Abstract
NOTCH signaling fulfills critical functions in intestinal epithelial cell lineage specification and the initiation of colorectal adenomas and colorectal cancers (CRCs). Because NOTCH signaling also plays important roles in the maintenance and self-renewal of cancer initiating cells in several types of malignancies, we studied the role of NOTCH signaling in colon cancer initiating cells (CCIC). CCIC form tumors that maintain many properties of the primary CRCs from which they were derived, such as glandular organization, cell polarity, gap junctions, and expression of characteristic CRC molecular markers. Furthermore, CCIC have the property of self-renewal. Here, we show that NOTCH signaling is 10-30 fold higher in CCIC compared to commonly used colon cancer cell lines. Using small molecule inhibition and shRNA knockdown, we demonstrate that NOTCH prevents CCIC apoptosis through repression of p27 and ATOH1. NOTCH also plays critical roles in the intrinsic maintenance of CCIC self-renewal, and the repression of secretory cell lineage differentiation genes such as MUC2. Our studies describe a novel human cell system to study NOTCH signaling in CRC tumor initiation and suggest inhibition of NOTCH signaling is likely to be an important mechanism to improve CRC chemoprevention and chemotherapy.
Introduction
Colorectal cancer (CRC) is the second leading cause of US cancer death (1). For metastatic CRC, the 5 year survival rate is ~10% (1). A mechanistic understanding of CRC initiation, recurrence and metastasis is therefore an important goal. A number of studies have shown that WNT and NOTCH pathways help maintain intestinal homeostasis, regulate cell fate decisions and play important roles in CRC tumorigenesis and progression (2-8). The majority of CRC tumors have increased WNT signaling (9). In normal intestinal homeostasis, WNT signaling stimulates intestinal stem and progenitor cell proliferation, but paradoxically also causes terminal differentiation into Paneth cells. One might therefore expect that CRC cells would attempt to differentiate terminally into Paneth cells. However, other signaling pathways active in CRC prevent terminal differentiation and maintain self-renewal capacity. A candidate is the NOTCH signaling pathway.
The role of NOTCH signaling in CRC is less well characterized than WNT. NOTCH signaling is triggered through the binding of a ligand on the membrane of one cell (Delta/Delta-like/Jagged/Serrate) to a receptor (NOTCH1/2/3/4) on the membrane of the contacting cell. This causes proteolytic cleavage of NOTCH receptors to release the cytoplasmic tail of NOTCH (NICD)(10). NICD translocates to the nucleus, associates with CSL transcription factors (CBF1/RBPJκ/Suppressor of Hairless/Lag-1) and co-activator Mastermind to turn on transcription of target genes (11). The best characterized targets of NOTCH are hairy/enhancer of split (HES) family of transcription factors, particularly HES1 in the intestine (12, 13). In normal mouse intestine, inhibition of NOTCH signaling results in exit from the proliferative compartment and differentiation into post-mitotic goblet cells (7). Similar results are seen in knockouts of other critical NOTCH signal transduction components including Hes1, Rbpjκ, Notch 1 and 2 receptors and Pofut1 knockouts for normal intestine (6-8, 12, 14). Apc mutant intestinal adenoma cells, which have elevated WNT signaling, also respond to NOTCH signaling inhibition by terminal differentiation into goblet cells, accompanied by cell cycle arrest and/or apoptosis(7, 15). Therefore, suppression of NOTCH signaling is a powerful mechanism for directing both normal intestinal enterocyte progenitors and Apc mutant intestinal cancer cells to differentiate down a secretory lineage.
NOTCH signaling plays an important role in intestinal tumor initiation but not progression in mice (15). Transgenic expression of NICD in the intestine leads to expansion of enterocyte progenitor cells (6) and increases the number of adenomas in ApcMin mice(15). In addition, inhibition of NOTCH by Jag1 deletion decreases adenoma initiation in ApcMin mice(16). In human CRCs, NOTCH signaling is high in adenomas and early stage CRCs (16, 17), but low in advanced, later stage and metastatic CRCs (15). The molecular mechanisms that cause NOTCH signaling to be important for early stage CRC initiation are not understood and dramatically fewer mechanistic studies of NOTCH signaling in human CRC cell lines have been performed. Pharmacological and siRNA mediated NOTCH signaling inhibition in colon cancer cell lines in vitro enhances sensitivity to cytotoxic chemotherapy. However, in the absence of cytotoxic chemotherapy, endogenous NOTCH signaling levels are present but generally low (18-20). In summary, while NOTCH signaling appears to be very important in adenoma formation and CRC tumorigenesis, the relatively low endogenous signaling levels in many commonly used CRC cell lines has limited mechanistic studies of NOTCH signaling in human CRC cells that could provide important insights into better ways to improve CRC chemoprevention and chemotherapy.
