Abstract
Background and Aims
Red or purple coloration of leaf margins is common in angiosperms, and is found in approx. 25 % of New Zealand Veronica species. However, the functional significance of margin coloration is unknown. We hypothesized that anthocyanins in leaf margins correspond with increased phenolic content in leaf margins and/or the leaf entire, signalling low palatability or leaf quality to edge-feeding insects.
Methods
Five species of Veronica with red leaf margins, and six species without, were examined in a common garden. Phenolic content in leaf margins and interior lamina regions of juvenile and fully expanded leaves was quantified using the Folin–Ciocalteu assay. Proportions of leaf margins eaten and average lengths of continuous bites were used as a proxy for palatability.
Key Results
Phenolic content was consistently higher in leaf margins compared with leaf interiors in all species; however, neither leaf margins nor more interior tissues differed significantly in phenolic content with respects to margin colour. Mean phenolic content was inversely correlated with the mean length of continuous bites, suggesting effective deterrence of grazing. However, there was no difference in herbivore consumption of red and green margins, and the plant species with the longest continuous grazing patterns were both red-margined.
Conclusions
Red margin coloration was not an accurate indicator of total phenolic content in leaf margins or interior lamina tissue in New Zealand Veronica. Red coloration was also ineffective in deterring herbivory on the leaf margin, though studies controlling for variations in leaf structure and biochemistry (e.g. intra-specific studies) are needed before more precise conclusions can be drawn. It is also recommended that future studies focus on the relationship between anthocyanin and specific defence compounds (rather than general phenolic pools), and evaluate possible alternative functions of red margins in leaves (e.g. antioxidants, osmotic adjustment).
Keywords: Anthocyanin, co-evolution, defence indication, herbivory, leaf edge, leaf margin, phenolic content, Veronica, Hebe
INTRODUCTION
The most frequently reported cases of anthocyanin synthesis in leaves involve reddening of entire leaf surfaces, e.g. autumn, juvenile and evergreen leaves (Hughes and Smith, 2007; Hughes et al., 2007; Archetti et al., 2009). However, anthocyanins also commonly occur in more localized areas of leaves, such as leaf veins, petioles and margins. These latter cases, though pervasive in the plant kingdom, have received virtually no attention in the literature, and their functional significance (if any) remains unknown.
The current study examined New Zealand species in the genus Veronica, of which approx. 25 % exhibit red/purple coloration of leaf margins due to anthocyanin pigments (Grayer-Barkmeijer, 1978). Anecdotal field observations of naturally occurring Veronica spp. in both the North and South Islands revealed considerable herbivore damage to leaf margins, with substantially less tissue damage to the leaf interior. Edge-feeding has been reported for numerous insects, including caterpillars, adult beetles and beetle larvae, grasshoppers and weevils (Cranshaw, 1998). The widespread occurrence of leaf margin damage in Veronica led to the hypothesis that red coloration in leaf margins might function in herbivory defence.
Previous researchers have proposed that red coloration of leaf surfaces in general might function to deter insects, either through signalling low leaf quality (Archetti, 2000; Hamilton and Brown, 2001; Archetti et al., 2009) or by causing the leaf to appear less visually attractive (Stone, 1979; Karageorgou and Manetas, 2006). Low leaf quality may be generally defined as anything that renders the plant an undesirable food or host choice for an insect, such as lower nutritional quality, greater chemical defence, or imminent senescence (Archetti et al., 2009). This explanation for red coloration in leaves is termed ‘co-evolution’ because both insect and plant host benefit from this warning system, as insects avoid low quality food/host sources, and plants incur less leaf damage (Archetti et al., 2009). Previous studies have provided some support for the co-evolution hypothesis in leaves that are entirely red. For example, an inverse relationship between redness and leaf quality (i.e. nitrogen) has been demonstrated in senescing leaves of deciduous tree species (Lee et al., 2003; Schaberg et al., 2003), winter leaves of Cistus creticus (an evergreen angiosperm; Kytridis et al., 2008), tomato leaves (e.g. Bongue-Bartelsman and Phillips, 1995) and arabidopsis leaves (Peng et al., 2008). A correlation between increased phenolics and anthocyanin has also been shown in senescing leaves of multiple tree species (Karageorgou et al., 2008) and juvenile leaves of Quercus coccifera (Karageorgou and Manetas, 2006). In insect-choice studies, red coloration has been shown to be less attractive to insects which lack a red visual receptor than green and/or yellow (Furuta, 1986, 1990; Archetti and Leather, 2005; Döring et al., 2009), and red leaves of some species have been observed to incur less herbivory than non-red conspecifics (Numata et al., 2004; Karageorgou and Manetas, 2006). Schaefer and Rolshausen (2007), however, did not observe a preference for red or green leaves by aphids, though their methods have been disputed (Döring and Hardie, 2007).
