Abstract
Purpose
The leukemias account for the largest number of cases of childhood cancer and remain the primary cause of cancer-related mortality among children in the United States. There is a need for novel antileukemia agents due to toxicity and resistant to existing chemotherapeutic agents. In this study, the effects of pseudolaric acid B (PAB) on three human leukemia cell lines, acute promyelocytic leukemia HL-60 cells, acute lymphoblastic leukemia CCRF-CEM cells, and human chronic myeloid leukemia blast-phase K562 cells were investigated in vitro, compared to normal human peripheral blood mononuclear cells (PBMC).
Methods
Cell viability was determined using CellTiter-Glo luminescent reagent. Colony formation was assessed by Microtitration cloning assay. Cell cycle analysis was carried out by flow cytometry. Tubulin polymerization was measured by recording the increase in absorbance. Inhibition of topoisomerase I (topo I) and topoisomerase II (topo II) enzyme activities was measured by DNA relaxation assay using topo I and II drug screening kit. Apoptosis was observed by DAPI staining assay and Caspase3/7 activities was measured using Caspase-Glo® 3/7 assay kit.
Results
Pseudolaric acid B selectively inhibited the growth of human leukemia HL-60, CCRF-CEM and K562 cells, but not normal PBMC. PAB suppressed colony formation in HL-60 cells. Cell cycle analysis showed that PAB blocked the cell cycle at G2/M phase in HL-60 cells, suggesting that it suppresses mitosis. DNA topo I and topo II were not inhibited, but tubulin polymerization was inhibited. PAB-induced apoptosis and activated caspase-3/7 activity.
Conclusions
This study indicates that PAB has a potential for use against leukemia and its effects might be mediated by inhibiting tubulin polymerization, preventing cell division and activating caspase-3, which leads to apoptosis.
Keywords: Pseudolaric acid B, Leukemia, Peripheral blood mononuclear cells, Cytotoxicity, Cell cycle, Apoptosis
Introduction
Among cancers, leukemias account for the largest number of cases of childhood cancer. Acute lymphoblastic leukemia (ALL) represents approximately three-fourths, whereas acute myeloma leukemia (AML) represents one-sixth of the cases. Despite recent advances in the treatment of childhood cancers, leukemias remain the primary cause of cancer-related mortality among children in the United States (Pui and Evans 2006; Pui and Jha 2007). Although ALL and AML are potentially curable diseases, with a 5-year survival of 70% for ALL and 40% for AML, once leukemias recurs, the outcome is dismal. On the other hand, adult leukemia patients are not transplantation, a more rational therapy not adequately defined. These individuals are treated with regimens that focus on chemotherapy. Therefore, there is a need for novel antileukemia agents, especially those that are effective for relapse leukemias resistant to existing chemotherapeutic agents.
Pseudolaric acids A and B (PAA and PAB, Fig. 1) are diterpenoids isolated from the Chinese herb Tujinpi, the bark of Pseudolarix kaemferi (Zhou et al. 1983). PAB has also been found in Pseudolarix amabilis (Li et al. 1999). PAA and PAB possess an unusual tricyclic core that includes a fused ring system (polyhydroazulene) with a trans substitution pattern at the junction sites, a structural framework that have never been found in any other natural products. The difference between them is that the allylic methyl group on the ring system in PAA is replaced with a carboxylic acid methyl ester in PAB (Fig. 1). Both compounds have been demonstrated to be potent antifertility and antifungal agents (Yang et al. 2003; Zhang et al. 1990). The antifungal activity of PAB was approximately as potent as amphotericin B in vitro, yet significantly less toxic (Li et al. 1995). Pan et al. (1990) reported that PAA and PAB exhibited potent anticancer activity against a panel of 60 human cancer cells. PAB was more potent than PAA. Further studies showed that PAB-induced cell cycle arrest and apoptosis in skin, uterus, liver and gastric carcinomas (Gong et al. 2004, 2005a, b; Li et al. 2005; Wong et al. 2005). Although PAB exhibits cytotoxic activity and induces apoptosis in several tumor cells, its action in leukemias is not clear. In the present study, the effects of PAB on the growth of three human leukemia cells: acute promyelocytic leukemia (HL-60), acute lymphoblastic leukemia (CCRF-CEM) and chronic myeloid leukemia blast-phase (K562) cells were evaluated, compared to human peripheral blood mononuclear cells (PBMC). Its effects on topoisomerases and tubulin polymerization were measured. Furthermore, the effects of PAB on cell cycle progression and induction of apoptosis were also investigated in HL-60 cells.
Fig. 1.
The chemical structures of PAA and PAB
Materials and methods
Samples and reagents
Pseudolaric acid B was isolated from Pseudolarix ambalis as described previously (Li et al. 1999). Taxol, interleukin-2 (IL-2), and all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO). CellTiter-Glo luminescent reagents and Caspase-Glo® 3/7 assay kit were purchased from Promega Co., Sydney, Australia. Stock solution of test compound was prepared in DMSO. The highest test concentrations of DMSO ranged from 0.2 to 0.5%.
