Abstract
Objectives. To characterize the baseline tendon friction rubs (TFRs) in early dcSSc and to evaluate the association of change in TFR over 6 and 12 months with changes in modified Rodnan skin score (MRSS) and HAQ-Disability Index (HAQ-DI) over 12 and 24 months, respectively.
Methods. We analysed data from the d-Pen study, a 2-year study in early dcSSc (⩽18 months from first non-Raynaud’s symptom). TFR was scored as present/absent at seven anatomical sites at baseline and every 6 months thereafter. Multivariable linear regression models assessed associations between TFR and change in MRSS, and change in the HAQ-DI, over 12 and 24 months, respectively. Covariates included baseline TFR, change in the TFR over 6 and 12 months, age, sex, duration of SSc, MRSS, and tender joint count and swollen joint count (SJC).
Results. Forty-nine (37%) of 134 patients had TFR at baseline, 50% had resolution of their TFR, whereas 21% developed new TFRs. Patients with baseline TFRs were likely to be Caucasian (86 vs 58%) and had a higher HAQ-DI score (P = 0.008). In regression analyses, change in TFR (P = 0.04) and baseline MRSS (P = 0.03) predicted change in MRSS over a 12-month period (Model R2 = 0.14). For the HAQ-DI model, independent predictors were change in TFR at 6 months (P = 0.008) and baseline SJC (P = 0.04, Model R2 = 0.19). Results were similar for 24-month models.
Conclusions. We document the presence of TFR very early in the course of dcSSc. Changes in TFR over 6 and 12 months predict changes in MRSS and HAQ-DI over 12 and 24 months, respectively.
Keywords: Early scleroderma, Diffuse SSc, D-Penicillamine study, Tendon friction rubs, Scleroderma clinical trial
Introduction
SSc is a chronic, multi-system CTD characterized by inflammation, autoimmunity, functional and structural abnormalities in small blood vessels resulting in fibrosis of the skin and visceral organs. SSc is usually categorized into two major subsets of SSc, lcSSc and dcSSc [1]. Since the natural course, morbidity and mortality vary markedly between dcSSc and lcSSc, it is very important to make an early diagnosis of SSc and to ascertain whether they are lcSSc or dcSSc [1]. Among other predictors, tendon friction rubs (TFRs) were a significant predictor of developing dcSSc in a large US scleroderma cohort [2]. TFR, a physical sign, was originally described in SSc by Westphal [3] in 1876 and further characterized by Shulman et al. [4] in 1961 as grating sensations to the fibrinous deposits on the surface of the tendon sheaths and overlying fascia. Rodnan and Medsger [5] described this finding as a ‘leathery crepitus’ on palpation of knees, wrists, fingers and ankles during motion.
Using a large US scleroderma cohort of both dcSSc and lcSSc with an average duration of ⩾3 years, Steen and colleagues [2, 6] showed that the presence of TFR is associated with severe skin thickening, joint contractures, cardiac and renal involvement and poor survival in dcSSc. However, these findings have not been validated in other observational SSc cohorts or in the context of a controlled clinical trial.
Therefore, our current objective was to assess the prevalence and characteristics of TFR in a large early dcSSc randomized controlled trial (RCT) and to determine whether baseline TFR and change in TFR was associated with a change in the modified Rodnan skin score (MRSS) [7] and HAQ-Disability Index (HAQ-DI) [8, 9]. We chose MRSS and HAQ-DI as our outcomes due to relatively short duration of the RCT. Our hypotheses were that baseline TFR will be associated with greater disease severity (as exemplified by higher baseline MRSS and HAQ-DI scores), and change in TFR will predict change in MRSS and HAQ-DI.
Methods
We analysed data from the d-Pen study, a 2-year, multi-centre US study that randomized 134 subjects with early diffuse SSc (⩽18 months from first non-Raynaud’s symptom) to receive 750–1000 mg/day of d-Pen vs 125 mg of d-Pen every other day [9]. Since there were no statistical differences in skin scores, lung function and mortality between high- and low-dose therapy, data from both treatment arms were pooled for this analysis. TFR were examined at seven anatomical sites at baseline and every 6 months thereafter, and were coded as present/absent at each site. These sites included hands, wrists, elbows, shoulders, knees, ankles and other sites where TFRs were noted (e.g. fingers). Regardless of whether TFRs were noted on one or both sides of the body, they were counted as one site. An improvement or worsening in TFR was defined as decrease or increase in the number of TFRs compared with baseline. Physical exam for the main outcome of TFR was standardized among the various study investigators and detection of TFR has been found to be easily reproducible [2].
