Abstract
Nicotinic acetylcholine receptors mediate fast synaptic transmission by fluxing ions across the membrane in response to neurotransmitter binding. We show here that during affinity purification of the nicotinic acetylcholine receptor from Torpedo, phosphatidic acid, but not other anionic or zwitterionic phospholipids, is hydrolyzed to diacylglycerol. The phospholipase C activity elutes with the acetylcholine receptor and is inhibited by a lipid phosphate phosphohydrolase inhibitor, sodium vanadate, but not a phosphatidate phosphohydrolase inhibitor, N-ethylmaleimide. Further, the hydrolysis product of phosphatidic acid, diacylglycerol, enhances the functional capabilities of the acetylcholine receptor in the presence of anionic lipids. We conclude that a phospholipase C activity, which appears to be specific for phosphatidic acid, is associated with the nicotinic acetylcholine receptor. The acetylcholine receptor may directly or indirectly influence lipid metabolism in a manner that enhances its own function.
Keywords: Lipid/Diacylglycerol, Lipid/Phosphatidic Acid, Membrane/Proteins, Receptors, Receptors/Neurotransmitters, Receptors/Structure-Function, Nicotinic Acetylcholine Receptors, Lipid-Protein Interactions, Phospholipase Activity, Synaptic Plasticity
Introduction
Cys-loop receptors mediate fast transmission at chemical synapses by transiently opening an ion channel across the cell membrane in response to neurotransmitter binding (1–6). The resultant influx of ions into the cell alters the membrane potential, leading to the generation or in some cases the inhibition of an action potential. Many endogenous and exogenous compounds influence the ability of Cys-loop receptors to flux ions, altering the efficiency of synaptic transmission. Cys-loop receptors play a dynamic role in communication within the nervous system.
The ability of Cys-loop receptors to flux ions is sensitive to membrane lipid composition (7–11). The effects of lipids on the function of the prototypical Cys-loop receptor, the Torpedo nicotinic acetylcholine receptor (nAChR),4 have been intensely studied, particularly in reconstituted membranes of “well defined” lipid compositions. A mixture of both anionic and neutral lipids is optimal for the nAChR to adopt an agonist-activatable conformation (9–11). Of the many anionic lipids, phosphatidic acid (PA) is particularly effective at stabilizing a large proportion of agonist-activatable resting nAChRs (10, 12).
Correlations between lipids and nAChR function are based on the assumption that the lipid composition of a reconstituted membrane is defined by the exogenous lipids supplied during affinity purification. Although this assumption holds in most cases, we show here that nAChR-reconstituted PC/PA membranes contain the unexpected lipid diacylglycerol (DAG). DAG appears during affinity purification of the nAChR as a result of the hydrolysis of PA. PA-specific phospholipase C activity co-purifies with the nAChR and is inhibited by vanadate but not N-ethylmaleimide. Also, the presence of DAG in a reconstituted membrane influences the ability of the nAChR to undergo agonist-induced conformational transitions. Our results support the novel hypothesis that nicotinic acetylcholine receptors influence cellular events by mechanisms that do not involve the flux of ions into the cell (13–17). The nAChR may be able to alter the lipid composition of its surrounding microenvironment in a manner that enhances its own function.
EXPERIMENTAL PROCEDURES
Materials
Frozen Torpedo californica electroplax tissue was obtained from Aquatic Research Consultants (San Pedro, CA). 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol (PG), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoserine (PS), and DAG were from Avanti Polar lipids, Inc. (Alabaster, AL). Ethidium bromide and carbamylcholine chloride (Carb) were from Sigma.
nAChR Purification and Reconstitution
The nAChR was affinity-purified on a bromoacetylcholine bromide-derivatized Affi-Gel 102 column (Bio-Rad) as described elsewhere (18, 19). The column-bound nAChR was washed extensively with successive solutions of dialysis buffer (100 mm NaCl, 10 mm Tris-HCl, 0.1 mm EDTA, 0.02% w/v NaN3, pH 7.8) containing 1% cholate supplemented with 1.3, 3.2 (referred to as the lipid A wash), and 0.13 mm of the desired lipid. The nAChR was then eluted from the column with a 0.13 mm lipid solution in 250 mm NaCl, 0.1 mm EDTA, 0.02% NaN3, 5 mm phosphate, pH 7.8, with 0.5% cholate and 10 mm Carb. After elution from the column, the nAChR was dialyzed five times against 2 liters of dialysis buffer with buffer change approximately once every 12 h. The reconstituted membranes typically have lipid-protein ratios in the 150–300 mol:mol range, as estimated by Fourier transform infrared (20) and enzymatic assays (12).
