Abstract
Evidence strongly suggests that excessive or protracted signaling, or both, by cell-surface or intracellular innate immune receptors is central to the pathogenesis of most autoimmune and autoinflammatory rheumatic diseases. The initiation of aberrant innate and adaptive immune responses in autoimmune diseases can be triggered by microbes and, at times, by endogenous molecules—particularly nucleic acids and related immune complexes—under sterile conditions. By contrast, most autoinflammatory syndromes are generally dependent on germline or de novo gene mutations that cause or facilitate inflammasome assembly. The consequent production of proinflammatory cytokines, principally interferon-α/β and tumor necrosis factor in autoimmune diseases, and interleukin-lβ in autoinflammatory diseases, leads to the creation of autoamplification feedback loops and chronicity of these syndromes. These findings have resulted in a critical reappraisal of pathogenetic mechanisms, and provide a basis for the development of novel diagnostic and therapeutic modalities for these diseases.
Introduction
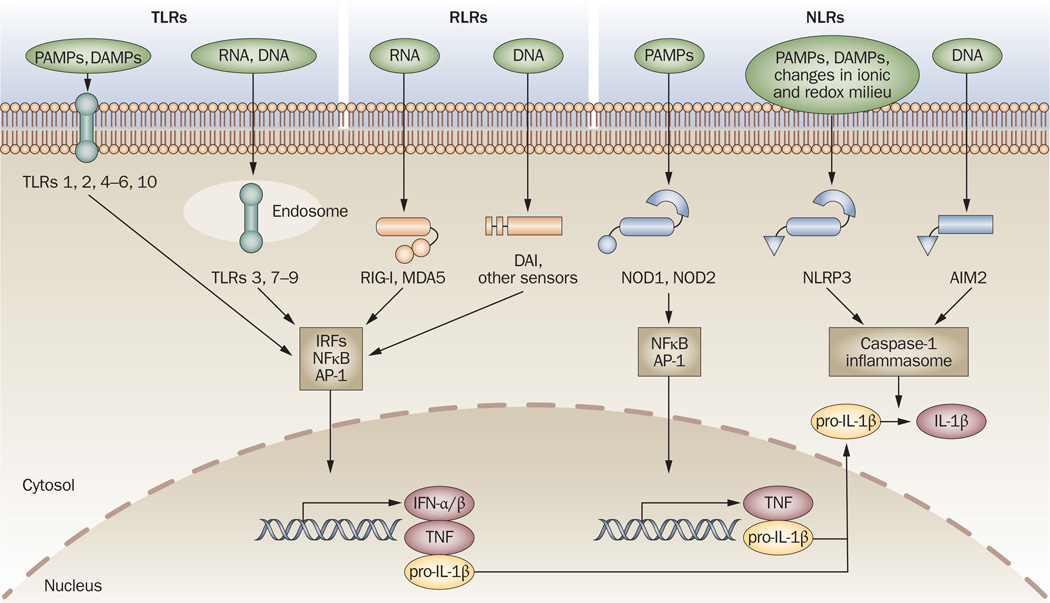
Mammalian cells sense danger signals, derived from both pathogens and damaged cells, through a collection of receptors expressed on cell membranes, in the cytoplasm and in endolysosomal compartments. Three main classes of innate immune sensors—Toll-like receptors (TLRs), retinoid acid inducible gene (RIG)-I-like receptors (RLRs), and nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRs)—are present in eukaryotes; their composition, ligands and downstream signaling pathways are reviewed elsewhere.1 The ultimate result of engaging these receptors is cellular activation and the subsequent production of proinflammatory cytokines, including type I interferons (IFN-α/β), tumor necrosis factor (TNF) and interleukin (IL)-1β, all of which contribute to the elimination of infection (Figure 1).
Figure 1.
Innate immune sensors. TLRs, RLRs (for example, RIG-I, MDA5, DAI and other sensors) and NLRs (for example, NODI, N0D2, NLRP3 and AIM2) are innate immune sensors that recognize danger signals derived from pathogens (PAMPs), damaged cells (DAMPs) or associated nucleic acids at the cell surface, in endolysosomes or in the cytoplasm. Signaling by these sensors promotes either the activation and nuclear translocation of transcription factors (IRFs, NFκB and AP-1) that drive expression of cytokines (IFN-α/β, TNF and pro-IL-lβ), or the assembly of the caspase-1 inflammasome and subsequent maturation of IL-1β from pro-IL-1β. Abbreviations: AIM2, absent in melanoma 2; AP-1, activator protein 1; DAMP, danger-associated molecular pattern; IFN, interferon; IL-1β, interleukin-1β; IRFs, interferon regulatory factors; MDA5, melanoma differentiation-associated gene-5; NFκB, nuclear factor κB; NLR, NOD-like receptor; NLRP, NLR with a pyrin domain; NOD, nucleotide-binding and oligomerization domain; PAMP, pathogen-associated molecular pattern; RIG-I, retinoid acid-inducible gene-l; RLR, RIG-l-like receptor; TLR, Toll-like receptor; TNF, tumor necrosis factor.
Although these responses have evolved to be beneficial—and, in most cases, they are—defective regulation might result in autoimmune or autoinflammatory diseases, many of which present with rheumatic manifestations. The major issues with regard to autoimmune diseases, such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA), center on the nature and relative contribution of endogenous versus exogenous stimuli, the abnormalities that override the normal discrimination between self and foreign antigens, and the interplay between innate and adaptive immune systems. By contrast, for most autoinflammatory diseases, the focus is on defining the exact genetic mutations that either provoke or permit uncontrolled activation of the innate immune system. As reviewed in this article, important advances towards resolving these issues have already been made and have had a considerable impact on disease diagnosis and the design of novel therapies.
Autoimmune diseases
Autoimmune rheumatic diseases, which can be caused by both genetic and environmental factors, are considered among the most intractable groups of disorders. Although impressive advances have been made in defining their immunopathogeneses, the exact triggers of autoimmunity have only emerged gradually over the past few years. Strong evidence that signaling through the innate sensors is often a prerequisite for destructive adaptive immune responses has now provided a new dimension for understanding autoimmune rheumatic diseases.
Systemic lupus erythematosus
SLE, the prototypic systemic autoimmune disease, is characterized by multiorgan involvement and the presence of autoantibodies, primarily against nuclear and cytoplasmic materials that contain RNA or DNA, or both. Although defects in mechanisms of B-cell and T-cell tolerance have been identified, discoveries relating to the biology of TLRs have provided a more-comprehensive understanding of the mechanisms by which this disease is initiated in genetically predisposed individuals.
Sensing of self nucleic acids in autoimmunity
Because indiscriminate recognition of endogenous (self) nucleic acids would be detrimental, a number of preventive mechanisms have evolved. First, free self nucleic acids are rapidly degraded by extracellular and intracellular nucleases (in contrast to viral nucleic acids, which are protected from nuclease-mediated degradation by a capsid). Self nucleic acids are also excluded from endolysosomes, where engagement of TLR3 (specific for double-stranded [ds]RNA), TLR7 or TLR8 (single-stranded [ss]RNA) and TLR9 (dsDNA) occurs. Furthermore, mammalian nucleic acids are methylated, which might decrease their recognition by TLRs.2 Finally, processed mammalian RNA is capped, in contrast to viral RNA, which retains exposed 5’ triphosphates required to engage cytosolic helicases.3
These mechanisms, however, are not infallible and can be breached, leading to direct or indirect stimulation of antigen-presenting dendritic cells (DCs) and autoreactive T cells and B cells (Figure 2). Early research identified two major pathways by which these barriers can be overcome in SLE. The first evidence of a potential link between TLR-induced immune activation and SLE came from studies by Ronnblom and associates,4 who showed that immune complexes comprising apoptotic or necrotic cell materials and SLE IgG could induce IFN-α following FcγRIIa-mediated uptake by plasmacytoid DCs (pDCs), although no connection between this effect and TLRs that recognize nucleic acids was made at the time. Subsequently, Marshak-Rothstein and associates5 observed that SLE-associated complexes of autoantibodies and chromatin promote B-cell proliferation through the combined engagement of B-cell receptors (BCR) with rheumatoid factor (RF) activity and, after phagocytosis and entry into endolysosomes, TLR9. Additional studies showed that pDC production of type I IFNs was attenuated when immune complexes of SLE IgG and apoptotic or necrotic materials were treated with RNase,6 which indicated a potential role for TLR7 or TLR8 in mediating this response. Subsequently, engagement of TLR7 or TLR8 was directly demonstrated using small nuclear ribonucleoprotein (snRNP), a major SLE autoantigen that contains uracil-rich small nuclear RNA (snRNA), complexed with specific autoantibodies and delivered to endolysosomes (reviewed elsewhere7,8). DNA-containing or chromatin-containing immune complexes also induced TLR9-dependent cytokine production by pDCs.9
Figure 2.