Here we adapt the co-evolution hypothesis to red leaf margins, with the hypothesis that anthocyanins function to deter insect herbivory occurring at the leaf margin specifically. Eleven species of Veronica were studied, five of which have anthocyanins in leaf margins and six which do not (Fig. 1). In New Zealand Veronica, marginal anthocyanins are epidermal (Fig. 2), and may be either lost or retained as leaves mature. First, signal honesty was tested with regards to phenolic content by determining whether species with red margins also exhibit greater phenolic content in either the leaf margin and/or the leaf interior. Phenolics are products of the phenylpropanoid pathway, and include many compounds involved in herbivory defence, including flavonoids, tannins, lignin, coumarin and their derivatives. Anthocyanins are synthesized in the phenolic pathway as well, and would therefore seem to be appropriate signalling molecules indicating relative investment in these defence compounds (i.e. ‘defence indication’). It should be noted that, here, defence indication is defined as a sub-category of the co-evolution hypothesis, specific to defence signalling, as opposed to signalling general leaf quality (Schaefer and Rolshausen, 2006). Secondly, the percentage of leaf edge eaten by insects in both juvenile leaves and the current season's growth was quantified for each species as a general proxy for leaf palatability.
Fig. 1.
Photographs of Veronica species grown in Otari Wilton's Bush used in this study. Species with red margins in juvenile leaves (A–E) were (A) V. bishopiana (aff. Hikurangi Swamp), (B) V. decumbens, (C) V. pinguifolia, (D) V. pubescens ssp. sejuncta and (E) V. speciosa. Species lacking red margins in juvenile leaves (F–K) included (F) V. elliptica, (G) V. glaucophylla, (H) V. macrocarpa, (I) V. rigidula, (J) V. townsonii and (K) V. urvilleana. Species which had red margins in mature leaves were (B) V. decumbens and (E) V. speciosa.
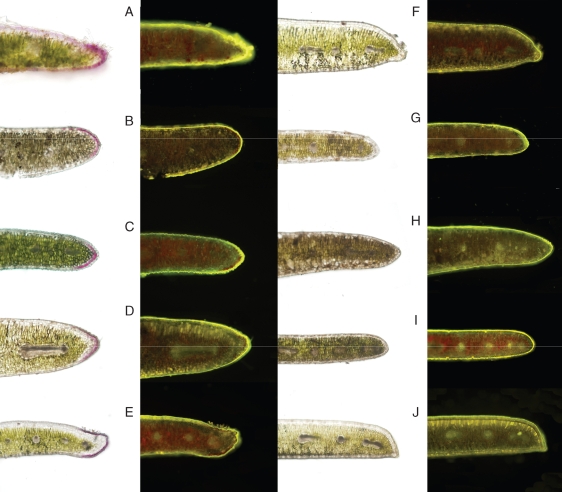
Fig. 2.
Bright-field images of juvenile leaf sections, next to the same section stained with Naturstoff Reagent A (viewed under an FITC filter); phenolics stain yellow (in black and white, the stain appears as bright white). Species depicted are (A) V. bishopiana (aff. Hikurangi Swamp), (B) V. decumbens, (C) V. pinguifolia, (D) V. pubescens ssp. sejuncta, (E) V. speciosa, (F) V. elliptica, (G) V. glaucophylla, (H) V. macrocarpa, (I) V. rigidula and (J) V. townsonii.
