Abstract
In many tissues, two or more types of stem cells share a niche, and how the stem cells coordinate their self-renewal and differentiation is poorly understood. In the Drosophila testis, germ line stem cells (GSCs) and somatic cyst progenitor cells (CPCs) contact each other and share a niche (the hub). The hub expresses a growth factor Unpaired (Upd) that activates the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway in GSCs to regulate the stem cell self-renewal. Here, we demonstrate that the JAK/STAT signaling also regulates CPCs self-renewal. We also show that a negative regulator, the suppressor of cytokine signaling 36E (SOCS36E), suppresses JAK/STAT signaling in somatic cells, preventing them from out-competing the GSCs. Furthermore, through selectively manipulating the JAK/STAT signaling level in either CPCs or GSCs, we demonstrate that the somatic JAK/STAT signaling is essential for self-renewal and maintenance of both CPCs and GSCs. These data suggest that a single JAK/STAT signal from the niche orchestrate the competitive and dependent co-existence of GSCs and CPCs in the Drosophila testis niche.
Keywords: Drosophila, stem cell, germline stem cells, somatic stem cells, signal transduction, JAK/STAT, stem cell interaction
Introduction
Stem cells are characterized by their ability to self-renew and to generate continuously differentiated cells to maintain tissue homeostasis (Takahashi et al., 2007). Stem cells have immense potential for therapeutic use in regenerative medicine and for developing anticancer therapies that specifically eliminate cancer stem cells (Reya et al., 2001). When stem cells divide, the daughter cells must first choose between maintenance of stem cell identity and or initiation of differentiation. Through asymmetric cell division, a stem cell in adult tissues can produce an offspring that will maintain the stem cell populations and a daughter cell that will differentiate into various short-lived cell types to replace damaged or dying cells (Yamashita et al., 2007; Fuller and Spradling, 2007, Morrison and Spradling, 2008). Specialized microenvironments, known as stem cell niches regulate the behavior of stem cells and its differentiating progeny (Xie and Spradling, 2000; Donovan and Gearhart, 2001; Ohlstein et al., 2004; Yamashita et al., 2007; Fuller and Spradling, 2007, Nystul and Spradling, 2007; Zahn et al., 2007; Xie et al., 2008; Jin et al., 2008; Morrison and Spradling, 2008). Two or more types of stem cells and stromal cells frequently co-exist in a particular microenvironment (Yamashita et al., 2007; Li and Xie, 2005; Decotto and Spradling, 2005; Fuller and Spradling, 2007). The various types of stem cells are coordinately regulated by a subset of neighboring stromal cells and extracellular substrates. The stromal cells secrete growth factors to provide a stem cell niche (Spradling et al., 2001; Ohlstein et al., 2004; Yamashita et al., 2007). However, how two or more types of stem cells share a niche and whether different types of stem cells regulate each other to coordinate their self-renewal and differentiation are topics not fully understood (Fuchs et al., 2004; Fuller and Spradling, 2007; Jin et al., 2008; Morrison and Spradling, 2008).
The Drosophila testis provides an excellent in vivo system to study stem cell–niche interactions at the cellular and molecular levels (Gilboa and Lehmann, 2004; Yamashita et al., 2007, Cheng et al. 2008, Sheng et al., 2009). At the tip of the Drosophila testis is a germinal proliferation center, which houses both the GSCs and CPCs (also known as somatic stem cells-SSCs), allowing spatially coordinated production of progeny cells that interact and co-differentiate to maintain normal spermatogenesis (Gönczy and Dinardo, 1996). Each adult fly testis has 7–9 GSCs that are encysted by two CPCs. Both GSCs and CPCs are physically attached to a group of 12 non-dividing somatic cells called the hub via adherens junctions (Fig. 3C Hardy et al. 1979; Wang et al., 2006; Fuller and, Spradling, 2007). Each GSC divides asymmetrically with the mitotic spindle orientated perpendicular to the hub (Yamashita et al. 2003, 2007). One of the daughter cells remains in contact with the hub, inherits the mother centriole, and retains GSC identity, whereas the other daughter cell, called gonialblast, lying one-cell diameter away from the hub, inherits the daughter centriole and initiates differentiation (Hardy et al., 1979; Yamashita et al., 2003, 2007). Similarly, CPCs self-renew and give rise to daughters that differentiate into somatic cyst cells (Hardy et al., 1979; Gönczy and DiNardo, 1996). The gonialblast will undergo four rounds of mitotic division with incomplete cytokinesis, resulting in the formation of 16 interconnected spermatogonia, while the somatic cyst cells will grow without further division, become elongate, and form a thin layer around the spermatogonial cyst (Lin, 2002; Gilboa and Lehmann, 2004; Yamashita et al., 2007; Voog et al., 2008). However, the germ cells become transcriptionally highly active and form spermatocytes that will increase in size and finally undergo meiosis and differentiate into sperm (Fuller and Spradling, 2007).
The JAK/STAT ligand Upd, which is exclusively expressed in the hub cells, maintains the GSCs and CPCs self-renewal by activation of the JAK/STAT pathway in GSCs and CPCs (Kiger et al., 2001; Tulina and Matunis, 2001; Leatherman and DiNardo, 2008). JAK/STAT signaling is involved in many developmental processes, including segmentation in embryogenesis, eye morphogenesis, hematopoiesis; stem cell maintenance and dedifferentiation of germline stem cells (Hou et al., 2002; Singh et al., 2005; Arbouzova and Zeidler, 2006; Singh et al., 2007; López-Onieva et al. 2008; Cronin et al., 2009; Jiang et al., 2009; Sheng et al., 2009). Several other signaling pathways or molecules are also involved in the process of self-renewal or differentiation of GSCs and CPCs in Drosophila testis (Schulz et al., 2004; Sarkar et al., 2007; Kiger et al., 2000; Tran et al., 2000; Shivdasani and Ingham, 2003; Bunt and Hime, 2004; Kawase et al., 2004; Singh et al., 2005, 2006). However, the regulation of CPCs and the interaction between CPCs and GSCs to coordinate their self-renewal or differentiation are processes that are not clearly understood. Here we show that a single JAK/STAT signal regulates the self-renewal of both GSCs and CPCs, and that the relative strength of the JAK/STAT signaling controls competitiveness for the niche and mutual dependence of GSCs and CPCs in the Drosophila testis niche.
Materials and Methods
Drosophila stocks
Oregon-R was used as wild type. The other fly stocks used in this study were described either in FlyBase or were otherwise specified as follows: stat92EF, a temperature-sensitive stat92E allele, was a gift from C. Dearolf through S. DiNardo. Nos-Gal4 (nanos-Gal4VP16), SM6, hs-Flp, TM3, Sb, hs-Flp, UAS-2XEYFP/FM7, FRT40A-tub-Gal80, FRT82B-tub-Gal80, FRT40A-w+, and FRT82B-w+ were obtained from the Bloomington stock center. FRT82B-stat92Ej6C8 and FRT82B-ras1c40bwere described previously (Hou et al., 1995, 1996; Chen et al. 2002); FRT82B-stat92E3R-105 and FRT82B-stat92E3R-180 were obtained from the Tübingen stock center (Luschnig et al., 2004). UAS-socs36E-45 was a gift from B. Mathey-Prevot. c587-Gal4 and yw hs-FLP; FRT40A arm-lacZ were provided by T. Xie (Kawase et al., 2004). UAS-upd was described previously (Hou et al., 2006). The 842 is a P-element enhancer trap that expresses LacZ in the hub, CPCs, and somatic cyst cells of the testis (provided by S. DiNardo). Transgenic lines of UAS-stat92E were generated by inserting stat92E cDNA into the pUAST (Brand and Perrimon, 1993). socs36E1, EY06665, was isolated in the gene disruption screen of the Berkeley Drosophila Genome Project (FlyBase). The deficiency Df(2L)T317 removes socs36E and several other genes in chromosome regions 36C–F and was obtained from the Bloomington stock center.
Flies were raised on standard Drosophila media at 25°C unless otherwise indicated. Chromosomes and mutations that are not described in the text can be found in FlyBase (http://flybase.bio.indiana.edu).
Characterization of socs36E mutant
Using Northern blot, RT-PCR, and anti-Socs36E antibody staining, we confirmed that socs36E1 is a null mutation of socs36E gene. Total RNAs were isolated from wild-type and socs36E1 homozygous mutant flies using Trizol (GibcoBRL), and purified with Qiagen RNeasy kit. To avoid DNA contamination, the RNA was first treated with 1 MU DNase I (2 U/ml) for 30 min at 37°C. Northern blotting was performed as described (Sambrook et al. 1989). 100 ng of total RNA was reverse transcribed using the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen). The following socs36E primers were used in this study: 5′-GCTGGTCAGGTGGAAAGTTTCGCCGAG-3′ and 5′-AAACTGGCTTGCTGCTGTGTTGCGTT-3′. PCR was performed as follows: 94°C for 3 min; 35 cycles of 94°C for 15 sec, 58°C for 30 sec, 68°C for 2 min; and 72°C for 10 min. The RT-PCR products were visualized in 1% agarose gel.