Breaching barriers for TLR recognition of self nucleic acids in systemic autoimmunity. Autoantibodies and autoreactive BCRs (for example, RFand anti-snRNP or anti-DNA antibodies) mediate the access of self nucleic acids to endolysosomal TLRs, leading to the production of type I IFNs from pDCs and autoantibodies of corresponding specificities from B cells. Accessory proteins such as HMGB1 (probably through RAGE) and LL37 can also facilitate DNA uptake and TLR engagement. The major snRNP antigen for anti-snRNP autoantibodies contains snRNA (red) and proteins (particle in yellow), whereas the antigenic targets of anti-DNA autoantibodies are apoptotic or necrotic materials containing DNA (blue), RNA (red) and other nucleic acid-binding molecules (particle in purple); accordingly, anti-RNP B cells are activated by TLR7 ligands, whereas anti-DNA or RF-producing B cells are activated by either TLR9 or TLR7 ligands. Abbreviations: BCR, B-cell receptor; FcγR, Feγ receptor; HMGB1, high mobility group box 1; IFN, interferon; pDC, plasmacytoid dendritic cell; RAGE, receptor for advanced glycosylation end-products; RF, rheumatoid factor; snRNA, small nuclear RNA; snRNP, small nuclear ribonucleoprotein; TLR, Toll-like receptor.
The uptake of nucleic acid–autoantibody complexes by pDCs and B cells might be facilitated by certain accessory molecules, such as the high mobility group box 1 protein (HMGB1), a nuclear DNA-binding protein released from necrotic or cytokine-stimulated cells that interacts with the receptor for advanced glycosylation end-products (RAGE).10 DNA uptake, TLR9 engagement and IFN-α/β production by pDCs are also promoted by LL37, an antimicrobial cathelicidin peptide released following skin injury11 The release of LL37 and subsequent induction of IFN-α/β has been implicated in the pathogenesis of psoriasis, and potential involvement in skin manifestations of SLE might also be considered. Thus, the uptake of immune complexes through FcγRs, BCRs or other accessory receptors facilitates the trafficking of self nucleic acids to endolysosomal compartments, leading to TLR engagement.
Evidence for the involvement of TLRs in SLE
Lupus-prone MRL-Faslpr mice that lack TLR9 showed considerable decreases in autoantibody titers to dsDNA, as measured by the highly specific Crithidia luciliae staining assay rather than the less-specific enzyme-linked immunosorbent assay.12 However, results from additional studies using this strain and other lupus-predisposed strains suggest that TLR9 suppresses disease (reviewed elsewhere7,8). Although the definitive reason for this suppression is unknown, insights from studies of TLR trafficking strongly indicate that a deficiency in TLR9 might lead to increased transport of TLR7 to endolysosomes, with the consequence of increased sensing of RNA-containing particles. This possibility stems from the finding that transport of both TLR7 and TLR9 to endolysosomes is mediated by the endoplasmic reticulum protein Unc93bl (see below). The ‘disease-protective’ effect of TLR9 might, therefore, be misleading, and both TLR9 and TLR7 might contribute to the overall disease, with TLR7 potentially having a greater impact.
Indeed, TLR7-deficient MRL-Faslpr mice show a considerable decrease in the levels of anti-Sm autoantibodies and a modest reduction in renal disease.12 The participation of TLR7 signaling in SLE has been clearly demonstrated in male BXSB mice bearing the Y-linked autoimmune accelerating (Yaa) locus. BXSB mice spontaneously develop a lupus-like syndrome; acceleration of disease in male BXSB mice has been attributed to an X to Y chromosomal translocation that causes duplication of Tlr7 and 16 other genes.13,14 Importantly, the Yaa SLE-like disease phenotype was abolished by a reduction of the Tlr7 gene dosage by half, whereas systemic autoimmunity developed to varying degrees in normal background transgenic mice with at least a 4-fold increase in the expression of TLR7 mRNA15 However, mice carrying both the BXSB Y chromosome and a Tlr7-null mutation on the X chromosome exhibited only a partial reduction in disease, implying contributions from other genes within the translocated segment.16,17 Furthermore, normal background mice consomic for the BXSB Y chromosome did not develop disease, nor did crosses between BXSB males and normal mice, unlike the disease-developing crosses between BXSB males and other lupus-predisposed strains.18 Therefore, a modest increase in TLR7 expression is insufficient to induce disease, but can act as an enhancer when complemented with additional lupus-predisposing abnormalities, as expected in this polygenic disorder.
Additional compelling evidence that recognition of self nucleic acids by endolysosomal TLRs has an important role in lupus pathogenesis has come from studies using C57BL/6-Faslpr and BXSB mice congenic for the 3d mutation of the Unc93bl gene; this mutation impairs signaling via TLR3, TLR7 and TLR9.19 Unc93bl encodes an endoplasmic reticulum (ER)-resident protein that physically associates with these TLRs and is required for their trafficking from the ER to endolysosomes, where they encounter cognate nucleic acid ligands (Figure 3).20 The 3d mutation markedly reduced all overt parameters of the disease, including the levels of antinuclear autoantibodies (ANAs) and IgM RF, lymphoproliferation, glomerulonephritis and mortality. Moreover, this requirement for nucleic-acid-sensing TLRs could not be substituted for by lipopolysaccharide-mediated TLR4 engagement and induction of IFN-α/β. The results definitively demonstrate that endosomal TLRs are necessary for the generation of autoantibodies to nucleic-acid-containing materials, and even the production of RFs, and, in conjunction with the aforementioned studies, explain the dominant presence of ANAs in SLE.
Figure 3.
Trafficking of nucleic-acid-sensing TLRs. The ER-resident protein Unc93bl physically associates with TLR3, TLR7, TLR8 and TLR9 in the ER and mediates the trafficking of these receptors to endolysosomes. Efficient signaling by some of these TLRs might require proteolytic cleavage of a portion of the LRR ectodomain by lysosomal cathepsins and other proteases. Bacteria, viruses and other materials enter the cell through the endocytic pathway. Phagosomes and endosomes undergo interconnected maturation and fuse with lysosomes, giving rise to phagolysosomes or endolysosomes, acidic organelles that are rich in hydrolytic enzymes, in which degradation of the engulfed materials occurs. TLR engagement by the released nucleic acids leads to activation of transcription factors and expression of genes encoding cytokines. Abbreviations: ER, endoplasmic reticulum; LRR, leucine-rich repeat; TLR, Toll-like receptor.
IFNs as pathogenetic effectors in SLE
As noted earlier, an important consequence of TLR engagement by nucleic acids is the production of type I IFNs (IFN-α/β), especially by pDCs. These pleiotropic cytokines affect almost every aspect of innate and adaptive immune responses, and strong evidence suggests that they are critically involved in the pathogenesis of systemic autoimmunity (reviewed elsewhere8,21). Indeed, patients with SLE show increased levels of type I IFNs in serum and increased expression of IFN-inducible genes (the ‘interferon signature’) in both peripheral blood cells and affected kidneys, frequently correlating with disease flares. In addition, immune complexes of nuclear or ribosomal extracts together with cerebrospinal fluid IgG from SLE patients with neuropsychiatric manifestations potently induced the production of IFN-α by pDCs,22 implying that IFN-α is involved in this adverse complication.
