Abstract
Skeletal integrity is tightly regulated by the activity of osteoblasts and osteoclasts that are both under the control of extracellular glycosaminoglycans (GAGs) through their interactions with endogenous growth factors and differentiation-promoting ligands. Receptor Activator of NF-kappa-B Ligand (RANKL), which is a Tumor Necrosis Factor (TNF)-related protein that is critical for osteoclast formation, is produced by osteoblasts and further modulated by certain types of GAGs. Using unfractionated osteoblast-derived GAGs that reflect the complex tissue microenvironment within which osteoclasts reside, we demonstrate that these GAGs block the osteoclastogenic activity of RANKL. Furthermore, RANKL significantly reduces ERK activity, a putative suppressor of osteoclastogenesis, but osteoblast-derived GAGs eliminate the inhibitory effects of RANKL on ERK activity. Notably, while imposing an anti-osteoclastic affect, these GAGs also enhanced the proliferation of osteoblasts. Thus, the osteoblast microenvironment is a potent source of GAGs that promote bone anabolic activities. The anti-osteoclastogenic and osteoblast-related mitogenic activities of these GAGs together may provide a key starting point for the development of selective sugar-based therapeutic compounds for the treatment of osteopenic disorders.
Keywords: Glycosaminoglycans, RANKL, ERK, Osteoclastogenesis
Introduction
Bone remodeling and maintenance of skeletal integrity are coordinated and dynamic processes that involve a balance between bone matrix synthesis by osteoblasts and bone resorption by osteoclasts. Multinucleated osteoclasts originate from monocyte / macrophage cells in bone marrow from the hematopoietic lineage [Suda et al., 1992]. Increased osteoclast activity has been observed in many osteopenic disorders, including osteoporosis, lytic bone metastases or rheumatoid arthritis, and ultimately results in abnormal bone resorption and bone fractures [Roodman, 1999]. Among the numerous factors affecting osteoclastogenesis, the receptor activator of NF-κB (RANK), its ligand RANKL and osteoprotegerin (OPG), the decoy receptor of RANKL, are essential for osteoclast development [Takahashi et al., 1999].
RANKL, a member of the TNF-ligand superfamily, is produced by osteoblasts and bone marrow stromal cells, whereas its cognate receptor RANK is expressed on the surface of pre-osteoclasts [Fuller et al., 1998]. The interaction between RANKL and RANK stimulates the maturation of targeted osteoclasts by activation of transcription factors that regulate osteoclastogenesis [Hsu et al., 1999; Lacey et al., 1998; Takayanagi et al., 2002]. RANKL is also required for the survival of mature osteoclasts [Katagiri and Takahashi, 2002]. OPG is produced by osteoblasts and acts as a decoy receptor to RANKL, thus inhibiting osteoclast differentiation and survival through competing with RANK for RANKL binding [Burgess et al., 1999; Khosla, 2001]. Evidence from genetic studies has shown that RANKL and RANK are essential for osteoclastogenesis. RANK or RANKL deficient mice exhibit marked osteopetrosis and a defect in tooth eruption caused by the diminishment of osteoclast formation [Dougall et al., 1999; Kim et al., 2000]. Mice lacking OPG have increased number and activity of osteoclasts and consequently suffer from severe osteoporosis [Bucay et al., 1998; Mizuno et al., 1998].
Accumulating clinical evidence has further consolidated the key role of the RANKL/RANK/OPG axis in bone metabolism and the direct relevance of these factors to human disease. Elevated RANKL levels and/or a decline of OPG has been demonstrated in several bone metabolic diseases, including postmenopausal osteoporosis, glucocorticoid-induced osteoporosis, multiple myeloma-associated osteolytic lesions and atherosclerotic disease associated vascular calcification. This imbalance may be a key mechanism responsible for osteoporosis [Eghbali-Fatourechi et al., 2003; Pearse et al., 2001; Sasaki et al., 2002; Schoppet et al., 2004]. Furthermore, inactivating mutations in the OPG gene and activating mutations in the RANK gene have been identified in genetic disorders of mineral metabolism [Hughes et al., 2000; Nakatsuka et al., 2003; Whyte and Hughes, 2002; Whyte et al., 2002]. All of these genetic abnormalities result in unopposed activation of RANK/RANKL signaling, which enhances osteoclastogenesis and consequently increases bone loss.
Recent studies have suggested that glycosaminoglycans (GAG) have important roles in the interaction and activity of RANK/RANKL. GAGs are polyanionic linear polysaccharides composed of repeating disaccharide units with a carboxyl group and one or more sulfates [Lamoureux et al., 2007]. Most GAGs are covalently attached to core proteins to form proteoglycans which are the major components of bone extracellular matrix [Iozzo, 1998]. Endogenous GAGs include heparin, heparan sulfate, chondroitin sulfate, dermatan sulfate, keratin sulfate and hyaluronic acid. GAGs regulate a wide variety of biological processes, including hemostasis, inflammation, angiogenesis, cytokine presentation/binding, cell adhesion and migration as well as the control of proliferation and differentiation [Gandhi and Mancera, 2008; Perrimon and Bernfield, 2000]. GAGs bind to a large number of protein ligands via interaction with protein heparin-binding domains and these interactions modify the biological activity of cell surface receptors. Recent studies have demonstrated that dermatan sulfate and heparin possess high affinity for RANKL and suppress osteoclast formation by obstructing the interaction between RANKL and RANK [Ariyoshi et al., 2008; Shinmyouzu et al., 2007].
Because osteoclastogenesis is largely controlled by factors produced by osteoblasts (i.e., RANKL), it is important to understand how osteoblast-specific GAGs regulate osteoclast formation via interactions with RANKL. GAGs synthesized and secreted by osteoblasts are attached to the cell surface and contained within the extracellular matrix as a mixture of species with varying structure and activity [Haupt et al., 2009; Jackson et al., 2007]. The integrity of GAGs is important to maintain the proliferation and differentiation of osteoblasts [Kumarasuriyar et al., 2009]. Furthermore, bone-derived heparan sulfates promote the proliferation and differentiation of osteoblasts. The activities of GAGs are fine-tuned by structural changes at different developmental stages during osteogenesis [Haupt et al., 2009; Jackson et al., 2007; Nurcombe et al., 2007] that are supported by changes in the expression of GAG-related enzymes and proteoglycans in response to the osteogenic master regulator RUNX2 [Haupt et al., 2009; Teplyuk et al., 2009].