Colon cancer initiating cells (CCIC) (21-24) maintain biological similarity to primary human CRCs. Like most primary CRCs and their metastases, CCIC can maintain an organized glandular structure, with preserved cell polarity, gap junctions and expression of differentiation markers typical for CRC such as CK20 or CEA that are often not highly expressed in commonly used CRC cell lines typically used for in vitro studies (21, 23, 24). CCIC have the property of self-renewal (25) and can form new tumors after serial transplantation (21, 23, 24). Because previous studies have shown that NOTCH signaling plays a role in T-cell leukemia (26), breast(27), brain(28-30) and pancreatic (31) cancer CIC, we studied NOTCH signaling in CCIC. Here, we show that NOTCH signaling is markedly higher in CCIC compared to commonly used CRC cell lines and plays important roles in the intrinsic maintenance of CCIC viability, tumor formation, self-renewal, and the alternate expression of enterocyte or secretory intestinal cell lineage differentiation markers. These data describe a novel human CRC cell system to study NOTCH signaling in CRC tumor initiation and they suggest that inhibition of NOTCH signaling is likely to be an important mechanism to improve CRC chemoprevention and chemotherapy.
Materials and Methods
Isolation and culture of CCIC
Human colon cancer samples were obtained from colon cancer resections and liver metastasis under protocols approved by the University of California – Irvine IRB. Fresh CRC tissue was extensively washed with PBS, minced and incubated at 37°C with collagenase. Cells were then strained through 40μm filter and used for in vitro culture or xenograft. CCIC cultures were derived essentially as described previously (22) and cultured as spheres in ultra low attachment flasks in DMEM/F12 containing non-essential amino acids, antibiotic-antimycotic, N2 supplement (Invitrogen), B27 supplement (Invitrogen), heparin 4ug/ml (Sigma), EGF (40ng/ml), bFGF (20ng/ml) at 37°C at 5% CO2. For xenograft assays primary tumor cells or CCIC were injected in NOD/SCID mice subcutaneously as a 1:1 mixture of matrigel and CCIC media. For DAPT treatment: Cells were treated with 10uM DAPT (EMD Biosciences) or DMSO for 48h and used for cell cycle analysis and western blotting. 3D culture of CCIC: Single CCIC were mixed with 2.5mg/ml collagen (BD Biosciences) and 7.5% matrigel (BD Biosciences) and plated in transwell dishes overlaid with media.
Culture of colon cancer cell lines
HCT116, DLD1, HT29 and SW480 were purchased from ATCC and cultured according to recommended media conditions at 37°C, 5% CO2.
Lentiviral constructs and infection
The lentiviral vectors pLKO.1-puro, pLKO.1-scrambled-shRNA (Addgene), and pLKO.1-shRNA that targets RBPJκ (TRCN0000016203, Sigma) were transfected into 293T cells along with pCMV and pMP2G.
Lentiviral RBPJκ /NOTCH reporter was purchased as a prepackaged lentivirus from SA Biosciences. CCIC and colon cancer cell lines were infected with lentivirus at an MOI of 50 in presence of polybrene (1μg/ml) and selected in puromycin according to manufacturer's protocol and similar to previous studies (32, 33). Concentrations for puromycin selection were determined by plotting a kill curve. Mock infected cells did not survive puromycin selection. Stable cell lines were then used for FACS analysis to assay for NOTCH reporter activity.
Flow cytometry
Flow cytometry was performed on single CCIC with CD133 antibody (Miltenyi Biotec), CD44 (BD Pharmingen) and ESA (Biomedia). Enzyme assay for ALDH1 was performed using the Aldeflour kit (Stem Cell technologies). For reporter assays, stable CCIC with reporter were analyzed for GFP expression. For cell cycle analysis CCIC were fixed and stained with propidium idodide and then analyzed on a BD Flowcytometer.
Immunohistochemistry
Immunohistochemistry and immunofluorescence was performed on paraffin embedded sections from xenografts and 3D cultures as previously described (34). Beta-catenin immunocytochemistry was performed on four CCIC lines to evaluate sub-cellular localization. Antibodies are listed in Supplementary Methods.
Gene expression profiling
Expression profiling was carried out using total RNA from 2 CCIC lines in quadruplicates on Affymetrix GeneChip Human Exon 1.0 ST Array. The Cyber-T program was used to determine statistically significant, differentially expressed genes compared to normal colon datasets (35). Differentially regulated genes were analyzed by Ingenuity Pathway Analysis. Expression data were confirmed by TaqMan in four CCIC lines (including the two that were profiled).
Protein Isolation and Western Blotting
Protein isolation and western analysis were carried out as previously described(34). Antibodies are listed in Supplementary Methods.
RNA isolation and quantitative RT-PCR
Total RNA was extracted using Qiagen RNeasy kit, reverse transcribed and gene expression was quantified on ABI PRISM HT7900. Expression levels are an average of 3 independent experiments in 2 CCIC lines. All expression levels are normalized to GAPDH and RPLPO.
Results
CCIC express CRC markers and retain tumor initiation and self-renewal capacity in long term culture
To study the role of NOTCH and other critical signal transduction pathways for human CRCs, we derived CCIC from colon cancers resected from ten patients (Supplementary Table S1). In agreement with other groups, we found that CCIC can form xenografts in NOD/SCID mice that maintain the histopathology of primary human CRCs (21, 23, 24, 36). Cytokeratin 20 (CK20) and Carcinoembryonic antigen (CEA) are CRC peptide markers commonly used in routine anatomic pathology studies of human CRC patient tumors. Xenografts derived from injection of CCIC in NOD/SCID mice are CK20 and CEA-positive and CK-7 negative (matching the marker pattern most commonly seen in patient CRC specimens) (Fig. 1A). At the same time, commonly used CRC cell lines that form tumors in xenograft assays do not recapitulate the glandular architecture, maintain cell polarity, or express the immunohistochemical markers for CRC typically used to confirm colonic origin in primary and metastatic CRC specimens (Supplementary Fig. S1A).
