Abstract
GA binding protein (GABP) consists of GABPα and GABPβ subunits. GABPα is a member of Ets family transcription factors and binds DNA via its conserved Ets domain, whereas GABPβ does not bind DNA but possesses transactivation activity. In T cells, GABP has been demonstrated to regulate the gene expression of interleukin-7 receptor α chain (IL-7Rα) and postulated to be critical in T cell development. To directly investigate its function in early thymocyte development, we used GABPα conditional knock-out mice where the exons encoding the Ets DNA-binding domain are flanked with LoxP sites. Ablation of GABPα with the Lck-Cre transgene greatly diminished thymic cellularity, blocked thymocyte development at the double negative 3 (DN3) stage, and resulted in reduced expression of T cell receptor (TCR) β chain in DN4 thymocytes. By chromatin immunoprecipitation, we demonstrated in DN thymocytes that GABPα is associated with transcription initiation sites of genes encoding key molecules in TCR rearrangements. Among these GABP-associated genes, knockdown of GABPα expression by RNA interference diminished expression of DNA ligase IV, Artemis, and Ku80 components in DNA-dependent protein kinase complex. Interestingly, forced expression of prearranged TCR but not IL-7Rα can alleviate the DN3 block in GABPα-targeted mice. Our observations collectively indicate that in addition to regulating IL-7Rα expression, GABP is critically required for TCR rearrangements and hence normal T cell development.
Keywords: Chromatin Immunoprecipitation (ChIP), DNA Repair, T Cell Receptor, Thymocyte, Transcription Factors, T Cell Development
Introduction
T cells are derived from multipotent lymphoid progenitors in the bone marrow and differentiate into mature T cells as a result of a series of lineage commitment steps occurring in the thymus and periphery (1, 2). The co-receptor molecules CD4 and CD8 can be used to distinguish four different populations of developing thymocytes, with the most immature thymocytes being double negative for CD4 and CD8 expression (termed DN thymocytes).2 DN cells then become double positive (DP) thymocytes expressing both CD4 and CD8 that undergo vigorous positive and negative selections, with subsequent differentiation into single positive (SP) thymocytes expressing either CD4 or CD8. The SP thymocytes leave the thymus and populate peripheral lymphoid organs, where they can be activated upon encountering foreign antigens. During their early developmental stages, DN thymocytes can be subdivided further into four sequential stages of differentiation, which are identified by their surface expression of CD44 and CD25: DN1 (CD44+CD25−), DN2 (CD44+CD25+), DN3 (CD44−CD25+), and DN4 (CD44−CD25−). Both DN1 and DN2 subsets retain the potential to become natural killer cells and dendritic cells, and complete commitment of early thymocytes to the T cell lineage occurs at the DN3 stage (3). Rearrangement of T cell receptor β (TCRβ) locus is initiated in DN3 thymocytes, and productive recombination and proper expression of TCRβ form a major checkpoint known as β-selection. At this selection stage, TCRβ pairs with pre-Tα chain and forms pre-TCR. Deficiency in critical components of V(D)J recombination machinery such as Rag recombinases blocks T cell development at the DN3 stage.
Successful commitment and further development of T lineage cells are orchestrated by a number of transcription factors, as revealed by germ line and conditional gene knock-out experiments. These includes Ikaros family factors, the “E proteins” E2A and HEB, Runx family factors, TCF family transcription factors activated by Wnt signaling, RBPSuh activated by Notch-Delta interaction, GATA-3, Myb, Gfi-1, and the Ets family transcription factor PU.1 (2, 3). Ikaros family factors and PU.1 are critical in specifying hematopoietic stem cells (HSCs) to lymphoid lineages (4, 5). Notch molecules and their direct transcriptional effector RBPSuh as well as E2A and HEB and their antagonists Id2 and Id3 have critical roles in establishing T cell identity and affecting successive T-lineage subspecializations (6–8). The T cell-specific transcription factors GATA3 and TCF-1 have nonredundant roles at multiple stages during T cell development (9, 10). In addition to PU.1, other Ets factors, including Ets-1, Fli-1, Tel, Elf-1, and GA binding protein (GABP), are expressed throughout most stages of T cell development (11). Ets factor functions may be partially redundant; for example, no lymphoid developmental phenotype has been observed in Elf-1 knock-out mice even though Elf-1 has been shown to bind to immunologically important genes (12).
Among more than 30 Ets factors, GABP is the only one that functions as a heterodimer of GABPα and GABPβ (13). GABPα contains the DNA-binding Ets domain that is conserved among all Ets factors, whereas GABPβ cannot bind DNA but has transactivation activities. GABP proteins are ubiquitously expressed and known to regulate genes that control basic cellular functions such as cell cycle progression (14, 15). Interestingly, GABP also participates in the regulation of tissue/cell type-specific genes, such as nicotinic acetylcholine receptor subunits δ and ϵ in neuromuscular synapses (16) and CD18 in myeloid cells (13, 17). In the immune system, we have demonstrated that GABP is critically required for normal expression of Pax5 and thus B cell development and that diminished GABPα expression causes severe defects in humoral responses (18). In T cells, GABP is critical for the expression of IL-7 receptor α chain (IL-7Rα) (19). Because IL-7Rα is indispensable for T cell development, survival, and homeostasis of naïve T cells, as well as for the maintenance and possibly the generation of memory T cells (20), ablation of GABP is postulated to have profound impact on multiple aspects in T cell biology. In this report, we investigated the roles of GABP in T cell development by targeting the GABPα subunit in developing thymocytes.
EXPERIMENTAL PROCEDURES
Mice
GABPαFL/+ and GABPα+/− mice were kindly provided by Dr. Steven Burden (Skirball Institute, New York University Medical School) (21). The targeting strategy was summarized in Fig. 1A, and the genotyping primers are as follows: Acondi1, 5′-cagccaagagcaacagatgaatg; Acondi2, 5′-tagtttgcattacaaagatgctg; and Acondi3, 5′-ccaaaggaattaggggaatctttcc. Lck-Cre transgenic mice were from Taconic, and OT-I TCR transgenic mice were from The Jackson Laboratory. IL-7Rα transgenic mice were previously described (22). All of the mice were handled in accordance with protocols approved by the Institutional Animal Use and Care Committee of the University of Iowa.
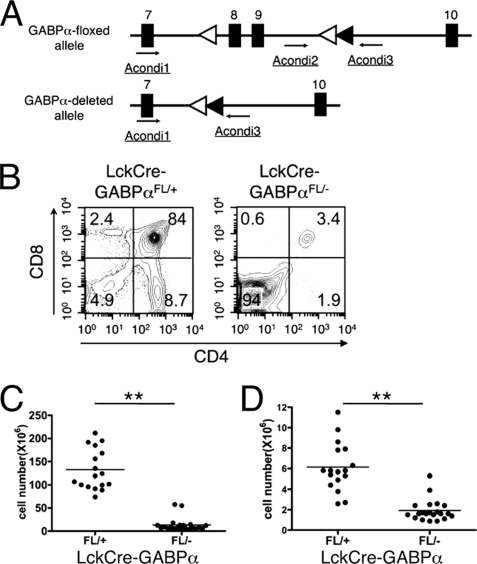
FIGURE 1.