Cell viability assay
HL-60, CCRF-CEM and K562 cells, obtained from ATCC were grown in RPMI 1640 medium supplemented with 10% FBS, penicillin (100 units/mL) and streptomycin (10 μg/mL) at 37°C in a humidified atmosphere containing 5% CO2. For the cell viability assay, HL-60, CCRF-CEM and K562 cells were seeded in white solid 96-well plates at a density of 2 × 104 cells/well in 90 μL medium. After 24 h incubation at 37°C, 10 μL drug solution with different concentrations of PAB (0, 001, 0.01, 0.1, 1, 10 μM) was added into each well. Cells were incubated for 48 h. At the end of incubation, cell viability was determined by adding 50 μL of CellTiter-Glo luminescent reagent (Promega Co., Australia) and by recording the luminescence for each well.
Human peripheral blood mononuclear cells were isolated from the blood of healthy adult volunteer donors by centrifuging venous blood on Ficoll-hypaque density gradients (Amersham Biosciences Inc., Piscataway, NJ). PBMC were seeded as above. Cells were stimulated with IL-2 (20 U/mL) and 2 h later, drug was added to the wells and further incubated for 48 h and cell viability was determined as above.
Clonogenic survival assay
HL-60 cells were set up at 5×104 cells/mL in 25-cm2 tissue culture flasks (8 mL/flask). Cells were allowed to grow undisturbed for 24 h before addition of PAB. After 2, 6 and 24 h incubation with PAB, cells were washed once and resuspended in drug-free medium. Cells were counted and the viable cells were diluted to the required cell number. Cloning efficiency was determined by plating twofold dilutions of viable cells ranging from 5 to 0.625 cells/well in 96-well plates. The plates were incubated in humidified atmosphere with 10% CO2, 5% O2 and 70% N2 and the wells were inspected for positive colonies after 8 days. Positive colonies were scored if wells contained 50 or more viable cells at 8 days. The cloning efficiency of the cells was calculated from the proportion of negative wells using Poisson statistic and X 2 minimization. Cloning results were expressed as colony forming units/mL, which was calculated from the percentage cloning efficiency times viable cell concentration of cultures at the time of cloning. The cloning efficiency of the control culture of HL-60 cells was 36.3% in this experiment.
Cell cycle analysis
Cell cycle analysis in HL-60 cells was performed by flow cytometry as described earlier (Ma et al. 2007). Cells were exposed to 1 μM PAB for 6–48 h. 2–4 × 105 cells were collected and resuspended in 1 mL of PBS buffer, followed by the immediate addition of 1 mL of ice-cold 70% ethanol. After fixing overnight at 4°C, cells were recovered by centrifugation, washed with PBS, and resuspended in 0.5 mL PBS containing 40 μg/mL RNase A and 50 μg/mL propidium iodide. DNA content was analyzed by flow cytometry and data was analyzed using Becton-Dickinson CELL Quest software.
Assay for tubulin polymerization
The assay for microtubule polymerization was performed as described previously (Ma et al. 2005). Briefly, lyophilized bovine brain tubulin [containing 90% tubulin and 10% microtubule associated proteins (MAPS)] was reconstituted (2 mg/mL) in G-PEM buffer consisting of 80 mM Pipes [piperazine-N,N′-bis(2-ethanesulfonic acid)] sequisodium salt, 2 mM MgCl2, 0.5 mM EGTA, and 1 mM GTP, pH 6.9. 100 μL of reconstituted tubulin was transferred to the well of pre-warmed plate and tubulin polymerization was measured at 37°C by recording the increase in absorbance at 340 nm for 30 min on a Bio-Tek Power wave XS plate reader.
