Abstract
Vitamin A is essential for the formation and maintenance of many body tissues. It is also important for embryonic growth and development and can act as a teratogen at critical periods of development. Retinoic acid (RA) is the biologically active form of vitamin A and its signaling is mediated by the RA and retinoid X receptors. In addition to its role as an important molecule during development, RA has also been implicated in clinical applications, both as a potential anti-tumor agent as well as for the treatment of skin diseases. This review presents an overview of how dietary retinoids are converted to RA, hence presenting the major players in RA metabolism and signaling, and highlights examples of treatment applications of retinoids. Moreover, we discuss the origin and diversification of the retinoid pathway, which are important factors for understanding the evolution of ligand-specificity among retinoid receptors.
Keywords: Retinoic acid signaling, Metabolism, Retinoids, Therapy, Evolution, Vertebrates, Invertebrates
Introduction: the importance of vitamin A
Vitamin A (retinol) is a fat-soluble vitamin essential for the formation and maintenance of many body tissues, such as skin, bone, and vasculature, as well as for the promotion of good vision and immune function [1]. Vitamin A also plays a role in reproduction and in embryonic growth and development. Vitamin A is converted to more active compounds, such as retinoic acid (RA), through which it exerts its multiple effects on embryonic development and organogenesis, tissue homeostasis, cell proliferation, differentiation, and apoptosis [2, 3]. In fact, RA was one of the first morphogens identified [4]. RA is a rapidly diffusing signaling molecule that can specify cell identities and control gene expression through the activation of specific nuclear receptors.
Deficiency of vitamin A (VAD) leads to blindness and infectious diseases, whereas less severe forms of VAD retard growth and intensify iron deficiency anemia [5]. Conversely, excess dietary vitamin A can result in toxicity to the liver, central nervous system (CNS), musculo-skeletal system, internal organs, and skin [1, 4]. This so-called hypervitaminosis A can lead to reduced mineral bone density, and therefore increased risk for hip fracture [6], and to malformations of the developing embryo [1].
In this review, we follow the path of retinoid metabolism from dietary intake to RA synthesis and signaling and discuss the different components of the pathway. We also discuss the applications of retinoids in the clinic and finally attempt to discover the evolutionary origins of retinoid signaling.
Retinoid metabolism and transport
Retinoid uptake and processing
Analogs of retinol, with or without biological activity, are called retinoids. Moreover, the definition of the term retinoid has been expanded to also include compounds not related to retinol structurally, but with retinoid activity [4]. The only source of retinoids in most animals is diet-derived as these compounds cannot be synthesized de novo.
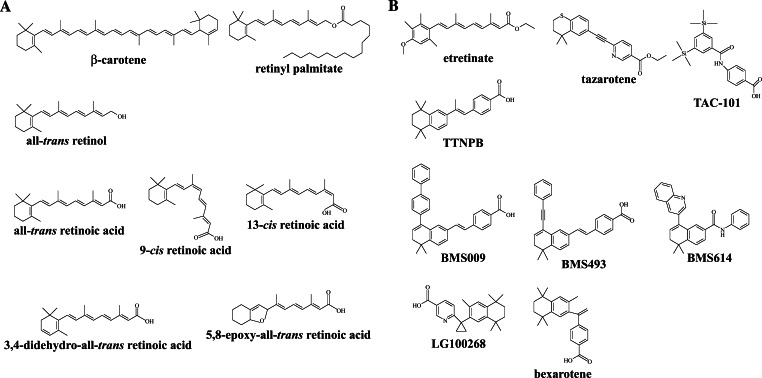
Plants and animals can cleave carotenoids to form retinoids [4]. Carotenoids are responsible for the yellow, red, orange or purple color of many vegetables, fruits, and flowers [4]. Carotenoids can also be absorbed by and stored in animal tissues. Of the 600 identified carotenoids, only 10% are vitamin A precursors with β-carotene being the most potent [7]. Alternatively, animals can obtain retinol by eating animal tissues that have already converted carotenoids to retinoids. Due to the tendency of retinyl esters to accumulate in the livers of mammals, birds, and fish, retinyl esters also contribute to the dietary intake of retinol. In animal tissues, the predominant retinoid is retinyl palmitate, but other fatty acids, such as retinyl oleate and retinyl stearate are also found [4]. The primary function of retinol and retinyl esters is to serve as precursors for the biosynthesis of active retinoids. Retinol has six known biologically-active isoforms: all-trans, 11-cis, 13-cis, 9,13-di-cis, 9-cis, and 11,13-di-cis with all-trans being the predominant physiological form. Endogenous retinoids with biological activity, although this functional distinction is not always very clear-cut, include: all-trans RA, 9-cis RA, 11-cis retinaldehyde, 3,4-didehydro RA, and perhaps 14-hydroxy-4,14-retro retinol, 4-oxo RA, and 4-oxo retinol (Fig. 1a) [8–10].
Fig. 1.
Natural and synthetic retinoids. a Structures of natural retinoids and of their catabolism products. b Synthetic agonists and antagonists of RAR and RXR
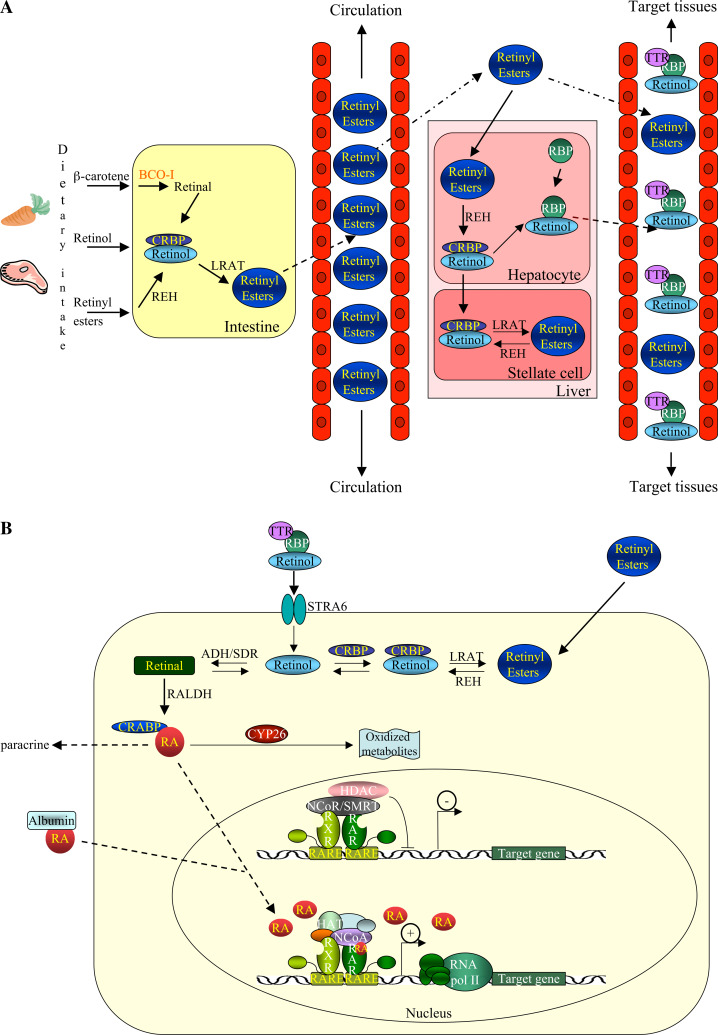
The precursors of RA are converted to the various active forms in the gut, liver, and target tissues (Fig. 2). As early as 1930, there were reports that β-carotene can be converted to retinoids in the small intestine [11], and shortly thereafter a central cleavage mechanism was proposed at the 15,15′ carbon double bond, which produces two molecules of all-trans-retinaldehyde [12]. The enzymatic activity of β,β-carotene-15,15′-monooxygenase (BCO-I) was described independently in two laboratories in 1965, but the cloning of the corresponding gene was only described in this decade [4]. The first BCO-I to be cloned was from the fruit fly Drosophila melanogaster and chicken, and more recently the gene was cloned in human, mouse, zebrafish, and the tunicate Ciona intestinalis [13]. Homologs of BCO have also been identified in the cephalochordate amphioxus [14]. BCO-I is involved in the production of retinaldehyde for photoreception, but its expression in multiple tissues in vertebrates makes it possible that the retinoids it produces are also involved in other processes (Fig. 3) [15].
Fig. 2.
Metabolism of vitamin A and transcriptional activation. a Dietary intake of retinoids and absorption in the intestine. In the intestine, retinoids are converted to retinol, which is bound by CRBP. This retinol is further processed to retinyl esters and exported into the circulation, where the retinyl esters are taken to the liver. In hepatocytes of the liver, retinyl esters are converted to retinol and bound to RBP for transport to target cells. In stellate cells of the liver, retinol is converted to retinyl esters for storage. In the bloodstream, the retinol:RBP complex is bound to TTR to avoid elimination by the kidney and to ensure delivery to target tissues. b Retinol is delivered to target cells in a complex with RBP and TTR and uptake occurs via the STRA6 receptor. In the target cell, free retinol is oxidized to retinaldehyde by ADH/SDR enzymes in a reversible reaction. A second, irreversible oxidation is catalyzed by RALDH and converts retinaldehyde to RA, which is bound by CRABP. RA enters the nucleus, where RAR/RXR heterodimers are bound to RAREs and associated with a co-repressor complex. Binding of RA induces a conformational change of the RAR/RXR heterodimer that results in release of co-repressors and recruitment of co-activators and initiation of transcription. RA is degraded and eliminated by CYP26 enzymes. ADH Alcohol dehydrogenase, BCO-I β,β-carotene-15,15′-monooxygenase, CRABP cellular retinoic acid binding protein, CRBP cellular retinol binding protein, CYP26 cytochrome P450 family 26, HAT histone acetyltransferase, HDAC histone deacetylase, LRAT lecithin:retinol acetyltransferase, NCoA co-activator complex, NCoR co-repressor complex, RA retinoic acid, RALDH retinaldehyde dehydrogenase, RAR retinoic acid receptor, RARE retinoic acid response element, RBP retinol binding protein, REH retinyl ester hydrolase, RNA pol II ribonucleic acid polymerase II, RXR retinoid X receptor, SDR short-chain dehydrogenase/reductase, SMRT silencing mediator of retinoic acid and thyroid hormone receptor, STRA6 stimulated by retinoic acid gene 6, TTR transthyretin
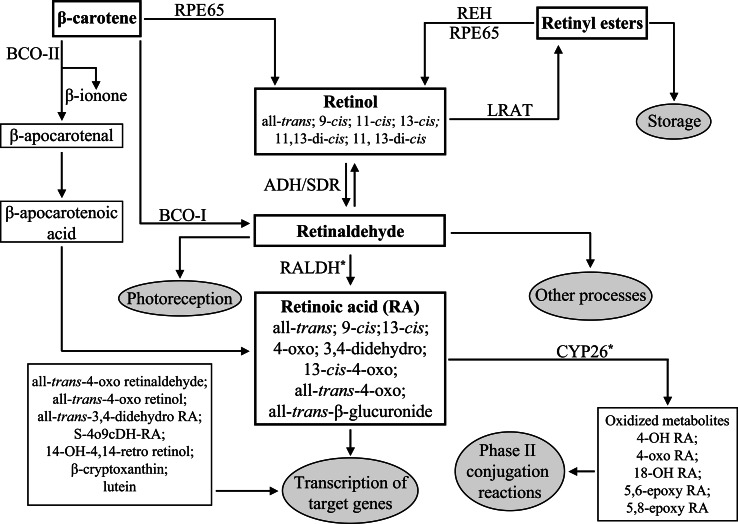
Fig. 3.
Biochemical pathway of retinoids. Endogenous retinoids are boxed and the major physiological endpoints of the metabolic pathway are indicated in gray circles. The enzymes responsible for conversion of the retinoids are indicated. The asterisks indicate that certain enzymes of the CYP family can catalyze the synthesis or degradation of RA in vitro. ADH Alcohol dehydrogenase (ADH1, 3, 4), BCO-I β,β-carotene-15,15′-monooxygenase, BCO-II β,β-carotene-9′,10′-dioxygenase, CYP26 cytochrome P450 family 26, LRAT lecithin:retinol acetyltransferase, RA retinoic acid, RALDH retinaldehyde dehydrogenases (RALDH1, 2, 3, 4), REH retinol ester hydrolase, RPE65 retinal pigment epithelium-specific protein 65 kDa, SDR short-chain dehydrogenase/reductase
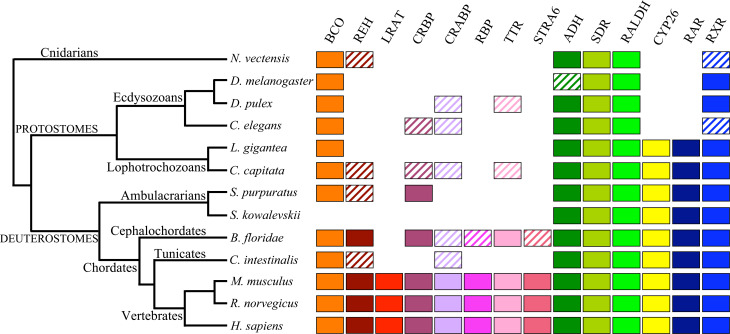
A second enzyme, β,β-carotene-9′10′-dioxygenase (BCO-II), has been demonstrated to have a role in RA synthesis. BCO-II cleaves other carotenoids in addition to β-carotene, leading to the formation of β-apocarotenal and β-ionone [4]. This is followed by the conversion of β-apocarotenal to β-apocarotenoic acid and a stepwise oxidation of β-apocarotenoic acid to RA in a process involving enzymes that have yet to be identified (Fig. 3). This alternative pathway is important as it implies that, in tissues expressing BCO-II, RA can be produced in the absence of “classical RA synthesis pathway” enzymes, such as alcohol dehydrogenase (ADH), short-chain dehydrogenase/reductase (SDR), retinaldehyde dehydrogenase (RALDH) [13] or certain cytochrome P450s [16]. In addition to BCO-I and BCO-II, another family member has been identified in vertebrates, retinal pigment epithelium-specific protein 65 kDa (RPE65), which functions in the visual cycle [13]. Putative BCO family members have been identified by BLAST searches in echinoderms (Stongylocentrotus purpuratus), ecdysozoans (Daphnia pulex, Caenorhabditis elegans), lophotrochozoans (Lottia gigantea, Capitella capitata), and cnidarians (Nematostella vectensis) (Fig. 4). It appears therefore that BCO function might be present in all metazoans, hence representing an ancient pathway for retinoid synthesis [13].
Fig. 4.
Molecular components of RA metabolism and signaling in different metazoans. Using vertebrate sequences for each component, the genomes of the respective species were searched using BLAST. The best hits were then used for reverse BLAST searches of the respective vertebrate genomes to verify the association of a given sequence with the protein family of the RA signaling component in question. Hatched boxes indicate that representatives of a given protein family might exist in a given animal group, but that our BLAST analyses were not conclusive. For example, for CRBP and CRABP, the hatched boxes indicate that members of the fatty acid binding protein family have been identified, and, for RXR, the hatched boxes highlight that this protein is absent from the assayed species, but present in other members of this phylum. The species are: Branchiostoma floridae (amphioxus), Caenorhabditis elegans (nematode worm), Capitella capitata (annelid worm), Ciona intestinalis (sea squirt), Daphnia pulex (water flea), Drosophila melanogaster (fruit fly), Homo sapiens (human), Lottia gigantea (gastropod snail), Mus musculus (mouse), Nematostella vectensis (sea anemone), Rattus norvegicus (rat), Saccoglossus kowalevskii (acorn worm), Strongylocentrotus purpuratus (sea urchin). ADH Alcohol dehydrogenase (ADH1, 3, 4), BCO BCO-I (β,β-carotene-15,15′-monooxygenase) or BCO-II (β,β-carotene-9′,10′-dioxygenase) or RPE65 (retinal pigment epithelium-specific protein 65 kDa), CRABP cellular retinoic acid binding protein, CRBP cellular retinol binding protein, CYP26 cytochrome P450 family 26, LRAT lecithin:retinol acetyltransferase, RALDH retinaldehyde dehydrogenases (RALDH1, 2, 3, 4), RAR retinoic acid receptor, RBP retinol binding protein, REH retinol ester hydrolase, RXR retinoid X receptor, SDR short-chain dehydrogenase/reductase, STRA6 stimulated by retinoic acid gene 6, TTR transthyretin
In the enterocytes of the intestine, retinol is bound to cellular retinol binding protein II (CRBP-II) (Fig. 2a). There are two additional CRBPs, CRBP-I and CRBP-III, that also bind retinol and are found in specific anatomic locations in embryo and adult. The CRBPs belong to the greater family of fatty acid binding proteins (FABPs). In addition to vertebrates, CRBPs are found in cephalochordates and echinoderms, whereas in other invertebrate species fatty acid binding proteins and lipid binding proteins may function in retinol binding [17]. The role of CRBPs may be to determine the levels of intracellular retinol accumulation and esterification [18]. Mice null for CRBP-I appear normal and their sole phenotype is low stores of hepatic retinyl esters [19]. The lipid droplets found in the livers of CRBP-I null mice appear to be smaller and less abundant than in wild-type littermates. The unaltered serum levels of retinol in CRBP-I null mice also demonstrated that CRBP-I does not play a role in the delivery of retinol to retinol binding protein (RBP) prior to secretion of retinol:RBP by the liver. Mice null for CRBP-II have impaired retinol uptake, but when kept on a vitamin A-enriched diet, they develop and reproduce normally albeit with reduced retinol stores [20]. Reduction of vitamin A in the maternal diet during gestation results in neonatal mortality right after birth of null mice, but not of wild-type littermates, suggesting that CRBP-II in both the mother and the fetus are required for adequate delivery of vitamin A to the developing fetus when the dietary intake of vitamin A is limiting [20]. CRBP-III null mice are viable, healthy, and display impairment of vitamin A incorporation into milk [21]. In addition, CRBP-I and CRBP-III are able to compensate for each other to maintain normal retinoid homeostasis, but under conditions of high retinoid demand such as lactation, the compensation is incomplete [21].
