Abstract
Parathyroid hormone (PTH) regulates calcium homeostasis and bone metabolism by activating PTH type I receptor (PTH1R). Here we show that transforming growth factor (TGF)-β type II receptor (TβRII) forms an endocytic complex with PTH1R in response to PTH and regulates signalling by PTH and TGF-β. TβRII directly phosphorylates the PTH1R cytoplasmic domain, which modulates PTH-induced endocytosis of the PTH1R–TβRII complex. Deletion of TβRII in osteoblasts increases the cell-surface expression of PTH1R and augments PTH signalling. Conditional knockout of TβRII in osteoblasts in mice results in a high bone mass with increased trabecular bone and decreased cortical bone, similar to the bone phenotype in mice expressing a constitutively active PTH1R. Disruption of PTH signalling by injection of PTH(7–34) or ablation of PTH1R rescues the bone phenotype of TβRII knockout mice. These studies reveal a previously unrecognized function for TβRII and a mechanism for integration of PTH and local growth factor at the membrane receptor level.
The bones of most mammals are continuously formed and resorbed through a process called bone remodelling1. The bone remodelling cycle is achieved through the coordinated activity of two cell types: osteoblasts, which deposit the calcified bone matrix; and osteoclasts, which resorb bone2. Osteoclasts probably evolved as an adaptive mechanism to regulate the mineral-ion homeostasis of terrestrial vertebrates. PTH is secreted by the parathyroid glands, which also first emerged in terrestrial vertebrates, presumably to regulate bone remodelling by acting directly on osteoblasts and indirectly on the osteoclast1. The interaction of PTH with locally osteotropic factors such as TGF-β and insulin-like growth factor (IGF)3, which are evolutionarily conserved in aquatic vertebrates4–7, orchestrates an anabolic signalling network for the coupling of bone resorption and formation. However, the mechanisms responsible for the interaction of these osteotropic factors are still unclear.
On binding to PTH1R8,9, a G-protein-coupled seven-transmembrane receptor (GPCR), PTH activates Gαs and Gαq, leading to the production of cyclic AMP, activating cAMP-dependent protein kinase (PKA) and stimulating phospholipase for the activation of protein kinase C (PKC)10–12. Signalling by PTH through PKA and PKC is rapidly shut off in association with the endocytosis of PTH1R. Phosphorylation at the cytoplasmic domain of PTH1R is crucial for the recruitment of arrestin proteins, which are required for the endocytic process13.
TGF-β1 is present abundantly in the bone matrix. Active TGF-β1 released during osteoclastic bone resorption induces the migration of bone mesenchymal stem cells to couple bone resorption with formation14. TGF-β elicits its cellular response through the ligand-induced formation of a heteromeric complex containing TGF-β types I (TβRI) and II (TβRII) kinase receptors15–17. TβRII is a constitutively active serine/threonine (S/T) kinase that transphosphorylates the GS motif of TβRI on ligand binding, resulting in subsequent phosphorylation of a subclass of intracellular signalling molecules called R-Smads. R-Smads then interact with Co-Smad (Smad4) and translocate into the nucleus, where they induce cellular responses by acting as transcription factors18–20. Several lines of evidence have indicated that PTH and TGF-β work in concert to exert their physiological activities in bone. For example, PTH increases the concentration of TGF-β in bone3. In addition, PTH induces bone resorption by directly activating osteoblasts1, which release osteotropic growth factors (including TGF-β) from the bone matrix. PTH requires TGF-β/Smad3 signalling to exert its anti-apoptotic effects in osteoblasts21. TGF-β has parathyroid hormone-related peptide (PTHrP)-dependent and PTHrP-independent effects on endochondral bone formation22. These factors may therefore work jointly to couple bone resorption to bone formation23,24.
Endocytosis of growth factors and GPCRs is known to integrate different signalling pathways25. We found that PTH induced the recruitment of TβRII as an endocytic activator. TβRII directly phosphorylated the cytoplasmic domain of PTH1R and facilitated PTH-induced endocytosis of the PTH1R–TβRII complex. In particular, the signalling of both receptors was coordinately regulated during endocytosis. Disruption of TGF-β in osteoblasts in mice increased the cell-surface expression of PTH1R and produced a bone phenotype that mimicked those seen in mice expressing a constitutively active PTH1R. These findings show a functional interaction between PTH and TGF-β receptors that integrates the activities of these two critical bone remodelling factors.
RESULTS
PTH induces endocytosis of TβRII
Endocytosis of seven-transmembrane receptors has been shown to integrate signals of different pathways. To test whether endocytosis of PTH1R coordinates the signals of PTH and TGF-β, we first examined the effect of PTH on internalization of TβRII. Flag-tagged TβRII was expressed in human embryonic kidney 293 (HEK293) cells or HEK293 cells stably expressing PTH1R (HEK293-PTH1R). In the absence of PTH, TβRII was present predominantly at the cell surface at 16 h post-transfection (Fig. 1a; Supplementary Information, Fig. S1a). When stimulated with PTH, TβRII was internalized in HEK293-PTH1R cells (Fig. 1a). To reveal PTH ligand, PTH was labelled with the red fluorophore tetramethylrhodamine (TMR) at Lys 13 (PTHTMR). PTHTMR bound only to cells expressing PTH1R and was internalized into intracellular vesicles within 30 min (Fig. 1b, top panels). Immunostaining showed that internalization of TβRII was induced by PTHTMR in a time-dependent fashion, similarly to that of PTHTMR (Fig. 1b, middle panels). In addition, the amount of cell-surface TβRII was decreased significantly in 30 min (Fig. 1c). In particular, the internalized TβRII was co-localized with PTHTMR in the cytoplasmic vesicles or in proximity to the membrane (Fig. 1b, bottom panels). Consistent with this observation, measurement of TβRII levels on the plasma membrane by cell-surface biotinylation showed that PTH decreased endogenous cell-surface TβRII by more than 80% in 20 min (Fig. 1d, e). In contrast, PTHTMR did not induce endocytosis of IGF type I receptor (IGF1R) and bone morphogenetic protein type II receptor (BMPRII) (Supplementary Information, Fig. S1b). To assess the physical interaction between PTH and TβRII directly, we employed fluorescence resonance energy transfer (FRET) between PTHTMR (acceptor) and TβRII fused with green fluorescent protein (TβRIIGFP, donor) (Fig. 1f; Supplementary Information, Fig. S2). The FRET efficiency determined by donor recovery after acceptor photobleaching was 10.57 ± 2.1% s.d., indicating that PTH recruited TβRII into close proximity despite the fact that the two fluorophores were separated by plasma membrane. The FRET efficiency between PTH and a mutant receptor (TβRII-K277R) devoid of kinase activity was less than 2.5%, suggesting that the kinase activity is involved in the interaction. Taken together, these results show that PTH induces PTH1R-dependent endocytosis of TβRII.
Figure 1.
PTH induces endocytosis of TβRII. (a, b) PTH induces endocytosis of TβRII in cells expressing PTH1R. HEK293 cells or HEK293-PTH1R cells were transfected with TβRII–Flag for 16 h. The cells were then stimulated with PTH or PTHTMR for the indicated periods and immunostained with anti-Flag antibody (green). TβRII is internalized after PTH treatment (a) and co-localized with PTHTMR (red) during endocytosis (b). Scale bars, 10 μm. (c) Quantitative assessment of endocytic trafficking of TβRII induced by PTH. The cells were classified by expressing TβRII into three types (membrane, membrane/vesicle and vesicle) as described in Methods. (d, e) PTH decreases the endogenous TβRII level at the cell surface. HEK293-PTH1R cells were stimulated with PTH for the indicated periods and then biotinylated at the cell surface. The cell lysates were immunoprecipitated (IP) with anti-TβRII antibody, and the immunoprecipitates were probed with streptavidin-labelled horseradish peroxidase to detect cell-surface TβRII, and with anti-TβRII antibody to detect total TβRII (d). The ratio of cell-surface TβRII to total TβRII was determined with the value at time zero being set to 100% (e). (f) PTH recruits TβRII into close proximity. FRET was performed between PTHTMR and TβRIIGFP or TβRII-K277RGFP. The efficiency of FRET was determined by donor (GFP) recovery after acceptor (TMR) photobleaching. Two asterisks, P < 0.01 versus the unbleached area. Results in panels c, e and f are shown as the mean ± s.d. (panels c, e, n = 3; panel f, n = 4). Uncropped images of blots are shown in Supplementary Information, Fig. S9.
