Abstract
Thyroid hormone (TH) affects diverse biological processes and can exert its effects through both gene regulation via binding the nuclear TH receptors (TRs) and non-genomic actions via binding to cell surface and cytoplasmic proteins. The critical importance of TH in vertebrate development has long been established, ranging from the formation of human cretins to the blockage of frog metamorphosis due the TH deficiency. How TH affects vertebrate development has been difficult to study in mammals due to the complications associated with the uterus-enclosed mammalian embryos. Anuran metamorphosis offers a unique opportunity to address such an issue. Using Xenopus as a model, we and others have shown that the expression of TRs and their heterodimerization partners RXRs (9-cis retinoic acid receptors) correlates temporally with metamorphosis in different organs in two highly related species, Xenopus laevis and Xenopus tropicalis. In vivo molecular studies have shown that TR and RXR are bound to the TH response elements (TREs) located in TH-inducible genes in developing tadpoles of both species. More importantly, transgenic studies in Xenopus laevis have demonstrated that TR function is both necessary and sufficient for mediating the metamorphic effects of TH. Thus, the non-genomic effects of TH have little or no roles during metamorphosis and likely during vertebrate development in general.
Keywords: thyroid hormone receptor, chromatin, histone acetylation, postembryonic development, metamorphosis, Xenopus laevis, Xenopus tropicalis
1. INTRODUCTION
Thyroid hormone (TH) plays an important role in vertebrate development and human pathology (Oppenheimer, 1979; Yen, 2001). The critical effects of TH on human development have been well documented. The most obvious and earliest known human abnormalities due to TH deficiency are the goiter (a lump in the neck due to thyroid gland enlargement) and cretinism (a form of severe mental deficiency together with retarded skeletal growth) (Hetzel, 1989). In humans, many of the developmental defects caused by TH deficiency prior to birth can be reversed if TH replacement is initiated shortly after birth (Larsen, 1989), indicating that TH influences neonatal development mainly by acting directly on the foetus, not through the mother. The most important period of TH action is the so-called postembryonic development, a few months before and several months after birth when TH levels are high (Howdeshell, 2002; Tata, 1993). This period bears many similarities to anuran metamorphosis (Shi, 1999; Tata, 1993), including the presence of high levels of TH. Such similarities coupled with the difficulties to manipulate the uterus-enclosed mammalian embryos have made anuran metamorphosis a highly valuable model to study TH action during vertebrate development. Here we will review some of the studies on the role of TH receptor (TR) during amphibian metamorphosis, with an emphasis on our own work in Xenopus laevis as presented at the meeting.
2. MECHANISMS OF TH ACTION
To affect target cells, circulating TH in the plasma needs to be taken up by cells through active transport (Hennemann et al., 2001; Shi et al., 2002). Upon entering the cells, TH can bind to a number of cytosolic proteins and enter the nucleus where it binds to TRs (Shi et al., 1996). In addition, TH can also bind to cell surface proteins, such as integrins (Bassett et al., 2003; Davis et al., 2005). Thus, it is not surprising that TH can affect cells through both the so-called non-genomic action via the binding to cell surface and cytoplasmic proteins and transcriptional regulation via TRs.
2.1. Non-genomic action of TH
TH affects diverse biological processes. Some of the effects of TH are too fast to be mediated through transcriptional regulation via TR in the nucleus (Bassett et al., 2003; Davis and Davis, 1996). For example, TH administration leads to acute improvement in cardiac output in human patients, TH can alter myocardial contractility and reduce systemic vascular resistance within minutes. At the cellular level, TH can affect cell morphology, respiration (mitochondrial function), and ion homeostasis, etc. TH appears to exert diverse non-genomic effects through multiple pathways (Bassett et al., 2003; Davis and Davis, 1996; Davis and Davis, 2002; Davis et al., 2005; Shi et al., 1996). First, it has long been known that TH can bind to cell surface proteins (Davis et al., 2005). The identities of these proteins are largely unknown except the integrin αVβ3, which binds strongly T4 (3, 3’, 5, 5’-tetraiodothyronine) and to a lesser extent T3 (3, 3’, 5-triiodothyronine) (Davis et al., 2005). This binding of TH to the integrin is expected to affect cell-extracellular matrix interactions and trigger intracellular signaling processes rapidly. Second, within the cell, a number of cytosolic proteins are known to bind to TH (Davis and Davis, 1996; Davis and Davis, 2002; Parkison et al., 1991; Shi et al., 1994; Shi et al., 1996). Most of these cytosolic proteins have additional functions, often as enzymes. TH binding may thus affect the enzymatic functions of these proteins and conversely, regulating their enzymatic activity may influence their binding to TH. For example, a cytosolic thyroid hormone binding protein is the monomer form of M2 pyruvate kinase (Parkison et al., 1991). TH binding prevents the formation of the enzymatically active tetramer and conversely, the formation of the tetramer inhibits its binding to TH (Ashizawa and Chen, 1992; Ashizawa et al., 1991). Finally, while TR is predominantly nuclearly localized even in the absence of TH, a small fraction is present in the cytoplasm. It has been shown that one of the two TR isoforms, TRβ, can form a complex with the signaling kinase MAPK in TH treated cells, which is likely responsible for the rapid activation of MAPK by TH (Davis et al., 2005). In addition, unliganded TRβ can interact with phosphatidylinosital 3 kinase (PI3K) to activate the signaling pathway (Guigon and Cheng, 2009; Storey et al., 2006). Thus, TRβ can also function as a mediator of the non-genomic effects of TH by interacting with these and other cytosolic proteins (Guigon and Cheng, 2009).