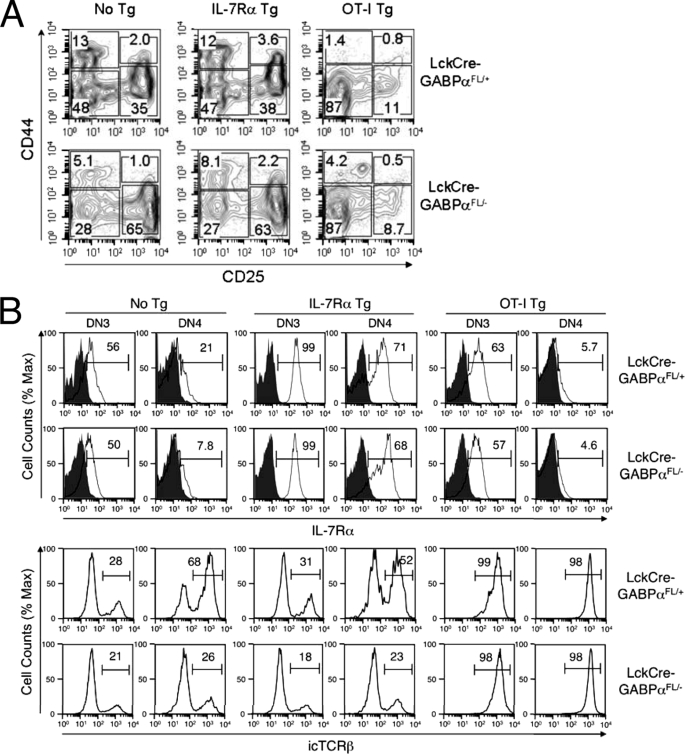
Ablation of GABPα blocks T cell development at DN stage. A, GABPα targeting strategy. In the Gabpa gene, exons 8 and 9, which encode most of the Est DNA-binding domain, were flanked with 2 LoxP sites (open triangles). The relative locations of genotyping primers are indicated. With these primer combinations, WT, floxed, and deleted Gabpa alleles were detected at 153, 417, and 600 bp, respectively. Filled triangles, Frt sites. A paired of Frt sites were used to flank a neomycin-resistant gene, which was excised by a Flippase transgene to minimize the disturbance of natural gene structure by gene targeting. After the Flippase excision, only one Frt site was left in the targeted intron. B, representative flow cytometric profile of thymocytes. LckCre-GABPαFL/+ and LckCre-GABPαFL/− mice were analyzed at an age of 6–8 weeks. The thymocytes from mice of indicated genotypes were stained with anti-CD4 and anti-CD8 antibodies. The percentages of each population are shown. C, total thymic cellularity. D, DN thymocyte numbers. The data are pooled from more than five independent experiments. **, p < 0.01.
Flow Cytometry
Single cell suspensions were prepared from thymi and stained with fluorochrome-conjugated antibodies, as described (19). All fluorochrome-conjugated antibodies were from either eBiosciences or BD Biosciences. For intracellular staining, the cells were sequentially surface-stained, fixed, and permeabilized with Cytofix/Cytoperm solution (BD Biosciences), followed by staining with anti-TCRβ. All of the data were acquired on a BD FACSCalibur flow cytometer and analyzed using FlowJo software (Tree Star Inc.).
Analysis of ChIP-Seq Data Using Site Identification from Short Sequence Reads (SISSRs) Algorithm
Genome-wide mapping of GABP binding locations in human Jurkat T cell lymphoma cells was performed using chromatin immunoprecipitation followed by massive parallel sequencing (ChIP-Seq) (23). The sequence tag reads from anti-GABPα antibody and control IgG samples were downloaded and analyzed using the SISSRs application (24). SISSRs v1.4 was run with option a (which retains one read/genomic position even if multiple reads were mapped to that position and thus avoids overrepresentation of one position caused by PCR amplification-generated bias) with fragment length F set to 200, p value set to 0.01, and the remaining parameters set to their default values. Each identified binding site is associated with a fold enrichment score, which is the ratio of the normalized number of GABP sequence tags supporting the inferred binding site to the normalized number of control IgG tags supporting the exact same site. Genome-wide distribution of GABPα-binding sites was determined with reference to RefSeq genes downloaded from the UCSC genome browser.
Chromatin Immunoprecipitation
DN thymocytes were isolated by depleting lineage-positive cells from total thymocytes using biotinylated antibodies and Dynabeads M280 Streptavidin (Invitrogen). Chromatin fragments of DN cells were prepared as previously described and immunoprecipitated with anti-GABPα antibody (H180; Santa Cruz Biotechnologies) or rabbit IgG using Dynabeads Protein A (Invitrogen) (18). To assess enrichment of selected chromatin fragments by the GABPα antibody, primers were designed to amplify the conserved region of a gene in the mouse genome based on GABP binding locations in the same gene in the human genome as revealed by ChIP-Seq in Jurkat cells. Each primer set was tested for linear amplification range with input DNA using SYBR Advantage qPCR premix (Clontech) on ABI 7300 Real Time PCR System (Applied Biosystems). For Rag1 and Rag2 gene loci where no GABP binding was found in ChIP-Seq, the primers were designed to amplify their promoter regions as negative controls. For calculation of enrichment of each selected gene/region, 2−ΔCt was used, where ΔCt is the difference of Ct (cross-over threshold) values detected in GABPα antibody- and IgG-precipitated samples. Primer sequences for the amplification of each genomic segment are as follows: Rag1, 5′-AGCTATCACTGGGAGGCAGA and 5′-GAGGAGGCAAGTCCAGACAG; Rag2, 5′-CACTCTACCCTGCAGCCTTC and 5′-TCTGCCCTCTTGTAGCCAGT; Atm, 5′-ATGCCGACTTCTTCCTTTGG and 5′-TGAGGAAAGATGGGCTCAGA; Atr, 5′-TTTGTTGGGTCGTCAGGTC and 5′-GAAATCCCAGAGGCGACAG; Prkdc, 5′-CTCCGCATGGTTAGACTGGT and 5′-CTAGCCAGCTCTTCCCTGAA; Xrcc6, 5′-GCGTGGTCCGGTAATAAGGT and 5′-AGGGTGAGAGGCTGGAGAAG; Xrcc5, 5′- CTGATTGGGCGAGAGACAAG and 5′-TCAACCACCAGTCCTGTTCC; Dclre1c, 5′-GCCAGTGCTACCCACAAACT and 5′-ACTAGACCCAAAGCCCACCT; and Lig4, 5′-CAGCGTGCGGATACAACTAA and 5′-CTGCTAAGCAAGTGGTGTGG.