PTH induces the formation and internalization of TβRII–PTH1R complexes
To gain information on the nature of the physical interaction between PTH1R and TβRII, yellow fluorescent protein (YFP)-based protein-fragment complementation assay (PCA) was used26,27. The YFP1 fragment was fused to the carboxy terminus of TβRII, and the YFP2 fragment was fused to the C terminus of PTH1R. The PCA strategy enables the visualization of protein–protein interactions in living cells based on the reconstitution of the YFP fluorophore when complementary YFP-labelled fragments come into close proximity (Fig. 2a). Expression of TβRII–YFP1 or PTH1R–YFP2 alone in the presence of PTH, or together without PTH, did not generate fluorescence (Fig. 2b, c). YFP fluorescence was induced on the plasma membrane when both plasmids were expressed in the presence of exogenous PTH, whereas no fluorescence was observed with exogenous TGF-β (Fig. 2b, c), indicating that PTH induces the interaction between TβRII and PTH1R. As expected, PTHTMR was predominantly co-localized with the reconstituted YFP fluorophores at the cell membrane by 30 s and became visible as intracellular puncta by 5 min (Fig. 2d). In contrast, the interaction of PTH1R with TβRII-K277R was not seen (Fig. 2c, d), even though TβRII-K277R was well expressed (Supplementary Information, Fig. S3). The formation of the complex of PTH1R with TβRII was confirmed in immunoprecipitation experiments with overexpressed proteins (Fig. 2e) or endogenous proteins in primary osteoblasts (Fig. 2f). Again, no interaction between PTH1R and TβRII-K277R was observed (Fig. 2e). Because several GPCRs have been reported to form dimers or oligomers capable of signalling28,29, we investigated whether interaction between PTH1R and TβRII was necessary for the dimerization or oligomerization of PTH1R. PCA was performed with PTH1R that had been fused with different YFP fragments at the same location on the cytoplasmic tail of PTH1R (Fig. 2g). PTH reconstituted YFP fluorescence by 30 s (Fig. 2h, upper panels) and PTHTMR was predominantly co-localized with the YFP fluorescence (Fig. 2h, lower panels), indicating PTH-induced formation of PTH1R dimer or oligomer. Most of the TβRII was co-localized with the YFP fluorophores of PTH1R dimer/oligomer (Fig. 2i), suggesting that TβRII can be recruited by, but may not be essential for, the dimerization or oligomerization of PTH1R. In line with these observations, PTH induced the recruitment and co-localization of TβRII with β-arrestin (Fig. 3), an adaptor protein involved in PTH endocytosis. Taken together, these results demonstrate that PTH induces the interaction of TβRII with PTH1R and the internalization of TβRII–PTH1R as a complex.
Figure 2.
PTH induces the formation and internalization of TβRII–PTH1R complexes. (a) Strategy of YFP-based PCA (YFP-PCA). (b, c) PTH, but not TGF-β, induces an interaction of PTH1R with TβRII as shown with YFP-PCA. HEK293 cells were transfected with indicated plasmids and then treated with PTH or TGF-β for 30 s. The reconstituted YFP fluorescence appears green. The cells were stained with 4,6-diamidino-2-phenylindole (DAPI) (blue)(b), and YFP fluorescence was quantified by fluorimetry normalized to untransfected cells. Scale bars, 10 μm. Two asterisks, P < 0.01 versus untreated (c). (d) Co-internalization of TβRII with PTH/PTH1R. HEK293 cells co-expressing TβRII–YFP1 and PTH1R–YFP2 were treated with PTHTMR for 30 s or 5 min. Reconstituted YFP fluorescence appears green and PTHTMR appears red. Co-localization of PTH and the TβRII–PTH1R complex appears yellow. Scale bars, 10 μm. (e, f) TβRII specifically interacts with PTH1R in response to PTH in immunoprecipitation assays. HEK293 cells co-transfected with indicated plasmids (e) or primary calvarial osteoblasts (f) were treated with PTH for 30 min. Cell extracts were immunoprecipitated (IP) and immunoblotted (IB) with the indicated antibodies. The asterisk indicates that the cells were lysed in IP buffer with 0.5% SDS for immunoblotting. HA, haemagglutinin. (g, h) PTH induces the formation of PTH1R dimer/oligomer as shown with YFP-PCA illustrated in g. HEK293 cells co-expressing PTH1R–YFP1 and PTH1R–YFP2 were treated with PTH and then stained with DAPI (blue). Reconstituted YFP fluorescence on formation of the PTH1R dimer/oligomer appears green (h, upper panel). Co-localization of PTHTMR (red) and the PTH1R dimer/oligomer (green) appears yellow (h, lower panel). Scale bars, 10 μm. (i) TβRII is mainly co-localized with PTH-induced PTH1R dimer/oligomer. HEK293 cells co-expressing PTH1R–YFP1, PTH1R–YFP2 and TβRII–Flag were treated with PTH for 5 min and then immunostained with anti-Flag antibody. Reconstituted YFP fluorescence appears green, TβRII appears red, and co-localization of TβRII and the PTH1R dimer/oligomer appears yellow. Results in panels c are shown as the mean ± s.d. (n = 3). Uncropped images of blots are shown in Supplementary Information, Fig. S9.
Figure 3.
PTH induces recruitment and co-localization of TβRII with β-arrestin. (a) PTHTMR (red)-induced recruitment of β-arrestin2–GFP (βarr–GFP; green) expressed in HEK293-PTH1R cells. The cells were treated with PTHTMR for 30 min (right three panels) or were untreated (left panel). (b) PTH induces co-localization of TβRII with β-arrestin2–GFP. The HEK293-PTH1R cells co-transfected with TβRII–Flag and β-arrestin2–GFP were treated with PTHTMR for 30 min. TβRII–Flag (blue) was immunostained with anti-Flag antibody. Co-localization of PTHTMR, TβRII and β-arrestin2–GFP in the merged image appears white. Scale bars, 10 μm.
Endocytosis of TβRII–PTH1R coordinates signalling of PTH and TGF-β
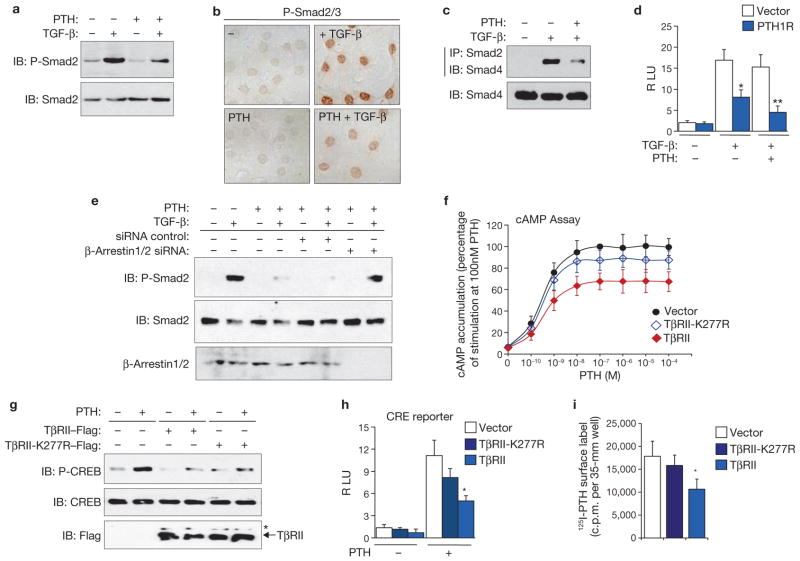
The results described above suggest that internalization of the PTH1R–TβRII complex regulates the signalling of PTH and TGF-β. Indeed, PTH decreased TGF-β-induced phosphorylation and the nuclear accumulation of Smad2/Smad3 (Fig. 4a, b) and blunted the interaction of Smad2 with Smad4 (Fig. 4c). Expression of PTH1R inhibited TGF-β-induced transcriptional activity of a Smad-binding element (SBE) reporter, and PTH enhanced the inhibition (Fig. 4d; Supplementary Information, Fig. S4a). In addition, inhibition of endocytosis by knocking down β-arrestin-1/2 relieved the inhibitory effect of PTH on the phosphorylation of Smad2 (Fig. 4e). These results indicate that PTH attenuates TGF-β/Smad signalling by inducing the endocytosis of PTH1R–TβRII as a complex. Conversely, expression of TβRII decreased PTH-induced cAMP accumulation (Fig. 4f) and inhibited the PTH-induced phosphorylation of cAMP-response-element-binding protein (CREB) (Fig. 4g) and the transcriptional activity of a CREB responsive element (CRE) reporter (Fig. 4h; Supplementary Information, Fig. S4b). Moreover, expression of TβRII decreased the cell-surface concentration of PTH1R in a 125I-PTH binding assay (Fig. 4i). The inhibitory effects of TβRII on PTH signalling were decreased by co-expression of TβRI (Supplementary Information, Fig. S5), probably as a result of decreased availability of TβRII through its interaction with TβRI (ref. 15). Indeed, TβRII-K277R also showed significantly decreased effects (Fig. 4f–i). These findings indicate that TβRII inhibits PTH signalling by inducing the endocytosis of PTH1R and decreasing cell-surface PTH1R. Taken together, these results indicate that PTH-induced endocytosis of PTH1R–TβRII modulates the signalling of both PTH and TGF-β.