Patients’ written consent was obtained according to the Declaration of Helsinki, and the design of the work was approved by local ethics committees in the USA by each centre.
We developed multi-variable linear regression models to assess associations between clinical variables with change in MRSS and change in the HAQ-DI over 12 and 24 months, respectively, and presented it as absolute change in units. The main independent variables of interest were baseline TFR and change in TFR (improvement or worsening) over 6 or 12 months, respectively. The associations between 12- and 24-month MRSS and HAQ-DI change with demographic variables such as age, sex and race; clinical variables such as baseline disease duration, maximum oral aperture, serum creatinine, haematocrit, MRSS, tender joint count (TJC) and swollen joint count (SJC), baseline TFR and change in TFR at 6 and 12 months, respectively, and change in SJC and TJC at 6 and 12 months were examined in bivariate analyses of association with 12- and 24-month MRSS and HAQ-DI changed scores using Student’s t-test and analysis of variance for categorical variables, and Pearson and Spearman correlations for continuous variables. Variables demonstrating significance at the 5% level (P ⩽ 0.05) in bivariate analyses were entered into forward and backward step-wise procedures in order to generate a list of candidate predictors for final models of MRSS change and HAQ-DI change. Variables selected by either forward or backward step-wise procedures (P = 0.15 for both) for each outcome variable became the candidate predictor set, which was then run through a best subsets regression procedure that orders each possible subset of the candidate set by its adjusted R2. The resulting covariates for the MRSS change model included baseline disease duration of SSc (defined from the first non-Raynaud’s sign or symptom typical of SSc), baseline MRSS, change in TJC over 6 and 12 months, and change in TFR over 6 and 12 months. HAQ-DI models included the aforementioned variables as well as baseline TJC, SJC, baseline HAQ-DI scores, change in TJC and SJC over 6 and 12 months and change in MRSS over 6 and 12 months. We included MRSS, SJC and TJC as they have an impact on functional disability [10]. All analyses were done using STATA 10.1 (College Station, TX, USA); P = 0.05 was indicative of statistical significance.
Results
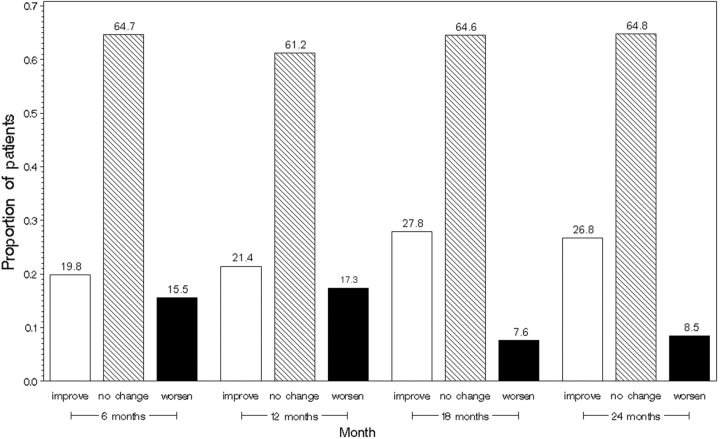
Baseline characteristics of these subjects are described in Table 1. The average age at presentation was 44 years; the majority of the patients were females (78%) and Caucasian (68%). The average disease duration was 9.5 months (s.d. 4.1), the average MRSS score was 21 (8.0) and HAQ-DI score was 1.04 (0.47) suggestive of moderate disease severity [11] and functional disability [12]. Forty-nine of 134 patients (37%) had TFR at baseline. The most common sites with TFR were hands (20%) then ankles (18%), wrists (14%), knees (12%), elbows (7.6%) and shoulders (2.3%). Approximately two-thirds of the patients had no TFR, whereas 13% had TFR at one site, 13% at two sites, 6% at three sites, 3% at four sites and 2% at five sites. Patients with TFR were likely to be Caucasian, and have a higher mean HAQ-DI score, a greater MRSS, a decreased oral aperture and a lower haematocrit exemplifying greater disease severity [11]. Compared with the baseline visit, 21.4 and 26.8% had improvement and 17.3 and 8.3% showed worsening (as defined by ⩾1 change from baseline TFR) in the number of sites with TFR at 12 and 24 months, respectively (Fig. 1). Of 49 patients at baseline with TFR, 17 (35%) had complete resolution of TFR (defined as no TFR at follow-up during the course of the study—8 at 6 months, 2 at 12 months, 4 at 18 months and 3 at 24 months), 27 (55%) did not complete the 2-year study and 5 patients did not have resolution of TFR by the end of the study. In contrast, in 85 patients without baseline TFR, 18 (21%) developed new TFR (defined as new TFR at follow-up visit—11 at 6 months, 6 at 12 months and 1 at 24 months), 33 (39%) did not develop TFRs during the study period and 33 (39%) did not complete the 2-year study.