Liganded-CRBP (holo-CRBP) is responsible for the delivery of retinol to lecithin:retinol acetyltransferase (LRAT) for esterification [22]. It appears that the role of CRBP-II is to solubilize the fat-soluble retinol and to protect it from degradation as well as to direct it to LRAT for conversion to palmitate and other retinyl esters (Fig. 2a) [23]. LRAT is broadly expressed in tissues and at relatively high levels in the intestine, liver, and eye in the retinal-pigmented epithelial cells. It is responsible for the conversion of all-trans retinol to all-trans retinyl esters. Mice null for lrat develop normally; however, the retinal function of these mice is impaired and slight changes in the retina were observed compared to wild-type littermates [24]. Null mice also had only trace levels of all-trans-retinyl esters in the liver, kidney, and lung, but elevated levels in adipose tissue compared to wild-type littermates [25]. Studies in lrat null mice led to the conclusion that LRAT is a key component of the visual cycle and is essential for the trapping of retinoids in the retinal pigmented epithelial cells of the eye [24]. In times of retinoid insufficiency, the stores of retinyl esters in the adipose tissue of lrat−/− mice are mobilized—an activity that is coupled with the upregulation of CRBP-III. In lrat−/− mice, retinol is mainly absorbed into the cell as free retinol, although retinyl esters (retinyl palmitate, retinyl linolate, retinyl oleate) are also found in circulation, which suggests that retinyl esters are synthesized via an acyl-CoA-dependent enzymatic process in the absence of LRAT. The acyl-CoA-dependent enzymatic activity is termed acyl-CoA:retinol acetyltransferase (ARAT), but its exact molecular functions remain to be defined [26].
The absorbed retinoids are secreted into the lymph either as chylomicrons or as unesterified retinol [4]. Chylomicrons consist of aggregates of triacylglycerol and phospholipids packed together with carotenoids, retinyl esters, small amounts of retinol, cholesteryl esters, and a few apolipoproteins [4]. The chylomicrons are then secreted from the enterocytes into the intestinal lymph (Fig. 2a) [27]. The chylomicrons enter the general circulation, where they are reduced to chylomicron remnants. The retinyl esters are part of the chylomicron remnants [27], which are transported either to target tissues or to hepatocytes for storage [4].
Hepatic retinoid metabolism
In the liver, the retinyl esters are hydrolyzed to retinol, which is then bound to RBP (Fig. 2a). RBP belongs to the lipocalin protein family and is able to bind to and protect retinol from being metabolized, due to a special hydrophobic pocket [28]. The liver is the main site of RBP synthesis [29], and it has been shown that RBP recycles extensively between the liver, the plasma, and extra-hepatic tissues [4]. The first demonstration of retinol complexed to RBP came as early as 1968 [30]. The levels of retinol bound to RBP are under strict regulation and are maintained at a steady state of 2 μM regardless of the daily vitamin A intake fluctuations. In severe VAD, the retinyl esters are depleted and the retinol:RBP levels also drop.
Mice null for RBP have been generated and exhibit various phenotypes [31]. The finding that rbp−/− mice were viable was surprising given that RBP is the sole known specific carrier for retinol in the circulation of adult animals and embryos. The null mice are fertile with impaired function of the retina. In addition, the retinol levels observed in the blood of rbp−/− mice are ten times less than those of wild-type littermates, and in the liver there is increased storage of retinyl esters [31]. By 5 months of age, rbp−/− mice accumulate much higher stores of retinol than their wild-type littermates, and their hepatic stores do not change after a short-term exposure to a VAD diet. Thus, RBP is required for mobilization and storage of retinol. The visual impairment of rbp−/− mice is not due to a developmental retinal defect, but probably related to retinol uptake, as the null mice regain their vision after 5 months when kept on a diet sufficient in vitamin A, although blood levels of retinol remain low [31].
The retinol:RBP complex is secreted into the plasma for delivery to target tissues (Fig. 2a) [32]. Most of the retinol:RBP is associated with transthyretin (TTR) to prevent elimination by the kidney [4]. TTR is a serum protein found in a 1:1 association with RBP and has also been suggested to play a role in retinol delivery to tissues. Mice null for TTR were found to be viable and healthy without any developmental defects. Plasma levels of retinol and RBP are practically zero in the ttr−/− mice compared to wild-type littermates. The hepatic levels of retinyl esters and of retinol are the same in the ttr−/− mice and their wild-type littermates indicating that TTR is not involved in retinol uptake or storage. Levels of retinol and retinyl esters in the testes, kidney, spleen, and eye of ttr−/− mice were normal indicating that retinoid delivery to these tissues is not TTR dependent. Injected hRBP appeared rapidly in the kidneys of TTR-null mice supporting a role for TTR in inhibition of glomerular filtration of RBP in the kidney [22].
A large portion of the unesterified retinol is delivered to the hepatic stellate cells (Fig. 2a) [4]. In vitamin A sufficient states, the chylomicron remnant retinyl esters are converted to retinol and stored in lipid droplets in the liver [4]. In fact, over 80% of the total retinol and retinyl esters in vertebrates are stored in the liver (Fig. 3). Hepatic stellate cells have high levels of CRBP-I and LRAT, which are key for the storage of retinyl esters as evidenced in studies of mice null for CRBP-I or LRAT [19, 24]. Some retinyl esters are stored outside the liver as lipid droplets in interstitial cells and in organs such as the lung, kidney, and intestine [4]. Extra-hepatic storage may be important for the supply of retinol to tissues with high retinoid demand.
In addition to retinol and retinyl esters, a number of retinoids are found in plasma at nanomolar concentrations. These retinoids include all-trans RA, 13-cis RA, 13-cis-4-oxo RA, all-trans-4-oxo RA, and all-trans retinoyl-β-glucuronide (Figs. 1a, 3) [4]. These retinoids, with the exception of all-trans retinoyl-β-glucuronide, are transported in plasma bound to albumin, and their plasma levels fluctuate with vitamin A intake. Although it is not known whether these retinoids reflect catabolism products or have biological functions, their in vitro activity leads to the assumption that they have functional roles [4]. However, the work of Niederreither et al. [33] provides genetic evidence that during mouse development (in vivo) these retinoids (e.g. all-trans-4-oxo-RA) are inactive breakdown products and not biologically active compounds.
Retinoids and target tissues
Cellular uptake
Holo-RBP delivers retinol to target tissues, where retinol undergoes multiple enzymatic reactions to products including active retinoids [8] (Fig. 2b). The existence of a specific cell surface receptor for RBP on retinal pigment epithelium and intestinal epithelial cells was shown in the 1970s, and since then there has been accumulating evidence for the existence of the RBP receptor in other tissues and cell types, such as the placenta, choroid plexus, testis, and macrophages [34]. This cell surface RBP receptor binds specifically to RBP and, in addition, mediates retinol uptake from holo-RBP [34]. The receptor was finally identified in 2007 as stimulated by retinoic acid gene 6 (STRA6) [34]. STRA6 is a widely expressed multitransmembrane protein and meets all criteria for a RBP receptor, namely, it mediates cellular uptake of retinol and is localized in cellular locations expected of the RBP receptor in native tissues [34]. STRA6 is broadly expressed in the murine embryo, but in the adult its expression becomes more restricted [35]. In mouse mammary epithelial cells, STRA6 expression can be upregulated by Wnt1 and retinoids. In addition, STRA6 mRNA levels are upregulated in mammary gland tumors and human colorectal tumors [35]. Importantly, while the absence of RBP in mice and humans gives rise to relatively mild phenotypes, the phenotypes observed for STRA6 absence are more severe suggesting that STRA6 might have other roles in mediating retinoid signaling beyond being a receptor for RBP [36]. Mutations in STRA6 have also been associated with Matthew–Wood syndrome, a disease associated with severe microphthalmia, pulmonary hypoplasia, and cardiac defects [37]. Outside of vertebrates, only the cephalochordate amphioxus possesses putative stra6, rbp, and ttr homologs suggesting that amphioxus might possess a vertebrate-like retinol transport system (Fig. 4).
Retinol processing
In the target tissue, retinol either associates with CRBP or serves as a substrate for several cytosolic and microsomal enzymes termed retinol dehydrogenases (RDH) that oxidize retinol to retinaldehyde (also called retinal) (Figs. 2b and 3) [22, 38]. These retinol dehydrogenases are members of the ADH or SDR families [38]. Retinol metabolism is catalyzed by the ubiquitously expressed ADH3 as well as by the tissue-restricted ADH1 and ADH4. All three can oxidize all-trans retinol to all-trans retinaldehyde with ADH4 being the most efficient. In contrast to vertebrates that have multiple ADHs, invertebrates have only one ADH, typically ADH3 (Fig. 4) [17].
Mice null for Adh3, which is ubiquitously expressed during development, display reduced viability and growth defects, which can be rescued by dietary supplementation with retinol. This indicates that, in the absence of ADH3, other retinol-oxidizing enzymes can carry out its function, provided that retinol levels are sufficiently high [39]. Adh3 null mice die when kept on a VAD diet indicating that, in cases of limited retinol supply, other retinol dehydrogenases cannot compensate for the absence of ADH3 [39].
There is no obvious phenotype associated with the loss of Adh1 and Adh4. However, the absence of Adh1 and Adh4 becomes evident when the respective null mice are fed with an excess of vitamin A (for ADH1) or subjected to a VAD diet (for ADH4) [40]. When large doses of retinol are administered to Adh1−/− mice, the mice are more sensitive to vitamin A toxicity [41]. The same toxicity was also observed in Adh3−/− mice on the same diet compared to wild-type littermates, but with a less severe phenotype than for Adh1−/− mice. These findings correlate well with the expression profiles of ADHs in the liver, the major detoxification organ, where ADH4 is absent and ADH1 is expressed at higher levels than ADH3 [40].
Absence of both Adh1 and Adh4 does not have additive effects. The compound null mice are not more sensitive to vitamin A toxicity than the Adh1−/− mice, and in VAD states they do not display higher lethality than the Adh4−/− mice suggesting separate roles for these two ADHs in retinoid metabolism [40]. Based on these studies, ADH1 is responsible for the reduction of vitamin A toxicity by facilitating the conversion of retinol to RA, whereas ADH4 is responsible for reducing the negative effects of VAD by ensuring that retinol metabolism to RA occurs at sufficiently high levels to provide ligand to the receptors. The function of ADH1 slightly overlaps with that of ADH3 in providing protection against vitamin A toxicity, and the function of ADH4 overlaps with that of ADH3 in providing protection from VAD [40]. It can therefore be concluded that Adh1 and Adh4 are necessary only in extreme vitamin A conditions such as excess or deficiency, whereas Adh3 functions as the ubiquitous ADH.
In addition to ADHs, SDRs also function in the oxidation of retinol to retinaldehyde (Fig. 3) [40]. While ADHs are cytosolic, SDRs are microsomal. The oxidation of retinaldehyde to RA is a cytosolic process and ADHs may function in RA synthesis, whereas the retinaldehyde produced by SDRs may also be required for processes other than RA synthesis [40]. SDRs have not been the subject of extensive genetic studies as to their possible role in RA synthesis, with RDH5 and RDH10 being the only SDRs, for which genetic studies have been undertaken. Mice null for Rdh5 are viable, but suffer a delay in dark adaptation consistent with a proposed role within the visual cycle in metabolism of 11-cis retinol to 11-cis retinaldehyde [40]. Therefore, although RDH5 is important for vision, it does not play an evident role in vivo in RA synthesis. In contrast, RDH10 is important for embryonic RA synthesis, functioning in embryonic patterning and morphogenesis, and its loss results in embryonic lethality [42, 43]. Like ADHs, SDRs are widely distributed in metazoans (Fig. 4). Although their ability to bind retinoids needs to be experimentally assessed, the presence of apparent homologs of ADHs and SDRs in cnidarians, ecdysozoans, lophotrochozoans, ambulacrarians, and chordates suggests that ADH/SDR-dependent retinol processing might be an evolutionary conserved process.
Retinaldehyde processing
Following the oxidation of retinol to retinaldehyde by ADH or SDR enzymes, the next step in RA synthesis is the irreversible oxidation of retinaldehyde to RA (Fig. 2b). This reaction can be performed by retinaldehyde dehydrogenases (RALDHs) (Fig. 3) [32]. Vertebrates generally have three RALDHs of the ALDH1A class (named ALDH1A1 or RALDH1, ALDH1A2 or RALDH2, and ALDH1A3 or RALDH3) and one RALDH of the ALDH8 class (called RALDH4). Rodents have an additional ALDH1A enzyme called ALDH1A4 in rat and ALDH1A7 in mouse [44]. For the synthesis of active retinoids, a variety of cytochrome P450s (CYPs) have also been implicated, at least in vitro. For example, CYP1A1, 1A2, 2B4, 2C3, and 2J4 have been shown to catalyze the oxidation of retinaldehyde to RA [1].
RALDH1 is expressed in the dorsal retina of embryos and in several epithelial tissues in the adult [45]. This protein has been proposed to function in dorsoventral patterning of the eye and axonal path finding of retinal ganglion cells [40]. The dorsal retina of raldh1−/− embryos display only minor effects suggesting that RALDH1 is not essential for RA synthesis but may instead be involved in the catabolism of excess retinol. In contrast, overexpression of RALDH1 in Xenopus embryos does lead to premature RA synthesis indicating that RALDH1 can function in RA synthesis in vivo [40].
RALDH2 expression occurs in multiple embryonic and adult tissues. Overexpression of raldh2 in Xenopus embryos leads to high levels of RA strongly suggesting a function for raldh2 in RA synthesis in vivo [45]. During mouse embryogenesis, RALDH2 expression is first detected at the same time as RA, at E7.5, and until midgestation it is localized in mesenchymal tissues, such as the proximal limb bud, trunk mesoderm, lung bud mesoderm, and heart [40]. The raldh2−/− mice die at midgestation, around day E8.75, due to defects in heart development. In addition, raldh2−/− embryos exhibit shortening of the anteroposterior axis, and the limb buds do not form due to lack of RA, as evidenced by reduced Hox expression in the mutants [46]. More recently, effects of RALDH2 deficiency on the development of the hindbrain and neural crest have been described [47]. The raldh2−/− embryos can be “rescued” to a considerable extent with external administration of RA, and this finding led to the conclusion that the function of RALDH2 is to provide RA for development [46].
RALDH3 is expressed in mouse and chicken retina, lens, and olfactory pit as well as in the ureteric buds and surface ectoderm adjacent to the developing forebrain [40]. RALDH3 has been demonstrated to oxidize retinaldehyde to RA in vitro [40]. Mice null for RALDH3 have defects in nasal and ocular development and die shortly after birth, due to respiratory distress [48]. The defects observed in RALDH3 mutants are in fact similar to those observed in VAD fetuses or mice lacking retinoid receptors indicating the importance of RALDH3 for RA synthesis [48].
RALDH4 is expressed in mouse liver and kidney and displays a preference for processing 9-cis retinaldehyde over all-trans retinaldehyde [49]. The expression of RALDH4 in fetal liver and expression in the adult kidney suggest that it could play a role in 9-cis RA biosynthesis [49].
Recently, RALDH-like genes have been identified in the genomes of various invertebrate phyla including cnidarians, arthropods, nematode and annelid worms, mollusks, acorn worms, amphioxus, and ascidian tunicates (Fig. 4) [50, 51]. These findings indicate that ALDH enzymes capable of synthesizing RA have originated before the protostome–deuterostome split and might even have been present earlier in metazoan evolution [17].
Newly synthesized RA is bound to cellular RA binding proteins types I and II (CRABP-I and CRABP-II) and can then either enter the nucleus to activate transcription (autocrine) or be transported to a nearby target cell (paracrine) (Fig. 2b). CRABPs are expressed in tissues sensitive to RA [52]. CRABP-II binds to all-trans RA with lower affinity than CRABP-I and both bind 9-cis RA with lower affinity than all-trans RA [8]. CRABP-II has been suggested to act as a facilitator of RA uptake and metabolism as well as to be a co-regulator of RA signaling [53]. In the absence of ligand, CRABP-II is found in the cytosol and in the presence of RA it quickly translocates to the nucleus, where the complex associates directly with retinoid receptors and mediates ligand transfer from binding protein to the receptor [54].
CRABP-I and CRABP-II double null mice are physiologically normal with the sole phenotype being postaxial forelimb polydactyly. Mice null for both CRABPs do not have altered sensitivity to teratology following retinoid administration [55]. It appears therefore that both CRABP-I and CRABP-II are redundant components of the RA signaling pathway [55] suggesting that their functions can be carried out by other family members, such as FABP5 (see "Non-canonical retinoid receptors"). Outside vertebrates, evidence for putative CRABPs is scarce. CRABP-like proteins of invertebrates, such as ascidian tunicates, amphioxus, annelids, nematodes or arthropods (Fig. 4), are members of the greater FABP family [56] without exhibiting a strong phylogenetic association with vertebrate CRABPs [17]. It is hence conceivable that RA binding of FABPs was acquired specifically in the lineage leading to extant vertebrates.