Figure 4.
Endocytosis of TβRII–PTH1R coordinates signalling of PTH and TGF-β. (a–c) PTH inhibits the TGF-β-induced phosphorylation of Smad2, the nuclear accumulation of phosphorylated Smad2/3 and the interaction between Smad2 and Smad4, as determined with immunoblotting (IB), immunostaining and immunoprecipitation (IP), respectively. C3H10T1/2 cells transduced with pMSCV-PTH1R–HA retrovirus were pretreated with PTH for 30 min, and then treated with TGF-β for 15 min for immunoblotting, or 30 min for immunostaining and for immunoprecipitation. (d) PTH represses TGF-β-induced SBE luciferase transcription activities in HEK293 cells. RLU, relative luciferase units. (e) Knocking down of β-arrestins eliminates the inhibitory effect of PTH on TGF-β-induced Smad2 phosphorylation in HEK293-PTH1R cells. (f) Expression of TβRII inhibits the PTH-induced accumulation of intracellular cAMP. UMR106 cells transfected with the indicated plasmids were stimulated with the indicated concentrations of PTH for 15 min. cAMP accumulation was detected, and the values were normalized to the level induced by 100 nM PTH, which was 74.0 ± 7.8 s.d. pmol per 35-mm well. Asterisk, P < 0.05 versus vector. (g) Expression of TβRII inhibits PTH-induced phosphorylation of CREB in UMR106 cells. The asterisk indicates that the cells were lysed in IP buffer with 0.5 % SDS for immunoblotting. (h) Expression of TβRII represses PTH-induced CRE luciferase transcription activities in UMR106 cells. RLU, luciferase units. (i) Expression of TβRII decreases the cell-surface PTH1R level. HEK293-PTH1R cells transfected with the indicated plasmids were treated with 125I-PTH for 30 min. The amount of 125I-PTH bound to the cell-surface PTH1R was determined with a scintillation counter. Asterisk, P < 0.05 versus vector; two asterisks, P < 0.01 versus vector. Results in panels d, f, h and i are shown as the mean ± s.d. (n = 3). Uncropped images of blots are shown in Supplementary Information, Fig. S9.
TβRII directly phosphorylates PTH1R to modulate their endocytosis
We next investigated the mechanism by which PTH1R and TβRII are internalized as a complex. TβRII possesses an S/T kinase that phosphorylates the S/T cluster region (GS motif) of TβRI in the activation of the Smad signalling pathway17. Four S/T clusters were identified in the PTH1R cytoplasmic domain (cPTH1R) (Fig. 5a, upper panel). One of these is highly homologous with the GS motif of TβRI (Fig. 5a, lower panel). To examine whether the TβRII kinase phosphorylates PTH1R directly, we prepared a native TβRII kinase, a cytoplasmic domain of TβRII (cTβRII)30, and a kinase-dead cTβRII-K277R, and then tested their potential phosphorylation activity with cPTH1R as a substrate. cTβRII showed auto-phosphorylation activity and phosphorylated cPTH1R (Fig. 5b), whereas cTβRII-K277R did not. To confirm these results, HEK293-PTH1R cells were transfected with TβRII or TβRII-K277R and labelled with 32P. Immunoprecipitation of the cell lysates showed that phosphorylated PTH1R was significantly increased by expression of TβRII but not by expression of TβRII-K277R (Fig. 5c). Kinase assay for cPTH1R (residues 461–551) showed that the phosphorylation sites are within the region containing four S/T clusters (Fig. 5a, d).
Figure 5.
TβRII directly phosphorylates PTH1R to modulate endocytosis of PTH1R/TβRII complex. (a) Amino acids in the PTH1R cytoplasmic domain from 461 to 593 contain four S/T clusters (underlined). Serines or threonines in these clusters (grey background) were replaced by alanines to generate four mutants (M1–M4) (upper panel). Residues 484–498 of PTH1R share conserved S/T residues with TβRI at residues 182–194 (lower panel). (b) TβRII kinase domain (cTβRII) directly phosphorylates the intracellular domain of PTH1R (cPTH1R) by in vitro kinase assay. Glutathione S-transferase (GST), cTβRII–GST or cTβRII-K277R–GST proteins alone (top panel), or in combination with PTH1R–GST (second panel), were pulled down by glutathione beads. The beads-bound proteins were incubated with [γ-32P]ATP, and the protein-associated radiolabel was detected by phosphorimager analysis. The GST-tagged proteins were revealed by Coomassie brilliant blue staining (lower two panels). (c) Phosphorylation of PTH1R was increased by expression of TβRII, but not by expression of TβRII-K277R, as determined by in vivo kinase assay. HEK293-PTH1R cells were transfected with plasmids as indicated and labelled with [γ-32P] ATP. The cell lysates were immunoprecipitated by anti-HA antibody, and the protein-associated radiolabel was detected by phosphorimager analysis. The asterisk indicates that the cells were lysed in IP buffer with 0.5% SDS for immunoblotting. (d) A fragment of PTH1R cytoplasmic domain containing residues 461–551, which contains four S/T clusters (cPTH1R (461–551)), was sufficient for phosphorylation by cTβRII. Asterisk, false-positive band. (e) Mutation of the S/T cluster in residues 484–498 of cPTH1R (cPTH1R-M2) disrupted the phosphorylation of cPTH1R by cTβRII. (f) TβRII kinase directly phosphorylates a synthesized small peptide (residues 477–506) of PTH1R. The peptide was resolved on a 4–20% Tris/Tricine/SDS gel, and the peptide-associated radiolabel was revealed by phosphorimager analysis (upper right). Mass spectrometry analysis showed two peaks, at m/z 3,399.7 and 3,479.7, respectively (lower, arrows), different by 80 units (the mass of a phosphate group) and 160 units from m/z 3319.7 (the mass of the peptide). (g, h) The M2 mutation (PTH1R-M2–HA) or a constitutively activated mutation (PTH1R-H223R–HA) disrupted the interaction between TβRII and PTH1R (g) and the inhibitory effect of PTH1R on the TGF-β-induced Smad2 phosphorylation (h). Uncropped images of blots are shown in Supplementary Information, Fig. S9.
To identify precisely which cluster was phosphorylated, we generated four individual mutations (M1–M4) for each of the S/T clusters (Fig. 5a). The phosphorylation of cPTH1R by TβRII was diminished by mutation (M2) of the S/T cluster, which is homologous with the GS motif of TβRI (Fig. 5e), indicating that the S/T cluster is required for the phosphorylation. Deletion or mutation of the region containing this GS-like cluster was previously shown to reduce receptor internalization markedly31,32. TβRII kinase directly phosphorylated a synthesized peptide motif containing the GS-like cluster (Fig. 5f, upper panel). Mass spectrometry analysis identified singly and doubly phosphorylated species (Fig. 5f, lower panel), indicating that TβRII kinase is able to phosphorylate the region at more than one site simultaneously. Moreover, the M2 mutation and a constitutively activated mutation33 of PTH1R significantly decreased the interaction with TβRII (Fig. 5g). Accordingly, the mutants relieved the inhibitory effect of PTH1R on TGF-β signalling (Fig. 5h) and showed increased CRE transcription activity (Supplementary Information, Fig. S6a). These results, together with the result that TβRII kinase decreased the cell-surface level of PTH1R other than the M2 mutant (Fig. 4i; Supplementary Information, Fig. S6b–d), indicate that the kinase activity of TβRII modulates endocytosis of PTH1R by phosphorylation of the PTH1R cytoplasmic domain.
