Abstract
Peripheral activation of the immune system by infectious agents triggers the brain-cytokine system causing sickness behaviors which profoundly impact well-being. Dietary fiber is a beneficial foodstuff that, from a gastrointestinal tract perspective, exists in both insoluble and soluble forms. We show that a diet rich in soluble fiber protects mice from endotoxin-induced sickness behavior by polarizing mice Th2 when compared to a diet containing only insoluble fiber. Mice fed soluble fiber became less sick and recovered faster from endotoxin-induced sickness behaviors than mice fed insoluble fiber. In response to intraperitoneal endotoxin, mice fed soluble fiber had up-regulated IL-1RA and reduced IL-1βand TNF-αin the brain as compared to mice fed insoluble fiber. Importantly, mice fed soluble fiber had a basal increase in IL-4 in the ileum and spleen which was absent in MyD88 knockout mice. Con A stimulated splenocytes from mice fed soluble fiber showed increased IL-4 and IL-5 and decreased IL-2, IL-12 and IFN-γwhen compared to mice fed insoluble fiber. Likewise, endotoxin-stimulated macrophages from mice fed soluble fiber demonstrated decreased IL-1β, TNF-α, IFN-γ, IL-12 and nitrate and increased IL-1RA, arginase 1 and Ym1 when compared to mice fed insoluble fiber. Finally, the behavioral protection afforded by feeding mice soluble fiber was reduced in IL-4 knockout mice, as was the impact of soluble fiber on Con A stimulated splenocytes and endotoxin activated macrophages. These data show that a diet rich in soluble fiber protects against endotoxin-induced sickness behavior by polarizing mice Th2 and promoting alternative activation of macrophages.
Keywords: Sickness behavior, cytokines, dietary fiber, Th1/Th2, IL-4, macrophage alternative activation
Introduction
A mechanism for inflammation-mediated sickness has recently been elucidated anchored by a new paradigm: the brain cytokine system, which can diffusely impact neuronal circuits and neurotransmitters that organize physiologic and pathologic behavior (Dantzer et al., 2008). A critical activator of this system is acute systemic inflammation often triggered by serious infections, such as sepsis. While some progress has been achieved in mitigating mortality and physical morbidity associated with serious infection, less success has been realized in the treatment of consequent behavioral sequela (Streck et al., 2008). Therefore, an important challenge in understanding neuroimmunity is whether activation of the neuroimmune system can be modulated so as to abrogate or ameliorate the development of neuroinflammation and its biobehavioral consequences.
Dietary fiber has gained significant recent attention and is now touted as “an essential part of a healthy diet” (Roediger, 1980). Currently, almost nothing is known about dietary fiber and its impact on neuroimmunity. In general, fiber can be divided into two types: insoluble, which remains undigested and unfermented in the gastrointestinal (GI) tract; and soluble fiber, which is resistant to digestion but fermentable (Groff and Gropper, 1999). Fermentation of soluble fiber by GI bacteria (primarily in the ileum/colon) generates short-chain fatty acids (SCFAs) (Scott, Duncan and Flint, 2008). These SCFAs are two- to five-carbon weak acids with butyrate appearing to have the greatest potential role in immunity due to its recently described palliative effect in inflammatory bowel diseases (Torres and Rios, 2008, Rose et al., 2007). Butyrate is a well recognized histone deacetylase inhibitor (Sealy and Chalkley, 1978) and transcription of certain cytokines appears reliant on acetylation of histones associated with their promoters (Bowen et al., 2008). Recently, Cox et al. have shown that monocytes treated ex vivo with SCFAs down-regulate MCP-1 and IL-10 (Cox et al, 2009) and Cavaglieri et al. reported that, after direct SCFA administration to cultured lymphocytes, IL-4 was unchanged (Cavaglieri et al., 2003). However, it is not clear if ex vivo stimulation of immune cells with either fiber or its fermentation products actually reflect what occurs in vivo. Notably, soluble fibers are dramatically altered in the GI tract by fermentation and these fermentation products can significantly change the intestinal microbiota (Scott, Duncan and Flint, 2008). Soluble fiber, for instance, has been shown to modify progression of immune-mediated type 1 diabetes in non-obese diabetic (NOD) mice (Wen et al., 2008). These findings suggest that the systemic innate immune system can vary with diet.
One way that diet could regulate the innate immune system is by changing T-helper (Th) cell polarization and impacting Th 1/2 determining cytokines. Critical to the Th2 phenotype, IL-4 is a potent anti-inflammatory cytokine whose expression is reliant on histone acetylation (Ansel et al., 2006). IL-4 is mainly secreted by CD4+ T-cells and is a cytokine necessary to the development of the Th-2 phenotype (Kindt, Goldsby and Osborne, 2007, Gilmour and Lavender, 2008). Importantly, Th2 cells are best suited for neutralization of soluble bacterial toxins, such as endotoxin, (Kindt, Goldsby and Osborne, 2007) and produce IL-5 and IL-13 in addition to IL-4 (Fowell, 2009). IL-4 also appears to directly repress Th1 activation via suppression of IFN-γ (Fowell, 2009). This is important because IFN-γis a defining cytokine of the Th1 phenotype and an inducer of classically activated macrophages. Classical activation is supported by IFN-γand classically activated macrophages prototypically elaborate IL-1β, IL-6 and TNF-α(Gordon, 2003, Martinez, Helming and Gordon, 2009). Furthermore, IL-1βand TNF-αare key mediators of the sickness behaviors associated with activation of the neuroimmune system (Dantzer, 2006). Finally, IL-4 drives alternative activation of macrophages (Martinez, Helming and Gordon, 2009). These macrophages are phenotypically distinct from classically activated macrophages and from macrophages deactivated by IL-10 (Gordon and Taylor, 2005, Varin and Gordon, 2009). Relevant to sickness behavior and neuroimmune system activation, alternatively activated macrophages have reduced responsivity to endotoxin (Re et al., 1994) and elaborate IL-1RA. IL-1RA directly antagonizes IL-1 in both the periphery and brain (Arend, Palmer and Gabay, 2008) and, as we have shown, can speed sickness recovery (Johnson et al. 2005, Johnson et al. 2007). Therefore, the hypothesis to be tested was whether sickness behavior induced by endotoxin can be reduced by the dietary soluble fiber, pectin when compared to the insoluble fiber, cellulose. To probe this posit, C56BL/6J mice were fed defined diets containing either cellulose or pectin, as the sole fiber source. Mice were then challenged with LPS to determine their sensitivity to innate immune challenge. Characterization of the immunological response included behavioral testing, T-cell profiling and macrophage activation analysis. MyD88 and IL-4 KO mice were utilized to determine how fiber regulated innate immunity.
Methods
Materials
All reagents and chemicals were purchased from Sigma-Aldrich except, FCS (0.05 ng/ml, 0.48 units/ml endotoxin), Atlanta Biologicals. Bio-Rad Protein Assay (500-0006), Bio-Rad. IL-1RA (MRA00) and IL-4 (M400B) ELISA, R&D Systems. Nylon Mesh (CMN-0210-D), Small Parts, Inc. Griess Reagent (G2930), Promega. High-Capacity cDNA Reverse Transcription Kit (PN 4374966), Applied Biosystems. Cytokine panels for BioPlex Multiplexing Platform, Bio-Rad.
Animals
Animal use was conducted in accordance with the Guide for the Care and Use of Laboratory Animals at the University of Illinois and as we have previously described (Johnson et al., 2007). C56BL/6J, Il4tm1Nnt (IL-4 knockout), and myeloid differentiation factor 88 (MyD88) knockout male mice were bred in house. All knockout mice were bred on a C57BL/6J background. Mice were housed in standard shoebox cages and allowed water and food ad libitum. Housing temperature (72°F) and humidity (45-55%) were controlled as was a 12/12-h reversed dark-light cycle (2000 - 0800). Where required, mice were sacrificed by CO2 asphyxiation.