Retinoic acid degradation
The balance between synthesis and catabolism allows the control of the levels of RA in cells and tissues. Catabolism of RA to oxidized metabolites, such as 4-hydroxy RA and 4-oxo RA, occurs mainly through enzymes of the CYP26 family and is thought to be initiated by hydroxylation of the C4 or C18 position of the β-ionone ring of RA (Fig. 3) [57]. In vertebrates, there are generally three CYP26 enzymes, called CYP26A1, CYP26B1, and CYP26C1. Similarly, other CYPs, such as CYP1A2, 2A4, 2A6, 1B1, 2B1, 2B6, 2C3, 2C7, 2C8, 2C9, 2D6, 2E1, 2E2, 2G1, 3A4/5, 3A6, 3A7, and 4A11 have been implicated in the catabolism of RA in vitro [58, 59]. Given that in most cases this implication was inferred from in vitro data, it is still difficult to assess the in vivo relevance of these observations.
The first CYP26 to be cloned was CYP26A1 from zebrafish in 1996, and shortly thereafter the human gene was cloned by the same group [60, 61]. In zebrafish, CYP26A1 is expressed in the hindbrain, pharyngeal arches, pectoral fin, and neural retina [62]. It is also expressed during gastrulation and in a defined pattern in epithelial cells of the regenerating caudal fin in response to exogenous RA [60]. When transfected into COS1 cells, it results in the metabolism of all-trans RA into more polar metabolites, such as 4-oxo RA and 4-hydroxy RA [60]. The human CYP26A1 is similarly responsible for the metabolism of all-trans RA to polar metabolites, such as 4-oxo RA, 4-hydroxy RA, 18-hydroxy RA, 5,6-epoxy RA, and 5,8-epoxy RA [61]. In certain human tumor lines, the expression of CYP26A1 is induced in the presence of RA. It was also found that RA-inducible metabolism correlates with CYP26A1 expression implicating this enzyme in RA metabolism [61]. In the mouse, CYP26A1 is expressed in the rostral neural plate and tail bud. In the chick, CYP26A1 is expressed in the hindbrain, mesoderm, and endoderm [62]. In Xenopus, CYP26A is found in the circumblastoporal ring and dorsal animal hemisphere, and its overexpression results in anteriorization [63]. Disruption of the murine CYP26A1 gene results in embryonic lethality. The CYP26A1 null mutants die during mid- to late gestation and display a number of morphogenetic defects, which closely resemble those observed in RA teratogenicity [64]. The most prominent defects were spina bifida and truncation of the lumbosacral region and, in very extreme cases, sirenomelia (also called mermaid tail) [64]. In addition, mutants displayed defects, such as abnormal patterning of the rostral hindbrain and posterior transformations of the cervical vertebrate, which correlates with the major sites of CYP26A1 expression, which are the rostral neural plate and the embryonic tail bud.
CYP26B1 was identified shortly after CYP26A1 and was shown to metabolize all-trans RA to the polar metabolites 4-oxo RA, 4-hydroxy RA, and 18-hydroxy-all-trans RA [65]. The expression pattern of CYP26B1 in the cerebellum and pons of the brain is different from that of CYP26A1, even though the two enzymes have similar catalytic activity [65]. In the developing mouse, CYP26B1 is expressed in the distal region of the developing limb bud. Mice null for CYP26B1 exhibit severe limb malformations [62]. The absence of CYP26B1 also results in the induction of proximodistal patterning defects in the developing limb characterized by expansion of proximal identity and restriction of distal identity as well as pronounced apoptosis and delayed chondrocyte maturation [62]. In the chick, CYP26B1 is expressed in the tail bud, anterior mesenchyme, heart, vasculature, eye, limb bud, hindgut, and the hindbrain in rhombomeres [62]. In zebrafish, CYP26B1 is expressed in the hindbrain, diencephalon, midbrain-hindbrain boundary, cerebellum, and pharyngeal arches [62].
A third CYP26 enzyme, CYP26C1, was identified more recently [66]. Transiently transfected cells expressing CYP26C1 are able to metabolize all-trans RA to polar metabolites similar to those generated by CYP26A1 and CYP26B1. In the developing mouse embryo, CYP26C1 is expressed in prospective rhombomeres 2 and 4, the first branchial arch, and along the lateral surface mesenchyme adjacent to the rostral hindbrain at early developmental stages. By midgestation, weak expression is detected in the cervical mesenchyme and in the maxillary component of the first branchial arch. Based on the observed expression pattern, CYP26C1 functions to protect the hindbrain, first branchial arch, developing ear, and tooth buds from RA exposure during embryonic development [62]. In chick, CYP26C1 is expressed in cranial mesoderm, rhombomeres, and neural crest [62]. In zebrafish, CYP26C1 is found at the presumptive hindbrain during the segmentation phase. At the 2-somite stage, it is expressed in rhombomeres 2-4 (r2-4), then as development progresses, it is detected in r2-6 and the pharyngeal arches in the 6-somite stage. After 24 h of development, gene expression is also detected in the telencephalon and diencephalon, and expression later extends to the eye, otic vesicle, and midbrain. By 48 h of development, expression is restricted to the otic vesicles, pharyngeal arches, and pectoral fins and overall expression levels are decreased [62]. In Xenopus, CYP26C1 expression was shown to be restricted to the anterior region of the neurula with expression extending to restricted sets of cranial nerves at the tadpole stage [63].
In general, the expression patterns of the three CYP26s are non-overlapping suggesting individual roles for each enzyme in RA catabolism [67]. This hypothesis is supported by the observation that both CYP26A1 and CYP26C1 activities are needed for correct anteroposterior patterning and production of migratory neural crest cells [68]. A recent triple knockdown of CYP26A1, CYP26B1, and CYP26C1 in zebrafish further demonstrated the importance of combinatorial CYP26 activity in hindbrain development as RA-responsive genes that are normally restricted to nested domains in the posterior hindbrain were expressed in the entire hindbrain [69].
CYP26s, like other major components of the RA signaling cascade, can also be found outside the vertebrates. CYP26s have initially been described in ascidian tunicates, cephalochordates, hemichordates, and echinoderms [50, 51]. More recent analyses have indicated the existence of CYP26 orthologs in lophotrochozoans, such as L. gigantea and C. capitata, but failed to identify CYP26s in ecdysozoans [44, 50]. It appears therefore that CYP26s were present in the last common ancestor of bilaterians and might have selectively been lost in the ecdysozoan lineage. The biochemical activity of these apparent orthologs as well as their in vivo function will have to be carefully assessed before any firm conclusions can be drawn.
Retinoid-dependent signaling
Canonical retinoid receptors
The effects of RA are mediated through its binding to retinoid receptors, which are members of the nuclear receptor family. The retinoid receptors share the same modular structure as other nuclear receptors with the main modules being the DNA binding domain (DBD), which confers sequence-specific DNA recognition, and a ligand-binding domain (LBD), which also harbors a ligand-dependent activation function AF-2 and a major dimerization interface [70]. Retinoid receptors can be divided into two subgroups, RA receptors (RARs) and retinoid X receptors (RXRs). In vertebrates, there are generally three RARs and three RXRs (α, β, and γ) with RARs binding to all-trans RA and 9-cis RA, and RXRs binding to 9-cis RA with high affinity, but not to all-trans RA. Although RXR was originally identified as an orphan receptor that responded specifically to retinoids [71], RXR is the heterodimeric partner of a number of nuclear receptors, such as RAR, thyroid hormone receptor (TR), vitamin D receptor (VDR) or peroxisome proliferator-activated receptor (PPAR) [72]. This fact raises the question whether RXR is a silent or an active transcription partner in such heterodimers. Through the study of RAR/RXR heterodimers, it was determined that RXR activity is silenced in the absence of RAR ligand and this is referred to as “RXR subordination”, a phenomenon also observed in other RXR heterodimers [73].
The transcriptional activation by RAR is dependent on the formation of the RAR/RXR heterodimer, which occurs on two interfaces: (1) the LBD interface, which is independent of the DNA binding activity of the complex, and (2) the DBD interface, which is involved in the recognition of response elements on DNA. The dimerization interface found in the LBD further stabilizes, but does not change, the binding repertoire directed by the DBDs [74, 75]. The precise heterodimerization surfaces of RAR and RXR in the DBD were determined by extensive structure–function analyses [76, 77]. Within the RXR protein, the second zinc finger forms a surface that interacts with the second zinc finger of RAR bound to a retinoic acid response element (RARE) on the target DNA [76]. The DNA response element for RAR/RXR consists of two or more degenerate copies of the (A/G)G(G/T)TCA or of the more relaxed (A/G)G(G/T)(G/T)(G/C)A half-site motif organized in direct repeats or palindromes normally separated by a nucleotide spacer [78]. In addition to DR5 (direct repeat with a five-nucleotide spacer), RAR/RXR heterodimers are able to regulate transcription from DR2 (two nucleotide spacer) and DR1 (one nucleotide spacer) elements [79, 80]. When bound to DR2 and DR5 elements, the 5′ half-site is occupied by RXR and the 3′ half-site by RAR [77, 81, 82]. In contrast, when bound to DR1 elements, the polarity of the heterodimer is inverted (5′-RAR-RXR-3′) and the complex is unresponsive to RA stimulation, probably due to the inability of RAR ligands to induce the dissociation of co-repressors [83].
RARα was cloned in 1987 independently by the Evans and Chambon laboratories [84, 85]. Mice null for RARα display some of the features of vitamin A deficiency with decreased viability, growth deficiency, and male sterility, due to degeneration of the seminiferous epithelium as well as other congenital malformations, such as webbed digits [86]. Most of the defects of RARα null mice can be reversed by treatment with RA.
RARβ was cloned by the Dejean laboratory and shown to be a RAR by the Dejean and Chambon laboratories [87, 88]. So far, five transcriptional variants have been described for RARβ, expressed from different promoters (P1, P2, and P3) [89, 90]. In the developing mouse embryo, RARβ expression is spatially and temporally restricted in various structures suggesting a role for RARβ in morphogenesis. RARβ−/− mice exhibit a selective loss of striosomal compartmentalization in the rostral striatum [91]. The RARβ−/− mice also display locomotor defects, which are correlated with dopamine signaling implicating retinoids in the regulation of brain function [92]. In addition, RARβ2−/− mice (mice null for isoform 2 of RARβ) display abnormalities in the vitreous humor of the eye, an effect that is more pronounced in RARβ2/RARγ2 null mice suggesting a role for RARβ2 in the formation of vitreous body [86]. RARβ is also the paradigm for RAR regulation as it is both a direct target of RA and highly activated by treatment with exogenous retinoids.
The third RAR, RARγ, was identified during the characterization of the murine RARα and RARβ and was found to be highly expressed in the skin [93, 94]. RARγ null mice display some defects associated with VAD, which can be rescued with RA treatment, indicating that RARγ mediates some of the retinoid functions in vivo [95]. Congenital defects establishing a link between RA and axial patterning were also observed, such as webbed digits, homeotic transformations, and malformations of cervical vertebrae [95]. In more recent studies, conditional mutants for RARγ expression in cartilage were generated to study the role of RARs in growth plate formation and skeletal growth [96].
The observations made with the single RAR knockout mice suggest a high degree of functional redundancy among the RARs. In contrast, the double knockout mice generated more dramatic phenotypes leading to reduced viability [97, 98]. The RAR double mutants die either in utero or shortly after birth and display a number of congenital abnormalities associated with VAD [86].
RXRs were identified and cloned by several independent groups [71, 99]. Mouse knockouts have been generated for all three RXRs [3]. The inactivation of RXRα is lethal during embryogenesis [86]. RXRα−/− mice exhibit hypoplastic development of the ventricular chamber of the heart and ocular malformations, defects that were also observed in VAD models, lending support to the idea that RXRα is involved in retinoid signaling in vivo [100]. Treatment of these embryos with vitamin A, which normally had teratogenic effects on limb development, has no effect on RXRα−/− mice thus implicating RXRα in the teratogenic response to RA [101]. RXRα also plays a role in RA-induced cleft palate as shown in a study where pregnant wild-type and RXRα−/− mice were treated with teratogenic doses of RA at midgestation [102]. The knockout for RXRβ resulted in 50% embryonic lethality at or before birth [103]. The surviving offspring appear normal, but the males are sterile due to oligo-astheno-teratozoospermia and failure of spermatid release [103]. RXRγ-deficient mice appear to be normal [104], but are resistant to thyroid hormone, which is consistent with RXRγ expression in the thyrotrope cells of the anterior pituitary gland [105]. Compound knockouts for the various RXRs showed that one copy of RXRα is sufficient to perform most of the functions of the RXRs [104].
While vertebrates generally have three RAR and three RXR paralogs (except teleost fish that have more), only one RAR has been identified in invertebrate chordates, such as ascidian tunicates and cephalochordates [44, 50]. More recent analyses of the genomes of protostomes and deuterostomes led to the identification of putative RARs in sea urchins, hemichordates, mollusks, and annelids (Fig. 4) [44, 50] lending further support to the hypothesis that RA signaling was already present in Urbilateria, the last common ancestor of protostomes and deuterostomes [44, 50]. In contrast, RXR, likely due to its property as a heterodimeric partner for multiple nuclear receptors, has been identified in virtually all metazoan species analyzed [44, 50], and its presence is therefore not diagnostic of the presence of RA signaling in a given taxon.
Non-canonical retinoid receptors
Recently, the possibility that retinoid signaling could be mediated by receptors other than the RAR and RXR couple has been raised. For example, one of the established roles of RA is the maintenance of skin integrity. However, experiments in knockout mice suggest that this effect of RA is not RAR-mediated [106]. RA was previously reported to be a ligand for the PPARβ/δ orphan receptor [107, 108], which was shown to induce differentiation and which displays anti-apoptotic activities mediated by regulation of the PDK-1/Akt survival pathway in keratinocytes [109, 110]. RA delivery to PPARβ/δ by FABP5 occurs in a similar fashion as CRABP-II delivery of RA to RAR. Since the binding affinity of CRABP-II/RAR to RA is much higher than that of FABP5/PPARβ/δ, in most cell types the classical RAR pathway will be predominantly activated [106]. In contrast, in cells with a high FABP5 to CRABP-II ratio, the PPARβ/δ pathway will be activated, hence abolishing the RA-triggered upregulation of RAR target genes. This RA signaling through PPARβ/δ leads to anti-apoptotic activities that overcome the growth-inhibitory activities of RAR. It appears therefore that, under certain circumstances, RA can serve as a physiological ligand for PPARβ/δ [111].
One of the RA receptor-related orphan receptors (RORβ) has also been proposed to be regulated by RA [112]. RORs are evolutionarily close to RARs and are thought to be involved in the regulation of genes that control the integration of sensory input as well as circadian rhythm [112]. The crystal structure of the rat RORβ LBD indicates that all-trans RA and several other retinoids bind to this receptor [112]. Moreover, incubation of RORβ LBD with all-trans RA or the synthetic retinoid ALRT1550 results in quantitative binding and complete replacement of stearate from the Escherichia coli host that co-purified with the RORβ LBD [112]. The in vivo relevance of this observation is still unclear, and further studies are required to determine its significance.
The chicken ovalbumin upstream promoter-transcription factors (COUP-TFI and II) are amongst the most conserved nuclear receptors. COUP-TFs play a role in organogenesis, angiogenesis, neuronal development, metabolic homeostasis, cell fate determination, and circadian rhythm [113, 114]. Recently, the crystal structure of the COUP-TFII LBD was determined, and it was demonstrated that COUP-TFII can be activated by micromolar concentrations of RA [115]. In cell culture experiments, both 9-cis RA and all-trans RA can serve as low affinity ligands for COUP-TFII [115]. However, the high concentrations of all-trans RA and 9-cis RA that are required to activate COUP-TFII make it unlikely that RA would be a physiological ligand for COUP-TFII. Thus, the relevance of this finding needs to be reexamined to determine its significance in vivo.
The possibility that RA might bind to and mediate effects of these three nuclear receptors opens up new avenues for understanding the diversity of effects of RA signaling in vivo.
Retinoid receptor ligands
RARs are able to bind both all-trans RA and 9-cis RA with similar high affinity although the kinetics of binding can differ for each RAR paralog. Kinetic studies in vitro and in whole cells suggest that there could be differences in the interactions of the RAR paralogs with the endogenous ligands under physiologic conditions [116].
In addition to RA, RARs are also activated by a number of physiologically-occurring retinoids, such as all-trans-4-oxo RA, all-trans-4-oxo retinaldehyde, all-trans-4-oxo retinol, and all-trans-3,4-didehydro RA [117]. All-trans retinol binds to all three RARs with a 4- to 7-fold lower affinity than all-trans RA, and this is not due to its metabolism to RA or to other active compounds [118]. All-trans-4-oxo RA binds to and activates RARβ and is a modulator of positional specification in early embryos [119]. Another active retinoid is 4-oxo retinol, which is also able to bind to RARs and activate their transcription [9]. Treatment of Xenopus embryos at the blastula stage with 4-oxo retinol causes axial truncation showing that this compound is a biologically active retinoid, presumably because of the ability of 4-oxo retinol to bind to and activate RARs [9]. The retinol metabolite 14-hydroxy-4,14-retro retinol was also shown to be active in vivo [10]. Another recently identified and characterized retinoid is the 9-cis-substituted RA metabolite S-4-oxo-9-cis-13,14-dihydro-RA (S-4o9cDH-RA) [120]. The endogenous levels of S-4o9cDH-RA were found to be higher than those of all-trans RA in mice and rats, and the compound is able to activate transcription of RAR target genes [120]. In vivo, S-4o9cDH-RA induces morphological changes in the chicken wing bud similar to those induced by all-trans RA [120]. Additional RAR ligands include β-cryptoxanthin and lutein, which act as RAR ligands in a yeast two-hybrid system and bind RA with lower affinity than all-trans RA [121].