Mixtures of GAGs may help regulate osteoclastogenesis in microenvironments where osteoblasts/osteoclasts co-reside. Therefore, we extracted GAGs from the cell surface of osteoblasts as well as those secreted into the media in soluble forms. Their binding affinity for RANKL and the effect on RANKL induced osteoclast differentiation was then examined. To reduce potential contaminating effects from endogenous RANKL activity, we used osteoclastic precursor cells (RAW264.7) that lack RANKL or OPG expression, but remain responsive to exogenous RANKL stimulation and possess osteoclastogenic potential [Collin-Osdoby et al., 2003]. Our study demonstrates that GAGs isolated from osteoblasts have affinity for RANKL and significantly inhibit RANKL-induced osteoclastogenesis by modulating ERK activity. These findings suggest that osteoblasts produce extracellular macromolecules to fine-tune osteoclast activity. Moreover the anti-osteoclastic activity of these osteoblast-derived GAGs supports their further development as substances for treating bone disorders with aberrantly elevated RANKL / osteoclast activity.
MATERIALS AND METHODS
Extraction of GAGs from Porcine Osteoblasts
GAGs were obtained from porcine primary osteoblast cultures as described previously [Manton et al., 2006] with strict adherence to the ethical guidelines of the Biomedical Research Council of Singapore and the National University of Singapore. Osteoblasts were collected from pre-confluent cultures and the expression of alkaline phosphatase used to confirm an osteoblastic phenotype. The osteoblasts obtained were then seeded at 5,000 cells/cm2 and cultured in αMEM containing 10% FCS for 8 days (~80% confluent) with a media change every 3 days prior to GAG extraction. We isolated and purified GAGs from primary porcine osteoblasts based on their localization on the cell surface, in the ECM or as soluble factors. However, the quantity of ECM derived GAG was significantly lower than the other two fractions (data not shown) and insufficient for further analysis.
The media containing the soluble GAG fraction was gently removed from the culture dishes without disturbing the cells. To remove any cell debris, the media were centrifuged at maximum speed and the supernatants passed through a 0.45 μm filter. The cell monolayer was then washed with PBS and removed by trypsin-EDTA. The resulting cell solution was boiled for 10 min to deactivate the trypsin and the lysate collected by centrifugation at maximum speed for 15 min. The supernatant containing the cell surface GAG fraction was then passed through a 0.45 μm filter to remove any debris.
Supernatants obtained from media (soluble fraction) or cells (cell surface fraction) were subjected to anionic-exchange chromatography on a CaptoQ-Sepharose column (50 ml) equilibrated in 50 mM PBS with 250 mM NaCl (low-salt buffer). Samples were loaded at a flow rate of 4 ml/min and the column washed with the same buffer until baseline was established. The bound material was eluted with 1 M NaCl (high-salt buffer) and the peak fractions pooled, concentrated, and desalted with distilled H2O using HiPrep Desalting 16/10 Columns (GE Life-Sciences) as per manufacturer’s instructions. The proteoglycan samples from the soluble and cell surface fractions were freeze-dried to be concentrated. The samples were then treated with neuraminidase (0.1 U) for 4 h followed by further digestion with 10 mg/ml Pronase (in 500 mM Tris–acetate, 50 mM calcium acetate, pH 8.0) and 5 volumes of 100 mM Tris–acetate (pH 8.0) at 37°C for 24 h. The entire mixture was then diluted 1:10 with low-salt buffer, passed through a CaptoQ-Sepharose column (50 ml) and eluted as described previously. The peak fractions were pooled, concentrated, and desalted with water, freeze-dried and stored at −20°C. The purified GAG chains from soluble and cell surface fractions were further characterized by size exclusion chromatography on a Sepharose CL-6B column (1 × 120 cm).
Cell Culture
The RAW264.7 murine monocytic cell line (ATCC TIB-71, USA) was routinely cultured in Dulbecco’s modified Eagle’s media (DMEM) supplemented with 10% fetal bovine serum (FBS) and 100 units/ml penicillin/streptomycin at 37°C in a humidified 5% CO2 incubator. For experiments, RAW 264.7 cells were seeded at 2 × 103/cm2.
Human primary osteoblasts were generously provided by Kerry Manton [Manton et al., 2006]. The cells were derived from explants of discarded calvarial bone obtained from a 19-year-old male donor after informed consent according to the ethical guidelines of the Biomedical Research Council of Singapore and the National University Hospital, Singapore. The culture was maintained in DMEM glucose supplemented with 10% FCS, 2 mM L-glutamine, 100 U/ml penicillin/streptomycin and 100 mg/ml Fungizone.
Tartrate-resistant Acid Phosphatase Staining
Monocytic RAW264.7 cells, which represent a widely used in vitro model for the study of osteoclastogenesis, can be differentiated into tartrate-resistant acid phosphatase (TRAP) positive multinucleated osteoclasts [Collin-Osdoby et al., 2003; Cool et al., 2007]. RAW264.7 cells were maintained in alpha-minimum essential medium (α-MEM) containing 10% FCS, 2 mM glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin in a humidified incubator (5% CO2 in air) at 37°C . For experiments, cells were seeded in 8-well chamber slides at a density of 2,000 cells/cm2 and cultured for 5 days with 10 ng/ml RANKL (PeproTech, UK) in the presence or absence of 5 μg/ml GAG (soluble or cell surface). The medium and test reagents were refreshed at day 3. At day 5, the adherent cells were fixed with 4 % paraformaldehyde in PBS at 4°C for 60 min, before being permeabilized with 0.2% Triton X-100 in PBS at room temperature for 5 min. After rinsing with PBS, the cells were then incubated with 0.01% naphthol AS-MX phosphate and 0.05% fast red violet LB salt (Sigma, USA) in the presence of 50 mM sodium tartrate and 90 mM sodium acetate (pH 5.0) for TRAP staining. TRAP-positive cells containing ten or more nuclei were considered to be osteoclasts and counted across 10 random fields at 10× magnification (Olympus IX81). All experiments were repeated three times and the average number of osteoclasts per 10 random fields calculated. Photomicrographs of TRAP stained colonies were taken using an Olympus IX81 microscope equipped with a Spot CCD camera and Image pro-plus v2.0) (Olympus, Japan).
