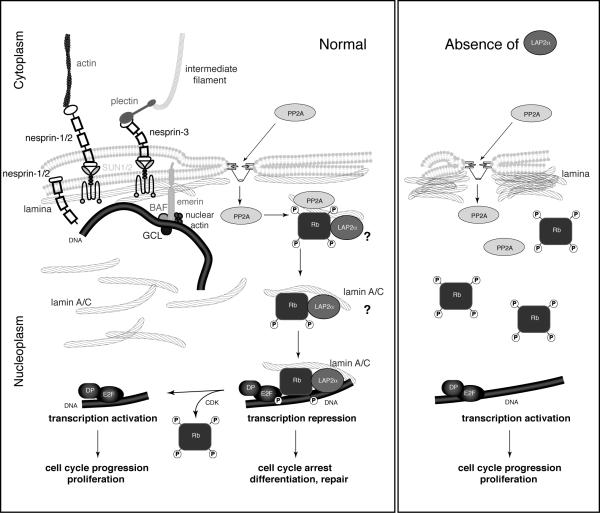
Figure 1. Overview of the localization of nuclear (envelope) proteins linked to dilated cardiomyopathy.
Schematic depiction of the structural and gene regulatory roles of lamins and other nuclear envelope proteins and their interaction with transcription factors to mediate gene regulation. (Left) In wildtype cells, lamins form a filamentous network, i.e., the lamina, lining the nucleoplasmic side of the nuclear envelope. They space nuclear pore complexes and interact with multiple integral nuclear membrane proteins such as emerin and SUN1/2-proteins. SUN-proteins interact across the perinuclear space with nesprins that can bind to actin stress fibers or, via plectin, to the intermediate filament network, thereby establishing a physical link between the nuclear interior and the cytoskeleton. Shorter nesprin isoforms can also be located on the inner nuclear membrane, where they may bind to lamins and emerin. Emerin, just as most integral Lamina Associated Polypeptide 2 (LAP2) family members, contains a transmembrane domain and a so-called LEM domain through which these proteins bind BAF and, thus, are involved in chromatin organization. Emerin can further bind nuclear actin and signaling factors such as the Drosophila melanogaster germ cell-less gene product (GCL). Lamins also make up a more diffuse network in the nucleoplasm that may serve as a scaffold for multiple transcription factors such as retinoblastoma protein pRb and LAP2α. The phosphatase PP2A dephosphorylates pRb in a complex that involves lamin A/C. It is yet unclear if LAP2α is part of this complex and if a complex of hypophosphorylated pRb-lamin A/C-LAP2α translocates to the E2F-target genes to repress transcription, leading to cell cycle arrest and potentially, DNA repair and differentiation. Cyclin dependent kinases (CDK) can phosphorylate pRb through which it is released from the E2F-target genes, resulting in transcription activation, cell cycle progression and proliferation. (Right) In the absence of LAP2α, lamin A/C is localized predominantly at the nuclear periphery, and pRb remains hyperphosphorylated and, therefore, cannot repress transcription of E2F-target genes. The result is increased proliferation and, potentially, early depletion of progenitor/stem cells. [Figure adapted from 16 and reproduced with permission from Bentham Science Publishers Ltd.]