2.2. Nuclear action of TH
There are two types TRs in all vertebrates, TRα and TRβ, both of which bind TH with high affinities (Davey et al., 1994; Puzianowsak-Kuznicka et al., 1996; Sap et al., 1986; Weinberger et al., 1986). TRs belong to the superfamily of nuclear hormone receptors (Evans, 1988; Laudet and Gronemeyer, 2002; Mangelsdorf et al., 1995; Tsai and O'Malley, 1994; Yen and Chin, 1994). TH can both activate and repress transcription through TRs. The mechanism for gene repression by TH is not well understood and thus will not be discussed here. Transcriptional activation by TH requires the binding of TRs, most likely as heterodimers with RXRs (9-cis-retinoic acid receptors), to the TH response elements (TREs) in TH-inducible genes. TR/RXR heterodimers bind to TREs constitutively, even in the context chromatin (Perlman et al., 1982; Tsai and O'Malley, 1994; Wong et al., 1995). They repress or activate transcription in the absence or presence of TH, respectively.
In vitro and cell culture studies involving different animal species by many laboratories have led to a fairly detailed understanding of the mechanisms of the gene regulation by TR. TR functions by recruiting cofactors. Many such cofactors have been isolated and characterized (Burke and Baniahmad, 2000; Glass and Rosenfeld, 2000; Huang et al., 2003; Ito and Roeder, 2001; Jones and Shi, 2003; McKenna et al., 1999; McKenna and O'Malley, 2001; Meng et al., 2003; Rachez and Freedman, 2000; 2001; Sato et al., 2009; Wahlstrom et al., 1999; Xu et al., 1999; Zhang and Lazar, 2000). In the absence of TH, TR recruits corepressors, such as the highly related proteins SMRT and N-CoR, which form multimeric complexes containing histone deacetylases (HDACs) (Burke and Baniahmad, 2000; Glass and Rosenfeld, 2000; Jones and Shi, 2003; Zhang and Lazar, 2000) (Fig. 1). This leads to the deacetylation of the promoter regions of the target genes to facilitate gene repression. When TH is present, the corepressor complexes are released and replaced by coactivator complexes. Many diverse groups of coactivators have been identified. Among them include ATP-dependent chromatin remodeling proteins, histone acetylases (HATs) such as p300 and SRCs, protein arginine methyltransferases, and TRAP/DRIP/mediator complex that associates with the recruitment and activation of RNA polymerase II (Chen et al., 1999; Demarest et al., 2002; Heimeier et al., 2008; Huang et al., 2003; Ito and Roeder, 2001; Li et al., 2000; Matsuda et al., 2009; Matsuda et al., 2007; McKenna and O'Malley, 2001; Rachez and Freedman, 2001; Sheppard et al., 2001; Yen, 2001; Zhang and Lazar, 2000) (Fig. 1). The recruitment of such cofactors to the target genes leads to histone acetylation, methylation, and chromatin remodeling, resulting in transcriptional activation.
Fig. 1.
Mechanisms of transcriptional regulation by TR. For TH-inducible genes, TR heterodimerized with RXR constitutively binds the TREs in their promoters or enhancers. In the absence of TH, TR binds corepressor complexes, such as those containing histone deacetylase HDAC3 and the highly related protein N-CoR or SMRT to inhibit transcription from the promoters. This is accomplished in part through deacetylation of lysine residues of histone H3 and H4 to induce a “closed” chromatin state, as suggested by the folding of histone tails (red beaded structure) on to the DNA helix, because of the charge-charge interaction between the positively charged histone tails and negatively charged DNA. The binding by TH induces a conformational change in TR, leading to the binding of coactivator complexes, such as those containing coactivators SRC and p300, which are histone acetyltransferases (HATs). They will acetylate histones H3 and H4, facilitating the formation of an “open” chromatin state, as diagramed by the unfolding of histone tails (red beaded structure) away from the DNA helix due to the neutralization of the positive charges on the histone tails by acetylation. Liganded TR can also recruit other coactivator complexes, such as chromatin remodeling complexes and mediator complex (also known as DRIP/TRAP complex), with the latter directly contacting RNA polymerase, to activate transcription.
3. ROLES OF TR IN XENOPUS METAMORPHOSIS
3.1. A model of TR in frog development
Early expression studies showed that the mRNA levels of TR, especially TRα, are upregulated shortly after hatching at stage 35 in Xenopus laevis, reaching peak levels by tadpole feeding stage (stage 45), when a free living tadpole is developed, although TRβ expression parallels with plasma TH concentrations (Fig. 2) (Shi et al., 1994; Yaoita and Brown, 1990). In addition, RXR genes, in particular, RXRα, are also expressed in premetamorphic Xenopus laevis tadpoles (Fig. 2) (Wong and Shi, 1995). Similar expression patterns for TR and RXR genes have also been observed in Xenopus tropicalis (Wang et al., 2008). Based on these and the transcriptional properties of TR/RXR heterodimers, we have previously proposed a dual function model for TR during Xenopus laevis development (Fig. 2) (Sachs et al., 2000; Shi et al., 1996). According to the model, the unliganded TR expressed in premetamorphic tadpoles between stage 45 when a free feeding tadpole is formed (Nieuwkoop and Faber, 1956) and stage 55, just when endogenous TH becomes detectable (Fig. 2) (Leloup and Buscaglia, 1977), forms a heterodimer with RXR and the TR-RXR heterodimer binds to the TREs of TH-inducible genes, leading to the repression of their expression. This then ensures proper tadpole growth before metamorphic organ transformations. After stage 55, availability of TH allows the binding of TH to chromatin-bound TR and the TH-bound TR-RXR then activates these target genes to initiate metamorphosis in different organs and tissues (Fig. 2).
Fig. 2.