Figure 1. Colon cancer initiating cells maintain histopathological properties of CRC in vitro.
(A) H&E, CEA, CK20 and CK7 staining in tumors generated by injection of CCIC in flank of NOD/SCID mice. One representative out of 8 tumors is shown (B) Flow cytometry analysis for CD133, CD44, ESA and ALDH1 on CCIC cultured in non-adherent culture conditions. Expression of these markers is conserved on long term culture in vitro. (C) H&E staining of xenografts generated in mice from CCIC cultured in vitro for indicated times showing that CCIC can maintain glandular differentiation and histologically similar tumors after long term culture. (D) H&E, CK20, β-catenin and CK7 staining of 3D cultures of single CCIC plated and cultured for 3 weeks showing CCIC can generate tumor foci in vitro.
CD133, CD44, ESA and ALDH1 have previously been shown to be important CCIC markers (21, 23, 24, 36). As expected, FACS analysis of the CCIC we derived also express CD133, CD44, ESA and ALDH1 (Fig. 1B). To evaluate CCIC capacity for self-renewal we injected CCIC spheres in the flank of NOD/SCID mice at regular intervals to assay its tumor forming ability. As expected, the CCIC we derived faithfully maintain the histology and marker expression in xenograft assays over multiple passages (Fig. 1C). To date we have tested up to six passages of CCIC lines that have maintained self-renewal capacity for more than one year. Consistent with previous studies (37), single CCIC cells can form carcinomatous glands in collagen 3D culture in vitro. Like the xenografts, the cells in these colonies maintain architectural features typical of differentiated CRC including the formation of gland lumens and the expression of typical CK7-/CK20+ cytokeratin pattern seen in bona fide primary CRC. (Fig. 1D). Some of the cells also have nuclear β-catenin staining indicative of elevated WNT signaling seen in CRCs (Fig. 1D). In contrast, commonly used CRC cell lines in 3D culture (Supplementary Fig. S1B) form solid nests of unpolarized cells that lack signs of glandular organization and other features of colon differentiation.
Elevated WNT and NOTCH signaling in CCIC as compared to normal colon tissue
The molecular mechanisms that sustain CCIC are poorly characterized. To explore critical biological pathways regulating CCIC, we generated gene expression profiles using Affymetrix whole genome exon arrays and compared them to normal colon profiles. The differentially expressed genes were then used in Ingenuity pathway analysis to identify functional categories and signaling pathways that are enriched. Because of their relevance to CRC and other CIC types, we focused on WNT and NOTCH signaling. Similar to previous studies showing high levels of WNT signaling in commonly used CRC cell lines, WNT targets such as MYC,CD44, CTNNB1, LEF1, TLE4 and CD44 are all expressed at significantly higher levels in CCIC compared to normal colon (Supplementary Table. S2). These data are also consistent with the presence of nuclear β-catenin protein seen in CCIC in vitro cultures, which is seen in ~50-90% of CCIC. Similarly, canonical NOTCH target genes such as HES1, HES4, HES6 and signaling components such as JAG1, JAG2 and NOTCH1 were all significantly higher in CCIC (Supplementary Table. S2). To validate the array data for NOTCH components and targets we performed q-PCR analysis in CCIC derived from nine CCIC lines. In the CCIC lines tested the canonical NOTCH target gene HES1 and pathway components JAG1 and NOTCH1 are significantly higher compared to normal colon epithelium (Fig. 2). In summary, CCIC have elevated levels of WNT and NOTCH target genes and signaling components compared to normal colon tissue.
Figure 2. NOTCH signaling is high in CCIC cultures.
Expression of NOTCH components JAG1, NOTCH1 and target HES1 in CCIC is higher as compared to normal colon crypts.
Colon cancer initiating cells have active NOTCH signaling
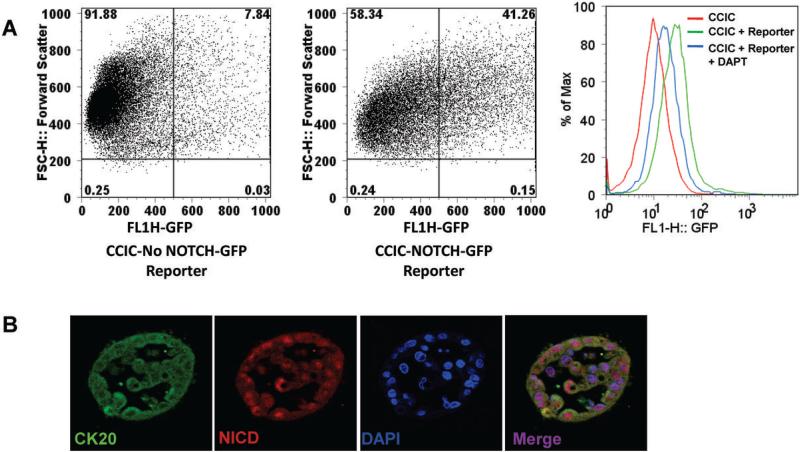
Because our gene expression analyses were exploratory and normal colon tissue was not cultured in the same way as CCIC (which could confound data interpretation), we used NOTCH reporter constructs to test directly whether CCIC have active NOTCH signaling. We infected CCIC and commonly used CRC cell lines with a lentiviral NOTCH GFP reporter construct for direct comparison. FACS analysis of CCIC showed high levels of NOTCH activity. Even in CCIC lines that are far from the highest in endogenous NOTCH target gene expression (such as CCIC-1, 2nd bar series in Figure 2C) more than 33% of the cells have active NOTCH signaling as indicated by high GFP expression (Fig. 3A). However, commonly used colon cancer cell lines have little (SW480) to no active NOTCH signaling (HCT116, DLD1 and Colo320) (all ≤3% of cells) (Supplementary Fig. S2). In summary, CCIC have ~10-30 fold more cells with high levels of NOTCH signaling than commonly used colon cancer cell lines. Similar results were seen with studies using a NOTCH signaling luciferase reporter gene (data not shown).