Further support for the role of type I IFNs in human SLE is the association of this disease, across ethnic groups, with certain alleles of IRF5, STAT4, TYK2 and IRAKI, as well as the occurrence of trisomy of the type I IFN gene cluster, in a few patients with SLE.23–25 Administration of ligands of TLR3, TLR4, TLR7 or TLR9 (all of which induce type I IFNs), recombinant IFN-α or a plasmid encoding this cytokine also exacerbated autoimmunity in predisposed mice, whereas deficiency of the IFN-α/β receptor in some (but not all) spontaneous models of lupus considerably reduced disease severity.8 Pristane-induced lupus in mice also depends on type I IFN production mediated by IRF9, STAT1 and enhanced TLR7 signaling.26 Moreover, deficiency in components that negatively regulate TLR signaling and type I IFN production (for example, the TAM [Tyro3, Axl, Mer] family of receptor tyrosine kinases or the single Ig IL-1 receptor [IL-R]-related molecule [SIGIRR]) promotes murine lupus.27,28 Although type I IFNs are major initiators of systemic autoimmune responses, once a broad spectrum of cells of the innate and adaptive branches of the immune system is activated, additional inflammatory cytokines and chemokines are produced, including type II IFN (IFN-γ), which is also a major pathogenetic effector in murine lupus.29,30
Defective clearance of apoptotic materials
Despite the evidence that nucleic acids complexed with IgG autoantibodies promote systemic autoimmunity through TLR-dependent type I IFN production, apoptotic materials themselves might also induce inflammatory cytokines and autoimmune responses. A number of observations support this possibility. First, it has been shown that dying cells taken up by conventional DCs trigger TLR-independent type I IFN production and adaptive immune responses.31 Second, human SLE is characterized by the inefficient removal of apoptotic materials,32 a defect also noted in humans and certain mouse strains deficient in the complement factor Clq33 or the TAM receptor protein tyrosine kinases,27 which develop lupus manifestations. Furthermore, injection of apoptotic cells or apoptosis-promoting agents in normal or lupus mice induced autoantibody production and kidney disease.34,35 Finally, disease was reduced in lupus-predisposed mice when clearance of apoptotic cells was increased by opsonization with adiponectin, a calreticulin-binding adipokine.36 Hence, apoptotic materials are likely to provide not only autoantigenic cargo, but also ligands that can induce proinflammatory cytokines.
Non-TLR sensors in SLE
RLRs and NLRs that sense cytosolic nucleic acids and other ‘danger’ molecules might also precipitate clinical manifestations of SLE. Thus, viral 5’-triphosphate RNA (which is recognized by RIG-I) aggravated lupus nephritis in MRL-Faslpr mice by increasing IFN signaling and decreasing the number of CD4+CD25+ regulatory T cells.37 Moreover, transient exposure of these mice to non-CpG DNA (a potential ligand for cytosolic DNA sensors) exacerbated glomerulonephritis, splenomegaly, lymphoproliferation and hypergammaglobulinemia.37 Inflammatory responses might also be triggered by DNA that has been incompletely digested either extracellularly by DNase I or intracellularly by DNase II, as inferred by the existence of a few SLE patients with function-impairing DNase I mutations,38 lupus development in mice deficient in DNase I,39 and anemia or arthritis in mice deficient in DNase II.40 Moreover, the 3’ repair exonuclease Trexl is mutated in patients with autoimmune Aicardi–Goutieres syndrome and chilblain lupus, which exhibit some clinical and biochemical overlaps with SLE. Trexl is known to metabolize reverse-transcribed RNA and to negatively regulate I FN-stimulatory DNA responses. Interestingly, Trexl-deficient cells accumulate ssDNA derived from endogenous retroelements, revealing a potentially novel mechanism for the initiation of systemic autoimmunity by cytosolic DNA.41 Mutations in SAM domain and HD domain protein 1 (SAMHD1), another negative regulator of innate immune responses, have also been shown to be associated with Aicardi–Goutières syndrome.42
Evidence for potential contributions of NLR-mediated induction of inflammasomes in SLE flares associated with sun exposure comes from the finding that UVB irradiation of keratinocytes induced IL-1β secretion.43 IL-1β levels are also increased in most mouse lupus models, and recombinant IL-1β aggravated nephritis in NZB/W mice, whereas treatment with a soluble IL-1 receptor reduced disease in MRL-Faslpr mice.44 Moreover, levels of IL-18, another inflammasome-induced cytokine, were increased in sera of MRL-Faslpr mice, and daily injections of this cytokine accelerated kidney pathology, whereas vaccination with pro-IL-18 to induce the generation of blocking autoantibodies conferred a protective effect.45
Absent in melanoma 2 (AIM2), a member of the hematopoietic IFN-inducible nuclear protein HIN-200 family, has been shown to be a sensor for cytoplasmic dsDNA and to trigger the assembly of a novel inflamma-some that causes IL-1β maturation (reviewed elsewhere1). Notably, p202, another DNA-binding member of the HIN-200 family, is hyperexpressed in lymphoid cells of lupus-predisposed NZB mice and has been suggested to be a disease-contributing factor in this model.46 However, p202 has no known ortholog in humans, cannot form an inflammasome owing to the lack of a pyrin domain, and functionally inhibits the AIM2–DNA interaction; these findings raise questions about the mechanisms by which hyperexpression of p202 promotes murine lupus.
SLE initiation and amplification
From the evidence outlined above, we formulated a two-phase paradigm to explain the interconnected contributions of the innate and adaptive immune systems in SLE pathogenesis.8 The first phase can be mediated by TLR-independent or TLR-dependent pathways triggered by endogenous or exogenous agents that result in the initial production of type I IFNs, activation of DCs, and engagement of previously quiescent non-deleted or non-edited autoreactive T cells and B cells. Once autoantibodies are produced, the second amplification phase is initiated, in which nucleic acids and related subcellular particles (for example, ribonucleoproteins, nucleosomes) complexed with autoantibodies are taken up by conventional DCs and pDCs through FcR and by B cells through BCRs, leading to the engagement of nucleic-acid-sensing endolysosomal TLRs, enhanced immunocyte activation, inflammatory cytokine production, spreading of the autoimmune response and, eventually, disease (Figure 4).
Figure 4.
Initiation and amplification stages in systemic autoimmunity. The pathogenesis of SLE might follow a two-phase process. In the first, initiation, phase (1), apoptotic or microbial materials, or both, induce TLR-independent or TLR-dependent production of type I IFNs by immature DCs, leading to DC maturation and efficient presentation of self-antigens, engagement of autoreactive T cells and B cells, and production of autoantibodies. In the second, self-sustaining amplification, phase (2), autoantibodies complexed with nucleic-acid-containing particles (RNP apoptotic or necrotic materials) are taken up by pDCs (via FcγR) and B cells (via BCR), leading to enhanced TLR-dependent type I IFN production, secretion of B-cell trophic factors, Ig class switching and perpetuation of the autoimmune response. Abbreviations: BCR, B-cell receptor; DC, dendritic cell; FcγR, Feγ receptor; IFN, interferon; pDC, plasmacytoid dendritic cell; RNP, ribonucleoprotein; SLE, systemic lupus erythematosus; TLR, Toll-like receptor.
Microbial triggers in systemic autoimmunity
It is well known that SLE flares are frequently associated with, or follow, viral infections, and Epstein–Barr virus (EBV) is considered a major environmental risk factor for this disease. Moreover, disease is enhanced in predisposed mice infected with certain viruses or given microbial or synthetic TLR ligands.8,18 Although viruses might enhance autoimmunity by inducing immune responses that crossreact with self-antigens, engagement of sensors of the innate immune system might be an alternative or additive contributor.
Indeed, in certain cases, a microbial stimulus might be necessary to trigger systemic inflammatory diseases, as exemplified in mice with a hypomorphic mutation of the Ptpn6 gene (termed ‘spin’ for spontaneous inflammation) induced by N-ethyl-N-nitrosourea.47 Ptpn6 encodes the Src-homology-2-domain-containing protein tyrosine phosphatase SHP-1, which downregulates signaling from TCRs, BCRs, TLRs, integrins, and receptors for several cytokines and chemokines. Spontaneous null and hypomorphic mutations of Ptpn6 are responsible for the classical motheaten (me) and the less-severe viable motheaten (mev) phenotypes. Homozygous spin-mutant mice displayed chronic inflammatory lesions affecting the feet, salivary glands and lungs, as well as anti-chromatin antibodies. Notably, while deficiency in components of IL-1R signaling (MyD88, IRAK4 or IL-1R) attenuated disease in spin mice, neither STAT1 deficiency (that is, no type I or type II IFN signaling) nor TNF deficiency had any effect, indicating that IL-1β is the primary effector cytokine in this model. Of great interest, this inflammatory pathology was not observed when spin mice were derived and bred in a germ-free environment, but disease emerged upon transfer to a conventional pathogen-free environment.47 These findings indicate that microbial stimuli, even from mucosal microbiota, can trigger or enhance autoimmune diseases in predisposed individuals by a TLR–MyD88–IRAK4-mediated induction of pro-IL-1β followed by inflammasome-mediated maturation and secretion of IL-1β. Binding of this cytokine to its receptor leads to autoamplification and perpetuation of the autoimmune or inflammatory process.48
Rheumatoid arthritis
TLR-mediated activation of macrophages and fibroblasts might be an important primary or secondary contributor to the pathogenesis of RA. So far, however, substantiating such an association in humans has proved difficult; stronger evidence has been obtained from animal models of spontaneous or induced arthritides.
