SUMMARY
One of the pathological hallmarks in brains of patients with Alzheimer’s disease (AD) is the presence of neuritic plaques, in which amyloid deposits are surrounded by reactive gliosis and dystrophic neurites. Within neuritic plaques, reticulon 3 (RTN3), a homolog of Nogo protein, appears to regulate the formation of both amyloid deposition via negative modulation of BACE1 activity and dystrophic neurites via the formation of RTN3 aggregates. Transgenic mice over-expressing RTN3, but not the other known markers of dystrophic neurites in AD brain, spontaneously develop RTN3-immunoreactive dystrophic neurites. The presence of dystrophic neurites impairs cognition. Blocking abnormal RTN3 aggregation will increase the available RTN3 monomer and is therefore a promising potential therapeutic strategy for enhancing cognitive function in AD patients.
Keywords: Alzheimer’s disease, neuritic plaques, dystrophic neurites, BACE1, reticulon, Nogo, RTN3, RTN3 aggregates, axonal transport, LTP, dendritic spines, Barnes maze, cognitive impairment
Introduction
Alzheimer’s disease (AD), the most common age-related neurodegenerative disorder associated with cognitive impairments, is characterized by the presence of extracellular neuritic plaques and intracellular neurofibrillary tangles (Selkoe, 2005). Neuritic plaques consist of amyloid deposits, which contain mostly insoluble aggregated Aβ peptides (Aβ40/Aβ42, 40 and 42 amino acids) (Glenner and Wong, 1984), reactive gliosis and dystrophic neurites (DNs). DNs, the third quantitatively altered neuronal architecture within neuritic plaques (Onorato et al., 1989), are typically recognizable as bulb and/or ring-like structures by using antibodies specific to ubiquitin or phosphorylated tau (Dickson and Vickers, 2001; Teter and Ashford, 2002). Neurofibrillary tangles contain tau aggregation (Brunden et al., 2009), and amyloid deposition may occur significantly earlier than tau aggregation in tangles and neurites (Tarawneh and Holtzman, 2009).
In this review, we summarize a dual role of reticulon 3 (RTN3) in forming two aspects of neuritic plaques: amyloid deposition and DNs. RTN3 and its family of proteins was initially identified as a negative modulator of BACE1 (He et al., 2004; Murayama et al., 2006; Wojcik et al., 2007). Interestingly, RTN3 also marks DNs, and RTN3-immunoreactive DNs (RIDNs) are more abundant than phosphorylated tau- or ubiquitin-positive DNs in AD brains, particularly the hippocampus (Hu et al., 2007). Remarkably, mice overexpressing RTN3 spontaneously develop DNs (Hu et al., 2007). This is the first in vivo evidence showing that DNs can be formed independent of either amyloid deposition or neurofibrillary tangles in animals. Clearly, RTN3 plays a dual role in AD pathogenesis.
Reticulon/Nogo Proteins
The reticulon (RTN) family has four homologous genes (RTN1 to RTN4) in mammals (Oertle and Schwab, 2003; Yan et al., 2006). Owing to multiple splicing events, the entire RTN protein family is larger. RTN proteins (RTNs) are distinguished by the highly conserved C-terminal reticulon-homology domain (RHD), which is composed of two putative transmembrane domains separated by one hydrophilic loop plus a hydrophilic tail. In silica mining uncovered RTN paralogous genes in invertebrates, plants and fungi (Nziengui et al., 2007).
Mice deficient in RTN4, also called Nogo, are healthy and fertile, but fail to yield the definitive function of Nogo in vivo (Lee et al., 2009). Instead, genetic studies using “model organisms” appear more advantageous in revealing the functions of RTNs. Complete deficiency of RTN genes in S. cerevisiae suggests that RTNs shape the tubular structure of the endoplasmic reticulum (ER) (Voeltz et al., 2006). RTNs have also been implicated in the regulation of nuclear envelope growth (Anderson and Hetzer, 2008; Kiseleva et al., 2007).
Although RTNs are largely present in the ER (Roebroek et al., 1996), confocal examination has also revealed the presence of these proteins on the cell surface, in the Golgi, lipid rafts, axons and growth cones (Chen et al., 2000; Dodd et al., 2005; GrandPre et al., 2000; Hu et al., 2007; Oertle et al., 2003). In accordance with their dynamic localizations, additional functions of RTNs such as regulation of the cellular trafficking of proteins and cholesterol (Harrison et al., 2009; Iwahashi and Hamada, 2003; Liu et al., 2007; Steiner et al., 2004; Wakana et al., 2005) as well as of protein translocation (Zhao and Jantti, 2009) have also been suggested.