Generating mutant CPCs clones
Clones of mutant CPCs were generated using the MARCM system (Lee and Luo 1999). For mutants on chromosome 3R, c587-Gal4.UAS-2XEYFP/FM7; FRT82B-tub-Gal80/TM3, Sb virgin females were mated with males of +/SM6, hs-Flp; FRT82B-stat92Ej6C8/TM3, Sb, +/SM6, hs-Flp; FRT82B-stat92E3R-105/TM3, Sb, +/SM6, hs-Flp; FRT82B-stat92E3R-180/TM3, Sb, +/SM6, hs-Flp; FRT82B-ras1c40b/TM3, Sb, or +/SM6, hs-Flp; FRT82B-w+/TM3, Sb. For mutants on chromosome 2L, c587-Gal4.UAS-2XEYFP/FM7; FRT40A-tub-Gal80/Cyo virgin females were mated with males of FRT40A-socs36E1/Cyo; +/TM3, Sb, hs-Flp or FRT40A- w+/Cyo; +/TM3, Sb, hs-Flp. Control clones were induced by crossing the c587-Gal4.UAS-2XEYFP/FM7; FRT40A-tub-Gal80/Cyo virgin females with males of FRT40A- w+/Cyo; +/TM3, Sb, hs-Flp. Adult males 1 or 2 days old and carrying a tub-Gal80 transgene in trans to the mutant-bearing chromosome were heat-shocked for 1 hr at 37°C four times (total 2 days) separated by 8 to 12 hr of interval on each heat shock. The males were transferred at 25°C to fresh food every day. The testes were removed 2, 4, or 6 days after the final heat-shock treatment, and then processed for antibody staining.
Generating mutant GSCs clones
Clones of mutant GSCs were generated as previously described (Kawase et al. 2004). In brief, FRT40A+ and FRT40A socs36E1/Cyo males were mated with virgin females of genotype yw hs-FLP; FRT40A arm-lacZ. Adult males 1 or 2 days old and carrying an arm-lacZ transgene in trans to the mutant-bearing chromosome were heat-shocked for 1 hr at 37°C six times, separated by 8 to 12 hr. The males were transferred at 25°C to fresh food every day. The testes were removed 5 or 11 days after the first heat-shock treatment, and then processed for antibody staining.
Immunofluorescence staining and microscopy
The immunofluorescence staining of the testes were performed as described (Singh et al. 2006; Singh and Hou, 2008). In brief the testes were dissected in Ringer’s solution and fixed for 40 min in 4% formaldehyde-PBX (PBS plus 0.1% Triton X-100), rinsed several times in PBX and blocked overnight at 4°C in PBX-2 (PBX plus 2% normal goat serum). The testes were rinsed once with PBX and then incubated overnight at 4°C in primary antibody diluted in PBX containing 0.5% BSA. After several washes with PBX, the testes were incubated with secondary antibodies (Molecular Probes), diluted in PBX containing 0.5% BSA for 2 hr at RT. After several more washes with PBX, and rinse with PBS twice, the testes were first treated with 0.04 mg/ml RNase A (Sigma) for 30 min and then incubated in 1 μg/ml DAPI (Molecular Probes) for 5 min. Following several more washes, the tissues were mounted in PBS: glycerol (1:1) solution. Confocal images were obtained by using a Zeiss LSM510 system and processed using Adobe Photoshop 7.0.
A Peptide (amino acids: RVKPIDQNTPMPYYGNV) corresponding to the C-terminals of Socs36E was used to produce antibodies in rabbits. Antiserum was purified using the peptide as affinity reagents. Socs36E staining was performed using the purified antisera at 1:1000 dilutions. The following antisera were also used: rabbit polyclonal anti-Vasa (1:5000; gift from R. Lehmann); mouse monoclonal anti-Hts mAb 1B1 (1:4; Developmental Studies Hybridoma Bank [DSHB]); mouse monoclonal anti-Armadillo (Arm) N7A1 (1:4; DSHB); mouse monoclonal anti-Fas III (1:10; DSHB); rabbit polyclonal anti-GFP (1:200; Molecular Probes); guinea pig polyclonal anti-Tj (1:500; gift from D. Godt; Li et al., 2003); rabbit polyclonal anti-Stat92E (1:1000); mouse monoclonal anti-Eya mAb 10H6 (1:1000; DSHB); anti-dPERK (1:1000, Sigma). The second antibodies were goat anti-mouse, goat anti-guinea pig, and goat anti-rabbit IgG conjugated to Alexa 488 or Texas Red (1:400; Molecular Probes). DAPI (Molecular Probes) was used to stain DNAs.
Detection of apoptosis
We used an Apop Tag Fluorescein Direct Detection Kit (Intergen) to detect cell death in the testes. The testes were dissected and fixed in 4% formaldehyde in PBX, as described above. The fixed testes were washed in PBX, and cell death was detected according to the manufacturer’s instructions.
Quantification of GSCs and CPCs
The number of GSCs/testis and CPCs/testis was determined using serial confocal reconstructions of the entire testis apex. Vasa-positive cells (with a spherical fusome) contacting the hub were scored as GSCs. Tj-positive cells contacting the hub were scored as CPCs.
Results
The JAK/STAT signaling regulates CPCs self-renewal and maintenance
To determine whether the JAK/STAT signaling directly regulates CPCs self-renewal, we generated stat92E null CPCs clones using the MARCM (mosaic analysis with a repressible cell marker) system (Lee and Luo, 1999) in combination with a c587-Gal4, which drives GFP expression only in CPCs and cyst cells (Kawase et al., 2004). Marked clones homozygous for wild-type control (Fig. 1, A, C, and E) and stat92Ej6C8 mutation (Fig. 1, B, D, and F) were generated in somatic cells by subjecting males of the appropriate genotype to heat-shock treatments and identified by GFP expression. The marked CPCs were identified according to the published criteria that they should directly contact the hub and encyst GSCs (Gönczy and DiNardo, 1996; Fig. 1A). To eliminate variation caused by heat-shock treatments, we counted only testes with GFP clones. Among these, the changes in the number of GFP-marked CPCs in each testis and the percentages of the testes carrying one or more marked CPCs were used to deduce whether a given gene is important for maintaining CPCs.
Fig. 1.
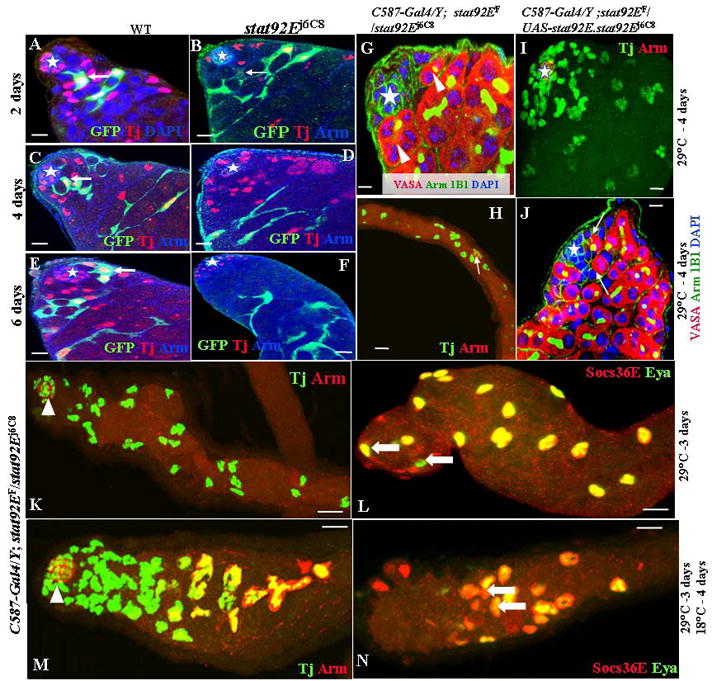
JAK/STAT signaling is required for CPCs self-renewal. (A) Testes are labeled for Tj (red); GFP (green); and Dapi (blue). (B–F) Testes are labeled for Tj (red); GFP (green); and Arm (blue). The hubs are marked by stars. Testes with wild-type (WT) GFP-marked clones are shown at 2 days (A), 4 days (C), and 6 days (E) ACI. Testes with stat92Ej6C8 homozygous mutant GFP-marked clones are shown at 2 days (B), 4 days (D), and 6 days (F) ACI. Testes of the following genotypes were shifted from RT to 29°C for 4 days: C587-Gal4/Y;stat92EF/stat92Ej6C8 (G, H); C587-Gal4/Y;stat92EF/UAS-stat92E.stat92Ej6C8 (C, D). (H, I) Testes were immunostained with anti-Tj (green) and anti-Arm (red). (G, J) Testes were immunostained with anti-Vasa (red), anti-Arm (green), mAb 1B1 (green), and DAPI (blue). The hub cells are marked by stars (G, I–J). Arrows point to GSCs (J). Arrowheads point to spermatogonia (G). GFP-marked CPCs are highlighted by arrows. (K, L) C587-Gal4/Y; stat92EF/stat92Ej6C8 testes shifted to 29°C for 3 days from RT. Testis in (K) is immunostained with anti-Tj (green) and anti-Arm (red). Testis in (L) is immunostained with anti-Socs36E (red) and anti-Eya (green). Tj-positive cells move away from the hub (arrowhead), and late-stage somatic cells (arrows in L) move up to the tip. (M, N) C587-Gal4/Y; stat92EF/stat92Ej6C8 testes shifted to 29°C for 3 days and then returned to 18°C for 4 days. Testis in (M) is immunostained with anti-Arm (red, arrowhead); anti-Tj (green); and anti-Eya (red). Testis in (N) is immunostained with anti-Socs36E (red) and anti-Eya (green). Tj-positive cells move back toward the hub (arrowhead), and late-stage somatic cells (arrows in N) move away from the hub. Scale bars represent 10 μm.