Early studies showed that stimulation of synovial fibroblasts with lipopolysaccharide, lipopeptides or peptidoglycan resulted in the upregulation of TLR2 and the production of proinflammatory cytokines, metallo-proteinases and chemokines—a profile similar to that in RA synovial fluid.49,50 In addition, CD16+ monocytes from the peripheral blood of RA patients produced TNF, IL-1, IL-6 and IL-8 on TLR2 stimulation, and macrophages with this phenotype are localized in the synovial lining layer.51 TLR3, TLR4, TLR7 and type I IFNs are also upregu-lated in the RA synovium, suggesting that a broader set of exogenous and/or endogenous ligands (especially those in synovial fluid) might provoke the induction of proinflammatory mediators and DC activation.52 Moreover, inhibitors of TLR8 reduced TNF production in RA synovial cultures.53
TLR involvement in autoimmune arthritis has been suggested by results from transgenic, knockout and induced models. As noted earlier, B cells that express BCRs with RF activity can be activated by the uptake of nucleic acid-antibody immune complexes and engagement of TLRs.7,8,19,54 Studies in the K/BxN arthritis model, which is mediated by anti-glucose-6-phosphate isomerase antibodies, also showed that lack of tolerance and subsequent T-cell help required efficient presentation of glucose-6-phosphate isomerase by TLR-stimulated B cells,55 and that synovitis in these animals was dependent on MyD88 and IL-1R signaling.56 Moreover, TLR4 deficiency or treatment with TLR4 antagonists suppressed disease in the collagen-induced model of arthritis, and deficiency in MyD88 or TLR2 reduced disease in streptococcal-cell-wall-induced arthritis.57 Spontaneous arthritis in mice that lack the IL-1R antagonist (IL-IRa) was also reduced in the absence of TLR4.58 Interestingly, IL-lRa-deficient mice required a microbial trigger for disease initiation, as do SKG mice, which carry a mutation in the gene encoding the protein kinase ZAP-70 and are prone to develop autoimmune arthritis.59 The involvement of nucleic-acid-sensing TLRs was also inferred by the finding that pharmacologic inhibition or targeted disruption of cathepsin K resulted in defective TLR9 signaling and attenuated autoimmune adjuvant-induced arthritis in rats, suggesting a novel therapeutic approach for human RA and other diseases mediated by endolysosomal TLRs.60 This finding is compatible with the requirement for cleavage of a portion of the TLR9 leucine-rich repeat (LRR) ectodomain by cathepsins and other proteases in endolysosomes for efficient signaling.61,62
Inefficient digestion of microbial or self DNA in macrophages might also lead to TLR-independent signaling and the induction of proinflammatory cytokines (for example, IFN-β, TNF) and autoimmunity, as suggested by the development of RA-like manifestations in DNase Il-deficient mice.40,63 These findings are consistent with earlier studies showing that injection of bacterial or mitochondrial DNA into joints induced arthritis mediated by macrophage-produced TNF,64,65 and that a polymorphism in the 5’-regulatory region of the gene encoding DNase II confers increased risk for RA.66
Several endogenous molecules in the synovial fluid of RA patients (for example, heat shock proteins, HMGB1, fibrinogen, fibronectin extra domain A, hyaluronan and nucleic acids) have also been reported to engage TLRs and might contribute to inflammation.67 Tenascin C, an extracellular matrix glycoprotein expressed in inflamed joints, induced TLR4-dependent production of proinflammatory cytokines by macrophages and fibroblasts isolated from RA synovia. Moreover, intra-articular injection of tenascin C promoted joint inflammation in mice, while its deficiency resulted in resolution of inflammation and protection from erosive arthritis in mouse models.68
Other autoimmune diseases
Salivary gland epithelial cells from patients with primary Sjogren’s syndrome show high constitutive or induced expression of TLR1, TLR2, TLR3, TLR4 and TLR7.69–71 These cells also show high levels of production of IFN-α and expression of genes encoding proteins in the IFN pathway. Moreover, complexes of snRNPs with autoantibodies and dsRNA viruses potently induced IFN-α in salivary-gland-infiltrating pDCs.72,73 These findings suggest that TLR and type I IFN signaling, perhaps induced by viral infection, might contribute to inflammatory responses in this disease.74 TLRs and type I IFNs have also been implicated in other rheumatic diseases with a potential autoimmune basis, including psoriatic arthritis,11,75 dermatomyositis76 and polymyositis.77
Autoinflammatory diseases
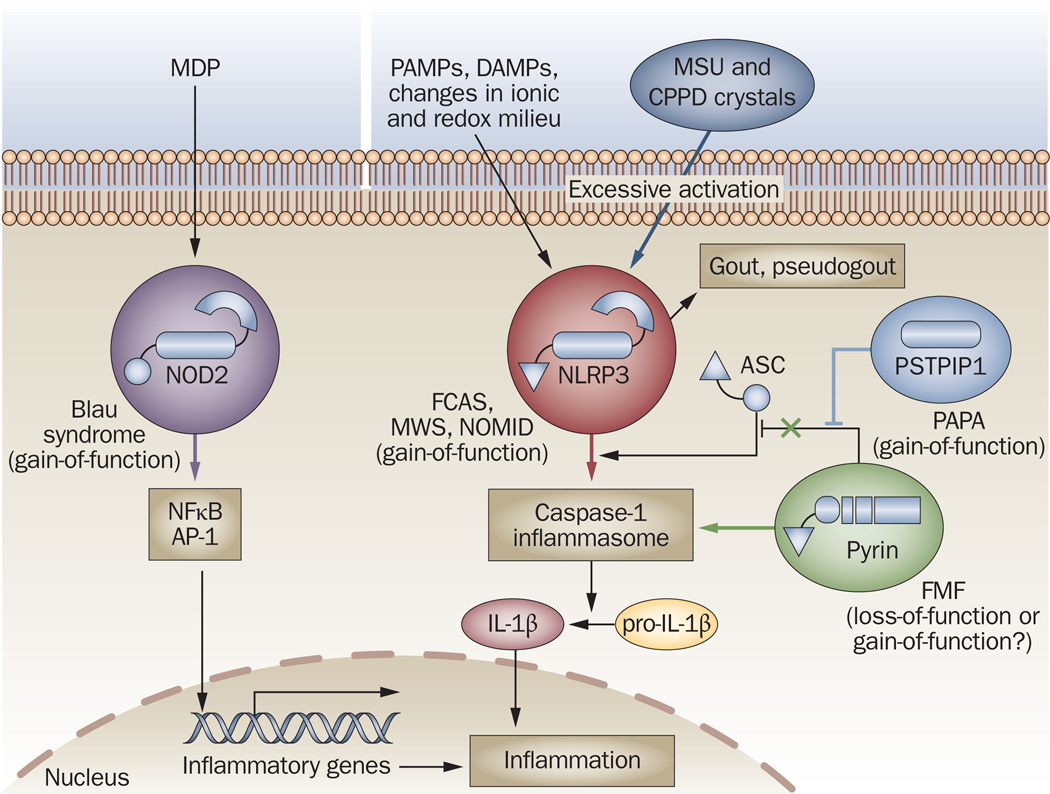
Unlike autoimmune diseases, in which pathogenesis explicitly requires the adaptive immune system, over-activation of the innate immune system alone is sufficient to induce ‘autoinflammatory’ diseases.78 Although some of these disorders might have complex modes of inheritance, they are frequently caused by germline or de novo mutations in the NLR signaling pathway, resulting in spontaneous and uncontrollable production of IL-1β (Figure 5), although exogenous or endogenous stimuli might provoke such responses. Below, we briefly describe those syndromes that might fall within the scope of rheumatology.
Figure 5.