Assay for topoisomerase activity
Inhibition of topoisomerase I (topo I) and topoisomerase II (topo II) enzyme activities was measured by DNA relaxation assay using topo I and II drug screening kit (Topogen, Columbus, OH) as described earlier (Ma et al. 2005; Khan et al. 2002). Measurement of the catalytic activity of topo I or II was based on the conversion of supercoiled DNA to relaxed DNA. The assay was performed in a total volume of 20 μL containing 250 ng kDNA, samples (3–70 μM), and 2 units of topo I or II in the assay buffer (50 mM Tris–HCl, pH 8.0, 120 mM KCl, 10 mM MgCl2, 0.5 mM ATP, 0.5 mM dithiothreitol, and 30 μg/mL BSA). The mixture was incubated at 37°C for 30 min and the reaction was terminated by the addition of 1/5 volume of 5× stop buffer (5% sarkosyl, 0.025% bromophenol blue, 25% glycerol). DNA was analyzed by electrophoresis on agarose gel (1%) in TAE buffer (40 mM Tris–acetate, 2 mM EDTA, pH 8.5). After staining with ethidium bromide, gel was analyzed on a Gel Doc system for quantitation of DNA. Enzyme activity was measured in terms of the percentage of substrate kDNA converted to product (decatenated kDNA). Cleavage complex stabilization activity was based on nicked DNA production. The assay was performed with 4 units of topo II in the cleavage buffer (30 mM Tris–HCl, pH 7.6, 120 mM NaCl, 8.0 mM Cl2, 3.0 mM ATP, and 15 mM β-mercaptoethanol). The reaction was terminated by addition of 2 μL SDS (10%) followed by addition of Proteinase K (50 μg/mL) and further incubation at 37°C for 30 min. The loading buffer (10×) was added, and DNA was extracted with an equal volume of CIA (chloroform/isoamyl alcohol, 24:1). DNA was analyzed by electrophoresis and photodocumented as already described. Cleavage buffer is optimized to detect formation of cleavage complexes, and the decatenation activity is less robust in this buffer.
DAPI staining
HL-60 cells were treated with 1 μM PAB or vehicle for 24 h. The cells were harvested by centrifugation and washed three times with PBS, fixed in a solution of 3.7% formaldehyde for 10 min, and then in 1 mL of methanol. Fixed cells were stained with 4 μg/mL DAPI for 10 min. The nuclear morphology of cells was observed by fluorescence microscopy (Ma et al. 2007).
Caspase-3/7 activity assay
Caspase activity was detected by using Caspase-Glo® 3/7 assay kit (Promega Co., Sydney, Australia). This assay is a luminescent assay which measures caspase-3 and -7 activities. The level of caspase activity is measured by the addition of a caspase-3/7 substrate, which is cleaved by the caspase-3/7 to release a substrate for luciferase, resulting in the luciferase reaction and the production of light. Briefly, HL-60 cells (2 × 104 cells/well) were seeded in a white solid 96 well plate and incubated for 24 h at 37°C. The time course of caspase activation was evaluated in an initial set of experiments. HL-60 cells were exposed to 0.1% DMSO or 1 μM PAB for 2, 6, 12, 24 and 48 h. 50 μL of caspase-3/7 reagent was added to each well and incubated for 1 h on rotary shaker at room temperature. Luminescence for each well was recorded.
Statistical analysis
All data are presented as mean ± SD from three separate experiments performed in duplicate. Statistical differences were calculated using the Student’s t test and considered significant at *P < 0.05 or **P < 0.01 level. Figures 4, 5, and 6 are representative of three independent experiments with similar pattern.
Fig. 4.
Flow cytometry histograms of cell cycle distribution of HL-60 cells of untreated control and of PAB treated group at 1 μM. The time course of the cell cycle progress showed that HL-60 cells were treated with PAB for 6–48 h, progressive reduction of cells in G0/G1 phase occurred with a concomitant increase of cells arrested in the G2/M phase and which were unable to proceed through mitosis to the next G1 phase, instead accumulating in the G2/M compartment. The proportion of cells in the S phase was comparatively unchanged by PAB treatment
Fig. 5.
Inhibition of tubulin polymerization of bovine tubulin in vitro by PAB. In vitro polymerization of purified bovine brain tubulin in the presence of pseudolaric acid B was assessed as described in “Materials and methods”. Results are plotted as the extent of tubulin polymerization (A 340 nm) as function of time
Fig. 6.

Induction of apoptosis by PAB in HL-60 cells. Cells were treated with vehicle (a) or with 1 μM PAB for 24 h (b). DAPI staining was conducted as described in “Materials and methods” and nuclear morphology was examined by using a fluorescent microscope. Exposure to 1 μM PAB for 24-h-induced apoptotic changes such as condensed chromatin, and fragmented nuclei (×1,000). Arrow indicates apoptotic cells with condensed and fragmented nuclei
Results
Selective inhibition of the growth of leukemia cells, but not normal cells
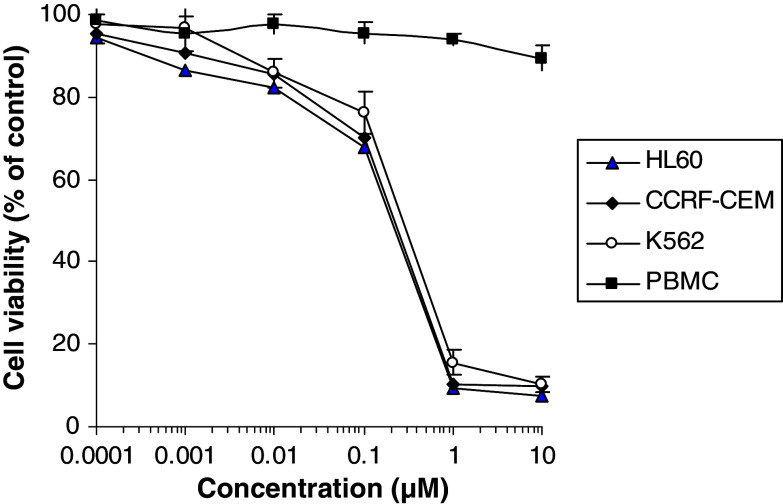
The effects of various concentrations of PAB on proliferation of leukemia cells and PBMC were tested using CellTiter-Glo®Tumor luminescent cell viability assay. As shown in Fig. 2, PAB inhibited the growths of HL-60, CCRF-CEM and K562 cells in dose-dependent manner with IC50 values 0.17 ± 0.01, 0.18 ± 0.02 and 0.28 ± 0.02 μM at 48 h, respectively. Meanwhile PAB was tested for selectivity using PBMC stimulated by IL-2. PAB revealed no cytotoxic effect on normal PBMC up to 10 μM concentration. Thus, PAB show at least 100-fold less toxicity on normal PBMC than leukemia cells. This indicates that PAB possessed selective antiproliferative activity towards leukemia cells.