A drawback of the MARCM technique is the persistence of the Gal80 protein (Kirilly et al. 2005). The daughter cell will inherit some functional Gal80 protein before it is eventually degraded. Therefore, we wait 48 hours before counting the clones. Two days after clone induction (ACI), 100% (61/61) of the testes carried one or more GFP-marked wild type CPCs and early cyst cells (Fig. 1A; Table 1). Four days ACI, 100% (57/57) of the testes carried one or more GFP-marked wild type CPCs and late cyst cells (Fig. 1C). Six days ACI, 92% (55/60) of the testes carried one or more GFP-marked wild type CPCs and late cysts cells (Fig. 1E; Table 1). However, the GFP-marked stat92Ej6C8 CPCs were extremely unstable. Even two days ACI, only 5% (4/76) of the testes carried one GFP-marked stat92Ej6C8 CPCs (Fig. 1B; Table 1). Four days ACI, none (67/67) of the testes carried GFP-marked stat92Ej6C8 CPCs and only late cyst cells were detected (Fig. 1D). Six days ACI, only very-late-stage GFP-marked stat92Ej6C8 somatic cysts were detected in all testes (Fig. 1F; Table 1). Similar results were obtained with the two other stat92E mutant alleles (Table 1). Therefore, the JAK/STAT signaling in CPCs regulates their self-renewal and maintenance in the fly testis.
Table 1.
stat92E, socs36E, ras1 regulate CPCs
| Genotyes | 2 days ACI |
6 days ACI |
||||
|---|---|---|---|---|---|---|
| Testes with GFP clone | % of Testes with CPCs clone | CPCs/testis | Testes with GFP clone | % of Testes with CPCs clone | CPCs/testis | |
| Wild-type (control) | 61 | 100 (61/61) | 1.3±0.2 | 60 | 92 (55/60) | 1.1±0.4 |
| stat92Ej6C8 | 76 | 5 (4/76) | 0.5±0.1 | 71 | 0 (0/71) | 0 |
| stat92E3R-105 | 65 | 8 (5/65) | 0.8±0.2 | 58 | 0 (0/58) | 0 |
| stat92E3R-180 | 59 | 7 (4/59) | 0.7±0.3 | 62 | 0 (0/62) | 0 |
| socs36E1 | 56 | 100 (56/56) | 1.6±0.4 | 51 | 100 (51/51) | 3.7±0.6 |
| ras1c40b | 48 | 100 (48/48) | 1.5±0.5 | 47 | 100 (47/47) | 3.4±0.7 |
Since the above MARCM system could induce stat92E, homozygous mutant clones in both the germ line and soma, we re-examined the role of the JAK/STAT pathway in somatic cells by using a temperature-sensitive (ts) allele of stat92E (stat92EF) (Baksa et al. 2002). Because this allele has a closely linked background lethal mutation, we used the stat92EF heterozygous with stat92Ej6C8. stat92EF/stat92Ej6C8 (referred as stat92Ets from now on) flies are lethal at 29°C, but viable and fertile at 18°C and room temperature (RT, 22°C), with testes indistinguishable from those of wild type (Brawley and Matunis, 2004; data not shown). To examine the effects of removing stat92E from somatic cells, we analyzed the testes from stat92Ets flies raised at RT, then shifted to 29°C. We then selectively rescued the stat92Ets mutation in somatic cells only [C587-Gal4/Y; stat92EF/UAS-stat92E.stat92Ej6C8 or CPCs-wt (wild type)+GSCs-ts]. We used anti-Tj antibody to mark the CPCs and early cyst cells, and we compared CPCs changes in the stat92Ets and CPCs-wt+GSCs-tstestes raised at RT and then shifted to 29°C.
After 4 days at 29°C, CPCs and GSCs were completely lost in all stat92Ets testes (34/34=100%, Fig. 1G, Table 1), and the hub moved to inside in most testes (Fig. 1G, H). Seventy percent of the testes retained some somatic cyst cells (Fig. 1G Table 1). However, all CPCs-wt+GSCs-ts testes (33/33=100%) possessed wild-type CPCs and somatic cyst cells after 4 days at 29°C (Fig. 1I; Table 2). Further, most GSCs were also restored in CPCs-wt+GSCs-ts testes (Fig. 1J). We conclude, therefore, that somatic JAK/STAT signaling regulates the self-renewal and maintenance of CPCs and positively affects the maintenance of GSCs.
Table 2.
GSCs and CPCs coordinate their self-renewal and differentiation through the JAK/STAT signal transduction pathway
| Genotype | 2 days at 29°C, % testes with |
4 days at 29°C, % testes with |
||||
|---|---|---|---|---|---|---|
| GSCs | Spermatogonia | Spermatocytes | GSCs | Spermatogonia | Spermatocytes | |
| Wild-type (control) | 100 (33/33)a | 100 (33/33) | 100 (33/33) | 100 (29/29) | 100 (29/29) | 100 (29/29) |
| +/Y;+/+;STAT92E6c8/STAT92EF | 20 (8/41) | 100 (41/41) | 100 (41/41) | 0 (0/28) | 36 (10/28) | 50 (14/28) |
| C587-Gal4/Y;UASsocs36E/+STAT92E6c8/STAT92EF | 0 (0/44) | 0 (0/44) | 0 (0/44) | – | – | – |
| C587-Gal4/Y;UAS-socs36E/+;UAS-STAT92E.STAT92E6c8/STAT92EF | 32 (11/34) | 100 (34/34) | 100 (34/34) | 25 (9/32) | 84 (27/32) | 84 (27/32) |
| +/Y;UAS-Socs36E/+;Nos-Gal4.STAT92E6c8/STAT92EF | 0 (0/36) | 11 (4/36) | 22 (8/36) | 0 (0/40) | 0 (0/40) | 0 (0/40) |
| C587-Gal4/Y;+/+;UAS-STAT92E.STAT92E6c8/STAT92EF | 40 (16/40) | 100 (40/40) | 100 (40/40) | 20 (5/25) | 100 (25/25) | 100 (25/25) |
| +/Y;UAS STAT92E./+; Nos-Gal4.STAT92E6c8/STAT92EF | 75 (30/40) | 88 (35/40) | 88 (35/40) | 44 (22/50) | 72 (36/50) | 80 (40/50) |
| Genotype | 2 days at 29°C, % testes with |
4 days at 29°C, % testes with |
||
|---|---|---|---|---|
| CPCs | Somatic cyst cells | CPCs | Somatic cyst cells | |
| Wild-type (control) | 100 (28/28)b | 100 (28/28)c | 100 (34/34) | 100 (34/34) |
| +/Y;+/+;STAT92E6c8/STAT92EF | 100 (32/32) | 100 (32/32) | 0 (0/34) | 70 (24/34) |
| C587-Gal4/Y;UASsocs36E/+STAT92E6c8/STAT92EF | 0 (0/39) | 0 (0/39) | – | – |
| C587-Gal4/Y;UAS-socs36E/+;UAS-STAT92E.STAT92E6c8/STAT92EF | – | – | 100 (33/33) | 100 (33/33) |
| +/Y;UAS-Socs36E/+;Nos-Gal4.STAT92E6c8/STAT92EF | 100 (25/25) | 100 (25/25) | 67 (18/27) | 100 (27/27) |
| C587-Gal4/Y;+/+;UAS-STAT92E. STAT92E6c8/STAT92EF | 100 (27/27) | 100 (27/27) | 100 (25/25) | 100 (24/24) |
| +/Y;UAS STAT92E./+; Nos-Gal4.STAT92E6c8/STAT92EF | 100 (36/36) | 100 (36/36) | 0 (44/44) | 86 (38/44) |
The percentage of testes carrying one or more GSCs.
The percentage of testes carrying one or more Tj-positive somatic cells that contact the hub.
The percentage of testes carrying Tj-positive cells that do not contact the hub
To further examine the behavior of somatic cells in stat92EF testes, we used anti-Tj antibody to mark the CPCs and early somatic cyst cells, and anti-eyes absent (Eya) and anti-Socs36E antibodies (the two antibodies have similar expression patterns, Fig. 2D, E) to mark late-stage somatic cyst cells. After shifting the stat92Ets flies to 29°C for 3 days, the Tj-positive cells moved down and away from the hub (Fig. 1K), while the Eya- and Socs36E-positive cells moved up to the hub (Fig. 1L), indicating that all CPCs were differentiated into daughter somatic cyst cell.
Fig. 2.