Molecular basis of autoinflammatory diseases. Mutations in several components of the NLR signaling pathways promote uncontrollable spontaneous or ligand (MDP, PAMP, DAMP)-induced activation of either transcription factors (NFκB, AP-1) or the caspase-1 inflammasome, leading to excessive production of IL-1β and inflammation. Gene alterations identified so far result in gain-of-function mutations of NOD2 (in Blau syndrome), NLRP3 (in FCAS, MWS and NOMID) or PSTPIP1 (in PAPA), and probably loss-of-function mutations in pyrin (in FMF), which lead to inefficient sequestration of ASC, an adaptor required for inflammasome assembly. In addition, activation of NLRP3 by MSU and CPPD crystals is associated with the pathogenesis of gout and pseudogout, respectively. Abbreviations: AP-1, activator protein 1; ASC, apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (CARD); CPPD, calcium pyrophosphate dihydrate; DAMP, danger-associated molecular pattern; FCAS, familial cold autoinflammatory syndrome; FMF, familial Mediterranean fever; IL-1β, interleukin-1β; MDP, muramyl dipeptide; MSU, monosodium urate; MWS, Muckle– Wells syndrome; NFκB, nuclear factor κB; NLR, NOD-like receptor; NLRR, NLR with a pyrin domain; NOD, nucleotide-binding and oligomerization domain; NOMID, neonatal onset multisystem inflammatory disease; PAPA, pyogenic arthritis with pyoderma gangrenosum and acne; PAMP, pathogen-associated molecular pattern; PSTPIP1, proline–serine–threonine–phosphatase-interacting protein 1.
Blau syndrome
Mutations in the NOD2 (CARD 15) gene that affect the LRR domain were initially identified as susceptibility traits in Crohn’s disease.79,80 Concurrently, distinct gain-of-function missense mutations that affect the NACHT domain of this gene, leading to increased basal activation of nuclear factor κB (NFκB), were shown to be associated with Blau syndrome,81 a rare autosomal-dominant disorder characterized by the triad of granulomatous arthritis, uveitis and skin rash with camptodactyly. Interestingly, similar NOD2 missense mutations are also associated with early onset sarcoidosis, which might explain some overlapping clinical features between these syndromes.82
Cryopyrinopathies
Cryopyrinopathies (also referred to as hereditary periodic fever syndromes or cryopyrin-associated periodic syndromes) are prototypic autoinflammatory diseases that mostly manifest at birth or in early infancy and encompass three related disorders. The mildest, familial cold autoinflammatory syndrome (FCAS), presents with cold-induced fevers, pseudourticarial skin rash and other generalized symptoms. Muckle–Wells syndrome (MWS), which is of intermediate severity and unrelated to cold exposure, manifests as fevers, hives, sensorineural hearing loss and arthritis. The most severe syndrome, neonatal onset multisystem inflammatory disease (NOMID, also known as chronic infantile neurologic cutaneous articular syndrome), presents with fever, urticaria, epiphyseal overgrowth of the long bones and chronic aseptic meningitis.
Cryopyrinopathies are caused by autosomal-dominant or de novo gain-of-function mutations in the protein NLRP3 (also known as NALP3, cryopyrin or CIAS1).83–85 More than 60 disease-associated mutations have been identified to date; most cluster in the NACHT domain and its flanking region, suggesting they might alter the function of this domain and potentially promote spontaneous oligomerization, inflammasome assembly and IL-1β secretion. Some correlation between genotype and disease severity has been reported, but additional genetic or environmental factors are likely to contribute to disease expression. Moreover, a considerable proportion of patients with NOMID and a few with FCAS or MWS lack NLRP3 mutations, suggesting that other proteins involved in IL-1β production might be affected. Consequently, a recombinant nonglycosylated homolog of the IL-IRa (anakinra), IL-1R fused to IgGlFc (rilonacept) and a human anti-IL-lβ antibody (cana-kinumab) have shown remarkable effectiveness in treating these syndromes—even in some patients without NLRP3 mutations.78–86
Familial Mediterranean fever and PAPA
Familial Mediterranean fever (FMF) is a periodic systemic autoinflammatory disease caused by mutations in the pyrin-encoding MEFV gene.78 Apparently unprovoked attacks of FMF, usually lasting 1–3 days and involving fever, abdominal or chest pain, monoarticular arthritis and erysipeloid rash, are associated with massive recruitment of polymorphonuclear leukocytes in the affected regions, neutrophilia and an acute-phase response. Some patients might also develop systemic amyloidosis, leading to kidney failure. Pyrin is composed of an N-terminal pyrin domain (PYD), a B-box zinc-finger domain, a coiled-coil domain and a C-terminal PrySpry domain. Pyrin is primarily expressed in neutrophils, eosinophils, monocytes and DCs but not in lymphocytes, and is also present in synovial, peritoneal and skin-derived fibroblasts.
The exact mechanisms by which pyrin mutations cause FMF and inflammation have not been fully elucidated, but current evidence is compatible with two possible interpretations. The first is that wild-type pyrin normally acts as a negative regulator of inflammasomes by sequestering the apoptosis-associated speck-like protein (ASC) adaptor through cognate PYD association, or by directly inhibiting caspase-1 through the PrySpry domain (in which most of the FMF-associated missense mutations are localized)87–89; in this scenario, the disease-associated mutations result in a loss of function and are recessive, the most common form of inheritance. Alternatively, wild-type pyrin might itself form an inflammasome complex, implying that the mutations are gain-of-function and are inherited dominantly.90 In addition, pyrin associates with microtubules and colocalizes with actin filaments, and might normally participate in regulating inflammatory responses at the level of leukocyte cytoskeletal organization.91 This function might be affected by FMF-associated mutations, providing a potential explanation for why the microtubule-destabilizing agent colchicine is the drug of choice in FMF, although anakinra might comprise a supplemental therapy in some patients.
Another autosomal-dominant disorder associated with compromised pyrin function is the pyogenic arthritis with pyoderma gangrenosum and acne (PAPA) syndrome. This rare condition is characterized by recurrent inflammatory episodes that eventually cause purulent skin lesions and cystic acne, an accumulation of sterile, pyogenic, neutrophil-rich materials in synovial fluid, and erosive destructive forms of arthritis. PAPA is caused by mutations in the gene encoding proline-serine-threonine-phosphatase-interacting protein 1 (PSTPIP1), which binds pyrin through its Src-homology-3 domain and participates in actin reorganization.92,93 These mutations seem to interfere with the dephosphorylation of PSTPIP1 by an interacting tyrosine phosphatase, resulting in increased binding to, and sequestration of, pyrin by the mutant PSTPIP1; this mechanism is compatible with pyrin acting as an inhibitor of inflammation. Additional pathophysiologic effects of the mutant PSTPIP1 might extend to the adaptive immune system, as this protein has been shown to interact with several T-cell molecules (Wiskott–Aldrich syndrome protein, CD2 and FasL [also known as TNF ligand superfamily member 6]) and to promote T-cell activation.94–96 Accordingly, although anakinra exerts some beneficial effects on the articular attacks of PAPA, it is not as effective as it is for cryopyrinopathies.97
The genes affected in two other likely autoinflammatory syndromes—chronic recurrent multifocal osteomyelitis and synovitis acne pustulosis hyperostosis osteitis—are currently unknown; however, the symptoms show certain similarities to two mouse models that have distinct mutations in the Pstpip2 gene, a homolog of PSTPIPI.98
Gout and pseudogout
Autoinflammation might also be triggered by endogenous substances that accumulate in certain metabolic and protein misfolding diseases. Of relevance to rheumatology are gout and pseudogout, in which monosodium urate crystals and calcium pyrophosphate dihydrate crystals, respectively, mediate inflammation through activation of the NLRP3 inflammasome and hyperproduction of IL-1β.99 Accordingly, anakinra has considerable therapeutic effects in these conditions.100
Other diseases
Other diseases that have a potential autoinflammatory origin and are of interest to rheumatologists are systemic-onset juvenile idiopathic arthritis, adult-onset Still’s disease, Schnitzler syndrome and Guadaloupe variant periodic fever syndrome (also known as FCAS2; caused by mutations in NLRP12), all of which respond well to anakinra treatment.78,101–103 Also relevant is TNFR-associated periodic syndrome (TRAPS), which is characterized by severe abdominal pain, pleurisy, arthritis, migratory skin rash and/or periorbital edema. This syndrome is caused by dominantly inherited mis-sense mutations that affect the extracellular region of TNFR1 (p55).104 These mutations were initially thought to impair ectodomain cleavage by metalloproteinases, an important mechanism of TNFR1 inactivation. However, subsequent studies suggest that TNFR1 mutations, often affecting cysteine residues involved in intramolecular disulfide bonds important for maintaining proper conformation, cause protein misfolding, which might either trigger an ER stress response, or constitutively induce intracellular signaling through aggregation of TNFR1 -associated death domains, recruitment of downstream signaling molecules and IL-lβ production.105,106 However, disease development depends on the presence of wild-type TNFR1, and therefore therapeutic regimens require both etanercept and anakinra.