A dual function model of TR in frog development. During embryogenesis, TH response genes are expressed at basal levels in the absence of TR and TH to facilitate embryonic organ development. After tadpole hatching at stage 35/36, TRα expression increases, reaching high levels by stage 45 when tadpole feeding begins (Yaoita and Brown, 1990). RXRα is also highly expressed by this time (Wong and Shi, 1995), the TR/RXR heterodimers bind to TH response genes to repress their expression due to the lack of TH, thus ensuring proper tadpole growth and preventing premature metamorphosis. When endogenous TH level rises after stage 55 (Leloup and Buscaglia, 1977), the TH-bound TR/RXR heterodimers then activate TH response genes, such as the TRβ genes, leading to metamorphosis.
3.2. TR binds to the TREs of endogenous target genes during frog development
We used the chromatin immunoprecipitation assays (ChIP) to analyze the binding of TR to target genes during Xenopus laevis development (Sachs and Shi, 2000). As the model predicted, there is little or no TR present at the TREs of two known direct TH-inducible genes, TRβ and TH/bZIP genes, in embryos but TR is present on the TREs in premetamorphic tadpoles when analyzed either in whole animals or in individual organs like the intestine and tail (Buchholz et al., 2005; Havis et al., 2003; Matsuda et al., 2009; Paul et al., 2005a; Paul et al., 2005b; Sachs et al., 2002; Sachs and Shi, 2000; Tomita et al., 2004). Furthermore, quantitative ChIP assay showed that during metamorphosis or after TH-treatment of premetamorphic tadpoles, the binding of TR to the TREs increases, especially on the TH/bZIP TRE, which has a weaker affinity to TRs compared to the TRE in the TRβ in direct DNA binding assays in vitro (Buchholz et al., 2005; Matsuda et al., 2009). Similar results were also observed in Xenopus tropicalis (Wang et al., 2008), a species highly related with Xenopus laevis. Thus, TR is bound to the TREs in premetamorphic tadpoles, especially those with high affinities for TR and during metamorphosis, the increase in TR expression, especially TRβ due to auto-regulation (Kanamori and Brown, 1992; Machuca et al., 1995; Ranjan et al., 1994; Shi et al., 1992; Yaoita and Brown, 1990), leads to increased binding of the TR to the TREs in target genes, especially those with weaker affinities for TR. Such observations also suggest that the different temporal expression profiles of TR genes in different organs should lead to organ-dependent variations in the regulation of target genes by TR, which may contribute to the temporal regulation of metamorphosis in different organs (Shi et al., 1994; Shi et al., 1996).
3.3. RXR is important for TR function during frog development
Our ChIP assay also showed that like TR, RXRα is also present at the TREs in premetamorphic tadpoles but not during embryogenesis, suggesting that TR/RXR heterodimers are the functional complexes for frog development (Sachs and Shi, 2000). By making use of the fact that early Xenopus embryos have very low levels of endogenous TR and TH (Banker et al., 1991; Kanamori and Brown, 1992; Wong and Shi, 1995) (although such low levels are important for at least some aspects of embryogenesis (Havis et al., 2006)), we studied the effect of overexpression of TR upon microinjection of its mRNA into fertilized eggs (Puzianowska-Kuznicka et al., 1997). The results showed that TRα over-expression had distinct effects on embryonic development depending on the presence or absence of TH. Interestingly, the developmental effects of TRα were greatly enhanced by co-over-expression of RXRα. TRα or RXRα alone had little effect on embryonic development both in the presence or absence of T3. Coexpression of TRα and RXRα led nearly 90% of the embryos with deformity in the absence of T3 and in the presence of T3, 100% had deformity and/or embryonic lethality. More importantly, overexpression of TR and RXR together, but not either one alone, repressed endogenous TH-inducible genes while the addition of TH resulted in the reversal of the repression and further activation of the TH response genes. Thus, RXR is important for TR functions during frog development.
3.4. TR is required for TH-induced metamorphosis
The development of a sperm-mediated transgenic method (Kroll and Amaya, 1996) has made it possible to genetically determine the role of TR in mediating the metamorphic effects of TH. Schreiber et al. (Schreiber et al., 2001) reported the first transgenic study on TR where they ubiquitously overexpressed a dominant negative TR (incapable of binding TH due to a small deletion at the C-terminus) in Xenopus laevis tadpoles and found that diverse aspects of TH-induced metamorphic changes were inhibited. The same conclusion was also reached in several labs in subsequent studies, where a dominant negative TR was overexpressed by using in vivo transfection or transgenesis with ubiquitous and tissue-specific promoters (Fig. 3) (Buchholz et al., 2003; Das et al., 2002; Nakajima and Yaoita, 2003; Schreiber and Brown, 2003; Schreiber et al., 2009). These in vivo studies also showed that the expression of the dominant negative TR inhibited TH-induced transcription of target genes. Mechanistically, we demonstrated that the dominant negative TR expressed in transgenic animals competed against endogenous TR for binding to endogenous target genes, leading to the retention of corepressors at the target genes even in the presence of TH (Buchholz et al., 2003). This caused a reduction in local histone acetylation, thus contributing to the inhibition of gene expression and frog metamorphosis. Thus, TR is essential for TH-induced gene regulation and metamorphosis.
Fig. 3.
TR is necessary for the metamorphic effects of TH. Transgenic expression of a dominant negative TR (dnTR) blocks TH-induced metamorphosis. Wild type animals treated with TH underwent characteristic changes, including gill resorption and limb morphogenesis (compared the middle panels to the ones on the left). The TH-treatment failed to induce such changes in the sibling transgenic animals (right panels, which resemble the left but not the middle ones) (Buchholz et al., 2003).