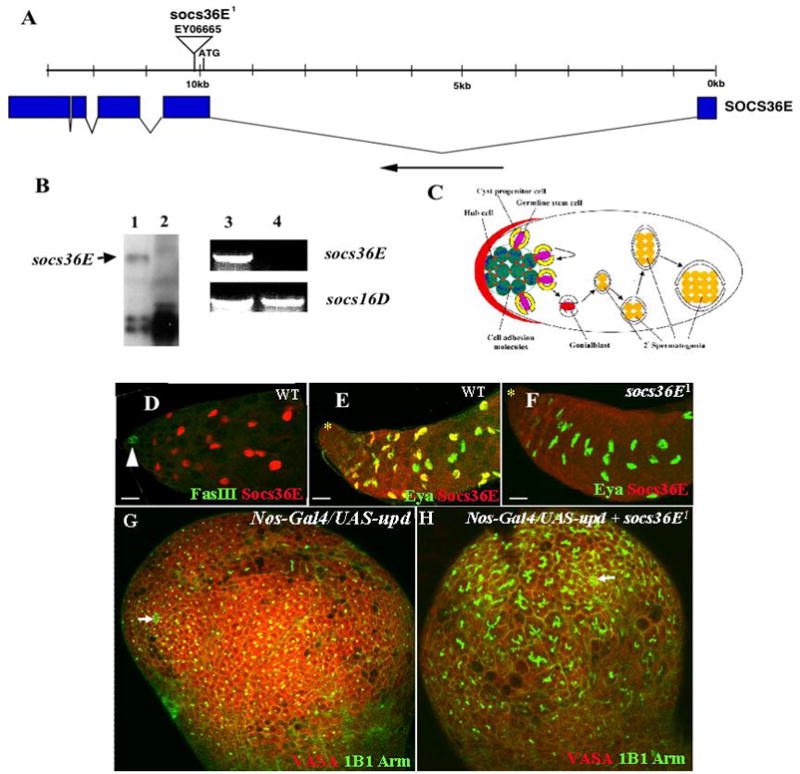
Analysis of socs36E mutation. (A) socs36E exons are indicated by filled boxes; breaks between the boxes indicate introns. Triangle indicates insertion site of the P element. The black arrow at the bottom of the figure indicates the orientation of the transcription. (B) Molecular analysis of socs36E1 mutant. Lanes 1 and 2 are representing results of using Northern blotting; the black arrow in lane 1 points to the band of the socs36E transcript, which is undetectable in socs36E1 mutant flies (lane 2). Lanes 3 and 4 represent results using RT-PCR. The socs36E transcript is undetectable in socs36E1 mutant flies (lane 4). socs16D serves as an internal control. (C) A sagittal section of the Drosophila testis apex is drawn schematically and leaves out most of the cells for clarity. Both the GSCs (pink) and the CPCs (yellow) are anchored around the hub (green) through adherens junctions. Asymmetric division of both stem cells results in spermatogenic cysts, in which each gonialblast (red) is encased by two somatic cyst cells (white). Four more consecutive divisions produce a cyst of 16 spermatogonia. (D) A wild-type testis was immunostained with anti-Fas III antibody to label the hub (green, arrowhead) and anti-Socs36E (red). Socs36E is expressed in somatic cyst cells. (E, F) Wild-type (E) and socs36E1 (F) testes were stained with anti-Eya (green) and anti-Socs36E (red). The expression of Socs36E and Eya partially overlaps. Socs36E is totally absent in the socs36E1 testis (F). The hubs are marked by stars. (G,H) Testes immunostained for germ cells (with anti-Vasa, red), the hub (with anti-Arm, green) fusomes (with mAb1B1, green). The hubs are marked by arrow. Scale bars in D, E, and F represent 10 μm and in G and H represent 30μm.
We next studied whether restoring stat92E activity would restore the normal somatic cell positions. When the stat92Ets flies that had been shifted to 29°C for 3 days were returned to 18°C for 3 days, the Tj-positive cells moved back toward the hub (Fig. 1M), while Eya and Socs36E positive cells moved back to their normal locations (Fig. 1N). The total number of somatic cells in these testes did not significantly change (data not shown), indicating that the JAK/STAT signaling does not regulate proliferation or death of somatic cells. These data suggest that daughter somatic cyst cells were able to de-differentiate back into CPCs once the JAK/STAT signaling was restored.
socs36E is the inhibitor and target of JAK/STAT signaling and is expressed in somatic cyst cells
Since JAK/STAT signaling is essential and sufficient for GSCs and CPCs self-renewal and maintenance in the Drosophila testis (Fig. 1 and Tables 1, 2 Kiger et al. 2001, Tulina and Matunis, 2001, Leatherman and Dinardo, 2008), studying targets of the signaling, would help to understand the regulatory mechanisms in one or both stem cell lineages. In mammalian tissues, the JAK/STAT cascade is regulated by both activators and inhibitors (O’Shea et al. 2002). Among the inhibitors are suppressors of cytokine signaling (SOCSs), which bind and inhibit the activity of the receptor, or JAK (Starr and Hilton 1999). The JAK/STAT is also responsible for socs gene induction; it forms part of a classical negative feedback circuit. In the Drosophila genome, there are three putative socs genes, socs36E, socs44A, and socs16D (Arbouzova and Zeidler, 2006). SOCS36E is most similar to mammalian SOCS5 whereas SOCS44A and SOCS16D are most similar to mammalian SOCS6 and 7. SOCS36E is the only JAK/STAT pathway repressor that is also a direct target gene of the pathway (Arbouzova and Zeidler, 2006) and was reported to suppress activities of EGF receptor signaling pathways once overexpressed in the wing disc (Callus and Mathey-Prevot, 2002). Although SOCS44A is not a direct target of the JAK/STAT pathway, it can suppress its activity in some tissues while it also appears to up-regulate EGFR signaling (Rawlings et al. 2004). The function of SOCS16D has not been characterized yet. We generated anti-Socs36E polyclonal antibodies and examined Socs36E expression in the wild-type testis. We found that Socs36E protein is highly expressed in somatic cyst cells, and partially overlaps with the Eya protein (Fig. 3D and E).
Fig. 3.
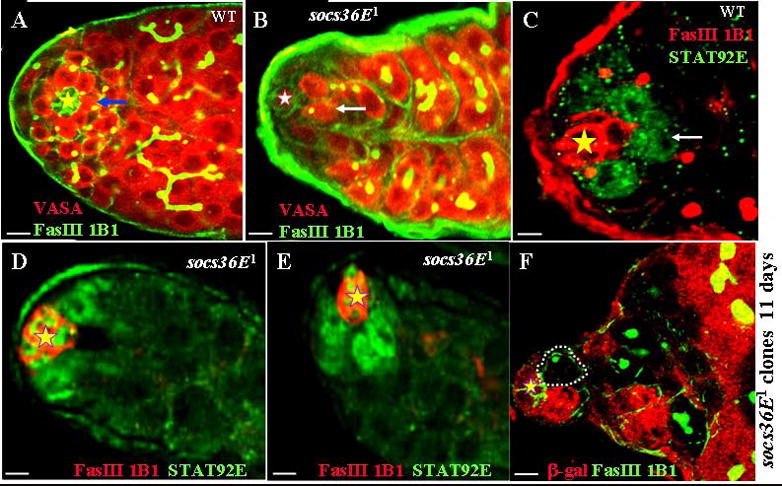
Socs36E nonautonomously regulates GSCs self-renewal and maintenance.
(A, B) Wild-type (A) and socs36E1 (B) testes were immunostained with anti-Fas III (green), mAb 1B1 (green), and anti-Vasa (red). Arrows point to GSCs. (C to E) Wild-type (C) and socs36E1 (D and E) testes were immunostained with anti-Fas III (red), mAb 1B1 (red), and anti-STAT92E (green). Arrow in C points to GSCs. (F) Confocal section through the apex of the testis containing socs36E1 clones 11 days ACI. The testis is immunostained with anti-β-galactosidase (red); anti-Fas III (green); and mAb1B1 (green). One socs36E null GSCs clone (outlined) and a number of socs36E null spermatogonia clones that are β-galactosidase–negative are found in this testis. The hubs are marked by stars. Scale bars represent 10 μm.
By searching available mutations in the FlyBase, we identified a P-element insertion in the socs36E gene (FlyBase gene CG15154; Fig. 2A). The P element EY06665 was inserted into the coding sequence of the socs36E gene 516 bp downstream of the ATG translation starting code (Fig. 2A). The P element over a deletion [Df(2L)T317] that covers the socs36E locus is viable; the male flies are partially sterile and have reduced fertility. We refer to EY06665 as socs36E1. In socs36E1 flies, the socs36E mRNA was undetectable in Northern blotting and RT-PCR analyses (Fig. 2B). The Socs36E protein is also undetectable in socs36E1 and socs36E1/Df(2L)T317 testes (Fig. 2F; data not shown), indicating that socs36E1 is a socs36E null mutation. Further, we found that socs36 block the overexpressed Upd phenotypes associated ectopic cells with GSCs and CPCs features in testis niche (compare Fig. 2G to 2H). The above results suggest that socs36E could be an excellent target and regulator of JAK/STAT pathway in the Drosophila testis niche.
Somatic socs36E nonautonomously regulates the self-renewal of GSCs
To explore further, we first examined Socs36E’s function in GSCs by staining the testes from the wild-type and sosc36E1 mutant flies with anti-Fasciclin III (Fas III-to mark the hub cells), mAb1B1 (to mark the fusome), and anti-Vasa (to mark the all germ cells). While around the hub an average of 8.3 GSCs (n = 56) could be clearly visualized in the wild-type testis (Fig. 3A), all testes of the sosc36E1 flies showed significant GSCs loss, with an average of only 3.4 GSCs (n = 53; Fig. 3B).