Knockout of TβRII in osteoblasts increases cell-surface expression of PTH1R and PTH signalling
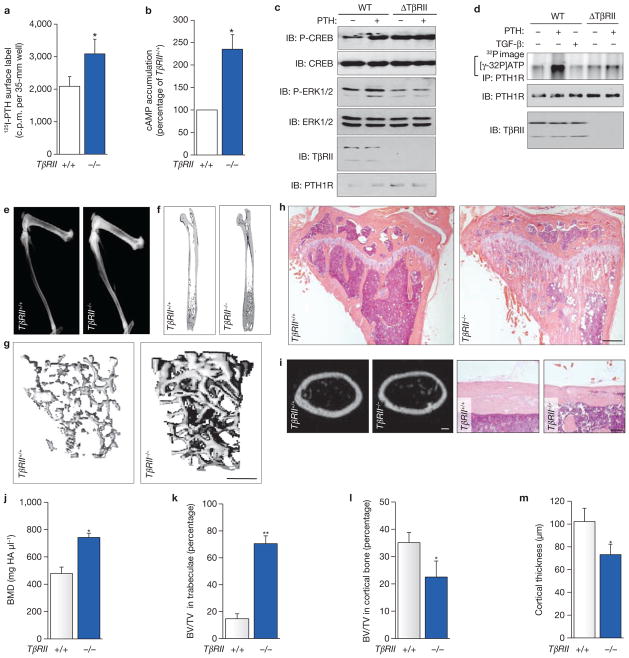
If TβRII kinase activity is required for PTH-induced endocytosis of PTH1R in osteoblasts, we reasoned that elimination of TβRII would enhance the cell-surface expression of PTH1R and PTH signalling. TβRII was selectively deleted in osteoblasts by crossing the mice homozygous for floxed TβRII (TβRIIflox/flox) with Cre-transgenic mice driven by an osteocalcin promoter (Supplementary Information, Fig. S7). This mutation resulted in upregulation of the cell-surface expression of PTH1R in osteoblasts, as assessed by a radioligand-binding assay (Fig. 6a). In accord with the increased PTH1R, basal cAMP accumulation was also increased (Fig. 6b). To investigate more precisely the effects of a loss of TβRII on osteoblasts, primary osteoblasts were isolated from mice homozygous for the TβRIIflox/flox gene and then infected with adenoviral Cre to remove TβRII. TβRII-null osteoblasts (ΔTβRII) showed an increase in basal-phosphorylated CREB, close to the level observed in wild-type osteoblasts treated with PTH (Fig. 6c). In these cells, PTH no longer stimulated the phosphorylation of CREB, probably because of saturation of PTH1R/cAMP signalling. Consistently, in the TβRII-null osteoblasts, PTH no longer induced the phosphorylation of PTH1R (Fig. 6d) and PTH had no effect on the phosphorylation of extracellular-signal-regulated kinase (ERK) (Fig. 6c), which is activated through the endocytosis of PTH1R34. These data indicate that the deletion of TβRII increases PTH signalling by disrupting PTH1R endocytosis, resulting in an increased cell-surface expression of PTH1R in TβRII−/− mice.
Figure 6.
Conditional disruption of TβRII in osteoblasts increases cell-surface expression of PTH1R and results in a bone phenotype consistent with overactivity of PTH1R. (a) Primary calvarial osteoblasts from TβRII−/− mice show increased binding of 125I-PTH. (b) Primary calvarial osteoblasts from TβRII−/− mice show increased basal accumulation of cAMP. The cAMP level in TβRII+/+ cells was 6.1 ± 5.3 s.d. pmol per 35-mm well. (c) Cre-mediated ablation of TβRII in primary TβRIIflox/flox calvarial osteoblast (ΔTβRII) activates CREB phosphorylation independently of ligand. (d) PTH-induced phosphorylation of PTH1R was inhibited in ΔTβRII cells. WT, wild type. (e) Faxitron radiographs of lower limbs from 8-week-old TβRII+/+ and TβRII−/− mice. (f) μCT images of femora. Scale bar, 1.0 mm. (g) Three-dimensional reconstruction of the proximal tibiae from μCT scans. Scale bar, 1.0 mm. (h) Micrographs from proximal tibiae stained with H&E. Scale bars, 0.5 mm. (i) Cross-sections of cortical bone at femora diaphysis from μCT scans (left two panels; scale bar, 200 μm) and H&E-stained sections of tibia diaphysis from TβRII+/+ and TβRII−/− mice (right two panels; scale bar, 50 μm). (j–l) Quantitative μCT analysis at distal femora: bone mineral density measurements (j), trabecular bone volume (k) and cortical bone volume (l). (m) Quantitative μCT for cortical bone thickness at mid-diaphysis of femora. Data shown represent means ± s.d. for five animals per group. Asterisk, P < 0.05 versus TβRII+/+; two asterisks, P < 0.01 versus TβRII+/+. Uncropped images of blots are shown in Supplementary Information, Fig. S9.
Osteoblast-specific TβRII knockout mice show increased bone formation, similar to the bone phenotype of mice expressing constitutively active PTH1R
Mice lacking TβRII were viable and indistinguishable from wild-type littermates (TβRII+/+) at birth. By 8 weeks of age, TβRII−/− mice showed a marked increase in bone mass and bone mineral density compared with that of their wild-type littermates (Fig. 6e–h, j). Haematoxylin and eosin (H&E) staining and microcomputed tomography (μCT) analysis revealed a substantial increase in trabeculation at the metaphyseal region of long bones (Fig. 6g, h, k), with a disorganized reticular pattern extending into the endosteal region such that the size of the bone marrow space was reduced. In contrast, cortical bone in the metaphyseal region was more porous than in controls and was reduced in volume (Fig. 6h, l). The thickness of cortical bone at diaphysis was also decreased (Fig. 6i, m). These data indicate that a loss of TβRII in osteoblasts affects the trabecular and cortical bone morphology differentially even though total bone mass is increased. These changes are strikingly similar to those of mice expressing a constitutively active PTH1R in cells of the osteoblastic lineage35.
Because PTH1R mediates the anabolic effect of PTH in bone and an excess of PTH signalling is characterized by large numbers of osteoclasts and rapid bone turnover, we analysed the numbers of osteoblasts and osteoclasts at the trabecular and endosteal surfaces of TβRII−/− mice by histomorphometry. Both parameters were substantially increased from 6 weeks to 12 weeks of age (Fig. 7a–d), except that the number of osteoclasts at trabeculae had returned to normal in mature mice at 12 weeks of age (Fig. 7c). Calcein double labelling of the TβRII−/− mice (Fig. 7e) indicated a disorganized pattern at trabecular and endosteal surface, consistent with greatly induced bone turnover and decreased periosteal mineral apposition, a major determinant of cortical bone formation. Additionally, the concentrations of serum markers, including osteocalcin for bone formation (Fig. 7f) and the C-terminal telopeptide of type I collagen (CTX) (Fig. 7g) for bone resorption, were significantly increased in TβRII−/− mice relative to their wild-type littermates. Moreover, the expression level of the receptor activator of nuclear factor-κB ligand (RANKL) was significantly increased in the TβRII-null osteoblasts (Fig. 7h). These results show greatly increased bone remodelling in the TβRII−/− mice. However, the effects are manifested differently in different bone compartments. In trabecular bone, the activity of osteoclasts seems to be overwhelmed by the robust osteoblast activity, particularly as the mice age. Conversely, at the endosteal surface, osteoclasts seem to remain active, which ultimately leads to cortical thinning, a phenomenon also seen in situations in which PTH1R is hyperactive.
Figure 7.