Diets
Chow was closed source NIH 5K52 LabDiet (Purina Mills). This diet, as described by the manufacturer, contains 15% dietary fiber of non-specific source and fermentability containing approximately 5% semi-fermentable fiber (hemi-cellulose) and 10% non-fermentable fiber (cellulose). Experimental diets were open source uniform-base diets containing a single source of dietary fiber, either soluble fiber (pectin) or insoluble fiber (cellulose). These single fiber diets contained either 5% cellulose (D12450B, Research Diets), 10% cellulose (D06082201, Research Diets), or 10% pectin (D06082202, Research Diets). Diets were fed to mice for 6-8 wks immediately post weaning. All diets were comparable in macronutrient composition (Table 1).
Table 1.
Macronutrient composition of diets
| Protein (% kcal) |
Fat (% kcal) |
Carbohydrate (% kcal) |
Fiber (% weight) |
kcal/gm | |
|---|---|---|---|---|---|
| Chow | 22 | 16 | 61 |
15% NDF
*
5% ADF # |
3.47 |
| 5% Cellulose | 20 | 10 | 70 | 5% Cellulose | 3.85 |
| 10% Cellulose | 20 | 10 | 70 | 10% Cellulose | 3.56 |
| 10% Pectin | 20 | 10 | 70 | 10% Pectin | 3.67 |
NDF = Neutral detergent fibers cellulose, hemi-cellulose, and lignin.
ADF = Acid detergent fibers cellulose and lignin
Sickness behavior
Social withdrawal, fever, food intake, and weight loss were used to measure endotoxin-induced sickness behavior, as we have previously described (Sherry, Kim and Freund, 2009, Sherry et al., 2009). To examine social withdrawal, mice were injected IP with 100 μg/kg lipopolysaccharide (LPS) (Escherichia coli, O127:B8) 250 L or 250 μL of saline. At the times indicated, a novel 3- to 4-wk old conspecific juvenile (challenge) mouse, enclosed in a 3 × 3 inch wire mesh cage, was placed in the home cage of the adult (test) mouse for 5 minutes. Duration of test mouse-initiated exploratory behavior of the challenge mouse was determined from the video records. To control for mouse-to-mouse variability in baseline social exploration and allow comparison of relative changes in social exploration levels, the pre-LPS exposure (0 h) measurement was used as an internal control for each mouse. Four independent studies were preformed to measure social exploratory behavior. Results are expressed as a percentage of the baseline measurement and shown as means ± SEM. In an independent study to measure fever, mice were implanted IP with calibrated temperature-sensitive XM-FH mouse-specific transmitters (Mini Mitter). In brief, transmitters were placed in mice anesthetized with a sodium ketamine hydrochloride/xylazine hydrochloride solution (80 mg/ml:12mg/ml, ketamine:xylazine) (1.5 ml/kg, IP). Mice were allowed to recover 1 wk and then administered LPS as above. Temperature was monitored pre-LPS administration (basal temperature) and every 30 min post-LPS for 22 h. Results are expressed as change from basal temperature ± SEM. In an independent study to measure food intake and weight loss, mice were injected IP with LPS as above. Food intake and body weight were measured pre-LPS and 24 h post LPS. Results are expressed as a change from pre-LPS ± SEM.
Short Chain Fatty Acid (SCFA) Analysis
Cecal contents were collected from sacrificed mice into 2N HCL (500 mg of cecal material/ml HCL). Samples were mixed (1:0.5) with 250 g/l metaphosphoric acid and allowed to sit at room temperature for 30 min. Samples were then centrifuged at 20,100 g for 20 min and supernatants collected and stored at −20 °C for subsequent analysis. Immediately prior to analysis, samples were thawed and centrifuged at 13,000 g for 10 min. Acetate, propionate and butyrate were determined in the supernatant by gas-liquid chromatography (Hewlett-Packard 5890A Series II) using a 180 cm × 64 mm internal diameter glass column packed with 10% SP-1200/1% H3PO4 on 80/100 mesh Chromosorb WAW (Supelco Inc.) as we have previously described (Kuzmuk et al., 2005).
Cytokine measurements
All cytokine measurements were performed using a Bio-Rad BioPlex Multiplexing Platform with a custom cytokine panel comprised of the cytokines indicated. Cytokine measurements were conducted following the manufactures’ instructions.IL-1RA was measured by ELISA as we have previously described (Sherry, Kim and Freund, 2009, Sherry et al., 2009). In brief, for blood cytokines, cytokines were measured in serum derived from the inferior vena cava. For tissue cytokines, 75 mg of spleen, ileum, cecum, colon or brain X-were collected from sacrificed mice, into 500 μl of ice-cold homogenization buffer (1% Triton X-100, 100 mM NaCl, 50 mM NaF, 1 mM DTT, 25 mM benzamidine, 1 mM PMSF, 1:1000 Protease Inhibitor Cocktail Set III (Calbiochem #539134), 2 mM sodium orthovanadate and 250 nM okadaic acid, 50 mM Tris, pH 7.4). Tissues were ground with a tissue tearor (BioSpec Productions) and the homogenates centrifuged at 10,000 g (4°C) for 10 min. Cytokine levels were measured in the clarified lysates and normalized to total tissue protein as measured by Bio-Rad Protein Assays. For macrophage and splenocyte generated cytokines, cytokine production was measured in supernatants and normalized to total cellular protein as measured by Bio-Rad Protein assay.
Real-time PCR
RNA isolation and real-time PCR was performed as we have previously described (Sherry et al., 2007). In brief, TaqMan Gene Expression primer for IL-1β (Mm0043228_m1), IL-1RA (Mm00446185_m1), TNF-α(Mm00443258_m1 ), IL-6 (Mm00446190_m1 ), IL-4 (Mm00445259_m1), Arg1 (Mm01190441_g1), and Ym1 (Mm00657889_mH) were used in real-time RT-PCR performed on a 7900 HT Fast Real-Time PCR System (Applied Biosystems) using TaqMan Universal PCR Master Mix. To normalize gene expression, a parallel amplification of endogenous GAPDH (Mm999999615_g1) was performed with TaqMan Gene Expression primer. Reactions with no reverse transcription and no template were included as negative controls. Relative quantitative evaluation of target gene levels was performed by comparing ΔCts, where Ct is the threshold concentration.
Splenocyte isolation and activation
Spleens from sacrificed mice were finely minced in growth media as described by Kruisbeek (Kruisbeek, 2000). Resulting tissue clumps were dispersed by passage (3 times) through a 19-G needle with the last expulsion through a 200 μm nylon mesh screen. Cells were then pelleted and resuspended in 10 ml of hypertonic RBC lysis (142 mM NaCl, 1 mM KHCO3 and 118 mM NaEDTA, pH 7.4) buffer at room temperature for 5 min then mixed 1:1 with growth medium (RPMI 1640 supplemented with 10% FCS, 2 g/l sodium bicarbonate, 110 mg/l sodium pyruvate, 62.1 mg/l penicillin, 100 mg/l streptomycin, and 10 mM HEPES, pH 7.4) and re-pelleted and resuspended at 37°C in growth media. Cells were then plated on plastic for 1 h to remove adherent cells. The resultant supernatant splenocytes were pelleted and resuspended at 1 × 106 cells/ml in growth media. For in vitro activation experiments, splenocytes were treated with 5 μg/ml of Con A as described by Mills et al. (Mills et al., 2000). After 24 h, splenocytes were pelleted and the surpernatant used for cytokine measurements and the cell pellet used for protein determination and real-time PCR.