3.5. TR is sufficient to mediate the metamorphic effects of TH
Given the ability of TH to affect cells through both non-genomic and nuclear pathways, it is important to determine whether TR is sufficient to mediate the metamorphic effects of TH. For this purpose, we generated a dominant positive TR that cannot bind to TH (due to a small deletion at the C-terminus) but constitutively activate transcription (due to the N-terminal fusion of the strong viral activator VP16) (Buchholz et al., 2004). As overexpression of liganded TR has adverse effects on early embryogenesis (see above), we placed the dominant positive TR under the control of a heat shock-inducible promoter for transgenesis. When transgenic tadpoles and their wild type siblings reached premetamorphic stages, we subjected them to daily heat shock treatment to induce the transgene expression and compared the effects of heat shock treatment with those due to TH treatment of the wild type sibling animals. As expected, TH-treatment led to typical metamorphic changes, including limb morphogenesis and gill resorption, etc. (Fig. 4). Interestingly, upon heat shock treatment, transgenic but not wild type tadpoles underwent the same changes as induced by TH treatment, including both external morphological changes such as limb morphogenesis and gill resorption (Fig. 4) as well as the remodeling of internal organs such as the intestine (Buchholz et al., 2004). Furthermore, gene expression studies showed that the expression of the dominant positive TR induced the expression of the same endogenous TH-inducible genes with the same organ-specificity as TH treatment (Buchholz et al., 2004). In addition, ChIP assays demonstrated the binding of the dominant positive TR to the TREs of endogenous TH-target genes (Buchholz et al., 2004). Thus, the binding of this constitutively active TR to these target genes activates them, leading to metamorphic changes in all organs/tissues. These results suggest that TR is sufficient to mediate the effects of TH during Xenopus metamorphosis, at least on all the parameters that were measured.
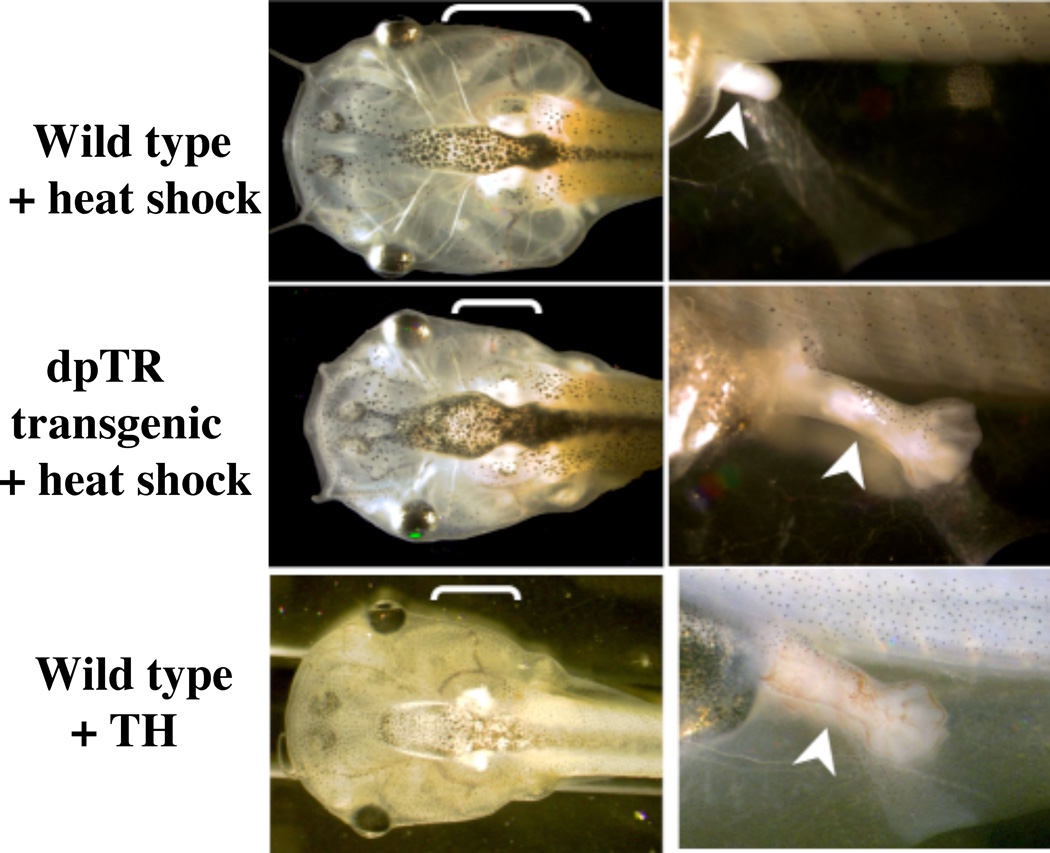
Fig. 4.
TR is sufficient to mediate the metamorphic effects of TH. Wild type tadpoles and sibling tadpoles transgenic for a dominant positive TR (dpTR) under the control of a heat shock-inducible promoter were reared together in methimazole to block endogenous T3 synthesis and were heat-shocked daily for 8 days. For comparison, wild type tadpoles were treated with TH for 3 days. Note that the heat shock induction of dpTR expression resulted in metamorphic events, including gill resorption (bracket) and limb outgrowth (arrowhead) in the transgenic (middle) but not wild type (top) animals, just like TH-treatment of wild type sibling animals (bottom) (Buchholz et al., 2004).
4. CONCLUSIONS
TH affects many biological processes and can elicit both fast, short-term effects as well as slow, long-term consequences. The fast effects have been generally observed and studied in cells and/or animal organs. It is generally believed that they are mediated through the non-genomic action of the hormone and that the underlying mechanisms likely vary depending upon the effects observed. It is unclear whether such non-genomic actions contribute to the developmental effects of the hormone, in part due to the lack of good model system. Studies on anuran metamorphosis have now provided strong evidence to show that TR is both necessary and sufficient to mediate the metamorphic effects of TH. Thus, the non-genomic action of TH appears to play little or no role during vertebrate development.