Immunoblot Analysis
RAW264.7 cells were washed in PBS twice before being lysed in RIPA buffer (150 mM NaCl, 10 mM Tris pH 7.4, 2 mM EDTA, 0.5% Nonidet P-40, 0.1% sodium dodecyl sulfate [SDS], 1% Triton X-100, protease inhibitor cocktail) at 4°C for 10 min. The protein concentration was determined using BCA protein assay kit (Pierce Biotechnology, USA) following the manufacturers’ instructions. Protein (20 μg) was then denatured and separated by SDS-PAGE. Proteins were transferred to nitrocellulose membranes that were blocked with 5% nonfat dry milk in PBS with 0.1% Tween-20 (PBST) for 1 h at room temperature. Blots were then incubated with primary antibody and the corresponding peroxide-conjugated goat anti-mouse / rabbit secondary antibody (Santa Cruz, USA) for 1 h at room temperature with three washes by PBST in between. The primary antibodies used were rabbit polyclonal phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (Cell Signaling Technology) and mouse monoclonal actin (Chemicon, USA). For reblotting, membranes were stripped at 50°C for 30 min in stripping buffer (62.5 mM Tris-HCl, pH 6.7, 2% SDS, and 0.7% β-mercaptoethanol). Blots were then washed for 30 min with three changes of PBST. Efficiency of stripping was determined by re-exposure of the membranes to ECL. Thereafter, membranes were blocked and immunoblotted for actin to verify the equivalent loading of samples. Immunoreactive bands were visualized by the enhanced chemiluminescence (ECL) kit according to the manufacturer’s instructions (Pierce, USA).
Confocal Laser Scanning Microscopy
Human primary osteoblasts were grown on chamber slides with or without GAGs for 2 days. Thereafter, the cells were rinsed and fixed in 4% paraformaldehyde for 10 min followed by permeabilization with 0.1% Triton X-100 for 5 min. After rinsing, the cells were blocked in 1% BSA for 30 min before being incubated with rhodamine phalloidin (Invitrogen, USA) for 20 min. The cells were then washed and mounted in VECTASHIELD® Mounting Medium with DAPI (Vector Laboratories, USA). Labeled cells were visualized with Zeiss Axio Imager Z1 system (Carl Zeiss MicroImaging, USA). Images were exported in the tagged-information-file format.
BrdU (5-bromo-2-deoxyuridine) Incorporation Assay
Primary human osteoblasts seeded at 5,000 cells/cm2 in 96-well microplate were synchronized by serum starvation for 24 h followed by treatment with GAG for another 24 h. Thereafter, the cells were pulsed with BrdU for 2 h at 37°C according to the manufacturers’ instructions for BrdU Incorporation Assay kit (Roche, Switzerland). The incorporated BrdU was detected with peroxidase-conjugated anti-BrdU antibody and the bound conjugate visualized with the soluble chromogenic substrate TMB and measured using an ELISA reader (BioRad, USA). The morphology of osteoblasts was also examined by phase-contrast microscopy (Olympus IX81, 10× objective) after 4 days growth in the various conditions.
RANKL/GAGs Binding Assay
To investigate the RANKL-binding properties to the GAGs extracted in this study, specially-prepared Heparin/GAG binding plates were obtained from Iduron (UK) and an enzyme-based assay performed according to the manufacturer’s instructions. The plates were first coated with 5 μg soluble or cell surface GAG overnight in standard assay buffer (100 mM NaCl, 50 mM sodium acetate, 0.2% Tween 20, pH 7.2). After blocking in 0.2% gelatin, 10 ng RANKL was applied to the plate surface. The GAG-coated wells not subjected to RANKL served as blank controls. Wells not pre-coated with GAGs but subjected to RANKL were used to show that RANKL does not bind to the plate surface. RANKL binding to soluble and cell surface GAG was then detected by its specific antibody conjugated with biotin (Abcam, UK). Biotin was further bound with ExtrAvidin-AP followed by p-nitrophenyl phosphate, which is the chromogenic substrate of AP and gives a yellow-color product detectable at 405 nm using a Victor3 multilabel plate reader (PerkinElmer, USA). The entire assay was performed at room temperature and protected from light, with each step followed by extensive washing to remove non-specific interactions. The experimental readings were adjusted by subtracting background (determined from blank readings) and analyzed.
Heparin Sepharose Beads Precipitation Assay
RANKL (10 ng) was incubated with 30 μl of a 50% heparin–Sepharose CL 6B beads slurry (Amersham Pharmacia Biotech, UK) in 100 μl of PBS in the presence or absence of competing heparin (5 μg) or GAG (5 μg) for 20 min at 4°C. The beads were then washed twice with PBS and the bound RANKL eluted by boiling in Laemmli buffer (Sigma, USA) and detected using anti-RANKL antibody (Santa Cruz, USA) by Western Blot analysis.
Statistic Analysis
Each experiment was repeated three times and data were expressed as means ± SEM. Differences among treatments were analyzed by Students’ t test or Analysis of Variances (ANOVA). Significant difference was set at P < 0.05.
RESULTS
Differences in the composition of soluble GAGs and cell surface GAGs
Skeletal homeostasis is controlled by the interplay between osteoblasts and osteoclasts. Both cell types contact one another and are influenced by factors residing in the osteogenic niche. Therefore, we investigated whether GAGs isolated from osteoblasts are capable of influencing physiological events in osteoclasts and/or osteoblasts. GAGs produced by osteoblasts are present on cell surfaces, secreted into the extracellular milieu or sequestered in the extracellular matrix (ECM). Because ECM derived GAGs are present in negligible quantities in primary calvarial osteoblasts under our experimental samples, our studies focus only on soluble and cell surface GAGs. Anion exchange chromatography using a step-gradient of 1M NaCl was performed to purify GAGs and GAG elution follows the conductivity of NaCl (Fig. 1A). The elution profiles of soluble and cell surface GAGs were similar, except that the magnitude of cell surface GAG peak was higher indicating that this fraction contains larger amounts of GAGs (Fig. 1A). For both soluble and cell surface GAGs we collected fractions 640 to 760 (Fig. 1A). We determined the size distribution of GAGs to assess whether there may be differences in the relative constituents between soluble and cell surface GAGs. We observed two major GAG species (peak 1 and 2) in the soluble fraction and only one major GAG species (peak 2) in the cell surface fraction (Fig. 1B). Chondroitin sulfate (CS) and heparan sulfate (HS) are the two most abundant GAG species in bone [Seibel et al., 2006]. CS has a higher molecular weight than HS suggesting that peak 1 contains CS and peak 2 contains HS. Although digestion with chondroitin- and heparin lyases is necessary to confirm this assessment, we did not discriminate between these two major GAG species because our studies are primarily concerned with maintaining a mixture of GAG that reflect the collective biological activity of osteoblast-derived GAG variants acting on osteoclasts in the bone microenvironment.
Figure 1.