Knockdown of GABPα Expression in EL-4 Cells and Quantitative Reverse Transcription-PCR
Small interference RNA constructs targeting GABPα (siGABPα) and control vector pBS/U6 were described previously (19). Murine thymoma EL-4 cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum, 50 μm 2-mercaptoethnaol, and penicillin/streptomycin. EL-4 cells were transiently transfected by electroporation as described (19). In brief, 4 × 106 EL-4 cells were suspended in RPMI 1640 containing 25 mm HEPES, mixed with 9 μg of pBS/U6 or siGABPα along with 1 μg of pEYFP-N1 (Clontech) in a GenePulser cuvette (4-mm gap; Bio-Rad) and then electroporated at 250 V using a GenePulser Xcell electroporator (Bio-Rad). Forty-eight hours later, EYFPhigh cells were isolated by cell sorting, and total RNA was extracted and reverse-transcribed using a QuantiTech reverse transcription kit (Qiagen). The resulting cDNA was then analyzed for expression of different genes using quantitative PCR as described above. Relative expression levels of genes of interest were normalized to that of a housekeeping gene, hypoxanthine phosphoribosyltransferase 1 (HPRT1). Primer sequences for each transcript are as follows: Gabpa, 5′-CCGCTACACCGACTACGATT and 5′-ACCTTCATCACCAACCCAAG; Il7r, 5′-AAAGCATGATGTGGCCTACC and 5′-GACTCCACTCGCTCCAGAAG; Tyms, 5′- GCCCAGTTTATGGTTTCCAA and 5′-CCAGGCACACATGATGATTC; Pola1, 5′-TTGAACTGGAAGTGCTGCTG and 5′-CACATATCATTCGGCCACAG; Atm, 5′-AGTGTGGACATGGAGAGCACA and 5′-CGGAATATGGATCAGCCTCAAG; Atr, 5′-TGGTTGGAGAATGCTGGCTAC and 5′- ATAAGCGCCTGGTGAACGTC; Prkdc, 5′-AAGGCAGAAGCCTGGACAAG and 5′-ATCCGCCAGTAGGTCAATGC; Xrcc6, 5′-GATCAGAACATTCAGGTGACTCC and 5′-GCCTTCATCTTGTCTATCTGCTC;Xrcc5, 5′-GACCTGAAGAAGCTGGTGATGTG and 5′-TAGGATGACGTCCAGAGCTTCAC;Delre1c, 5′-CACCATCTCACAACGGACAG and 5′-TGGTAGATGGCTTGATGCTG; Lig4, 5′-TCGTGTCCTGATGCTTAGTTG and 5′-AGGAAGCCATAGAAGCGACAA; and Hprt1, 5′-GCGTCGTGATTAGCGATGATG and 5′-CTCGAGCAAGTCTTTCAGTCC.
RESULTS
Ablation of GABPα Blocks T Cell Development at DN3 Stage
In our previous studies, we have generated GABPα-deficient mice using a gene trap strategy, yielding hypomorphic GABPα alleles (GABPαtp/tp) (19). In contrast to preimplantation lethality resulting from a complete GABPα-null mutation, hypomorphic expression of GABPα prolonged the survival of GABPαtp/tp embryos up to embryonic day 14.5 (19). By transferring GABPαtp/tp fetal liver cells, which contain hematopoietic stem cells, into sublethally irradiated Rag2−/− mice, we observed partial blocks of T cell development at DN or DP stages. These data were presented as supplemental information in a previous report (18). The incomplete developmental block may be accounted for by the hypomorphic nature of the GABPα+/tp allele, where the levels of leaky expression of the WT GABPα protein may vary. To circumvent this caveat, we used conditionally targeted GABPα+/FL mice, in which exons 8 and 9 (encoding the DNA binding Ets domain) were flanked with LoxP sites (Fig. 1A) (21). The floxed GABPα allele was converted to a deleted allele (GABPα+/−) by crossing to EIIa-Cre transgenic mice.
To inactivate GABPα in early developing thymocytes, we crossed GABPα+/FL and GABPα+/− to Lck-Cre transgenic mice to obtain LckCre-GABPαFL/+ and LckCre-GABPαFL/− progeny. LckCre-GABPαFL/+ mice showed expected normal DN, DP, and SP subsets. In contrast, LckCre-GABPαFL/− mice manifested a block at the DN stage in thymocyte development, with concomitant reduction of DP and SP cells (Fig. 1B). In some of the LckCre-GABPαFL/− mice, DP and SP thymocytes can be detected as more discrete populations. Regardless of the presence of DP and SP cells, all LckCre-GABPαFL/− mice had similar greatly diminished thymocyte cellularity (Fig. 1C). Despite the increased frequency of DN thymocytes, their absolute numbers were still significantly reduced (Fig. 1D), suggesting an absolute requirement of GABP for normal thymocyte maturation.
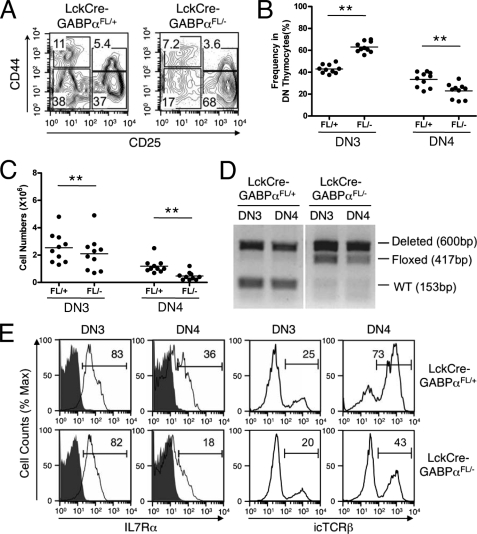
We next examined early T cell development at the DN stages. LckCre-GABPαFL/− mice all exhibited increased frequency of DN3 subsets and a corresponding reduction in DN4 cells compared with LckCre-GABPαFL/+ controls (Fig. 2, A and B). The absolute counts of both DN3 and DN4 thymocytes were diminished in LckCre-GABPαFL/− mice, despite the increased DN3 frequency (Fig. 2C). The Lck-Cre transgene initiates excision of the floxed sequences at the DN2 stage and completes the excision at the DN3 stage (25). To determine the excision efficiency, we sorted DN3 and DN4 thymocytes from LckCre-GABPαFL/+ and LckCre-GABPαFL/− mice and used PCR to detect the conversion of a floxed allele to a deleted allele. In LckCre-GABPαFL/+ DN3 and DN4, the excision of the floxed allele was almost complete as expected, indicating the accessibility of the floxed GABPα allele and efficiency of LckCre-mediated excision (Fig. 2D). Contrary to the expectation that the floxed allele in LckCre-GABPαFL/− DN3 and DN4 cells should be converted to a deleted allele, it was still detectable, although the signal was weaker in DN4 cells. Our observations suggest that the excision of the floxed sequence is efficient in the presence of protection by a WT allele, converting GABPαFL/+ DN3 and DN4 thymocytes to GABPα−/+. On the other hands, in LckCre-GABPαFL/− DN3 and DN4 subsets, there is strong selection against “double deleted” GABPα−/− cells, leading to greatly diminished thymic cellularity. As a result, a considerable portion, if not all, of the remaining DN3 and DN4 thymocytes in LckCre-GABPαFL/− mice is an accumulation of those that escaped the complete excision and were detected as “undeleted” cells. This is not an unprecedented scenario. For example, when Mcl-1, a pro-survival Bcl-2 family member, was conditionally inactivated, profound reduction of thymocytes and peripheral T cells was observed, and the residual T cells found in the mice all contained a protective undeleted allele, indicating that the double deleted cells are not viable (26). Strong selection against Mcl-1-null cells also occurred when it was inactivated in B lineage cells (26) or HSCs (27). Similarly, lymphoblastomic leukemia 1 (Lyl1) and T cell acute lymphocytic leukemia 1 (Tal1/Scl), members of the basic helix-loop-helix class transcription factors, are recently shown to be absolutely required for HSC survival, and Tal1−/−Lyl1FL/FL HSC-derived myeloid colonies always contained at least one Lyl1-floxed allele after type I interferon-induced, Mx1-Cre-mediated excision (28).
FIGURE 2.