Peritoneal macrophage isolation and activation
As we have previously described (Sherry et al., 2007), peritoneal cells were collected from sacrificed mice by peritoneal lavage using 10 ml of ice-cold growth medium. Lavage cells were pelleted and resuspended in 10 ml of RBC lysis buffer at room temperature for 5 min then mixed 1:1 with growth medium and re-pelleted and resuspended at 37°C in growth media. Cells were plated on plastic at 0.5 × 106 cells/ml and after 1 h plates were washed twice to remove non-adherent cells, resulting in >80% macrophages as confirmed by CD11b staining and morphology. Cells were incubated in fresh growth media cultured at 37°C in a 5% CO2 environment. For in vitro activation experiments measuring cytokines, peritoneal macrophages were treated with 10 ng/ml LPS (Escherichia coli, O127:B8) or carrier (PBS) for 2 h. For in vitro experiments measuring nitric oxide production, peritoneal macrophages were treated with 100 ng/ml LPS or carrier (PBS) for 24 h. Nitrate was then measured in the supernatant by the Griess reagent method as previously described (Mills et al., 2000). After, the indicated times, surpernatant was used for cytokine measurements and the adherent cells used for protein determination and real-time PCR.
Statistical analysis
Data are presented as mean ± SEM. The experimental design for behavioral experiments was a completely randomized design, with a 2 × 2 factorial arrangement of treatments (2 levels of pretreatment and 2 levels of treatment). All data were analyzed using SAS (Cary, NC) Inst PROC MIXED procedures of SAS. The statistical model for social withdrawal included the effects of diet/phenotype × LPS × time. Post-hoc comparisons of individual group means were performed with the Tukey’s test. Where indicated, experimental data were analyzed by ANOVA using SAS. Statistical significance was denoted at p<0.05.
Results
Soluble fiber protects against endotoxin-induced sickness behavior
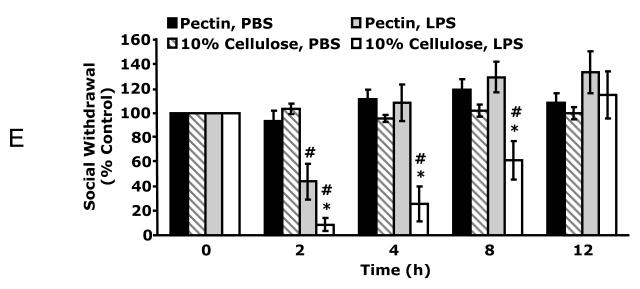
Defined diets containing 10% fat + 5% cellulose (cellulose) are used extensively in mice as control diets for the study of diet-induced obesity (El-Haschimi et al., 2000). Fig. 1A demonstrates that mice fed cellulose were 32% more withdrawn and recovered 50% slower from LPS-induced social withdrawal than mice fed commercial mouse chow (chow). At 2 h and 4 h post intraperitoneal (IP) LPS administration, mice fed chow were 55.9 ± 8.2% and 13.6 ± 14.9% withdrawn, respectively, compared to mice fed a cellulose diet, which were 87.4 ± 7.5% and 71.5 ± 9.6% withdrawn at the same time points. Three-way ANOVA (LPS × diet × time) of social withdrawal revealed significant LPS × diet × time [F (12,212) = 1.96, p < 0.05], LPS × time [F (4,212) = 23.65, p < 0.001], and diet × time [F (12,212) = 2.34, p < 0.005] interactions. To determine which ingredient in chow was most likely responsible for the observed endotoxin resistance, defined diets were formulated that were identical in base composition to the cellulose diet but contained either 5% cellulose or 10% pectin (pectin). Fig. 1B shows that mice fed pectin got 32% less sick and recovered 50% faster from LPS-induced social withdrawal than mice fed cellulose. At 2 h post IP LPS administration, mice fed pectin were 55.8 ± 14.7% withdrawn, respectively, compared to mice fed cellulose which were 87.4 ± 7.5% withdrawn. Moreover, mice fed cellulose were still 71.5 ± 11.7% withdrawn at 4 h post LPS as compared to mice fed pectin, which were fully recovered. To observe this effect, mice were required to be on diet for at least 5 wks (data not shown). Three-way ANOVA (LPS × diet × time) of social withdrawal revealed significant LPS × diet × time [F (12,212) = 1.96, p < 0.05], LPS × time [F (4,212) = 23.65, p < 0.001], and diet × time [F (12,212) = 2.34, p < 0.005] interactions. To further examine the impact of pectin on sickness behavior; fever, weight loss and food intake were measured. Fig 1C demonstrates that post IP LPS mice fed pectin recovered 44% faster from fever (11.5 h vs. 8 h) as compared to mice fed cellulose. Two-way ANOVA (diet × time) of fever revealed significant time effect [F (46,368) = 1.85, p < 0.005]. Fig. 1D shows that at 24 h post LPS, mice fed pectin lost 40% less weight (1.5 ± 0.16 vs 0.9 ± 0.13g, F (1,10) = 5.1, p < 0.05) and had a 229% increase in food intake (0.37 ± 0.09 vs 1.22 ± 0.18g, F (1,10) = 24.5, p < 0.0005) as compared to mice fed cellulose. To determine if the difference in LPS responsiveness was due to a deficiency in dietary fiber between the cellulose and pectin diets, mice were fed diets containing 10% pectin and 10% cellulose. Fig. 1E demonstrates that mice fed 10% cellulose were 35% more withdrawn and recovered 66% slower from LPS-induced social withdrawal than mice fed pectin. At 2 h, 4 h and 8 h post IP LPS, mice fed 10% cellulose were 91.1 ± 5.3%, 74.2 ± 14.4% and 38.8 ± 15.7% withdrawn, respectively, compared to mice fed pectin, which were 55.7 ± 14.7% withdrawn at 2 h post LPS and fully recovered at 4 and 8 h post LPS. Three-way ANOVA (LPS × diet × time) of social withdrawal revealed significant LPS × diet × time [F (12,212) = 1.96, p < 0.05], LPS × time [F (4,212) = 23.65, p < 0.001], and diet × time [F (12,212) = 2.34, p < 0.005] interactions. Taken together these finding indicate that a diet rich in soluble fiber affords protection from endotoxin-induced sickness behavior.
Fig. 1. Soluble fiber protects against endotoxemia.
A, Chow and 5% cellulose (cellulose) fed mice were administered carrier (PBS) or LPS IP and social withdrawal measured prior to LPS (0 h) and at 2, 4, 8 and 12 h post LPS. Results are expressed as percentage change from the 0 h measurement, means ± SEM; n = 8; *p<0.05, main effect of diet, #p<0.05, treatment effect of LPS. B, 10% pectin (pectin) and cellulose fed mice were administered LPS IP and social withdrawal measured prior to LPS (0 h) and at 2, 4, 8 and 12 h post LPS. Results are expressed as percentage change from the 0 h measurement, means ± SEM; n = 8; *p<0.05, main effect of diet, #p<0.05, treatment effect of LPS. C, Pectin and cellulose fed mice were administered LPS IP (0 h) and temperature measured. Results are expressed as a change from pre-LPS (0 h) measurement, means ± SEM; n = 4; arrowp<0.05, main effect of time. D, Pectin and cellulose fed mice were administered LPS IP and food intake and body weight measured 24 h post LPS. Results are expressed as percentage change from the pre-LPS measurement, means ± SEM; n = 4; *p<0.05, main effect of diet. E, Pectin and 10% cellulose fed mice were administered LPS IP and social withdrawal measured prior to LPS (0 h) and at 2, 4, 8 and 12 h post LPS. Results are expressed as percentage change from the 0 h measurement, means ± SEM; n = 8; *p<0.05, main effect of diet, #p<0.05, treatment effect of LPS
Soluble fiber up-regulates IL-1RA
IL-1RA is important in counter-regulating sickness symptoms induced by endotoxin (O’Connor et al., 2005). Fig. 2A demonstrates that mice fed pectin when compared to mice fed cellulose had a 2.5-fold (589.13 ± 146.63 vs 1501.48 ± 316.00 ΔmRNA, p < 0.05) increase in brain-based IL-1RA mRNA expression in response to IP LPS. In addition, LPS-induced IL-1βand TNF-αexpression was reduced 1.8-fold (32.83 ± 5.27 vs 18.31 ± 1.87 ΔmRNA, p < 0.05) and 1.7-fold (34.91 ± 5.19 vs 21.11 ± 3.17 ΔmRNA, p < 0.05), respectively, in brains of mice fed pectin compared to mice fed cellulose (Fig. 2B). IL-6 expression was not impacted by diet (19.7 ± 3.9 v 18.4 ± 1.9 ΔmRNA) (Fig. 2B). Taken together these finding indicate that soluble fiber results in dampened brain-based pro-inflammation and enhanced anti-inflammation during endotoxin administration.