Anion exchange chromatography of soluble and cell surface GAGs from primary osteoblasts. The crude GAGs were purified from porcine primary osteoblast culture media (soluble fraction) or cell extracts (cell surface fraction). A, Samples were applied to a 50 ml CaptoQ-Sepharose column at 4 ml/min in the presence of 1 M NaCl. During washing, a small amount of GAG eluted at absorbance of 232 nm and generated a peak from fraction number 640 to 760. B, Sepharose CL-6B size-exclusion column chromatogram of the GAGs isolated from soluble and cell surface fractions. Size fractions of soluble GAG chains displayed two peaks (1 and 2). Cell surface GAG chains displayed only one peak (2). The deflections below baseline represent monitor artifacts.
Affinity of GAGs for RANKL
To determine whether the soluble and cell surface GAGs bind to RANKL, we immobilized a fixed amount of GAG (5 μg) on the surface of heparin/GAG binding plates. RANKL (ranging from 10 to 250 ng) was incubated with GAGs pre-bound to the plate surface and RANKL affinity for GAGs was determined and quantified by ELISA using a biotinylated RANKL antibody. RANKL binding was observed to both cell surface and soluble GAGs and increased dose-dependently from 10 to 250 ng/ml (Fig. 2A). The RANKL titration also indicated that a GAG amount of 5 μg suffices to bind 10 ng RANKL. Therefore, we administered GAGs (5 μg/ml) and RANKL (10 ng/ml) to RAW264.7 cells at the indicated concentrations to examine the biological effect of GAGs on RANKL-induced osteoclastogenesis (see Fig. 3). Comparison of GAG fractions revealed that soluble and cellular GAGs, which each contain Peak 2 components (i.e., presumably HS), have comparable affinity for RANKL (Fig. 2A).
Figure 2.
Binding ability of GAGs to RANKL. A, Different amounts of RANKL (10 ng, 50 ng and 250 ng) were added to the plate coated with 5 μg soluble or cell surface GAG. An enzyme based assay was performed to detect the amount of RANKL bound to the immobilized GAG. Data represents the mean ± S.E. of triplicate experiments. B, RANKL (10 ng) was added to heparin-Sepharose beads with or without 5 μg/ml free heparin or GAG. Lane 1 was RANKL protein as a positive control. RANKL bound to the beads was visualized by immunoblot analysis and the amount of RANKL protein was determined by densitometric analysis. Data represent the average of three independent experiments.
Figure 3.
The effect of GAGs on RANKL-induced osteoclast formation. RAW264.7 cells were cultured for 5 days in the presence of 10 ng/ml RANKL, or 10 ng/ml RANKL together with either 5 μg/ml soluble GAG or 5 μg/ml surface GAG. The cells without any treatment served as the control. Osteoclastogenesis was monitored by TRAP. A, RAW264.7 monocytic cells were negative in TRAP staining (control). After 5 days of treatment with 10 ng/ml RANKL, numerous TRAP positive (purple), multinucleated cells were observed in all cultures with RANKL. All images are from randomly chosen fields using a 10× objective. The data are representative of three independent experiments. B, GAGs from osteoblasts inhibited RANKL-induced osteoclastic differentiation of RAW264.7 cells. Data represent the mean ± S.E. of triplicate experiments.
Heparin, a highly sulfated HS species, has recently been reported to bind to RANKL and to inhibit RANKL activity [Ariyoshi et al., 2008]. Therefore we used heparin-conjugated Sepharose beads, which are routinely used to isolate heparin-binding proteins, to precipitate RANKL. We then performed competition assays with excess free GAGs to assess the relative efficiency by which heparin, cell surface GAGs and soluble GAGs can prevent RANKL precipitation (Fig. 2B). These competition assays will thus reveal the relative binding affinity of either GAG to RANKL. The exogenous administration of excess free heparin diminishes RANKL precipitation indicating that RANKL is in a specific, dynamic and reversible binding equilibrium with heparin-Sepharose. Importantly, unbound soluble and cell surface GAGs prevent binding of RANKL to heparin-Sepharose indicating that GAGs in either of these two fractions directly bind RANKL (Fig. 2B). Moreover, cell surface and soluble GAGs exhibit similar competitive binding to RANKL based on densitometric quantification (Fig. 2B), consistent with the results from the ELISA-based heparin binding assays (Fig. 2A).
GAGs inhibited RANKL induced osteoclast formation
We next examined the effects of soluble and cell surface GAGs on RANKL-induced osteoclastic differentiation of RAW264.7 cells. Cells cultured in the absence of RANKL failed to differentiate into TRAP-positive, multinucleated osteoclasts, but 5-day treatment with 10 ng/ml RANKL dramatically changed the morphology of RAW264.7 cells and resulted in the appearance of multinucleated TRAP-positive osteoclast-like cells (Fig. 3A). Although RANKL produced large multinucleated TRAP-positive osteoclasts, when combined with osteoblast-specific GAGs from either the soluble or cell surface fraction, the size and number of multinucleated, TRAP-positive cells was greatly reduced (Fig. 3A). Furthermore, the average number of osteoclasts per 10 random fields was 73 ± 11 when cells were treated by RANKL alone (Fig. 3B). In the presence of 5μg/ml of osteoblasts-derived GAG, the number of osteoclasts induced by RANKL was reduced by 69% (soluble GAGs) and 41% (cell surface GAGs) respectively (Fig. 3B). Therefore, GAGs produced by osteoblasts inhibit RANKL-induced osteoclastogenesis.
GAGs antagonize inhibition of ERK activity by RANKL in osteoclasts
Extracellular signal-regulated protein kinase (ERK; p44/42 MAPK) is a central node in cell signaling pathways that mediate cell proliferation and survival. ERK is activated in osteoclasts upon RANKL stimulation and returns to basal levels by 4 h after RANKL stimulation [Hotokezaka et al., 2002]. This elevated ERK activity is apparently related to the survival of osteoclasts, but not their differentiation, because inhibition of the ERK pathway by PD98059 or U0126 does not suppress osteoclast formation [Matsumoto et al., 2000], but rather increases osteoclastogenesis [Hotokezaka et al., 2002]. Consistent with the notion that ERK negatively regulates osteoclast differentiation, we observed that long-term treatment with RANKL diminishes the activity of ERK (Fig. 4). We further examined the effect of osteoblast-derived GAGs on ERK activity in the presence of RANKL; we selected soluble GAGs for these experiments because this fraction appears to be somewhat more potent at inhibiting RANKL-induced osteoclastogenesis than cell surface GAGs. The results show that osteoblast-related GAGs abrogate suppression of ERK activity by RANKL in RAW264.7 osteoclasts (Fig. 4). The ability of osteoblast-derived GAGs to block the RANKL-dependent suppression of ERK signaling may be functionally linked to inhibition of osteoclastogenesis.