Early T cell development in LckCre-GABPαFL/− mice. A–C, ablation of GABPα blocks T cell development at DN3 stage. A, representative flow cytometric profile of DN thymocytes. DN thymocytes from mice of indicated genotypes were further fractionated based on CD25 and CD44 expression, and percentages of DN1-DN4 subsets in the DN population were shown. B, frequency of DN3 and DN4 thymocytes in DN population. C, absolute numbers of DN3 and DN4 thymocytes. **, p < 0.01. D, excision of the floxed GABPα allele in LckCre-GABPαFL/+ and LckCre-GABPαFL/− mice. DN3 and DN4 thymocytes were isolated by cell sorting, and genomic DNA extracted from these cells was analyzed for WT, floxed, and deleted GABPα allele by PCR using primers shown in Fig. 1A. The size of expected PCR products is marked in parentheses. The data are representative from three independent sorting and PCR assessments. E, the expression of IL-7Rα and TCRβ in DN3 and DN4 thymocytes. Cell surface expression of IL-7Rα and intracellular expression of TCRβ were measured in indicated mice, and the percentages of positive population are marked. For IL-7Rα staining, isotype controls are shown as shaded histograms. The data are representative of at least three independent experiments with more than 10 animals of each genotype examined.
We previously demonstrated that GABP critically regulates the expression of IL-7Rα in developing and mature T cells (19). Although LckCre-GABPαFL/− DN3 thymocytes expressed similar levels of IL-7Rα compared with LckCre-GABPαFL/+ DN3 cells, the IL-7Rα expression was reduced in LckCre-GABPαFL/− DN4 cells (Fig. 2E). β-Selection occurs at the DN3 stage, which requires productive rearrangements of the TCRβ locus and successful pre-TCR signaling. The block at the DN3 stage in LckCre-GABPαFL/+ mice is reminiscent of failed TCRβ rearrangements, for example, because of deficiency in Rag recombinases. To determine whether GABPα has IL-7Rα-independent roles in early T cell development, we measured TCRβ expression by intracellular staining. As shown in Fig. 2E, although reduction of intracellular TCRβ was small in DN3 cells, LckCre-GABPαFL/− DN4 thymocytes exhibited more apparent decrease in intracellular TCRβ expression. These observations are consistent with the notion that DN3 and DN4 thymocytes in LckCre-GABPαFL/− mice may consist of a mixture of undeleted (FL/−) and double deleted (−/−) cells. The lack of apparent defects in IL-7Rα and TCRβ expression in DN3 might be explained by the fact that there are fewer double deleted cells because of stronger selection at this developmental stage. On the other hand, DN4 thymocytes might be less stringently dependent on GABPα activity for survival, and thus more double deleted DN4 thymocytes are present, allowing a window for observation of the effects derived from GABPα deficiency and thus revealing critical roles for GABP in supporting the normal expression of IL-7Rα and TCRβ in developing thymocytes.
GABP Directly Regulates Expression of Genes Critical for V(D)J Recombination
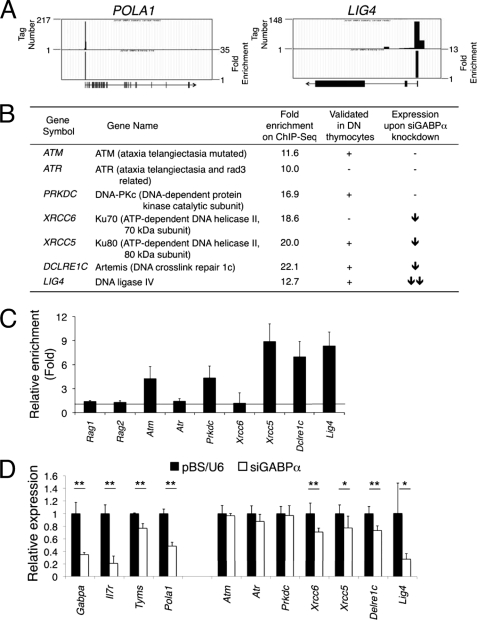
Diminished TCRβ expression in LckCre-GABPαFL/− DN4 thymocytes raised the intriguing possibility that GABP may contribute to the regulation of V(D)J recombination at the TCR loci in addition to its direct role in activating IL-7Rα transcription. High throughput analyses of GABP-associated genes have been performed on human Jurkat T cell lymphoma cells using ChIP coupled with DNA microarrays (ChIP-on-chip) (29) and more recently ChIP coupled with massive parallel sequencing (ChIP-Seq) (23). The ChIP-Seq approach covers the entire genome and has enhanced sensitivity, and we therefore downloaded the ChIP-Seq data of GABPα antibody or control IgG-enriched sequence tags and reanalyzed the data using the SISSRs algorithm (24). Our analysis revealed ∼13,000 GABP binding locations where GABPα-associated sequences are significantly enriched. Focusing on protein-coding genes that harbor GABP-binding sites within 2 kb of their transcription initiation sites (TISs), we found that GABP is associated with 8308 unique RefSeq genes. It was reported that GABP has a unique role in inducing cell cycle re-entry through direct regulation of thymidylate synthase and p180 catalytic subunit of DNA polymerase α (encoded by TYMS and POLA1, respectively) (14). These genes were found to have enriched GABPα binding at their TISs, validating the usefulness of the ChIP-Seq data (GABP binding at the POLA1 locus was shown as an example in Fig. 3A).
FIGURE 3.
GABP directly regulates key genes involved in V(D)J recombination. A, GABP binding at selected gene loci inferred from ChIP-Seq. Sequence tags enriched by GABPα antibody in ChIP-Seq of Jurkat T cells were mapped to human genome on the UCSC genome browser. The raw data are displayed as accumulative tag numbers within a 200-bp-wide window as shown in the upper part of each panel, and the tag numbers are marked on the left. The ChIP-Seq data with GABPα and control IgG samples were further analyzed using the SISSR algorithm to calculate the relative enrichment in individual locations. The data are displayed as enrichment peaks in the lower part of each panel, with their heights (y axis) corresponding to fold enrichment of GABP sequence tags over control tags, as marked on the right of each panel. The gene symbols are as marked, and the gene structure and direction of transcription are shown under each panel, with vertical lines/boxes denoting exons. B, summary of GABPα binding at the TISs of genes involved in V(D)J recombination, including gene symbols, commonly used gene names, and fold enrichment of GABP binding at each gene locus based on the Jurkat ChIP-Seq data. The results from C and D were also summarized in the last two columns. C, validation of GABP binding in DN thymocytes. Chromatin fragments were prepared from murine DN thymocytes and immunoprecipitated with either anti-GABPα antibody or a control IgG. Gene regulatory regions overlapping with TISs of indicated genes were detected by quantitative PCR using primers designed based on the Jurkat ChIP-Seq results. The relative enrichment by the GABPα antibody versus control IgG was shown for each gene. D, effect of knocking down GABPα on the expression of GABP-bound genes. EL-4 cells were transfected with siGABPα or control pBS/U6 vector alone with pEYFP-N1, and EYFP-positive cells were sorted, RNA extracted, and reverse-transcribed. Expression of genes of interest was measured by quantitative PCR. The data are the means ± S.D. of four samples from two independent experiments. *, p < 0.05; **, p < 0.01 by t test.