The initial connection of RTNs to AD pathogenesis was made with the finding that RTNs interact with Alzheimer’s β-secretase (He et al., 2004). The increased interaction between these two proteins reduces the proteolytic activity of β-secretase (He et al., 2004; Murayama et al., 2006; Wojcik et al., 2007). β-secretase, now widely recognized as BACE1 (Hussain et al., 1999; Lin et al., 2000; Sinha et al., 1999; Vassar et al., 1999; Yan et al., 1999), initiates cleavage of APP to release Aβ, and its production is almost abolished in mice deficient in BACE1, (Vassar et al., 2009). Although BACE1 interacts with all four RTNs in cultured cells, immunohistochemical staining of brain sections shows that RTN3 is mainly co-localized with BACE1 in various neurons (He et al., 2004). Based on this observation, the studies of RTNs in AD have currently focused on RTN3.
RTN3 as a negative modulator of BACE1 activity
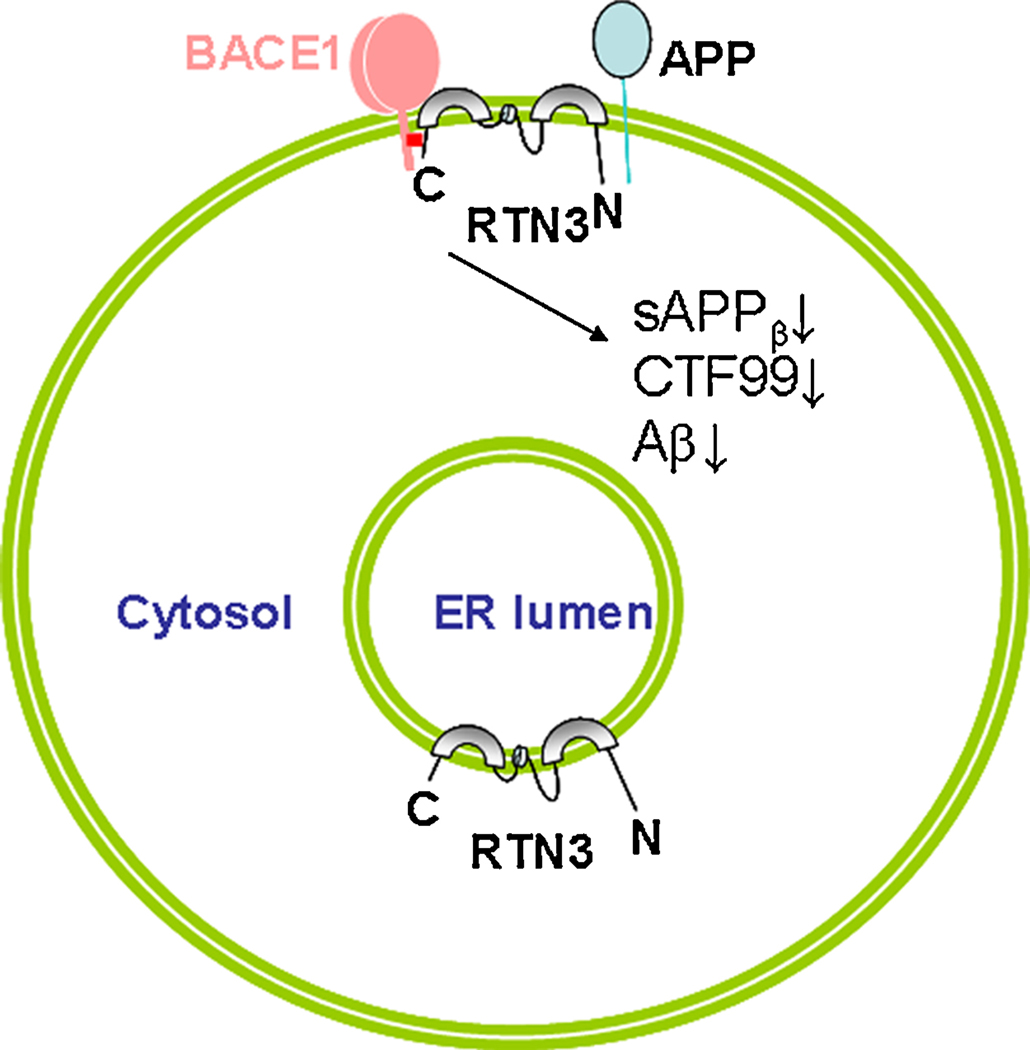
Despite the suggested presence of multiple spliced isoforms (Cai et al., 2005; di Scala et al., 2005), the RTN3 isoform encoding 231 amino acids is the most predominant isoform and is expressed by many cells, including neurons. A membrane topological study as illustrated in Figure 1 revealed that RTN3 adopts a ω-shape structure in which two long hydrophobic segments partially pass within the lipid bilayer and both the N- and C-terminal domains face the cytosolic side of the membrane (He et al., 2007). This topology is similar to the reported membrane topology of RTN4-C (Voeltz et al., 2006). A subtle change in membrane sequence can disrupt the docking of RTN3 within the membrane (He et al., 2007). For instance, the first membrane-anchoring domain of RTN3 possesses a signal peptide sequence that governs the proper insertion of this protein into the lipid bilayer. Mutant RTN3 with a deletion of this domain will be mostly degraded due to misfolding (He et al., 2007). Disruption of the second long hydrophobic stretch may still insert RTN3 into the membrane, but the mutant protein is unstable and degraded rapidly (He et al., 2007).