Fig. 2.
Effects of PAB on the growths of human leukemia HL-60, CCRF-CEM and K562 cells, and normal PBMC. Cells were treated with 1 μM PAB or vehicle for 48 h and cell viability was determined by CellTiter-Glo® luminescent cell viability assay, the results shown were mean ± SD (bars) of three experiments
Inhibition of colony formation of HL-60 cells
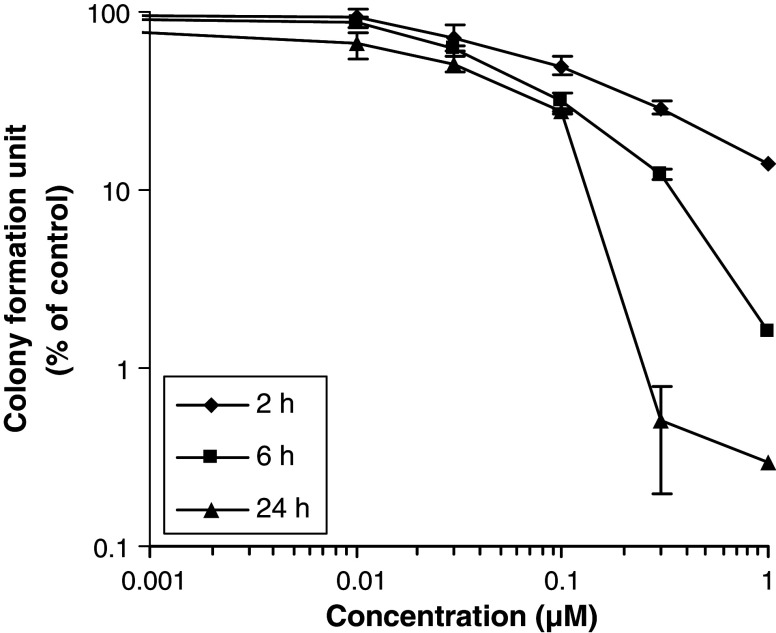
To determine if the cell growth inhibitory activity of PAB is cytotoxic rather than only cytostatic, colony formation assays were performed. The effect on clonogenicity of HL-60 cells upon exposure to PAB for 2, 6 and 24 h was studied. The result is shown in Fig. 3. It was found that PAB inhibited colony formation in a concentration- and time-dependent manner. At 2 h exposure to PAB, colony formation units decreased gradually with increasing concentration of PAB. After 24 h exposure, the cytotoxicity was increased significantly (Fig. 3).
Fig. 3.
Effect of PAB on colony formation of HL-60 cells, after exposure for 2, 6 and 24 h, as determined by the microtitration cloning assay. Results are mean ± SD (bars) from three experiments
Induction of cell cycle arrest in G2/M phase
To determine if the antiproliferative effects of PAB involved growth arrest at specific phases of the cell cycle, flow cytometric studies were performed. Cells were exposed to 1 μM PAB for different time periods; the cell number percentage in each phase (G0/Gl, S and G2/M) of the cell cycle was calculated by flow cytometric analysis (Fig. 4). PAB induced the inhibition of cell growth and cell cycle progression mainly by inducing arrest of the G2/M phase in HL-60 cells and its effect was in a time-dependent manner. After 6 h of PAB treatment, there was a significant increase of cells in G2/M phase, with a corresponding decrease in G1 phase. The number of G2/M cells reached a maximum at 12 h. These results suggest that cells in the G2 or M phase were unable to proceed through mitosis to the next G1 phase, accumulating instead in the G2/M compartment. After 24 h, a significant increase occurred in the percentage of hypodiploid cells (peak prior to G1 phase) while the proportion of cells at G2/M decreased relatively. By 48 h, most of the cells had reached G2/M and died. G1, S, G2/M phase cells decreased at the higher drug concentrations. The hypodiploid cells are likely dead cells with degraded and/or apoptotic DNA. The polyploid DNA content reflects cells, which have not separated at mitosis and hence contain greater DNA content.