The origin of the phospholipase activity was examined in more detail by running columns, as described above, except that the nAChR fractions were extracted either immediately or 1 week after elution from the affinity column. The one-week samples were maintained under N2 at 4 °C prior to extraction. The data in Figs. 3 and 4 were from columns eluted in 0.39 mm as opposed to 0.13 mm lipids.
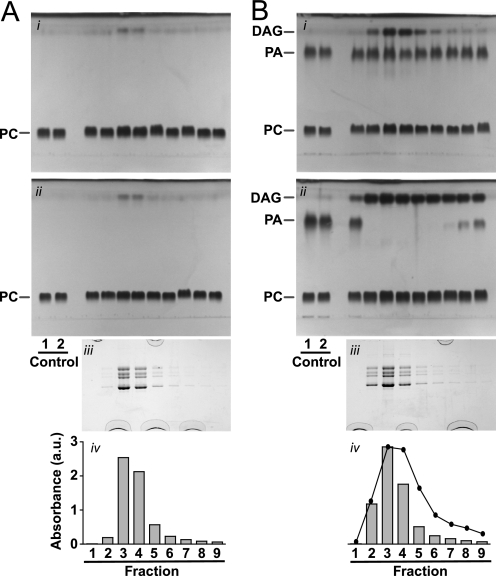
FIGURE 3.
PA-specific phospholipase activity is associated with the nAChR. Affinity purification of the nAChR in the presence of PC and PA either without (A) or with (B) prior treatment of the solubilized nAChR with ∼8 μm α-bungarotoxin, the latter to diminish nAChR binding to the affinity resin. Panels i and ii are TLCs developed in chloroform:methanol:formic acid:H2O (50:37.5:3.5:2, v/v/v/v) of lipid extracts from the control lipid solutions and the detergent-solubilized nAChR fractions eluted from the affinity column. The control lipids are extracts of the stock lipid A solution, as described for Fig. 2. Fractions 1–6 were collected upon elution of the solubilized nAChR from each affinity column with Carb. Panels i, the lipid extractions were performed immediately after running the column. Panels ii, the lipid extractions were performed 1 week later (samples maintained at 4 °C under N2). Panels iii, SDS-PAGE of each of the collected nAChR fractions. Panels iv, A280 of each of the eluted nAChR fractions.
FIGURE 4.
The effects of N-ethylmaleimide and vanadate on the nAChR-associated phospholipase activity. Affinity purification of the nAChR in the presence PC and PA in the absence of inhibitor (A) or in the presence of 4.2 mm N-ethylmaleimide (B) or 1 mm sodium vanadate (C). Panels i and ii are TLCs developed in chloroform:methanol:formic acid:H2O (50:37.5:3.5:2, v/v/v/v) of lipid extracts from the control lipid solutions and the detergent-solubilized nAChR fractions eluted from the affinity column. In each case, Control 1 is an extract of the stock lipid A solution. Control 2 is an extract of the same lipid A solution after it has been washed through the affinity column with the column-bound nAChR. Fractions 1–6 were collected upon elution of the solubilized nAChR from each affinity column with Carb. Panels i, lipid extractions performed immediately after running the column. Panels ii, lipid extractions performed 1 week later (samples maintained at 4 °C under N2). Panels iii are SDS-PAGEs of each of the collected nAChR fractions. Panels iv, the A280 of each of the eluted nAChR fractions. Note that sodium vanadate absorbs at 280 nm, making the absolute A280 readings somewhat unreliable.
Thin Layer Chromatography
The lipids were extracted using a modified Bligh and Dyer extraction protocol (21). Aliquots containing 500 μg of PC (phospholipid C assay; Wako Chemicals USA, Richmond, VA) were diluted to 1.6 ml with dialysis buffer and mixed with 6 ml of CHCl3:CH3OH 1:2 (v/v). After shaking vigorously, an additional 2 ml of both CHCl3 and dialysis buffer were added (final ratio, CHCl3:CH3OH:dialysis buffer 1:1:0.9 (v/v/v)), and the extraction tubes were centrifuged at 500 × g for 10 min. The bottom organic phase (∼4 ml) was removed, and the extraction was sometimes repeated by adding an additional 4 ml of CHCl3. The pooled organic phases were evaporated under N2 and dissolved in a small volume of CHCl3.