Figure 1. Interaction of RTN3 with BACE1 on the membrane.
RTN3 adopts a ω-shape membrane topology. The C-terminal region specified in red block mediates the interaction between BACE1 and RTN3. The sequence with two transmembrane domains affects the folding of RTN3 and is consequently important for proper interaction to occur. Increased expression of RTN3 will not only cause reduced levels of BACE1 on the cell surface, but will also create a spatial hindrance between BACE1 and its APP substrate. Both can result in reduction of sAPPβ, CTF99 and Aβ.
A physical interaction between RTN3 and BACE1 in the cellular membrane is a prerequisite for RTN3 to exert its inhibition of BACE1 activity (Figure 1). The highly conserved QID motif located at the C-terminal tail may mediate the interaction between RTN3 and BACE1, as deletion of this motif significantly weakens the interaction (He et al., 2006). Disrupting RTN3 docking on the membrane by mutations in RTN3 transmembrane domains also affects its interaction with BACE1 (Kume et al., 2009b). Consistently, the sequences near the transmembrane domain of BACE1 within the C-terminus mediate the optimal BACE1/RTN3 interaction (He et al., 2006).
Increased expression of RTNs substantially decreases processing of APP by BACE1, resulting in decreased release of Aβ (He et al., 2004; Murayama et al., 2006; Wojcik et al., 2007). This inhibitory modulation is achieved via two pathways. First, an increased interaction between RTN3 and BACE1 creates a spatial hindrance that prevents BACE1 from gaining access to its APP substrate for catalytic cleavage (Figure 1). Increased expression of RTN3 indeed reduces the interaction between BACE1 and APP (He et al., 2004). Second, BACE1 is biosynthesized in the ER and matures in the Golgi, where the prodomain is removed and carbohydrate moieties are added to four Asp residues (Vassar et al., 2009). Subcellular fractionation and surface labeling experiments show that increased expression of RTN3 in cells can arrest BACE1 in the ER compartments, thereby reducing transport of BACE1 to the endosomal compartments and cell surface (Shi et al., 2009b). Normally, the endosomal compartment has a more acidic environment in which the proteolytic processing of APP by BACE1 is favored. The increased retention of BACE1 in the ER compartment would therefore also reduce Aβ production because of the near neutral pH environment in the ER. Lowering the expression of RTN3 by RNAi not only increases the exit of BACE1 into late secretory compartments, but also allows the enhanced interaction of BACE1 with APP. Thus increased generation of Aβ was indeed seen in cells as a result of knocking down RTN3 expression (He et al., 2004).
The above in vitro experiments were further substantiated by utilizing transgenic mice over-expressing the human RTN3 transgene under the control of a murine prion promoter (Tg-RTN3) (Shi et al., 2009b). These transgenic mice express about five fold more RTN3 than wild-type mice and exhibit decreased processing of APP, mostly in cortical regions. When this line of Tg-RTN3 mice was crossed with “bi-transgenic” mice expressing Swedish mutant APP and PS1ΔE9 (Borchelt et al., 1997), amyloid deposition in the cerebral cortex was significantly reduced in the generated “triple transgenic” Tg-R3PA mice, compared to the “bi-transgenic” APP/PS1ΔE9 mice. Both Aβ1–40 and Aβ1–42 levels were significantly lowered in the same area, consistent with the reduction of amyloid plaques.
The role of RTN3 in forming DNs
The reduction of amyloid deposition in Tg-R3PA mice is not uniform, as it is less substantial in the hippocampal region (Shi et al., 2009b). This sub-regional disparity appears counterintuitive if another important phenotype of Tg-RTN3 mice (to be discussed below) is not taken into consideration.
Previous immunohistochemical examinations show that antibodies specific to RTN3 mark abundant DNs surrounding amyloid plaques, more evident in the hippocampal region (Hu et al., 2007). Compared to other common DN markers discussed earlier such as phosphorylated tau or ubiquitin, RIDNs are apparently more abundant because each AT8-positive (phosphorylated tau) or ubiquitinpositive DNs also contain RTN3, whereas many RIDNs do not contain phosphorylated tau or ubiquitin (Figure 2). Notably, another RTN3 antibody failed to label DNs (Kume et al., 2009a) and this discrepancy is perhaps related to the detection sensitivity of these antibodies. Nevertheless, Nogo antibody appears to also mark DNs (Park et al., 2006).
Figure 2. RIDNs are abundantly present in brains of AD cases.