Conditional disruption of TβRII in osteoblasts increases bone remodelling. (a, b) The number of osteoblasts was measured at the metaphyseal trabecular bone (a) and the endosteal cortical bone (b) of tibiae. The number of osteoblasts per bone perimeter (N.ob/B.Pm) is shown. (c, d) The number of osteoclasts was measured at the metaphyseal trabecular bone (c) and the endosteal cortical bone (d) of tibiae. The number of osteoclasts per bone perimeter (N.oc/B.Pm) was shown. (e) Calcein double labelling in the metaphyseal trabecular bone (upper panel; scale bar, 50 μm) and the diaphyseal cortical bone (lower panel; scale bar, 25 μm) of tibiae from 8-week-old TβRII+/+ and TβRII−/− mice. (f, g) Eight-week-old TβRII−/− mice show increased osteocalcin (f) and CTX (g) in serum. (h) Basal mRNA level of RANKL was increased in ΔTβRII cells. Data shown represent means ± s.d. for five animals per group. Asterisk, P < 0.05 versus TβRII+/+; two asterisks, P < 0.01 versus TβRII+/+.
Injection of PTH(7–34) or decrease in PTH1R expression rescues the bone phenotype of the TβRII−/− mice
To determine whether the increased bone mass in TβRII−/− mice was a result of stimulation of PTH signalling, we injected PTH(7–34), a PTH antagonist36–38, subcutaneously into the TβRII−/− mice. The bone phenotype of TβRII−/− mice was rescued by increasing doses of PTH(7–34), as shown by reduced trabecular bone volume and increased cortical thickness (Fig. 8a (upper panels), b). Calcein double labelling showed that the highly enhanced incorporation of calcein in TβRII−/− mice returned to a level comparable to that of their wild-type littermates (Fig. 8a, lower panels). Moreover, the high bone turnover in TβRII−/− mice was suppressed by injection of PTH(7–34), as demonstrated by decreased serum levels of calcium, osteocalcin and CTX, and decreased numbers of osteoblasts and osteoclasts at the trabeculae, whereas the circulating PTH level was not significantly changed (Fig. 8c–h). These parameters indicate downregulated bone remodelling by antagonist-mediated disruption of PTH action. Finally, we ablated PTH1R in osteoblasts simultaneously with TβRII by crossing the TβRII−/− mice with PTH1R+/− mice. The osteoblast-specific TβRII−/−/PTH1R+/− mice showed a substantial decrease in bone formation compared with the TβRII−/− mice, and produced a similar bone phenotype to that observed in TβRII−/− mice with injection of PTH(7–34) (Fig. 8i, j). These results demonstrate that hyperactive PTH signalling through PTH1R leads to the bone phenotype observed in the TβRII−/− mice.
Figure 8.
Injection of PTH(7–34) or disruption of PTH1R in osteoblasts rescues the bone phenotype exhibited in the TβRII−/− mice. (a) 1-month-old TβRII−/− male mice were injected subcutaneously with vehicle or PTH(7–34) at indicated dosages each day for 4 weeks. Representative μCT images of cross-sections at the distal femora (scale bar, 500 μm) (upper) and calcein double labelling in the metaphyseal trabecular bone at distal femora (scale bar, 25 μm) (lower) are shown. (b) Quantitative μCT analysis at the distal femora. BV/TV indicates trabecular bone volume expressed as a percentage of tissue volume. (c–f) Quantitative analysis of the serum markers. Calcium (c), osteocalcin (d), CTX (e) and intact PTH (f) were measured in serum collected at the time at which the mice were killed. (g, h) Histomorphometric analysis of the number of osteoblasts (g) or osteoclasts (h) per bone perimeter at the metaphyseal trabecular bone of tibiae. (i, j) Seven-week-old TβRII−/−/PTH1R+/− mice reproduced the bone phenotype observed in TβRII−/− mice with injection of PTH(7–34). BV/TV at the distal femora (i) and representative μCT images of cross-sections at the distal femora (scale bar, 500 μm) (j; upper panels) and calcein double labelling in the metaphyseal trabecular bone at distal femora (scale bar, 25 μm) (j; lower panels) are shown. (k) Model of the integration of the PTH and TGF-β signalling pathway at the level of PTH1R and TβRII receptors through endocytosis. Data shown represent means ± s.d. for three animals per group. Asterisk, P < 0.05 versus TβRII−/− with vehicle, or versus TβRII−/−/PTH1R+/+; two asterisks, P < 0.01 versus TβRII−/− with vehicle.
DISCUSSION
Endocytosis of cell-surface receptors is a regulatory mechanism for the integration of different signalling pathways25. We have shown that PTH induces an interaction between TβRII and PTH1R to facilitate their endocytosis as a complex. Phosphorylation of PTH1R by TβRII modulates endocytosis, leading to downregulation of PTH signalling. Similarly, the recruitment of TβRII to PTH1R by PTH dampens TGF-β signalling. Conversely, PTH signalling is stimulated in the TβRII knockout mice, producing a bone phenotype strikingly similar to that observed in animals bearing a constitutively active PTH1R. We therefore propose a working model in which PTH-induced endocytosis of a TβRII–PTH1R complex provides a platform for integrating the signals of TGF-β and PTH (Fig. 8k).
Activation of PTH1R differentially affects cortical and trabecular bone surfaces. Patients with Jansen’s metaphyseal chondrodysplasia, caused by constitutively activating mutations of PTH1R, show ligand-independent cAMP accumulation, loss of cortical bone, and augmentation of trabecular bone33. These bone phenotypes are reproduced in mice with osteoblastic expression of Jansen’s PTH1R mutant35. In contrast, PTH1R knockout mice show decreased trabecular bone and increased thickness of cortical bone39. The skeletal abnormalities with constitutively activating PTH1R are similar to the phenotype in the TβRII−/− mice. In particular, PTH ligand binding, cAMP production and CREB phosphorylation are increased in untreated TβRII−/− osteoblasts. In addition, intermittent injection of PTH in TβRII−/− mice no longer enhanced the bone phenotype exhibited in TβRII−/− mice (data not shown). The sustained PTH1R signalling contributed to the cAMP activation in the TβRII−/− mice, resulting in increased bone mass and the differential regulation of trabecular versus cortical bone. The observation was validated by the rescue of bone phenotype of TβRII−/− mice by the injection of PTH(7–34) or by the disruption of PTH1R expression. The bone phenotype of the TβRII−/− mice is apparently not simply generated by the loss of Smad signalling, which regulates the mechanical properties of bone matrix40,41. Indeed, TβRII is internalized dynamically (Supplementary Information, Fig. S8) and is a target of both PTH and TGF-β. Thus, TGF-β/Smad signalling probably occurs through the signalling cascade integrated in the effect of PTH on bone. Actually, PTH activates other signalling pathways such as the canonical Wnt signalling42.
PTH stimulates the bone remodelling process by regulating bone formation and bone resorption. PTH glands probably developed in terrestrial vertebrates to provide an additional ligand to activate the PTH1R, which evolved earlier in evolution to service locally produced PTHrP. The fact that PTH arrives at PTH1R through the systemic circulation provides a means for the rapid and integrated regulation of osteoblasts and osteoclasts in physiological settings of increased calcium demands. We further speculate that the mechanisms described in this study that involve interaction between PTH1R and TβRII could serve as a platform for integrating signals from local osteotropic factors for the rapid skeletal adjustments imposed by the increased demands for calcium and bone remodelling required of terrestrial vertebrates. Therefore, direct phosphorylation of the cytoplasmic domain of PTH1R by TβRII integrates PTH signalling for bone remodelling and TGF-β1 signalling for coupling bone resorption and formation14,43. Finally, these findings raise the possibility of manipulating this pathway with pharmaceuticals that would more effectively couple osteoclast and osteoblast activity.