To further verify the GSCs loss phenotype of socs36E mutation, we examined the expression of STAT92E protein that is expressed specifically in GSCs and gonialblasts (Brawley and Matunis, 2004; Wang et al. 2006, Fig. 3C) in wild-type testes. Unexpectedly, we found that STAT92E protein is strongly expressed in hub cells as well as all the cells that surround the hub cells in socs36E mutant testes ((Fig. 3D and E). Some of these surrounding cells are likely CPCs because only a few GSCs are left in socs36E mutant testes (Fig. 3B), and many CPCs are tightly associated with the hub (described below in Fig. 4B) in socs36E mutant testes. Since STAT92E protein level is readout of the JAK/STAT signaling (Chen et al. 2002), the above results indicate that socs36E normally represses the JAK/STAT signaling in the hub and somatic cells.
Fig. 4.
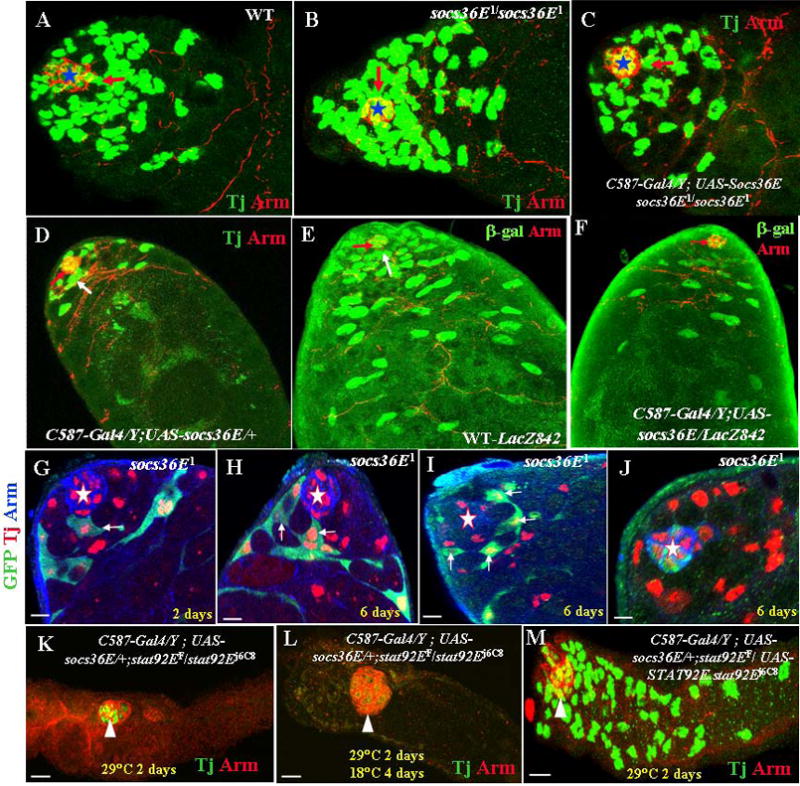
Socs36E regulates CPCs self-renewal through the JAK/STAT signal transduction pathway. (A to C) Testes immunostained with anti-Tj antibody to label somatic cells (green) and anti-Arm antibody to label the hub (red, stars). Their genotypes are (A) wild-type: CPCs (red arrow) contact the Arm-positive hub. (B) A socs36E1/socs36E1; Increased numbers of CPCs contact the hub. (C) C587-Gal4/Y; UAS-socs36E socs36E1/socs36E1. Expression of socs36E in somatic cells rescued the phenotype of socs36E1 to wild-type. (D) A C587-Gal4/Y;UAS-socs36E/+ testis. Overexpression of socs36E (C587-Gal4/UAS-socs36E) resulted in a decrease of Tj-positive somatic cells. (E–F). Testes immunostained with anti-β-galactosidase (green) from an 842 marker and anti-Arm (red, white arrowheads). (E) A wild-type testis with the 842 marker. β-galactosidase (green) is expressed in the hub, CPCs (red arrow), and early somatic cyst cells. (F) A C587-Gal4Y;/UAS-socs36E/842 testis. β-galactosidase stainings are significantly reduced in CPCs and early somatic cyst cells. (G–J) Testes are labeled for Tj (red), GFP (green), and Arm (blue). The hubs are marked by stars. (G) Testis with GFP-marked homozygous socs36E1 mutant clones 2 days ACI. (H, I, and J) Testes with GFP-marked homozygous socs36E1 mutant clones 6 days ACI. GFP-marked CPCs are highlighted by arrows. (K–M) Testes are immunostained with anti-Arm (red) and anti-Tj (green). The hubs are marked by arrowheads. (K) A C587-Gal4/Y; UAS-socs36E/+; stat92EF/stat92Ej6C8 testis shifted to 29°C for 2 days. (L) A C587-Gal4/Y; UAS-socs36E/+; stat92EF/stat92Ej6C8 testis shifted to 29°C for 2 days and then returned to 18°C for 4 days. (M) A C587-Gal4/Y; UAS-socs36E/+; stat92EF/UAS-stat92E.stat92Ej6C8 testis shifted to 29°C for 2 days. Scale bars represent 10 μm.
To determine whether the Socs36E function is required in the germ line or in the surrounding somatic cells for GSC maintenance, we generated socs36E mutant clones in GSCs. 5 and eleven days ACI, comparable numbers of marked wild type and socs36E mutant GSCs were detected. Eleven days ACI, one or more marked socs36E mutant GSCs (Fig. 3F) and daughter cells were found in 62% (18/29) of the testes. In all cases, spermatogenesis was normal, as judged by normal cysts with 16 differentiating spermatocytes. Thus, the socs36E function is not required in the germ cells, and somatic Socs36E regulates the self-renewal of GSCs.
Somatic socs36E regulates CPCs Self-Renewal
We then examined the somatic cell fates in socs36E mutant testes by comparing CPCs change in the wild-type and socs36E mutant testes. In the wild-type testis, there is an average of 18.3 CPCs (n = 60), and the CPCs make only limited contacts to the hub cells through their cytoplasm extension (Fig. 1A and 4A). In socs36E mutant testes, there is an average of 20.2 CPCs (n = 55), and the CPCs directly contact the hub, forming a tight circle around the hub (Fig. 4B). Expressing UAS-socs36E specifically in somatic cells using a C587-Gal4 driver rescued the socs36E1 mutant phenotype, and the CPCs returned to their normal position (Fig. 4C). Therefore, socs36E mutation does not significantly change the total number of CPCs in each testis, rather enhances their physical contact to the hub. Further, overexpressing UAS-socs36E in wild-type testes using the C587-Gal4 driver resulted in a dramatic loss of Tj-expressing cells (Fig. 4D). In wild-type testes, the enhancer trap line 842 drives β-galactosidase expression in the hub, CPCs, and early somatic cysts (Fig. 4E; Fabrizio et al. 2003). In the C587-Gal4/Y; UAS-socs36E/842 testis, the number of β-galactosidase–positive cells decreased drastically (Fig. 4F). In the somatic cell lineage, only CPC are mitotic active (Voog et al. 2008). The increase or decrease of somatic cells is determined by changes in CPCs. Taken together, the above data suggest that Socs36E restricts the self-renewal of CPCs.
To determine whether the Socs36E directly regulates CPCs, we generated socs3E null CPCs clones using the MARCM system (Lee and Luo, 1999) in combination with a c587-Gal4. Two days ACI, 100% (56/56) of the testes carried one or more GFP-marked CPCs and early cyst cells (Fig. 4G; Table 1), and each testis had an average of 1.6 CPCs (Table 1). Six days ACI, 100% (51/51) of the testes carried one or more GFP-marked CPCs and late cyst cells (Fig. 4H and I; Table 1), and each testis had an average 3.7 of CPCs (Table 1). Further, we found that six days ACI some GFP-marked somatic cells were merged into the hub (Fig. 4J). These data suggest that socs36E mutant CPCs have the advantage of staying in the niche or even becoming a part of the niche.
To further examine the interaction between Socs36E and the JAK/STAT signal transduction pathway in CPCs, we expressed UAS-socs36E specifically in somatic cells using a C587-Gal4 driver (C587-Gal4/Y; UAS-socs36E/+) in the stat92Ets mutant background. To our surprise, after shifting the C587-Gal4/Y; UAS-socs36E/+; stat92Ets flies to 29°C from RT for 2 days, the hub moved to the middle of the testis, and all Tj-positive somatic cells had disappeared (Fig. 5K; Table 2). When we returned the C587-Gal4/Y; UAS-socs36E/+; stat92Ets flies to 18°C for 4 days after 2 days at 29°C, the hub remained in the middle of the testis, and none of the Tj-positive somatic cells were restored (Fig. 5L). However, simultaneously expressing UAS-stat92E with UAS-socs36E in somatic cells in the stat92Ets testes (Fig. 5M; Table 2) completely rescued the quick-loss phenotype of somatic cells in the C587-Gal4/Y; UAS-socs36E/+; stat92Ets testes (compare Fig. 5M with Fig. 5K). These data suggest that Socs36E negatively regulates the self-renewal and maintenance of CPCs through the JAK/STAT signal transduction pathway. JAK/STAT signaling is necessary and sufficient for regulating self-renewal and proliferation of CPCs. Expression of socs36E in the stat92Ets testes further reduced the signaling to a nearly null condition and resulted in the rapid loss of somatic cells.
Fig. 5.