TLCs were performed as described (11) using high performance silica gel plates (60 Å, 4.5-μm particle size; Whatman), except in Fig. 2 where silica gel 60 WF2548 plates (EM Science) were used. TLC plates were typically developed using chloroform:methanol:formic acid:H2O (50:37.5:3.5:2, v/v/v/v) as a solvent system, except in Fig. 1 (B and D), where benzene:2-propanol:ethyl acetate:formic acid (72.5:3.5:22:2) was used.
FIGURE 2.
PA-specific phospholipase activity elutes with the nAChR from the bromoacetylcholine affinity column. A, affinity purification of the nAChR in the presence of PC. B, affinity purification of the nAChR in the presence of PC and PA. Panels i and ii are TLCs developed in chloroform:methanol:formic acid:H2O (50:37.5:3.5:2, v/v/v/v) of lipid extracts from the control lipid solutions and the detergent-solubilized nAChR fractions eluted from the affinity column. In each case, Control 1 is an extract of the stock lipid A solution. Control 2 is an extract of the same lipid A solution after it has been washed through the affinity column while the nAChR is bound to the column. Fractions 1–9 were collected upon elution of the solubilized nAChR from each affinity column with Carb. Panels i, the lipid extractions were performed immediately after eluting the nAChR from the column. Panels ii, the lipid extractions were performed 1 week later (samples maintained at 4 °C under N2). Panels iii are SDS-PAGE of each of the collected nAChR fractions. Panels iv, the A280 of each of the eluted nAChR fractions. B, panel iv also shows a densitometric analysis of the DAG bands observed on the TLC in panel i of B.
FIGURE 1.
Characterization of lipids in reconstituted nAChR membranes. Thin layer chromatography shows that the lipid compositions of most nAChR reconstitutions are as expected based on the lipids supplied during affinity purification of the nAChR, except that some of the PA in PC/PA-nAChR is hydrolyzed to DAG. A, lanes 1, 3, 5, and 7 are lipid standards. Lanes 2, 4, 6, and 8 are lipid extracts from PC-nAChR, PC/PG-nAChR, PC/PS-nAChR, and PC/PA-nAChR. The TLC plate was developed using a chloroform:methanol:formic acid:H2O (50:37.5:3.5:2, v/v/v/v) solvent system. B, the same as A except that the TLC plate was developed using a less polar solvent system (benzene:2-propanol:ethyl acetate:formic acid (72.5:3.5:22:2)) that resolves neutral lipids while phospholipids remain at the origin. C, lane 2, dipalmitin (a mixture of 1,2-DAG and 1,3-DAG with palmitoyl acyl chains); lane 3, 1-palmitoyl-2-oleoyl-DAG; lane 4, PC/PA-nAChR; lane 5, cholesterol (Chol); lane 6, cholesterol ester, lane 7, free fatty acid (FFA)-oleic acid. The same solvent system as in B was used. D, representative SDS-PAGE from two nAChR reconstitutions. Lane 1, standards; lane 2, PC-nAChR; lane 3, PC/PA-nAChR. The SDS-PAGE samples were from the purifications used for the lipid extractions in A–C.
Ethidium Fluorescence Measurements
All of the fluorescence experiments were performed on a Cary Eclipse fluorescence spectrophotometer equipped with the Cary Eclipse v1.1 software package (Varian, Inc.). The fluorescence emission intensity at 590 nm was monitored as a function of time (2.0-s sampling time), whereas ethidium bromide was continuously excited at 530-nm excitation. The excitation and emission slits were 5 and 20 nm, respectively. For each experiment, 1.8 ml of 0.3 μm (1 × KD) ethidium bromide in Torpedo Ringer buffer (5 mm Tris, 250 mm NaCl, 5 mm KCl, 2 mm MgCl2, and 3 mm CaCl2, pH 7.0) was equilibrated at 22.5 °C inside the spectrophotometer. At the indicated times, 30 μg of nAChR suspended in 200 μl of Torpedo Ringer buffer was added, followed by 10 μl of 100 mm carbamylcholine and then 20 μl of 100 mm dibucaine (i.e. ∼500 μm and 1 mm final concentrations, respectively for the two ligands).
RESULTS
nAChR-reconstituted PC/PA Membranes Contain DAG
Protocols are well established for the affinity purification and reconstitution of the nAChR into membranes with a defined lipid composition (18, 19, 22, 23). Typically, the nAChR is solubilized with cholate and bound to an affinity column, where it is washed extensively with cholate-solubilized lipids to both remove nonspecifically bound protein and replace the natural Torpedo lipids with the “lipids of interest.” The nAChR is then eluted in the presence of the same cholate-solubilized lipids and reconstituted into membrane vesicles by cholate dialysis.