MATERIALS AND METHODS
Plant material
The Veronica species studied were grown in a common garden in Otari Wilton's Bush, a native plant reserve in Wellington, New Zealand. Plants received full sunlight throughout most of the day. Five to seven individuals of each species were grown together (i.e. not randomized) in one garden at one site, which the authors acknowledge might limit the application of these results. However, this experimental design also allowed environmental conditions and insect exposure to be controlled, which would have been substantially more difficult in the field, especially given the diverse habitats to which these species are endemic (Bayly and Kellow, 2006).
Species that lacked anthocyanin coloration in leaf margins throughout leaf ontogeny were Veronica macrocarpa (Vahl.), V. townsonii (Cheesem.), V. glaucophylla (Cockayne), V. elliptica (Forst.), V. rigidula (Cheesem.) and V. urvilleana (W.R.B. Oliv.). Species that exhibited red margin coloration in juvenile leaves were V. pubescens ssp. sejuncta (Bayly et de Lange) Garn.-Jones, V. bishopiana aff. Hikurangi Swamp (Petrie) Hatch, V. pinguifolia (Hook. f.) Cockayne et Allan, V. speciosa (R. Cunn.), V. decumbens (J.B. Armstr.); of these, V. speciosa and V. decumbens also exhibited reddening in mature leaves. All experiments were conducted in March and April 2009.
Phenolic assays
One juvenile leaf (most apical node) and one mature leaf (3rd node below the first fully expanded leaf) were excised from five different individuals, placed in air-tight plastic bags, transported on ice, and analysed within 4 h of collection (Waterman and Mole, 1994). Preliminary analyses showed phenolic content to be stable during this period. A scalpel was used to cut tissue sections <1 mm wide from both leaf margins and lamina interior (i.e. the area halfway between midrib and leaf edge). Five milligrams of material were extracted in 1·5 mL of 70 % methanol at 40 °C for 2 h. Extracts were stored at –80 °C until analysis, whereupon they were incubated for an additional hour at 40 °C. Total phenolics were measured according to Waterman and Mole (1994) using Folin–Ciocalteu assay. The Folin–Ciocalteu assay detects phenolics via oxidation of the phenol ion by a phosphotungstic–phosphomolybdic complex, resulting in blue coloration of the reduced chromophore. Fifteen microlitres of extract were added to 1·85 mL of de-ionized H2O and 75 µL of Folin–Ciocalteu reagent (Sigma Chemical Co., St Louis, MO, USA), followed by 225 µL sodium carbonate after 1–8 min. After 2 h incubation at room temperature, absorbance of the solution at 760 nm was measured using a Helios Gamma Spectrophotometer (Unicam, Cambridge, UK), and compared with that of a condensed tannin standard (Sigma Chemical Co.).
To image phenolics in vivo, transverse sections through fresh leaves were stained with diphenylboric acid–ethanolamine (Naturstoff Reagent A) (Sigma Chemical Co.) and 1 % solutions of AlCl3 (Markham, 1982; Harborne, 1989). Epifluorescence micrographs were obtained with an Olympus AX70 photomicroscope equipped with a 460–490 nm excitation band pass filter, a 510 emission barrier filter, and a 505 nm dichromatic mirror.
Herbivory
In some species only current-year growth was present on shoots, while in others, leaves from the previous season were also present. Current-season growth (i.e. spring through summer) was determined visually as the nodes where leaves began expanding in size (leaves developing during colder months are smaller). One branch was randomly excised from ten different individuals, and leaves from the current season's growth were removed and scanned. Leaf perimeter was quantified using LAMINA Leaf Shape Determination Software (Bylesjö et al., 2008). Lengths of individual bites were measured using a ruler on scaled, magnified images. Bite data for Veronica bishopiana (aff. Hikurangi Swamp) could not be derived due to damage to the electronic files, which was not identified until the end of the field season. Therefore data for this species were not included in this analysis. However, it had been noted in the field notebook that there was no observed damage in the first node (juvenile leaves) of any of the branches collected for V. bishopiana (aff. Hikurangi Swamp), so it was possible to include these data in juvenile leaf analyses (Fig. 4).
Fig. 4.