The importance of retinoids has led to the development of paralog-specific agonists and antagonists [122]. One of the most widely known and used pan-RAR (i.e. targeting all three RAR paralogs) agonists is (E)-4-[2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-2-naphthalenyl)-1-propenyl]benzoic acid (TTNPB) (Fig. 1b) [123]. TTNPB is an arotenoid, an aromatic retinoid, in which the RA structure is fixed in the cisoid geometric structure [124]. The teratogenic potency of TTNPB is threefold higher than that of all-trans RA in mice, rats, and rabbits [125]. TTNPB binds to RARs with less affinity than all-trans RA, but two factors contribute to the potency of TTNPB compared to all-trans RA: (1) the lower binding affinity for CRABPs, and (2) the decreased catabolism [126]. A variant of TTNPB, (E)-4-[2-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthalenyl)-1-propenyl]benzoic acid (3-methyl TTNPB), is able to bind and activate both RARs and RXRs. Known pan-RAR antagonists include BMS009, BMS493, and BMS614 (Fig. 1b) [127]. Whereas BMS009 and BMS493 act as inverse RAR agonists by enhancing co-repressor interactions with RARs, BMS614 acts as an antagonist by inhibiting co-activator recruitment [127].
The natural in vivo ligand for RXR still remains elusive. RXRα was found to bind 9-cis RA, an isomerization product of all-trans RA, which was subsequently shown to have high affinity for all three RXR subtypes [128, 129]. However, the biological significance of 9-cis RA as the in vivo RXR ligand remains controversial as no significant levels of 9-cis RA could be detected in vivo in different vertebrates [130]. However, 9-cis RA was shown to be functional in other organisms, such as in the CNS of the mollusk Lymnaea stagnalis, where the presence of both all-trans RA and 9-cis RA has been shown [131]. Similarly, 9-cis RA was detected along with all-trans RA in locust embryos suggesting a functional role for retinoids in insects [132]. In addition, 9-cis RA has been detected in regenerating limb blastemas of both urodele amphibians and fiddler crabs suggesting a role in limb regeneration in both vertebrates and crustaceans [133, 134]. It would appear therefore that the question of whether 9-cis RA is a natural endogenous ligand for RXR is one that will continue to be debated, but based on current information, it would appear that 9-cis RA is detectable and functional at least in certain ecdysozoans and lophotrochozoans as well as during amphibian regeneration. Excepting the regenerating blastemas of amphibians, vertebrates generally show very small levels of endogenous 9-cis RA that are too low to activate RXRs. Adding to this debate is the fact that oleic acid was found in the LBD of mammalian RXRα when heterodimerized to RARα suggesting that this fatty acid might be an endogenous ligand for RXR [135]. Moreover, the fatty acid docosahexaenoic acid (DHA) has been shown to bind mammalian RXRα and to activate RXR-dependent transcription [136]. Since RXRs are heterodimeric partners of several nuclear receptors that participate in fatty acid biosynthesis, metabolism, and transport of vertebrates, it is conceivable that the natural ligand of vertebrate RXRs is indeed a fatty acid [137]. However, since DHA does not activate RXR-dependent transcription in amphioxus, fatty acid-dependent activation of RXRs might represent a vertebrate-specific innovation [138].
Since RAR ligands are usually potent teratogens and RXR ligands are non-teratogenic, research turned to synthetic compounds that recognize only RXR in order to decipher the functions of RXRs [139]. The most potent of these so-called rexinoids are SR11217 and SR11237, which recognize and activate only RXR homodimers. Similar compounds include bexarotene [123] and LG100268 (Fig. 1b) [140]. Compounds that are antagonists of RXR homodimers, but agonists of specific RXR heterodimers, have also been described [127] and have allowed a better understanding of the relationships between each partner in the heterodimeric complex and a reassessment of the transcriptional activity of RXRs [141].
Target genes
Some of the first RAR target genes to be discovered include RARβ itself, laminin B1, CRBP, and CRABP [142]. The mouse CRBP-I promoter was shown to possess a DR2 response element, which confers RA inducibility [80]. While the mouse CRBP-II promoter contains DR1 and DR2 response elements [79], the human CRBP-II gene is regulated through a DR5 response element [143]. Moreover, proteins involved in RA metabolism, such as CYP26, are also directly regulated by RA [62, 142]. Some of the most well-known target genes of RARs are the Hox genes. Hox genes are key players in the patterning of the anteroposterior axis during development [144]. Administration of excess RA to developing embryos has profound effects on axial patterning and specification of regional identities in a number of structures such as the CNS, axial skeleton, and limbs [50, 145–147]. This is accompanied by repatterning of Hox expression domains suggesting a specific role for Hox gene products as molecular transducers of the morphogenetic signals of retinoids [50, 145–147]. Functional RAREs have been identified in the Hox clusters of vertebrates, such as mice, chicken, frogs, and zebrafish [148] as well as in the single Hox cluster of amphioxus [149, 150]. Recently, a functional RARE has also been described in the regulatory region of the Hox1 gene of the ascidian tunicate Ciona intestinalis [151]. This suggests that direct regulation of Hox genes by RA is a feature common to all chordates.
RARs also regulate a number of factors involved in metabolism, for example the 17β-hydroxysteroid dehydrogenase EDH17B2, which is implicated in steroidogenesis, the alcohol dehydrogenase ADH1C, and the liver bile acid transporter NTCP [142]. Over the last few decades, over 500 genes have been put forth as being regulatory targets of RA. In some cases, direct regulation was demonstrated driven by liganded RAR/RXR heterodimers bound to RAREs. In most cases, though, the regulation of the proposed gene target is indirect occurring through intermediate transcription factors or non-classical associations of receptors with other proteins. Of the target genes suggested, 27 were found to be direct targets of the classical RAR/RXR-dependent RARE pathway and another 100 are good candidates for being direct targets [4]. Moreover, a microarray-based screen in zebrafish embryos identified both positive and negative RA targets in vivo [152]. This screen identified genes previously reported to be regulated by RA as well as novel RA-responsive genes, such as Dhrs3a (a member of the SDR family), which functions to limit RA signaling in the CNS by catalyzing the reduction of retinaldehyde to retinol [152]. Finally, using a chromatin immuno-precipitation on chip (ChIP on chip) approach in MCF-7 breast cancer cells, Hua et al. recently identified genomic RAR targets [153]. With this ChIP on chip analysis, Hua et al. [153] defined a total of 1,413 genes that were significantly regulated by either RA or paralog-specific RA agonists. Analysis of the distribution of RAR binding sites showed that the vast majority of these novel RAREs are located either in introns or in so-called promoter-distal intergenic regions located at a distance from the actual RA target gene [153].
Non-genomic signaling
Non-genomic retinoid signaling is independent of gene transcription mediated by RAR/RXR heterodimers. While some of these actions are the result of RA binding to retinoid receptors, non-genomic effects of RA can also occur in the absence of retinoid receptors. An example for the first category is the RA-dependent regulation of a type of homeostatic synaptic plasticity in neurons, a process that requires dendritically-localized RARα and that is independent of transcription [154, 155]. It has been shown that unliganded RARα is actively exported from the nucleus and that in the cytoplasm RARα acts as an RNA-binding protein that associates with mRNAs, such as the mRNAs encoding glutamate receptor 1 (GluR1). This association results in repression of GluR1 translation. RA binding to RARα reduces its association with the GluR1 mRNA and relieves translational repression [154, 155].
RARβ has also been implicated in mediating non-genomic actions of RA [156]. In Xenopus cell culture, RA has been shown to act through RARβ to induce a rapid increase in the frequency of spontaneous transmitter release at developing neuromuscular synapses [156]. This process is mediated by changes in intracellular Ca2+ levels, which are triggered by the phospholipase Cγ (PLCγ) and phosphatidylinositol-3-kinase (PI3K) signaling pathways and by v-src sarcoma (SRC) tyrosine kinase activation [157]. The PI3K signaling pathway has also been implicated in conveying non-genomic, RAR-mediated RA signals in SH-SY5Y neuroblastoma cells, where rapid activation of the PI3K signaling pathway is required for RA-induced differentiation [158].
RARα, RARβ, and RARγ are also involved in the retinoid-dependent repression of AP-1, which leads to inhibition of AP-1-dependent cell proliferation without requiring transcriptional activation [159]. The activity of the transcription factor NFκB is also regulated non-genomically by RA, probably in a RAR-dependent manner. NFκB is an important component of the immune and inflammatory systems and deregulation of NFκB is a feature of chronic inflammatory diseases, atherosclerosis and some cancers [160]. In mice, NFκB activity is repressed by RA and elevated in a VAD background [161]. Moreover, RA decreases lipopolysaccharide-induced activation of NFκB and transcription of its target genes, while VAD mice exhibit a stronger NFκB response after lipopolysaccharide challenge [162]. The defective immune and inflammatory responses under VAD conditions might hence be mediated by NFκB deregulation [4].
Non-genomic effects of RA can also occur in the absence of retinoid receptors. For example, in human bronchial epithelial cells, rapid activation of cAMP response element-binding (CREB) protein by RA is independent of RAR/RXR [163]: RA activates CREB in cells, in which the RAR/RXR heterodimers are silenced with small interfering RNAs or deactivated by antagonists [163]. In addition, RA-dependent induction of CREB requires protein kinase C (PKC), extracellular regulated kinase (ERK), and p90 ribosomal S6 kinase (RSK) activity [163]. RA might also modulate the activity of PKC. It has been suggested that the PKC protein contains a RA binding site and that the RA-dependent modulation of PKCα activity is controlled by competitive binding of PKCα to all-trans RA and acidic phospholipids [164].
A recent study in the adult mollusk L. stagnalis has demonstrated yet another candidate mechanism for the non-genomic actions of RA. Using central neurons from adult animals, Farrar et al. [165] showed that RA-induced positive growth cone turning was maintained even in the presence of actinomycin D, an inhibitor of transcription, thereby showing that this process is independent of transcription. This RA-dependent process requires local protein synthesis and Ca2+ influx suggesting that these chemo-attractive effects of RA involve a novel, localized mechanism that is independent of retinoid receptor activity, at least in the nucleus [165].
Evolution of retinoid-dependent signaling
The continuous sequencing of metazoan genomes has led to the proposal that RA signaling is not a chordate innovation, but has its origins much earlier in bilaterian evolution, as evidenced by the discovery of key components of the RA genetic machinery in the genomes of sea urchins, acorn worms, annelids, and mollusks (Fig. 4) [13, 44, 50, 51, 147]. In examining the available information from different bilaterian animals, certain conclusions can be drawn. In comparison to the vertebrate RA signaling complement, the cephalochordate amphioxus, of all invertebrates, appears to have the most vertebrate-like set with putative RA storage, mobilization, and transport components being present. The importance of RA signaling during amphioxus development supports this hypothesis: in amphioxus, RA controls regional patterning of the pharyngeal endoderm [166–168], patterning, and neuronal specification in the CNS [169, 170], and anteroposterior distribution of sensory neurons in general ectoderm [171]. Tunicates, which constitute the other invertebrate chordate group, appear to have lost at least the retinoid mobilization and transport components. Moreover, the appendicularian tunicate Oikopleura dioica has lost its RA signaling complement almost in its entirety, with only RXR being present [50, 51]. At least in ascidian tunicates, RA signaling appears to be active with roles in specification of ectoderm and endoderm [172, 173], development and morphogenesis of anterior CNS [50, 174] and adhesive organs (palps) [173, 175], and in whole body regeneration [176].
Outside the chordate lineage, evidence for functional roles of RA signaling becomes scarcer. Although the basic components for synthesis and degradation of endogenous RA as well as the retinoid receptors RAR and RXR are present in ambulacrarians (such as hemichordates and sea urchins) and lophotrochozoans (such as annelids and mollusks), molecular evidence for a functional RA pathway remains elusive. Nonetheless, it has been shown that RA treatment results in delay of embryonic development of the sea urchin Paracentrotus lividus [177], and in the mollusk L. stagnalis RA is apparently involved in neuronal differentiation and neurite outgrowth [178]. Based on these effects of RA in mollusks, it has been hypothesized that, if this action of RA on neurons is mediated by a functional RAR/RXR heterodimer, this function of RA signaling might be conserved between mollusks and vertebrates, because in vertebrates it is well established that the RA pathway controls neuronal specification, differentiation, and outgrowth. This role for RA in neuronal survival and outgrowth might thus have already been present in the last common ancestor of protostomes and deuterostomes.
Retinoids in disease and therapy
The role of retinoids in carcinogenesis and disease processes as well as their therapeutic value has been the subject of extensive study starting with the studies of Wolbach and colleagues in 1925 [179, 180], who were the first to showcase retinoids as anti-cancer agents. Retinoids are thought to be effective chemopreventors, due to their anti-proliferation, pro-differentiation properties. Since those early studies, a wealth of preclinical, clinical, and epidemiologic data have suggested a role for retinoids in cancer prevention and treatment [181, 182].
The most well-known implication of RARs in disease is APL (acute promyelocytic leukemia), which is caused by translocations of the RARα gene [183]. In APL, the DBD and LBD of the RARα gene are fused with the N-terminus of the PML (promyelocytic leukemia) gene and result in a fusion protein called PML-RARα [184, 185]. The fusion protein interferes with normal retinoid-mediated transactivation through its ability to either homodimerize or heterodimerize with RXR or PML through its coiled-coil domain [186–188]. PML-RARα remains RA-responsive, and treatment with RA results in partial remissions in APL patients. The RA treatment therapy allows the reactivation of normal signaling pathways that are disrupted in tumor cells [122]. While the major cause of APL is the translocation and fusion of the RARα gene with PML, other translocations are also involved. These include the NPM-RARα, NuMA-RARα, PLZF-RARα, and the Stat5-RARα translocations [189]. These proteins have varying responses to all-trans RA treatments. A follow-up to a randomized study showed that treatment of APL with all-trans RA and chemotherapy is better than chemotherapy alone [190]. In fact, the use of all-trans RA has rendered APL the most curable subtype of acute myeloid leukemia in adults [191]. PML-RARα transgenic mice develop leukemias that recapitulate the features of APL in humans [192]. Although the studies in knockout mice did not uncover a role for RAR in myeloid development, retinoid-deficient embryos develop leukopenia and treatment of their myeloid ex vivo with all-trans RA increases myeloid growth indicating that RAR can at least indirectly modulate myeloid development [193].
Histopathological studies have shown that downregulation of RARα during tumor progression coincides with loss of response to RAR ligand [194] and that loss of RARβ is associated with a variety of malignancies [195]. Various studies led to the conclusion that RARβ may act as a solid tumor repressor, which is supported by the fact that RARβ expression is lost from many neoplasmic tissues, such as non-small cell lung carcinoma (NSCLC), squamous cell carcinomas of head and neck or breast cancer [196–198]. Two RARβ isoforms (RARβ2 and RARβ4) have been shown to have antagonistic roles in the development of lung cancer [199]. RARs have also been linked to skin diseases, a finding that is supported by studies in knockout and transgenic mice [200]. Moreover, exposure to UV light reduces the levels of RARγ and RXRα and results in loss of RA induction of RAR target genes [200].
RA is thought to inhibit tumor growth by inhibiting uncontrolled cell proliferation, inducing apoptosis of abnormal cells, and promoting normal cell proliferation [182]. Thus, epidemiological studies show that lower vitamin A intake results in higher risk to develop cancer, which is in agreement with what is observed in laboratory animals with VAD, which display increased susceptibility to chemical carcinogens [195]. VAD in experimental animals has been associated with a higher incidence of cancer and with increasing susceptibility to chemical carcinogens.
Pioneering efforts showed retinoids to be effective inhibitors of tumorigenesis of the skin and in the respiratory, mammary, buccal, and stomach epithelia of rodents [201]. In a phase I trial of all-trans RA for the treatment of solid tumors, patients experienced side effects normally associated with retinoid treatments, such as skin reactions and nausea as well as transient elevations of liver enzymes and triglycerides [202]. In preclinical studies, 9-cis RA was shown to be effective in the chemoprevention of mammary and prostate cancer. Retinoids, and 13-cis RA in particular, have been shown to be effective for the prevention of head and neck cancer. Large studies, such as the CARET (β-carotene and retinol efficacy trial) and ATBC (α-tocopherol, β-carotene) cancer prevention trials, suggest that retinoids reduce tumor occurrence and mortality in non-smokers, show some evidence of benefit for former smokers, and reveal an elevated risk of lung cancer in smokers. RA therapy has also been shown to improve the survival of children with neuroblastoma by suppressing residual disease after chemotherapy and now forms an important part of the treatment for the disease [203].
Synthetic retinoids, such as TAC-101 (Taiho Pharmaceutical, Tokyo, Japan) and tazarotene (AVAGETM) (Allergan, Irvine, CA) (Fig. 1b), also entered phase I trials for the treatment of solid tumors. TAC-101 was associated with hypertriglyceridemia, myalgia, and arthralgia in a dose-escalation study; however, no dose-limiting toxicities were reported [204]. In a separate dose-escalation study, tazarotene was also associated with dry skin, cheilitis, headache, and hypertriglyceridemia, and in heavily pretreated patients anemia and thrombocytopenia were also reported [204]. In more recent studies, tazarotene has been shown to have chemopreventive efficacy against anti-basal cell carcinomas [205]. In addition, tazarotene has been proposed to act as an anti-neoplastic agent, when used in conjunction with rexinoids [206].