We have examined the lipid compositions of numerous reconstituted nAChR membranes, as shown in Fig. 1A for the nAChR reconstituted into membranes composed of PC (PC-nAChR), PC/phosphatidylglycerol (PC/PG-nAChR), PC/phosphatidylserine (PC/PS-nAChR), and PC/PA (PC/PA-nAChR). This TLC, developed using the solvent system chloroform:methanol:formic acid:H2O (referred to as the polar solvent system), shows that in all but one case, the lipid composition of the resulting membrane matches that of the exogenous lipids used to wash and elute the nAChR from the affinity column. For example, lipid extracts from PC-nAChR contain one lipid that runs adjacent to the PC standard. Lipid extracts from PC/PG-nAChR, PC/PS-nAChR, and PC/PA-nAChR each contain two lipids corresponding to PC and the expected anionic lipid, both at or very close to the expected PC/anionic lipid ratio. The lipid extracts from PC/PA-nAChR, however, contain a relatively intensely staining band that runs slightly below the effective solvent front. The migration distance of this band suggests a neutral, as opposed to a phospho-lipid.
The unexpected neutral lipid observed in the lipid extracts from PC/PA-nAChR is resolved from the solvent front on a TLC plate developed using the solvent system, benzene:2-propanol:ethyl acetate:formic acid (Fig. 1B) (referred to as the nonpolar solvent system). The relative migration of the neutral lipid does not match that of cholesterol, cholesterol-ester, or free fatty acid (Fig. 1C). The lipid runs slightly above the 1,2-dipalmitoyl form of dipalmitin (a mixture of 1,2-dipalmitoylglycerol and 1,3-dipalmitoylglycerol) and corresponds precisely to the 1-palmitoyl-2-oleoylglycerol (referred to here as DAG) standard. Analysis of the neutral lipid extracted from the TLC by mass spectrometry confirms that it is the palmitoyl-oleoyl form of DAG (supplemental Fig. S1). DAG stains intensely with Coomassie Blue, even though control TLCs suggest that it represents less than 10 mol% of the total lipid in PC/PA-nAChR (30).
The TLC developed with the nonpolar solvent system (Fig. 1B) shows that DAG is not present in PC-nAChR, PC/PG-nAChR, or PC/PS-nAChR, although on TLCs overloaded with lipid, a very faint band caused by DAG can be observed in the reconstitutions containing PG and PS. Further, DAG is not evident in reconstitutions containing the anionic lipids phosphatidylinositol or cardiolipin, or the zwitterionic lipid, phosphatidylethanolamine (data not shown). Given that DAG is only prominent in lipid extracts from PC/PA-nAChR and that only the 1-palmitoyl-2-oleoyl isomer of DAG is present, it seems likely that DAG appears as a result of hydrolysis of PA during affinity purification of the nAChR.
Note that each of the lipid extractions (Fig. 1A, lanes 2, 4, 6, and 8) contains an additional faint band that runs at the solvent front slightly above DAG on TLCs developed using the polar solvent system (Fig. 1A, lanes 2, 4, 6, and 8). No distinct band with equivalent staining intensity is observed on TLC plates developed using the nonpolar solvent system (Fig. 1B, lanes 2, 4, 6, and 8). In our experience, trace organic contaminants that arise during lipid extraction/storage concentrate at the solvent front on TLC plates developed using the polar solvent system. These contaminants and DAG both stain intensely with Coomassie Blue. The identity of this(these) trace compound(s) was not studied further, the important point being that DAG is prominent in PC/PA-nAChR but is essentially lacking in PC-nAChR, PC/PG-nAChR, and PC/PS-nAChR, i.e. those reconstitutions lacking PA.
DAG Forms via Hydrolysis of PA
We next determined the origin of DAG in our nAChR-reconstituted PC/PA membranes by examining the affinity purification protocol. Affinity columns were run in the presence of either cholate-solubilized PC or PC/PA. In each case, the lipid compositions of the stock cholate-solubilized lipid solution (lipid A; see “Experimental Procedures”) was examined both before and after passing the lipids through the affinity column; the latter passed through the affinity column both before (data not shown) and while the solubilized nAChR was bound to the resin (Fig. 2, A and B, panels i and ii, Controls 1 and 2). In all cases, these control lipid solutions contained only PC or PC/PA. None of the incubation conditions led to the formation of DAG. This result shows that DAG formation is not the result of an inherent instability of PC or PA prior to or after contact with the affinity resin (see also below).