Figure 3. NOTCH signaling is active in CCIC.
(A) FACS analyses of CCIC-1 with NOTCH/ RBPJκ GFP reporter. 33% of have high levels of GFP as compared to control population without reporter. Histogram of CCIC alone, CCIC with reporter and CCIC with reporter treated with DAPT 10μM for 24h. NOTCH inhibition in CCIC decreases expression of GFP from the reporter. (B) Immunofluorescence for NICD in 3D cultures. Tumor foci formed from CCIC express cytoplasmic CK20 (green) and nuclear NICD (red). Sections were counterstained with DAPI (blue). Merged image shows that NICD localizes to the nucleus of tumor foci.
Gamma-secretase (γ-secretase) is a critical component of NOTCH signal transduction at the cell membrane. DAPT is a γ-secretase inhibitor that inhibits NOTCH signaling by blocking the γ-secretase complex responsible for the S3 cleavage to release the NICD domain(38). To further validate that CCIC have elevated NOTCH signaling, we transiently treated CCIC with DAPT. Consistent with inhibition of NOTCH signaling, DAPT caused a ≥50% reduction in the number of GFP+ CCIC (Fig. 3A). To investigate if active NOTCH signaling is present in CCIC in 3D culture we performed immunofluorescence for NICD with an antibody that recognizes NICD. NICD staining is seen in the nucleus of CCIC in 3D in vitro cultures indicating active NOTCH signaling (Fig. 3B).
Gamma-secretase inhibitors induce goblet cell lineage markers and cause apoptosis in colon cancer initiating cells
Since NOTCH signaling is active in CCIC, we tested whether NOTCH is required for CCIC tumor formation. CCIC treated with DAPT had no NICD detectable by western analysis (Fig. 4A right). Similarly, DAPT treated CCIC downregulated NOTCH targets HES1 and HES5 (Fig. 4A left). In the mouse intestine, Hes1 represses Atoh1, a bHLH transcription factor that promotes intestinal stem cell differentiation towards secretory lineages (39). DAPT treatment of CCIC increases expression of ATOH1 and expression of goblet cell marker MUC2 (40, 41), likely caused by lower HES1 levels (Fig. 4B).
Figure 4. DAPT blocks NOTCH signaling in CCIC and causes expression of goblet cell markers.
(A) Expression of HES1 and HES5 in CCIC decreases on treatment with DAPT (left). DAPT treatment inhibits cleavage of NOTCH by γ-secretase as NICD is not detected by immunoblotting in CCIC (right). (B) DAPT relieves repression caused by expression of HES1 on MATH1, MUC2 and CDK inhibitor p27. (C) MUC2 + cells is increased by 2.5 fold on transient treatment with DAPT. Counted MUC2+ cells in 100 colonies in 5 independent experiments.
In addition, in the mouse intestine Hes1 represses transcription of CDK inhibitors p27/CDKN1B and p57/CDKN1C and arrests intestinal progenitor cells that differentiate down the goblet cell lineage (8). Q-PCR analysis of human CCIC shows that NOTCH pathway inhibition with DAPT relieves repression of p27 but not p57 (Fig. 4B and data not shown). In addition, we treated CCIC that had been cultured in 3D collagen matrix for 7-10 days and had already formed adenocarcinomatous glands. Consistent with studies of NOTCH inhibitors in Apc mice where NOTCH inhibition causes Apc mutant intestinal adenoma cells to differentiate terminally into post-mitotic goblet cells (7, 15), DAPT increased the number of MUC2 immunoreactive cells in previously formed CCIC glands (Fig. 4C, Supplementary Fig S3).
Next, we examined if NOTCH signaling is required at earlier stage for CCIC to initiate 3D culture tumor formation. Single CCIC plated in 3D culture and treated with DAPT could no longer form adenocarcinomatous glands but only disorganized CCIC clusters that were MUC2-negative and had no self-renewal capacity (Fig. 5A). In contrast DAPT did not affect proliferation and colony formation in HCT116 cells which have low levels of NOTCH signaling (Supplementary Fig S4). To test if NOTCH inhibition causes CCIC to undergo apoptosis we treated CCIC in non-adherent cultures with DAPT and performed cell cycle analysis. FACS analysis showed an increase in the sub-G1 population indicating that CCIC were undergoing apoptosis (Fig. 5B). To further validate whether DAPT induces CCIC apoptosis we performed western blots for cleaved caspase-3. DAPT increased cleaved caspase-3 in CCIC, consistent with a triggered apoptosis response (Fig. 5C). In summary, human CCIC in both non-adherent and 3D culture have active NOTCH signaling and express markers of NOTCH signaling such as nuclear NICD, HES1 and HES5. NOTCH signaling is maintained in CCIC adenocarcinomatous glands in 3D culture and express CRC markers such as CK20 and CEA. Treatment with the NOTCH inhibitor DAPT induces CCIC apoptosis and eliminates the CCIC capacity for self-renewal and formation of new tumors.