Fig. 2. Soluble fiber up-regulates IL-1RA.
10% pectin (pectin) and 5% cellulose (cellulose) fed mice were administered carrier (PBS) or LPS IP. At 2 h post LPS, real-time RT-PCR was used to quantify IL-1RA (A), IL-1β(B), TNF-α(B) and IL-6 (B) mRNAs from brain. Results are expressed as relative change in mRNA expression ( mRNA), means ± SEM; n = 6-8; *p<0.05 main effect of diet, #p<0.05 treatment effect of LPS.
IL-4 production is augmented by soluble fiber
We (O’Connor et al., 2007) and others (Mosser, 2003) have shown that elaboration of IL-1RA by macrophages is increased by IL-4. Fig 3A demonstrates that mice fed pectin had a 3.4-fold (1.0 ± 0.1 vs 3.5 ± 0.9 ΔmRNA, p < 0.01), 2.1-fold (1.2 ± 0.4 vs 2.4 ± 0.2 ΔmRNA, p < 0.005), 92.4-fold (0.7 ± 0.2 vs 62.6 ± 27.4 ΔmRNA, p < 0.005), 12.6-fold (1.3 ± 0.5 vs 16.4 ± 10.4 ΔmRNA, p < 0.05), and 4.4-fold (1.4 ± 0.7 vs 6.1 ± 1.9 ΔmRNA, p < 0.005) increase in basal IL-4 message in the brain, spleen, ileum, cecum, and colon, respectively, as compared to mice fed cellulose. Liver, white adipose tissue, duodenum and jejunum showed no significant difference in IL-4 mRNA expression (1.1 ± 0.4 vs 0.8 ± 0.5, 1.3 ± 0.6 vs. 0.7 ± 0.3, 1.4 ± 0.8 vs. 1.5 ± 0.2, 1.3 ± 0.6 vs 14.9 ± 7.2 ΔmRNA, pectin vs cellulose, p = ns, liver, white adipose tissue (WAT), duodenum, and jejunum, respectively). IL-4 protein expression was also examined and Fig. 3B shows that pectin fed mice had a 2-fold (1.6 ± 0.4 vs 3.1 ± 0.6 pg/mg protein, p < 0.05) and 3-fold (2.4 ± 1.2 vs 7.7 ± 2.3 pg/mg protein, p < 0.05) increase in spleen and ileum IL-4, respectively, as compared to mice fed cellulose. Brain and cecum showed no pectin-dependent increase in IL-4 protein (1.9 ± 0.5 vs 2.9 ± 1.9 pg/mg protein cecal IL-4, pectin vs cellulose). Fig.3C demonstrates that MyD88 KO mice fail to up-regulate splenic and ileal IL-4 message when compared to wild type mice fed pectin (5.82 ± 1.56 vs 0.87 ± 0.22, mRNA, p < 0.05 and 54.20 ± 10.22 vs 0.36 ± 0.21 mRNA, p < 0.005, pectin fed C57BL/6J vs MyD88 KO, spleen and ileum, respectively). Taken together these findings indicate that soluble fiber augments IL-4 expression in a manner dependent on MyD88.
Fig. 3. IL-4 production is augmented by soluble fiber.
Mice were fed 10% pectin (pectin) or 5% cellulose (cellulose) for 6 wks. A, Real-time RT-PCR was used to quantify IL-4 mRNAs from the indicated tissues. Results are expressed as relative change in mRNA expression (ΔmRNA), means ± SEM; n = 6-8; *p<0.05 main effect of diet. B, ELISA was used to quantify IL-4 from the indicated tissues. Results are expressed as means ± SEM; n = 5-7; *p<0.05 main effect of diet. C, Wild type (WT) and MyD88 KO mice were fed 10% pectin (pectin) or 5% cellulose (cellulose) for 6 wks. Real-time RT-PCR was used to quantify IL-4 mRNAs from the indicated tissues. Results are expressed as relative change in mRNA expression (ΔmRNA), means ± SEM; n = 3; *p<0.05 main effect of diet.
Soluble fiber polarizes mice towards a Th2 phenotype
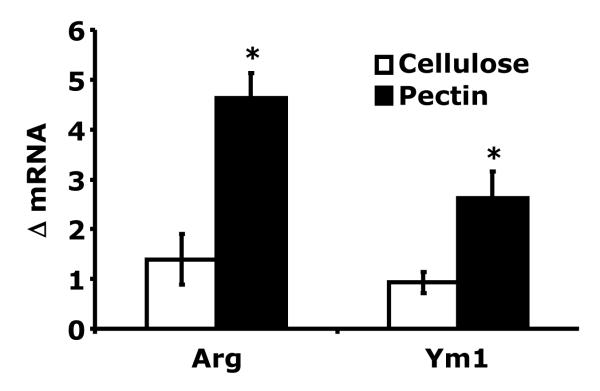
Alternatively activated macrophages result from Th2 polarization (Martinez, Helming and Gordon, 2009). Table 2 demonstrates that isolated peritoneal macrophages from mice fed pectin had a dampened pro-inflammatory response to LPS when compared to mice fed cellulose. Macrophages from mice fed pectin had a 228% and 188% increase in basal arginase 1 (1.4 ± 0.5 vs 4.6 ± 0.5 ΔmRNA Arg1, p < 0.001) and Ym1 (0.9 ± 0.2 vs 2.6 ± 0.5 ΔmRNA Ym1, p < 0.01) mRNA expression, respectively, compared to mice fed cellulose (Fig. 4). In addition, macrophages from mice fed chow had similar post LPS up-regulation of TNF-αand IL-1RA as mice fed pectin (6093 ± 294 vs 5919 ± 630 pg TNF-α/mg total protein, chow vs pectin: 1840 ± 451 vs 1337 ± 81 pg IL-1RA/mg total protein, chow vs pectin, p = ns). T cell production of IL-4 is a hallmark of the Th2 phenotype (Ansel et al., 2006). Table 3 shows that Con-A activated splenocytes from mice fed pectin as compared to mice fed cellulose, had a 188% and 387% increase in IL-4 and IL-5, and a 60%, 80% and 52% decrease in IL-2, IL-12 and IFN-γproduction, respectively. In addition, splenocytes from mice fed chow had similar post Con A up-regulation of IL-4 and IFN-γas mice fed pectin (127 ± 25 vs 103 ± 40 pg IL-4/mg total protein, chow vs pectin: 69 ± 5 vs 89 ± 84 pg IFN-γ/mg total protein, chow vs pectin, p = ns). Since IL-4 expression is enhanced by histone acetylation (Valapour et al., 2002) and a bi-product of pectin fermentation is the histone deacytelase inhibitor butyrate (Han et al., 2007), SCFAs in the gut lumen were examined. Table 4 demonstrates that a pectin containing diet increased cecal acetate, propionate, and butyrate: 3.1-fold, 3.3-fold and 3.5-fold, respectively. In-vitro butyrate treatment of splenocytes from mice fed a cellulose diet, however, did not show increased IL-4 production (data not shown). Taken together these findings indicate that mice fed pectin are polarized Th2 and have alternatively activated macrophages when compared to mice fed cellulose. Butyrate, however, does not appear sufficient to polarize splenocytes Th2.