Inhibition of tubulin polymerization in vitro
Since most of the known mitotic inhibitors have been shown to interfere with the microtubular dynamics (Gerber 2008), we assessed the effect of PAB on polymerization of bovine brain tubulin in vitro. As shown in Fig. 5, PAB inhibited both rates and extents of tubulin polymerization in dose-dependent fashion.
Effect of PAB on topo I and topo II
Next we investigated the effects of PAB on the catalytic activities of purified human topo I and topo II. PAB did not inhibit the DNA relaxation activity of topo I and topo II up to a highest test concentration of 200 μΜ. PAB did not show any cleavage complex stabilization activity either with topo I or topo II and did not cause the formation of nicked DNA (data not shown).
Induction of apoptosis in HL-60 cells
To investigate whether antiproliferative activity was due to induction of apoptosis in HL 60 cells, DAPI staining assay and flow cytometry assay was performed. The cell cycle analysis indicated that PAB induced a marked, time-dependent increase in the percentage of sub-G1 population in HL-60 cells at 24 and 48 h after drug treatment (Fig. 4). DAPI staining assay showed that cells treated with 1 μM PAB display condensed and fragmented nuclei, which were indicative of apoptosis. Thus in total, these results indicate that PAB-induced apoptosis in HL-60 cells (Fig. 6).
Activation of caspase-3/7
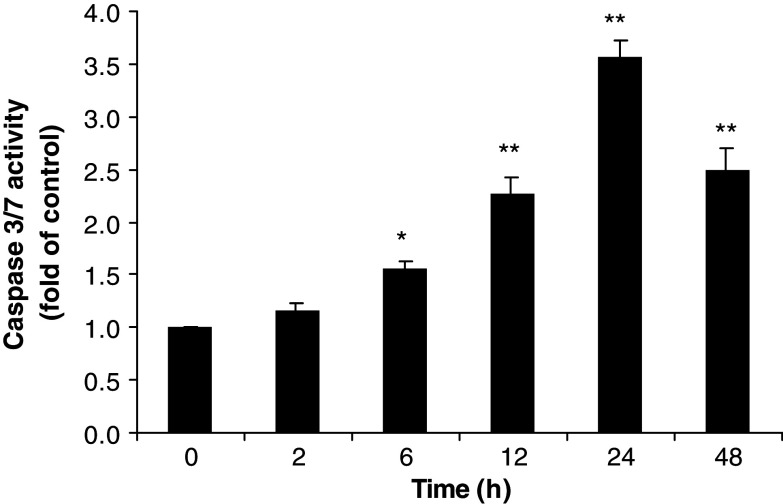
Caspase-3 and -7 play crucial roles in apoptosis, the main type of programmed cell death, in mammalian cells and are activated in a sequential cascade of cleavages from their inactive forms. After activation, caspases can cleave their substrates. To detect the enzymatic activity of caspase-3/7 during PAB-induced apoptosis, HL-60 cells were exposed to 1 μM PAB for 2, 6, 12, 24 and 48 h and caspase activity was measured by using Caspase-Glo® 3/7 assay kit. Figure 7 showed that PAB induced a time dependent increase in caspase-3/7 activities in HL-60 cells. Caspase-3/7 were activated at 6 h after 1 μM PAB treatment. The maximum activity was seen at 24 h. Caspase-3/7 activities gradually decreased at 48 h.
Fig. 7.
Time course of caspase-3 activation after treatment with PAB. HL-60 cells were treated with or without PAB (1 μM) for 2, 6, 12, 24, and 48 h. Enzymatic activity of caspase-3 were determined by adding 50 μL of caspase reagents. The activity of caspase-3 is expressed as the value relative to untreated cells. The results shown are mean ± SD (bars) of triplicate experiments. *P < 0.05; **P < 0.01
Discussion
Use of chemotherapeutic agents has been shown to improve the outcomes of patients with leukemia. Moreover, numerous new anticancer agents have been shown to have anticancer activity, either alone or in combination with other anticancer agents (Gerber 2008; Larson and Stock 2008). However, for most cancer patients, currently available therapies have been only temporarily successful because they frequently lead to resistance or unacceptable levels of toxicity. Therefore, alternative therapeutic agents that kill cancer cells selectively are highly desirable. In present study, we selected three human leukemia cell lines, i.e. acute promyelocytic leukemia HL-60 cells, acute lymphoblastic leukemia CCRF-CEM cells, and human chronic myeloid leukemia blast-phase K562 cells to represent acute and chronic leukemia to study the effects of PAB on human leukemias. The results showed that PAB significantly inhibited the growths of human leukemia HL-60, CCRF-CEM and K562 cells in vitro. Acute leukemia HL-60 (IC50 = 0.17 μM), CCRF-CEM (IC50 = 0.18 μM) cells are more sensitive to PAB than chronic leukemia K562 (IC50 = 0.28 μM) cells, which indicates that PAB may be more active against acute leukemia. PAB had little effect on the growth of normal human PBMC at the 100 times higher than IC50 values used for human leukemia cells, suggesting PAB might selectively induce cytotoxicity in human leukemia cells.