Conclusions
Rheumatic diseases have long been regarded as genetic or environmental, or a combination thereof. The heritability of autoimmune phenotypes, albeit in a non-Mendelian manner, argues in favor of the occurrence of an unlucky combination of causative alleles at multiple loci. On the other hand, the variable penetrance of the disease pheno-type and the limited concordance of autoimmunity in identical twins has highlighted that environmental or epigenetic factors, or both, are also influential. Following the identification of the molecular pathways that bring about inflammation, we now know that the innate immune sensors and signaling systems that evolved to detect infection or cellular damage and initiate tissue repair are sometimes subverted, and can instead contribute to the initiation or propagation of an autoimmune or autoinflammatory disease. Microbes might function as triggers or long-term drivers of pathology, but might be absent, indicating that some of these syndromes might truly arise from the dysfunction of ‘self’ mechanisms. We do not yet know precisely where the problems arise in each disease, but we are developing a real insight into how inflammatory and autoimmune disorders can be suppressed. This insight is far more specific than it used to be, and immunosuppression in the future will probably not involve eliminating entire classes of immune cells, or disrupting their function. Despite the biochemical complexity of inflammation, it should be possible to discern the most sensitive, nonredundant targets for inhibition and, in certain instances, to use them for clinical benefit with few adverse effects.
Key points.
-
▪
Toll-like receptors (TLRs), retinoid acid inducible gene-l-like receptors (RLRs) and nucleotide-binding and oligomerization domain-like receptors (NLRs) can detect the presence of pathogens and products of damaged tissues
-
▪
Responses by these receptors usually benefit the host, but when inappropriately engaged by self molecules, or insufficiently inhibited, they can cause long-lasting immunopathology
-
▪
In certain autoimmune rheumatic diseases, such as systemic lupus erythematosus, recognition of self nucleic acids by TLRs seems to be the major pathogenetic mechanism
-
▪
In other diseases, such as rheumatoid arthritis and Sjögren’s syndrome, recognition of products from microbes and damaged tissues by these or other innate sensors are likely to contribute
-
▪
In autoinflammatory diseases, uncontrollable activation of the innate immune system is caused by mutations in components of the NLR system leading to inflammasome induction
-
▪
These findings explain the efficacy of blocking proinflammatory cytokines in these diseases, and suggest that additional therapeutic targets will be identified within the signaling pathways of the innate immune sensors
Acknowledgments
The work of the authors is supported by National Institute of Health grants. Space limitations precluded citation of many original publications, and we apologize for these omissions.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Baccala R, et al. Sensors of the innate immune system: their mode of action. Nat. Rev. Rheumatol. 2009;5:448–456. doi: 10.1038/nrrheum.2009.136. [DOI] [PubMed] [Google Scholar]
- 2.Ishii KJ, Akira S. TLR ignores methylated RNA? Immunity. 2005;23:111–113. doi: 10.1016/j.immuni.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Schlee M, et al. Approaching the RNA ligand for RIG-I? Immunol. Rev. 2009;227:66–74. doi: 10.1111/j.1600-065X.2008.00724.x. [DOI] [PubMed] [Google Scholar]
- 4.Bave U, Vallin H, Alm GV, Ronnblom L. Activation of natural interferon-alpha producing cells by apoptotic U937 cells combined with Iupus IgG and its regulation by cytokines. J. Autoimmun. 2001;17:71–80. doi: 10.1006/jaut.2001.0519. [DOI] [PubMed] [Google Scholar]
- 5.Leadbetter EA, et al. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 6.Lovgren T, Eloranta ML, Bave U, Alm GV, Ronnblom L. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 2004;50:1861–1872. doi: 10.1002/art.20254. [DOI] [PubMed] [Google Scholar]
- 7.Marshak-Rothstein A, Rifkin IR. mmunologically active autoantigens: the role of toll-like receptors in the development of chronic nflammatory disease. Annu. Rev. Immunol. 2007;25:419–441. doi: 10.1146/annurev.immunol.22.012703.104514. [DOI] [PubMed] [Google Scholar]
- 8.Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR- ndependent pathways of type I interferon nduction in systemic autoimmunity. Nat. Med. 2007;13:543–551. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- 9.Boule MW, et al. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J. Exp. Med. 2004;199:1631–1640. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian J, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat. Immunol. 2007;8:487–96. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 11.Lande R, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 12.Christensen SR, et al. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–A28. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Pisitkun P, et al. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 14.Subramanian S, et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc. Natl Acad.Sci. USA. 2006;103:9970–9975. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deane JA, et al. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27:801–810. doi: 10.1016/j.immuni.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santiago-Raber ML, et al. Evidence for genes in addition to Tlr7 in the Yaa translocation linked with acceleration of systemic lupus erythematosus. J. Immunol. 2008;181:1556–1562. doi: 10.4049/jimmunol.181.2.1556. [DOI] [PubMed] [Google Scholar]
- 17.Fairhurst AM, et al. Yaa autoimmune phenotypes are conferred by overexpression of TLR7. Eur. J. Immunol. 2008;38:1971–1978. doi: 10.1002/eji.200838138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv. Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- 19.Kono DH, et al. Endosomal TLR signaling is required for anti-nucleic acid and rheumatoid factor autoantibodies in lupus. Proc. Natl Acad. Sci. USA. 2009;106:12061–12066. doi: 10.1073/pnas.0905441106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature. 2008;452:234–238. doi: 10.1038/nature06726. [DOI] [PubMed] [Google Scholar]
- 21.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–392. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Santer DM, Yoshio T, Minota S, Moller T, Elkon KB. Potent induction of IFN-alpha and chemokines by autoantibodies in the cerebrospinal fluid of patients with neuropsychiatric lupus. J. Immunol. 2009;182:1192–1201. doi: 10.4049/jimmunol.182.2.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harley IT, Kaufman KM, Langefeld CD, Harley JB, Kelly JA. Genetic susceptibility to SLE: new insights from fine mapping and genome-wide association studies. Nat. Rev. Genet. 2009;10:285–290. doi: 10.1038/nrg2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graham RR, Horn G, Ortmann W, Behrens TW. Review of recent genome-wide association scans in lupus. J. Intern. Med. 2009;265:680–688. doi: 10.1111/j.1365-2796.2009.02096.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhuang H, et al. Lupus-like disease and high nterferon levels corresponding to trisomy of the type I interferon cluster on chromosome 9p. Arthritis Rheum. 2006;54:1573–1579. doi: 10.1002/art.21800. [DOI] [PubMed] [Google Scholar]
- 26.Lee PY, et al. TLR7-dependentend FcgammaR-ndependent production of type I interferon in experimental mouse lupus. J. Exp. Med. 2008;205:2995–3006. doi: 10.1084/jem.20080462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat. Rev. Immunol. 2008;8:327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lech M, et al. TirS/Sigirr prevents murine lupus by suppressing the immunostimulatory effects of lupus autoantigens. J. Exp. Med. 2008;205:1879–1888. doi: 10.1084/jem.20072646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baccala R, Kono DH, Theofilopoulos AN. Interferons as pathogenic effectors in autoimmunity. Immunol. Rev. 2005;204:9–26. doi: 10.1111/j.0105-2896.2005.00252.x. [DOI] [PubMed] [Google Scholar]