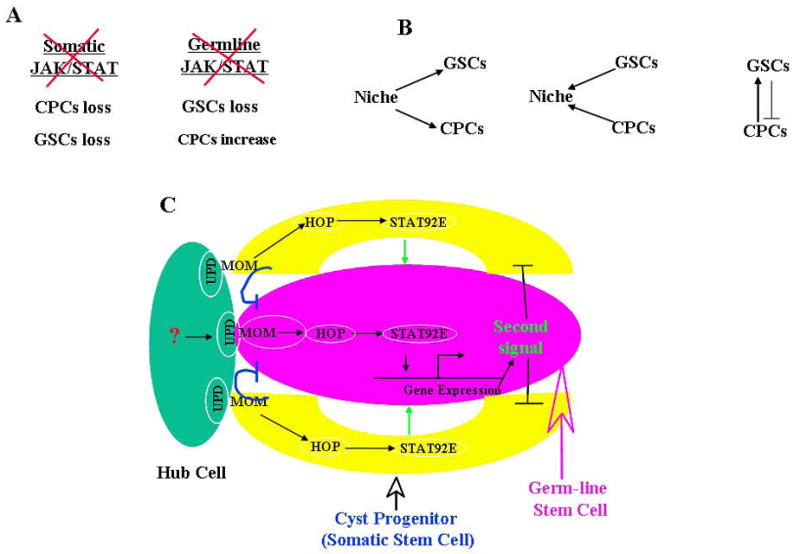
Three regulatory levels of GSCs and CPCs. See text for detail. In (C), the blue inhibitory lines represent CPCs competing with GSCs for the niche; the black inhibitory lines represent GSCs negatively regulating CPCs proliferation.
Somatic socs36E does not regulate the EGF receptor signal transduction pathway
Since it has been shown that disruption of the EGF receptor/Ras/Raf signaling pathway in somatic cells results in GSC overproliferation (Kiger et al. 2000; Tran et al. 2000) and overexpression of socs36E suppresses the EGF receptor signaling in the wing disc (Callus and Mathey-Prevot, 2002), it is possible that the somatic EGF receptor signaling is turned on in the socs36E mutant testis, which will stimulate GSCs to differentiate and vacate the niche for the CPCs. We call this “GSCs-out and CPCs-in” model. However, overexpression of socs36E in somatic cells does not result in GSC overproliferation (data not shown). This result suggests that socs36E in does not suppress the EGF receptor signaling in somatic cells, and Socs36E in the testis may not function through the EGF receptor signal transduction pathway.
To further explore the interaction between the Socs36E and EGF receptor signal transduction pathway in CPCs, we generated ras1 null CPCs clones using the MARCM system (Lee and Luo, 1999) in combination with a c587-Gal4. If Socs36E suppresses the EGF receptor/Ras/Raf signaling in the somatic cells, we would expect the socs36E and ras1 mutant CPCs to have opposite phenotypes. However, we found that socs36E and ras1 mutant CPCs have similar phenotypes. Two days ACI, 100% (48/48) of the testes carried one or more GFP-marked ras1 mutant CPCs and very-early-stage somatic cysts (Supplementary Fig. S3A), and each testis had an average 1.5 of CPCs (Table 1). Six days ACI, 100% (51/51) of the testes carried one or more GFP-marked ras1 mutant CPCs and late-stage somatic cysts (Supplementary Fig. S3B); each testis had an average 3.4 of CPCs (Table 1). Some of the GFP-marked somatic cells were also merged into the hub at six days ACI (Fig. S3C). Further, we stained wild-type and socs36E mutant testes with anti-dpERK and found similar expression pattern (Supplementary Fig. S3 D–E). These data further suggest that Socs36E in the testis does not function through the EGF receptor signal transduction pathway and the “GSC-out and CPCs-in” model is not correct.
Our findings on Socs36E can be summarized in the following five points: (1) Socs36E protein is expressed in somatic cyst cells and may keep the JAK/STAT signaling downregulated in these cells. (2) In wild-type testis, the JAK/STAT signaling is activated in both GSCs and CPCs; in socs36E mutant testis, the JAK/STAT signaling is activated in GSCs, CPCs as well as in the hub cells; socs36E may normally suppress the signaling in the CPCs and hub. (3) In socs36E mutant testis, GSCs are reduced, while CPCs are more tightly associated with the hub; with elevated JAK/STAT signaling, the CPCs may push GSCs out to get closer to the niche. (4) In testes with clones of CPCs lacking socs36E, the number of socs36E mutant CPCs gradually increases over time, and some of the mutant CPCs merge into the hub, indicating that socs36E mutant CPCs have the advantage of staying in the niche or even becoming a part of the niche. (5) Somatic socs36E may not regulate GSCs and CPCs through the EGF receptor signal transduction pathway.
We still do not understand how the somatic cyst Socs36E suppresses the JAK/STAT signaling in CPCs. One possible explanation is that our Socs36E antibody is not sensitive enough to detect low-level Socs36E in CPCs, and the low-level Socs36E is sufficient to keep the JAK/STAT signaling down in CPCs. Another possibility is that Socs36E is expressed only in somatic cyst cells, and, when the JAK/STAT signaling is activated in these cells by generating a socs36E mutation, some of them move up to the niche, push out the wild-type GSCs and CPCs, and become CPCs, or even hub cells. A previous study has demonstrated that daughter cyst cells can re-enter the cell cycle to proliferate in the absence of germ cells, and some of these cyst cells switch to the hub cell fates (Gönczy and DiNardo, 1996), which are similar to the phenotypes of the socs36E mutant testes.
GSCs and CPCs coordinate their self-renewal through the JAK/STAT signaling
To understand how GSCs and CPCs coordinate their self-renewal or differentiation, we manipulated the level of the JAK/STAT signaling in either GSCs or CPCs using the stat92Ets mutation in combination with overexpression of UAS-socs36E, or selectively rescuing Stat92E activity by UAS-stat92E in just one stem cell type. To simplify the description, we referred to C587-Gal4/Y; UAS-socs36E/+; stat92Ets as CPC-null+GCS-ts, which means that in this genotype, the JAK/STAT signaling is null in CPCs and the ts mutation in GSCs. When expressing UAS-socs36E specifically in germ cells using the Nos-Gal4 driver in the stat92Ets mutant background (UAS-socs36E/+; stat92EF/Nos-Gal4.stat92Ej6C8), we referred to the testis as CPC-ts+GSC-null. Similarly, C587-Gal4/Y; UAS-socs36E/+; stat92EF/UAS-stat92E.stat92Ej6C8 was referred to as CPC-wt+GSC-ts. The results are described in detail in supporting online text and briefly summarized in the following two paragraphs:
The somatic JAK/STAT signaling is essential for self-renewal and maintenance of both CPCs and GSCs
We first examined the CPC-null+GCS-ts testes through overexpression of socs36E in somatic cells in the stat92Ets mutant background and found that both CPCs (Fig. 5K) and GSCs (Supplementary fig. 1B and D) were lost quickly. Selectively rescuing stat92E activity in somatic cells (CPC-wt+GSC-ts) not only completely rescued the loss of somatic cells (Fig. 5M), but also significantly rescued the rapid loss of germ cells (Supplementary Fig. S1G). Therefore, removing the JAK/STAT signaling in somatic cells resulted in somatic cell loss, which, in turn, accelerated the differentiation of GSCs and spermatogonia (Fig. 5A). Restoring the JAK/STAT signaling in somatic cells rescued the loss of CPCs and partially rescued the loss of GSCs; the somatic JAK/STAT signaling is essential for self-renewal and maintenance of both CPCs and GSCs.
The germ line JAK/STAT signaling is required for GSC self-renewal and maintenance but negatively regulates these functions in CPCs
We also examined the CPC-ts+GSC-null testes through overexpression of socs36E in germ cells in the stat92Ets mutant background. We found that further reducing the JAK/STAT signaling in germ cells through overexpressing socs36E accelerated germ cell loss (Supplementary Fig. S2D) but caused an increase in somatic cells (Supplementary Fig. S2C). Therefore, accelerated germ cell loss resulted in somatic cell increase (Fig. 5A); the germ line JAK/STAT signaling is required for GSC self-renewal and maintenance, but negatively regulates these functions in CPCs.
Discussion
Niches are restricted and specialized tissue microenvironments that integrate local and systemic signals for the regulation and maintenance for resident stem cells (Morrison, Spradling, 2008). In many animal tissues, many types of stem cells reside together to coordinate proliferation and differentiation. However, the regulatory molecular mechanism is poorly understood due to the difficulty of studying this process in complex mammalian tissues. The Drosophila testis provides an ideal model system for studying this mechanism at cellular and molecular levels (Song and Xie, 2002; Yamashita et al., 2003; Yamashita et al. 2007). In this study, we demonstrated that a single JAK/STAT signal from the niche regulates the self-renewal of both GSCs and CPCs and controls competitiveness for the niche and mutual dependence of GSCs and CPCs in the Drosophila testis niche.