The fact that mutations in both lamin A/C and LAP2α result in DCM suggests a major role for the lamin A/C-LAP2α complex in cardiac function. Gotic et al tried to further dissect the cardiac function role of this complex and specifically looked at the role of LAP2α in recently created LAP2α–/– mice7 and in a conditional knockout mouse model. In their studies, young male LAP2α–/– mice developed ventricular systolic dysfunction characterized by significantly decreased fractional shortening and ejection fraction; 10-12 months old male mice showed similar defects in systolic function without further progression. Interestingly, 3 out of 11 old male mice showed cardiac fibrosis. Although these numbers are not quite statistically significant, they could indicate an increased susceptibility to cardiac fibrosis in LAP2α–/– mice. In contrast to the male mice, female LAP2α–/– mice did not develop any overt phenotype. This finding is reminiscent of LmnaH222P/H222P mice, a model for Emery-Dreifuss muscular dystrophy, where female mice developed a milder phenotype, which suggests yet-to-be identified sex differences. Because of the systolic dysfunction observed in LAP2α–/– mice, Gotic at al studied the expression of transcription factors crucial in cardiac development and hypertrophic response. MEF2c expression was significantly decreased in newborn LAP2α–/– hearts, though reached normal levels by the age of 10 weeks. GATA4, Myocardin A and Striated Muscle Activator of Rho Signaling (STARS) was found to be repressed in old LAP2α–/– hearts only. Brain Natriuretic Peptide (BNP), which is one of the downstream effectors of MEF2c and GATA4, showed a reduced expression in both young and old LAP2α–/– hearts. Since BNP has an inhibitory effect on fibroblast proliferation and extracellular matrix production, its reduced expression could add to the sporadically observed extensive cardiac fibrosis in male LAP2α–/– hearts. In an attempt to evoke a more pronounced cardiac phenotype, the authors subjected LAP2α–/– mice to treatment with a β-adrenergic agonist, isoproterenol, which increases heart rate and contractility. Whereas wild-type animals had a significant increase in systolic left ventricular diameter and end-systolic left ventricular volume after isoproterenol infusion for 7 days, the increase in LAP2α–/– over baseline was not statistically significant. However, absolute differences between wild-type and LAP2α–/– mice were comparable after isoproterenol treatment, indicating that the effect of LAP2α on the β-adrenergic response was rather small. The authors attribute this attenuated stress response to reduced expression of β-adrenergic receptor 2 in LAP2α–/– hearts. Unfortunately, without analysis of the expression for β-adrenergic receptor-1, it is hard to draw definitive conclusions in this matter.
The authors recently demonstrated that LAP2α regulates the homeostasis and number of progenitor cells by modulating localization of lamins A/C and affecting pRb activity.7 To address whether the observed systolic dysfunction in male LAP2α–/– mice was a consequence of loss of LAP2α during early development or later on in life, the authors used a conditional LAP2α–/– mouse that abolishes LAP2α expression in mature cardiomyocytes only. These mice, however, did not develop any overt cardiac phenotype, suggesting that the major role of LAP2α is restricted to early stages of cardiac development or during cardiac homeostasis later on in life. This is consistent with previous findings that LAP2α is primarily involved in the regulation of early progenitor cell proliferation in regenerative tissues in vivo7 and is only moderately expressed in the adult heart.6 It would be interesting to test the opposite scenario, i.e., whether depletion of LAP2α only in early stages of cardiac development or in progenitor cells is sufficient to cause systolic dysfunction. A role of LAP2α in cardiac progenitor cells could also have important implications in adult hearts. A recent study provided strong evidence for cardiomyocyte renewal in humans.17 Evaluation of the integration of carbon-14, generated by nuclear bomb tests during the Cold War, into DNA of cardiomyocyte nuclei showed that about half of the cardiomyocytes are exchanged during normal life span. Cardiomyocyte renewal does decrease by age from 1% at the age of 25 to 0.45% at the age of 75. In view of the previously established role of LAP2α in the proliferation of tissue progenitor cells, its absence could negatively affect the cardiac regenerative potential resulting in heart failure and, eventually, lead to dilated cardiomyopathy.
In light of these facts, what's the outlook of DCM caused by mutations in nuclear envelope proteins? There is increasing evidence for a major role of defective gene regulation in the development of DCM associated with mutations in nuclear envelope proteins. Emerin-null and LmnaH222P/H222P mice, two models for Emery-Dreifuss muscular dystrophy that is associated with severe DCM in humans, show significant activation of the ERK1/2-branch of the mitogen activated protein kinase (MAPK) pathway before clinical signs or molecular markers of cardiomyopathy become apparent.18, 19 These findings showed promise for pharmacological interventions to treat or prevent cardiomyopathy in the context of Emery-Dreifuss muscular dystrophy. Indeed, treatment of LmnaH222P/H222P mice with PD98059, an inhibitor of ERK phosphorylation, delayed the development of left ventricular dilatation.20 Although, Gotic et al did not address any putative alterations in the MAPK pathway directly, significant changes in some major cardiac transcription factors such as GATA4 and MEF2c support a primary role for defective gene regulation in the pathogenesis of DCM. This is consistent with the reduced activation of hypertrophic genes despite severe DCM in lamin A/C–null mice21 and the recent finding that mice that are haploinsufficient for lamin A/C (Lmna+/–) have an attenuated hypertrophic response and reduced induction of the cardiac hypertrophy gene Egr-1 in response to pressure overload.22 Although the impaired activation of mechanosensitive genes Egr-1 and Iex-1 in mouse fibroblasts lacking lamins A and C may suggest that the reduced nuclear stiffness associated with loss of lamin A/C may contribute to this impaired gene activation,23 it is also likely that transcriptional regulation and nuclear structural support are separate—albeit potentially overlapping—functions of lamins. In the case of nuclear envelope proteins, DCM could arise from defects in either function, depending on the specific nature of the mutation. Mutations that effect both structural and signaling properties, or complete loss of lamin function, would then result in additive effects and more severe DCM, as seen in lamin A/C–null mice. The pathogenic changes in gene expression causing DCM could be manageable with pharmacological interventions. However, it will be more challenging to intervene with the loss of nuclear and cellular integrity causing DCM.