Leaf margin herbivory quantification. The graphs on the left represent herbivory on entire season's growth averaged (for species with red margins only in juvenile leaves, this would include both red- and non-red-margined leaves). The graphs on the right represent herbivory on the first node leaves only. The percentage of leaf margin missing due to herbivory is depicted in (A) and (C), average length of perimeter missing per bite (in centimetres) in (B) and (D). Bars represent means of herbivory for ten randomly selected branches. Error bars represent the s.e.
Statistics
Phenolic content of both red and green margin leaves was transformed by log10 for normality, and compared using a nested, random effects ANOVA, with species nested within colour (species being the random effect). Edge versus interior phenolic content for each species was analysed using a paired, one-tailed t-test. The difference in phenolic content between edges and leaf interiors for red and green-margined species was compared using a nested, random-effects model. The association between herbivory (average length of individual bite marks per branch and average leaf perimeter missing per branch), margin colour and mean margin phenolics was assessed using a MANOVA. Statistical significance for all tests was determined at P < 0·05.
RESULTS
Phenolics
Leaf margins had significantly greater phenolic content than interior parts of the leaf in both juvenile (P < 0·05, t > 2·5, d.f. = 4 for all species except V. rigdula, for which P > 0·05) and adult leaves (P < 0·05, t > 2·5, d.f. = 4 for all species except V. glaucophylla and V. bisopiana aff. Hikurangi Swamp; P > 0·05 for both of these; Fig. 3). Because edge phenolics and interior phenolics were correlated (r2 > 0·85), only edges were compared statistically. The difference in mean phenolic content between edges and interiors of leaves did not differ significantly between red and green leaves (P > 0·75, d.f. = 1 for both juvenile and adult leaves, F ratio = 0·031 and 0·082, respectively). There was also no significant association between colour and phenolic content in leaf margins of juvenile leaves (P = 0·93, d.f. = 1, F ratio = 0·0077) or mature leaves (P = 0·91, d.f. = 1, F ratio = 0·0142); there was a significant species effect in both juvenile and adult models (P < 0·0001, d.f. = 10 for both; F ratio = 51·4 and 51·8 for juvenile and mature leaf models, respectively).
Fig. 3.
Total phenolic content of leaf margins and more central tissues of the leaves, reported here as tannic acid equivalents. The left panel represents phenolic content of juvenile leaves (i.e. first node), while the right panel represents mature leaves (>5 node). Bars represent means + s.d. of five replicates.
Herbivory
The mean percentage of perimeter missing per individual bite was positively correlated with leaf phenolic content. Species with the least phenolics had the longest continuous bites on average (P = 0·038, d.f. = 1, F = 6·5), suggesting that tissues with the least phenolics were more likely to be continuously grazed, whereas tissues with the most phenolics were merely ‘sampled’. Leaf margin colour had a less significant association with bite size (P = 0·057, d.f. = 1, F = 5·15), and red-margined species had the longest continuous bite marks on average (Fig. 4). The average percentage of leaf edge lost to herbivory was not significantly related to either margin colour (P = 0·47, F = 0·597) (Fig. 4) or phenolics (P = 0·77, F = 0·094; Fig. 3). In all species except for V. macrocarpa, tissue lost occurred exclusively at the leaf margins.
DISCUSSION
Leaf margins in all Veronica examined (including juvenile and adult leaves) contained significantly higher concentrations of total phenolics than interior regions of the leaf lamina (Fig. 3). Leaf microscopy revealed the highest phenolic content in epidermal cells, trichomes and hypodermal cells, when present, all of which were more abundant in leaf margins compared with more-central lamina regions (Fig. 2). Higher concentrations of phenolics in these cells could be due to increased secondary chemical defences at a common site of insect attack (Gutterman and Chauser-Volfson, 2000; Wittstock and Gershenzon, 2002), and/or protection from ultra-violet light (in the case of epidermal cells) (Caldwell et al., 1983; Tevini et al., 1991; Jordan, 1996). Microscopic analyses did not indicate higher concentrations of phenolics in anthocyanic epidermal cells relative to adjacent non-anthocyanic epidermal cells, though these observations were purely qualitative.