Figure 5. DAPT inhibits formation of tumor foci and causes apoptosis in CCIC.
(A) Single cells plated in 3D culture and treated with 10μM DAPT continuously are unable to initiate to tumor foci as compared to control. (B) Cell cycle analysis of cells treated DAPT shows increase in sub-G1 population indicating apoptosis. (C) CCIC treated with DAPT express cleaved caspase-3, show decrease in HES1 and absence of NICD consistent with apoptosis.
Knockdown of RBPj leads to expression of goblet cell markers, a decrease in enterocyte markers and tumor foci
DAPT inhibits γ-secretase which acts on NOTCH as well as other signal transduction pathways in the cell (42, 43). To test whether inhibition of NOTCH signaling specifically causes CCIC to express goblet cell differentiation markers and initiate apoptosis, we transduced CCICs with a lentivirus expressing shRNA for the critical NOTCH effector RBPJκ/CBF-1/CSL. Q-PCR and western analysis confirmed efficient knockdown of RBPJκ (Fig. 6A left and middle). Consistent with NOTCH inhibition, RBPJκ knockdown decreased RBPJκ /NOTCH target gene HES1 (Fig. 6A left). Q-PCR analysis showed that RBPJκ knockdown led to increased expression of ATOH1 and the goblet cell marker MUC2 (Fig. 6A right). CK20, a marker of the enterocyte lineage, decreases consistent with the effect of inhibiting NOTCH signaling (Fig. 6A right). In addition, as seen with DAPT treatment, RBPJκ shRNA knockdown relieved repression of the cell cycle inhibitor p27 but not p57 (Fig. 6A right). In summary, the effect of RBPJκ knockdown on the expression of target genes in CCIC is essentially the same as blocking NOTCH signaling pharmacologically with DAPT. Likewise, effects on colon cell marker expression are consistent with changes induced by NOTCH pathway inhibition in the mouse intestine.
Figure 6. RBPJκ knockdown causes expression of goblet cell markers and apoptosis in CCIC.
(A) Lentiviral infection of shRNA against RBPJκ suppresses expression of RBPJκ mRNA and causes downregulation of NOTCH target HES1 (left). shRNA knockdown decreases RBPJκ protein levels (middle). Knockdown of RBPJκ increases expression of secretory lineage markers MATH1, MUC2, decreases enterocyte marker CK20 and increases expression of CDK inhibitor p27 compared to control (right). (B) CCIC infected with scrambled or shRNA for RBPJκ and plated in 3D culture for 3 weeks. CCIC expressing RBPJκ shRNA cannot initiate tumor foci in 3D culture. (C) Cell cycle analysis of cells expressing RBPJκ shRNA shows gradual increase in sub-G1 peak post infection indicating apoptosis. (D) Apoptosis in CCIC by RBPJκ shRNA is confirmed by increase in cleaved caspase-3.
To analyze if RBPJκ knockdown affects CCIC tumor focus formation in 3D culture, we plated cells transduced with either shRNA for RBPJκ or a scrambled control and selected for puromycin resistant colonies. Cells expressing shRNA against RBPJκ infected cells were unable to form colonies as compared to control (Fig. 6B). To test whether RBPJκ knockdown induced apoptosis in CCIC, we performed cell cycle analysis. Cell cycle analysis revealed that up to 4 days post infection the scrambled and RBPj KD populations had similar FACS profiles (Fig. 6C). However, after 4 days a Sub-G1 peak starts appearing only in the RBPJκ KD population, consistent with apoptosis. At 8 days post-infection, 60% of RBPJκ KD cells undergo apoptosis and by 15 days this number increases to 90% (Fig. 6C). Cleaved caspase-3 also increases post RBPJκ KD, confirming that the cells are apoptotic (Fig. 6D). In summary, these data are consistent with a requirement for NOTCH signaling for CCIC survival (due to suppression of apoptosis), proliferation, and subsequent tumor formation and self-renewal.
Discussion
Previous work has shown that NOTCH signaling is highly active in cancer initiating cells in human T cell leukemia, gliomas, medulloblastoma and pancreatic cancers (26, 28, 29, 31). Here, we show that NOTCH signaling is also highly active in human CCIC. NOTCH signaling is significantly higher than commonly used CRC cell lines, with a ~10-30 fold increase in the number of cells with active NOTCH signaling (Fig. 3 and Supplementary Fig. S2). NOTCH therefore plays an important role in the intrinsic maintenance of CCIC viability, tumor formation, self-renewal, and the alternate expression of enterocyte (HES1, HES5, CK20, CEA) or secretory cell lineage (MUC2 and ATOH1) differentiation markers (Fig. 4, Fig. 6 and Supplementary Fig. S3).