Figure 4.

The effect of soluble GAGs on RANKL-induced ERK activity. RAW264.7 cells were cultured for 5 days in the presence of 10 ng/ml RANKL, or 10 ng/ml RANKL together with 5 μg/ml soluble GAG. Immunoblot analysis reveals that RANKL antagonizes ERK phosphorylation, whereas the osteoblast-derived soluble GAG reduces the inhibitory effect of RANKL on ERK activity. The blot is representative of three independent biological replicates.
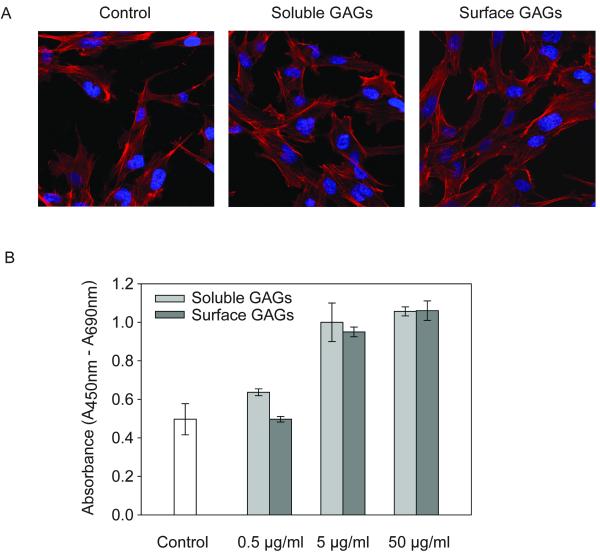
GAGs increased the proliferation of osteoblasts
GAGs secreted by osteoblasts may not only generate paracrine effects on osteoclasts, but also may have autocrine mitogenic and osteogenic effects on osteoblasts [Ling et al., 2006; Manton et al., 2006; Teplyuk et al., 2009]. Therefore, we assessed whether osteoblast-specific GAGs control osteoblast morphology and/or proliferation (Fig. 5). Treatment with GAGs does not have appreciable effects on osteoblast morphology and cells exhibited a characteristic spindle-like morphology with numerous filopodia extending from the cells (Fig. 5A). More importantly, the data show that both soluble and cell surface GAGs dose-dependently increase osteoblast proliferation (Fig. 5B). GAGs administered at doses that significantly inhibit osteoclastogenesis (5 μg/ml) enhance osteoblast proliferation by nearly 2 fold. We conclude that osteoblast derived GAGs have bone anabolic potential not only by inhibiting RANKL dependent osteoclastogenesis but also by stimulating the proliferative potential of osteoblasts.
Figure 5.
The effect of GAGs on osteoblast proliferation. A, Primary human osteoblasts were grown for 2 days with or without 5 μg/ml soluble or cell surface GAG. Cells left untreated served as control. The actin cytoskeleton and the nuclei of the cells were stained with rhodamine phalloidin or DAPI, respectively and the images obtained by confocal microscopy. B, Primary human osteoblasts were deprived of serum to synchronize and arrest the cell growth for 24 hr before treatment with 0.5, 5, 50 μg/ml soluble or cell surface GAGs. After 24 hr, cell proliferation was determined by measuring BrdU incorporation. The cells without any treatment served as the control. Data represent the mean ± S.E. of triplicate experiments.
DISCUSSION
Bone growth and remodeling is regulated by cytokines, growth factors and extracellular components, such as GAGs which are associated to the cell surface or localized in the extracellular matrix [Cool and Nurcombe, 2006; Sims and Gooi, 2008]. GAGs functionally interact with growth factors/cytokines in the microenvironment where osteoblasts and osteoclasts co-reside to orchestrate the process of bone remodeling [Ariyoshi et al., 2008; Jackson et al., 2006; Shinmyouzu et al., 2007; Theoleyre et al., 2006]. RANKL/OPG are key growth factors secreted by osteoblasts to control the activity of osteoclasts and like other growth factors, GAGs present in the microenvironment largely modulate their activity. To increase our understanding of the interplay between osteoblast-derived GAGs and RANKL, we used a homogenous population of RAW264.7 cells that have osteoclastic potential. Use of this cell line avoids complexities of studies with bone marrow stromal cells that contain mixed populations of cells including osteoblasts which can also be the targets of RANKL. Studies using different types of GAGs have yielded apparently contradictory results regarding the functions of these complex sugars [Ariyoshi et al., 2008; Ariyoshi et al., 2005; Irie et al., 2007; Lamoureux et al., 2007; Shinmyouzu et al., 2007; Theoleyre et al., 2006]. The differences in the outcome and interpretation of these studies may be attributed to the fact that GAGs are a mixture of polysaccharides with diverse structures and biological activities.
In bone tissue, osteoclasts are surrounded by complex mixtures of GAGs synthesized by osteoblasts, which are potent regulators of osteoclast function. Our data clearly demonstrate that osteoblast-derived GAGs attenuate RANKL dependent differentiation of osteoclasts, at least in part by direct binding to RANKL and by blocking RANKL mediated downregulation of ERK activity. The inhibitory potency of these GAGs to block osteoclast function (i.e., ~69 % reduction using soluble GAGs) is comparable to the in vitro effects of bisphosphonate (i.e., ~75 % reduction by 10−5 M neridronic acid), which is a highly effective drug widely used for the prevention of bone resorption and treatment of postmenopausal osteoporosis [Nicolin et al., 2007; Watts et al., 1990].
Our data demonstrate that osteoblast-derived GAGs inhibit RANKL-induced osteoclastogenesis by direct binding to RANKL. These GAGs are expected to contain a mixture of heparan sulfate, chondroitin sulfate, dermatan sulfate, keratin sulfate and hyaluronic acid [Ecarot-Charrier and Broekhuyse, 1987; Hunter et al., 1983; Ling et al., 2006; Nakamura et al., 2001], but not heparin which is produced in basophils and mast cells [Braunsteiner and Thumb, 1963]. Chondroitin sulfate has recently been shown to reduce the numbers of TRAP positive multinucleated cells induced by RANKL, indicating that it antagonizes RANKL activity [Ariyoshi et al., 2008]. Likewise, dermatan sulfate also binds to RANKL and is able to prevent interactions between RANKL and RANK [Shinmyouzu et al., 2007]. Hyaluronic acid (HA) meanwhile has no effect on osteoclast differentiation, though low-molecular-weight HA has been shown to enhance osteoclastogenesis via up-regulation of RANK protein levels [Ariyoshi et al., 2005]. Similar to our study, these prior reports also used cultured RAW264.7 cells.