METHODS
Plasmids and antibodies
The TβRII-Flag/pcDNA3.0 and PTH1R-HA/pcDNA3.0 plasmids were cloned from human TβRII30 and PTH1R44. Zipper-YFP1/pcDNA3.1, zipper-YFP2/pcDNA3.126, and luciferase reporters SBE45 and CRE (Stratagene, La Jolla, CA) have been described previously. PTH1R-HA was introduced into pMSCVneo retroviral expression vector (BD Biosciences, San Jose, CA) to generate pMSCV-PTH1R-HA. TβRII-YFP1, PTH1R-YFP1, and PTH1R-YFP2 were generated by replacing the zippers in zipper-YFP1 or zipper-YFP2. Cytoplasmic domain of TβRII (amino acids 192-566, cTβRII) and cytoplasmic domain of PTH1R (amino acids 461-593, cPTH1R) were fused with GST at N-terminals using pGEX-KG prokaryotic gene fusion vector (Pharmacia Corp., Pfizer Inc., New York, NY). The mutants TβRII-K277R-Flag, TβRII-K277R-YFP1, PTH1R-H223R-YFP2, PTH1R-M2-YFP2, PTH1R-H223R-HA, PTH1R-M2-HA, TβRII-K277R-GST, cPTH1R-M1-GST, cPTH1R-M2-GST, cPTH1R-M3-GST, and cPTH1R-M4-GST were generated by primer-mediated PCR mutagenesis and verified via DNA sequencing. Primary antibodies used for immunoblotting, immunoprecipitation, or immunostaining included mouse Flag antibody M2 (IF, 1:500; IB, 1:2000) (Sigma-Aldrich Corp., St. Louis, MO); mouse HA antibody 16B12 (IB, 1:2000) and rabbit PTH1R antibody PRB-640P (IB, 1:500) and PRB-630P (IB, 1:500) (Covance, Princeton, NJ); mouse TβRII antibody C-4 (IF, 1:250; IB, 1:500), rabbit CREB antibody (IB, 1:1000), and rabbit P-CREB antibody (IB, 1:1000) (Upstate Biotechnology, Inc., Lake Placid, NY); Rabbit PTH(1-84) antibody (IB, 1:500) (GeneTex, Inc., Irvine, CA); mouse ERK antibody C-16 (IB, 1:1000), mouse P-ERK antibody E-4 (IB, 1:800), and mouse Smad4 antibody B-8 (IB, 1:1000) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); mouse Smad2 antibody L16D3 (IB, 1:1000) and mouse P-Smad2 antibody (Ser465/467) (IB, 1:500) (Cell Signaling Technology, Danvers, MA).
Cells, transfection and reagents
The HEK293, C3H10T1/2 and UMR106 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and maintained in DMEM or α-minimal essential medium supplemented with 10% fetal bovine serum (FBS; Invitrogen Corp., Carlsbad, CA). HEK293-PTH1R cells were generated by stable expression of PTH1R–HA with pMSCV-neo retrovirus transduction. The transfection of DNA plasmids were performed with Lipofectamine reagent (Invitrogen Corp.). The knockdown of β-arrestins was performed with siRNAs against β-arrestin1 and β-arrestin2 (Santa Cruz Biotechnology, Inc.). Human PTH(1–34) (here termed PTH) and PTH(7–34) was purchased from Bachem, Inc. (Torrance, CA), and the radioligand [125I]-[Nle-8,21,Tyr-34]-human PTH(1–34)-OH (here termed 125I-PTH) was labelled at University of Alabama. [Lys-13(Nε-5-carboxy-TMR)]-PTH(1–34)-NH2 (here termed PTHTMR] and PTH1R small peptide (residues 476–506) was synthesized by Genemed Synthesis, Inc. (San Antonio, TX); 100 nM of PTH, PTHTMR, 125I-PTH or PTH(1–84), or 2 ng of TGF-β1, was used for cell treatment.
Immunoprecipitation, immunoblotting analysis and luciferase reporter assay
The cells were lysed in IP buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate) containing protease inhibitors. The lysates were immunoprecipitated by incubation with the appropriate antibodies, followed by absorption on Protein G-Sepharose. The immunoprecipitates were separated by SDS–PAGE and blotted onto a nitrocellulose membrane. Immunoblots were characterized by using the SuperSignal West Femto Substrate system (Pierce, Thermo Fisher Scientific, Inc., Rockford, IL). To perform immunoblotting for TβRII-K277R, the cells were lysed in IP buffer with 0.5% SDS. Luciferase activities were assayed with the Dual-Luciferase Reporter Assay System (Promega Corp., Madison, WI).
TβRII trafficking
The cells expressing TβRII were classified into three types: first, those in which TβRII was dominantly present at the cell surface (membrane), second, those that located to the cell surface and vesicles in the cytosol (membrane/vesicle), and third, those that mainly localized to the cytosol as vesicles (vesicle). Endocytic trafficking was assessed as described46. For cell-surface TβRII biotinylation, HEK293-PTH1R cells treated with PTH at different time points were suspended in PBS containing 1 mM Sulfo-NHS–SS-Biotin, and rocked at 37 °C for 30 min. After quenching of excess biotin, the cells were lysed; the cell lysates were then immunoprecipitated with anti-TβRII antibody and immunoblotted with anti-TβRII antibody (total TβRII) or streptavidin-tagged horseradish peroxidase (cell-surface TβRII). Chemiluminescent density was quantified with a Storm 860 scanner (Molecular Dynamics, Sunnyvale, CA). Values of cell-surface TβRII were normalized to total TβRII. The curve for cell-surface level was determined by comparing the values at different time points, with that at time zero being set to 100%. Data correspond to means ± s.d. from three independent experiments.
Ligand binding, receptor internalization, cAMP assay and real-time PCR
Ligand binding and receptor internalization were performed as described13, with minimal modifications. Cells grown in 35-mm six-well plates were incubated in serum-free DMEM for 1 h at 0 °C, followed by a 45-min incubation with the buffer containing 125I-PTH (100,000 c.p.m. per well) as a radioligand. For the binding study, cell-associated 125I-PTH was extracted with 0.8 M NaOH and quantified in a scintillation counter. For cAMP assays, cells grown in 35-mm six-well plates were stimulated with PTH at 37 °C for 15 min. Cellular cAMP was extracted and measured with the Biotrak enzymeimmunoassay system (GE Healthcare, Inc., Princeton, NJ). For real-time PCR, total RNA was isolated from calvarial osteoblasts with TRIzol reagent (Invitrogen). cDNA synthesis was performed with TaqMan Reverse Transcription reagent (Applied Biosystems, Foster, CA). PCR was performed with TaqMan Gene Expression assays (Applied Biosystems) with the primer–probe set mm0041908-m1 for RANKL and mm99999915-g1 for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Values were normalized to GAPDH mRNA levels.
Fusion protein expression and kinase assay
The GST-tagged cytoplasmic domains of PTH1R (residues 461–593) and TβRII (residues 192–567) were expressed in BL21 (DE3) cells, then pulled down by glutathione-agarose beads and added into kinase reaction that contained 50 mM Tris-HCl pH 7.5, 10 mM MgCl2 and 200 mCi of [γ-32P]ATP. The products were analysed by 10% SDS–PAGE and the protein-associated radiolabel was determined by the Storm 860 scanner. Mass spectrometric analysis was performed at the Prometic Core Facility of Johns Hopkins University.
Confocal microscopy, FRET measurements and YFP-PCA analysis
Cells plated on glass coverslips were washed with cold PBS, fixed with 4% paraformaldehyde for 10 min, and then carefully permeabilized with 0.1% Triton X-100 for 6 ± 2 s.d. min. The cells were mounted with Vectashield medium (Vector Laboratories) and imaged with a Leica TCS SP1 or SP2 confocal microscope. FRET experiments were performed as described47. In brief, HEK293-PTH1R cells were transfected with TβRIIGFP (donor) and then treated with PTHTMR (acceptor) for 15 min. FRET efficiency between PTHTMR and TβRIIGFP was determined by donor (GFP) recovery after acceptor (TMR) photobleaching. The TβRII-K277RGFP expressed on the plasma membrane was examined as a control. The YFP-PCA analysis was performed as described previously26,27. The results shown are means ± s.d. from four independent experiments.
Generation of conditional TβRII knockout mice and PTH1R TβRII double knockout mice
The genotype of transgenic mice was determined by PCR analyses of genomic DNA isolated from mouse tails. Mice with osteoblast-specific inactivation of TβRII (TβRII−/−) were generated by crossing OC-Cre mice48 with mice homozygous for a floxed TβRII allele49. Genotyping for the Cre transgene was performed by PCR with the primers Cre 5′ (5′-CAAATAGCCCTGGCAGAT-3′) and Cre 3′ (5′-TGATACAAGGGACATCTTCC-3′). The floxed TβRII allele was identified with the primers50 lox1F (5′-TAAACAAGGTCCGGAGCCCA-3′) and lox1R (5′-ACTTCTGCAAGAGGTCCCCT-3′). Recombination and subsequent loss of the floxed TβRII allele were analysed with primers lox1F and lox2R (5′-AGAGTGAAGCCGTGGTAGGT-3′). Mice with osteoblast-specific disruption of PTH1R (PTH1R+/−) were generated by crossing OC-Cre mice with mice homozygous for a floxed PTH1R allele51. The mice with disruption of PTH1R in a TβRII knockout background (TβRII−/−/PTH1R+/−) were generated by crossing PTH1R+/− mice with TβRII−/− mice. The floxed PTH1R allele was analysed with the primers as described51. All mice were given a standard chow diet and water. Procedures involving mice were approved by the Institutional Animal Care and Use Program of the Johns Hopkins University.