In serum-free non-adherent culture conditions, where CCIC have minimal expression of intestinal enterocyte/secretory lineage differentiation marker genes (44) and are in a more “stem cell/early progenitor- like” state, NOTCH signaling is highly active and suppresses both CCIC apoptosis and cell cycle arrest (Fig. 4 and 6). Inhibition of NOTCH signaling activates the intrinsic apoptosis pathway, causing cleavage of caspase-3 (Fig. 5 and 6). Additionally, repression of NOTCH signaling inhibits CK20 expression and increases expression of HATH1 (which causes secretory lineage committed intestinal cells to exit the cell cycle) and the cyclin-dependent kinase inhibitor p27/CDKN1B, which causes cell cycle arrest. Repression of NOTCH does not affect p57/CDKN1C expression. In normal mouse intestine, NOTCH inhibition causes de-repression of Atoh1, p27 and p57. In this context, individual p27 and p57 knockouts have little effect and knockout of both p27 and p57 are required for the conversion of enterocyte progenitors into post-mitotic goblet cells (8). Therefore, the effect of NOTCH inhibition to arrest human CCIC but not affect p57 levels appears to reflect a lack of redundancy between p27 and p57, or a different mechanistic requirement for cell cycle inhibitors than that used in normal mouse intestine. Future experiments to study NOTCH inhibition in cultured normal human intestinal stem cells and Apc+/−;p27−/−;p57−/− intestinal tumors may help to resolve whether this difference is due to species (human vs. mouse) or cell type (CCIC vs. normal intestinal stem cells).
In 3D solid-phase culture conditions, individual CCIC form tumor foci. Under these conditions, CCIC express differentiated enterocyte lineage markers such as HES1 and CK20. In this system, endogenous NOTCH signaling suppresses goblet cell differentiation markers and promotes proliferation. NOTCH inhibition decreases the number of CCIC expressing CK20 and simultaneously increases CCIC expressing secretory lineage markers such as MUC2. Overall our data in CCIC are consistent with mouse studies showing that blocking NOTCH in the context of overactive WNT signaling can cause mouse intestinal cancer cells expressing enterocyte lineage markers to trans-differentiate into post-mitotic goblet cells and reduce tumor growth (7, 16). Functionally, NOTCH inhibition causes CCIC to initiate apoptosis, lose the capacity to form tumors and lose the property of self-renewal. An interesting aspect of CCIC with NOTCH inhibition by either RPBJκ knockdown or DAPT treatment in 3D culture is that the cells expressing secretory differentiation markers such as MUC2 are always seen individually surrounded by CK20+ cells and not adjacent to one another (Fig. 4C and data not shown). In multiple metazoan developmental systems ranging from Drosophila to mice, NOTCH signaling is thought to be mediated by ligand-receptor cell-cell interactions whereby a cell that is beginning to differentiate prevents its neighbors from differentiating in the same way at the same time by “lateral inhibition.” Therefore, future studies of CCIC in 3D culture may help our understanding of the mechanistic roles of NOTCH signal transduction components in the process of lateral inhibition of differentiation, with particular focus on the intestinal cell lineages and gland formation.
In the mouse, over-expression of NOTCH signaling alone does not cause intestinal tumor formation. However, activation of both WNT and NOTCH pathways, such as in Apc mutant mouse intestine, increases the number of adenomas that initiate compared to WNT activation alone. In the context of inhibited WNT signaling (such as Tcf4-/- mice), NOTCH activation is not sufficient to initiate tumors (15). Human CCIC have high levels of both WNT and NOTCH signaling. Similar to the studies in mice and commonly used CRC cell lines, blocking WNT activity in CCIC (such as by expression of the WNT inhibitor DKK1) significantly inhibits the number and size of CCIC tumors that form (Supplementary Fig. S5). In the context of WNT inhibition however, CCIC viability and proliferation is sufficiently impaired that we cannot readily study the effect of combined WNT/NOTCH inhibition on expression of enterocyte or secretory lineage differentiation markers because of extensive cell cycle arrest and apoptosis. In summary, consistent with mouse and commonly used human CRC cell line studies, our data in CCIC indicate that high NOTCH activity is insufficient to allow CCIC to overcome WNT inhibition.
Overall, our results in human CCIC are largely consistent with the mouse phenotypes observed when NOTCH signaling is blocked (7, 8). CCIC maintain the capacity to express differentiation markers of multiple lineages and depend on self renewal pathways like NOTCH. It is possible that in human colon active NOTCH signaling is required in the initial stages of tumor formation. In the absence of NOTCH signaling, a colon enterocyte progenitor cell with active WNT signaling would undergo terminal differentiation and not give rise to an adenoma, similar to mouse model studies (15).
Epidemiologic, pre-clinical and clinical studies show non-steroidal anti-inflammatory drugs (NSAIDs) such as Sulindac are effective in decreasing the number of colon adenomas that initiate in patients (45-48). Two direct targets of NSAIDs are of COX1 and COX2 enzymes. However, other targets of Sulindac may also play a role in inhibition of CRC. For example, a recent study showed that amide derivatives of Sulindac that do not inhibit COX1 or COX2 can inhibit CRC cell line growth (49). In parallel, clinical trials of sulindac sulfide and other NSAIDs are also being performed to block the formation of β-amyloid plaques in Alzheimer disease patients (50). The proposed mechanism of action for NSAIDs to inhibit β-amyloid plaque formation is through inhibition of the γ-secretase complex (50). In cell culture and mice, sulindac inhibits cleavage of NOTCH receptors (50). Therefore, it is tempting to speculate that (in addition to COX1/2 inhibition) an important mechanism of NSAID colon adenoma chemopreventive activity is the inhibition of the γ-secretase complex and NOTCH signaling in CCIC as they arise in the colon. In the future, it will be important to conduct correlative studies of NOTCH signaling inhibition in NSAID colon adenoma chemoprevention trials to address this question.