Postmortem brain sections were stained with antibody R458 for RTN3 (green), ubiquitin (red) or AT8 for phosphorylated tau (red). Under the staining conditions, both ubiquitin and AT8 antibodies recognized neurofibrillary tangles and dystrophic neurites. However, RTN3 immunoreactive dystrophic neurites were obviously more abundant in the hippocampal region of AD cases. (The merged figures were previously published in Hu et al. (Hu et al., 2007))
RTN3 is not just a more encompassing marker for DNs in AD brains because transgenic mice overexpressing RTN3 can produce RIDNs in their hippocampus and more in the CA1 region (Hu et al., 2007). The formation of DNs in Tg-RTN3 mice is an active process, as it relies on expression levels of RTN3, has a preferred location and aging process, and can occur completely independent of amyloid deposition or neurofibrillary tangles. The occurrence of RIDNs in Tg-RTN3 mice is not attributable to an overexpression artifact because similar RIDNs are found in the hippocampus of normal elderly mice (Shi et al., 2009a).
When looking at the elderly mouse brain, age-dependent RIDNs can actually be classified into two populations: dispersed and clustered DNs. RTN3 is present in both of these populations, whereas ubiquitin, neurofilament and APP only mark clustered DNs (Shi et al., 2009a). The morphology of dispersed DNs is similar to that of RIDNs in Tg-RTN3 mice, while the morphology of clustered DNs more resembles the DNs seen surrounding amyloid plaques. The results from studies of aged mouse brain not only support the high abundance of RIDNs in AD brains, but also suggest that RTN3 may play an important role in forming both types of DNs. The clustered RIDNs in aged brains may potentially evolve from dispersed RIDNs.
RIDNs contain RTN3 aggregates
Ultrastructural examination of RIDNs in AD brains by pre-embedding immuno-electron microscopy (EM) revealed a recognizable aggregative structure, suggesting aggregation of RTN3 (Hu et al., 2007). Examinations of Tg-RTN3 mouse samples by EM further confirmed the presence of RTN3-positive aggregates in DNs (Hu et al., 2007). These RTN3 aggregates are commonly found in close proximity to the axonal terminus where normal synaptic vesicles and synapses are visible, but not in areas of typical dendritic structures (Hu et al., 2007), suggesting their origins of axonal swelling.
Biochemically, the high molecular weight RTN3 (HMW-RTN3) aggregates are identifiable on a western blot in samples from AD brains and Tg-RTN3 mouse brain, and their levels are correlative with the abundance of RIDNs (Hu et al., 2007). In vitro biochemical studies further confirm that RTN3 is capable of forming RTN3 oligomers (Hu et al., 2007; Shibata et al., 2008). Together, these data suggest that excessive aggregates of RTN3 are accumulated in axonal regions and cause swelling of axons in their morphology.
The effects of RIDNs on amyloid deposition
If RIDNs are formed prior to amyloid plaques, as shown in the case of Tg-R3PA mouse hippocampus, then preformed RIDNs appear to adversely affect the process of amyloid deposition in the hippocampus (Shi et al., 2009b). Although high BACE1 levels were observed in an annulus around Aβ42-positive plaque cores (Zhao et al., 2007), RIDNs did not overlap with this annulus BACE1 immunoreactivity (Shi et al., 2009b), suggesting partial segregation of BACE1 and aggregated RTN3 in neuritic regions. If RTN3 forms aggregates and accumulates in the neuritic regions, less RTN3 is expected to modulate BACE1 activity. Such a reduction in available RTN3 may potentially offset effects of overexpressed RTN3 on Aβ generation and amyloid deposition in Tg-R3PA hippocampus as discussed earlier. Hence, the presence of RIDNs prior to the formation of amyloid plaques will interfere with the negative modulation of BACE1 activity. The study is the first to demonstrate that preformed RIDNs, largely in the CA1 region of R3PA mice, affect the process of amyloid deposition.
The presence of RIDNs impairs cognitive function
Three lines of evidence demonstrate impaired hippocampal function upon formation of RIDNs in Tg-RTN3 hippocampi. First, in 10-month-old Tg-RTN3 CA1 region, dendritic segments, apical trees and spines were visibly fewer compared to the control, and basal and apical dendritic aborizations were also significantly less extended (Shi et al., 2009a). These defects appear more restricted to the CA1 region where RIDNs are predominantly present. Second, 10-month-old Tg-RTN3 performed poorly compared to their wild-type littermates on the Barnes maze test, which has been shown to be dependent on a functional hippocampus (Barnes et al., 1990), revealing their impaired learning and memory (Hu et al., 2007). Third, Tg-RTN3 mice exhibit reduced LTP as recorded on hippocampal slices (Hu et al., 2007). A reduction in LTP together with the reduced dendritic spine density indicate impairment in synaptic plasticity, and helps explain the impaired spatial learning and memory observed in Tg-RTN3 mice in the Barnes maze task. Collectively, these data suggest that DNs in Tg-RTN3 mice produce impairments in spatial learning and memory as well as synaptic plasticity, implying that the occurrence of abundant RIDNs may contribute to cognitive dysfunction in AD. Since Tg-RTN3 mice mimic and accelerate the natural occurrence of RIDNs in normal elderly mice, this animal model will be particularly useful for testing drugs aimed at improving cognitive function in AD patients with cognitive impairments.