Quantitative analyses of methanolic extracts clearly showed that leaves with anthocyanic margins did not contain higher concentrations of total phenolics, either in the leaf margin or more central parts of the leaf (Fig. 3). In juvenile leaves, red- and non-red margined species exhibited a similar range of total phenolics; in adult leaves, the two red-margined species represented plants with the highest (V. decumbens) and lowest (V. speciosa) levels of total phenolics. The apparent uncoupling of anthocyanin concentration from the total phenolic pool suggests that anthocyanin synthesis occurs independently of increases in total phenolics. Although a linear relationship between anthocyanin and total phenolics has been found in some species (e.g. Karageorgou et al., 2008), this is clearly not the case universally (Pirie and Mullins, 1976; Gould et al., 2000; Konczak-Islam et al., 2003; Sývacý and Sökmen, 2004; Karageorgou et al., 2006). However, it is possible that regulatory pathways exist which up-regulate anthocyanin concentrations concomitantly with specific phenolics functioning in defence, but levels of these defence phenolics were simply undetectable against the larger background pool of non-defensive phenolics. Indeed, it is known that at least some phenolics involved in anti-herbivory defence (e.g. formyl phloroglucinol) comprise only a small part of the total phenolic pool (<1 %), and do not correlate with total phenolic levels (Lawler et al., 1998, 1999). If the defence indication hypothesis is to be maintained for anthocyanins and phenolic defences, a more focused investigation involving specific defence compounds is needed, because the argument that anthocyanins should fluctuate concomitantly with carbon fluxes through the general phenolic pathway does not appear to be a reliable assumption. Analyses considering co-regulation of anthocyanin and non-phenolic defences (e.g. alkaloids, terpenoids, cyanogenic glycosides) would also be helpful (e.g. Hanley et al. 2009), as well as bioassays measuring the overall nutritional quality of red- and non-red-margined leaves.
Regarding the possibility that red margins might signal lower overall nutritional quality, we note that a pilot bioassay study was conducted using a New Zealand generalist caterpillar (Ctenopseustis obliquana). After 7 d of feeding on either red- or green-margined leaves (same species as those listed previously), no significant difference in caterpillar growth (i.e. length) was observed between caterpillars fed red-margined species and green-margined species (C. Bean and E. Cooke, Wellington, New Zealand, unpubl. res.). While this study did not quantify preference or ‘choice’ for red/green margins, it does suggest that red-margined tissues are no less nutritious than green-margined tissues to a generalist feeder, which would undermine the honesty of the signal with regards to overall leaf quality. However, again, such a study would be much more informative if carried out with red/non-red-margined morphs of the same species, and with both specialist and generalist feeders.
Regardless of whether or not anthocyanins correlate with phenolics or leaf quality, the more general question remains – do anthocyanins in leaf margins reduce insect herbivory? The present assessment of leaf margin herbivory in the common garden showed that red and green-margined species exhibited a similar percentage perimeter loss due to herbivory, both in juvenile leaves (Fig. 4C) and when all leaves of the current season's growth were considered (Fig. 4A). The results also showed a significant inverse relationship between phenolic content and continuous bite length, indicating that tissues with the least phenolics were more likely to be continuously grazed, whereas tissues with the most phenolics were merely ‘sampled’. Inconsistent with our hypothesis, however, the two species that showed the most continuous grazing were both species with red margins (V. speciosa and V. pubescens; Fig. 4B). Thus, one may conclude that red-margins are, at the very least, not universal deterrents. However, before drawing conclusions any more specific than this, some important factors should be considered.
Although a common garden study allowed for control of a range of environmental and ecological variables, this type of study system also had many limitations. For example, the Veronica species in the garden naturally occur in diverse habitats within New Zealand (Bayly and Kellow, 2006) and therefore are likely to differ in defence adaptations to co-occurring endemic insects and specialist feeders, or in the amount and diversity of herbivores that attack them. Perhaps the species which were least attacked in the garden corresponded to those best adapted to the insect community present in the garden, irrespective of margin colour. It is also unknown which insects were responsible for the damage observed, which limits the application of these results to the native habitats of the plants. Hamilton and Brown (2001) ascertained that specialist insects should be the driving force behind the evolution of signalling, as they are often responsible for the most damage to their hosts (Coley and Barone, 1996). Therefore, future studies may consider examining interactions between specialist feeders and their natural host plants, ideally comparing relative herbivory on red- and non-red-margined phenotypes within a single species.