Based on the phenotypic analysis of LckCre-GABPαFL/− mice, we hypothesized that GABPα may have critical roles in TCRβ rearrangement, which is initiated by expression of RAG1 and RAG2 recombinases that introduce DNA double-strand breaks (DSBs). The DSBs are dangerous lesions that can be sensed and amended in cells with great efficiency to avoid possible chromosomal abnormalities, cell death, or neoplastic transformation. Sensing the DSBs leads to activation of at least three phosphatidyl-inositol 3 kinase-like protein kinases, ATM, ATR, and DNA-dependent protein kinase catalytic subunit (DNA-PKcs) (30). Repair of the DSBs is achieved by nonhomologous end joining (NHEJ) mechanism, which involves Ku70- and Ku80-containing DNA-PK complex, Artemis, Cernunnos, and DNA ligase IV (31). Analysis of these gene loci in the Jurkat ChIP-Seq data revealed that whereas no GABP binding was associated with the RAG1, RAG2, and NHEJ1 (encoding Cernunnos) loci, enriched GABP binding was found at the TISs of ATM, ATR, DCLRE1C (encoding Artemis), LIG4 (encoding DNA ligase IV), PRKDC, XRCC6, and XRCC5 (encoding the catalytic subunit, Ku70, and Ku80 components in the DNA-PK complex, respectively) genes (actual GABP binding at the LIG4 TIS shown in Fig. 3A, and fold enrichment of GABP binding summarized in Fig. 3B). To determine whether such GABP binding occurs in developing thymocytes, we isolated WT DN thymocytes and performed ChIP using an anti-GABPα antibody. The direct binding of GABPα was confirmed in the TISs of Atm Prkdc, Xrcc5, Dclre1c, and Lig4 genes (Fig. 3C), supporting a potential role for GABP in regulating key factors involved in the rearrangements of TCR loci.
It has been known that binding of a transcription factor to gene regulatory regions as found in ChIP-Seq or ChIP-on-chip is not necessarily correlated with its direct activation/repression of the bound genes, as seen previously with Foxp3 and in yeast (32, 33). We next investigated whether the expression levels of GABP-associated genes were altered by diminished GABPα expression. As discussed above, LckCre-GABPαFL/− DN4 thymocytes contained double deleted cells exhibiting reduced levels of IL-7Rα and intracellular TCRβ (Fig. 2E), but they are also mixed with nondeleted FL/− cells that escaped excision because of the selection pressure against the double deleted cells (Fig. 2D). In sorted DN4 thymocytes from LckCre-GABPαFL/+ and LckCre-GABPαFL/− mice, with the exception that GABPα transcript was modestly decreased in LckCre-GABPαFL/− DN4 cells, all other gene transcripts examined did not show consistent changes (data not shown), which is likely explained by obscuration caused by those nondeleted cells. To circumvent this problem, we used siGABPα to knock down its expression in EL-4 thymoma cells (19). After co-transfection of siGABPα or control pBS/U6 vector along with pEYFP-N1 expressing enhanced yellow fluorescent protein into EL-4 cells, we sorted for EYFP-positive cells 48 h later and analyzed gene expression by quantitative reverse transcription-PCR. Consistent with our previous findings (19), the siGABPα constructs effectively reduced the Gabpa transcript and its T cell target gene Il7r (Fig. 3D). In addition, knockdown of Gabpa resulted in decreased expression of Tyms and Pola1, other known GABP targets in regulating cell cycle progression (14) (Fig. 3D). Among the V(D)J recombination-related genes, Lig4 expression was most reduced, and Xrcc6, Xrcc5, and Dclre1c exhibited moderate but consistent reduction upon knockdown of Gabpa, whereas Atm, Atr, and Prkdc expression was not affected (Fig. 3D). Coupled with validated direct binding of GABPα at the TISs in DN thymocytes, our observations suggest that GABP directly regulates the expression of DNA ligase IV (Lig4), Ku80 (Xrcc5), and Artemis (Dclre1c), highlighting its direct involvement in TCRβ rearrangements during early T cell development.
Forced Expression of Prearranged TCR but Not IL-7Rα Can Alleviate DN3 Block Caused by GABPα Ablation
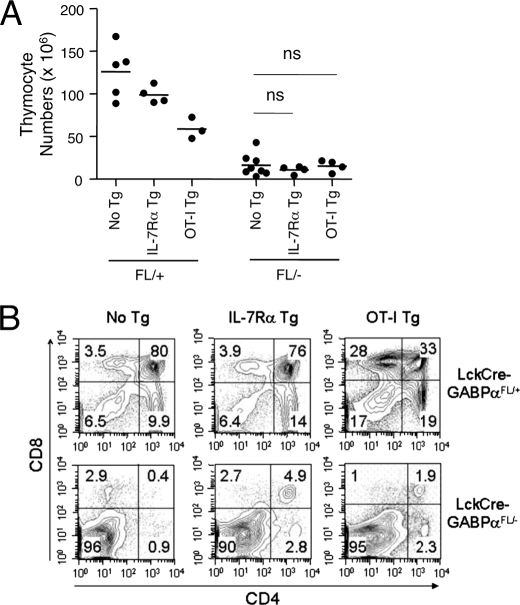
Our biochemical studies identified DNA ligase IV, Ku80, and Artemis as direct GABP target genes in developing T cells in addition to IL-7Rα. Because IL-7-derived signals are critical for TCRγ but not TCRβ rearrangements (34, 35), our findings suggest that GABP regulates at least two independent pathways, IL-7Rα and TCRβ, during the early thymocyte developmental stages. We next asked whether forced expression of IL-7Rα or prearranged TCR could alleviate T cell developmental block caused by GABPα deficiency. Previously we generated IL-7Rα transgenic mice in which the transgene was driven by a CD4 promoter and enhancer, with an intronic suppressor sequence deleted and thus resulting in a pan-T cell overexpression of IL-7Rα (22). IL-7Rα-Tg mice showed an approximately one-third reduction in total thymocytes (Fig. 4A). This can be partly explained by overconsumption of IL-7 because of the forced expression of IL-7Rα in DP thymocytes in the transgenic mice, where under normal conditions IL-7Rα expression in DP cells is completely turned off (34, 36). OT-I TCR transgenic mice express a TCR specific for Ova257–264 epitope, consisting of prearranged Vα2 and Vβ5 chains. Forced expression of a single TCR also reduced total thymic cellularity, which may reflect either enhanced negative selection on self-ligands, reduced positive selection caused by the avidity of TCR/MHC interactions, or reductions in thymocyte expansion caused by more rapid developmental transitions (Fig. 4A) (37). Introduction of either IL-7Rα or TCR transgene did not increase the total thymic cellularity in LckCre-GABPαFL/− mice (Fig. 4A), and the DN block in LckCre-GABPαFL/− mice remained despite expression of either transgene (Fig. 4B).
FIGURE 4.
Forced expression of IL-7Rα or OT-I TCR transgene did not improve thymic cellularity in LckCre-GABPαFL/− mice. A, total thymic cellularity in the presence of IL-7Rα or OT-I transgene. LckCre-GABPαFL/+ and LckCre-GABPαFL/− mice were crossed to IL-7Rα or OT-I TCR transgenic mice to acquire the transgenes. The data are pooled results from two independent experiments with three to eight animals analyzed. ns, not significant. B, representative flow cytometric profiles of thymocytes with CD4 and CD8 staining, and the percentages of each population are shown.