Table 2.
Macrophage Activation Profile
| Cellulose (ng/mg protein) | Pectin (ng/mg protein) | |||
|---|---|---|---|---|
| PBS | LPS | PBS | LPS | |
| IL-1β | 0.93 ± 0.07 | 1.31 ± 0.05 # | 0.82 ± 0.03 | 0.94 ± 0.06 * |
| TNF-α | 11.53 ± 1.23 | 16.93 ± 0.58 # | 7.16 ± 0.25 * | 9.83 ± 0.41 * |
| IFN-γ | 0.39 ± 0.03 | 0.62 ± 0.007 # | 0.37 ± 0.006 | 0.41 ± 0.02 * |
| IL-12 | 0.64 ± 0.007 | 1.03 ± 0.08 # | 0.45 ± 0.03 * | 0.63 ± 0.05 * |
| IL-1RA | 0.02 ± 0.001 | 0.18 ± 0.05 # | 0.07 ± 0.02 | 0.59 ± 0.06 # * |
| Nitrite | N/D | 10.4 ± 2.5 # | N/D | N/D |
Mice fed 10% pectin (pectin) or 5% cellulose (cellulose) and peritoneal macrophages were isolated in treated in vitro with either LPS or PBS for 2 hrs and cytokines were measured in the supernant. Nitrite was measured by Greiss reagent method. Results are expressed as ng/mg protein, means ± SEM, n= 4
p<0.05 main effect of diet
p<0.05 treatment effect of LPS
Fig. 4. Arginase and Ym1 expression in peritoneal macrophages.
Mice were fed 10% pectin (pectin) or 5% cellulose (cellulose) for 6 wks. Real-time RT-PCR was used to quantify arginase (Arg) and Ym1 mRNAs from peritoneal macrophages. Results are expressed as relative change in mRNA expression (ΔmRNA), means ± SEM; n = 6-8; *p<0.05 main effect of diet.
Table 3.
Th1/Th2 Polarization in Splenocytes
| Cellulose (pg/mg protein) |
Pectin (pg/mg protein) |
|
|---|---|---|
| IL-4 | 43.5 ± 12.7 | 124.4 ± 17.7 * |
| IL-5 | 8.4 ± 1.6 | 48.7 ± 6.9 * |
| IL-13 | 12.7 ± 1.2 | 24.1 ± 4.6 |
| IFN-γ | 8298 ± 543 | 3979 ± 353 * |
| IL-12 | 48.8 ± 6.9 | 23.7 ± 2.5 * |
| IL-2 | 23605 ± 4117 | 13994 ± 3600 * |
Mice were fed 10% pectin (pectin) or 5% cellulose (cellulose) for 6 wks. Cytokines were measured from Con A treated splenocytes. Results are expressed in pg/mg protein, means ± SEM, n=4
p<0.05, main effect of diet.
Table 4.
Cecal Short Chain Fatty Acids
| Cellulose (μg/g) | Pectin (μg/g) | |
|---|---|---|
| Acetate | 4927 ± 264 | 14,994 ± 1238 * |
| Propionate | 887 ± 46 | 2920 ± 326 * |
| Butyrate | 1249 ± 180 | 4357 ± 411 * |
| Total | 7062 ± 398 | 22,271 ± 1834 * |
Mice were fed 10% pectin (pectin) or 5% cellulose (cellulose) for 6 wks. SCFAs were measured from cecum. Results are expressed on a wt/wt fecal sample basis, means ± SEM, n=4
p<0.05, main effect of diet.
IL-4 knockout (KO) blunts the ability of soluble fiber to down-regulate endotoxin-induced sickness behavior
Since alternatively activated macrophages are resistant to LPS (Gordon, 2003), we examined the impact of pectin on IL-4 KO mice. Fig. 5A shows that IL-4 KO mice fed pectin were 3-fold more withdrawn after LPS administration than wild type mice fed pectin (53.3 ± 2.2% vs 16.4 ± 3.8%) and were as withdrawn as wild type (2.9 ± 1.4%) and IL-4 KO (9.9 ± 3.5%) mice fed cellulose. Three-way ANOVA (LPS × diet × time) revealed significant LPS × diet × time [F (3,30) = 12.89, p < 0.001], and diet × time [F (3,30) = 32.32, p < 0.001] interactions. Tables 5 and 6 show that Con A stimulated splenocytes and LPS stimulated peritoneal macrophages from IL-4 KO mice fed cellulose and pectin had similar cytokine profiles indicative of Th1 polarization and classical macrophage activation. Fig. 5B demonstrates that peritoneal macrophages from IL-4 KO mice fed pectin had a 69% increase in arginase 1 (1.1 ± 0.2 vs 3.5 ± 0.3 ΔmRNA Arg1, p < 0.0001) and no significant increase in Ym1 (1.2 ± 0.4 vs 0.8 ± 0.2 ΔmRNA Ym1, p = ns) mRNA expression, confirming that these macrophages are not alternatively activated. Taken together these findings indicate that IL-4 KO blunts the impact of soluble fiber on endotoxin-induced social withdrawal by dampening Th2 polarization and alternative macrophage activation.
Fig. 5. IL-4 knockout (KO) blunts the impact of soluble fiber on endotoxemia resistance.
A, 10% pectin (pectin) and 5% cellulose (cellulose) fed wild type (WT) and IL-4 KO mice were administered carrier (PBS) or LPS IP and social withdrawal measured at 2 h post LPS. Results are expressed as percentage change from cellulose PBS WT mice, means ± SEM; n = 3-4; #p<0.05, treatment effect of LPS, *p<0.05 main effect of diet, $p<0.05 phenotype effect. B, IL-4 KO mice were fed 10% pectin (pectin) or 5% cellulose (cellulose) for 6 wks. Real-time RT-PCR was used to quantify arginase (Arg) and Ym1 mRNAs from peritoneal macrophages. Results are expressed as relative change in mRNA expression (ΔmRNA), means ± SEM; n = 3; *p<0.05 main effect of diet.
Table 5.
Th1/Th2 Polarization in Splenocytes from IL-4 KO Mice
| Cellulose (pg/mg protein) |
Pectin (pg/mg protein) |
|
|---|---|---|
| IL-4 | N/D | N/D |
| IL-5 | 30.1 ± 6.3 | 26.8 ± 9.0 |
| IL-13 | 133 ± 28 | 118 ± 12 |
| IFN-γ | 7.8 ± 3.3 | 4.5 ± 1.1 |
| IL-12 | 1703 ± 466 | 891 ± 145 |
| IL-2 | 22552 ± 4766 | 9212 ± 2067 * |
IL-4 KO mice were fed 10% pectin (pectin) or 5% cellulose (cellulose) for 6 wks. Cytokines were measured from Con A treated splenocytes. Results are expressed in pg/ml/protein, means ± SEM, n=4
p<0.05, main effect of diet.
Table 6.