In the present study, we show that soluble GAG is somewhat more potent than cell surface GAG at antagonizing RANKL. Examination of the size distribution of these two GAG fractions revealed molecular weight differences (presumably due to CS that is absence from cell surface GAG). Albeit not the most straightforward interpretation, it is conceivable that CS in the soluble GAG fraction may contribute to inhibitory effects on RANKL, because CS has been shown to inhibits osteoclast formation [Ariyoshi et al., 2008]. Our finding that osteoblast derived GAGs can be used to block osteoclastogenesis will provide the pre-translational impetus to conduct a more detailed enzymatic examination of specific GAG species in future studies.
To begin examining the mechanism by which osteoblast-derived GAGs affect osteoclastogenesis, we assessed the role of these GAGs in mediating RANKL decreased ERK signaling [Hotokezaka et al., 2002]. ERK is a well-known mitogenic kinase essential for cell proliferation and its activity regulates RANKL induced osteoclast formation [Hotokezaka et al., 2002; Lee et al., 2002; Matsumoto et al., 2000]. As such, ERK appears to play a dual role during osteoclastogenesis wherein activated ERK drives proliferation and enhances the survival of osteoclasts, whilst also inhibiting osteoclast differentiation [Hotokezaka et al., 2002]. Thus, sustained ERK activation blocks osteoclast differentiation. Consistent with this concept, we show that stimulation of RAW264.7 cells with RANKL, a known pro-osteoclastic differentiation factor, results in down regulation of ERK activity. However when RAW264.7 cells are co-treated with both RANKL and GAG, ERK activity remains at levels similar to non-RANKL treat cells. Hence, the inhibitory effect of GAGs on RANKL induced osteoclastogenesis may be mediated by maintaining active ERK signaling.
Local communication between bone cells is critical for control of bone remodeling and formation [Henriksen et al., 2009]. Osteoblasts play a central role by manufacturing autocrine or paracrine factors to regulate osteoclastogenesis (i.e. by RANKL/OPG) and also their own activity (i.e. FGF2 and BMP2). FGF2 and BMP2, important growth factors for osteoblast growth and differentiation, together with other growth and adhesive factors are controlled by HS, an abundant GAG present in the local extracellular microenvironment [Jackson et al., 2006; Takada et al., 2003]. We have found in this study that osteoblast-derived total GAGs promote the proliferation of human osteoblasts. GAGs yield stimulatory or inhibitory responses on osteogenesis depending on their chemical composition [Haupt et al., 2009; Ling et al., 2006; Manton et al., 2006]. Soluble or cell surface GAGs are each a mixture of several different GAG species which vary from each other in their structure and functions. However, the overall effect of GAGs on human osteoblasts is stimulatory. Together with the ability of these GAGs to inhibit osteoclastogenesis, our results suggest that bone-specific GAGs shift the balance of bone remodeling towards bone formation by favoring osteoblastogenesis while antagonizing osteoclastogenesis.
Collectively, our results demonstrate that in the absence of OPG, the decoy and inhibitory receptor for RANKL, GAGs reduce the osteoclastogenic ability of RANKL in RAW264.7 cells. The potency of this anti-osteoclastic effect apparently depends on the composition of the GAGs. Our results show that osteoblast derived GAGs maintain active ERK signaling which is normally inhibitory for osteoclastogenesis and silenced in differentiating osteoclasts by RANKL signaling. The inhibitory effect of GAGs on osteoclastogenesis suggests that GAGs have bone anabolic activities and that these natural compounds have considerable therapeutic potential in the treatment of osteoporosis and other osteopenic diseases.
Acknowledgments
We thank all members of the Stem Cells and Tissue Repair group in the Institute of Medical Biology for stimulating discussions. In particular, we thank Kerry Manton and Phua Zer Cheng for providing the primary human osteoblasts and experimental assistance, respectively. This study was supported by funding from Singapore’s Agency for Science Technology and Research (A*STAR), the Biomedical Research Council (BMRC) of Singapore and the Institute of Medical Biology (IMB), Singapore. This work was also supported in part by National Institutes of Health Grants R01 AR49069 (to AJvW), as well as R01 AR39588, P01 AR48818 and P01 CA82834 (to GSS).
REFERENCES
- Ariyoshi W, Takahashi T, Kanno T, Ichimiya H, Shinmyouzu K, Takano H, Koseki T, Nishihara T. Heparin inhibits osteoclastic differentiation and function. J Cell Biochem. 2008;103:1707–17. doi: 10.1002/jcb.21559. [DOI] [PubMed] [Google Scholar]
- Ariyoshi W, Takahashi T, Kanno T, Ichimiya H, Takano H, Koseki T, Nishihara T. Mechanisms involved in enhancement of osteoclast formation and function by low molecular weight hyaluronic acid. J Biol Chem. 2005;280:18967–72. doi: 10.1074/jbc.M412740200. [DOI] [PubMed] [Google Scholar]
- Braunsteiner H, Thumb N. Mast cells, basophils and heparin liberation. Bibl Haematol. 1963;15:9–15. [PubMed] [Google Scholar]
- Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–8. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess TL, Qian Y, Kaufman S, Ring BD, Van G, Capparelli C, Kelley M, Hsu H, Boyle WJ, Dunstan CR, Hu S, Lacey DL. The ligand for osteoprotegerin (OPGL) directly activates mature osteoclasts. J Cell Biol. 1999;145:527–38. doi: 10.1083/jcb.145.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin-Osdoby P, Yu X, Zheng H, Osdoby P. RANKL-mediated osteoclast formation from murine RAW 264.7 cells. Methods Mol Med. 2003;80:153–66. doi: 10.1385/1-59259-366-6:153. [DOI] [PubMed] [Google Scholar]