- 30.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type 1 interferons (alpha/beta) in mmunity and autoimmunity. Annu. Rev. Immunol. 2005;23:307–335. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 31.Janssen E, et al. Efficient T cell activation via a Toll-lnterleukin 1 Receptor-independent pathway. Immunity. 2006;24:787–799. doi: 10.1016/j.immuni.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 32.Viorritto IC, Nikolov NP, Siegel RM. Autoimmunity versus tolerance: can dying cells tip the balance? Clin. Immunol. 2007;122:125–134. doi: 10.1016/j.clim.2006.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis MJ, Botto M. Complement deficiencies in humans and animals: links to autoimmunity. Autoimmunity. 2006;39:367–378. doi: 10.1080/08916930600739233. [DOI] [PubMed] [Google Scholar]
- 34.Mevorach D, Zhou JL, Song X, Elkon KB. Systemic exposure to irradiated apoptotic cells nduces autoantibody production. J. Exp. Med. 1998;188:387–392. doi: 10.1084/jem.188.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Denny MF, et al. Accelerated macrophage apoptosis induces autoantibody formation and organ damage in systemic lupus erythematosus. J. Immunol. 2006;176:2095–2104. doi: 10.4049/jimmunol.176.4.2095. [DOI] [PubMed] [Google Scholar]
- 36.Takemura Y, et al. Adiponectin modulates nflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J. Clin. Invest. 2007;117:375–386. doi: 10.1172/JCI29709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allam R, et al. Viral 5’-triphosphate RNA and non-CpG DNA aggravate autoimmunity and lupus nephritis via distinct TLR-independent immune responses. Eur. J. Immunol. 2008;38:3487–3498. doi: 10.1002/eji.200838604. [DOI] [PubMed] [Google Scholar]
- 38.Yasutomo K, et al. Mutation of DNASE1 in people with systemic lupus erythematosus. Nat Genet. 2001;28:313–314. doi: 10.1038/91070. [DOI] [PubMed] [Google Scholar]
- 39.Napirei M, et al. Features of systemic lupus erythematosus in Dnasel-deficient mice. Nat Genet. 2000;25:177–181. doi: 10.1038/76032. [DOI] [PubMed] [Google Scholar]
- 40.Nagata S. Rheumatoid polyarthritis caused by a defect in DNA degradation. Cytokine Growth Factor Rev. 2008;19:295–302. doi: 10.1016/j.cytogfr.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 41.Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic nitiation of autoimmunity. Cell. 2008;134:587–598. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rice GI, et al. Mutations involved in Aicardi-Goutieres syndrome implicate SAMHD1 as regulator of the innate immune response. Nat Genet. 2009;41:829–832. doi: 10.1038/ng.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feldmeyer L, et al. The inflammasome mediates UVB-induced activation and secretion of nterleukin-lbeta by keratinocytes. Curr. Biol. 2007;17:1140–1145. doi: 10.1016/j.cub.2007.05.074. [DOI] [PubMed] [Google Scholar]
- 44.Schorlemmer HU, Kanzy EJ, Langner KD, Kurrle R. Immunoregulation of SLE-like disease by the IL-1 receptor: disease modifying activity on BDF1 hybrid mice and MRL autoimmune mice. Agents Actions. 1993;39:C117–C120. doi: 10.1007/BF01972740. (Spec. No.) [DOI] [PubMed] [Google Scholar]
- 45.Bossu P, et al. IL-18 cDNA vaccination protects mice from spontaneous lupus-like autoimmune disease. Proc. Natl Acad. Sci. USA. 2003;100:14181–14186. doi: 10.1073/pnas.2336094100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rozzo SJ, et al. Evidence for an interferon-nducible gene, Ifi202, in the susceptibility to systemic lupus. Immunity. 2001;15:435–443. doi: 10.1016/s1074-7613(01)00196-0. [DOI] [PubMed] [Google Scholar]
- 47.Croker BA, et al. Inflammation and autoimmunity caused by a SHP1 mutation depend on IL-1, MyD88, and a microbial trigger. Proc. Natl Acad. Sci. USA. 2008;105:15028–15033. doi: 10.1073/pnas.0806619105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beutler B. Microbe sensing, positive feedback loops, and the pathogenesis of inflammatory diseases. Immunol. Rev. 2009;227:248–263. doi: 10.1111/j.1600-065X.2008.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seibl R, et al. Expression and regulation of Tollike receptor 2 in rheumatoid arthritis synovium. Am. J. Pathol. 2003;162:1221–1227. doi: 10.1016/S0002-9440(10)63918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kyburz D, et al. Bacterial peptidoglycans but not CpG oligodeoxynucleotides activate synovial fibroblasts by toll-like receptor signaling. Arthritis Rheum. 2003;48:642–650. doi: 10.1002/art.10848. [DOI] [PubMed] [Google Scholar]
- 51.Iwahashi M, et al. Expression of Toll-like receptor 2 on CD16+ blood monocytes and synovial tissue macrophages in rheumatoid arthritis. Arthritis Rheum. 2004;50:1457–1467. doi: 10.1002/art.20219. [DOI] [PubMed] [Google Scholar]
- 52.Roelofs MF, et al. Type I interferons might form the link between Toll-like receptor (TLR) 3/7 and TLR4 mediated synovial inflammation in rheumatoid arthritis (RA) Ann. Rheum. Dis. 2009;68:1486–1493. doi: 10.1136/ard.2007.086421. [DOI] [PubMed] [Google Scholar]
- 53.Sacre SM, et al. Inhibitors of TLR8 reduce TNF production from human rheumatoid synovial membrane cultures. J. Immunol. 2008;181:8002–8009. doi: 10.4049/jimmunol.181.11.8002. [DOI] [PubMed] [Google Scholar]
- 54.Herlands RA, Christensen SR, Sweet RA, Hershberg U, Shlomchik MJ. T cell-independent and toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity. 2008;29:249–260. doi: 10.1016/j.immuni.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shih FF, Racz J, Allen PM. Differential MHC class II presentation of a pathogenic autoantigen during health and disease. J. Immunol. 2006;176:3438–3448. doi: 10.4049/jimmunol.176.6.3438. [DOI] [PubMed] [Google Scholar]
- 56.Choe JY, Crain B, Wu SR, Corr M. Interleukin 1 receptor dependence of serum transferred arthritis can be circumvented by tolllike receptor 4 signaling. J. Exp. Med. 2003;197:537–542. doi: 10.1084/jem.20021850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Joosten LA, et al. Toll-like receptor 2 pathway drives streptococcal cell wall-induced joint inflammation: critical role of myeloid differentiation factor 88. J. Immunol. 2003;171:6145–6153. doi: 10.4049/jimmunol.171.11.6145. [DOI] [PubMed] [Google Scholar]
- 58.Abdollahi-Roodsaz S, et al. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J. Clin. Invest. 2008;118:205–216. doi: 10.1172/JCI32639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoshitomi H, et al. A role for fungal β-glucans and their receptor Dectin-1 in the induction of autoimmune arthritis in genetically susceptible mice. J. Exp. Med. 2005;201:949–960. doi: 10.1084/jem.20041758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Asagiri M, et al. Cathepsin K-dependent toll-like receptor 9 signaling revealed in experimental arthritis. Science. 2008;319:624–627. doi: 10.1126/science.1150110. [DOI] [PubMed] [Google Scholar]
- 61.Ewald SE, et al. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature. 2008;456:658–662. doi: 10.1038/nature07405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park B, et al. Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll-like receptor 9. Nat Immunol. 2008;9:1407–1414. doi: 10.1038/ni.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Okabe Y, Sano T, Nagata S. Regulation of the innate immune response by threonine-phosphatase of Eyes absent. Nature. 2009;460:520–524. doi: 10.1038/nature08138. [DOI] [PubMed] [Google Scholar]
- 64.Deng GM, Nilsson IM, Verdrengh M, Collins LV, Tarkowski A. Intra-articularly localized bacterial DNA containing CpG motifs induces arthritis. Nat Med. 1999;5:702–705. doi: 10.1038/9554. [DOI] [PubMed] [Google Scholar]
- 65.Collins LV, Hajizadeh S, Holme E, Jonsson IM, Tarkowski A. Endogenously oxidized mitochondrial DNA induces in vivo and in vitro inflammatory responses. J. Leukoc. Biol. 2004;75:995–1000. doi: 10.1189/jlb.0703328. [DOI] [PubMed] [Google Scholar]
- 66.Rossol M, et al. Homozygosity for DNASE2 single-nucleotide polymorphisms in the 5’ regulatory region is associated with rheumatoid arthritis. Ann. Rheum. Dis. 2009;68:1498–1503. doi: 10.1136/ard.2008.092239. [DOI] [PubMed] [Google Scholar]