Primary osteoblast isolation and adenovirus infection
Calvarial osteoblasts were isolated as described previously52. The cells from TβRII−/− mice were cultured in osteogenic medium (0.1 M dexamethasome, 10 M glycerol phosphate, 0.05 M L-ascorbic acid in α-MEM) with 5% FBS for 3 days to ensure the deletion of TβRII through osteocalcin-driven expression of Cre. For adenovirus infection, monolayer osteoblasts were infected with Cre recombinase virus M1 (Ad-CreM1) or control adenovirus (Ad-GFP) (Vector Laboratories, Burlingame, CA) at a multiplicity of infection of 100 and were harvested for immunoblotting analysis after 48 h. For treatment with PTH, calvarial osteoblasts were pre-cultured in osteogenic medium for 3 days.
Microcomputed tomography, radiographic analysis and histomorphometric analysis
Tibiae and femora obtained from mice were dissected free of soft tissue and analysed by a high-resolution μCT imaging system, MicroCT-40 (Scanco, Southeastern, PA), with a voltage of 55 kilovolt peak (kVp) and a current of 109 μA. Three-dimensional trabecular structural parameters, including bone mineral density, trabecular and cortical bone volume, and cortical thickness in the secondary spongiosa, were measured as described previously53. Radiographs were taken with a Faxitron X-ray machine (Faxitron X-Ray LLC, Lincolnshire, IL), under constant conditions (19 kV, 10 s exposure). To examine the dynamic bone formation, two sequential doses of calcein (Sigma-Aldrich Corp.; 10 mg kg−1 in 2% sodium bicarbonate in sterile saline) was injected into mice 3 and 9 days before they were killed. Calcein double labelling was then observed under a fluorescence microscope. The numbers of osteoblasts and osteoclasts were determined by quantitative histomorphometric analysis conducted in a blinded fashion with OsteoMeasureXP software (OsteoMetrics, Inc., Decatur, GA). Four randomly selected visual fields per specimen, in three specimens per mouse in each group, were measured.
PTH(7–34) injection and serum analysis
PTH(7–34) or vehicle (equivalent volume of 1 mM acetic acid in sterile PBS) in a final volume of 100 μl was given daily by subcutaneous injection for 4 weeks to 1-month-old male mice (three per group). Serum and bone were collected for levels of calcium, osteocalcin, CTX and PTH, and for mineralized bone histology and histomorphometry, respectively. Calcium was determined with a Calcium Colorimetric Assay Kit (BioVision, Inc., Mountain View, CA). Serum levels of intact PTH were measured with a mouse PTH enzyme-linked immunosorbent assay (ELISA) kit (Alpco Diagnostics, Salem, NH). Osteocalcin and CTX were determined with a mouse osteocalcin ELISA kit (Biomedical Technologies, Inc., Stoughton, MA) and a rat/mouse RatLaps ELISA kit (Nordic Bioscience Diagnostics, Herlev, Denmark), respectively.
Statistical analysis
Statistical differences between two groups of data were analysed with Student’s t-test. Data are presented as means ± s.d.
Supplementary Material
Acknowledgments
We gratefully acknowledge Stephen Michnick and Odyssey Thera, Inc., for YFP-based PCA constructs; Harold Moses for TβRIIflox/flox mice; Henry Kronenberg for PTH1Rflox/flox mice; Lijuan Pang and Hui Deng for technical assistance; Robert Cole and Tatiana Boronina for mass spectrometry analysis; and Elaine Henz for editorial service. This work was supported by National Institutes of Health grant DK080898 and DK057501 (X.C).
Footnotes
Note: Supplementary Information is available on the Nature Cell Biology website.
AUTHOR CONTRIBUTIONS
T.Q. performed most of the experiments, analysed data and prepared the manuscript. X.W. performed the most of the in vivo experiments and analysed in vivo data. F.Z. maintained the mice and collected tissue samples. C.T. provided suggestions for the project. M.W. prepared the manuscript. X.C. supervised the project and wrote most of the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/
References
- 1.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 2.Zaidi M. Skeletal remodeling in health and disease. Nature Med. 2007;13:791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- 3.Pfeilschifter J, et al. Parathyroid hormone increases the concentration of insulin-like growth factor-I and transforming growth factor β1 in rat bone. J Clin Invest. 1995;96:767–774. doi: 10.1172/JCI118121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardie LJ, et al. Isolation of the first piscine transforming growth factor β gene: analysis reveals tissue specific expression and a potential regulatory sequence in rainbow trout (Oncorhynchus mykiss) Cytokine. 1998;10:555–563. doi: 10.1006/cyto.1997.0334. [DOI] [PubMed] [Google Scholar]
- 5.Reinecke M, Collet C. The phylogeny of the insulin-like growth factors. Int Rev Cytol. 1998;183:1–94. doi: 10.1016/s0074-7696(08)60142-4. [DOI] [PubMed] [Google Scholar]
- 6.Laing KJ, Pilstrom L, Cunningham C, Secombes CJ. TGF-β3 exists in bony fish. Vet Immunol Immunopathol. 1999;72:45–53. doi: 10.1016/s0165-2427(99)00116-6. [DOI] [PubMed] [Google Scholar]
- 7.Zhan Y, Jimmy K. Molecular isolation and characterisation of carp transforming growth factor β1 from activated leucocytes. Fish Shellfish Immunol. 2000;10:309–318. doi: 10.1006/fsim.1999.0239. [DOI] [PubMed] [Google Scholar]
- 8.Juppner H, et al. A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science. 1991;254:1024–1026. doi: 10.1126/science.1658941. [DOI] [PubMed] [Google Scholar]
- 9.Abou-Samra AB, et al. Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc Natl Acad Sci USA. 1992;89:2732–2736. doi: 10.1073/pnas.89.7.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segre GV, Goldring SR. Receptors for secretin, calcitonin, parathyroid hormone (PTH)/PTH-related peptide, vasoactive intestinal peptide, glucagonlike peptide 1, growth hormone-releasing hormone, and glucagon belong to a newly discovered G-protein-linked receptor family. Trends Endocrinol Metab. 1993;4:309–314. doi: 10.1016/1043-2760(93)90071-l. [DOI] [PubMed] [Google Scholar]
- 11.Juppner H. Molecular cloning and characterization of a parathyroid hormone/parathyroid hormone-related peptide receptor: a member of an ancient family of G protein-coupled receptors. Curr Opin Nephrol Hypertens. 1994;3:371–378. [PubMed] [Google Scholar]
- 12.Potts JT, Jr, Gardella TJ, Juppner H, Kronenberg H. The history of parathyroid hormone and its receptor: structure-based design of parathyroid hormone analogues. Osteoporos Int. 1997;7 (Suppl 3):S169–S173. doi: 10.1007/BF03194366. [DOI] [PubMed] [Google Scholar]
- 13.Vilardaga JP, et al. Internalization determinants of the parathyroid hormone receptor differentially regulate β-arrestin/receptor association. J Biol Chem. 2002;277:8121–8129. doi: 10.1074/jbc.M110433200. [DOI] [PubMed] [Google Scholar]
- 14.Tang Y, et al. TGF-β1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nature Med. 2009;15:757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wrana JL, et al. TGFβ signals through a heteromeric protein kinase receptor complex. Cell. 1992;71:1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- 16.Franzen P, et al. Cloning of a TGFβ type I receptor that forms a heteromeric complex with the TGFβ type II receptor. Cell. 1993;75:681–692. doi: 10.1016/0092-8674(93)90489-d. [DOI] [PubMed] [Google Scholar]
- 17.Wrana JL, Attisano L, Wieser R, Ventura F, Massagué J. Mechanism of activation of the TGF-β receptor. Nature. 1994;370:341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- 18.Derynck R, Zhang Y, Feng XH. Smads: transcriptional activators of TGF-β responses. Cell. 1998;95:737–740. doi: 10.1016/s0092-8674(00)81696-7. [DOI] [PubMed] [Google Scholar]