Acknowledgements
VLRMV is supported by the American Heart Association [09POST2080264]; JL is supported by the National Institutes of Health [R01 HL082792 and R01 NS059348] and the American Heart Association [0635359N].
Footnotes
Conflict of Interest Disclosure:
None
References
- 1.Malhotra R, Mason PK. Lamin A/C deficiency as a cause of familial dilated cardiomyopathy. Curr Opin Cardiol. 2009;24(3):203–208. doi: 10.1097/HCO.0b013e32832a11c6. [DOI] [PubMed] [Google Scholar]
- 2.Parks SB, Kushner JD, Nauman D, Burgess D, Ludwigsen S, Peterson A, Li D, Jakobs P, Litt M, Porter CB, Rahko PS, Hershberger RE. Lamin A/C mutation analysis in a cohort of 324 unrelated patients with idiopathic or familial dilated cardiomyopathy. Am Heart J. 2008;156(1):161–169. doi: 10.1016/j.ahj.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perrot A, Hussein S, Ruppert V, Schmidt HH, Wehnert MS, Duong NT, Posch MG, Panek A, Dietz R, Kindermann I, Bohm M, Michalewska-Wludarczyk A, Richter A, Maisch B, Pankuweit S, Ozcelik C. Identification of mutational hot spots in LMNA encoding lamin A/C in patients with familial dilated cardiomyopathy. Basic Res Cardiol. 2009;104(1):90–99. doi: 10.1007/s00395-008-0748-6. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Q, Bethmann C, Worth NF, Davies JD, Wasner C, Feuer A, Ragnauth CD, Yi Q, Mellad JA, Warren DT, Wheeler MA, Ellis JA, Skepper JN, Vorgerd M, Schlotter-Weigel B, Weissberg PL, Roberts RG, Wehnert M, Shanahan CM. Nesprin-1 and -2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum Mol Genet. 2007;16(23):2816–2833. doi: 10.1093/hmg/ddm238. [DOI] [PubMed] [Google Scholar]
- 5.Holaska JM. Emerin and the nuclear lamina in muscle and cardiac disease. Circ Res. 2008;103(1):16–23. doi: 10.1161/CIRCRESAHA.108.172197. [DOI] [PubMed] [Google Scholar]
- 6.Taylor MR, Slavov D, Gajewski A, Vlcek S, Ku L, Fain PR, Carniel E, Di Lenarda A, Sinagra G, Boucek MM, Cavanaugh J, Graw SL, Ruegg P, Feiger J, Zhu X, Ferguson DA, Bristow MR, Gotzmann J, Foisner R, Mestroni L. Thymopoietin (lamina-associated polypeptide 2) gene mutation associated with dilated cardiomyopathy. Hum Mutat. 2005;26(6):566–574. doi: 10.1002/humu.20250. [DOI] [PubMed] [Google Scholar]
- 7.Naetar N, Korbei B, Kozlov S, Kerenyi MA, Dorner D, Kral R, Gotic I, Fuchs P, Cohen TV, Bittner R, Stewart CL, Foisner R. Loss of nucleoplasmic LAP2alphalamin A complexes causes erythroid and epidermal progenitor hyperproliferation. Nat Cell Biol. 2008;10(11):1341–1348. doi: 10.1038/ncb1793. [DOI] [PubMed] [Google Scholar]
- 8.Gotic I, Leschnik M, Kolm U, Markovic M, Haubner BJ, Biadasiewicz K, Metzler B, Stewart CL, Foisner R. LAP2alfa loss impairs heart function and stress response in mice. Circ Res. 2009:XXX–XXX. doi: 10.1161/CIRCRESAHA.109.205724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivorra C, Kubicek M, Gonzalez JM, Sanz-Gonzalez SM, Alvarez-Barrientos A, O'Connor JE, Burke B, Andres V. A mechanism of AP-1 suppression through interaction of c-Fos with lamin A/C. Genes Dev. 2006;20(3):307–320. doi: 10.1101/gad.349506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson BR, Nitta RT, Frock RL, Mounkes L, Barbie DA, Stewart CL, Harlow E, Kennedy BK. A-type lamins regulate retinoblastoma protein function by promoting subnuclear localization and preventing proteasomal degradation. Proc Natl Acad Sci U S A. 2004;101(26):9677–9682. doi: 10.1073/pnas.0403250101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lloyd DJ, Trembath RC, Shackleton S. A novel interaction between lamin A and SREBP1: implications for partial lipodystrophy and other laminopathies. Hum Mol Genet. 2002;11(7):769–777. doi: 10.1093/hmg/11.7.769. [DOI] [PubMed] [Google Scholar]