Macrophage Activation Profile in IL-4 KO Mice
| Cellulose (pg/mg protein) | Pectin (pg/mg protein) | |||
|---|---|---|---|---|
| PBS | LPS | PBS | LPS | |
| IL-1β | 10.6 ± 1.2 | 35.3 ± 8.1 | 14.9 ± 1.2 | 41.1 ± 11.6 |
| TNF-α | 113.1 ± 12.7 | 2815 ± 286 | 134 ± 43 | 1600 ± 465 |
| IFN-γ | N/D | N/D | N/D | N/D |
| IL-12 | 6.8 ± 1.2 | 29.5 ± 4.5 | 8.9 ± 0.9 | 26.6 ± 1.6 |
| IL-1RA | 371 ± 84 | 952 ± 120 | 673 ± 94 | 1333 ± 168 |
IL-4 KO mice fed 10% pectin (pectin) or 5% cellulose (cellulose) and peritoneal macrophages were isolated in treated in vitro with either LPS or PBS for 2 hrs and cytokines were measured in the supernant. Results are expressed as pg/mg protein, means ± SEM, n= 4
Discussion
Endotoxin is a powerful peripheral activator of the neuroimmune system inducing a myriad of sickness symptoms and behaviors that include social withdrawal, fever, anorexia and weight loss (Dantzer, 2006). Numerous clinical trials aimed at neutralizing the effects of endotoxin and the adverse consequences of sepsis have been conducted. Unfortunately, none of these trials have been particularly successful and sepsis remains a worrisome clinical problem (Rittirsch, Flierl and Ward, 2008). This is not entirely surprising due to the frequently observed aggressive feed-forward of activated pro-inflammatory cytokine cascades (Horn, 1998) and late phase immunosuppression that is inherent to endotoxemia (Rittirsch, Flierl and Ward, 2008). In addition, recent studies have revealed that patients who survive sepsis present later with long-term cognitive impairment, depression and anxiety (Streck et al., 2008). Therefore, strategies aimed at inducing relative resistance to endotoxin in the neuroimmune system may have benefit. We found that mice fed a diet rich in soluble fiber were neurobehavioral protected and recovered faster from endotoxin when compared to mice fed a diet comprised predominantly of insoluble fiber. The beneficial impact of soluble fiber was observed in mice fed a commercial diet with variable fiber solubility and source, as well as, in an open source diet where soluble fiber was provided by pectin. Interestingly, susceptibility to endotoxin was even worse in animals fed a diet containing 10% insoluble fiber when compared to 5% insoluble fiber. These results suggest that lack of dietary soluble fiber and amount of insoluble fiber are important to endotoxin susceptibility in the neuroimmune system. Overall, the amount of fiber fed was, on a relative dry weight basis, within the range recommended for humans (25-38 g) (Marlett, McBurney and Slavin, 2002) and not of uncommon source for human dietary supplementation (Knopp et al., 1999). Currently, no work has shown an impact of fiber on neuroimmunity but recent work has demonstrated that soluble fiber can impact colitis by decreasing proinflammatory cytokines and mucosal damage (Kanauchi et al., 2008) and irritable bowel syndrome (Galvez, Rodriguez-Cabezas and Zarzuelo, 2005).
In mouse models of endotoxemia, we and others have found that brain-based IL-1RA is important in counter-regulating the behavioral symptoms associated with sickness especial that of social withdrawal (Johnson et al., 2005) and food intake (Burgess et al., 1998). Social withdrawal is a powerful tool for studying sickness behavior in that it is easy to perform, very reproducible, non-lethal and a phenotypic expression of brain-based pro-inflammatory cytokine dysregulation (Dantzer, 2004). Here we demonstrated that a diet rich in soluble fiber increased endotoxin-dependent up-regulation of IL-1RA while blunting expression of brain-based IL-1β and TNF-α. Since this cytokine profile suggests alternative macrophage activation, IL-4 expression was examined and showed that IL-4 message was significantly increased basally in the brain, spleen and lower GI tract of mice fed soluble fiber. This increase in GI IL-4 was associated with a marked increase in cecal SCFAs. While soluble fiber-dependent SCFA up-regulation was expected (Wong et al., 2006), increased IL-4 expression, especially as measurable protein, in spleen and ileum was not. IL-4 has been examined after pectin (Lim et al., 2003) and probiotic (Jain, Yadav and Sinha, 2009) feeding and after direct SCFA administration to cultured lymphocytes (Cavaglieri et al., 2003). None of these studies demonstrated up-regulation of IL-4 and work using probiotic treatments has shown either no change or a down-regulation of IL-4 (Jain et al., 2009, Ghadimi et al., 2008). In the study by Lim et al., mice were fed a diet containing 5% pectin as compared to 10% in our study. In addition, their mice were fed a pectin diet for 2 wks. We found no significant impact of pectin on sickness behavior until at least 5 wks of pectin feeding (data not shown). Taken together these findings indicate that soluble fiber as a prebiotic requires an extended course of administration to impact the neuroimmune system.
IL-4 is mainly generated by CD4+ T cells and mast cells and is vital for differentiation of naïve T-helper cells to Th2 cells (Kindt, Goldsby and Osborne, 2007). We found that splenocytes from mice fed a soluble fiber diet polarize Th2 with marked up-regulation of IL-4 and IL-5 in response to Con A. Important to IL-4 expression is chromatin remodeling of its gene locus (Ansel et al., 2006). SCFAs, especially butyrate, have been shown to induce chromatin remodeling via acetylation of histones (Sealy and Chalkley, 1978) and histone acetylation can augment transcription of IL-4 (Yamashita et al., 2004). Sodium butyrate can increase IL-5 production in Jurkat cells via hyperacetylation of H3 and H4 on the IL-5 promoter impacting the NFAT3, YY1 and GATA3 binding sites (Han et al., 2007) and GATA3 is a transcriptional regulator of IL-4 in T-cells (Gilmour and Lavender, 2008). Neither we nor Cavaglieri et al. showed a direct effect of butyrate on lymphocyte-dependent IL-4 production. This is not entirely surprising in that SCFAs are rapidly metabolized almost exclusively in the GI tract with butyrate being a key fuel for GI epithelium (Gassull, 2006).
Further evidence against a direct effect of SCFAs on IL-4 production is that MyD88 KO mice fed pectin failed to up-regulate IL-4 in ileum and spleen (Fig. 3C). These findings indicate that soluble fiber-dependent up-regulation of IL-4 is dependent on MyD88 implicating Toll-like receptors (TLR) and/or IL-1 receptor family members (Kenny and O’Neill, 2008) as a key bridge to augmenting IL-4. This relationship between MyD88 and IL-4 is not entirely surprising because in studies investigating the up-regulation of Th2 cytokines by LPS (Mukherjee et al., 2009) lack of MyD88 inhibits LPS simulated macrophages from up-regulating IL-4. Interestingly, Wen et al. recently demonstrated that NOD mice lacking MyD88 have decreased incidence of type 1 diabetes (T1D) and that this reduction in T1D was due to alterations in gut microbiota (Wen et al., 2008).
IL-4 is vital to alternative macrophage activation (Gordon, 2003, Martinez, Helming and Gordon, 2009, Varin and Gordon, 2009), and we found macrophage activation profiles consistent with alternative activation in mice fed soluble fiber. This finding is important because, currently, how macrophages become alternatively activated in vivo is not clearly defined (Martinez, Helming and Gordon, 2009). Most work on alternatively activated macrophages has centered on helminth infection response and asthma. Non-pathologic skewing of macrophage activation to alternative, as demonstrated here, appears to be a novel finding especially since it is diet-induced. Furthermore, as mentioned above, our work is in stark contrast to those using a probiotic approach to immune regulation, which show a propensity to skew the immune system Th1.
IL-4 KO mice were behaviorally resistant to the impact of soluble fiber. Fig.5A shows that 2 h after administration of LPS wild type mice fed pectin were resistant to LPS (similar to the results demonstrated in Fig.1B). IL-4 KO mice fed pectin, however, demonstrated LPS-induced sickness comparable to wild type mice fed cellulose indicating that IL-4 is critical to pectin-mediated LPS resistance. In support, IL-4 KO mice could not be polarized Th2 with a pectin diet (Table 5) nor could macrophages be driven to an alternative activation phenotype (Table 6 and Fig.5B). This conclusion is not, however, absolute. Fig.5B demonstrates that arginine 1 mRNA was up-regulated in macrophages from IL-4 KO mice fed pectin indicating that other factors beside IL-4 may be important to certain markers of alternative macrophage activation. One of these candidates could be IL-13. As expected the Th2 response is markedly dampened in IL-4 KO mice because T cells appear to lack appropriate receptors for IL-13 (Wynn, 2003). Table 5 shows that in IL-4 KO mice pectin suppressed Con A-stimulated IL-2 production compared to cellulose (as it does in wild type mice, Table 3). Overall, the role of cytokines other than IL-4 in Th2 polarization is not entirely defined but IL-2 has been reported to be required, though it likely needs co-existent IL-4 stimulation (Cote-Sierra, 2004). Thus, pectin-dependent down-regulation of IL-2 in IL-4 wild type and IL-4 KO mice suggests an impact of pectin outside of its ability to simply down-regulate IL-4. Overall, the cytokine profile of peritoneal macrophages and splenocytes is modulated by the absence of IL-4 in IL-4 KO mice, which is to be expected given the importance of IL-4 in mediating Th cell polarization and macrophage activation (Wynn, 2003).