Axonal dystrophy of RIDNs
How increased expression of RTN3 in hippocampal neurons facilitates formation of RIDNs is intriguing. Although RTN3 is largely localized in the ER compartment, its presence in the axon and growth cone suggests axonal transport of this protein. Accumulation of RTN3 aggregates in axonal termini raises an interesting hypothesis that the transport of RTN3 exists as a delicate, bidirectional equilibrium and accelerated transport via the anterograde pathway but not the retrograde pathway causes excessive accumulation of RTN3 in axonal terminus.
This hypothesis was supported by examining axonally transported proteins in ligated sciatic nerves from wild-type and Tg-RTN3 mice. Unlike the favored anterograde transport of APP and synapsin-1 in wild-type sciatic nerves, RTN3 is transported normally in both directions (Shi et al., 2009a). However, in Tg-RTN3 sciatic nerves, high levels of RTN3 apparently increase the anterograde transport of RTN3, APP and synapsin-1. Perhaps, RTN3 is a structural membrane protein of a vesicle that is transported via kinesin-1-mediated machinery. When the quantity of RTN3-containing vesicles is increased, these vesicles can then be more readily transported to the axonal terminus and thus accumulated RTN3 may form aggregates and cause swelling of the neuritic terminus (see illustration in Figure 3). This leads to axon degeneration and consequently reduced synaptic transmission, which ultimately leads to cognitive impairment.
Figure 3. Hypothetical formation of RIDNs.
RTN3 is normally localized along the axon and in the growth cone. Its transport in the axon is expected to be bidirectional. When the levels of RTN3 are high, RTN3 is more transported in the anterograde direction but perhaps not in the retrograde direction. This imbalance causes an accumulation of RTN3 in the axonal region, which may form aggregates and cause axonal swelling. Transport of nutrients and other essential materials along the axon may therefore be impaired, eventually causing axonal degeneration. These altered or swollen axonal termini containing either RTN3 (green) alone or a combination of RTN3 and other dystrophic neurite marker proteins such as APP and ubiquitin (red) are a component of neuritic plaques and may lead to deficits in synaptic transmission and plasticity. Such deficits manifest as cognitive impairment in disease states.
Why RIDNs are predominantly formed in the CA1 region is another interesting question to be answered. Whether CA1 RTN3 is more readily prone to becoming clogged during axonal transport remains to be tested in future experiments. Alternatively, there may be a CA1-enriched factor that regulates the balanced transport of RTN3 in this region, and therefore increased expression of RTN3 may disrupt this regulation. In any case, altered or swollen axonal termini may in turn lead to deficits in synaptic transmission and plasticity (Hu et al., 2007; Rosenzweig and Barnes, 2003; Shi et al., 2009a).
Concluding remarks
Brains of AD patients often show altered axonal structures, the occurrence of dystrophic neurites, synaptic loss, and neuronal loss, and these alterations are attributable to the excessive accumulation of Aβ (Hardy and Selkoe, 2002). Ideally, therapeutic blockage of Aβ production could reduce all of the above changes. However, the results from Aβ vaccination trials with AN-1792 showed that, despite an observed reduction in plaques, DNs remained and cerebral amyloid angiopathy persisted in postmortem brains (Nicoll et al., 2006). This observation is perhaps not surprising, given that RTN3 aggregate-containing RIDNs are unlikely to be reduced by this treatment. Similarly, the occurrence of neurofibrillary tangles is also regarded as a downstream event of amyloid deposition, but the reduction of Aβ alone is not sufficient to ameliorate behavioral deficits if tau aggregation is still present (LaFerla and Oddo, 2005) (Oddo et al., 2006). Hence, effective therapeutic treatment of AD will require a “cocktail” approach that includes inhibition of aggregation of Aβ, tau and RTN3. We anticipate that compound drugs that can reduce these aggregates will be more effective at improving cognitive functions in AD patients. The current focus on RIDNs offers a new approach for the future discovery of drugs that inhibit the formation of neuritic plaques or delay the possible transition from diffuse plaques to neuritic plaques. Therefore pharmacological inhibition of the formation of RIDNs may be an effective strategy for ameliorating cognitive decline in the elderly and in patients with AD.