- 12.Lammerding J, Fong LG, Ji JY, Reue K, Stewart CL, Young SG, Lee RT. Lamins A and C but not lamin B1 regulate nuclear mechanics. J Biol Chem. 2006;281(35):25768–25780. doi: 10.1074/jbc.M513511200. [DOI] [PubMed] [Google Scholar]
- 13.Worman HJ, Fong LG, Muchir A, Young SG. Laminopathies and the long strange trip from basic cell biology to therapy. J Clin Invest. 2009;119(7):1825–1836. doi: 10.1172/JCI37679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broers JL, Machiels BM, van Eys GJ, Kuijpers HJ, Manders EM, van Driel R, Ramaekers FC. Dynamics of the nuclear lamina as monitored by GFP-tagged A-type lamins. J Cell Sci. 1999;112(Pt 20):3463–3475. doi: 10.1242/jcs.112.20.3463. [DOI] [PubMed] [Google Scholar]
- 15.Moir RD, Yoon M, Khuon S, Goldman RD. Nuclear lamins A and B1: different pathways of assembly during nuclear envelope formation in living cells. J Cell Biol. 2000;151(6):1155–1168. doi: 10.1083/jcb.151.6.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verstraeten VL, Broers JL, Ramaekers FC, van Steensel MA. The nuclear envelope, a key structure in cellular integrity and gene expression. Curr Med Chem. 2007;14(11):1231–1248. doi: 10.2174/092986707780598032. [DOI] [PubMed] [Google Scholar]
- 17.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324(5923):98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muchir A, Pavlidis P, Bonne G, Hayashi YK, Worman HJ. Activation of MAPK in hearts of EMD null mice: similarities between mouse models of X-linked and autosomal dominant Emery Dreifuss muscular dystrophy. Hum Mol Genet. 2007;16(15):1884–1895. doi: 10.1093/hmg/ddm137. [DOI] [PubMed] [Google Scholar]
- 19.Muchir A, Pavlidis P, Decostre V, Herron AJ, Arimura T, Bonne G, Worman HJ. Activation of MAPK pathways links LMNA mutations to cardiomyopathy in Emery-Dreifuss muscular dystrophy. J Clin Invest. 2007;117(5):1282–1293. doi: 10.1172/JCI29042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muchir A, Shan J, Bonne G, Lehnart SE, Worman HJ. Inhibition of extracellular signal-regulated kinase signaling to prevent cardiomyopathy caused by mutation in the gene encoding A-type lamins. Hum Mol Genet. 2009;18(2):241–247. doi: 10.1093/hmg/ddn343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikolova V, Leimena C, McMahon AC, Tan JC, Chandar S, Jogia D, Kesteven SH, Michalicek J, Otway R, Verheyen F, Rainer S, Stewart CL, Martin D, Feneley MP, Fatkin D. Defects in nuclear structure and function promote dilated cardiomyopathy in lamin A/C-deficient mice. J Clin Invest. 2004;113(3):357–369. doi: 10.1172/JCI19448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cupesi M, Yoshioka J, Gannon J, Kudinova A, Stewart CL, Lammerding J. Attenuated hypertrophic response to pressure overload in a lamin A/C haploinsufficiency mouse. J Mol Cell Cardiol. 2009 doi: 10.1016/j.yjmcc.2009.10.024. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, Stewart CL, Lee RT. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest. 2004;113(3):370–378. doi: 10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]