- Cool SM, Kenny B, Wu A, Nurcombe V, Trau M, Cassady AI, Grondahl L. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) composite biomaterials for bone tissue regeneration: in vitro performance assessed by osteoblast proliferation, osteoclast adhesion and resorption, and macrophage proinflammatory response. J Biomed Mater Res A. 2007;82:599–610. doi: 10.1002/jbm.a.31174. [DOI] [PubMed] [Google Scholar]
- Cool SM, Nurcombe V. Heparan sulfate regulation of progenitor cell fate. J Cell Biochem. 2006;99:1040–51. doi: 10.1002/jcb.20936. [DOI] [PubMed] [Google Scholar]
- Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR, Armstrong A, Shen V, Bain S, Cosman D, Anderson D, Morrissey PJ, Peschon JJ, Schuh J. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13:2412–24. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecarot-Charrier B, Broekhuyse H. Proteoglycans synthesized by cultured mouse osteoblasts. J Biol Chem. 1987;262:5345–51. [PubMed] [Google Scholar]
- Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. 2003;111:1221–30. doi: 10.1172/JCI17215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller K, Wong B, Fox S, Choi Y, Chambers TJ. TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts. J Exp Med. 1998;188:997–1001. doi: 10.1084/jem.188.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi NS, Mancera RL. The structure of glycosaminoglycans and their interactions with proteins. Chem Biol Drug Des. 2008;72:455–82. doi: 10.1111/j.1747-0285.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- Haupt LM, Murali S, Mun FK, Teplyuk N, Mei LF, Stein GS, van Wijnen AJ, Nurcombe V, Cool SM. The heparan sulfate proteoglycan (HSPG) glypican-3 mediates commitment of MC3T3-E1 cells toward osteogenesis. J Cell Physiol. 2009;220:780–91. doi: 10.1002/jcp.21825. [DOI] [PubMed] [Google Scholar]
- Henriksen K, Neutzsky-Wulff AV, Bonewald LF, Karsdal MA. Local communication on and within bone controls bone remodeling. Bone. 2009;44:1026–33. doi: 10.1016/j.bone.2009.03.671. [DOI] [PubMed] [Google Scholar]
- Hotokezaka H, Sakai E, Kanaoka K, Saito K, Matsuo K, Kitaura H, Yoshida N, Nakayama K. U0126 and PD98059, specific inhibitors of MEK, accelerate differentiation of RAW264.7 cells into osteoclast-like cells. J Biol Chem. 2002;277:47366–72. doi: 10.1074/jbc.M208284200. [DOI] [PubMed] [Google Scholar]
- Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, Wang L, Xia XZ, Elliott R, Chiu L, Black T, Scully S, Capparelli C, Morony S, Shimamoto G, Bass MB, Boyle WJ. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci U S A. 1999;96:3540–5. doi: 10.1073/pnas.96.7.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AE, Ralston SH, Marken J, Bell C, MacPherson H, Wallace RG, van Hul W, Whyte MP, Nakatsuka K, Hovy L, Anderson DM. Mutations in TNFRSF11A, affecting the signal peptide of RANK, cause familial expansile osteolysis. Nat Genet. 2000;24:45–8. doi: 10.1038/71667. [DOI] [PubMed] [Google Scholar]
- Hunter GK, Heersche JN, Aubin JE. Isolation of three species of proteoglycan synthesized by cloned bone cells. Biochemistry. 1983;22:831–7. doi: 10.1021/bi00273a019. [DOI] [PubMed] [Google Scholar]
- Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem. 1998;67:609–52. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- Irie A, Takami M, Kubo H, Sekino-Suzuki N, Kasahara K, Sanai Y. Heparin enhances osteoclastic bone resorption by inhibiting osteoprotegerin activity. Bone. 2007;41:165–74. doi: 10.1016/j.bone.2007.04.190. [DOI] [PubMed] [Google Scholar]
- Jackson RA, Murali S, van Wijnen AJ, Stein GS, Nurcombe V, Cool SM. Heparan sulfate regulates the anabolic activity of MC3T3-E1 preosteoblast cells by induction of Runx2. J Cell Physiol. 2007;210:38–50. doi: 10.1002/jcp.20813. [DOI] [PubMed] [Google Scholar]
- Jackson RA, Nurcombe V, Cool SM. Coordinated fibroblast growth factor and heparan sulfate regulation of osteogenesis. Gene. 2006;379:79–91. doi: 10.1016/j.gene.2006.04.028. [DOI] [PubMed] [Google Scholar]
- Katagiri T, Takahashi N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Dis. 2002;8:147–59. doi: 10.1034/j.1601-0825.2002.01829.x. [DOI] [PubMed] [Google Scholar]
- Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142:5050–5. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- Kim N, Odgren PR, Kim DK, Marks SC, Jr., Choi Y. Diverse roles of the tumor necrosis factor family member TRANCE in skeletal physiology revealed by TRANCE deficiency and partial rescue by a lymphocyte-expressed TRANCE transgene. Proc Natl Acad Sci U S A. 2000;97:10905–10. doi: 10.1073/pnas.200294797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumarasuriyar A, Lee I, Nurcombe V, Cool SM. De-sulfation of MG-63 cell glycosaminoglycans delays in vitro osteogenesis, up-regulates cholesterol synthesis and disrupts cell cycle and the actin cytoskeleton. J Cell Physiol. 2009;219:572–83. doi: 10.1002/jcp.21700. [DOI] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- Lamoureux F, Baud’huin M, Duplomb L, Heymann D, Redini F. Proteoglycans: key partners in bone cell biology. Bioessays. 2007;29:758–71. doi: 10.1002/bies.20612. [DOI] [PubMed] [Google Scholar]
- Lee SE, Woo KM, Kim SY, Kim HM, Kwack K, Lee ZH, Kim HH. The phosphatidylinositol 3-kinase, p38, and extracellular signal-regulated kinase pathways are involved in osteoclast differentiation. Bone. 2002;30:71–7. doi: 10.1016/s8756-3282(01)00657-3. [DOI] [PubMed] [Google Scholar]
- Ling L, Murali S, Dombrowski C, Haupt LM, Stein GS, van Wijnen AJ, Nurcombe V, Cool SM. Sulfated glycosaminoglycans mediate the effects of FGF2 on the osteogenic potential of rat calvarial osteoprogenitor cells. J Cell Physiol. 2006;209:811–25. doi: 10.1002/jcp.20760. [DOI] [PubMed] [Google Scholar]