Current uses of tazarotene include the treatment of psoriasis of the skin, which represents one of the better-established target tissues for retinoid therapy [204]. RA treatment of xeroderma pigmentosum results in a reduction of new and recurrent skin tumors [207]. Accutane™ and Retin A™ (13-cis RA) are used for the treatment of cystic acne, and etretinate is an efficient substance for the treatment of psoriasis (Fig. 1b). In addition to cystic acne, 13-cis RA has also been tested as a therapeutic agent in other diseases. In an early phase I study, patients suffering from head and neck malignancies treated with 13-cis RA experienced intense headaches, urethritis, and dermatitis [204]. In combination with ifosfamide and cisplatin, 13-cis RA was used in a phase I/II study in patients with advanced or recurrent squamous cell carcinoma of the head and neck with a response rate of 72% [204]. In combination with interferon α and cisplatin, 13-cis RA has demonstrated activity in advanced squamous cell carcinoma and recurrent cervical cancer [204]. It is clear from these and other studies that 13-cis RA has potential for the treatment of advanced cancers.
Preclinical studies have shown that rexinoids targeting RXRs have the same chemopreventive potential as retinoids, but without the toxic effects of the latter. Upregulation of RXRα is associated with malignant transformation, and RXR paralogs are overexpressed in more than 66% of human breast cancer and in situ lesions [208]. Overexpression of RXRα results in sensitization of tumors to the anti-growth effects of rexinoids both in vitro and in vivo as well as in induction of cellular differentiation and control of aberrant cell growth [209]. In contrast, ablation of RXRα results in hyperplasia of the prostate epithelium and skin [208]. Rexinoids have also been reported to potentiate the apoptotic and anti-proliferative effects of PPAR ligands on breast cancer cell lines [210] and have been shown to reduce insulin resistance in obese and diabetic mice, an effect that can be enhanced by combination treatment with PPARγ agonists [211, 212]. Finally, rexinoids, when paired with cAMP elevating drugs, such as phosphodiesterase inhibitors, exert anti-leukemic effects [213, 214].
A new rexinoid NRX194204 (NuRx Pharmaceuticals, Irvine, CA) with anti-inflammatory and growth inhibitory properties has been tested for its ability to prevent and/or treat lung and breast cancer in vivo and was found to reduce both size and number of adenocarcinomas [215]. In addition to its effects in lung tumors, this rexinoid is also effective in the prevention and treatment of mammary tumors [215], and phase I clinical trials have been initiated for this compound. Recent studies have also shown that rexinoids, when combined with a selective estrogen receptor modulator (SERM), have emergent properties that effectively kill breast cancer cells. Targretin (bexarotene) (Fig. 1b) is already approved by the FDA for treatment of cutaneous T cell lymphoma, due to its ability to induce a 50% inhibitory response in patients with minimal toxicity when administered orally or topically [208]. Bexarotene was also shown to suppress both estrogen-dependent and estrogen-independent tumor development in mouse models [216, 217]. In another study, using mouse xenograft models with human melanoma cell lines, bexarotene was tested alone or in combination with TTNPB or rosiglitazone (a PPARγ agonist) for the treatment of melanoma and was shown to be able to reduce tumor size [218].
The rexinoid LG100268 (Ligand Pharmaceuticals, San Diego, CA) (Fig. 1b) has been shown to inhibit cell survival pathways involving PI3K and NFκB [219]. When used in conjunction with the SERM arzoxifene, LG100268 was shown to promote apoptosis in a rat model of estrogen-dependent breast carcinoma and in human breast cancer cells in culture [219]. In addition, a dramatic reduction in tumor volume occurs in tumor-bearing rats after three courses of treatment with LG100268 and arzoxifene. These results open up new avenues in chemoprevention with the ability to administer high doses of drugs for short-term use with high potency and minimal side effects.
Despite promising results in preclinical studies, in clinical studies rexinoids do not perform up to expectations for the treatment of lung and breast carcinomas. In a clinical study of bexarotene in patients with metastatic breast cancer, the rexinoid showed limited activity with only a 20% partial response in women with hormone-refractory or chemotherapy-refractory disease [208]. In a more recent phase III trial, bexarotene was used in combination with more classical chemotherapy agents, such as cisplatin or carboplatin, where it failed to meet primary end points in advanced non-small cell lung carcinoma, but it did increase the overall survival of certain patient subgroups (i.e. males, smokers, and patients with stage IV disease) [208]. The key to understanding the seemingly conflicting results of preclinical and clinical studies may lie in deciphering the downstream targets of RXR action in vivo.
Cumulatively, these data demonstrate that retinoids and their receptors play critical roles in development and homeostasis of a variety of tissues and that perturbation of either the ligand or receptor function can have both disease and therapeutic implications. Future experimental work will have to address the intricate relationship(s) between the receptors and their ligands. Moreover, further comparative analyses of the gene networks controlled by RAR and RXR will help to decipher the evolution of the RA signaling cascade and the functions of RA in different species. This will in turn provide new insights into the structure and function of the vertebrate pathways, which might ultimately lead to the development of new paralog-specific ligands with implications in a clinical setting.
Acknowledgments
The authors would like to thank Gabriel V. Markov, Jasmin Schulz, and Gérard Benoit for critical reading of the manuscript, and the ANR (ANR-07-BLAN-0038 and ANR-09-BLAN-0262-02), the CNRS, and CRESCENDO (a European Union Integrated Project of FP6) for providing financial support. Furthermore, we would like to apologize to all colleagues, whose original work and contributions could not be cited in this article due to space limitations.
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s00018-010-0361-3
References
- 1.Collins MD, Mao GE. Teratology of retinoids. Annu Rev Pharmacol Toxicol. 1999;39:399–430. doi: 10.1146/annurev.pharmtox.39.1.399. [DOI] [PubMed] [Google Scholar]
- 2.Morriss-Kay GM, Ward SJ. Retinoids and mammalian development. Int Rev Cytol. 1999;188:73–131. doi: 10.1016/S0074-7696(08)61566-1. [DOI] [PubMed] [Google Scholar]
- 3.Kastner P, Mark M, Chambon P. Nonsteroid nuclear receptors: what are genetic studies telling us about their role in real life? Cell. 1995;83:859–869. doi: 10.1016/0092-8674(95)90202-3. [DOI] [PubMed] [Google Scholar]
- 4.Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006;66:606–630. doi: 10.1002/neu.20242. [DOI] [PubMed] [Google Scholar]
- 5.Sommer A. Xerophthalmia and vitamin A status. Prog Retin Eye Res. 1998;17:9–31. doi: 10.1016/S1350-9462(97)00001-3. [DOI] [PubMed] [Google Scholar]
- 6.Melhus H, Michaelsson K, Kindmark A, Bergstrom R, Holmberg L, Mallmin H, Wolk A, Ljunghall S. Excessive dietary intake of vitamin A is associated with reduced bone mineral density and increased risk for hip fracture. Ann Intern Med. 1998;129:770–778. doi: 10.7326/0003-4819-129-10-199811150-00003. [DOI] [PubMed] [Google Scholar]
- 7.Silveira ER, Moreno FS. Natural retinoids and β-carotene: from food to their actions on gene expression. J Nutr Biochem. 1998;9:446–456. doi: 10.1016/S0955-2863(98)00040-0. [DOI] [Google Scholar]
- 8.Napoli JL. Biochemical pathways of retinoid transport, metabolism, and signal transduction. Clin Immunol Immunopathol. 1996;80:S52–S62. doi: 10.1006/clin.1996.0142. [DOI] [PubMed] [Google Scholar]
- 9.Achkar CC, Derguini F, Blumberg B, Langston A, Levin AA, Speck J, Evans RM, Bolado J, Jr, Nakanishi K, Buck J, Gudas LJ. 4-Oxoretinol, a new natural ligand and transactivator of the retinoic acid receptors. Proc Natl Acad Sci USA. 1996;93:4879–4884. doi: 10.1073/pnas.93.10.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buck J, Derguini F, Levi E, Nakanishi K, Hammerling U. Intracellular signaling by 14-hydroxy-4,14-retro-retinol. Science. 1991;254:1654–1656. doi: 10.1126/science.1749937. [DOI] [PubMed] [Google Scholar]
- 11.Moore T. Vitamin A and carotene: the absence of the liver oil vitamin A from carotene. VI. The conversion of carotene to vitamin A in vivo. Biochem J. 1930;24:692–702. doi: 10.1042/bj0240692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karrer P, Helfenstein A, Wehrli H, Wettstein A (1930) Uber die Konstitution des Lycopins und Carotins. Helv Chim Acta 13
- 13.Simoes-Costa MS, Azambuja AP, Xavier-Neto J. The search for non-chordate retinoic acid signaling: lessons from chordates. J Exp Zool B Mol Dev Evol. 2008;310:54–72. doi: 10.1002/jez.b.21139. [DOI] [PubMed] [Google Scholar]
- 14.Holland LZ, Albalat R, Azumi K, Benito-Gutierrez E, Blow MJ, Bronner-Fraser M, Brunet F, Butts T, Candiani S, Dishaw LJ, Ferrier DE, Garcia-Fernandez J, Gibson-Brown JJ, Gissi C, Godzik A, Hallbook F, Hirose D, Hosomichi K, Ikuta T, Inoko H, Kasahara M, Kasamatsu J, Kawashima T, Kimura A, Kobayashi M, Kozmik Z, Kubokawa K, Laudet V, Litman GW, McHardy AC, Meulemans D, Nonaka M, Olinski RP, Pancer Z, Pennacchio LA, Pestarino M, Rast JP, Rigoutsos I, Robinson-Rechavi M, Roch G, Saiga H, Sasakura Y, Satake M, Satou Y, Schubert M, Sherwood N, Shiina T, Takatori N, Tello J, Vopalensky P, Wada S, Xu A, Ye Y, Yoshida K, Yoshizaki F, Yu JK, Zhang Q, Zmasek CM, de Jong PJ, Osoegawa K, Putnam NH, Rokhsar DS, Satoh N, Holland PW. The amphioxus genome illuminates vertebrate origins and cephalochordate biology. Genome Res. 2008;18:1100–1111. doi: 10.1101/gr.073676.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Lintig J, Hessel S, Isken A, Kiefer C, Lampert JM, Voolstra O, Vogt K. Towards a better understanding of carotenoid metabolism in animals. Biochim Biophys Acta. 2005;1740:122–131. doi: 10.1016/j.bbadis.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, Howald WN, Juchau MR. Biosynthesis of all-trans-retinoic acid from all-trans-retinol: catalysis of all-trans-retinol oxidation by human P-450 cytochromes. Drug Metab Dispos. 2000;28:315–322. [PubMed] [Google Scholar]
- 17.Albalat R. The retinoic acid machinery in invertebrates: ancestral elements and vertebrate innovations. Mol Cell Endocrinol. 2009;313:23–35. doi: 10.1016/j.mce.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 18.Levin MS. Cellular retinol-binding proteins are determinants of retinol uptake and metabolism in stably transfected Caco-2 cells. J Biol Chem. 1993;268:8267–8276. [PubMed] [Google Scholar]
- 19.Ghyselinck NB, Bavik C, Sapin V, Mark M, Bonnier D, Hindelang C, Dierich A, Nilsson CB, Hakansson H, Sauvant P, Azais-Braesco V, Frasson M, Picaud S, Chambon P. Cellular retinol-binding protein I is essential for vitamin A homeostasis. EMBO J. 1999;18:4903–4914. doi: 10.1093/emboj/18.18.4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.E X, Zhang L, Lu J, Tso P, Blaner WS, Levin MS, Li E. Increased neonatal mortality in mice lacking cellular retinol-binding protein II. J Biol Chem. 2002;277:36617–36623. doi: 10.1074/jbc.M205519200. [DOI] [PubMed] [Google Scholar]
- 21.Piantedosi R, Ghyselinck N, Blaner WS, Vogel S. Cellular retinol-binding protein type III is needed for retinoid incorporation into milk. J Biol Chem. 2005;280:24286–242892. doi: 10.1074/jbc.M503906200. [DOI] [PubMed] [Google Scholar]
- 22.Gottesman ME, Quadro L, Blaner WS. Studies of vitamin A metabolism in mouse model systems. Bioessays. 2001;23:409–419. doi: 10.1002/bies.1059. [DOI] [PubMed] [Google Scholar]
- 23.Herr FM, Ong DE. Differential interaction of lecithin–retinol acyltransferase with cellular retinol binding proteins. Biochemistry. 1992;31:6748–6755. doi: 10.1021/bi00144a014. [DOI] [PubMed] [Google Scholar]
- 24.Batten ML, Imanishi Y, Maeda T, Tu DC, Moise AR, Bronson D, Possin D, Van Gelder RN, Baehr W, Palczewski K. Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem. 2004;279:10422–10432. doi: 10.1074/jbc.M312410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Byrne SM, Wongsiriroj N, Libien J, Vogel S, Goldberg IJ, Baehr W, Palczewski K, Blaner WS. Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT) J Biol Chem. 2005;280:35647–35657. doi: 10.1074/jbc.M507924200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross AC. Retinol esterification by rat liver microsomes. Evidence for a fatty acyl coenzyme A: retinol acyltransferase. J Biol Chem. 1982;257:2453–2459. [PubMed] [Google Scholar]
- 27.Blomhoff R, Green MH, Berg T, Norum KR. Transport and storage of vitamin A. Science. 1990;250:399–404. doi: 10.1126/science.2218545. [DOI] [PubMed] [Google Scholar]
- 28.Zanotti G, Berni R. Plasma retinol-binding protein: structure and interactions with retinol, retinoids, and transthyretin. Vitam Horm. 2004;69:271–295. doi: 10.1016/S0083-6729(04)69010-8. [DOI] [PubMed] [Google Scholar]
- 29.Soprano DR, Soprano KJ, Goodman DS. Retinol-binding protein messenger RNA levels in the liver and in extrahepatic tissues of the rat. J Lipid Res. 1986;27:166–171. [PubMed] [Google Scholar]
- 30.Kanai M, Raz A, Goodman DS. Retinol-binding protein: the transport protein for vitamin A in human plasma. J Clin Invest. 1968;47:2025–2044. doi: 10.1172/JCI105889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, Freeman S, Cosma MP, Colantuoni V, Gottesman ME. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J. 1999;18:4633–4644. doi: 10.1093/emboj/18.17.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134:921–931. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niederreither K, Abu-Abed S, Schuhbaur B, Petkovich M, Chambon P, Dolle P. Genetic evidence that oxidative derivatives of retinoic acid are not involved in retinoid signaling during mouse development. Nat Genet. 2002;31:84–88. doi: 10.1038/ng876. [DOI] [PubMed] [Google Scholar]
- 34.Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315:820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- 35.Blaner WS. STRA6, a cell-surface receptor for retinol-binding protein: the plot thickens. Cell Metab. 2007;5:164–166. doi: 10.1016/j.cmet.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Pasutto F, Sticht H, Hammersen G, Gillessen-Kaesbach G, Fitzpatrick DR, Nurnberg G, Brasch F, Schirmer-Zimmermann H, Tolmie JL, Chitayat D, Houge G, Fernandez-Martinez L, Keating S, Mortier G, Hennekam RC, von der Wense A, Slavotinek A, Meinecke P, Bitoun P, Becker C, Nurnberg P, Reis A, Rauch A. Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am J Hum Genet. 2007;80:550–560. doi: 10.1086/512203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Golzio C, Martinovic-Bouriel J, Thomas S, Mougou-Zrelli S, Grattagliano-Bessieres B, Bonniere M, Delahaye S, Munnich A, Encha-Razavi F, Lyonnet S, Vekemans M, Attie-Bitach T, Etchevers HC. Matthew-Wood syndrome is caused by truncating mutations in the retinol-binding protein receptor gene STRA6. Am J Hum Genet. 2007;80:1179–1187. doi: 10.1086/518177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pares X, Farres J, Kedishvili N, Duester G. Medium- and short-chain dehydrogenase/reductase gene and protein families: medium-chain and short-chain dehydrogenases/reductases in retinoid metabolism. Cell Mol Life Sci. 2008;65:3936–3949. doi: 10.1007/s00018-008-8591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molotkov A, Fan X, Deltour L, Foglio MH, Martras S, Farres J, Pares X, Duester G. Stimulation of retinoic acid production and growth by ubiquitously expressed alcohol dehydrogenase Adh3 . Proc Natl Acad Sci USA. 2002;99:5337–5342. doi: 10.1073/pnas.082093299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duester G, Mic FA, Molotkov A. Cytosolic retinoid dehydrogenases govern ubiquitous metabolism of retinol to retinaldehyde followed by tissue-specific metabolism to retinoic acid. Chem Biol Interact. 2003;143–144:201–210. doi: 10.1016/S0009-2797(02)00204-1. [DOI] [PubMed] [Google Scholar]