As noted earlier, brains from mice fed soluble fiber demonstrated a basal up-regulation of IL-4 mRNA and a doubling of endotoxin-induced IL-1RA when compared to mice fed insoluble fiber. These findings indicate that the impact of soluble fiber goes beyond the GI tract and peripheral immune system and affects the neuroimmune system. These findings also suggest that the brain may be able to be polarized Th2. This assertion is supported by the brain-based pro-inflammatory cytokine data which showed that mice fed soluble fiber had reduced IL-1βand TNF-αmRNA production in response to endotoxin while brain-based IL-1RA production was up. These data underscore that generalized loss of responsiveness to endotoxin was not the key difference between mice fed soluble verses insoluble fiber (clearly reflected by the macrophage activation profiles) and that functional differences in response to endotoxin were present. It is important to note that hyper-activation of the Th2 response can be deleterious because of increased susceptibility to infection. In turn, a Th2 polarization can augment the development of asthma and allergies. However, it is unlikely that the Th2 response induced by feeding a high pectin diet would lead to such complications because it appears to mimic what occurs in “normal” diets. Fig.1 shows that animals fed chow behaviorally recovery from LPS much faster than animals fed cellulose. Our data indicate that soluble fiber is a key to this process. In support, cytokine data from macrophages and splenocytes from chow fed mice are comparable to pectin fed mice. Overall, our data show that a diet comprised entirely of unfermentable fiber, like cellulose, is detrimental to neuroimmune recovery post LPS. However, almost any diet which contains plant material includes both fermentable and unfermentable fiber because all plant cell walls are a variable combination of cellulose and pectin. Thus, diets lacking any fermentable fiber are rare, unless actively sought out or administered. Another limitation of our work is that we restricted it to inflammatory cytokines and focused our examination to a relatively short period of time post LPS. Further work is needed to determine if cellulose or pectin diets impact constituents induced by LPS that manifest at substantially later time points. In sum, our data show the importance of pectin and IL-4 to neuroimmune recovery from endotoxin and the importance of pectin to Th2 polarization and alternative macrophage activation. Our data also highlights the potential detrimental nature of a diet lacking pectin especially as related to the response of the brain to infectious disease.
Acknowledgments
Support: This research was supported by grants from the National Institutes of Health (DK064862,NS058525 and AA019357 to G.G.F.), American Heart Association (Predoctoral Fellowship to C.L.S.) USDA National Institute of Food and Agriculture, Hatch project number #ILLU-971-32 , U.S. Department of Homeland Security, Assistance to Firefighters Grants Office, Research and Development Grants (EMW-2006-FP-02459) and Ruth Kirschstein Institutional NRSA 5T32 DK059802 to the Division of Nutritional Sciences (Predoctoral Fellowship to M.L.M)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ansel KM, Djuretic I, Tanasa B, Rao A. Regulation of Th2 differentiation and Il4 locus accessibility. Annu Rev Immunol. 2006;24:607–656. doi: 10.1146/annurev.immunol.23.021704.115821. [DOI] [PubMed] [Google Scholar]
- Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- Bowen H, Kelly A, Lee T, Lavender P. Control of cytokine gene transcription in Th1 and Th2 cells. Clin Exp Allergy. 2008;38:1422–1431. doi: 10.1111/j.1365-2222.2008.03067.x. [DOI] [PubMed] [Google Scholar]
- Burgess W, Gheusi G, Yao J, Johnson RW, Dantzer R, Kelley KW. Interleukin-1beta-converting enzyme-deficient mice resist central but not systemic endotoxin-induced anorexia. Am J Physiol. 1998;274:R1829–33. doi: 10.1152/ajpregu.1998.274.6.R1829. [DOI] [PubMed] [Google Scholar]
- Cavaglieri CR, Nishiyama A, Fernandes LC, Curi R, Miles EA, Calder PC. Differential effects of short-chain fatty acids on proliferation and production of pro- and anti-inflammatory cytokines by cultured lymphocytes. Life Sci. 2003;73:1683–1690. doi: 10.1016/s0024-3205(03)00490-9. [DOI] [PubMed] [Google Scholar]
- Cote-Sierra J, Foucras G, Guo L, Chiodetti L, Young HA, Hu-Li J, Zhu J, Paul WE. Interleukin 2 plays a central role in Th2 differentiation. Proc Natl Acad Sci U S A. 2004;101:3880–3885. doi: 10.1073/pnas.0400339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox M-AA, Jackson J, Stanton M, Rojas-Triana A, Bober L, Laverty M, Yang X, Zhu F, Liu J, Wang S, Monsma F, Vassileva G, Maguire M, Gustafson E, Bayne M, Chou C-CC, Lundell D, Jenhby C-HH. Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines. World Journal of Gastroenterology. 2009;44:5549–5557. doi: 10.3748/wjg.15.5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. Cytokine, sickness behavior, and depression. Neurol Clin. 2006;24:441–460. doi: 10.1016/j.ncl.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behaviour: A neuroimmune response to activation of innate immunity. Eur J Pharmacol. 2004;500:399–411. doi: 10.1016/j.ejphar.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowell DJ. Signals for the execution of Th2 effector function. Cytokine. 2009;46:1–6. doi: 10.1016/j.cyto.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez J, Rodriguez-Cabezas ME, Zarzuelo A. Effects of dietary fiber on inflammatory bowel disease. Mol Nutr Food Res. 2005;49:601–608. doi: 10.1002/mnfr.200500013. [DOI] [PubMed] [Google Scholar]
- Gassull MA. Review article: The intestinal lumen as a therapeutic target in inflammatory bowel disease. Aliment Pharmacol Ther. 2006;24(Suppl 3):90–95. doi: 10.1111/j.1365-2036.2006.03067.x. [DOI] [PubMed] [Google Scholar]
- Ghadimi D, Folster-Holst R, de Vrese M, Winkler P, Heller KJ, Schrezenmeir J. Effects of probiotic bacteria and their genomic DNA on TH1/TH2-cytokine production by peripheral blood mononuclear cells (PBMCs) of healthy and allergic subjects. Immunobiology. 2008;213:677–692. doi: 10.1016/j.imbio.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Gilmour J, Lavender P. Control of IL-4 expression in T helper 1 and 2 cells. Immunology. 2008;124:437–444. doi: 10.1111/j.1365-2567.2008.02845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Groff JL, Gropper SS. Advanced nutrition and human metabolism. Wadsworth; Belmont, CA: 1999. [Google Scholar]
- Han S, Lu J, Zhang Y, Cheng C, Li L, Han L, Huang B. HDAC inhibitors TSA and sodium butyrate enhanced the human IL-5 expression by altering histone acetylation status at its promoter region. Immunol Lett. 2007;108:143–150. doi: 10.1016/j.imlet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Horn KD. Evolving strategies in the treatment of sepsis and systemic inflammatory response syndrome (SIRS) QJM. 1998;91:265–277. doi: 10.1093/qjmed/91.4.265. [DOI] [PubMed] [Google Scholar]
- Jain S, Yadav H, Sinha PR. Probiotic dahi containing lactobacillus casei protects against salmonella enteritidis infection and modulates immune response in mice. J Med Food. 2009;12:576–583. doi: 10.1089/jmf.2008.0246. [DOI] [PubMed] [Google Scholar]