Supplementary Material
Acknowledgements
This work was supported by NCI grants CA09878 and CA108697.
REFERENCES
- 1.Jemal A. Cancer Statistics CA: A Cancer Journal for Clinicians. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Korinek V, Barker N, Moerer P, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nature Genetics. 1998;19:379–83. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 3.Korinek V, Barker N, Morin PJ, et al. Constitutive Transcriptional Activation by a beta -Catenin-Tcf Complex in APC−/− Colon Carcinoma. Science. 1997;275:1784–7. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 4.Sansom OJ, Reed KR, Hayes AJ, et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes & Development. 2004;18:1385–90. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andreu P, Colnot S, Godard C, et al. Crypt-restricted proliferation and commitment to the Paneth cell lineage following Apc loss in the mouse intestine. Development. 2005;132:1443–51. doi: 10.1242/dev.01700. [DOI] [PubMed] [Google Scholar]
- 6.Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964–8. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- 7.van Es JH, van Gijn ME, Riccio O, et al. Notch/[gamma]-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–63. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 8.Riccio Orbicia, Gijn MEv, Bezdek AC, et al. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Reports. 2008;9:373–83. doi: 10.1038/embor.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinzler KW, Vogelstein B. Lessons from Hereditary Colorectal Cancer. Cell. 1996;87:159–70. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 10.Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–6. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 11.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–89. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 12.Jensen J, Pedersen EE, Galante P, et al. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- 13.Heitzler P, Bourouis M, Ruel L, Carteret C, Simpson P. Genes of the Enhancer of split and achaete-scute complexes are required for a regulatory loop between Notch and Delta during lateral signalling in Drosophila. Development. 1996;122:161–71. doi: 10.1242/dev.122.1.161. [DOI] [PubMed] [Google Scholar]
- 14.Guilmeau S, Flandez M, Bancroft L, et al. Intestinal Deletion of Pofut1 in the Mouse Inactivates Notch Signaling and Causes Enterocolitis. Gastroenterology. 2008;135:849–60. e6. doi: 10.1053/j.gastro.2008.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fre S, Pallavi SK, Huyghe M, et al. Notch and Wnt signals cooperatively control cell proliferation and tumorigenesis in the intestine. Proceedings of the National Academy of Sciences. 2009;106:6309–14. doi: 10.1073/pnas.0900427106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodilla Vn, Villanueva A, Obrador-Hevia A, et al. Jagged1 is the pathological link between Wnt and Notch pathways in colorectal cancer. Proceedings of the National Academy of Sciences. 2009;106:6315–20. doi: 10.1073/pnas.0813221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reedijk M, Odorcic S, Zhang H, et al. Activation of Notch signaling in human colon adenocarcinoma. International journal of oncology. 2008;33:1223–9. doi: 10.3892/ijo_00000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng RD, Shelton CC, Li Y-M, et al. {gamma}-Secretase Inhibitors Abrogate Oxaliplatin-Induced Activation of the Notch-1 Signaling Pathway in Colon Cancer Cells Resulting in Enhanced Chemosensitivity. Cancer Res. 2009;69:573–82. doi: 10.1158/0008-5472.CAN-08-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akiyoshi T, Nakamura M, Yanai K, et al. [gamma]-Secretase Inhibitors Enhance Taxane-Induced Mitotic Arrest and Apoptosis in Colon Cancer Cells. Gastroenterology. 2008;134:131–44. doi: 10.1053/j.gastro.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Ye Y, Bai Z, Wang S. The COX-2 selective inhibitor-independent COX-2 effect on colon carcinoma cells is associated with the Delta1/Notch1 pathway. Dig Dis Sci. 2008;53:2195–203. doi: 10.1007/s10620-007-0139-0. [DOI] [PubMed] [Google Scholar]
- 21.Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–5. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 22.Cammareri P, Lombardo Y, Francipane MG, Bonventre S, Todaro M, Stassi G. Isolation and culture of colon cancer stem cells. Methods Cell Biol. 2008;86:311–24. doi: 10.1016/S0091-679X(08)00014-9. [DOI] [PubMed] [Google Scholar]
- 23.Dalerba P, Dylla SJ, Park IK, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158–63. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–10. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 25.Cho RW, Clarke MF. Recent advances in cancer stem cells. Curr Opin Genet Dev. 2008 doi: 10.1016/j.gde.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 26.Armstrong F, de la Grange PB, Gerby B, et al. NOTCH is a key regulator of human T-cell acute leukemia initiating cell activity. Blood. 2009;113:1730–40. doi: 10.1182/blood-2008-02-138172. [DOI] [PubMed] [Google Scholar]
- 27.Gabriela Dontu MA-H, Abdallah Wissam M., Clarke Michael F., Wicha Max S. Stem cells in normal breast development and breast cancer. Cell Proliferation. 2003;36:59–72. doi: 10.1046/j.1365-2184.36.s.1.6.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan X, Matsui W, Khaki L, et al. Notch Pathway Inhibition Depletes Stem-like Cells and Blocks Engraftment in Embryonal Brain Tumors. Cancer Res. 2006;66:7445–52. doi: 10.1158/0008-5472.CAN-06-0858. [DOI] [PubMed] [Google Scholar]