Further analysis of DN subsets revealed that although IL-7Rα transgene increased its expression in both DN3 and DN4 thymocytes, the DN3 block remained in LckCre-GABPαFL/− mice (Figs. 5A and 6A). IL-7Rα expression did not improve TCRβ expression either (Fig. 5B), consistent with previous observations that IL-7Rα-derived signals are not required for TCRβ rearrangement (34). Forced expression of prearranged TCRβ facilitated β selection at the DN3 stage, resulting in a relative accumulation of DN4 thymocytes (Figs. 5A and 6, C and D). Interestingly, TCR expression alleviated DN3 block in LckCre-GABPαFL/− mice, thus supporting the notion that GABP is critically required for normal TCRβ expression and that bypassing such a requirement can substantially improve T cell development despite the lack of GABPα. In the presence of TCR transgene, however, the expression of IL-7Rα in both LckCre-GABPαFL/+ and LckCre-GABPαFL/− DN4 thymocytes was considerably lower than that without the TCR transgene (Fig. 5B). This may be explained by excessive TCR signaling that is known to lead to the down-regulation of IL-7Rα in T cells (38, 39).
FIGURE 5.
Effect of IL-7Rα or OT-I TCR transgene on early thymocyte development. A, representative flow cytometric profiles of DN thymocytes based on CD25 and CD44 fractionation in mice of indicated genotypes. The percentages of each subset are shown. B, detection of cell surface IL-7Rα and intracellular TCRβ expression in the presence or absence of transgenes. Percentages of the positive populations are indicated. The data are representative from two independent experiments with three to eight animals analyzed.
FIGURE 6.
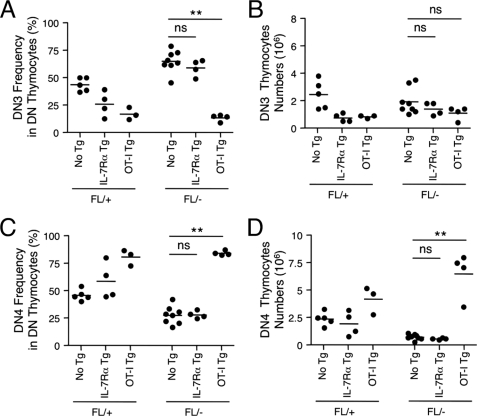
Frequency and numbers of DN3 and DN4 thymocytes in the presence of IL-7Rα or OT-I TCR transgene. DN3 frequency (A) and numbers (B) and DN4 frequency (C) and numbers (D) are shown. ns, not significant; **, p < 0.01. The data are the pooled results for two independent experiments with three to eight animals analyzed.
In terms of cellularity, DN3 thymocytes in LckCre-GABPαFL/− mice in the presence of IL-7Rα or TCR transgene remained diminished compared with those in LckCre-GABPαFL/+ counterparts (Fig. 6B), despite apparent changes in DN3 frequencies (Fig. 6A). On the other hand, the expression of IL-7Rα or TCR transgene resulted in the reduction of DN3 thymocytes in LckCre-GABPαFL/+ mice as well, and this is likely secondary to overall reduction in total thymocytes in the presence of these transgenes (Fig. 4A). In the DN4 compartment, whereas the IL-7Rα transgene did not improve its frequency and numbers, the OT-I TCR increased DN4 frequency as well as numbers (Fig. 6, C and D). It should be noted that early expression of a prearranged TCRα chain has been reported to impact the proliferation and survival of DN4 thymocytes and partially block their development to the DP stage (40, 41). Although OT-I TCR had the smallest impact compared with other TCR transgenes such as C2 and HY (41), these side effects should be taken into account for the interpretation of increased DN4 subset. Nonetheless, our observations using IL-7Rα and TCR transgenes collectively provided corroborating evidence for a critical role of GABPα in TCRβ rearrangements.
DISCUSSION
The GABPα/β complex has versatile roles in regulating basic cellular functions as well as tissue-specific genes (13, 42, 43). Gene targeting studies of the GABPα DNA-binding subunit have revealed its critical roles during embryogenesis (44), during re-entry of cell cycle (14), and at the neuro-muscular junction (16, 45). Our previous studies on the gene regulation of IL-7Rα have identified GABP as a nonredundant factor in supporting transcription of IL-7Rα in T cells (19). In this study, we found that ablation of GABPα in developing T cells by Lck-Cre transgene resulted in severe reduction of thymic cellularity, a substantial block at the DN3 stage, and diminished intracellular expression of TCRβ in DN4 thymocytes. By biochemical analysis, we demonstrated a link between GABP and genes critical for V(D)J recombination, and we further found that forced expression of a prearranged TCR but not IL-7Rα alleviated the DN3 block caused by GABPα inactivation. These findings revealed an unexpected contribution of GABP to normal TCRβ rearrangements, in addition to its direct regulation of IL-7Rα, which provides survival signals to developing thymocytes.
Assessment of conversion of the floxed GABPα allele to a deleted one in DN3 and DN4 thymocytes of LckCre-GABPαFL/− mice revealed a strong selection against double deleted, i.e. GABPα-null cells. Such strong selection against cells lacking genes that are essential for cell survival and/or proliferation have been observed in multiple lineages including T and B lymphocytes as well as HSCs when Mcl-1, a pro-survival Bcl-2 family member, was targeted (26, 27). Similar selection against double deleted cells was also observed in HSCs lacking another Ets family transcription factor Etv6 (46) or Tal1/Scl and Lyl1 (28). The strong selection against GABP-null cells thus suggests an essential role for GABP in thymocyte survival and/or proliferation. Indeed, it has been demonstrated in murine embryonic fibroblasts that GABP directly regulated gene expression of Pola1 and Tyms (14). Binding of GABP to these gene loci was found in Jurkat T cells (23) and validated in DN thymocytes in this study. We further showed decreased expression of Pola1 and Tyms upon knockdown of GABPα in EL-4 cells. Because DN3 and DN4 thymocytes are highly proliferative, their expansion is conceivably severely impaired when GABPα is ablated, thus at least partly explaining the greatly reduced thymic cellularity. The requirement of GABP in cell proliferation may be extended to DP thymocytes and later stages, which may explain why forced expression of OT-I TCR, which consists of both prearranged TCRα and TCRβ chains, failed to promote further thymocyte maturation and improve overall thymic numbers. It should be noted that the role of GABP in promoting cell proliferation is more general in various cell types, unlike its more T cell-specific regulation of IL-7Rα and V(D)J recombination at the TCR loci.
Because of the strong selection against double deleted cells, DN3 and DN4 thymocytes in LckCre-GABPαFL/− mice contained both −/− and FL/− phenotype cells, and these FL/− cells likely accounted for the positively detected intracellular TCRβ expression in the DN3 and DN4 thymocytes. This precluded a direct measurement of V(D)J recombination status in the TCRβ locus in the complete absence of GABPα. A contribution of the GABP complex to V(D)J recombination is supported by our findings that GABP directly regulates DNA ligase IV and other key molecules involved in this process and that forced expression of a prearranged TCR alleviated DN3 block caused by GABPα inactivation. However, more direct assessment of TCRβ recombination awaits future studies using different animal models, such as conditional targeting of GABPβ isoforms or GABPα knock-in, where thymocyte proliferation/survival may be less dependent on an intact GABP complex.