In addition to the variability in the herbivore population involved in this study, leaf traits also were not controlled. Leaf structure (e.g. leaf area and thickness, presence/absence of trichomes on leaf margins, waxes, etc.), growth form and secondary defence metabolites other than phenolics were certainly varied between the species of Veronica studied here, which also may have confounded the effects of leaf margin coloration (Coley and Kursar, 1996; Peeters, 2002). For example, the two species which exhibited the greatest vulnerability to continuous grazing (4-fold longer marks from attacks on average) also had several layers of clear, hypodermal cells at the leaf margin; this type of anatomy was absent in all other species (Fig. 2), and may have played some role in herbivory preference. Again, we emphasize the importance of intra-specific studies for controlling these types of variables, and re-iterate that the only conclusions that can really be drawn regarding herbivory in this study are that red margins are not universally avoided (Fig. 4).
In conclusion, the lack of a relationship between anthocyanin concentration and margin or leaf phenolics suggests that anthocyanins are not likely to be reliable indicators of increased phenolic content in leaf margins of New Zealand Veronica. Future studies testing the defence indication hypotheses might focus on quantifying specific chemical defences rather than general biochemical pools (phenolics). Furthermore, the idea that anthocyanins deter herbivory at the leaf edge was not supported, and additional physiological and ecological functions should certainly be explored. Future avenues of research may consider the possibilities that anthocyanins reduce vulnerability to ice formation or drought stress at leaf extremities (i.e. an osmotic function). Marginal anthocyanins might also provide photoprotection (light-attenuation and/or antioxidant function) in areas of the leaf more vulnerable to photoinhibition, as leaf margins have lower relative water content than tissues in closer proximity to major veins, resulting in desiccation and stomatal closure (Nejad et al., 2006). Ecologically, anthocyanins in leaf margins may also play a role in attracting animal insectivores to leaves by making leaf damage at the margin easier to see (S. Lev-Yadun, University of Haifa, Israel, pers. comm.), or possibly a signalling and/or deterrent role against (now extinct) herbivorous bird species (Hanley et al., 2009).
ACKNOWLEDGEMENTS
This work was supported by the Richter Fellowship for International Research, Wake Forest University (to N.M.H.).
LITERATURE CITED
- Archetti M. The origin of autumn colours by coevolution. Journal of Theoretical Biology. 2000;205:625–630. doi: 10.1006/jtbi.2000.2089. [DOI] [PubMed] [Google Scholar]
- Archetti M, Leather S. A test for the co-evolutionary theory of autumn colours: colour preference of Rhopalosiphum padi on Prunus padus. Oikos. 2005;110:39–343. [Google Scholar]
- Archetti M, Döring TF, Hagen SB, et al. Adaptive explanations for autumn colours: an interdisciplinary approach. Trends in Ecology and Evolution. 2009;24:166–173. doi: 10.1016/j.tree.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Bayly M, Kellow A. An illustrated guide to New Zealand hebes. Wellington, New Zealand: Te Papa Press; 2006. [Google Scholar]
- Bongue-Bartelsman M, Phillips DA. Nitrogen stress regulates gene expression of enzymes in the flavonoid biosynthetic pathway of tomato. Plant Physiology and Biochemistry. 1995;33:539–546. [Google Scholar]
- Bylesjö M, Segura V, Soolanayakanahally RY, et al. LAMINA: a tool for rapid quantification of leaf size and shape parameters. BMC Plant Biology. 2008;8:82. doi: 10.1186/1471-2229-8-82. doi:10.1186/1471-2229-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell MM, Robberecht R, Flint SD. Internal filters: prospects for UV-acclimation in higher plants. Physiologia Plantarum. 1983;58:445–450. [Google Scholar]