- 41.Molotkov A, Fan X, Duester G. Excessive vitamin A toxicity in mice genetically deficient in either alcohol dehydrogenase Adh1 or Adh3 . Eur J Biochem. 2002;269:2607–2612. doi: 10.1046/j.1432-1033.2002.02935.x. [DOI] [PubMed] [Google Scholar]
- 42.Sandell LL, Sanderson BW, Moiseyev G, Johnson T, Mushegian A, Young K, Rey JP, Ma JX, Staehling-Hampton K, Trainor PA. RDH10 is essential for synthesis of embryonic retinoic acid and is required for limb, craniofacial, and organ development. Genes Dev. 2007;21:1113–1124. doi: 10.1101/gad.1533407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strate I, Min TH, Iliev D, Pera EM. Retinol dehydrogenase 10 is a feedback regulator of retinoic acid signalling during axis formation and patterning of the central nervous system. Development. 2009;136:461–472. doi: 10.1242/dev.024901. [DOI] [PubMed] [Google Scholar]
- 44.Albalat R, Canestro C. Identification of Aldh1a, Cyp26 and RAR orthologs in protostomes pushes back the retinoic acid genetic machinery in evolutionary time to the bilaterian ancestor. Chem Biol Interact. 2009;178:188–196. doi: 10.1016/j.cbi.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 45.Haselbeck RJ, Hoffmann I, Duester G. Distinct functions for Aldh1 and Raldh2 in the control of ligand production for embryonic retinoid signaling pathways. Dev Genet. 1999;25:353–364. doi: 10.1002/(SICI)1520-6408(1999)25:4<353::AID-DVG9>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niederreither K, Subbarayan V, Dolle P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- 47.Niederreither K, Vermot J, Schuhbaur B, Chambon P, Dolle P. Retinoic acid synthesis and hindbrain patterning in the mouse embryo. Development. 2000;127:75–85. doi: 10.1242/dev.127.1.75. [DOI] [PubMed] [Google Scholar]
- 48.Dupe V, Matt N, Garnier JM, Chambon P, Mark M, Ghyselinck NB. A newborn lethal defect due to inactivation of retinaldehyde dehydrogenase type 3 is prevented by maternal retinoic acid treatment. Proc Natl Acad Sci USA. 2003;100:14036–14041. doi: 10.1073/pnas.2336223100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin M, Zhang M, Abraham M, Smith SM, Napoli JL. Mouse retinal dehydrogenase 4 (RALDH4), molecular cloning, cellular expression, and activity in 9-cis-retinoic acid biosynthesis in intact cells. J Biol Chem. 2003;278:9856–9861. doi: 10.1074/jbc.M211417200. [DOI] [PubMed] [Google Scholar]
- 50.Campo-Paysaa F, Marletaz F, Laudet V, Schubert M. Retinoic acid signaling in development: tissue-specific functions and evolutionary origins. Genesis. 2008;46:640–656. doi: 10.1002/dvg.20444. [DOI] [PubMed] [Google Scholar]
- 51.Canestro C, Postlethwait JH, Gonzalez-Duarte R, Albalat R. Is retinoic acid genetic machinery a chordate innovation? Evol Dev. 2006;8:394–406. doi: 10.1111/j.1525-142X.2006.00113.x. [DOI] [PubMed] [Google Scholar]
- 52.Napoli JL. Retinoic acid biosynthesis and metabolism. FASEB J. 1996;10:993–1001. doi: 10.1096/fasebj.10.9.8801182. [DOI] [PubMed] [Google Scholar]
- 53.Delva L, Bastie JN, Rochette-Egly C, Kraiba R, Balitrand N, Despouy G, Chambon P, Chomienne C. Physical and functional interactions between cellular retinoic acid binding protein II and the retinoic acid-dependent nuclear complex. Mol Cell Biol. 1999;19:7158–7167. doi: 10.1128/mcb.19.10.7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dong D, Ruuska SE, Levinthal DJ, Noy N. Distinct roles for cellular retinoic acid-binding proteins I and II in regulating signaling by retinoic acid. J Biol Chem. 1999;274:23695–23698. doi: 10.1074/jbc.274.34.23695. [DOI] [PubMed] [Google Scholar]
- 55.Lampron C, Rochette-Egly C, Gorry P, Dolle P, Mark M, Lufkin T, LeMeur M, Chambon P. Mice deficient in cellular retinoic acid binding protein II (CRABPII) or in both CRABPI and CRABPII are essentially normal. Development. 1995;121:539–548. doi: 10.1242/dev.121.2.539. [DOI] [PubMed] [Google Scholar]
- 56.Jackman WR, Mougey JM, Panopoulou GD, Kimmel CB. crabp and maf highlight the novelty of the amphioxus club-shaped gland. Acta Zool (Stockholm) 2004;85:91–99. doi: 10.1111/j.0001-7272.2004.00161.x. [DOI] [Google Scholar]
- 57.Petkovich PM. Retinoic acid metabolism. J Am Acad Dermatol. 2001;45:S136–S142. doi: 10.1067/mjd.2001.113715. [DOI] [PubMed] [Google Scholar]
- 58.Roberts ES, Vaz AD, Coon MJ. Role of isozymes of rabbit microsomal cytochrome P-450 in the metabolism of retinoic acid, retinol, and retinal. Mol Pharmacol. 1992;41:427–433. [PubMed] [Google Scholar]
- 59.Marill J, Capron CC, Idres N, Chabot GG. Human cytochrome P450s involved in the metabolism of 9-cis- and 13-cis-retinoic acids. Biochem Pharmacol. 2002;63:933–943. doi: 10.1016/S0006-2952(01)00925-X. [DOI] [PubMed] [Google Scholar]
- 60.White JA, Guo YD, Baetz K, Beckett-Jones B, Bonasoro J, Hsu KE, Dilworth FJ, Jones G, Petkovich M. Identification of the retinoic acid-inducible all-trans-retinoic acid 4-hydroxylase. J Biol Chem. 1996;271:29922–29927. doi: 10.1074/jbc.271.47.29922. [DOI] [PubMed] [Google Scholar]
- 61.White JA, Beckett-Jones B, Guo YD, Dilworth FJ, Bonasoro J, Jones G, Petkovich M. cDNA cloning of human retinoic acid-metabolizing enzyme (hP450RAI) identifies a novel family of cytochromes P450. J Biol Chem. 1997;272:18538–18541. doi: 10.1074/jbc.272.30.18538. [DOI] [PubMed] [Google Scholar]
- 62.White RJ, Schilling TF. How degrading: Cyp26s in hindbrain development. Dev Dyn. 2008;237:2775–2790. doi: 10.1002/dvdy.21695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tanibe M, Michiue T, Yukita A, Danno H, Ikuzawa M, Ishiura S, Asashima M. Retinoic acid metabolizing factor xCyp26c is specifically expressed in neuroectoderm and regulates anterior neural patterning in Xenopus laevis . Int J Dev Biol. 2008;52:893–901. doi: 10.1387/ijdb.082683mt. [DOI] [PubMed] [Google Scholar]
- 64.Abu-Abed S, Dolle P, Metzger D, Beckett B, Chambon P, Petkovich M. The retinoic acid-metabolizing enzyme, CYP26A1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures. Genes Dev. 2001;15:226–240. doi: 10.1101/gad.855001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.White JA, Ramshaw H, Taimi M, Stangle W, Zhang A, Everingham S, Creighton S, Tam SP, Jones G, Petkovich M. Identification of the human cytochrome P450, P450RAI-2, which is predominantly expressed in the adult cerebellum and is responsible for all-trans-retinoic acid metabolism. Proc Natl Acad Sci USA. 2000;97:6403–6408. doi: 10.1073/pnas.120161397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taimi M, Helvig C, Wisniewski J, Ramshaw H, White J, Amad M, Korczak B, Petkovich M. A novel human cytochrome P450, CYP26C1, involved in metabolism of 9-cis and all-trans isomers of retinoic acid. J Biol Chem. 2004;279:77–85. doi: 10.1074/jbc.M308337200. [DOI] [PubMed] [Google Scholar]
- 67.Reijntjes S, Gale E, Maden M. Generating gradients of retinoic acid in the chick embryo: Cyp26C1 expression and a comparative analysis of the Cyp26 enzymes. Dev Dyn. 2004;230:509–517. doi: 10.1002/dvdy.20025. [DOI] [PubMed] [Google Scholar]
- 68.Uehara M, Yashiro K, Mamiya S, Nishino J, Chambon P, Dolle P, Sakai Y. CYP26A1 and CYP26C1 cooperatively regulate anterior-posterior patterning of the developing brain and the production of migratory cranial neural crest cells in the mouse. Dev Biol. 2007;302:399–411. doi: 10.1016/j.ydbio.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 69.Hernandez RE, Putzke AP, Myers JP, Margaretha L, Moens CB. Cyp26 enzymes generate the retinoic acid response pattern necessary for hindbrain development. Development. 2007;134:177–187. doi: 10.1242/dev.02706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laudet V, Gronemeyer H. The nuclear receptor facts book. San Diego: Academic; 2002. [Google Scholar]
- 71.Mangelsdorf DJ, Ong ES, Dyck JA, Evans RM. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature. 1990;345:224–229. doi: 10.1038/345224a0. [DOI] [PubMed] [Google Scholar]
- 72.Leid M, Kastner P, Chambon P. Multiplicity generates diversity in the retinoic acid signalling pathways. Trends Biochem Sci. 1992;17:427–433. doi: 10.1016/0968-0004(92)90014-Z. [DOI] [PubMed] [Google Scholar]
- 73.Chambon P. The nuclear receptor superfamily: a personal retrospect on the first two decades. Mol Endocrinol. 2005;19:1418–1428. doi: 10.1210/me.2005-0125. [DOI] [PubMed] [Google Scholar]
- 74.Mader S, Chen JY, Chen Z, White J, Chambon P, Gronemeyer H. The patterns of binding of RAR, RXR and TR homo- and heterodimers to direct repeats are dictated by the binding specificites of the DNA binding domains. EMBO J. 1993;12:5029–5041. doi: 10.1002/j.1460-2075.1993.tb06196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perlmann T, Umesono K, Rangarajan PN, Forman BM, Evans RM. Two distinct dimerization interfaces differentially modulate target gene specificity of nuclear hormone receptors. Mol Endocrinol. 1996;10:958–966. doi: 10.1210/me.10.8.958. [DOI] [PubMed] [Google Scholar]
- 76.Zechel C, Shen XQ, Chambon P, Gronemeyer H. Dimerization interfaces formed between the DNA binding domains determine the cooperative binding of RXR/RAR and RXR/TR heterodimers to DR5 and DR4 elements. EMBO J. 1994;13:1414–1424. doi: 10.1002/j.1460-2075.1994.tb06395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zechel C, Shen XQ, Chen JY, Chen ZP, Chambon P, Gronemeyer H. The dimerization interfaces formed between the DNA binding domains of RXR, RAR and TR determine the binding specificity and polarity of the full-length receptors to direct repeats. EMBO J. 1994;13:1425–1433. doi: 10.1002/j.1460-2075.1994.tb06396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Balmer JE, Blomhoff R. A robust characterization of retinoic acid response elements based on a comparison of sites in three species. J Steroid Biochem Mol Biol. 2005;96:347–354. doi: 10.1016/j.jsbmb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 79.Durand B, Saunders M, Leroy P, Leid M, Chambon P. All-trans and 9-cis retinoic acid induction of CRABPII transcription is mediated by RAR-RXR heterodimers bound to DR1 and DR2 repeated motifs. Cell. 1992;71:73–85. doi: 10.1016/0092-8674(92)90267-G. [DOI] [PubMed] [Google Scholar]
- 80.Smith WC, Nakshatri H, Leroy P, Rees J, Chambon P. A retinoic acid response element is present in the mouse cellular retinol binding protein I (mCRBPI) promoter. EMBO J. 1991;10:2223–2230. doi: 10.1002/j.1460-2075.1991.tb07758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Perlmann T, Rangarajan PN, Umesono K, Evans RM. Determinants for selective RAR and TR recognition of direct repeat HREs. Genes Dev. 1993;7:1411–1422. doi: 10.1101/gad.7.7b.1411. [DOI] [PubMed] [Google Scholar]
- 82.Predki PF, Zamble D, Sarkar B, Giguere V. Ordered binding of retinoic acid and retinoid-X receptors to asymmetric response elements involves determinants adjacent to the DNA-binding domain. Mol Endocrinol. 1994;8:31–39. doi: 10.1210/me.8.1.31. [DOI] [PubMed] [Google Scholar]
- 83.Kurokawa R, DiRenzo J, Boehm M, Sugarman J, Gloss B, Rosenfeld MG, Heyman RA, Glass CK. Regulation of retinoid signalling by receptor polarity and allosteric control of ligand binding. Nature. 1994;371:528–531. doi: 10.1038/371528a0. [DOI] [PubMed] [Google Scholar]
- 84.Giguere V, Ong ES, Segui P, Evans RM. Identification of a receptor for the morphogen retinoic acid. Nature. 1987;330:624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- 85.Petkovich M, Brand NJ, Krust A, Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987;330:444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- 86.Mark M, Ghyselinck NB, Chambon P. Function of retinoic acid receptors during embryonic development. Nucl Recept Signal. 2009;7:e002. doi: 10.1621/nrs.07002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brand N, Petkovich M, Krust A, Chambon P, de The H, Marchio A, Tiollais P, Dejean A. Identification of a second human retinoic acid receptor. Nature. 1988;332:850–853. doi: 10.1038/332850a0. [DOI] [PubMed] [Google Scholar]
- 88.Dejean A, Bougueleret L, Grzeschik KH, Tiollais P. Hepatitis B virus DNA integration in a sequence homologous to v-erb-A and steroid receptor genes in a hepatocellular carcinoma. Nature. 1986;322:70–72. doi: 10.1038/322070a0. [DOI] [PubMed] [Google Scholar]
- 89.Peng X, Maruo T, Cao Y, Punj V, Mehta R, Das Gupta TK, Christov K. A novel RARβ isoform directed by a distinct promoter P3 and mediated by retinoic acid in breast cancer cells. Cancer Res. 2004;64:8911–8918. doi: 10.1158/0008-5472.CAN-04-1810. [DOI] [PubMed] [Google Scholar]
- 90.Zelent A, Mendelsohn C, Kastner P, Krust A, Garnier JM, Ruffenach F, Leroy P, Chambon P. Differentially expressed isoforms of the mouse retinoic acid receptor β generated by usage of two promoters and alternative splicing. EMBO J. 1991;10:71–81. doi: 10.1002/j.1460-2075.1991.tb07922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liao WL, Tsai HC, Wang HF, Chang J, Lu KM, Wu HL, Lee YC, Tsai TF, Takahashi H, Wagner M, Ghyselinck NB, Chambon P, Liu FC. Modular patterning of structure and function of the striatum by retinoid receptor signaling. Proc Natl Acad Sci USA. 2008;105:6765–6770. doi: 10.1073/pnas.0802109105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Krezel W, Ghyselinck N, Samad TA, Dupe V, Kastner P, Borrelli E, Chambon P. Impaired locomotion and dopamine signaling in retinoid receptor mutant mice. Science. 1998;279:863–867. doi: 10.1126/science.279.5352.863. [DOI] [PubMed] [Google Scholar]
- 93.Krust A, Kastner P, Petkovich M, Zelent A, Chambon P. A third human retinoic acid receptor, hRARγ. Proc Natl Acad Sci USA. 1989;86:5310–5314. doi: 10.1073/pnas.86.14.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zelent A, Krust A, Petkovich M, Kastner P, Chambon P. Cloning of murine α and β retinoic acid receptors and a novel receptor γ predominantly expressed in skin. Nature. 1989;339:714–717. doi: 10.1038/339714a0. [DOI] [PubMed] [Google Scholar]
- 95.Lohnes D, Kastner P, Dierich A, Mark M, LeMeur M, Chambon P. Function of retinoic acid receptor γ in the mouse. Cell. 1993;73:643–658. doi: 10.1016/0092-8674(93)90246-M. [DOI] [PubMed] [Google Scholar]
- 96.Williams JA, Kondo N, Okabe T, Takeshita N, Pilchak DM, Koyama E, Ochiai T, Jensen D, Chu ML, Kane MA, Napoli JL, Enomoto-Iwamoto M, Ghyselinck N, Chambon P, Pacifici M, Iwamoto M. Retinoic acid receptors are required for skeletal growth, matrix homeostasis and growth plate function in postnatal mouse. Dev Biol. 2009;328:315–327. doi: 10.1016/j.ydbio.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lohnes D, Mark M, Mendelsohn C, Dolle P, Dierich A, Gorry P, Gansmuller A, Chambon P. Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development. 1994;120:2723–2748. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- 98.Mendelsohn C, Lohnes D, Decimo D, Lufkin T, LeMeur M, Chambon P, Mark M. Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development. 1994;120:2749–2771. doi: 10.1242/dev.120.10.2749. [DOI] [PubMed] [Google Scholar]