Fractions of the detergent-solubilized nAChR eluted from the affinity column in the presence of PC were essentially free of DAG, with only a faint band running near the solvent in fractions containing substantial amounts of the nAChR (Fig. 2A, panel i, Fractions 1–9). As noted above, this band may be due to trace organic contaminants or possibly some neutral lipid(s) that co-purifies and/or co-extracts with the nAChR. Significantly, prolonged incubation of the fractions at 4 °C for several days did not change the intensities of this weakly staining band and did not lead to the appearance of DAG. This result confirms that PC is not hydrolyzed to DAG in the presence of the nAChR (Fig. 2A, panel ii, Fractions 1–9).
In contrast, fractions of the affinity-purified nAChR eluted in the presence of PC/PA exhibit more intense staining of DAG (Fig. 2B, panel i, Fractions 1–9). The amount of DAG varied proportionally to the amount of the nAChR eluted in each fraction (Fig. 2B, panel iv). Of enormous significance, further incubation of the nAChR-containing fractions at 4 °C under nitrogen led to the loss of PA with the concomitant formation of DAG (Fig. 2B, panel ii, Fractions 1–9). In fact, prolonged incubation at 4 °C led to complete hydrolysis of PA to DAG in most fractions. As noted above, DAG was not formed in control lipid A solutions that were incubated under identical conditions (but lacking the nAChR) (Fig. 2B, panels i and ii, Controls 1 and 2). In addition, the hydrolysis of PA to DAG was less in those fractions containing minimal eluted affinity-purified nAChR (Fig. 2B, panels i and ii, Fractions 1, 8, and 9). These results show unequivocally that DAG is formed via hydrolysis of PA. Although it is not yet possible to precisely quantify the phospholipase C activity, densitometric analysis of the DAG production in Fig. 2B (panel i) suggests a close correlation between the phospholipase C activity elution profile and that of the nAChR (Fig. 2B, panel iv). These correlations suggest that the phospholipase activity is either intrinsic to the nAChR itself or due to a tightly nAChR-associated protein. An alternative interpretation, however, is that the phospholipase C activity is a distinct protein that exhibits a high affinity for the acetylcholine affinity resin. A distinct protein with high affinity for the acetylcholine affinity resin could co-elute with the nAChR upon the addition of Carb.
The PA-specific Phospholipase C Activity Is Associated with the Affinity-purified nAChR
To test the possibility that a distinct protein with phospholipase C activity exhibits an intrinsic affinity for the acetylcholine affinity resin, we ran parallel affinity columns in the presence of PC/PA as in Fig. 2B, except that one of the solubilized nAChR samples was treated with α-bungarotoxin prior to application to the affinity column. α-Bungarotoxin is a competitive antagonist that binds to Torpedo nAChR with high affinity. α-Bungarotoxin treatment is sufficient to eliminate or at least greatly reduce the level of nAChR binding to the acetylcholine affinity resin. Consequently, minimal if any protein elutes from the affinity resin upon exposure to Carb (Fig. 3B, panel iii).
Significantly, the Carb eluates from the column run after α-bungarotoxin treatment show minimal if any PA-specific phospholipase activity. This control experiment shows unequivocally that the PA-specific phospholipase activity is not due to a distinct protein that associates independently with the acetylcholine affinity resin. Our results show that for the phospholipase activity to associate with the affinity resin, the nAChR must also be bound. The phospholipase C activity is therefore either intrinsic to the nAChR itself or it resides in a protein that associates tightly with the nAChR.
The PA-specific Phospholipase C Activity Is Vanadate-inhibitable
Two different PA-specific phospholipases have been identified in mammalian tissues. Cytosolic phosphatidate phosphatases are sensitive to N-ethylmaleimide (24, 25). Membrane-bound lipid phosphate phosphohydrolases are inhibited by sodium vanadate (26). We repeated the nAChR affinity purifications with PC/PA in the presence of either N-ethylmaleimide or 1 mm vanadate. N-Ethylmaleimide had no inhibitory effect on the observed phospholipase activity (Fig. 4B). In contrast, vanadate inhibits the hydrolysis of PA, even after prolonged incubation of the nAChR with the cholate-solulibilized lipid (Fig. 4C). The affinity-purified nAChR phospholipase C activity is thus vanadate-inhibitable. The phospholipase activity is similar to that of membrane-bound lipid phosphate phosphohydrolase.