While the conclusions above are based on studies of frog development, there are many similarities between mammalian postembryonic development and anuran metamorphosis (Shi, 1999; Tata, 1993). For example, during mammalian development, TRs are also expressed prior to the synthesis of endogenous TH and TH levels in the plasma peaks during postembryonic development (Howdeshell, 2002; Tata, 1993). These and other conservations argue that in mammals as well as other vertebrates, the developmental effects of TH is most likely mediated by TR with non-genomic effects of TH contributing little. Such a conclusion may not be surprising given the slow and lengthy process of vertebrate development compared to the time scale of the non-genomic effects of TH.
An important question to be addressed is how TR mediates the developmental effects of TH in various tissues/organs. Here, studies in the Xenopus laevis model have offered some clues. In premetamorphic tadpoles in the absence of TH, corepressor complexes, such as those containing N-CoR and SMRT (Fig. 1) are present at the TREs of TH-inducible genes and are released upon TH-treatment or during natural metamorphosis when endogenous TH levels are high (Sachs et al., 2002; Tomita et al., 2004). More importantly, transgenic studies with a dominant negative corepressor have shown that unliganded TR recruits corepressors to regulate the timing for the initiation of metamorphosis (Sato et al., 2007). During metamorphosis, liganded TR recruits coactivator complexes containing SRC/p300 to the promoters to activate their transcription (Fig. 1) (Havis et al., 2003; Matsuda et al., 2009; Paul et al., 2005a; Paul et al., 2005b). Genetic studies indicate that TR needs to recruit coactivators to target genes in order to activate their transcription and induce metamorphosis (Paul et al., 2005a; Paul et al., 2005b) and that the levels of coactivators regulate the rate of metamorphic progression. It is tempting to speculate that organ-specific variations in cofactor concentrations and/or utilization of different cofactors, may be an important factor in determining the organ-specific temporal regulation of metamorphosis, such as the early completion of the limb development but the late resorption of the tail resorption (Shi, 1999; Shi et al., 1996). This is clearly one of the important questions that need to be addressed in the future. A possible approach to this is to use a combination of genetic and molecular analyses. This will involve the identification of tissue specific TH target genes and the cofactors participating in their regulation by using techniques such as genome-wide ChIP analyses of cofactor recruitment by TR in different organs, followed by genetic studies such as transgenic overexpression or knockdown to determine the role of cofactors in the metamorphosis of different organs. Parallel studies in other vertebrate species such as mouse will show whether such developmental mechanisms are conserved.
ACKNOWLEDGMENT
This work has been supported by the Intramural Research Program of NICHD, NIH and by a JSPS (Japan Society for the Promotion of Science) fellowship to Kenta Fujimoto.
Abbreviations
- TH
Thyroid hormone
- TR
Thyroid hormone receptor
- HDAC
Histone deacetylase
- HAT
Histone acetyl transferase
- RXR
9-cis retinoic acid receptor
- TRE
TH response element
- ChIP
chromatin immunoprecipitation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ashizawa K, Chen S-Y. Regulation of thyroid hormone receptor-mediated transcription by a cytosol protein. Proc Natl Acad Sci USA. 1992;89:9277–9281. doi: 10.1073/pnas.89.19.9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashizawa K, McPhie P, Lin KH, Cheng SY. An in vitro novel mechanism of regulating the activity of pyruvate kinase M2 by thyroid hormone and fructose 1, 6-bisphosphate. Biochemistry. 1991;30:7105–7111. doi: 10.1021/bi00243a010. [DOI] [PubMed] [Google Scholar]
- Banker DE, Bigler J, Eisenman RN. The thyroid hormone receptor gene (c-erbA alpha) is expressed in advance of thyroid gland maturation during the early embryonic development of Xenopus laevis. Mol Cell Biol. 1991;11:5079–5089. doi: 10.1128/mcb.11.10.5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett JH, Harvey CB, Williams GR. Mechanisms of thyroid hormone receptorspecific nuclear and extra nuclear actions. Mol Cell Endocrinol. 2003;213:1–11. doi: 10.1016/j.mce.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Buchholz DR, Hsia VS-C, Fu L, Shi Y-B. A dominant negative thyroid hormone receptor blocks amphibian metamorphosis by retaining corepressors at target genes. Mol. Cell. Biol. 2003;23:6750–6758. doi: 10.1128/MCB.23.19.6750-6758.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz DR, Paul BD, Shi YB. Gene-specific changes in promoter occupancy by thyroid hormone receptor during frog metamorphosis. Implications for developmental gene regulation. J Biol Chem. 2005;280:41222–41228. doi: 10.1074/jbc.M509593200. [DOI] [PubMed] [Google Scholar]
- Buchholz DR, Tomita A, Fu L, Paul BD, Shi Y-B. Transgenic analysis reveals that thyroid hormone receptor is sufficient to mediate the thyroid hormone signal in frog metamorphosis. Mol. Cell. Biol. 2004;24:9026–9037. doi: 10.1128/MCB.24.20.9026-9037.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke LJ, Baniahmad A. Co-repressors 2000. FASEB J. 2000;14:1876–1888. doi: 10.1096/fj.99-0943rev. [DOI] [PubMed] [Google Scholar]