Interaction of niche, GSCs and CPCs in the Drosophila testis
Drosophila GSCs studies demonstrate that two types of stem cells (GSCs and CPCs in testes and GSCs and ESCs in ovary) reside in a close proximity are attached to the same niche cells, the hub. To maintain proper spermatogenesis, the communication between niche, GSCs and CPCs is crucial and need to be coordinated (Hardy et al. 1979; Decotto and Spradling, 2005; Leatherman and DiNardo, 2008). The hub, which express upd, serves as a docking site for GSCs and CPCs (Yamashita et al., 2003) activates the JAK/STAT pathway in GSCs and CPCs for its self-renewal and maintenance (Kiger et al., 2001; Tulina and Matunis, 2001; Brawley and Matunis 2004; Singh et al. 2006; Leatherman and DiNardo, 2008). It has been demonstrated that Bmp signals from the somatic cells maintain GSCs by repressing bam expression in the Drosophila testis (Kawase et al., 2004) and that Jak/STAT signaling leads to production of the BMP ligand Dpp (Wang et al., 2008). Early germ cells send signals to enclosed somatic cells that require the activity of stem cell tumor (stet), a rhomboid-like membrane protease, which activates Egfr ligands in the CPCs (Schulz, et al. 2002) to restrict GSCs self-renewal (Kiger et al. 2000; Tran et al. 2000). Recently, it has been identified that the transcriptional repressor Zfh-1 is a somatic target of Jak/STAT signaling and is necessary and sufficient to maintain CPCs; and GSCs depend on the CPCs for their normal function (Leatherman and DiNardo, 2008). Further, when hopTumL, an activated allele of JAK is expressed in CPCs they observed the accumulation of CPCs and GSCs in testis (Leatherman and DiNardo, 2008). This is similar to female GSCs as its maintenance depends on STAT signaling within the escort cells (Decotto and Spradling, 2005). Furthermore, dlg (discs large) is essentially required in CPCs for their survival, expansion, and differentiation, and for the encapsulation of the GSCs (Papagiannoul and Mechler, 2009).
In addition to above studies in Drosophila testis stem cells and niche interaction, our finding suggests that there are at least three regulatory levels of GSCs and CPCs in the Drosophila testis niche (Fig. 5, B and C). First, the JAK/STAT signaling from the niche regulates the both GSCs and CPCs self-renewal. Second, GSCs and CPCs compete for the niche, and their competitiveness is regulated by the relative strength of the JAK/STAT signaling in the two types of stem cells; SOCS36E, a negative regulator of the JAK/STAT signaling, down-regulates the JAK/STAT signaling in somatic cells, and possibly prevent the somatic cells from out-competing GSCs. Third, GSCs and CPCs mutually regulate and are dependent on each other. The somatic JAK/STAT signal is essential for self-renewal and maintenance of both CPCs and GSCs; the germ line JAK/STAT signal is required for GSCs self-renewal and maintenance, but negatively regulates these functions in CPCs; GSCs are dependent on somatic cells but also restrict somatic cell proliferation to prevent somatic cells from out-competing GSCs.
However, we still do not know how the stem cells regulate each other. The above phenotypes suggest that GSCs may be dependent on CPCs to anchor to their niche, but CPCs may not require GSCs to anchor to their niche. If this is the case, cell adhesion molecules may be the potential factors that mediate the CPCs’ function in helping GSCs anchor to their niche (Wang et al. 2006; Tanentzapf et al. 2007). Removal of CPCs may release GSCs from their anchors. We also demonstrated that germ cells negatively regulate CPCs’ self-renewal and proliferation through the JAK/STAT signal transduction pathway. One possible explanation for this action is that the JAK/STAT signaling in GSCs turns on a secreting factor, which negatively regulates CPCs self-renewal and proliferation (Fig. 5C).
Together, our data suggest that a single JAK/STAT signal can orchestrate the competitive and dependent co-existence of GSCs and CPCs in the Drosophila testis niche, which is consistent with previous study by Issigonis et al. (2009). Recent investigations in other niches increasingly suggest the existence of such complex interactions. In the Drosophila ovary, a feedback loop between stem cells and niche cells has recently been discovered (Song et al., 2007). Similar to Drosophila testis, in mammalian hair follicles, both hair follicle epithelial stem cells and melanocyte stem cells are located in the bulge region of the hair follicle and reside each other during the process of proliferation and maturation (Nishimura et al., 2005; Morrison and Spradling, 2008; Zhang and Li, 2008). In hematopoietic organs, stem cells reside in two distinct niches; interact with either osteoblasts or endothelial cells (Perry and Li, 2007). The competitive and dependent co-existence model of stem cells that we described in the Drosophila testis niche may also apply to these stem cell systems. The fact that two types of stem cells maintain close interaction during development and differentiation suggests the importance of cell coordination to facilitate organogenesis (Zhang and Li, 2008). Whether our findings may be extended to higher vertebrates remains an open question. Nevertheless, in mammals like in Drosophila, spermatogenesis depends on interactions between supportive Sertoli cells and germ cells (Griswold, 1998). Recent studies have demonstrated, however, that the distribution of undifferentiated spermatogonia, which probably includes CPCs, is not random. These cells appear to be preferentially localized close to the vascular network and interstitial cells that exist between adjacent tubules. The close association of stem cells with their niches could be important to expose these cells to systemic factors that may promote their survival, regulate self-renewal and differentiation potential, and communicate damage signals to activate their proliferation (Tavazoie et al. 2008).
Supplementary Material
Acknowledgments
We thank B. Mathey-Prevot, T. Xie, S. DiNardo, C. Dearolf, and the Bloomington stock center for fly stocks; D. Godt, R. Lehmann, and the Developmental Study Hybridoma Bank for antibodies; Stephen Lockett for help on confocal microscope; N. Parrish for help on preparation of the manuscript. This research was supported by the Intramural Research Program of NIH, National Cancer Institute.
References
- Arbouzova NI, Zeidler MP. JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions. Development. 2006;133:2605–2616. doi: 10.1242/dev.02411. [DOI] [PubMed] [Google Scholar]
- Baksa K, Parke T, Dobens LL, Dearolf CR. The Drosophila STAT protein, Stat92E, regulates follicle cell differentiation during oogenesis. Dev Biol. 2002;243:166–175. doi: 10.1006/dbio.2001.0539. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Brawley C, Matunis E. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science. 2004;304:1331–1334. doi: 10.1126/science.1097676. [DOI] [PubMed] [Google Scholar]
- Bunt SM, Hime GR. Ectopic activation of Dpp signalling in the male Drosophila germline inhibits germ cell differentiation. Genesis. 2004;39:84–93. doi: 10.1002/gene.20030. [DOI] [PubMed] [Google Scholar]
- Callus BA, Mathey-Prevot B. SOCS36E, a novel Drosophila SOCS protein, suppresses JAK/STAT and EGF-R signaling in the imaginal wing disc. Oncogene. 2002;21:4812–4821. doi: 10.1038/sj.onc.1205618. [DOI] [PubMed] [Google Scholar]
- Chen HW, Chen X, Oh S, Marinissen MJ, Gutkind JS, Hou XS. mom identifies a receptor for the Drosophila JAK/STAT signal transduction pathway and encodes a protein distantly related to the mammalian cytokine receptor family. Genes Dev. 2002;16:388–398. doi: 10.1101/gad.955202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Turkel N, Hemati N, Fuller MT, Hunt AJ, Yamashita YM. Centrosome misorientation reduces stem cell division during ageing. Nature. 2008;456:599–604. doi: 10.1038/nature07386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin SJ, Nehme NT, Limmer S, Liegeois S, Pospisilik JA, Schramek D, Leibbrandt A, Simoes Rde M, Gruber S, Puc U, Ebersberger I, Zoranovic T, Neely GG, von Haeseler A, Ferrandon D, Penninger JM. Genome-wide RNAi screen identifies genes involved in intestinal pathogenic bacterial infection. Science. 2009;325:340–343. doi: 10.1126/science.1173164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decotto E, Spradling AC. The Drosophila ovarian and testis stem cell niches: similar somatic stem cells and signals. Dev Cell. 2005;9:501–510. doi: 10.1016/j.devcel.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- Fuller MT, Spradling AC. Male and female Drosophila germline stem cells: Two versions of immortality. Science. 2007;316:402–404. doi: 10.1126/science.1140861. [DOI] [PubMed] [Google Scholar]
- Gilboa L, Lehmann R. How different is Venus from Mars? The genetics of germ-line stem cells in Drosophila females and males. Development. 2004;131:4895–4905. doi: 10.1242/dev.01373. [DOI] [PubMed] [Google Scholar]
- Gönczy P, DiNardo S. The germ line regulates somatic cyst cell proliferation and fate during Drosophila spermatogenesis. Development. 1996;122:2437–2447. doi: 10.1242/dev.122.8.2437. [DOI] [PubMed] [Google Scholar]
- Griswold MD. The central role of Sertoli cells in spermatogenesis. Semin Cell Dev Biol. 1998;9:411–416. doi: 10.1006/scdb.1998.0203. [DOI] [PubMed] [Google Scholar]