Pseudolaric acid B was further studied to examine its mode of action in HL60 cells. Firstly, the effect of PAB on cell clonogenic survival was studied. Cell growth inhibition tests are convenient and usually quick and easy to perform, but they only reveal changes in viability of cells during exposure to the drug. On the other hand, clonogenic assays demonstrate the metabolic or proliferative capacity of cells after exposure to the drug. PAB significantly inhibited colony formation of HL-60 cells in a time- and dose-dependent manner, suggesting that the effect of PAB is cytotoxic rather than just cytostatic. The selective cytotoxic effect of PAB may have been the result of cellular differences in drug uptake and metabolism or biochemical and physiologic differences in activation, transduction, and duration of various signaling pathways. To determine whether PAB can be used to treat human leukemia, we further characterized the molecular mechanism of PAB by studying the effect on cell cycle progression in HL-60 cells. Many anticancer drugs exhibit their activity by inhibiting cell cycle progression, for example, vinblastine and taxol block cell cycle at G2/M and cisplatin blocks cell cycle at G1/M phase, while methotrexate inhibits S phase (Cragg et al. 1997; Jordan et al. 1991; Shi et al. 1998). PAB-induced cell cycle arrest at G2/M phase in HL-60 cells, which indicates that the anticancer activity is related to inhibition of mitosis. This observation is consistent with previous studies that PAB-induced G2/M arrest in several types of human cancer cell lines (Li et al. 2005; Wong et al. 2005).
Microtubules are major cellular dynamic structural components that play a crucial role in many biological processes, including mitosis, intracellular transport, exocytosis, and cell growth. An essential function of microtubules is to partition duplicated chromosomes into daughter cells during cell division. Microtubule dynamics are dramatically increased during mitosis, are very sensitive to interferences, and thereby constitute an important target in cancer chemotherapy. Examples of drugs that inhibit microtubule polymerization include Vinca alkaloids, cryptophycins, halichodrins, estramustine, and colchicines. Another group of drugs that stimulates microtubule polymerization and stabilizes microtubules at high concentrations, includes paclitaxel (taxol), eleuthrobins, laulimalide, and discodermolide (Jordan et al. 1991; Shi et al. 1998; Cragg and Suffness 1988; Jordan 2002). PAB inhibits microtubule polymerization, and its antimiotic action seems to be related to inhibition of tubulin polymerization. This result is consistent with previous report (Wong et al. 2005). However, we found that the concentration responsible for inhibition of microtubule polymerization is higher than the concentration of inhibition of cell growth, which indicates the involvement of other pathways in the mechanism of action of PAB. Therefore, we further studied if PAB inhibited topoisomerases (topos) since they are intricately involved in maintaining the topographic structure of circular DNA during translation, transcription, and mitosis (Wang et al. 1997). Two major types of topos (topo I and topo II) exist in prokaryotic and eukaryotic cells and are classified by their mechanisms of DNA breakage. These breaks are essential for uncoiling of the DNA helix during replication and for relieving torsional tension along the fork of replicating DNA before strand resealing. Our result showed that PAB did not inhibit either topo I or topo II, indicating that the antimitotic activity of PAB is not related to inhibition of topoisomerases.
Cell deaths have two characteristics, apoptosis and necrosis. The major difference between both is the active participation of the cells in the process (Sherr 1996). Apoptosis is a highly organized physiological mechanism for destroying injured and abnormal cells (Sen and D’Incalci 1992). Some anticancer drugs, when applied in vivo, are able to intercalate with DNA in cancer cells and do arrest cancer cells in the cell cycle (Cohen 1997). Furthermore, mitochondrial cytochrome c releases an important control point in caspase activation and apoptosis (Lowe and Lin 2000). It is suggested that susceptibility to apoptosis-inducing effects of chemotherapeutic drugs may depend on the intrinsic ability of tumor cells to respond by apoptosis (Sherr 1996; Desagher and Martinou 2000; Tseng et al. 2002). In this study, apoptosis was detected within 48 h of 1 μM PAB treatment. PAB-induced extensive nuclear condensation and DNA fragmentation. Flow cytometry analysis showed that there were many hypodiploid debris, perhaps from apoptotic cells. These investigations indicate that the anticancer mechanism of PAB might be related to induction of apoptosis and inhibition of tubulin polymerization may serve as an inducing signal triggering the apoptotic cell death of leukemia cells exposed to PAB.