Table 1.
Baseline characteristics of the participants stratified by the presence and absence of TFRs
| Variables | All (n = 134) | No TFR (n = 85) | TFR (n = 49) | P-value |
|---|---|---|---|---|
| Age, years | 43.69 (12.39) | 42.34 (11.88) | 46.02 (13.01) | 0.098 |
| Female, n (%) | 104 (77.61) | 65 (76.47) | 39 (79.59) | 0.676 |
| Ethnicity | ||||
| Caucasian, n (%) | 91 (67.91) | 49 (57.65) | 42 (85.71) | |
| African American, n (%) | 26 (19.4) | 22 (25.88) | 4 (8.16) | 0.004 |
| Other, n (%) | 17 (12.69) | 14 (16.47) | 3 (6.12) | |
| Disease duration, months | 9.5 (4.1) | 9.75 (4.36) | 9.14 (3.66) | 0.416 |
| MRSS (0–51) | 21.04 (8.01) | 20.12 (7.78) | 22.65 (8.21) | 0.077 |
| HAQ-DI (0–3) | 1.04 (0.67) | 0.92 (0.62) | 1.24 (0.7) | 0.008 |
| Maximum oral aperture | 45.29 (10.03) | 47.11 (9.94) | 42.15 (9.49) | 0.006 |
| Right hand extension | 174.59 (28.55) | 178.73 (28.28) | 167.52 (27.88) | 0.03 |
| Left hand extension | 179.72 (25.72) | 183.06 (26.36) | 174 (23.78) | 0.052 |
| Tender joint count | 1.51 (2.36) | 1.6 (2.43) | 1.37 (2.24) | 0.584 |
| Total musculoskeletal assessment (SJC; 0–8) | 0.9 (1.56) | 0.72 (1.5) | 1.2 (1.62) | 0.082 |
| Diffusing capacity, per cent predicted | 75.34 (18.37) | 77.47 (19.68) | 71.55 (15.21) | 0.077 |
| Forced vital capacity, per cent predicted | 83.62 (16.89) | 83.7 (17.57) | 83.48 (15.77) | 0.944 |
| Total number of cutaneous ulcers | 0.11 (0.32) | 0.12 (0.32) | 0.1 (0.31) | 0.785 |
| Serum creatinine | 0.89 (0.19) | 0.91 (0.19) | 0.84 (0.19) | 0.041 |
| Systolic blood pressure, mmHg | 120.18 (19.27) | 120.21 (18.76) | 120.12 (20.33) | 0.98 |
| Diastolic blood pressure, mmHg | 72.99 (10.39) | 73.31 (9.95) | 72.45 (11.19) | 0.647 |
| Haematocrit | 39 (3.76) | 39.7 (3.51) | 37.79 (3.9) | 0.004 |
Data are represented as mean (s.d.), unless otherwise indicated. P-values are for comparison between presence of TFRs and absence of TFRs.
Fig. 1.
Improvement or worsening of TFRs from baseline at 6, 12, 18 and 24 months.