Acknowledgements
Authors wish to thank Dr. Chris Nelson for the critical reading of this manuscript. This work is partially supported by NIH grants to RY (AG025493), and awards from Ralph Wilson Foundation and Alzheimer’s Association to RY. A postdoctoral fellowship from American Health Assistance Foundation is awarded to MP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Anderson DJ, Hetzer MW. Reshaping of the endoplasmic reticulum limits the rate for nuclear envelope formation. J. Cell Biol. 2008;182:911–924. doi: 10.1083/jcb.200805140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA, Markowska AL, Ingram DK, Kametani H, Spangler EL, Lemken VJ, Olton DS. Acetyl-1-carnitine. 2: Effects on learning and memory performance of aged rats in simple and complex mazes. Neurobiol. Aging. 1990;11:499–506. doi: 10.1016/0197-4580(90)90110-l. [DOI] [PubMed] [Google Scholar]
- Borchelt DR, Ratovitski T, van Lare J, Lee MK, Gonzales V, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997;19:939–945. doi: 10.1016/s0896-6273(00)80974-5. [DOI] [PubMed] [Google Scholar]
- Brunden KR, Trojanowski JQ, Lee VM. Advances in tau-focused drug discovery for Alzheimer's disease and related tauopathies. Nat. Rev. Drug Discov. 2009;8:783–793. doi: 10.1038/nrd2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Saiyin H, Lin Q, Zhang P, Tang L, Pan X, Yu L. Identification of a new RTN3 transcript, RTN3-A1, and its distribution in adult mouse brain. Brain Res. Mol. Brain Res. 2005;138:236–243. doi: 10.1016/j.molbrainres.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- di Scala F, Dupuis L, Gaiddon C, de Tapia M, Jokic N, Gonzalez de Aguilar JL, Raul JS, Ludes B, Loeffler JP. Tissue specificity and regulation of the N-terminal diversity of reticulon 3. Biochem. J. 2005;385:125–134. doi: 10.1042/BJ20040458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson TC, Vickers JC. The morphological phenotype of beta-amyloid plaques and associated neuritic changes in Alzheimer's disease. Neuroscience. 2001;105:99–107. doi: 10.1016/s0306-4522(01)00169-5. [DOI] [PubMed] [Google Scholar]
- Dodd DA, Niederoest B, Bloechlinger S, Dupuis L, Loeffler JP, Schwab ME. Nogo-A, -B, and -C are found on the cell surface and interact together in many different cell types. J. Biol. Chem. 2005;280:12494–12502. doi: 10.1074/jbc.M411827200. [DOI] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- GrandPre T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Harrison KD, Miao RQ, Fernandez-Hernando C, Suarez Y, Davalos A, Sessa WC. Nogo-B receptor stabilizes Niemann-Pick type C2 protein and regulates intracellular cholesterol trafficking. Cell Metab. 2009;10:208–218. doi: 10.1016/j.cmet.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Hu X, Shi Q, Zhou X, Lu Y, Fisher C, Yan R. Mapping of Interaction Domains Mediating Binding between BACE1 and RTN/Nogo Proteins. J. Mol. Biol. 2006;363:625–634. doi: 10.1016/j.jmb.2006.07.094. [DOI] [PubMed] [Google Scholar]
- He W, Lu Y, Qahwash I, Hu XY, Chang A, Yan R. Reticulon family members modulate BACE1 activity and amyloid-beta peptide generation. Nat. Med. 2004;10:959–965. doi: 10.1038/nm1088. [DOI] [PubMed] [Google Scholar]
- He W, Shi Q, Hu X, Yan R. The membrane topology of RTN3 and its effect on binding of RTN3 to BACE1. J. Biol. Chem. 2007;282:29144–29151. doi: 10.1074/jbc.M704181200. [DOI] [PubMed] [Google Scholar]
- Hu X, Shi Q, Zhou X, He W, Yi H, Yin X, Gearing M, Lev A, Yan R. Transgenic mice overexpressing reticulon 3 develop neuritic abnormalities. EMBO J. 2007;26:2755–2767. doi: 10.1038/sj.emboj.7601707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain I, Powell D, Howlett DR, Tew DG, Meek TD, Chapman C, Gloger IS, Murphy KE, Southan CD, Ryan DM, Smith TS, Simmons DL, Walsh FS, Dingwall C, Christie G. Identification of a novel aspartic protease (Asp 2) as beta-secretase. Mol. Cell Neurosci. 1999;14:419–427. doi: 10.1006/mcne.1999.0811. [DOI] [PubMed] [Google Scholar]
- Iwahashi J, Hamada N. Human reticulon 1-A and 1-B interact with a medium chain of the AP-2 adaptor complex. Cell Mol. Biol. 2003:OL467–OL471. (Noisy. -le-grand) 49 Online Pub, [PubMed] [Google Scholar]