- Chen D, Ma H, Hong H, Koh SS, Huang SM, Schurter BT, Aswad DW, Stallcup MR. Regulation of transcription by a protein methyltransferase. Science. 1999;284:2174–2177. doi: 10.1126/science.284.5423.2174. [DOI] [PubMed] [Google Scholar]
- Das B, Schreiber AM, Huang H, Brown DD. Multiple thyroid hormone-induced muscle growth and death programs during metamorphosis in Xenopus laevis. P NATL ACAD SCI USA. 2002;99:12230–12235. doi: 10.1073/pnas.182430599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey JC, Schneider MJ, Galton VA. Cloning of a thyroid hormone-responsive Rana catesbeiana c-erbA-beta gene. Dev Genet. 1994;15:339–346. doi: 10.1002/dvg.1020150405. [DOI] [PubMed] [Google Scholar]
- Davis PJ, Davis FB. Nongenomic actions of thyroid hormone. Thyroid. 1996;6:497–504. doi: 10.1089/thy.1996.6.497. [DOI] [PubMed] [Google Scholar]
- Davis PJ, Davis FB. Nongenomic actions of thyroid hormone on the heart. Thyroid. 2002;12:459–466. doi: 10.1089/105072502760143827. [DOI] [PubMed] [Google Scholar]
- Davis PJ, Davis FB, Cody V. Membrane receptors mediating thyroid hormone action. Trends Endocrinol Metab. 2005;16:429–435. doi: 10.1016/j.tem.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Demarest SJ, Martinez-Yamout M, Chung J, Chen H, Xu W, Dyson HJ, Evans RM, Wright PE. Mutual synergistic folding in recruitment of CBP/p300 by p160 nuclear receptor coactivators. Nature. 2002;415:549–553. doi: 10.1038/415549a. [DOI] [PubMed] [Google Scholar]
- Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000;14:121–141. [PubMed] [Google Scholar]
- Guigon CJ, Cheng SY. Novel non-genomic signaling of thyroid hormone receptors in thyroid carcinogenesis. Mol Cell Endocrinol. 2009;308:63–69. doi: 10.1016/j.mce.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havis E, Le Mevel S, Dubois GM, Shi D-L, Scanlan TS, Demeneix BA, Sachs LM. Unliganded thyroid hormone receptor is essential for Xenopus laevis eye development. EMBO J. 2006;25:4943–4951. doi: 10.1038/sj.emboj.7601356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havis E, Sachs LM, Demeneix BA. Metamorphic T3-response genes have specific co-regulator requirements. EMBO Reports. 2003;4:883–888. doi: 10.1038/sj.embor.embor908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimeier RA, Hsia VS-C, Shi Y-B. Participation of BAF57 and BRG1- Containing Chromatin Remodeling Complexes in Thyroid Hormone-Dependent Gene Activation during Vertebrate Development. Mol. Endocrinol. 2008;22:1065–1077. doi: 10.1210/me.2007-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennemann G, Docter R, Friesema EC, de Jong M, Krenning EP, Visser TJ. Plasma membrane transport of thyroid hormones and its role in thyroid hormone metabolism and bioavailability. Endocr Rev. 2001;22:451–476. doi: 10.1210/edrv.22.4.0435. [DOI] [PubMed] [Google Scholar]
- Hetzel BS. The story of iodine deficiency: An international challenge in nutrition. Oxford: Oxford University Press; 1989. [Google Scholar]
- Howdeshell KL. A model of the development of the brain as a construct of the thyroid system. Environ Health Perspect. 2002;110:337–348. doi: 10.1289/ehp.02110s3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z-Q, Li J, Sachs LM, Cole PA, Wong J. A role for cofactor–cofactor and cofactor–histone interactions in targeting p300, SWI/SNF and Mediator for transcription. EMBO J. 2003;22:2146–2155. doi: 10.1093/emboj/cdg219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Roeder RG. The TRAP/SMCC/Mediator complex and thyroid hormone receptor function. Trends Endocrinol Metab. 2001;12:127–134. doi: 10.1016/s1043-2760(00)00355-6. [DOI] [PubMed] [Google Scholar]
- Jones PL, Shi Y-B. N-CoR-HDAC corepressor complexes: Roles in transcriptional regulation by nuclear hormone receptors. In: Workman JL, editor. Current Topics in Microbiology and Immunology: Protein Complexes that Modify Chromatin. Berlin: Springer-Verlag; 2003. pp. 237–268. [DOI] [PubMed] [Google Scholar]
- Kanamori A, Brown DD. The regulation of thyroid hormone receptor beta genes by thyroid hormone in Xenopus laevis. J Biol Chem. 1992;267:739–745. [PubMed] [Google Scholar]
- Kroll KL, Amaya E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development. 1996;122:3173–3183. doi: 10.1242/dev.122.10.3173. [DOI] [PubMed] [Google Scholar]
- Larsen PR. Maternal thyroxine and congenital hypothyroidism. N Engl J Med. 1989;321:44–46. doi: 10.1056/NEJM198907063210108. [DOI] [PubMed] [Google Scholar]
- Laudet V, Gronemeyer H. The nuclear receptor FactsBook. San Diego: Academic Press; 2002. [Google Scholar]
- Leloup J, Buscaglia M. La triiodothyronine: hormone de la métamorphose des amphibiens. C.R. Acad. Sci. 1977;284:2261–2263. [Google Scholar]