In multi-variable models, significant predictors of change in MRSS over 12 months were baseline MRSS [unadjusted regression coefficient (β) = −0.23, P = 0.03] and change in TFR over the first 6 months (β = 1.41, P = 0.04), while significant predictors of change in MRSS over 24 months were baseline MRSS (β = −0.34, P = 0.004), change in TFR over the first 12 months (β = 2.26, P = 0.004) and change in TJC over 12 months (β = 0.96, P = 0.03). For HAQ-DI at 12 months, significant predictors were change in TFR over the first 6 months (β = 0.12, P = 0.008) and baseline SJC (β = −0.12, P = 0.04), whereas significant predictors of change in HAQ-DI over 24 months were change in TFR over the first 12 months (β = 0.13, P = 0.008), baseline MRSS (β = −0.017, P = 0.02), SJC (β = −0.35, P = 0.001), TJC (β = 0.085, P = 0.02), change in SJC over 12 months (β = −0.22, P = 0.01) and change in TJC over 12 months (β = 0.13, P = 0.008). In other words, controlling for other covariates, an improvement (worsening) of 1 U in the TFR during the first 6 months was associated with an improvement (worsening) of 1.41 U in MRSS over 12 months. For the HAQ-DI model, an improvement (worsening) of 1 U in the TFR during first 6 months was associated with an improvement (worsening) of 0.12 points in HAQ-DI.
Discussion
TFR is a physical sign that is associated with increased morbidity and mortality in early dcSSc [2, 13]. Using a large RCT in early dcSSc, we show and validate that the presence of baseline TFR is associated with greater disease severity and functional disability at baseline. Whereas only 37% of the patients had TFR, the majority of those had TFR of hands, wrists and ankles, a finding that is similar to the observational study by Steen and Medsger [2]. We also show the dynamic nature of TFR: only 10% of patients with TFR at baseline continued to have TFR at subsequent visits, whereas 21% developed new TFR. Extending the findings of Steen and Medsger [2], the change in TFR over 6 and 12 months predicted changes in skin thickening and functional disability over 12 and 24 months. In other words, an improvement/worsening of TFR at 6 and 12 months predicts an improvement/worsening in skin thickening and functional disability at 12 and 24 months, respectively. Baseline TFR (presence or absence) was not predictive of change in MRSS/HAQ-DI.
The current analysis confirms and complements the study published by Steen and Medsger in patients with early disease and documents changes in TFR over the next 2 years. Our patient population had early dcSSc (mean 9.4 months) and participated in a large RCT, whereas Steen and Medsger recruited patients with longer disease duration with a mean of 3.0 years from a single university practice. A previous analysis from the d-Pen data set showed that an improvement of ⩾5.3 U in MRSS and ⩾0.14 in HAQ-DI is clinically meaningful [14]. In the current analysis, an improvement in TFR by 3–4 U is associated with a clinically meaningful improvement of ⩾5.3 U in MRSS, whereas an improvement in TFR by 2 U is associated with a clinically meaningful improvement of ⩾0.14 in HAQ-DI.
Our study had its limitations. First, this population with dcSSc was recruited for the RCT with pre-defined inclusion and exclusion criteria, and does not mirror the general population of dcSSc per se. Secondly, our analysis is post hoc rather than based on a priori hypothesis. Since the study had a follow-up of only 4 years, a longer follow-up would have been desirable to assess the impact on internal organs and mortality. Thirdly, after entering the RCT, the majority of the patients experienced an improvement in their MRSS and HAQ-DI. The improvement in MRSS and HAQ-DI was considered to be related to the natural history of SSc rather than any drug effect [15]. Our study was not powered to assess predictors of worsening of MRSS and HAQ-DI and hence may explain the reason why baseline TFR did not predict change in MRSS and HAQ-DI. Hence, further investigation into the utility of change in TFR and its relationship to internal organ involvement/mortality is warranted.
How can these results be used in clinical practice? The presence and finding of TFR during routine examination has been said to predate and to predict development of SSc and has been recommended to be used diagnostically [2]. Our analyses show that TFR is a frequent physical finding in patients with early dcSSc that should be routinely assessed when a patient presents with the constellation of signs and symptoms that suggest SSc, including recent onset arthritis, RP and puffy, swollen fingers. The presence of TFR permits earlier confirmation and classification of dcSSc and may result in earlier treatment of the disease. Assessment of TFR at multiple standard sites should be done during routine follow-up visits as changes may predict the future course of MRSS and HAQ-DI. TFR may be useful in clinical trials in early SSc, as changes in TFR reflect changes in disease severity and may be helpful when assessing its prognosis. In conclusion, TFR in early dcSSc is associated with greater disease severity and functional disability, and assessment of changes in TFR at different sites during routine visits may be useful in predicting the future course of MRSS and HAQ-DI.