- Coley PD, Barone JA. Herbivory and plant defence in tropical forests. Annual Review of Ecology and Systematics. 1996;27:305–335. [Google Scholar]
- Coley PD, Kursar TA. Anti-herbivore defenses of young tropical leaves: physiological constraints and ecological tradeoffs. In: Mulkey SS, Chazdon R, Smith AP, editors. Tropical forest plant ecophysiology. New York, NY: Chapman and Hall; 1996. pp. 305–336. [Google Scholar]
- Cranshaw W. Pests of the west. 2nd edn. Golden, CO: Fulcrum Publishing; 1998. [Google Scholar]
- Döring TF, Hardie J. Host finding in aphids and the handicap of trapping methods. Biology Letters. 2007;3:150–151. doi: 10.1098/rsbl.2006.0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döring TF, Archetti M, Hardie J. Autumn leaves seen through herbivore eyes. Proceedings of the Royal Society of London B, Biological Sciences. 2009;276:121–127. doi: 10.1098/rspb.2008.0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta K. Host preference and population dynamics in an autumnal population of the maple aphid, Periphyllus californiensis Shinji (Homoptera, Aphididae) Journal of Applied Entomology. 1986;102:93–100. [Google Scholar]
- Furuta K. Early budding of Acer palmatum caused by the shade; intraspecific heterogeneity of the host for the maple aphid. Bulletin of the Tokyo University Forests. 1990;82:137–145. [Google Scholar]
- Gould KS, Markham KR, Smith RH, Goris JJ. Functional role of anthocyanins in the leaves of Quintinia serrata A. Cunn. Journal of Experimental Botany. 2000;51:1107–1115. doi: 10.1093/jexbot/51.347.1107. [DOI] [PubMed] [Google Scholar]
- Grayer-Barkmeijer RJ. Flavonoids in Parahebe and Veronica: a chemosystematic study. Biochemical Systematics and Ecology. 1978;6:131–137. [Google Scholar]
- Gutterman Y, Chauser-Volfson E. The distribution of the phenolic metabolites barbaloin, aloeresin, and aloenin as a peripheral defense strategy in the succulent leaf parts of Aloe arborescens. Biochemical Systematics and Ecology. 2000;28:825–838. doi: 10.1016/s0305-1978(99)00129-5. [DOI] [PubMed] [Google Scholar]
- Hamilton WD, Brown SP. Autumn tree colours as a handicap signal. Proceedings of the Royal Society of London B, Biological Sciences. 2001;268:1489–1493. doi: 10.1098/rspb.2001.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley ME, Lamont BB, Armbruster WS. Pollination and plant defence traits co-vary in Western Australian Hakeas. New Phytologist. 2009;182:251–260. doi: 10.1111/j.1469-8137.2008.02709.x. [DOI] [PubMed] [Google Scholar]
- Harborne JB. General procedures and measurement of total phenolics. In: Harborne JB, editor. Methods of plant biochemistry. Vol. 1. London: Academic Press; 1989. pp. 1–28. [Google Scholar]
- Hughes NM, Smith WK. Seasonal photosynthesis and anthocyanin production in ten broadleaf evergreen species. Functional Plant Biology. 2007;34:1072–1079. doi: 10.1071/FP07205. [DOI] [PubMed] [Google Scholar]
- Hughes NM, Morley CB, Smith WK. The coordination of anthocyanin decline and photosynthetic maturation in developing leaves of three deciduous tree species. New Phytologist. 2007;175:675–685. doi: 10.1111/j.1469-8137.2007.02133.x. [DOI] [PubMed] [Google Scholar]
- Jordan BR. The effects of ultraviolet-B radiation on plants: a molecular perspective. Advances in Botanical Research. 1996;22:97–162. [Google Scholar]
- Karageorgou P, Manetas Y. The importance of being red when young: anthocyanins and the protection of young leaves of Quercus coccifera from insect herbivory and excess light. Tree Physiology. 2006;26:613–621. doi: 10.1093/treephys/26.5.613. [DOI] [PubMed] [Google Scholar]
- Karageorgou P, Buschmann C, Manetas Y. Red leaf color as a warning signal against insect herbivory: honest or mimetic? Flora. 2008;203:648–652. [Google Scholar]
- Konczak-Islam I, Okunu S, Yoshimoto M, Yamakawa O. Composition of phenolics and anthocyanins in a sweet potato cell suspension culture. Biochemical Engineering Journal. 2003;14:155–161. [Google Scholar]