- Li J, O'Malley BW, Wong J. p300 requires its histone acetyltransferase activity and SRC-1 interaction domain to facilitate thyroid hormone receptor activation in chromatin. Mol. And Cell Biol. 2000;20:2031–2042. doi: 10.1128/mcb.20.6.2031-2042.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machuca I, Esslemont G, Fairclough L, Tata JR. Analysis of structure and expression of the Xenopus thyroid hormone receptor b gene to explain its autoregulation. Mol. Endocrinol. 1995;9:96–107. doi: 10.1210/mend.9.1.7760854. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Paul BD, Choi CY, Hasebe T, Shi Y-B. Novel functions of protein arginine methyltransferase 1 in thyroid hormone receptor-mediated transcription and in the regulation of metamorphic rate in Xenopus laevis. Mol. Cell. Biol. 2009;29:745–757. doi: 10.1128/MCB.00827-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Paul BD, Choi CY, Shi Y-B. Contrasting effects of two alternative splicing forms of coactivator-associated arginine methyltransferase 1 on thyroid hormone receptor-mediated transcription in Xenopus laevis. Mol. Endocrinology. 2007;21:1082–1094. doi: 10.1210/me.2006-0448. [DOI] [PubMed] [Google Scholar]
- McKenna NJ, Lanz RB, O'Malley BW. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- McKenna NJ, O'Malley BW. Nuclear receptors, coregulators, ligands, and selective receptor modulators: making sense of the patchwork quilt. Ann N Y Acad Sci. 2001;949:3–5. doi: 10.1111/j.1749-6632.2001.tb03997.x. [DOI] [PubMed] [Google Scholar]
- Meng X, Yang YF, Cao X, Govindan MV, Shuen M, Hollenberg AN, Mymryk JS, Walfish PG. Cellular context of coregulator and adaptor proteins regulates human adenovirus 5 early region 1A-dependent gene activation by the thyroid hormone receptor. Mol Endocrinol. 2003;17:1095–1105. doi: 10.1210/me.2002-0294. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Yaoita Y. Dual mechanisms governing muscle cell death in tadpole tail during amphibian metamorphosis. Dev Dyn. 2003;227:246–255. doi: 10.1002/dvdy.10300. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of Xenopus laevis. 1st ed. Amsterdam, The Netherlands: North Holland Publishing; 1956. [Google Scholar]
- Oppenheimer JH. Thyroid hormone action at the cellular level. Science. 1979;203:971–979. doi: 10.1126/science.218285. [DOI] [PubMed] [Google Scholar]
- Parkison C, Ashizawa K, McPhie P, Lin KH, Cheng SY. The monomer of pyruvate kinase, subtype M1, is both a kinase and a cytosolic thyroid hormone binding protein. Biochem Biophys Res Commun. 1991;179:668–674. doi: 10.1016/0006-291x(91)91424-b. [DOI] [PubMed] [Google Scholar]
- Paul BD, Buchholz DR, Fu L, Shi Y-B. Tissue- and gene-specific recruitment of steroid receptor coactivator-3 by thyroid hormone receptor during development. J. Biol. Chem. 2005a;280:27165–27172. doi: 10.1074/jbc.M503999200. [DOI] [PubMed] [Google Scholar]
- Paul BD, Fu L, Buchholz DR, Shi Y-B. Coactivator recruitment is essential for liganded thyroid hormone receptor to initiate amphibian metamorphosis. Mol. Cell. Biol. 2005b;25:5712–5724. doi: 10.1128/MCB.25.13.5712-5724.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman AJ, Stanley F, Samuels HH. Thyroid hormone nuclear receptor. Evidence for multimeric organization in chromatin. J Biol Chem. 1982;257:930–938. [PubMed] [Google Scholar]
- Puzianowsak-Kuznicka M, Wong J, Kanamori A, Shi Y-B. Functional characterization of a mutant thyroid hormone receptor in Xenopus laevis. J. Biol. Chem. 1996;271:33394–33403. doi: 10.1074/jbc.271.52.33394. [DOI] [PubMed] [Google Scholar]
- Puzianowska-Kuznicka M, Damjanovski S, Shi Y-B. Both thyroid Hormone and 9-cis Retinoic Acid receptors are Required to Efficiently mediate the Effects of Thyroid Hormone on Embryonic Development and Specific Gene Regulation in Xenopus laevis. Mol. And Cell Biol. 1997;17:4738–4749. doi: 10.1128/mcb.17.8.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachez C, Freedman LP. Mechanisms of gene regulation by vitamin D(3) receptor: a network of coactivator interactions. Gene. 2000;246:9–21. doi: 10.1016/s0378-1119(00)00052-4. [DOI] [PubMed] [Google Scholar]
- Rachez C, Freedman LP. Mediator complexes and transcription. Curr Opin Cell Biol. 2001;13:274–280. doi: 10.1016/s0955-0674(00)00209-x. [DOI] [PubMed] [Google Scholar]
- Ranjan M, Wong J, Shi YB. Transcriptional repression of Xenopus TR beta gene is mediated by a thyroid hormone response element located near the start site. J Biol Chem. 1994;269:24699–24705. [PubMed] [Google Scholar]
- Sachs LM, Damjanovski S, Jones PL, Li Q, Amano T, Ueda S, Shi YB, Ishizuya-Oka A. Dual functions of thyroid hormone receptors during Xenopus development. Comp Biochem Physiol B Biochem Mol Biol. 2000;126:199–211. doi: 10.1016/s0305-0491(00)00198-x. [DOI] [PubMed] [Google Scholar]
- Sachs LM, Jones PL, Havis E, Rouse N, Demeneix BA, Shi Y-B. N-CoR recruitment by unliganded thyroid hormone receptor in gene repression during Xenopus laevis development. Mol. Cell. Biol. 2002;22:8527–8538. doi: 10.1128/MCB.22.24.8527-8538.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs LM, Shi Y-B. Targeted chromatin binding and histone acetylation in vivo by thyroid hormone receptor during amphibian development. PNAS. 2000;97:13138–13143. doi: 10.1073/pnas.260141297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sap J, Munoz A, Damm K, Goldberg Y, Ghysdael J, Leutz A, Beug H, Vennstrom B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986;324:635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Sato Y, Buchholz DR, Paul BD, Shi Y-B. A role of unliganded thyroid hormone receptor in postembryonic development in Xenopus laevis. Mechanisms of Development. 2007;124:476–488. doi: 10.1016/j.mod.