- 29.Garzia L, Andolfo I, Cusanelli E, et al. MicroRNA-199b-5p Impairs Cancer Stem Cells through Negative Regulation of HES1 in Medulloblastoma. PLoS ONE. 2009;4:e4998. doi: 10.1371/journal.pone.0004998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Purow BW, Haque RM, Noel MW, et al. Expression of Notch-1 and Its Ligands, Delta-Like-1 and Jagged-1, Is Critical for Glioma Cell Survival and Proliferation. Cancer Res. 2005;65:2353–63. doi: 10.1158/0008-5472.CAN-04-1890. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z, Li Y, Kong D, et al. Acquisition of Epithelial-Mesenchymal Transition Phenotype of Gemcitabine-Resistant Pancreatic Cancer Cells Is Linked with Activation of the Notch Signaling Pathway. Cancer Res. 2009;69:2400–7. doi: 10.1158/0008-5472.CAN-08-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hartman J, Edvardsson K, Lindberg K, et al. Tumor Repressive Functions of Estrogen Receptor {beta} in SW480 Colon Cancer Cells. Cancer Res. 2009;69:6100–6. doi: 10.1158/0008-5472.CAN-09-0506. [DOI] [PubMed] [Google Scholar]
- 33.Kaeser MD, Pebernard S, Iggo RD. Regulation of p53 Stability and Function in HCT116 Colon Cancer Cells. Journal of Biological Chemistry. 2004;279:7598–605. doi: 10.1074/jbc.M311732200. [DOI] [PubMed] [Google Scholar]
- 34.Pan Zishu, Sikandar Shaheen, Witherspoon Mavee, et al. Impaired placental trophoblast lineage differentiation in Alkbh1−/− mice. Developmental Dynamics. 2008;237:316–27. doi: 10.1002/dvdy.21418. [DOI] [PubMed] [Google Scholar]
- 35.Gardina P, Clark T, Shimada B, et al. Alternative splicing and differential gene expression in colon cancer detected by a whole genome exon array. BMC Genomics. 2006;7:325. doi: 10.1186/1471-2164-7-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang EH, Hynes MJ, Zhang T, et al. Aldehyde Dehydrogenase 1 Is a Marker for Normal and Malignant Human Colonic Stem Cells (SC) and Tracks SC Overpopulation during Colon Tumorigenesis. Cancer Res. 2009;69:3382–9. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Todaro M, Alea MP, Di Stefano AB, et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 38.De Strooper B, Annaert W, Cupers P, et al. A presenilin-1-dependent [gamma]- secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–22. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 39.Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for Secretory Cell Lineage Commitment in the Mouse Intestine. Science. 2001;294:2155–8. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- 40.Longman RJ, Douthwaite J, Sylvester PA, et al. Coordinated localisation of mucins and trefoil peptides in the ulcer associated cell lineage and the gastrointestinal mucosa. Gut. 2000;47:792–800. doi: 10.1136/gut.47.6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Klinken BJ-W, Einerhand AWC, Duits LA, et al. Gastrointestinal expression and partial cDNA cloning of murine Muc2. Am J Physiol Gastrointest Liver Physiol. 1999;276:G115–24. doi: 10.1152/ajpgi.1999.276.1.G115. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Rahn JJ, Lun X, et al. Gamma-Secretase Represents a Therapeutic Target for the Treatment of Invasive Glioma Mediated by the p75 Neurotrophin Receptor. PLoS Biol. 2008;6:e289. doi: 10.1371/journal.pbio.0060289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hemming ML, Elias JE, Gygi SP, Selkoe DJ. Proteomic Profiling of γ-Secretase Substrates and Mapping of Substrate Requirements. PLoS Biol. 2008;6:e257. doi: 10.1371/journal.pbio.0060257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vermeulen L, Todaro M, de Sousa Mello F, et al. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0805706105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cancer IAfRo. Handbook of Cancer Prevention: Non-Steroidal Anti-Inflammatory Drugs; Lyon, France: 1997. [Google Scholar]
- 46.Giardiello FM, Hamilton SR, Krush AJ, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–6. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 47.Labayle D, Fischer D, Vielh P, et al. Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology. 1991;101:635–9. doi: 10.1016/0016-5085(91)90519-q. [DOI] [PubMed] [Google Scholar]
- 48.Nugent KP, Farmer KC, Spigelman AD, Williams CB, Phillips RK. Randomized controlled trial of the effect of sulindac on duodenal and rectal polyposis and cell proliferation in patients with familial adenomatous polyposis. Br J Surg. 1993;80:1618–9. doi: 10.1002/bjs.1800801244. [DOI] [PubMed] [Google Scholar]
- 49.Piazza GA, Keeton AB, Tinsley HN, et al. A novel sulindac derivative that does not inhibit cyclooxygenases but potently inhibits colon tumor cell growth and induces apoptosis with antitumor activity. Cancer Prev Res (Phila Pa) 2009;2:572–80. doi: 10.1158/1940-6207.CAPR-09-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takahashi Y, Hayashi I, Tominari Y, et al. Sulindac Sulfide Is a Noncompetitive gamma -Secretase Inhibitor That Preferentially Reduces Abeta 42 Generation. J Biol Chem. 2003;278:18664–70. doi: 10.1074/jbc.M301619200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.