- 99.Hamada K, Gleason SL, Levi BZ, Hirschfeld S, Appella E, Ozato K. H-2RIIBP, a member of the nuclear hormone receptor superfamily that binds to both the regulatory element of major histocompatibility class I genes and the estrogen response element. Proc Natl Acad Sci USA. 1989;86:8289–8293. doi: 10.1073/pnas.86.21.8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kastner P, Messaddeq N, Mark M, Wendling O, Grondona JM, Ward S, Ghyselinck N, Chambon P. Vitamin A deficiency and mutations of RXRα, RXRβ and RARα lead to early differentiation of embryonic ventricular cardiomyocytes. Development. 1997;124:4749–4758. doi: 10.1242/dev.124.23.4749. [DOI] [PubMed] [Google Scholar]
- 101.Sucov HM, Izpisua-Belmonte JC, Ganan Y, Evans RM. Mouse embryos lacking RXRα are resistant to retinoic-acid-induced limb defects. Development. 1995;121:3997–4003. doi: 10.1242/dev.121.12.3997. [DOI] [PubMed] [Google Scholar]
- 102.Nugent P, Sucov HM, Pisano MM, Greene RM. The role of RXRα in retinoic acid-induced cleft palate as assessed with the RXRα knockout mouse. Int J Dev Biol. 1999;43:567–570. [PubMed] [Google Scholar]
- 103.Kastner P, Mark M, Leid M, Gansmuller A, Chin W, Grondona JM, Decimo D, Krezel W, Dierich A, Chambon P. Abnormal spermatogenesis in RXRβ mutant mice. Genes Dev. 1996;10:80–92. doi: 10.1101/gad.10.1.80. [DOI] [PubMed] [Google Scholar]
- 104.Krezel W, Dupe V, Mark M, Dierich A, Kastner P, Chambon P. RXRγ null mice are apparently normal and compound RXRα +/−/RXRβ −/−/RXRγ −/− mutant mice are viable. Proc Natl Acad Sci USA. 1996;93:9010–9014. doi: 10.1073/pnas.93.17.9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brown NS, Smart A, Sharma V, Brinkmeier ML, Greenlee L, Camper SA, Jensen DR, Eckel RH, Krezel W, Chambon P, Haugen BR. Thyroid hormone resistance and increased metabolic rate in the RXR-γ-deficient mouse. J Clin Invest. 2000;106:73–79. doi: 10.1172/JCI9422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129:723–733. doi: 10.1016/j.cell.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Berry DC, Noy N. Is PPARβ/δ a retinoid receptor? PPAR Res. 2007;2007:73256. doi: 10.1155/2007/73256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shaw N, Elholm M, Noy N. Retinoic acid is a high affinity selective ligand for the peroxisome proliferator-activated receptor β/δ. J Biol Chem. 2003;278:41589–41592. doi: 10.1074/jbc.C300368200. [DOI] [PubMed] [Google Scholar]
- 109.Di-Poi N, Tan NS, Michalik L, Wahli W, Desvergne B. Antiapoptotic role of PPARβ in keratinocytes via transcriptional control of the Akt1 signaling pathway. Mol Cell. 2002;10:721–733. doi: 10.1016/S1097-2765(02)00646-9. [DOI] [PubMed] [Google Scholar]
- 110.Tan NS, Michalik L, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor-β as a target for wound healing drugs. Expert Opin Ther Targets. 2004;8:39–48. doi: 10.1517/14728222.8.1.39. [DOI] [PubMed] [Google Scholar]
- 111.Wolf G. Retinoic acid as cause of cell proliferation or cell growth inhibition depending on activation of one of two different nuclear receptors. Nutr Rev. 2008;66:55–59. doi: 10.1111/j.1753-4887.2007.00006.x. [DOI] [PubMed] [Google Scholar]
- 112.Stehlin-Gaon C, Willmann D, Zeyer D, Sanglier S, Van Dorsselaer A, Renaud JP, Moras D, Schule R. All-trans retinoic acid is a ligand for the orphan nuclear receptor RORβ. Nat Struct Biol. 2003;10:820–825. doi: 10.1038/nsb979. [DOI] [PubMed] [Google Scholar]
- 113.Park JI, Tsai SY, Tsai MJ. Molecular mechanism of chicken ovalbumin upstream promoter-transcription factor (COUP-TF) actions. Keio J Med. 2003;52:174–181. doi: 10.2302/kjm.52.174. [DOI] [PubMed] [Google Scholar]
- 114.Pereira FA, Qiu Y, Zhou G, Tsai MJ, Tsai SY. The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev. 1999;13:1037–1049. doi: 10.1101/gad.13.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kruse SW, Suino-Powell K, Zhou XE, Kretschman JE, Reynolds R, Vonrhein C, Xu Y, Wang L, Tsai SY, Tsai MJ, Xu HE. Identification of COUP-TFII orphan nuclear receptor as a retinoic acid-activated receptor. PLoS Biol. 2008;6:e227. doi: 10.1371/journal.pbio.0060227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Allenby G, Janocha R, Kazmer S, Speck J, Grippo JF, Levin AA. Binding of 9-cis-retinoic acid and all-trans-retinoic acid to retinoic acid receptors α, β, and γ. Retinoic acid receptor γ binds all-trans-retinoic acid preferentially over 9-cis-retinoic acid. J Biol Chem. 1994;269:16689–16695. [PubMed] [Google Scholar]
- 117.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 118.Repa JJ, Hanson KK, Clagett-Dame M. All-trans-retinol is a ligand for the retinoic acid receptors. Proc Natl Acad Sci USA. 1993;90:7293–7297. doi: 10.1073/pnas.90.15.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pijnappel WW, Hendriks HF, Folkers GE, van den Brink CE, Dekker EJ, Edelenbosch C, van der Saag PT, Durston AJ. The retinoid ligand 4-oxo-retinoic acid is a highly active modulator of positional specification. Nature. 1993;366:340–344. doi: 10.1038/366340a0. [DOI] [PubMed] [Google Scholar]
- 120.Schuchardt JP, Wahlstrom D, Ruegg J, Giese N, Stefan M, Hopf H, Pongratz I, Hakansson H, Eichele G, Pettersson K, Nau H. The endogenous retinoid metabolite S-4-oxo-9-cis-13, 14-dihydro-retinoic acid activates retinoic acid receptor signalling both in vitro and in vivo. FEBS J. 2009;276:3043–3059. doi: 10.1111/j.1742-4658.2009.07023.x. [DOI] [PubMed] [Google Scholar]
- 121.Matsumoto A, Mizukami H, Mizuno S, Umegaki K, Nishikawa J, Shudo K, Kagechika H, Inoue M. β-Cryptoxanthin, a novel natural RAR ligand, induces ATP-binding cassette transporters in macrophages. Biochem Pharmacol. 2007;74:256–264. doi: 10.1016/j.bcp.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 122.Altucci L, Leibowitz MD, Ogilvie KM, de Lera AR, Gronemeyer H. RAR and RXR modulation in cancer and metabolic disease. Nat Rev Drug Discov. 2007;6:793–810. doi: 10.1038/nrd2397. [DOI] [PubMed] [Google Scholar]
- 123.Boehm MF, Zhang L, Badea BA, White SK, Mais DE, Berger E, Suto CM, Goldman ME, Heyman RA. Synthesis and structure–activity relationships of novel retinoid X receptor-selective retinoids. J Med Chem. 1994;37:2930–2941. doi: 10.1021/jm00044a014. [DOI] [PubMed] [Google Scholar]
- 124.de Lera AR, Bourguet W, Altucci L, Gronemeyer H. Design of selective nuclear receptor modulators: RAR and RXR as a case study. Nat Rev Drug Discov. 2007;6:811–820. doi: 10.1038/nrd2398. [DOI] [PubMed] [Google Scholar]
- 125.Kistler A. Limb bud cell cultures for estimating the teratogenic potential of compounds. Validation of the test system with retinoids. Arch Toxicol. 1987;60:403–414. doi: 10.1007/BF00302382. [DOI] [PubMed] [Google Scholar]
- 126.Pignatello MA, Kauffman FC, Levin AA. Multiple factors contribute to the toxicity of the aromatic retinoid, TTNPB (Ro 13–7410): binding affinities and disposition. Toxicol Appl Pharmacol. 1997;142:319–327. doi: 10.1006/taap.1996.8047. [DOI] [PubMed] [Google Scholar]
- 127.Germain P, Gaudon C, Pogenberg V, Sanglier S, Van Dorsselaer A, Royer CA, Lazar MA, Bourguet W, Gronemeyer H. Differential action on coregulator interaction defines inverse retinoid agonists and neutral antagonists. Chem Biol. 2009;16:479–489. doi: 10.1016/j.chembiol.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 128.Heyman RA, Mangelsdorf DJ, Dyck JA, Stein RB, Eichele G, Evans RM, Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992;68:397–406. doi: 10.1016/0092-8674(92)90479-V. [DOI] [PubMed] [Google Scholar]
- 129.Mangelsdorf DJ, Borgmeyer U, Heyman RA, Zhou JY, Ong ES, Oro AE, Kakizuka A, Evans RM. Characterization of three RXR genes that mediate the action of 9-cis retinoic acid. Genes Dev. 1992;6:329–344. doi: 10.1101/gad.6.3.329. [DOI] [PubMed] [Google Scholar]
- 130.Wolf G. Is 9-cis-retinoic acid the endogenous ligand for the retinoic acid-X receptor? Nutr Rev. 2006;64:532–538. doi: 10.1111/j.1753-4887.2006.tb00186.x. [DOI] [PubMed] [Google Scholar]
- 131.Dmetrichuk JM, Carlone RL, Jones TR, Vesprini ND, Spencer GE. Detection of endogenous retinoids in the molluscan CNS and characterization of the trophic and tropic actions of 9-cis retinoic acid on isolated neurons. J Neurosci. 2008;28:13014–13024. doi: 10.1523/JNEUROSCI.3192-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nowickyj SM, Chithalen JV, Cameron D, Tyshenko MG, Petkovich M, Wyatt GR, Jones G, Walker VK. Locust retinoid X receptors: 9-cis retinoic acid in embryos from a primitive insect. Proc Natl Acad Sci USA. 2008;105:9540–9545. doi: 10.1073/pnas.0712132105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hopkins P. Limb regeneration in the fiddler crab, Uca pugilator: hormonal and growth factor control. Am Zool. 2001;41:389–398. doi: 10.1668/0003-1569(2001)041[0389:LRITFC]2.0.CO;2. [DOI] [Google Scholar]
- 134.Viviano CM, Horton CE, Maden M, Brockes JP. Synthesis and release of 9-cis retinoic acid by the urodele wound epidermis. Development. 1995;121:3753–3762. [Google Scholar]
- 135.Bourguet W, Vivat V, Wurtz JM, Chambon P, Gronemeyer H, Moras D. Crystal structure of a heterodimeric complex of RAR and RXR ligand-binding domains. Mol Cell. 2000;5:289–298. doi: 10.1016/S1097-2765(00)80424-4. [DOI] [PubMed] [Google Scholar]
- 136.de Urquiza AM, Liu S, Sjoberg M, Zetterstrom RH, Griffiths W, Sjovall J, Perlmann T. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science. 2000;290:2140–2144. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- 137.Goldstein JT, Dobrzyn A, Clagett-Dame M, Pike JW, DeLuca HF. Isolation and characterization of unsaturated fatty acids as natural ligands for the retinoid-X receptor. Arch Biochem Biophys. 2003;420:185–193. doi: 10.1016/j.abb.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 138.Tocchini-Valentini GD, Rochel N, Escriva H, Germain P, Peluso-Iltis C, Paris M, Sanglier-Cianferani S, Van Dorsselaer A, Moras D, Laudet V. Structural and functional insights into the ligand-binding domain of a nonduplicated retinoid X nuclear receptor from the invertebrate chordate amphioxus. J Biol Chem. 2009;284:1938–1948. doi: 10.1074/jbc.M805692200. [DOI] [PubMed] [Google Scholar]
- 139.Lehmann JM, Jong L, Fanjul A, Cameron JF, Lu XP, Haefner P, Dawson MI, Pfahl M. Retinoids selective for retinoid X receptor response pathways. Science. 1992;258:1944–1946. doi: 10.1126/science.1335166. [DOI] [PubMed] [Google Scholar]
- 140.Boehm MF, Zhang L, Zhi L, McClurg MR, Berger E, Wagoner M, Mais DE, Suto CM, Davies JA, Heyman RA, Nadzan AM. Design and synthesis of potent retinoid X receptor selective ligands that induce apoptosis in leukemia cells. J Med Chem. 1995;38:3146–3155. doi: 10.1021/jm00016a018. [DOI] [PubMed] [Google Scholar]
- 141.Minucci S, Leid M, Toyama R, Saint-Jeannet JP, Peterson VJ, Horn V, Ishmael JE, Bhattacharyya N, Dey A, Dawid IB, Ozato K. Retinoid X receptor (RXR) within the RXR-retinoic acid receptor heterodimer binds its ligand and enhances retinoid-dependent gene expression. Mol Cell Biol. 1997;17:644–655. doi: 10.1128/mcb.17.2.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res. 2002;43:1773–1808. doi: 10.1194/jlr.R100015-JLR200. [DOI] [PubMed] [Google Scholar]
- 143.Astrom A, Pettersson U, Chambon P, Voorhees JJ. Retinoic acid induction of human cellular retinoic acid-binding protein-II gene transcription is mediated by retinoic acid receptor-retinoid X receptor heterodimers bound to one far upstream retinoic acid-responsive element with 5-base pair spacing. J Biol Chem. 1994;269:22334–22339. [PubMed] [Google Scholar]
- 144.McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68:283–302. doi: 10.1016/0092-8674(92)90471-N. [DOI] [PubMed] [Google Scholar]
- 145.Kessel M. Respecification of vertebral identities by retinoic acid. Development. 1992;115:487–501. doi: 10.1242/dev.115.2.487. [DOI] [PubMed] [Google Scholar]
- 146.Kessel M, Gruss P. Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell. 1991;67:89–104. doi: 10.1016/0092-8674(91)90574-I. [DOI] [PubMed] [Google Scholar]
- 147.Marletaz F, Holland LZ, Laudet V, Schubert M. Retinoic acid signaling and the evolution of chordates. Int J Biol Sci. 2006;2:38–47. doi: 10.7150/ijbs.2.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Langston AW, Thompson JR, Gudas LJ. Retinoic acid-responsive enhancers located 3′ of the Hox A and Hox B homeobox gene clusters. Functional analysis. J Biol Chem. 1997;272:2167–2175. doi: 10.1074/jbc.272.4.2167. [DOI] [PubMed] [Google Scholar]
- 149.Manzanares M, Wada H, Itasaki N, Trainor PA, Krumlauf R, Holland PW. Conservation and elaboration of Hox gene regulation during evolution of the vertebrate head. Nature. 2000;408:854–857. doi: 10.1038/35048570. [DOI] [PubMed] [Google Scholar]
- 150.Wada H, Escriva H, Zhang S, Laudet V. Conserved RARE localization in amphioxus Hox clusters and implications for Hox code evolution in the vertebrate neural crest. Dev Dyn. 2006;235:1522–1531. doi: 10.1002/dvdy.20730. [DOI] [PubMed] [Google Scholar]
- 151.Kanda M, Wada H, Fujiwara S. Epidermal expression of Hox1 is directly activated by retinoic acid in the Ciona intestinalis embryo. Dev Biol. 2009;335:454–463. doi: 10.1016/j.ydbio.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 152.Feng L, Hernandez RE, Waxman JS, Yelon D, Moens CB. Dhrs3a regulates retinoic acid biosynthesis through a feedback inhibition mechanism. Dev Biol. 2010;338:1–14. doi: 10.1016/j.ydbio.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hua S, Kittler R, White KP. Genomic antagonism between retinoic acid and estrogen signaling in breast cancer. Cell. 2009;137:1259–1271. doi: 10.1016/j.cell.2009.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen N, Onisko B, Napoli JL. The nuclear transcription factor RARα associates with neuronal RNA granules and suppresses translation. J Biol Chem. 2008;283:20841–20847. doi: 10.1074/jbc.M802314200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Poon MM, Chen L. Retinoic acid-gated sequence-specific translational control by RARα. Proc Natl Acad Sci USA. 2008;105:20303–20308. doi: 10.1073/pnas.0807740105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liao YP, Ho SY, Liou JC. Non-genomic regulation of transmitter release by retinoic acid at developing motoneurons in Xenopus cell culture. J Cell Sci. 2004;117:2917–2924. doi: 10.1242/jcs.01153. [DOI] [PubMed] [Google Scholar]
- 157.Liou JC, Ho SY, Shen MR, Liao YP, Chiu WT, Kang KH. A rapid, nongenomic pathway facilitates the synaptic transmission induced by retinoic acid at the developing synapse. J Cell Sci. 2005;118:4721–4730. doi: 10.1242/jcs.02603. [DOI] [PubMed] [Google Scholar]
- 158.Masia S, Alvarez S, de Lera AR, Barettino D. Rapid, nongenomic actions of retinoic acid on phosphatidylinositol-3-kinase signaling pathway mediated by the retinoic acid receptor. Mol Endocrinol. 2007;21:2391–2402. doi: 10.1210/me.2007-0062. [DOI] [PubMed] [Google Scholar]
- 159.Fanjul A, Dawson MI, Hobbs PD, Jong L, Cameron JF, Harlev E, Graupner G, Lu XP, Pfahl M. A new class of retinoids with selective inhibition of AP-1 inhibits proliferation. Nature. 1994;372:107–111. doi: 10.1038/372107a0. [DOI] [PubMed] [Google Scholar]
- 160.Baeuerle PA, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/S0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 161.Austenaa LM, Carlsen H, Ertesvag A, Alexander G, Blomhoff HK, Blomhoff R. Vitamin A status significantly alters nuclear factor-κB activity assessed by in vivo imaging. FASEB J. 2004;18:1255–1257. doi: 10.1096/fj.03-1098fje. [DOI] [PubMed] [Google Scholar]