Acknowledgements
We thank the investigators of the D-Penicillamine study for recruiting the patients.
Disclosure statement: D.K. was supported by a National Institutes of Health Award (NIAMS K23 AR053858-03) and the Scleroderma Foundation (New Investigator Award). P.P.K. was supported by Ruth L. Kirschstein National Research Service Award (NRSA) Institutional Research Training Grant NIAMS 1 T32 AR053463 and American College of Rheumatology Research and Education Foundation Clinical Investigator Fellowship Award 2009–2011. All other authors have declared no conflicts of interest.
References
- 1.Medsger TA., Jr Natural history of systemic sclerosis and the assessment of disease activity, severity, functional status, and psychologic well-being. Rheum Dis Clin North Am. 2003;29:255–73, vi. doi: 10.1016/s0889-857x(03)00023-1. [DOI] [PubMed] [Google Scholar]
- 2.Steen VD, Medsger TA., Jr The palpable tendon friction rub: an important physical examination finding in patients with systemic sclerosis. Arthritis Rheum. 1997;40:1146–51. doi: 10.1002/1529-0131(199706)40:6<1146::AID-ART19>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Westphal C. Zwei Falle Von Schlerodermie. Chariete Annalen. 1876;3:341–60. [Google Scholar]
- 4.Shulman LE, Kurban AK, Harvey AM. Tendon friction rubs in progressive system sclerosis (scleroderma) Trans Assoc Am Physician. 1961;74:378–88. [PubMed] [Google Scholar]
- 5.Rodnan GP, Medsger TA. The rheumatic manifestaions of progressive systemic sclerosis (scleroderma) Clin Orthop Relat Res. 1968;57:81–93. [PubMed] [Google Scholar]
- 6.Steen VD, Powell DL, Medsger TA., Jr Clinical correlations and prognosis based on serum autoantibodies in patients with systemic sclerosis. Arthritis Rheum. 1988;31:196–203. doi: 10.1002/art.1780310207. [DOI] [PubMed] [Google Scholar]
- 7.Clements PJ, Lachenbruch PA, Seibold JR, et al. Skin thickness score in systemic sclerosis: an assessment of interobserver variability in 3 independent studies. J Rheumatol. 1993;20:1892–6. [PubMed] [Google Scholar]
- 8.Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23:137–45. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- 9.Clements PJ, Furst DE, Wong WK, et al. High-dose versus low-dose D-penicillamine in early diffuse systemic sclerosis: analysis of a two-year, double-blind, randomized, controlled clinical trial. Arthritis Rheum. 1999;42:1194–203. doi: 10.1002/1529-0131(199906)42:6<1194::AID-ANR16>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 10.Clements PJ, Wong WK, Hurwitz EL, et al. The Disability Index of the Health Assessment Questionnaire is a predictor and correlate of outcome in the high-dose versus low-dose penicillamine in systemic sclerosis trial. Arthritis Rheum. 2001;44:653–61. doi: 10.1002/1529-0131(200103)44:3<653::AID-ANR114>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 11.Medsger TA, Jr, Silman AJ, Steen VD, et al. A disease severity scale for systemic sclerosis: development and testing. J Rheumatol. 1999;26:2159–67. [PubMed] [Google Scholar]
- 12.Khanna D, Clements PJ, Postlethwaite AE, Furst DE. Does incorporation of aids and devices make a difference in the score of the Health Assessment Questionnaire-Disability Index? Analysis from a Scleroderma Clinical Trial. J Rheumatol. 2008;35:466–8. [PubMed] [Google Scholar]
- 13.Akesson A, Fiori G, Krieg T, van den Hoogen FH, Seibold JR. Assessment of skin, joint, tendon and muscle involvement. Clin Exp Rheumatol. 2003;21(3 Suppl. 29):S5–8. [PubMed] [Google Scholar]
- 14.Khanna D, Furst DE, Hays RD, et al. Minimally important difference in diffuse systemic sclerosis- results from the D-penicillamine study. Ann Rheum Dis. 2006;65:1325–9. doi: 10.1136/ard.2005.050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amjadi S, Maranian P, Furst DE, et al. Course of the modified Rodnan skin thickness score in systemic sclerosis clinical trials: analysis of three large multicenter, double-blind, randomized controlled trials. Arthritis Rheum. 2009;60:2490–8. doi: 10.1002/art.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]