- Kytridis V-P, Karageorgou P, Levizou E, Manetas Y. Intra-species variation in transient accumulation of leaf anthocyanins in Cistus creticus during winter: evidence that anthocyanins may compensate for an inherent photosynthetic and photoprotective inferiority of the red-leaf phenotype. Journal of Plant Physiology. 2008;165:952–959. doi: 10.1016/j.jplph.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Lawler I, Eschler B, Schliebs DM, Foley WJ. Relationship between chemical functional groups on Eucalyptus secondary metabolites and their effectiveness as marsupial antifeedants. Journal of Chemical Ecology. 1999;25:2561–2573. [Google Scholar]
- Lawler IR, Foley WJ, Eschler BM, Pass DM, Handasyde K. Intraspecific variation in Eucalyptus secondary metabolites determines food intake by folivorous marsupials. Oecologia. 1998;116:160–169. doi: 10.1007/s004420050575. [DOI] [PubMed] [Google Scholar]
- Lee DW, O'Keefe J, Holbrook NM, Feild TS. Pigment dynamics and autumn leaf senescence in a New England deciduous forest, eastern USA. Ecological Research. 2003;18:677–694. [Google Scholar]
- Markham KR. Techniques of flavonoid identification. London: Academic Press; 1982. [Google Scholar]
- Nejad AR, Harbinson J, van Meeteren U. Dynamics in spatial heterogeneity of stomatal closure in Tradescantia virginiana altered by growth at high relative air humidity. Journal of Experimental Botany. 2006;57:3669–3678. doi: 10.1093/jxb/erl114. [DOI] [PubMed] [Google Scholar]
- Numata S, Kachi N, Okuda T, Manokaran N. Delayed greening, leaf expansion, and damage to sympatric Shorea species in a lowland rain forest. Journal of Plant Research. 2004;117:19–25. doi: 10.1007/s10265-003-0126-2. [DOI] [PubMed] [Google Scholar]
- Peeters PJ. Correlations between leaf structural traits and the densities of herbivorous insect guilds. Biological Journal of the Linnean Society. 2002;77:43–65. [Google Scholar]
- Peng M, Hudson D, Schofield A, et al. Adaptation of Arabidopsis to nitrogen limitation involves induction of anthocyanin synthesis which is controlled by the NLA gene. Journal of Experimental Botany. 2008;59:2933–2944. doi: 10.1093/jxb/ern148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirie A, Mullins MG. Changes in anthocyanin and phenolics content of grapevine leaf and fruit tissues treated with sucrose, nitrate, and abscisic acid. Plant Physiology. 1976;58:468–472. doi: 10.1104/pp.58.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaberg PG, van den Berg AK, Murakami PF, Shane JB, Donnelly JR. Factors influencing red expression in autumn foliage of sugar maple trees. Tree Physiology. 2003;23:325–333. doi: 10.1093/treephys/23.5.325. [DOI] [PubMed] [Google Scholar]
- Schaefer HM, Rolshausen G. Plants on red alert: do insects pay attention? Bioessays. 2006;28:65–71. doi: 10.1002/bies.20340. [DOI] [PubMed] [Google Scholar]
- Schaefer HM, Rolshausen G. Aphids do not attend to leaf colour as visual signal, but to the handicap of reproductive investment. Biology Letters. 2007;3:1–4. doi: 10.1098/rsbl.2006.0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone BC. Protective coloration of young leaves in certain Malaysian palms. Biotropica. 1979;11:126. [Google Scholar]
- Sývacý A, Sökmen M. Seasonal changes in antioxidant activity, total phenolic and anthocyanin constituent of the stems of two Morus species (Morus alba L. and Morus nigra L.) Plant Growth Regulation. 2004;44:251–256. [Google Scholar]
- Tevini M, Braun J, Fieser G. The protective function of the epidermal layer of rye seedlings against ultraviolet-B radiation. Photochemistry and Photobiology. 1991;53:329–333. [Google Scholar]
- Waterman PG, Mole VH. Analysis of phenolic plant metabolites. Oxford: Blackwell; 1994. [Google Scholar]
- Wittstock U, Gershenzon J. Constitutive plant toxins and their role in defense against herbivores and pathogens. Current Opinion in Plant Biology. 2002;5:300–307. doi: 10.1016/s1369-5266(02)00264-9. [DOI] [PubMed] [Google Scholar]