- Hardy RW, Tokuyasu KT, Lindsley DL, Garavito M. The germinal proliferation center in the testis of Drosophila melanogaster. J Ultrastruct Res. 1979;69:180–190. doi: 10.1016/s0022-5320(79)90108-4. [DOI] [PubMed] [Google Scholar]
- Hou XS, Chou TB, Melnick MB, Perrimon N. The torso receptor tyrosine kinase can activate Raf in a Ras-independent pathway. Cell. 1995;81:63–71. doi: 10.1016/0092-8674(95)90371-2. [DOI] [PubMed] [Google Scholar]
- Hou XS, Melnick MB, Perrimon N. marelle acts downstream of the Drosophila hop/JAK kinase and encodes a protein similar to the mammalian STATs. Cell. 1996;84:411–419. doi: 10.1016/s0092-8674(00)81286-6. [DOI] [PubMed] [Google Scholar]
- Hou SX, Zheng Z, Chen X, Perrimon N. The Jak/STAT pathway in model organisms: emerging roles in cell movement. Dev Cell. 2002;3:765–78. doi: 10.1016/s1534-5807(02)00376-3. [DOI] [PubMed] [Google Scholar]
- Issigonis M, Tulina N, De Cuevas M, Brawley C, Sandler L, Matunis E. JAK-STAT signal inhibition regulates competition in the Drosophila testis stem cell niche. Science. 2009;326:153–156. doi: 10.1126/science.1176817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Kirilly D, Weng C, Kawase E, Song X, Smith S, Schwartz J, Xie T. Differentiation-defective stem cells outcompete normal stem cells for niche occupancy in the Drosophila ovary. Cell Stem Cell. 2008;2:39–49. doi: 10.1016/j.stem.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase E, Wong MD, Ding BC, Xie T. Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the Drosophila testis. Development. 2003;131:1365–1375. doi: 10.1242/dev.01025. [DOI] [PubMed] [Google Scholar]
- Kiger AA, White-Cooper H, Fuller MT. Somatic support cells restrict germline stem cell self-renewal and promote differentiation. Nature. 2000;407:750–754. doi: 10.1038/35037606. [DOI] [PubMed] [Google Scholar]
- Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 2001;294:2542–2545. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- Kirilly D, Spana EP, Perrimon N, Padgett RW, Xie T. BMP signaling is required for controlling somatic stem cell self-renewal in the Drosophila ovary. Dev Cell. 2005;9:651–662. doi: 10.1016/j.devcel.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Leatherman JL, DiNardo S. Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell Stem Cell. 2008;3:44–54. doi: 10.1016/j.stem.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–31. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- Lin H. The stem-cell niche theory: lessons from flies. Nat Rev Genet. 2002;3:931–940. doi: 10.1038/nrg952. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Onieva L, Fernández-Miñán A, González-Reyes A. Jak/Stat signalling in niche support cells regulates dpp transcription to control germline stem cell maintenance in the Drosophila ovary. Development. 2008;35:533–540. doi: 10.1242/dev.016121. [DOI] [PubMed] [Google Scholar]
- Luschnig S, Moussian B, Krauss J, Desjeux I, Perkovic J, Nüsslein-Volhard C. An F1 genetic screen for maternal-effect mutations affecting embryonic pattern formation in Drosophila melanogaster. Genetics. 2004;167:325–342. doi: 10.1534/genetics.167.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science. 2005;307:720–724. doi: 10.1126/science.1099593. [DOI] [PubMed] [Google Scholar]
- Nystul T, Spradling A. An epithelial niche in the Drosophila ovary undergoes long-range stem cell replacement. Cell Stem Cell. 2007;1:277–285. doi: 10.1016/j.stem.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Kai T, Decotto E, Spradling A. The stem cell niche: theme and variations. Curr Opin Cell Biol. 2004;16:693–699. doi: 10.1016/j.ceb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- O’Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002, new surprises in the Jak/Stat pathway. Cell. 2002;109:S121–S131. doi: 10.1016/s0092-8674(02)00701-8. [DOI] [PubMed] [Google Scholar]
- Papagiannouli F, Mechler BM. discs large regulates somatic cyst cell survival and expansion in Drosophila testis. Cell Res. 2009;19:1139–1149. doi: 10.1038/cr.2009.71. [DOI] [PubMed] [Google Scholar]
- Perry JM, Li L. Disrupting the stem cell niche: good seeds in bad soil. Cell. 2007;129:1045–1047. doi: 10.1016/j.cell.2007.05.053. [DOI] [PubMed] [Google Scholar]
- Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J Cell Sci. 2004;117:1281–1283. doi: 10.1242/jcs.00963. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Sarkar A, Parikh N, Hearn SA, Fuller MT, Tazuke SI, Schulz C. Antagonistic roles of Rac and Rho in organizing the germ cell microenvironment. Curr Biol. 17:1253–1258. doi: 10.1016/j.cub.2007.06.048. [DOI] [PubMed] [Google Scholar]
- Schulz C, Kiger AA, Tazuke SI, Yamashita YM, Pantalena- Filho LC, Wood CG, Jones DL, Fuller MT. A misexpression screen reveals effects of bag-of-marbles and TGF-β class signaling on the Drosophila male germ line stem cell lineage. Genetics. 2004;167:707–723. doi: 10.1534/genetics.103.023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng XR, Brawley CM, Matunis EL. Dedifferentiating spermatogonia outcompete somatic stem cells for niche occupancy in the Drosophila testis. Cell Stem Cell. 2009;5:191–203. doi: 10.1016/j.stem.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivdasani AA, Ingham PW. Regulation of stem cell maintenance and transit amplifying cell proliferation by TGF-β signaling in Drosophila spermatogenesis. Curr Biol. 2003;13:2065–2072. doi: 10.1016/j.cub.2003.10.063. [DOI] [PubMed] [Google Scholar]
- Singh SR, Chen X, Hou SX. JAK/STAT signaling regulates tissue outgrowth and male germline stem cell fate in Drosophila. Cell Res. 2005;15:1–5. doi: 10.1038/sj.cr.7290255. [DOI] [PubMed] [Google Scholar]
- Singh SR, Zhen W, Zheng Z, Wang H, Oh SW, Liu W, Zbar B, Schmidt LS, Hou SX. The Drosophila homolog of the human tumor suppressor gene BHD interacts with the JAK-STAT and Dpp signaling pathways in regulating male germline stem cell maintenance. Oncogene. 2006;25:5933–5941. doi: 10.1038/sj.onc.1209593. [DOI] [PubMed] [Google Scholar]
- Singh SR, Liu W, Hou SX. The adult Drosophila malpighian tubules are maintained by multipotent stem cells. Cell Stem Cell. 2007;1:191–203. doi: 10.1016/j.stem.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SR, Hou SX. Immunohistological techniques for studying the Drosophila male germline stem cell. Methods Mol Biol. 2008;450:45–59. doi: 10.1007/978-1-60327-214-8_3. [DOI] [PubMed] [Google Scholar]
- Song X, Xie T. DE-cadherin-mediated cell adhesion is essential for maintaining somatic stem cells in the Drosophila ovary. Proc Natl Acad Sci USA. 2002;99:14813–14818. doi: 10.1073/pnas.232389399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Call GB, Kirilly D, Xie T. Notch signaling controls germline stem cell niche formation in the Drosophila ovary. Development. 2007;134:1071–80. doi: 10.1242/dev.003392. [DOI] [PubMed] [Google Scholar]
- Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- Starr R, Hilton DJ. Negative regulation of the JAK/STAT pathway. Bioessays. 1999;21:47–52. doi: 10.1002/(SICI)1521-1878(199901)21:1<47::AID-BIES6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tanentzapf G, Devenport D, Godt D, Brown NH. Integrin-dependent anchoring of a stem-cell niche. Nat Cell Biol. 2007;9:1413–1418. doi: 10.1038/ncb1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–88. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran J, Brenner TJ, DiNardo S. Somatic control over the germline stem cell lineage during Drosophila spermatogenesis. Nature. 2000;407:754–757. doi: 10.1038/35037613. [DOI] [PubMed] [Google Scholar]
- Tulina N, Matunis E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science. 2001;294:2546–2549. doi: 10.1126/science.1066700. [DOI] [PubMed] [Google Scholar]
- Voog J, D’Alterio C, Jones DL. Multipotent somatic stem cells contribute to the stem cell niche in the Drosophila testis. Nature. 2008;454:1132–1136. doi: 10.1038/nature07173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Singh SR, Zheng Z, Oh SW, Chen X, Edwards K, Hou SX. Rap-GEF signaling controls stem cell anchoring to their niche through regulating DE-cadherin-mediated cell adhesion in the Drosophila testis. Dev Cell. 2006;10:117–26. doi: 10.1016/j.devcel.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Wang L, Li Z, Cai Y. The JAK/STAT pathway positively regulates DPP signaling in the Drosophila germline stem cell niche. J Cell Biol. 2008;180:721–728. doi: 10.1083/jcb.200711022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290:328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- Xie T, Song X, Jin Z, Pan L, Weng C, Chen S, Zhang N. Interactions between stem cells and their niche in the Drosophila ovary. Cold Spring Harb Symp Quant Biol. 2008;73:39–47. doi: 10.1101/sqb.2008.73.014. [DOI] [PubMed] [Google Scholar]
- Yamashita YM, Jones DL, Fuller MT. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 2003;301:1547–1550. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- Yamashita YM, Mahowald AP, Perlin JR, Fuller MT. Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science. 2007;315:518–521. doi: 10.1126/science.1134910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn J, Doormann P, Dorn A, Dorn DC. Apoptosis of male germ-line stem cells after laser ablation of their niche. Stem Cell Res. 2007;1:75–85. doi: 10.1016/j.scr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li L. Stem cell niche: microenvironment and beyond. J Bio Chem. 2008;283:9499–9503. doi: 10.1074/jbc.R700043200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