Caspase-3 is the most prevalent caspase within cells, responsible for the majority of apoptotic effects (Cohen 1997). The activation of caspase-3 induces poly (ADP-ribose) polymerase (PARP) cleavage, chromosomal DNA breaks and finally lead to apoptosis (Lowe and Lin 2000). Our results showed PAB-induced caspase-3 activation in time-dependent manner, suggesting that caspase-3 might have a critical role in the apoptosis induced by PAB. PAB initiates activation of caspases and in turn the caspase activation leads to apoptotic cell death. These results provide a correlation between caspase-3 activity and PAB-induced apoptosis in HL-60 cells.
In conclusion, PAB selectively inhibited the growth of HL-60 cells. This inhibitory effect is cytotoxic. PAB blocked cells at G2/M phase of cell cycle. The antileukemia activity could be related to inhibition of tubulin polymerization and activation of caspase-3 that lead to cell cycle arrest and apoptosis. Although cell proliferation and apoptosis are complex processes, which involve many pathways including tubulin polymerization, inhibition of tubulin polymerization could be one of the mechanisms involved in the inhibition of cell proliferation and induction of apoptosis by PAB. Possibility of involvement of other pathways could not be ruled out. Further investigation on the exact mechanisms of antileukemia effects of PAB and its in vivo therapeutic efficacy and toxicity are warranted.
Acknowledgments
This study was supported in part by USDA Agricultural Research Service Specific Cooperative Agreement No. 58-6408-2-0009.
Conflict of interest statement
We declare that we have no conflict of interest.
References
- Cohen GM (1997) Caspases: the executioners of apoptosis. Biochem J 326:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg G, Suffness M (1988) Metabolism of plant-derived anticancer agents. Pharmacol Ther 37:425–461. doi:10.1016/0163-7258(88)90006-X [DOI] [PubMed] [Google Scholar]
- Cragg GM, Newman DJ, Snader KM (1997) Natural products in drug discovery and development. J Nat Prod 60:52–60. doi:10.1021/np9604893 [DOI] [PubMed] [Google Scholar]
- Desagher S, Martinou JC (2000) Mitochondria as the central control point of apoptosis. Trends Cell Biol 10:369–377. doi:10.1016/S0962-8924(00)01803-1 [DOI] [PubMed] [Google Scholar]
- Gerber DE (2008) Targeted therapies: a new generation of cancer treatments. Am Fam Physician 77:311–319 [PubMed] [Google Scholar]
- Gong XF, Wang MW, Wu Z, Tashiro S, Onodera S, Ikejima T (2004) Pseudolaric acid B induces apoptosis via activation of c Jun N-terminal kinase and caspase-3 in hela cells. Exp Mol Med 31:551–556 [DOI] [PubMed] [Google Scholar]
- Gong XF, Wang MW, Tashiro S, Onodera S, Ikejima T (2005a) Pseudolaric acid B induces apoptosis through P53 and Bax/Bcl-2 Pathways in human melanoma A 375-S2 cells. Arch Pharm Res 28:68–72. doi:10.1007/BF02975138 [DOI] [PubMed] [Google Scholar]
- Gong XF, Wang MW, Tashiro S, Onodera S, Ikejima T (2005b) Pseudolaric acid B induces human melanoma A 375-S2 cell apoptosis in vitro. Zhongguo Zhong Yao Za Zhi 30:55–57 [PubMed] [Google Scholar]
- Jordan MA (2002) Mechanism of action of anticancer drugs that interact with microtubule and tubulin. Curr Med Chem Anticancer Agents 2:1–17 [DOI] [PubMed] [Google Scholar]
- Jordan MA, Thrower D, Wilson L (1991) Mechanism of inhibition of cell proliferation by Vinca alkaloids. Cancer Res 51:2212–2221 [PubMed] [Google Scholar]
- Khan SI, Nimrod AC, Mehrpooya M, Nitiss JL, Walker LA, Clark AM (2002) Antifungal activity of eupolauridine and its action on DNA topoisomerases. Antimicrob Agents Chemother 46:1785–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson S, Stock W (2008) Progress in the treatment of adults with acute lymphoblastic leukemia. Curr Opin Hematol 15:400–407. doi:10.1097/MOH.0b013e3283034697 [DOI] [PubMed] [Google Scholar]