- Jain S, Yadav H, Sinha PR, Marotta F. Modulation of cytokine gene expression in spleen and peyer’s patches by feeding dahi containing probiotic lactobacillus casei in mice. J Dig Dis. 2009;10:49–54. doi: 10.1111/j.1751-2980.2008.00362.x. [DOI] [PubMed] [Google Scholar]
- Johnson DR, O’Connor JC, Dantzer R, Freund GG. Inhibition of vagally mediated immune-to-brain signaling by vanadyl sulfate speeds recovery from sickness. Proc Natl Acad Sci U S A. 2005;102:15184–15189. doi: 10.1073/pnas.0507191102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DR, O’Connor JC, Hartman ME, Tapping RI, Freund GG. Acute hypoxia activates the neuroimmune system which diabetes exacerbates. J Neurosci. 2007;27:1161–1166. doi: 10.1523/JNEUROSCI.4560-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanauchi O, Oshima T, Andoh A, Shioya M, Mitsuyama K. Germinated barley foodstuff ameliorates inflammation in mice with colitis through modulation of mucosal immune system. Scand J Gastroenterol. 2008;43:1346–1352. doi: 10.1080/00365520802245411. [DOI] [PubMed] [Google Scholar]
- Kenny EF, O’Neill LA. Signalling adaptors used by toll-like receptors: An update. Cytokine. 2008;43:342–349. doi: 10.1016/j.cyto.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Kindt TJ, Goldsby RA, Osborne BA. Immunology. W. H. Freeman and Company; New York, NY: 2007. [Google Scholar]
- Knopp RH, Superko HR, Davidson M, Insull W, Dujovne CA, Kwiterovich PO, Zavoral JH, Graham K, O’Connor RR, Edelman DA. Long-term blood cholesterol-lowering effects of a dietary fiber supplement. Am J Prev Med. 1999;17:18–23. doi: 10.1016/s0749-3797(99)00039-2. [DOI] [PubMed] [Google Scholar]
- Kruisbeek AM. Isolation of mouse mononuclear cells. In: Coligan JE, Bierer B, Margulies DH, Shevach EM, Strober W, Coico R, editors. Current protocols in immunology. Wiley; 2000. [Google Scholar]
- Kuzmuk KN, Swanson KS, Tappenden KA, Schook LB, Fahey GC., Jr Diet and age affect intestinal morphology and large bowel fermentative end-product concentrations in senior and young adult dogs. J Nutr. 2005;135:1940–1945. doi: 10.1093/jn/135.8.1940. [DOI] [PubMed] [Google Scholar]
- Lim BO, Lee SH, Park DK, Choue RW. Effect of dietary pectin on the production of immunoglobulins and cytokines by mesenteric lymph node lymphocytes in mouse colitis induced with dextran sulfate sodium. Biosci Biotechnol Biochem. 2003;67:1706–1712. doi: 10.1271/bbb.67.1706. [DOI] [PubMed] [Google Scholar]
- Marlett JA, McBurney MI, Slavin JL. Position of the american dietetic association health implications of dietary fiber. J Am Diet Assoc. 2002;102:993–1000. doi: 10.1016/s0002-8223(02)90228-2. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: An immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.1701141. [DOI] [PubMed] [Google Scholar]
- Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- O’Connor JC, Sherry CL, Guest CB, Freund GG. Type 2 diabetes impairs insulin receptor substrate-2-mediated phosphatidylinositol 3-kinase activity in primary macrophages to induce a state of cytokine resistance to IL-4 in association with overexpression of suppressor of cytokine signaling-3. J Immunol. 2007;178:6886–6893. doi: 10.4049/jimmunol.178.11.6886. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Chen LY, Papadimos TJ, Huang S, Zuraw BL, Pan ZK. Lipopolysaccharide-driven Th2 cytokine production in macrophages is regulated by both MyD88 and TRAM. J Biol Chem. 2009;284:29391–8. doi: 10.1074/jbc.M109.005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Satpathy A, Hartman ME, Horvath EM, Kelley KW, Dantzer R, Johnson RW, Freund GG. IL-1beta-mediated innate immunity is amplified in the db/db mouse model of type 2 diabetes. J Immunol. 2005;174:4991–4997. doi: 10.4049/jimmunol.174.8.4991. [DOI] [PubMed] [Google Scholar]
- Re F, Muzio M, De Rossi M, Polentarutti N, Giri JG, Mantovani A, Colotta F. The type II “receptor” as a decoy target for interleukin 1 in polymorphonuclear leukocytes: Characterization of induction by dexamethasone and ligand binding properties of the released decoy receptor. J Exp Med. 1994;179:739–743. doi: 10.1084/jem.179.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 2008;8:776–787. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger WE. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980;21:793–798. doi: 10.1136/gut.21.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose DJ, DeMeo MT, Keshavarzian A, Hamaker BR. Influence of dietary fiber on inflammatory bowel disease and colon cancer: Importance of fermentation pattern. Nutr Rev. 2007;65:51–62. doi: 10.1111/j.1753-4887.2007.tb00282.x. [DOI] [PubMed] [Google Scholar]
- Scott K, Duncan S, Flint H. Dietary fibre and the gut microbiota. Nutr Bull. 2008;33:201. [Google Scholar]
- Sealy L, Chalkley R. The effect of sodium butyrate on histone modification. Cell. 1978;14:115–121. doi: 10.1016/0092-8674(78)90306-9. [DOI] [PubMed] [Google Scholar]
- Sherry CL, Kim SS, Freund GG. Accelerated recovery from acute hypoxia in obese mice is due to obesity-associated up-regulation of interleukin-1 receptor antagonist (IL-1RA) Endocrinology. 2009 doi: 10.1210/en.2008-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry CL, Kramer JM, York JM, Freund GG. Behavioral recovery from acute hypoxia is reliant on leptin. Brain Behav Immun. 2009;23:169–175. doi: 10.1016/j.bbi.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry CL, O’Connor JC, Kramer JM, Freund GG. Augmented lipopolysaccharide-induced TNF-α production by peritoneal macrophages in type 2 diabetic mice is dependent on elevated glucose and requires p38 map kinase. J Immunol. 2007;178:663–670. doi: 10.4049/jimmunol.178.2.663. [DOI] [PubMed] [Google Scholar]
- Streck EL, Comim CM, Barichello T, Quevedo J. The septic brain. Neurochem Res. 2008;33:2171–2177. doi: 10.1007/s11064-008-9671-3. [DOI] [PubMed] [Google Scholar]
- Torres MI, Rios A. Current view of the immunopathogenesis in inflammatory bowel disease and its implications for therapy. World J Gastroenterol. 2008;14:1972–1980. doi: 10.3748/wjg.14.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valapour M, Guo J, Schroeder JT, Keen J, Cianferoni A, Casolaro V, Georas SN. Histone deacetylation inhibits IL4 gene expression in T cells. J Allergy Clin Immunol. 2002;109:238–245. doi: 10.1067/mai.2002.121145. [DOI] [PubMed] [Google Scholar]
- Varin A, Gordon S. Alternative activation of macrophages: Immune function and cellular biology. Immunobiology. 2009 doi: 10.1016/j.imbio.2008.11.009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, Gordon JI, Chervonsky AV. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. IL-13 effector functions. Annual Review of Immunology. 2003;21:425–456. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: Fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Ukai-Tadenuma M, Miyamoto T, Sugaya K, Hosokawa H, Hasegawa A, Kimura M, Taniguchi M, DeGregori J, Nakayama T. Essential role of GATA3 for the maintenance of type 2 helper T (Th2) cytokine production and chromatin remodeling at the Th2 cytokine gene loci. J Biol Chem. 2004;279:26983–26990. doi: 10.1074/jbc.M403688200. [DOI] [PubMed] [Google Scholar]