DAG Influences the Ability of the nAChR to Undergo Agonist-induced Conformational Transitions
Given that the PA-specific phospholipase activity purifies with the nAChR, we next tested whether the hydrolysis product of PA (i.e. DAG) influences nAChR activity. We examined whether DAG influences the ability of the nAChR to undergo agonist-induced conformational transitions using the conformationally sensitive probe ethidium bromide (Fig. 5). Ethidium is an open channel blocker that binds with high affinity to a hydrophobic site within the ion channel pore of the desensitized (KD ≅ 0.3 μm) but not the resting (KD ≅ 1 mm) nAChR (27). Relative to aqueous ethidium, nAChR-bound ethidium exhibits a greater fluorescence emission intensity, and its emission maximum shifts from 605 (aqueous solution) to 590 nm (nAChR-bound). The lipid-dependent uncoupled conformation of the nAChR in PC membranes also binds ethidium with low affinity (11).
FIGURE 5.
DAG influences the ability of the nAChR to undergo Carb-induced conformational transitions. The fluorescence emission of ethidium at 590 nm was monitored in the presence of the nAChR reconstituted into 3:2 PC/PA (trace i), PC (trace ii), 3:1 PC/DAG (trace iii), 3:2 PC/PS (trace iv), and 3:1:1 PC/PS/DAG membranes (trace v). A, at the indicated times ∼50 nm nAChR, 500 μm Carb, and 500 μm dibucaine (Dib) were added to a 0.3 μm ethidium solution. The sharp spikes in each fluorescence emission trace reflect the scattering of light upon insertion of the pipette into the cuvette. Note the large increase in fluorescence upon addition of 3:1 PC/DAG to ethidium is due primarily to vesicle scattering. B, dibucaine-displaceable ethidium fluorescence emission intensity at 590 nm in the presence (+) or absence (−) of Carb. The error bars are the standard errors.
Addition of PC/PA-nAChR to the 0.3 μm aqueous solution of ethidium leads to a slow increase in dibucaine-displaceable fluorescence (Fig. 5A, trace i). At this concentration (∼KD for the desensitized state), the slow increase in fluorescence is due to the slow binding of ethidium to the ion pore of nAChRs that pre-exist in the desensitized state. In contrast, no changes in fluorescence are observed upon addition of PC-nAChR to ethidium (Fig. 5A, trace ii), as PC-nAChR adopts a low affinity ethidium-binding conformation referred to as the lipid-dependent uncoupled state (11). Addition of PC/DAG-nAChR to ethidium leads to a rapid increase in fluorescence, but this increase is not dibucaine-displaceable and is due primarily to light scattering from the reconstituted membrane vesicles (Fig. 5A, trace iii).
The addition of 500 μm Carb to PC/PA-nAChR leads to a rapid and further increase in dibucaine-displaceable ethidium fluorescence (Fig. 5A, trace i). The Carb-induced increase in fluorescence intensity is due to increased ethidium binding to the newly desensitized nAChR. The rapid rate of Carb-induced ethidium binding is likely due to increased accessibility to the ion pore as the nAChR transitions from the resting to the open and then the desensitized states (12). In contrast, DAG alone in a PC membrane is not sufficient to stabilize an agonist-responsive resting nAChR. Although the inclusion of DAG in a PC membrane shifts some receptors into the desensitized conformation, DAG alone is not sufficient to impart on the nAChR a strong ability to undergo agonist-induced conformational transitions.
The effects of DAG on membranes containing anionic lipids, however, are substantial. The membranes composed of PC/PS are relatively ineffective at stabilizing an agonist-responsive nAChR. Consistent with this finding, the addition of PC/PS-nAChR to 0.3 μm ethidium leads to the slow binding of ethidium to a large population of pre-existing desensitized nAChRs (Fig. 5A, trace iv). Carb has little effect on ethidium fluorescence. PC/PS-nAChR likely stabilizes primarily desensitized and uncoupled conformations (12).
Relative to PC/PS-nAChR, the addition of PC/PS/DAG-nAChR to 0.3 μm ethidium leads to a less intense increase in fluorescence caused by ethidium binding to nAChRs pre-existing in the desensitized conformation (Fig. 5A, trace v). The addition of DAG therefore decreases the proportion of desensitized nAChRs in a PC/PS membrane. There is also a large and rapid increase in fluorescence upon the addition of Carb. The presence of DAG in a PC/PS membrane thus leads to the stabilization of a large proportion of resting nAChRs that likely undergo both channel gating and desensitization upon Carb-binding.