- Manton KJ, Sadasivam M, Cool SM, Nurcombe V. Bone-specific heparan sulfates induce osteoblast growth arrest and downregulation of retinoblastoma protein. J Cell Physiol. 2006;209:219–29. doi: 10.1002/jcp.20727. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Sudo T, Saito T, Osada H, Tsujimoto M. Involvement of p38 mitogen-activated protein kinase signaling pathway in osteoclastogenesis mediated by receptor activator of NF-kappa B ligand (RANKL) J Biol Chem. 2000;275:31155–61. doi: 10.1074/jbc.M001229200. [DOI] [PubMed] [Google Scholar]
- Mizuno A, Amizuka N, Irie K, Murakami A, Fujise N, Kanno T, Sato Y, Nakagawa N, Yasuda H, Mochizuki S, Gomibuchi T, Yano K, Shima N, Washida N, Tsuda E, Morinaga T, Higashio K, Ozawa H. Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem Biophys Res Commun. 1998;247:610–5. doi: 10.1006/bbrc.1998.8697. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Hirata A, Tsuji T, Yamamoto T. Immunolocalization of keratan sulfate proteoglycan in rat calvaria. Arch Histol Cytol. 2001;64:109–18. doi: 10.1679/aohc.64.109. [DOI] [PubMed] [Google Scholar]
- Nakatsuka K, Nishizawa Y, Ralston SH. Phenotypic characterization of early onset Paget’s disease of bone caused by a 27-bp duplication in the TNFRSF11A gene. J Bone Miner Res. 2003;18:1381–5. doi: 10.1359/jbmr.2003.18.8.1381. [DOI] [PubMed] [Google Scholar]
- Nicolin V, Bareggi R, Baldini G, Bortul R, Martinelli B, Narducci P. Effects of neridronic acid on osteoclasts derived by physiological dual-cell cultures. Acta Histochem. 2007;109:397–402. doi: 10.1016/j.acthis.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Nurcombe V, Goh FJ, Haupt LM, Murali S, Cool SM. Temporal and functional changes in glycosaminoglycan expression during osteogenesis. J Mol Histol. 2007;38:469–81. doi: 10.1007/s10735-007-9123-4. [DOI] [PubMed] [Google Scholar]
- Pearse RN, Sordillo EM, Yaccoby S, Wong BR, Liau DF, Colman N, Michaeli J, Epstein J, Choi Y. Multiple myeloma disrupts the TRANCE/ osteoprotegerin cytokine axis to trigger bone destruction and promote tumor progression. Proc Natl Acad Sci U S A. 2001;98:11581–6. doi: 10.1073/pnas.201394498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrimon N, Bernfield M. Specificities of heparan sulphate proteoglycans in developmental processes. Nature. 2000;404:725–8. doi: 10.1038/35008000. [DOI] [PubMed] [Google Scholar]
- Roodman GD. Cell biology of the osteoclast. Exp Hematol. 1999;27:1229–41. doi: 10.1016/s0301-472x(99)00061-2. [DOI] [PubMed] [Google Scholar]
- Sasaki N, Kusano E, Ando Y, Nemoto J, Iimura O, Ito C, Takeda S, Yano K, Tsuda E, Asano Y. Changes in osteoprotegerin and markers of bone metabolism during glucocorticoid treatment in patients with chronic glomerulonephritis. Bone. 2002;30:853–8. doi: 10.1016/s8756-3282(02)00742-1. [DOI] [PubMed] [Google Scholar]
- Schoppet M, Al-Fakhri N, Franke FE, Katz N, Barth PJ, Maisch B, Preissner KT, Hofbauer LC. Localization of osteoprotegerin, tumor necrosis factor-related apoptosis-inducing ligand, and receptor activator of nuclear factor-kappaB ligand in Monckeberg’s sclerosis and atherosclerosis. J Clin Endocrinol Metab. 2004;89:4104–12. doi: 10.1210/jc.2003-031432. [DOI] [PubMed] [Google Scholar]
- Shinmyouzu K, Takahashi T, Ariyoshi W, Ichimiya H, Kanzaki S, Nishihara T. Dermatan sulfate inhibits osteoclast formation by binding to receptor activator of NF-kappa B ligand. Biochem Biophys Res Commun. 2007;354:447–52. doi: 10.1016/j.bbrc.2006.12.221. [DOI] [PubMed] [Google Scholar]
- Sims NA, Gooi JH. Bone remodeling: Multiple cellular interactions required for coupling of bone formation and resorption. Semin Cell Dev Biol. 2008;19:444–51. doi: 10.1016/j.semcdb.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Suda T, Takahashi N, Martin TJ. Modulation of osteoclast differentiation. Endocr Rev. 1992;13:66–80. doi: 10.1210/edrv-13-1-66. [DOI] [PubMed] [Google Scholar]
- Takada T, Katagiri T, Ifuku M, Morimura N, Kobayashi M, Hasegawa K, Ogamo A, Kamijo R. Sulfated polysaccharides enhance the biological activities of bone morphogenetic proteins. J Biol Chem. 2003;278:43229–35. doi: 10.1074/jbc.M300937200. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Udagawa N, Suda T. A new member of tumor necrosis factor ligand family, ODF/OPGL/TRANCE/RANKL, regulates osteoclast differentiation and function. Biochem Biophys Res Commun. 1999;256:449–55. doi: 10.1006/bbrc.1999.0252. [DOI] [PubMed] [Google Scholar]
- Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- Teplyuk NM, Haupt LM, Ling L, Dombrowski C, Mun FK, Nathan SS, Lian JB, Stein JL, Stein GS, Cool SM, van Wijnen AJ. The osteogenic transcription factor Runx2 regulates components of the fibroblast growth factor/proteoglycan signaling axis in osteoblasts. J Cell Biochem. 2009;107:144–54. doi: 10.1002/jcb.22108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoleyre S, Kwan Tat S, Vusio P, Blanchard F, Gallagher J, Ricard-Blum S, Fortun Y, Padrines M, Redini F, Heymann D. Characterization of osteoprotegerin binding to glycosaminoglycans by surface plasmon resonance: role in the interactions with receptor activator of nuclear factor kappaB ligand (RANKL) and RANK. Biochem Biophys Res Commun. 2006;347:460–7. doi: 10.1016/j.bbrc.2006.06.120. [DOI] [PubMed] [Google Scholar]
- Watts NB, Harris ST, Genant HK, Wasnich RD, Miller PD, Jackson RD, Licata AA, Ross P, Woodson GC, 3rd, Yanover MJ, et al. Intermittent cyclical etidronate treatment of postmenopausal osteoporosis. N Engl J Med. 1990;323:73–9. doi: 10.1056/NEJM199007123230201. [DOI] [PubMed] [Google Scholar]
- Whyte MP, Hughes AE. Expansile skeletal hyperphosphatasia is caused by a 15-base pair tandem duplication in TNFRSF11A encoding RANK and is allelic to familial expansile osteolysis. J Bone Miner Res. 2002;17:26–9. doi: 10.1359/jbmr.2002.17.1.26. [DOI] [PubMed] [Google Scholar]
- Whyte MP, Obrecht SE, Finnegan PM, Jones JL, Podgornik MN, McAlister WH, Mumm S. Osteoprotegerin deficiency and juvenile Paget’s disease. N Engl J Med. 2002;347:175–84. doi: 10.1056/NEJMoa013096. [DOI] [PubMed] [Google Scholar]