- Kiseleva E, Morozova KN, Voeltz GK, Allen TD, Goldberg MW. Reticulon 4a/NogoA locates to regions of high membrane curvature and may have a role in nuclear envelope growth. J. Struct. Biol. 2007;160:224–235. doi: 10.1016/j.jsb.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume H, Konishi Y, Murayama KS, Kametani F, Araki W. Expression of reticulon 3 in Alzheimer's disease brain. Neuropathol. Appl. Neurobiol. 2009a;35:178–188. doi: 10.1111/j.1365-2990.2008.00974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume H, Murayama KS, Araki W. The two-hydrophobic domain tertiary structure of reticulon proteins is critical for modulation of beta-secretase BACE1. J. Neurosci. Res. 2009b;13:2963–2972. doi: 10.1002/jnr.22112. [DOI] [PubMed] [Google Scholar]
- LaFerla FM, Oddo S. Alzheimer's disease: Abeta, tau and synaptic dysfunction. Trends Mol. Med. 2005;11:170–176. doi: 10.1016/j.molmed.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Lee JK, Chan AF, Luu SM, Zhu Y, Ho C, Tessier-Lavigne M, Zheng B. Reassessment of corticospinal tract regeneration in Nogo-deficient mice. J. Neurosci. 2009;29:8649–8654. doi: 10.1523/JNEUROSCI.1864-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Koelsch G, Wu S, Downs D, Dashti A, Tang J. Human aspartic protease memapsin 2 cleaves the beta-secretase site of beta-amyloid precursor protein. Proc. Natl. Acad. Sci. U. S. A. 2000;97:1456–1460. doi: 10.1073/pnas.97.4.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Vidensky S, Ruggiero AM, Maier S, Sitte HH, Rothstein JD. Reticulon RTN2B regulates trafficking and function of neuronal glutamate transporter EAAC1. J. Biol. Chem. 2008;283:6561–6571. doi: 10.1074/jbc.M708096200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama KS, Kametani F, Saito S, Kume H, Akiyama H, Araki W. Reticulons RTN3 and RTN4-B/C interact with BACE1 and inhibit its ability to produce amyloid beta-protein. Eur. J. Neurosci. 2006;24:1237–1244. doi: 10.1111/j.1460-9568.2006.05005.x. [DOI] [PubMed] [Google Scholar]
- Nicoll JA, Barton E, Boche D, Neal JW, Ferrer I, Thompson P, Vlachouli C, Wilkinson D, Bayer A, Games D, Seubert P, Schenk D, Holmes C. Abeta species removal after abeta42 immunization. J. Neuropathol. Exp. Neurol. 2006;65:1040–1048. doi: 10.1097/01.jnen.0000240466.10758.ce. [DOI] [PubMed] [Google Scholar]
- Nziengui H, Bouhidel K, Pillon D, Der C, Marty F, Schoefs B. Reticulon-like proteins in Arabidopsis thaliana: structural organization and ER localization. FEBS Lett. 2007;581:3356–3362. doi: 10.1016/j.febslet.2007.06.032. [DOI] [PubMed] [Google Scholar]
- Oddo S, Vasilevko V, Caccamo A, Kitazawa M, Cribbs DH, LaFerla FM. Reduction of soluble Abeta and tau, but not soluble Abeta alone, ameliorates cognitive decline in transgenic mice with plaques and tangles. J. Biol. Chem. 2006;281:39413–39423. doi: 10.1074/jbc.M608485200. [DOI] [PubMed] [Google Scholar]
- Oertle T, Schwab ME. Nogo and its paRTNers. Trends Cell Biol. 2003;13:187–194. doi: 10.1016/s0962-8924(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Oertle T, van der Haar ME, Bandtlow CE, Robeva A, Burfeind P, Buss A, Huber AB, Simonen M, Schnell L, Brosamle C, Kaupmann K, Vallon R, Schwab ME. Nogo-A inhibits neurite outgrowth and cell spreading with three discrete regions. J. Neurosci. 2003;23:5393–5406. doi: 10.1523/JNEUROSCI.23-13-05393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onorato M, Mulvihill P, Connolly J, Galloway P, Whitehouse P, Perry G. Alteration of neuritic cytoarchitecture in Alzheimer disease. Prog. Clin. Biol. Res. 1989;317:781–789. [PubMed] [Google Scholar]
- Park JH, Gimbel DA, GrandPre T, Lee JK, Kim JE, Li W, Lee DH, Strittmatter SM. Alzheimer precursor protein interaction with the Nogo-66 receptor reduces amyloid-beta plaque deposition. J. Neurosci. 2006;26:1386–1395. doi: 10.1523/JNEUROSCI.3291-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebroek AJ, Ayoubi TA, van de Velde HJ, Schoenmakers EF, Pauli IG, Van de Ven WJ. Genomic organization of the human NSP gene, prototype of a novel gene family encoding reticulons. Genomics. 1996;32:191–199. doi: 10.1006/geno.1996.0105. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog. Neurobiol. 2003;69:143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Defining molecular targets to prevent Alzheimer disease. Arch. Neurol. 2005;62:192–195. doi: 10.1001/archneur.62.2.192. [DOI] [PubMed] [Google Scholar]