These simple studies show that the effects of DAG on nAChR function are complex and depend upon the lipid environment surrounding the nAChR. In the presence of PS, DAG increases substantially the proportion of agonist-responsive nAChRs. Although the levels of DAG studied here are higher than the levels of PA or DAG expected in vivo, the effects of DAG on nAChR function observed here are also much larger than required to have a profound influence on synaptic transmission in vivo. Even subtle changes in nAChR gating open times in human muscle nAChRs have pathological consequences (5). It is possible that even small amounts of DAG, particularly if concentrated in an nAChR-associated raft or on one leaflet of the bilayer, could alter gating kinetics in manners that influence synaptic communication. Further studies are required to fully elucidate the potential effects of DAG on nAChR function in vivo.
Finally, it should be noted that the kinetics of ethidium displacement from the nAChR observed here and elsewhere (11, 12) are much faster than those observed upon displacement of ethidium from the nAChR using either the local anesthetic phencyclidine or other cations (28). We also observe much slower ethidium displacement with the local anesthetic, proadifen (data not shown). The displacement of ethidium from the nAChR by dibucaine may thus involve complex mechanisms.
DISCUSSION
It is well established that nAChRs influence cellular activity by fluxing ions across the membrane in response to neurotransmitter binding. Increasing evidence suggests, however, that some nicotinic receptors play additional roles in cellular function not related to the flux of ions (13, 14). Homomeric α7 nAChRs function in nonexcitable cells, such as endothelial cells, keratinocytes, and T cells (15). Microglial α7 nAChRs couple with phospholipase C activation and Ca2+ mobilization from IP3-sensitive Ca2+ stores, leading to the suppression of neuroinflammation (16). Nicotinic agonists exert anti-inflammatory effects on macrophages that can be countered by α7 nAChR-selective antagonists. Also, α3, α4, and α5 nAChR subunits associate with phosphatidylinositol 3-kinase in monocytes and macrophages leading to the stimulation of phospholipase C activity (17).
We report here the novel finding that phospholipase C activity co-purifies with affinity-purified nAChR from Torpedo. This phospholipase activity is specific for PA over other anionic (PS, PG, 1,2-diacyl-sn-glycero-3-phosphoinositol, and cardiolipin) and zwitterionic (PC) phospholipids. The phospholipase activity is inhibited by sodium vanadate but not N-ethylmaleimide. The phospholipase activity elutes from an acetylcholine-linked nAChR affinity column with the same profile as the nAChR. SDS-PAGE shows that the eluted fractions contain four subunits corresponding to the α, β, γ, and δ subunits of the nAChR. No species corresponding in molecular mass to either cytosolic phosphatidate phosphatases (∼100 kDa) or membrane-bound lipid phosphate phosphohydrolases (∼35 kDa) are observed (24–26) (Figs. 1–3), although we cannot rule out the existence of trace amounts of these proteins. We therefore conclude that either the nAChR itself or a protein that closely associates with the nAChR, and that binds tightly with the nAChR on the affinity resin, exhibits phospholipase C activity.
The finding that either the nAChR itself or a tightly associated protein exhibits phospholipase activity provides further evidence that the nAChR can influence cellular function via mechanisms that do not involve the flux of ions. This phospholipase activity could generate DAG to influence cellular metabolism. The presence of DAG in the lipid microenvironment surrounding the nAChR could also directly influence nAChR function. It is interesting to note that discharge of Torpedo electric organ leads to the turnover of PA (29), possibly because of PA hydrolysis to DAG. The possibility that the nAChR can alter its lipid microenvironment in a manner that influences nAChR function with important biological consequences requires further investigation.
Supplementary Material
Acknowledgments
We thank Martin Pelchat for the use of the fluorescence spectrometer. We also thank David Brindley and Zemin Yao for insightful comments.
This work was supported by a grant from the Canadian Institutes of Health Research (to J. E. B.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- nAChR
- nicotinic acetylcholine receptor
- PA
- 1,2-diacyl-sn-glycero-3-phosphate
- PC
- 1,2-diacyl-sn-glycero-3-phosphocholine
- PG
- 1,2-diacyl-sn-glycero-3-phosphoglycerol
- PS
- 1,2-diacyl-sn-glycero-3-[phospho-l-Serine]
- Carb
- carbamylcholine chloride
- DAG
- diacylglycerol.