There are >30 members in the Ets transcription factor family in mammalian genome, all containing the conserved DNA-binding Ets domain, which recognizes an invariant GGA(A/T) consensus sequence (29, 42). Many Ets factors including GABPα are expressed ubiquitously, and some are often co-expressed in the same tissue/cell type (47, 48), yet each Ets factor demonstrated unique biological functions, as evidenced by distinct phenotypes in mice with individual factor targeted (48). Thus, it remains a major challenge as to how redundancy of these Ets factors is resolved in vivo to achieve specificity of an individual factor. Recent technological advances permitted global mapping of transcription factor occupancy by means of ChIP-on-chip or ChIP-Seq, with Ets-1, Elf-1, and GABPα binding locations mapped in Jurkat T cells (23, 29) and Elk-1 binding locations mapped in HeLa cells (49). Comparative analysis of these high throughput data revealed not only specific binding targets for each factor but also unexpected co-occupancy of the same genomic regions by multiple Ets proteins (29, 49). These observations thus offer one plausible explanation to certain dissociation of transcription factor binding from actual transcription status of the bound genes. Among V(D)J recombination-related genes analyzed in this study, most GABPα binding in Jurkat T cells was validated in primary DN thymocytes. However, only DNA ligase IV exhibited substantial decreased expression, similar to IL-7Rα and POLA1, in cells transfected with siGABPα, whereas other GABPα-bound genes showed moderate but consistent decrease (Ku80 and Artemis) or no expression changes (ATM and DNA-PKc). Thus, GABPα-associated genes can be roughly grouped into two subsets, with one group being more strictly dependent on GABPα (such as DNA ligase IV, IL-7Rα, and POLA1) and the other less dependent. Searching the existing genome-wide binding data by Ets factors indeed showed that the LIG4 gene was void of other Ets factor binding, whereas the XRCC5 gene was bound by Ets1, Elf-1, and Elk-1, and the ATM gene was bound by Elf-1 as well (29, 49). Certainly, selective regulation of target genes by GABP is not solely determined by co-occupancy by other transcription factors, and additional contributing factors at least contain a relative abundance of other Ets proteins (48), epigenetic modification of target genes (50), and interaction with tissue-specific partner proteins (51). Our results thus highlight the usefulness of genome-wide transcription factor binding data and the importance and necessity to individually evaluate biological function-related genes in a cell type of interest.
Development of B cells from pluripotent hematopoietic precursors is guided by three transcription factors, E2A, early B cell factor, and Pax5, and these factors act in a relatively simple gene regulatory cascade in B lineage specification and commitment (52, 53). In contrast, no equivalent T cell-specific transcription factors have unequivocal positive roles in instructing T lineage commitment and/or maintaining T cell identity. Rather, multiple transcription factors, T cell-specific or nonspecific, act in concert to direct the progressive commitment and maturation of T cells (2). Systematic analysis of transcription factors, with demonstrated essential roles or as yet undefined function in T cell development, identified different expression patterns throughout early T cell developmental stages (11). Along with Myb, Ikaros, Gfi-1, Stat5b, Oct1, and TOX, GABPα was classified in the group of “legacy” transcription factors, showing minimally changing transcript levels. Their stable expression during early T cell development contrasts remarkably with the massive changes in T-lineage gene expression. It is plausible to assume that their stable expression forms a critical platform for modulation of and/or interaction with “up-regulated” or “down-regulated” transcription factors, thus creating a genetic environment optimal for T cell commitment. The molecular details and dynamic changes in the regulatory circuitry at different thymocyte developmental stages await further investigation. Nonetheless, our current studies revealed GABP as a key component in such a regulatory circuit, which has essential and multifaceted roles in programming normal T cell development.
Acknowledgments
We are grateful to Dr. Steven Burden (Skirball Institute, New York University Medical School) for providing the GABPα-targeted mice and George Rasmussen and Heath Vignes for cell sorting (Flow Cytometry Facility, University of Iowa). We thank Dr. Keji Zhao (Laboratory of Molecular Immunology, NHLBI, National Institutes of Health) for useful discussion on analysis of ChIP-Seq data.
This work was supported, in whole or in part, by National Institutes of Health Grants AI077504 and HL095540 (to H.-H. X.). This work was also supported by the Intramural Research Program of the NIEHS, National Institutes of Health (to R. J.).
- DN
- double negative
- DP
- double positive
- SP
- single positive
- GABP
- GA binding protein
- IL-7Rα
- interleukin-7 receptor α chain
- TCR
- T cell receptor
- HSC
- hematopoietic stem cells
- ChIP
- chromatin immunoprecipitation
- TIS
- transcription initiation site
- DNA-PK
- DNA-dependent protein kinase
- EYFP
- enhanced yellow fluorescent protein
- SISSRs
- Site Identification from Short Sequence Reads
- siGABPα
- small interference RNA constructs targeting GABPα
- WT
- wild type
- DSB
- double-strand break.
REFERENCES
- 1.Bhandoola A., von Boehmer H., Petrie H. T., Zúñiga-Pflücker J. C. (2007) Immunity 26, 678–689 [DOI] [PubMed] [Google Scholar]
- 2.Rothenberg E. V., Taghon T. (2005) Annu. Rev. Immunol. 23, 601–649 [DOI] [PubMed] [Google Scholar]
- 3.Rothenberg E. V., Moore J. E., Yui M. A. (2008) Nat. Rev. Immunol. 8, 9–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott E. W., Simon M. C., Anastasi J., Singh H. (1994) Science 265, 1573–1577 [DOI] [PubMed] [Google Scholar]
- 5.Georgopoulos K. (2002) Nat. Rev. Immunol. 2, 162–174 [DOI] [PubMed] [Google Scholar]
- 6.Maillard I., Fang T., Pear W. S. (2005) Annu Rev. Immunol. 23, 945–974 [DOI] [PubMed] [Google Scholar]
- 7.Robey E. A., Bluestone J. A. (2004) Curr. Opin. Immunol. 16, 360–366 [DOI] [PubMed] [Google Scholar]
- 8.Engel I., Murre C. (2001) Nat. Rev. Immunol. 1, 193–199 [DOI] [PubMed] [Google Scholar]
- 9.Staal F. J., Clevers H. C. (2005) Nat. Rev. Immunol. 5, 21–30 [DOI] [PubMed] [Google Scholar]
- 10.Pai S. Y., Truitt M. L., Ting C. N., Leiden J. M., Glimcher L. H., Ho I. C. (2003) Immunity 19, 863–875 [DOI] [PubMed] [Google Scholar]