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Ding A, Heimeier RA, Yousef AF, Mymryk JS, Walfish PG, Shi Y-B. The adenoviral E1A protein displaces corepressors and relieves gene repression by unliganded thyroid hormone receptors in vivo. Cell Res. 2009;19:783–792. doi: 10.1038/cr.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber AM, Brown DD. Tadpole skin dies autonomously in response to thyroid hormone at metamorphosis. P NATL ACAD SCI USA. 2003;100:1769–1774. doi: 10.1073/pnas.252774999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber AM, Das B, Huang H, Marsh-Armstrong N, Brown DD. Diverse developmental programs of Xenopus laevis metamorphosis are inhibited by a dominant negative thyroid hormone receptor. PNAS. 2001;98:10739–10744. doi: 10.1073/pnas.191361698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber AM, Mukhi S, Brown DD. Cell-cell interactions during remodeling of the intestine at metamorphosis in Xenopus laevis. Dev Biol. 2009;331:89–98. doi: 10.1016/j.ydbio.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard HM, Harries JC, Hussain S, Bevan C, Heery DM. Analysis of the steroid receptor coactivator 1 (SRC1)-CREB binding protein interaction interface and its importance for the function of SRC1. Mol Cell Biol. 2001;21:39–50. doi: 10.1128/MCB.21.1.39-50.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y-B. Amphibian Metamorphosis: From morphology to molecular biology. New York: John Wiley & Sons, Inc.; 1999. [Google Scholar]
- Shi Y-B, Liang VC-T, Parkison C, Cheng S-Y. Tissue-dependent developmental expression of a cytosolic thyroid hormone protein gene in Xenopus: its role in the regulation of amphibian metamorphosis. FEBS Letters. 1994;355:61–64. doi: 10.1016/0014-5793(94)01173-7. [DOI] [PubMed] [Google Scholar]
- Shi Y-B, Ritchie J, Taylor PM. Complex regulation of thyroid hormone action: Multiple opportunities for pharmacological intervention. Pharmacology & Therapeutics. 2002;94:235–251. doi: 10.1016/s0163-7258(02)00219-x. [DOI] [PubMed] [Google Scholar]
- Shi Y-B, Wong J, Puzianowska-Kuznicka M, Stolow M. Tadpole competence and tissue-specific temporal regulation of amphibian metamorphosis: roles of thyroid hormone and its receptors. BioEssays. 1996;18:391–399. doi: 10.1002/bies.950180509. [DOI] [PubMed] [Google Scholar]
- Shi Y-B, Yaoita Y, Brown DD. Genomic organization and alternative promoter usage of the two thyroid hormone receptor β genes in Xenopus laevis. J. Biol. Chem. 1992;267:733–788. [PubMed] [Google Scholar]
- Storey NM, Gentile S, Ullah H, Russo A, Muessel M, Erxleben C, Armstrong DL. Rapid signaling at the plasma membrane by a nuclear receptor for thyroid hormone. Proc Natl Acad Sci U S A. 2006;103:5197–5201. doi: 10.1073/pnas.0600089103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata JR. Gene expression during metamorphosis: an ideal model for post-embryonic development. Bioessays. 1993;15:239–248. doi: 10.1002/bies.950150404. [DOI] [PubMed] [Google Scholar]
- Tomita A, Buchholz DR, Shi Y-B. Recruitment of N-CoR/SMRT-TBLR1 corepressor complex by unliganded thyroid hormone receptor for gene repression during frog development. Mol. Cell. Biol. 2004;24:3337–3346. doi: 10.1128/MCB.24.8.3337-3346.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MJ, O'Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Ann Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Wahlstrom GM, Vennstrom B, Bolin MB. The adenovirus E1A protein is a potent coactivator for thyroid hormone receptors. Mol Endocrinol. 1999;13:1119–1129. doi: 10.1210/mend.13.7.0316. [DOI] [PubMed] [Google Scholar]
- Wang X, Matsuda H, Shi Y-B. Developmental regulation and function of thyroid hormone receptors and 9-cis retinoic acid receptors during Xenopus tropicalis metamorphosis. Endocrinology. 2008;149:5610–5618. doi: 10.1210/en.2008-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger C, Thompson CC, Ong ES, Lebo R, Gruol DJ, Evans RM. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986;324:641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- Wong J, Shi Y-B. Coordinated regulation of and transcriptional activation by Xenopus thyroid hormone and retinoid X receptors. J Biol Chem. 1995;270:18479–18483. doi: 10.1074/jbc.270.31.18479. [DOI] [PubMed] [Google Scholar]
- Wong J, Shi YB, Wolffe AP. A role for nucleosome assembly in both silencing and activation of the Xenopus TR beta A gene by the thyroid hormone receptor. Genes Dev. 1995;9:2696–2711. doi: 10.1101/gad.9.21.2696. [DOI] [PubMed] [Google Scholar]
- Xu L, Glass CK, Rosenfeld MG. Coactivator and corepressor complexes in nuclear receptor function. Current Opinion in Genetics & Development. 1999;9:140–147. doi: 10.1016/S0959-437X(99)80021-5. [DOI] [PubMed] [Google Scholar]
- Yaoita Y, Brown DD. A correlation of thyroid hormone receptor gene expression with amphibian metamorphosis. Genes Dev. 1990;4:1917–1924. doi: 10.1101/gad.4.11.1917. [DOI] [PubMed] [Google Scholar]
- Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81:1097–1142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- Yen PM, Chin WW. New advances in understanding the molecular mechanisms of thyroid hormone action. Trends Endocrinol.Metab. 1994;5:65–72. doi: 10.1016/1043-2760(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Zhang J, Lazar MA. The mechanism of action of thyroid hormones. Annu. Rev. Physiol. 2000;62:439–466. doi: 10.1146/annurev.physiol.62.1.439. [DOI] [PubMed] [Google Scholar]