- 19.Massagué J, Wotton D. Transcriptional control by the TGF-β/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng XH, Derynck R. Specificity and versatility in TGF-β signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 21.Sowa H, et al. Parathyroid hormone-Smad3 axis exerts anti-apoptotic action and augments anabolic action of transforming growth factor β in osteoblasts. J Biol Chem. 2003;278:52240–52252. doi: 10.1074/jbc.M302566200. [DOI] [PubMed] [Google Scholar]
- 22.Serra R, Karaplis A, Sohn P. Parathyroid hormone-related peptide (PTHrP)-dependent and -independent effects of transforming growth factor β (TGF-β) on endochondral bone formation. J Cell Biol. 1999;145:783–794. doi: 10.1083/jcb.145.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003;423:349–355. doi: 10.1038/nature01660. [DOI] [PubMed] [Google Scholar]
- 24.Martin TJ, Sims NA. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med. 2005;11:76–81. doi: 10.1016/j.molmed.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Polo S, Di Fiore PP. Endocytosis conducts the cell signaling orchestra. Cell. 2006;124:897–900. doi: 10.1016/j.cell.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 26.Remy I, Montmarquette A, Michnick SW. PKB/Akt modulates TGF-β signalling through a direct interaction with Smad3. Nature Cell Biol. 2004;6:358–365. doi: 10.1038/ncb1113. [DOI] [PubMed] [Google Scholar]
- 27.Qiu T, Grizzle WE, Oelschlager DK, Shen X, Cao X. Control of prostate cell growth: BMP antagonizes androgen mitogenic activity with incorporation of MAPK signals in Smad1. EMBO J. 2007;26:346–357. doi: 10.1038/sj.emboj.7601499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terrillon S, Bouvier M. Roles of G-protein-coupled receptor dimerization. EMBO Rep. 2004;5:30–34. doi: 10.1038/sj.embor.7400052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasmussen SG, et al. Crystal structure of the human β2 adrenergic G-protein-coupled receptor. Nature. 2007;450:383–387. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- 30.Lin HY, Wang XF, Ng-Eaton E, Weinberg RA, Lodish HF. Expression cloning of the TGF-β type II receptor, a functional transmembrane serine/threonine kinase. Cell. 1992;68:775–785. doi: 10.1016/0092-8674(92)90152-3. [DOI] [PubMed] [Google Scholar]
- 31.Tawfeek HA, Qian F, Abou-Samra AB. Phosphorylation of the receptor for PTH and PTHrP is required for internalization and regulates receptor signaling. Mol Endocrinol. 2002;16:1–13. doi: 10.1210/mend.16.1.0760. [DOI] [PubMed] [Google Scholar]
- 32.Huang Z, Chen Y, Nissenson RA. The cytoplasmic tail of the G-protein-coupled receptor for parathyroid hormone and parathyroid hormone-related protein contains positive and negative signals for endocytosis. J Biol Chem. 1995;270:151–156. doi: 10.1074/jbc.270.1.151. [DOI] [PubMed] [Google Scholar]
- 33.Schipani E, Kruse K, Juppner H. A constitutively active mutant PTH-PTHrP receptor in Jansen-type metaphyseal chondrodysplasia. Science. 1995;268:98–100. doi: 10.1126/science.7701349. [DOI] [PubMed] [Google Scholar]
- 34.Syme CA, Friedman PA, Bisello A. Parathyroid hormone receptor trafficking contributes to the activation of extracellular signal-regulated kinases but is not required for regulation of cAMP signaling. J Biol Chem. 2005;280:11281–11288. doi: 10.1074/jbc.M413393200. [DOI] [PubMed] [Google Scholar]
- 35.Calvi LM, et al. Activated parathyroid hormone/parathyroid hormone-related protein receptor in osteoblastic cells differentially affects cortical and trabecular bone. J Clin Invest. 2001;107:277–286. doi: 10.1172/JCI11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sebastian EM, Suva LJ, Friedman PA. Differential effects of intermittent PTH(1–34) and PTH(7–34) on bone microarchitecture and aortic calcification in experimental renal failure. Bone. 2008;43:1022–1030. doi: 10.1016/j.bone.2008.07.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friedman PA, Goodman WG. PTH(1–84)/PTH(7–84): a balance of power. Am J Physiol Renal Physiol. 2006;290:F975–F984. doi: 10.1152/ajprenal.00336.2005. [DOI] [PubMed] [Google Scholar]
- 38.Langub MC, et al. Administration of PTH-(7–84) antagonizes the effects of PTH-(1–84) on bone in rats with moderate renal failure. Endocrinology. 2003;144:1135–1138. doi: 10.1210/en.2002-221026. [DOI] [PubMed] [Google Scholar]
- 39.Lanske B, et al. Ablation of the PTHrP gene or the PTH/PTHrP receptor gene leads to distinct abnormalities in bone development. J Clin Invest. 1999;104:399–407. doi: 10.1172/JCI6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balooch G, et al. TGF-β regulates the mechanical properties and composition of bone matrix. Proc Natl Acad Sci USA. 2005;102:18813–18818. doi: 10.1073/pnas.0507417102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Filvaroff E, et al. Inhibition of TGF-β receptor signaling in osteoblasts leads to decreased bone remodeling and increased trabecular bone mass. Development. 1999;126:4267–4279. doi: 10.1242/dev.126.19.4267. [DOI] [PubMed] [Google Scholar]
- 42.Wan M, et al. Parathyroid hormone signaling through low-density lipoprotein-related protein 6. Genes Dev. 2008;22:2968–2979. doi: 10.1101/gad.1702708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janssens K, ten Dijke P, Janssens S, Van Hul W. Transforming growth factor-β1 to the bone. Endocr Rev. 2005;26:743–774. doi: 10.1210/er.2004-0001. [DOI] [PubMed] [Google Scholar]
- 44.Schipani E, et al. Identical complementary deoxyribonucleic acids encode a human renal and bone parathyroid hormone (PTH)/PTH-related peptide receptor. Endocrinology. 1993;132:2157–2165. doi: 10.1210/endo.132.5.8386612. [DOI] [PubMed] [Google Scholar]
- 45.Shi Y, et al. Crystal structure of a Smad MH1 domain bound to DNA: insights on DNA binding in TGF-β signaling. Cell. 1998;94:585–594. doi: 10.1016/s0092-8674(00)81600-1. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto H, Komekado H, Kikuchi A. Caveolin is necessary for Wnt-3a-dependent internalization of LRP6 and accumulation of β-catenin. Dev Cell. 2006;11:213–223. doi: 10.1016/j.devcel.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 47.Castro M, Nikolaev VO, Palm D, Lohse MJ, Vilardaga JP. Turn-on switch in parathyroid hormone receptor by a two-step parathyroid hormone binding mechanism. Proc Natl Acad Sci USA. 2005;102:16084–16089. doi: 10.1073/pnas.0503942102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang M, et al. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277:44005–44012. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- 49.Chytil A, Magnuson MA, Wright CV, Moses HL. Conditional inactivation of the TGF-β type II receptor using Cre:Lox. Genesis. 2002;32:73–75. doi: 10.1002/gene.10046. [DOI] [PubMed] [Google Scholar]
- 50.Baffi MO, et al. Conditional deletion of the TGF-β type II receptor in Col2a expressing cells results in defects in the axial skeleton without alterations in chondrocyte differentiation or embryonic development of long bones. Dev Biol. 2004;276:124–142. doi: 10.1016/j.ydbio.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 51.Kobayashi T, et al. PTHrP and Indian hedgehog control differentiation of growth plate chondrocytes at multiple steps. Development. 2002;129:2977–2986. doi: 10.1242/dev.129.12.2977. [DOI] [PubMed] [Google Scholar]
- 52.Zhang F, et al. Sustained BMP signaling in osteoblasts stimulates bone formation by promoting angiogenesis and osteoblast differentiation. J Bone Miner Res. 2009;24:1224–1233. doi: 10.1359/JBMR.090204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang Y, et al. Recombinant human parathyroid hormone (1–34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res. 2003;18:1932–1941. doi: 10.1359/jbmr.2003.18.11.1932. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.