- 67.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev. Immunol. 2006;6:823–835. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Midwood K, et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med. 2009;15:774–780. doi: 10.1038/nm.1987. [DOI] [PubMed] [Google Scholar]
- 69.Spachidou MP, et al. Expression of functional Toll-like receptors by salivary gland epithelial cells: increased mRNA expression in cells derived from patients with primary Sjogren’s syndrome. Clin. Exp. Immunol. 2007;147:497–503. doi: 10.1111/j.1365-2249.2006.03311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kawakami A, et al. Toll-like receptor in salivary glands from patients with Sjogren’s syndrome: functional analysis by human salivary gland cell line. J. Rheumatol. 2007;34:1019–1026. [PubMed] [Google Scholar]
- 71.Ittah M, et al. Viruses induce high expression of BAFF by salivary gland epithelial cells through TLR- and type-l IFN-dependent and -independent pathways. Eur. J. Immunol. 2008;38:1058–1064. doi: 10.1002/eji.200738013. [DOI] [PubMed] [Google Scholar]
- 72.Bave U, et al. Activation of the type I interferon system in primary Sjogren’s syndrome: a possible etiopathogenic mechanism. Arthritis Rheum. 2005;52:1185–1195. doi: 10.1002/art.20998. [DOI] [PubMed] [Google Scholar]
- 73.Gottenberg JE, et al. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjogren’s syndrome. Proc. Natl Acad. Sci. USA. 2006;103:2770–2775. doi: 10.1073/pnas.0510837103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nordmark G, Aim GV, Ronnblom L. Mechanisms of Disease: primary Sjogren’s syndrome and the type I interferon system. Nat Clin. Pract. Rheumatol. 2006;2:262–269. doi: 10.1038/ncprheum0173. [DOI] [PubMed] [Google Scholar]
- 75.Nestle FO, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon- alpha production. J. Exp. Med. 2005;202:135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baechler EC, et al. An interferon signature in the peripheral blood of dermatomyositis patients is associated with disease activity. Mol. Med. 2007;13:59–68. doi: 10.2119/2006-00085.Baechler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eloranta ML, et al. A possible mechanism for endogenous activation of the type I interferon system in myositis patients with anti-Jo-1 or anti-Ro 52/anti-Ro 60 autoantibodies. Arthritis Rheum. 2007;56:3112–3124. doi: 10.1002/art.22860. [DOI] [PubMed] [Google Scholar]
- 78.Masters SL, Simon A, Aksentijevich I, Kastner DL. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease. Annu. Rev. Immunol. 2009;27:621–668. doi: 10.1146/annurev.immunol.25.022106.141627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hugot JP, et al. Association of N0D2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 80.Ogura Y, et al. A frameshift mutation in N0D2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 81.Miceli-Richard C, et al. CARD15 mutations in Blau syndrome. Nat Genet. 2001;29:19–20. doi: 10.1038/ng720. [DOI] [PubMed] [Google Scholar]
- 82.Kanazawa N, et al. Early-onset sarcoidosis and CARD15 mutations with constitutive nuclear factor-kappaB activation: common genetic etiology with Blau syndrome. Blood. 2005;105:1195–1197. doi: 10.1182/blood-2004-07-2972. [DOI] [PubMed] [Google Scholar]
- 83.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aksentijevich I, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 2002;46:3340–3348. doi: 10.1002/art.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Feldmann J, et al. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am. J. Hum. Genet. 2002;71:198–203. doi: 10.1086/341357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lachmann HJ, et al. Use of canakinumab in the cryopyrin-associated periodic syndrome. N. Engl. J. Med. 2009;360:2416–2425. doi: 10.1056/NEJMoa0810787. [DOI] [PubMed] [Google Scholar]
- 87.Richards N, et al. Interaction between pyrin and the apoptotic speck protein (ASC) modulates ASC-induced apoptosis. J. Biol. Chem. 2001;276:39320–39329. doi: 10.1074/jbc.M104730200. [DOI] [PubMed] [Google Scholar]
- 88.Chae JJ, et al. The B30.2 domain of pyrin, the familial Mediterranean fever protein, interacts directly with caspase-lto modulate IL-lbeta production. Proc. Natl Acad. Sci. USA. 2006;103:9982–9987. doi: 10.1073/pnas.0602081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Papin S, et al. The SPRY domain of Pyrin, mutated in familial Mediterranean fever patients, interacts with inflammasome components and inhibits prolL-lbeta processing. Cell Death Differ. 2007;14:1457–1466. doi: 10.1038/sj.cdd.4402142. [DOI] [PubMed] [Google Scholar]
- 90.Yu l.YJ, et al. Cryopyrin and pyrin activate caspase-1, but not NF-kappaB, via ASC oligomerization. Cell Death Differ. 2006;13:236–249. doi: 10.1038/sj.cdd.4401734. [DOI] [PubMed] [Google Scholar]
- 91.Mansfield E, et al. The familial Mediterranean fever protein, pyrin, associates with microtubules and colocalizes with actin filaments. Blood. 2001;98:851–859. doi: 10.1182/blood.v98.3.851. [DOI] [PubMed] [Google Scholar]
- 92.Wise CA, et al. Mutations in CD2BP1 disrupt binding to PTP PEST and are responsible for PAPA syndrome, an autoinflammatory disorder. Hum. Mol. Genet. 2002;11:961–969. doi: 10.1093/hmg/11.8.961. [DOI] [PubMed] [Google Scholar]
- 93.Shoham NG, et al. Pyrin binds the PSTPIP1/ CD2BP1 protein, defining familial Mediterranean fever and PAPA syndrome as disorders in the same pathway. Proc. Natl Acad. Sci. USA. 2003;100:13501–13506. doi: 10.1073/pnas.2135380100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Badour K, et al. Fyn and PTP-PEST-mediated regulation of Wiskott-Aldrich syndrome protein (WASp) tyrosine phosphorylation is required for coupling T cell antigen receptor engagement to WASp effector function and T cell activation. J. Exp. Med. 2004;199:99–112. doi: 10.1084/jem.20030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang H, Reinherz EL. CD2BP1 modulates CD2-dependent T cell activation via linkage to protein tyrosine phosphatase (PTP)-PEST. J. Immunol. 2006;176:5898–5907. doi: 10.4049/jimmunol.176.10.5898. [DOI] [PubMed] [Google Scholar]
- 96.Baum W, et al. Binding of the intracellular Fas igand (FasL) domain to the adaptor protein PSTPIP results in a cytoplasmic localization of FasL. J. Biol. Chem. 2005;280:40012–40024. doi: 10.1074/jbc.M502222200. [DOI] [PubMed] [Google Scholar]
- 97.Dierselhuis MP, Frenkel J, Wulffraat NM, Boelens JJ. Anakinra for flares of pyogenic arthritis in PAPA syndrome. Rheumatology (Oxford) 2005;44:406–408. doi: 10.1093/rheumatology/keh479. [DOI] [PubMed] [Google Scholar]
- 98.El-Shanti HI, Ferguson PJ. Chronic recurrent multifocal osteomyelitis: a concise review and genetic update. Clin. Orthop. Relat. Res. 2007;462:11–19. doi: 10.1097/BLO.0b013e3180986d73. [DOI] [PubMed] [Google Scholar]
- 99.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 100.McGonagle D, et al. Management of treatment resistant inflammation of acute on chronic tophaceous gout with anakinra. Ann. Rheum. Dis. 2007;66:1683–1684. doi: 10.1136/ard.2007.073759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jeru I, et al. Mutations in NALP12 cause hereditary periodic fever syndromes. Proc. Natl Acad. Sci. USA. 2008;105:1614–1619. doi: 10.1073/pnas.0708616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J. Exp. Med. 2005;201:1479–1486. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Church LD, Cook GP, McDermott M. F Primer: inflammasomes and interleukin 1beta in inflammatory disorders. Nat Clin. Pract. Rheumatol. 2008;4:34–42. doi: 10.1038/ncprheum0681. [DOI] [PubMed] [Google Scholar]
- 104.McDermott MF, et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell. 1999;97:133–144. doi: 10.1016/s0092-8674(00)80721-7. [DOI] [PubMed] [Google Scholar]
- 105.Lobito AA, et al. Abnormal disulfide-linked oligomerization results in ER retention and altered signaling by TNFR1 mutants in TNFRl-associated periodic fever syndrome (TRAPS) Blood. 2006;108:1320–1327. doi: 10.1182/blood-2005-11-006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rebelo SL, et al. Modeling of tumor necrosis factor receptor superfamily 1A mutants associated with tumor necrosis factor receptor-associated periodic syndrome indicates misfolding consistent with abnormal function. Arthritis Rheum. 2006;54:2674–2687. doi: 10.1002/art.21964. [DOI] [PubMed] [Google Scholar]