- Shi Q, Hu X, Prior M, Yan R. The occurrence of aging-dependent reticulon 3 immunoreactive dystrophic neurites decreases cognitive function. J. Neurosci. 2009a;29:5108–5115. doi: 10.1523/JNEUROSCI.5887-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Prior M, He W, Tang X, Hu X, Yan R. Reduced amyloid deposition in mice overexpressing RTN3 is adversely affected by preformed dystrophic neurites. J. Neurosci. 2009b;29:9163–9173. doi: 10.1523/JNEUROSCI.5741-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata Y, Voss C, Rist JM, Hu J, Rapoport TA, Prinz WA, Voeltz GK. The reticulon and DP1/Yop1p proteins form immobile oligomers in the tubular endoplasmic reticulum. J. Biol. Chem. 2008;283:18892–18904. doi: 10.1074/jbc.M800986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Anderson JP, Barbour R, Basi GS, Caccavello R, Davis D, Doan M, Dovey HF, Frigon N, Hong J, Jacobson-Croak K, Jewett N, Keim P, Knops J, Lieberburg I, Power M, Tan H, Tatsuno G, Tun,g J, Schenk D, Seubert P, Suomensaari SM, Wang S, Walker D, Zhao J, McConlogue L, John V. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature. 1999;402:537–540. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]
- Steiner P, Kulangara K, Sarria JC, Glauser L, Regazzi R, Hirling H. Reticulon 1-C/neuroendocrine-specific protein-C interacts with SNARE proteins. J. Neurochem. 2004;89:569–580. doi: 10.1111/j.1471-4159.2004.02345.x. [DOI] [PubMed] [Google Scholar]
- Tarawneh R, Holtzman DM. Critical issues for successful immunotherapy in Alzheimer's disease: development of biomarkers and methods for early detection and intervention. CNS. Neurol. Disord. Drug Targets. 2009;8:144–159. doi: 10.2174/187152709787847324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teter B, Ashford JW. Neuroplasticity in Alzheimer's disease. J. Neurosci. Res. 2002;70:402–437. doi: 10.1002/jnr.10441. [DOI] [PubMed] [Google Scholar]
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- Vassar R, Kovacs DM, Yan R, Wong PC. The {beta}-Secretase Enzyme BACE in Health and Alzheimer's Disease: Regulation, Cell Biology, Function, and Therapeutic Potential. J. Neurosci. 2009;29:12787–12794. doi: 10.1523/JNEUROSCI.3657-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell. 2006;124:573–586. doi: 10.1016/j.cell.2005.11.047. [DOI] [PubMed] [Google Scholar]
- Wakana Y, Koyama S, Nakajima K, Hatsuzawa K, Nagahama M, Tani K, Hauri HP, Melancon P, Tagaya M. Reticulon 3 is involved in membrane trafficking between the endoplasmic reticulum and Golgi. Biochem. Biophys. Res. Commun. 2005;334:1198–1205. doi: 10.1016/j.bbrc.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Wojcik S, Engel WK, Yan R, McFerrin J, Askanas V. NOGO is increased and binds to BACE1 in sporadic inclusion-body myositis and in AbetaPP-overexpressing cultured human muscle fibers. Acta Neuropathol. (Berl) 2007;114:517–526. doi: 10.1007/s00401-007-0281-y. [DOI] [PubMed] [Google Scholar]
- Yan R, Bienkowski MJ, Shuck ME, Miao H, Tory MC, Pauley AM, Brashier JR, Stratman NC, Mathews WR, Buhl AE, Carter DB, Tomasselli AG, Parodi LA, Heinrikson RL, Gurney ME. Membrane-anchored aspartyl protease with Alzheimer's disease beta-secretase activity. Nature. 1999;402:533–537. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- Yan R, Shi Q, Hu X, Zhou X. Reticulon proteins: emerging players in neurodegenerative diseases. Cell Mol. Life Sci. 2006;63:877–889. doi: 10.1007/s00018-005-5338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Fu Y, Yasvoina M, Shao P, Hitt B, O'Connor T, Logan S, Maus E, Citron M, Berry R, Binder L, Vassar R. Beta-site amyloid precursor protein cleaving enzyme 1 levels become elevated in neurons around amyloid plaques: implications for Alzheimer's disease pathogenesis. J. Neurosci. 2007;27:3639–3649. doi: 10.1523/JNEUROSCI.4396-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Jantti J. Functional characterization of the trans-membrane domain interactions of the Sec61 protein translocation complex beta-subunit. BMC. Cell Biol. 2009;10:76. doi: 10.1186/1471-2121-10-76. [DOI] [PMC free article] [PubMed] [Google Scholar]