- 162.Austenaa LM, Carlsen H, Hollung K, Blomhoff HK, Blomhoff R. Retinoic acid dampens LPS-induced NF-κB activity: results from human monoblasts and in vivo imaging of NF-κB reporter mice. J Nutr Biochem. 2009;20:726–734. doi: 10.1016/j.jnutbio.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 163.Aggarwal S, Kim SW, Cheon K, Tabassam FH, Yoon JH, Koo JS. Nonclassical action of retinoic acid on the activation of the cAMP response element-binding protein in normal human bronchial epithelial cells. Mol Biol Cell. 2006;17:566–575. doi: 10.1091/mbc.E05-06-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ochoa WF, Torrecillas A, Fita I, Verdaguer N, Corbalan-Garcia S, Gomez-Fernandez JC. Retinoic acid binds to the C2-domain of protein kinase Cα. Biochemistry. 2003;42:8774–8779. doi: 10.1021/bi034713g. [DOI] [PubMed] [Google Scholar]
- 165.Farrar NR, Dmetrichuk JM, Carlone RL, Spencer GE. A novel, nongenomic mechanism underlies retinoic acid-induced growth cone turning. J Neurosci. 2009;29:14136–14142. doi: 10.1523/JNEUROSCI.2921-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Escriva H, Holland ND, Gronemeyer H, Laudet V, Holland LZ. The retinoic acid signaling pathway regulates anterior/posterior patterning in the nerve cord and pharynx of amphioxus, a chordate lacking neural crest. Development. 2002;129:2905–2916. doi: 10.1242/dev.129.12.2905. [DOI] [PubMed] [Google Scholar]
- 167.Holland LZ, Holland ND. Expression of AmphiHox-1 and AmphiPax-1 in amphioxus embryos treated with retinoic acid: insights into evolution and patterning of the chordate nerve cord and pharynx. Development. 1996;122:1829–1838. doi: 10.1242/dev.122.6.1829. [DOI] [PubMed] [Google Scholar]
- 168.Schubert M, Yu JK, Holland ND, Escriva H, Laudet V, Holland LZ. Retinoic acid signaling acts via Hox1 to establish the posterior limit of the pharynx in the chordate amphioxus. Development. 2005;132:61–73. doi: 10.1242/dev.01554. [DOI] [PubMed] [Google Scholar]
- 169.Onai T, Lin HC, Schubert M, Koop D, Osborne PW, Alvarez S, Alvarez R, Holland ND, Holland LZ. Retinoic acid and Wnt/β-catenin have complementary roles in anterior/posterior patterning embryos of the basal chordate amphioxus. Dev Biol. 2009;332:223–233. doi: 10.1016/j.ydbio.2009.05.571. [DOI] [PubMed] [Google Scholar]
- 170.Schubert M, Holland ND, Laudet V, Holland LZ. A retinoic acid-Hox hierarchy controls both anterior/posterior patterning and neuronal specification in the developing central nervous system of the cephalochordate amphioxus. Dev Biol. 2006;296:190–202. doi: 10.1016/j.ydbio.2006.04.457. [DOI] [PubMed] [Google Scholar]
- 171.Schubert M, Holland ND, Escriva H, Holland LZ, Laudet V. Retinoic acid influences anteroposterior positioning of epidermal sensory neurons and their gene expression in a developing chordate (amphioxus) Proc Natl Acad Sci USA. 2004;101:10320–10325. doi: 10.1073/pnas.0403216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hinman VF, Degnan BM. Retinoic acid disrupts anterior ectodermal and endodermal development in ascidian larvae and postlarvae. Dev Genes Evol. 1998;208:336–345. doi: 10.1007/s004270050189. [DOI] [PubMed] [Google Scholar]
- 173.Katsuyama Y, Saiga H. Retinoic acid affects patterning along the anterior-posterior axis of the ascidian embryo. Dev Growth Differ. 1998;40:413–422. doi: 10.1046/j.1440-169X.1998.t01-2-00006.x. [DOI] [PubMed] [Google Scholar]
- 174.Fujiwara S. Retinoids and nonvertebrate chordate development. J Neurobiol. 2006;66:645–652. doi: 10.1002/neu.20240. [DOI] [PubMed] [Google Scholar]
- 175.Yagi K, Makabe KW. Retinoic acid differently affects the formation of palps and surrounding neurons in the ascidian tadpole. Dev Genes Evol. 2002;212:288–292. doi: 10.1007/s00427-002-0239-y. [DOI] [PubMed] [Google Scholar]
- 176.Rinkevich Y, Paz G, Rinkevich B, Reshef R. Systemic bud induction and retinoic acid signaling underlie whole body regeneration in the urochordate Botrylloides leachi . PLoS Biol. 2007;5:e71. doi: 10.1371/journal.pbio.0050071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sciarrino S, Matranga V. Effects of retinoic acid and dimethylsulfoxide on the morphogenesis of the sea urchin embryo. Cell Biol Int. 1995;19:675–680. doi: 10.1006/cbir.1995.1116. [DOI] [PubMed] [Google Scholar]
- 178.Dmetrichuk JM, Carlone RL, Spencer GE. Retinoic acid induces neurite outgrowth and growth cone turning in invertebrate neurons. Dev Biol. 2006;294:39–49. doi: 10.1016/j.ydbio.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 179.Fields AL, Soprano DR, Soprano KJ. Retinoids in biological control and cancer. J Cell Biochem. 2007;102:886–898. doi: 10.1002/jcb.21530. [DOI] [PubMed] [Google Scholar]
- 180.Wolbach SB, Howe PR. Tissue changes following deprivation of fat-soluble A vitamin. Nutr Rev. 1925;36:16–19. doi: 10.1111/j.1753-4887.1978.tb03675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dragnev KH, Rigas JR, Dmitrovsky E. The retinoids and cancer prevention mechanisms. Oncologist. 2000;5:361–368. doi: 10.1634/theoncologist.5-5-361. [DOI] [PubMed] [Google Scholar]
- 182.Niles RM. Recent advances in the use of vitamin A (retinoids) in the prevention and treatment of cancer. Nutrition. 2000;16:1084–1089. doi: 10.1016/S0899-9007(00)00436-6. [DOI] [PubMed] [Google Scholar]
- 183.Huang ME, Ye YC, Chen SR, Chai JR, Lu JX, Zhoa L, Gu LJ, Wang ZY. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988;72:567–572. [PubMed] [Google Scholar]
- 184.Borrow J, Goddard AD, Sheer D, Solomon E. Molecular analysis of acute promyelocytic leukemia breakpoint cluster region on chromosome 17. Science. 1990;249:1577–1580. doi: 10.1126/science.2218500. [DOI] [PubMed] [Google Scholar]
- 185.de The H, Chomienne C, Lanotte M, Degos L, Dejean A. The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor α gene to a novel transcribed locus. Nature. 1990;347:558–561. doi: 10.1038/347558a0. [DOI] [PubMed] [Google Scholar]
- 186.de The H, Lavau C, Marchio A, Chomienne C, Degos L, Dejean A. The PML-RARα fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell. 1991;66:675–684. doi: 10.1016/0092-8674(91)90113-D. [DOI] [PubMed] [Google Scholar]
- 187.Kakizuka A, Miller WH, Jr, Umesono K, Warrell RP, Jr, Frankel SR, Murty VV, Dmitrovsky E, Evans RM. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RARα with a novel putative transcription factor, PML. Cell. 1991;66:663–674. doi: 10.1016/0092-8674(91)90112-C. [DOI] [PubMed] [Google Scholar]
- 188.Kastner P, Perez A, Lutz Y, Rochette-Egly C, Gaub MP, Durand B, Lanotte M, Berger R, Chambon P. Structure, localization and transcriptional properties of two classes of retinoic acid receptor alpha fusion proteins in acute promyelocytic leukemia (APL): structural similarities with a new family of oncoproteins. EMBO J. 1992;11:629–642. doi: 10.1002/j.1460-2075.1992.tb05095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hummel JL, Zhang T, Wells RA, Kamel-Reid S. The retinoic acid receptor α (RARα) chimeric proteins PML-, PLZF-, NPM-, and NuMA-RARα have distinct intracellular localization patterns. Cell Growth Differ. 2002;13:173–183. [PubMed] [Google Scholar]
- 190.Fenaux P, Chevret S, Guerci A, Fegueux N, Dombret H, Thomas X, Sanz M, Link H, Maloisel F, Gardin C, Bordessoule D, Stoppa AM, Sadoun A, Muus P, Wandt H, Mineur P, Whittaker JA, Fey M, Daniel MT, Castaigne S, Degos L. Long-term follow-up confirms the benefit of all-trans retinoic acid in acute promyelocytic leukemia. European APL group. Leukemia. 2000;14:1371–1377. doi: 10.1038/sj.leu.2401859. [DOI] [PubMed] [Google Scholar]
- 191.Tallman MS, Nabhan C, Feusner JH, Rowe JM. Acute promyelocytic leukemia: evolving therapeutic strategies. Blood. 2002;99:759–767. doi: 10.1182/blood.V99.3.759. [DOI] [PubMed] [Google Scholar]
- 192.Brown D, Kogan S, Lagasse E, Weissman I, Alcalay M, Pelicci PG, Atwater S, Bishop JM. A PMLRARα transgene initiates murine acute promyelocytic leukemia. Proc Natl Acad Sci USA. 1997;94:2551–2556. doi: 10.1073/pnas.94.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Licht JD. Reconstructing a disease: What essential features of the retinoic acid receptor fusion oncoproteins generate acute promyelocytic leukemia? Cancer Cell. 2006;9:73–74. doi: 10.1016/j.ccr.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 194.Seewaldt VL, Johnson BS, Parker MB, Collins SJ, Swisshelm K. Expression of retinoic acid receptor β mediates retinoic acid-induced growth arrest and apoptosis in breast cancer cells. Cell Growth Differ. 1995;6:1077–1088. [PubMed] [Google Scholar]
- 195.Sun SY, Lotan R. Retinoids and their receptors in cancer development and chemoprevention. Crit Rev Oncol Hematol. 2002;41:41–55. doi: 10.1016/S1040-8428(01)00144-5. [DOI] [PubMed] [Google Scholar]
- 196.Castillo L, Milano G, Santini J, Demard F, Pierrefite V. Analysis of retinoic acid receptor β expression in normal and malignant laryngeal mucosa by a sensitive and routine applicable reverse transcription-polymerase chain reaction enzyme-linked immunosorbent assay method. Clin Cancer Res. 1997;3:2137–2142. [PubMed] [Google Scholar]
- 197.Picard E, Seguin C, Monhoven N, Rochette-Egly C, Siat J, Borrelly J, Martinet Y, Martinet N, Vignaud JM. Expression of retinoid receptor genes and proteins in non-small-cell lung cancer. J Natl Cancer Inst. 1999;91:1059–1066. doi: 10.1093/jnci/91.12.1059. [DOI] [PubMed] [Google Scholar]
- 198.Widschwendter M, Berger J, Daxenbichler G, Muller-Holzner E, Widschwendter A, Mayr A, Marth C, Zeimet AG. Loss of retinoic acid receptor β expression in breast cancer and morphologically normal adjacent tissue but not in the normal breast tissue distant from the cancer. Cancer Res. 1997;57:4158–4161. [PubMed] [Google Scholar]
- 199.Berard J, Gaboury L, Landers M, De Repentigny Y, Houle B, Kothary R, Bradley WE. Hyperplasia and tumours in lung, breast and other tissues in mice carrying a RARβ4-like transgene. EMBO J. 1994;13:5570–5580. doi: 10.1002/j.1460-2075.1994.tb06894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wang Z, Boudjelal M, Kang S, Voorhees JJ, Fisher GJ. Ultraviolet irradiation of human skin causes functional vitamin A deficiency, preventable by all-trans retinoic acid pre-treatment. Nat Med. 1999;5:418–422. doi: 10.1038/10577. [DOI] [PubMed] [Google Scholar]
- 201.De Luca LM. Retinoids and their receptors in differentiation, embryogenesis, and neoplasia. FASEB J. 1991;5:2924–2933. [PubMed] [Google Scholar]
- 202.Lee JS, Newman RA, Lippman SM, Huber MH, Minor T, Raber MN, Krakoff IH, Hong WK. Phase I evaluation of all-trans-retinoic acid in adults with solid tumors. J Clin Oncol. 1993;11:959–966. doi: 10.1200/JCO.1993.11.5.959. [DOI] [PubMed] [Google Scholar]
- 203.Reynolds CP. Differentiating agents in pediatric malignancies: retinoids in neuroblastoma. Curr Oncol Rep. 2000;2:511–518. doi: 10.1007/s11912-000-0104-y. [DOI] [PubMed] [Google Scholar]
- 204.Rigas JR, Dragnev KH. Emerging role of rexinoids in non-small cell lung cancer: focus on bexarotene. Oncologist. 2005;10:22–33. doi: 10.1634/theoncologist.10-1-22. [DOI] [PubMed] [Google Scholar]
- 205.So PL, Fujimoto MA, Epstein EH., Jr Pharmacologic retinoid signaling and physiologic retinoic acid receptor signaling inhibit basal cell carcinoma tumorigenesis. Mol Cancer Ther. 2008;7:1275–1284. doi: 10.1158/1535-7163.MCT-07-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yen A, Fenning R, Chandraratna R, Walker P, Varvayanis S. A retinoic acid receptor β/γ-selective prodrug (tazarotene) plus a retinoid X receptor ligand induces extracellular signal-regulated kinase activation, retinoblastoma hypophosphorylation, G0 arrest, and cell differentiation. Mol Pharmacol. 2004;66:1727–1737. doi: 10.1124/mol.104.003475. [DOI] [PubMed] [Google Scholar]
- 207.Kraemer KH, DiGiovanna JJ, Peck GL. Chemoprevention of skin cancer in xeroderma pigmentosum. J Dermatol. 1992;19:715–718. doi: 10.1111/j.1346-8138.1992.tb03766.x. [DOI] [PubMed] [Google Scholar]
- 208.Tanaka T, De Luca LM. Therapeutic potential of “rexinoids” in cancer prevention and treatment. Cancer Res. 2009;69:4945–4947. doi: 10.1158/0008-5472.CAN-08-4407. [DOI] [PubMed] [Google Scholar]
- 209.Tanaka T, Suh KS, Lo AM, De Luca LM. p21WAF1/CIP1 is a common transcriptional target of retinoid receptors: pleiotropic regulatory mechanism through retinoic acid receptor (RAR)/retinoid X receptor (RXR) heterodimer and RXR/RXR homodimer. J Biol Chem. 2007;282:29987–29997. doi: 10.1074/jbc.M701700200. [DOI] [PubMed] [Google Scholar]
- 210.Crowe DL, Chandraratna RA. A retinoid X receptor (RXR)-selective retinoid reveals that RXRα is potentially a therapeutic target in breast cancer cell lines, and that it potentiates antiproliferative and apoptotic responses to peroxisome proliferator-activated receptor ligands. Breast Cancer Res. 2004;6:R546–R555. doi: 10.1186/bcr913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mukherjee R, Davies PJ, Crombie DL, Bischoff ED, Cesario RM, Jow L, Hamann LG, Boehm MF, Mondon CE, Nadzan AM, Paterniti JR, Jr, Heyman RA. Sensitization of diabetic and obese mice to insulin by retinoid X receptor agonists. Nature. 1997;386:407–410. doi: 10.1038/386407a0. [DOI] [PubMed] [Google Scholar]
- 212.Singh Ahuja H, Liu S, Crombie DL, Boehm M, Leibowitz MD, Heyman RA, Depre C, Nagy L, Tontonoz P, Davies PJ. Differential effects of rexinoids and thiazolidinediones on metabolic gene expression in diabetic rodents. Mol Pharmacol. 2001;59:765–773. doi: 10.1124/mol.59.4.765. [DOI] [PubMed] [Google Scholar]
- 213.Benoit G, Altucci L, Flexor M, Ruchaud S, Lillehaug J, Raffelsberger W, Gronemeyer H, Lanotte M. RAR-independent RXR signaling induces t(15;17) leukemia cell maturation. EMBO J. 1999;18:7011–7018. doi: 10.1093/emboj/18.24.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ruchaud S, Duprez E, Gendron MC, Houge G, Genieser HG, Jastorff B, Doskeland SO, Lanotte M. Two distinctly regulated events, priming and triggering, during retinoid-induced maturation and resistance of NB4 promyelocytic leukemia cell line. Proc Natl Acad Sci USA. 1994;91:8428–8432. doi: 10.1073/pnas.91.18.8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Liby K, Royce DB, Risingsong R, Williams CR, Wood MD, Chandraratna RA, Sporn MB. A new rexinoid, NRX194204, prevents carcinogenesis in both the lung and mammary gland. Clin Cancer Res. 2007;13:6237–6243. doi: 10.1158/1078-0432.CCR-07-1342. [DOI] [PubMed] [Google Scholar]
- 216.Wu K, Kim HT, Rodriquez JL, Hilsenbeck SG, Mohsin SK, Xu XC, Lamph WW, Kuhn JG, Green JE, Brown PH. Suppression of mammary tumorigenesis in transgenic mice by the RXR-selective retinoid, LGD1069. Cancer Epidemiol Biomarkers Prev. 2002;11:467–474. [PubMed] [Google Scholar]
- 217.Wu K, Zhang Y, Xu XC, Hill J, Celestino J, Kim HT, Mohsin SK, Hilsenbeck SG, Lamph WW, Bissonette R, Brown PH. The retinoid X receptor-selective retinoid, LGD1069, prevents the development of estrogen receptor-negative mammary tumors in transgenic mice. Cancer Res. 2002;62:6376–6380. [PubMed] [Google Scholar]
- 218.Klopper JP, Sharma V, Berenz A, Hays WR, Loi M, Pugazhenthi U, Said S, Haugen BR. Retinoid and thiazolidinedione therapies in melanoma: an analysis of differential response based on nuclear hormone receptor expression. Mol Cancer. 2009;8:16. doi: 10.1186/1476-4598-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Rendi MH, Suh N, Lamph WW, Krajewski S, Reed JC, Heyman RA, Berchuck A, Liby K, Risingsong R, Royce DB, Williams CR, Sporn MB. The selective estrogen receptor modulator arzoxifene and the rexinoid LG100268 cooperate to promote transforming growth factor β-dependent apoptosis in breast cancer. Cancer Res. 2004;64:3566–3571. doi: 10.1158/0008-5472.CAN-04-0234. [DOI] [PubMed] [Google Scholar]