- 11.David-Fung E. S., Butler R., Buzi G., Yui M. A., Diamond R. A., Anderson M. K., Rowen L., Rothenberg E. V. (2009) Dev. Biol. 325, 444–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrett-Sinha L. A., Dahl R., Rao S., Barton K. P., Simon M. C. (2001) Blood 97, 2908–2912 [DOI] [PubMed] [Google Scholar]
- 13.Rosmarin A. G., Resendes K. K., Yang Z., McMillan J. N., Fleming S. L. (2004) Blood Cells Mol. Dis 32, 143–154 [DOI] [PubMed] [Google Scholar]
- 14.Yang Z. F., Mott S., Rosmarin A. G. (2007) Nat. Cell Biol. 9, 339–346 [DOI] [PubMed] [Google Scholar]
- 15.Crook M. F., Olive M., Xue H. H., Langenickel T. H., Boehm M., Leonard W. J., Nabel E. G. (2008) FASEB J. 22, 225–235 [DOI] [PubMed] [Google Scholar]
- 16.O'Leary D. A., Noakes P. G., Lavidis N. A., Kola I., Hertzog P. J., Ristevski S. (2007) Mol. Cell. Biol. 27, 3470–3480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosmarin A. G., Luo M., Caprio D. G., Shang J., Simkevich C. P. (1998) J. Biol. Chem. 273, 13097–13103 [DOI] [PubMed] [Google Scholar]
- 18.Xue H. H., Bollenbacher-Reilley J., Wu Z., Spolski R., Jing X., Zhang Y. C., McCoy J. P., Leonard W. J. (2007) Immunity 26, 421–431 [DOI] [PubMed] [Google Scholar]
- 19.Xue H. H., Bollenbacher J., Rovella V., Tripuraneni R., Du Y. B., Liu C. Y., Williams A., McCoy J. P., Leonard W. J. (2004) Nat. Immunol. 5, 1036–1044 [DOI] [PubMed] [Google Scholar]
- 20.Bradley L. M., Haynes L., Swain S. L. (2005) Trends Immunol. 26, 172–176 [DOI] [PubMed] [Google Scholar]
- 21.Jaworski A., Smith C. L., Burden S. J. (2007) Mol. Cell. Biol. 27, 5040–5046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haring J. S., Jing X., Bollenbacher-Reilley J., Xue H. H., Leonard W. J., Harty J. T. (2008) J. Immunol. 180, 2855–2862 [DOI] [PubMed] [Google Scholar]
- 23.Valouev A., Johnson D. S., Sundquist A., Medina C., Anton E., Batzoglou S., Myers R. M., Sidow A. (2008) Nat. Methods 5, 829–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jothi R., Cuddapah S., Barski A., Cui K., Zhao K. (2008) Nucleic Acids Res. 36, 5221–5231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee P. P., Fitzpatrick D. R., Beard C., Jessup H. K., Lehar S., Makar K. W., Pérez-Melgosa M., Sweetser M. T., Schlissel M. S., Nguyen S., Cherry S. R., Tsai J. H., Tucker S. M., Weaver W. M., Kelso A., Jaenisch R., Wilson C. B. (2001) Immunity 15, 763–774 [DOI] [PubMed] [Google Scholar]
- 26.Opferman J. T., Letai A., Beard C., Sorcinelli M. D., Ong C. C., Korsmeyer S. J. (2003) Nature 426, 671–676 [DOI] [PubMed] [Google Scholar]
- 27.Opferman J. T., Iwasaki H., Ong C. C., Suh H., Mizuno S., Akashi K., Korsmeyer S. J. (2005) Science 307, 1101–1104 [DOI] [PubMed] [Google Scholar]
- 28.Souroullas G. P., Salmon J. M., Sablitzky F., Curtis D. J., Goodell M. A. (2009) Cell Stem Cell 4, 180–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollenhorst P. C., Shah A. A., Hopkins C., Graves B. J. (2007) Genes Dev. 21, 1882–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Callén E., Nussenzweig M. C., Nussenzweig A. (2007) Oncogene 26, 7759–7764 [DOI] [PubMed] [Google Scholar]
- 31.Soulas-Sprauel P., Rivera-Munoz P., Malivert L., Le Guyader G., Abramowski V., Revy P., de Villartay J. P. (2007) Oncogene 26, 7780–7791 [DOI] [PubMed] [Google Scholar]
- 32.Harbison C. T., Gordon D. B., Lee T. I., Rinaldi N. J., Macisaac K. D., Danford T. W., Hannett N. M., Tagne J. B., Reynolds D. B., Yoo J., Jennings E. G., Zeitlinger J., Pokholok D. K., Kellis M., Rolfe P. A., Takusagawa K. T., Lander E. S., Gifford D. K., Fraenkel E., Young R. A. (2004) Nature 431, 99–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marson A., Kretschmer K., Frampton G. M., Jacobsen E. S., Polansky J. K., MacIsaac K. D., Levine S. S., Fraenkel E., von Boehmer H., Young R. A. (2007) Nature 445, 931–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazzucchelli R., Durum S. K. (2007) Nat. Rev. Immunol. 7, 144–154 [DOI] [PubMed] [Google Scholar]
- 35.Durum S. K., Candèias S., Nakajima H., Leonard W. J., Baird A. M., Berg L. J., Muegge K. (1998) J. Exp. Med. 188, 2233–2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munitic I., Williams J. A., Yang Y., Dong B., Lucas P. J., El Kassar N., Gress R. E., Ashwell J. D. (2004) Blood 104, 4165–4172 [DOI] [PubMed] [Google Scholar]
- 37.Brabb T., Huseby E. S., Morgan T. M., Sant'Angelo D. B., Kirchner J., Farr A. G., Goverman J. (1997) Eur. J. Immunol. 27, 136–146 [DOI] [PubMed] [Google Scholar]
- 38.Kaech S. M., Tan J. T., Wherry E. J., Konieczny B. T., Surh C. D., Ahmed R. (2003) Nat. Immunol. 4, 1191–1198 [DOI] [PubMed] [Google Scholar]
- 39.Schluns K. S., Kieper W. C., Jameson S. C., Lefrançois L. (2000) Nat. Immunol. 1, 426–432 [DOI] [PubMed] [Google Scholar]
- 40.Borowski C., Li X., Aifantis I., Gounari F., von Boehmer H. (2004) J. Exp. Med. 199, 607–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lacorazza H. D., Tucek-Szabo C., Vasović L. V., Remus K., Nikolich-Zugich J. (2001) J. Immunol. 166, 3184–3193 [DOI] [PubMed] [Google Scholar]
- 42.Sharrocks A. D. (2001) Nat. Rev. Mol. Cell Biol. 2, 827–837 [DOI] [PubMed] [Google Scholar]
- 43.Gallant S., Gilkeson G. (2006) Arch. Immunol. Ther. Exp. 54, 149–163 [DOI] [PubMed] [Google Scholar]
- 44.Ristevski S., O'Leary D. A., Thornell A. P., Owen M. J., Kola I., Hertzog P. J. (2004) Mol. Cell. Biol. 24, 5844–5849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ravel-Chapuis A., Vandromme M., Thomas J. L., Schaeffer L. (2007) EMBO J. 26, 1117–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hock H., Meade E., Medeiros S., Schindler J. W., Valk P. J., Fujiwara Y., Orkin S. H. (2004) Genes Dev. 18, 2336–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galang C. K., Muller W. J., Foos G., Oshima R. G., Hauser C. A. (2004) J. Biol. Chem. 279, 11281–11292 [DOI] [PubMed] [Google Scholar]
- 48.Hollenhorst P. C., Jones D. A., Graves B. J. (2004) Nucleic Acids Res. 32, 5693–5702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boros J., Donaldson I. J., O'Donnell A., Odrowaz Z. A., Zeef L., Lupien M., Meyer C. A., Liu X. S., Brown M., Sharrocks A. D. (2009) Genome Res. 19, 1963–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lucas M. E., Crider K. S., Powell D. R., Kapoor-Vazirani P., Vertino P. M. (2009) J. Biol. Chem. 284, 14698–14709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wallerman O., Motallebipour M., Enroth S., Patra K., Bysani M. S., Komorowski J., Wadelius C. (2009) Nucleic Acids Res. 37, 7498–7508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Busslinger M. (2004) Annu Rev. Immunol. 22, 55–79 [DOI] [PubMed] [Google Scholar]
- 53.Medina K. L., Pongubala J. M., Reddy K. L., Lancki D. W., Dekoter R., Kieslinger M., Grosschedl R., Singh H. (2004) Dev. Cell 7, 607–617 [DOI] [PubMed] [Google Scholar]