- Li E, Clark AM, Hufford CD (1995) Antifungal evaluation of pseudolaric acid B, a major constituent of Pseudolarix kaempferi. J Nat Prod 58:57–67. doi:10.1021/np50115a007 [DOI] [PubMed] [Google Scholar]
- Li XC, Elsohly HN, Nimrod AL, Clark AM (1999) Two auronols from Pseudolarix amabilis. J Nat Prod 62:767–7669. doi:10.1021/np980469w [DOI] [PubMed] [Google Scholar]
- Li KS, Gu XF, Li P, Zhang Z, Zhao YS, Yao ZJ, Qu NQ, Wang BY (2005) Effect of pseudolaric acid B on gastric cancer cells: inhibition of proliferation and induction of apoptosis. World J Gastroenterol 11:7555–7559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe SW, Lin AW (2000) Apoptosis in cancer. Carcinogenesis 21:485–495. doi:10.1093/carcin/21.3.485 [DOI] [PubMed] [Google Scholar]
- Ma G, Khan SI, Mustafa J, Walker LA, Khan IA (2005) Anticancer activity and possible mode of action of 4-O-podophyllotoxinyl 12-hydroxyl-ocadec-Z-9-enoate. Lipids 40:303–308. doi:10.1007/s11745-005-1386-0 [DOI] [PubMed] [Google Scholar]
- Ma G, Khan SI, Fisher NH, Khan IA, Pasco DS (2007) Inhibition of NF-κB-mediated transcription and induction of apoptosis by melampolides and repandolides. Cancer Chemother Pharmacol 60:35–43. doi:10.1007/s00280-006-0344-0 [DOI] [PubMed] [Google Scholar]
- Pan D, Li ZL, Hu CQ, Chen K, Chang JJ, Lee KH (1990) Antitumor agents: 109. The cytotoxic principles of Pseudolarix kaempferi: pseudolaric acid A and B and related derivatives. Planta Med 56:383–385. doi:10.1055/s-2006-960989 [DOI] [PubMed] [Google Scholar]
- Pui CH, Evans WE (2006) Treatment of childhood acute lymphoblastic leukemia. N Engl J Med 354:166–178. doi:10.1056/NEJMra052603 [DOI] [PubMed] [Google Scholar]
- Pui CH, Jha S (2007) New therapeutic strategies for the treatment of acute lymphoblastic leukaemia. Nat Rev Drug Discov 6:149–165. doi:10.1038/nrd2240 [DOI] [PubMed] [Google Scholar]
- Sen S, D’Incalci M (1992) Apoptosis: biochemical events and relevance to cancer chemotherapy. FEBS Lett 307:122–127. doi:10.1016/0014-5793(92)80914-3 [DOI] [PubMed] [Google Scholar]
- Sherr CJ (1996) Cancer cell cycles. Science 274:1672–1677. doi:10.1126/science.274.5293.1672 [DOI] [PubMed] [Google Scholar]
- Shi Q, Chen KC, Morrist-Natschke SL, Lee KH (1998) Recent progress in the development of tubulin inhibitors as antimitotic antitumor agents. Curr Pharm Des 4:219–248 [PubMed] [Google Scholar]
- Tseng CJ, Wang YJ, Liang YC, Jeng JH, Lee WS, Lin JK (2002) Microtubule damaging agents induce apoptosis in HL-60 cells and G2/M cell arrest in HT 29 cells. Toxicology 175:123–142. doi:10.1016/S0300-483X(02)00073-2 [DOI] [PubMed] [Google Scholar]
- Wang HK, Morris-Natschke SL, Lee KH (1997) Recent advances in the discovery and development of topoisomerase inhibitors as antitumor agents. Med Res Rev 4:367–425. doi:10.1002/(SICI)1098-1128(199707)17:4%3c367:AID-MED3%3e3.0.CO;2-U [DOI] [PubMed] [Google Scholar]
- Wong VKW, Chiu P, Chung SSM, Chow LMC, Zhao YZ, Yang BB, Ko BCB (2005) Pseudolaric acid B, a novel microtubule-destabilizing agent that circumvents multidrug resistance phenotype and exhibits antitumor activity in vivo. Clin Cancer Res 11:6002–6011. doi:10.1158/1078-0432.CCR-05-0209 [DOI] [PubMed] [Google Scholar]
- Yang SP, Dong L, Wang Y, Wu Y, Yue JM (2003) Antifungal diterpenoids of Pseudolarix kaempferi, and their structure-activity relationship study. Bioorg Med Chem 11:4577–4584. doi:10.1016/S0968-0896(03)00531-5 [DOI] [PubMed] [Google Scholar]
- Zhang YL, Lu RZ, Yan AL (1990) Inhibition of ova fertilizability by pseudolaric acid B in hamster. Acta Pharmacol Sin 11:60–62 [PubMed] [Google Scholar]
- Zhou BN, Ying BP, Song GQ, Chen ZX, Han J, Yan YF (1983) Pseudolaric acids from Pseudolarix kaempferi. Planta Med 47:35–38. doi:10.1055/s-2007-969944 [DOI] [PubMed] [Google Scholar]