REFERENCES
- 1.Dougherty D. A. (2008) Chem. Rev. 108, 1642–1653 [DOI] [PubMed] [Google Scholar]
- 2.Goetz T., Arslan A., Wisden W., Wulff P. (2007) Prog. Brain Res. 160, 21–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sine S. M., Engel A. G. (2006) Nature 440, 448–455 [DOI] [PubMed] [Google Scholar]
- 4.Thompson A. J., Lummis S. C. (2006) Curr. Pharm. Des. 12, 3615–3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen X. M., Deymeer F., Sine S. M., Engel A. G. (2006) Ann. Neurol. 60, 128–136 [DOI] [PubMed] [Google Scholar]
- 6.Karlin A. (2002) Nat. Rev. Neurosci. 3, 102–114 [DOI] [PubMed] [Google Scholar]
- 7.Criado M., Eibl H., Barrantes F. J. (1984) J. Biol. Chem. 259, 9188–9198 [PubMed] [Google Scholar]
- 8.Fong T. M., McNamee M. G. (1986) Biochemistry 25, 830–840 [DOI] [PubMed] [Google Scholar]
- 9.Baenziger J. E., Morris M. L., Darsaut T. E., Ryan S. E. (2000) J. Biol. Chem. 275, 777–784 [DOI] [PubMed] [Google Scholar]
- 10.Hamouda A. K., Sanghvi M., Sauls D., Machu T. K., Blanton M. P. (2006) Biochemistry 45, 4327–4337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.daCosta C. J., Baenziger J. E. (2009) J. Biol. Chem. 284, 17819–17825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.daCosta C. J., Medaglia S. A., Lavigne N., Wang S., Carswell C. L., Baenziger J. E. (2009) J. Biol. Chem. 284, 33841–33849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kihara T., Shimohama S., Sawada H., Honda K., Nakamizo T., Shibasaki H., Kume T., Akaike A. (2001) J. Biol. Chem. 276, 13541–13546 [DOI] [PubMed] [Google Scholar]
- 14.de Jonge W. J., Ulloa L. (2007) Br. J. Pharmacol. 151, 915–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma G., Vijayaraghavan S. (2002) J. Neurobiol. 53, 524–534 [DOI] [PubMed] [Google Scholar]
- 16.Suzuki T., Hide I., Matsubara A., Hama C., Harada K., Miyano K., Andrä M., Matsubayashi H., Sakai N., Kohsaka S., Inoue K., Nakata Y. (2006) J. Neurosci. Res. 83, 1461–1470 [DOI] [PubMed] [Google Scholar]
- 17.Blanchet M. R., Israël-Assayag E., Daleau P., Beaulieu M. J., Cormier Y. (2006) Am. J. Physiol. Lung Cell Mol. Physiol. 291, L757–L763 [DOI] [PubMed] [Google Scholar]
- 18.daCosta C. J., Ogrel A. A., McCardy E. A., Blanton M. P., Baenziger J. E. (2002) J. Biol. Chem. 277, 201–208 [DOI] [PubMed] [Google Scholar]
- 19.daCosta C. J., Wagg I. D., McKay M. E., Baenziger J. E. (2004) J. Biol. Chem. 279, 14967–14974 [DOI] [PubMed] [Google Scholar]
- 20.daCosta C. J., Baenziger J. E. (2003) Acta Crystallogr. D Biol. Crystallogr. 59, 77–83 [DOI] [PubMed] [Google Scholar]
- 21.Bligh E. G., Dyer W. J. (1959) Can J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 22.Ellena J. F., Blazing M. A., McNamee M. G. (1983) Biochemistry 22, 5523–5535 [DOI] [PubMed] [Google Scholar]
- 23.McCarthy M. P., Moore M. A. (1992) J. Biol. Chem. 267, 7655–7663 [PubMed] [Google Scholar]
- 24.Reue K., Brindley D. N. (2008) J. Lipid Res. 49, 2493–2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simon M. F., Rey A., Castan-Laurel I., Grés S., Sibrac D., Valet P., Saulnier-Blache J. S. (2002) J. Biol. Chem. 277, 23131–23136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brindley D. N., Waggoner D. W. (1998) J. Biol. Chem. 273, 24281–24284 [DOI] [PubMed] [Google Scholar]
- 27.Herz J. M., Johnson D. A., Taylor P. (1987) J. Biol. Chem. 262, 7238–7247 [PubMed] [Google Scholar]
- 28.Herz J. M., Kolb S. J., Erlinger T., Schmid E. (1991) J. Biol. Chem. 266, 16691–16698 [PubMed] [Google Scholar]
- 29.Bleasdale J. E., Hawthorne J. N., Widlund L., Heilbronn E. (1976) Biochem. J. 158, 557–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sturgeon R. M., Baenziger J. E. (2010) Biophys. J. 98, 989–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.