Abstract
Purpose
Dasatinib is a dual Src/Abl inhibitor, recently approved for Bcr-Abl+ leukemias with resistance or intolerance to prior therapy. Because Src kinases contribute to multiple blood cell functions by triggering a variety of signaling pathways, we hypothesized that their molecular targeting might lead to growth inhibition in acute myeloid leukemia (AML).
Experimental Design
We studied growth factor-dependent and independent leukemic cell lines, including three cell lines expressing mutants of receptor tyrosine kinases (Flt3 or c-Kit) as well as primary AML blasts for responsiveness to dasatinib.
Results
Dasatinib resulted in the inhibition of Src family kinases in all cell lines and blast cells at ~10−9 M. It also inhibited mutant Flt3 or Kit tyrosine phosphorylation at ~10−6 M. Mo7e cells expressing the activating mutation (codon 816) of c-Kit were most sensitive to growth inhibition with a GI50 5×10−9 M. Primary AML blast cells exhibited growth inhibition < 10−6 M. Cell lines which showed growth inhibition at ~10−6 M demonstrated a G1 cell cycle arrest and correlated with accumulation of p21 and p27 protein. Addition of rapamycin or cytotoxic agents enhanced the growth inhibition. Dasatinib also caused the apoptosis of Mo7e cells expressing oncogenic Kit.
Conclusions
While all of the precise targets for dasatinib are not known, this multi-kinase inhibitor causes either growth arrest or apoptosis in molecularly heterogeneous AML. Addition of cytotoxic or targeted agents can enhance its effects.
INTRODUCTION
The treatment of acute myeloid leukemia (AML) remains challenging (1). Molecular profiling has correlated well with its phenotypic diversity (2). Nonetheless, AML results from two profound disturbances in hematopoiesis: a gain-of-function in proliferation and a loss-of-function in complete differentiation (3). A mutation in a transcription factor commonly blocks myeloid differentiation, while aberrant tyrosine kinase activity promotes excessive proliferation and survival (4). The clinical efficacy of imatinib mesylate in chronic myeloid leukemia (CML) has encouraged research on tyrosine kinases and their inhibitors in hematologic malignancies (5). Receptor tyrosine kinases (RTK) mediate cytokine effects to the intracellular signaling pathways, whereas cytoplasmic protein tyrosine kinases are activated by cytokine receptors. Tyrosine kinase signaling cascades play a major role in both benign and malignant hematopoietic cell signaling (6). One of the most common genetic abnormalities in AML is a gain-of-function mutation in the receptor tyrosine kinase Flt3 (FMS-like tyrosine kinase-3) due to internal tandem duplication (ITD). The constitutively active Flt3-ITD is associated with inferior prognosis and is present in approximately 30% of AML (5). Point mutations in kinase domains confer gain of function for Kit (Stem Cell Factor Receptor) and Flt3. Thus, approximately half of adult AML cases possess aberrant RTK activity. Recent sequencing of tyrosine kinase domains have not revealed mutations to account for the other half (7, 8). However, leukemic cell proliferative growth may be conferred by cryptic translocations, mutations outside of the sequenced kinase domains, or aberrant activation of accessory kinases. We and others have previously shown that activation of Flt3 leads to Src-family kinase (SFK) Lyn activation(9),(10). Another leukemia associated gain-of-function mutation in an RTK occurs with mutations of c-Kit, e.g., at codon 816 (11-13), and may also be associated with a poor outcome (14, 15). Lyn contributes also to Kit signaling (16, 17). Lyn activates the phosphatidylinositol 3′-kinase (PI 3′K)/Akt and Ras-ERK1/2 pathways, which are essential for proliferation and inhibition of apoptosis (6). Inhibition of Flt3 with small-molecule inhibitors has decreased leukemic burden in clinical trials (18) and improved survival in mouse models of mutant Flt3 leukemias (19).
Dasatinib is a small-molecule kinase inhibitor used for the treatment of Bcr-Abl+ CML and ALL with imatinib resistance or intolerance to previous therapy(20, 21). Dasatinib inhibits Bcr-Abl kinase activity in the low-nanomolar range and inhibits all clinically relevant imatinib-resistant forms with the exception of the common T315I mutation. It has been shown that targeting Src kinases with dasatinib may delay transition of CML chronic phase to blast crisis, and may be effective in treating Ph+ B-ALL (22). Dasatinib is also being studied in adults with solid tumors with preliminary results suggesting activity for dasatinib in gastrointestinal stromal cell tumors, which possess an activating Kit mutation (23). Patients with mastocytosis due to c-Kit mutations also benefit from dasatinib (24).
We sought to study the efficacy of dasatinib on the growth of leukemic cell lines expressing gain-of-function mutations in an RTK, cytokine-driven proliferation and survival via the IL-3/GM-CSF receptor, and factor-independency. We also studied dasatinib on primary AML blasts. Our results indicate that dasatinib can inhibit SFK at 10−9 M, as well as leukemic cell line growth at ~10−6 M concentrations and primary AML cell growth at < 10−6 M. Cells expressing the D816H mutant of Kit were the most sensitive. Because growth inhibition occurred typically at micromolar concentrations, additional kinases or pathways are relevant. Proteomic array suggests additional targets. Cell cycle arrest could be observed which correlated with increased levels of p27 and p21. Combining dasatinib with other compounds such as rapamycin or cytarabine may be advantageous.
MATERIALS AND METHODS
Reagents
Bristol-Myers (Princeton, NJ) provided the Src/Abl kinase inhibitor dasatinib, and its stock solution was prepared by dissolving the compound in DMSO at 10 mM and stored at −20°C. Rapamycin and etoposide (Calbiochem, San Diego, CA) were dissolved in DMSO and cytarabine (Sigma-Aldrich, St. Louis, MO) in water. Trypan blue was purchased from Sigma-Aldrich. The MTT ([3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) cell proliferation assay was purchased from America Type Culture Collection (ATCC, Manassas, VA).
Leukemic cells
Ba/F3-Flt3ITD, engineered to express the Flt3 internal tandem duplication mutation as described elsewhere(9), is an IL-3 independent murine pro-B cell line. MV4-11 is human acute monocytic leukemia cell line expressing Flt3ITD, which was obtained from the ATCC. Mo7e is a human acute megakaryoblastic leukemia cell line, dependent on either human Interleukin-3 (IL-3), Stem Cell Factor (SCF), or Granulocyte/Macrophage Colony-Stimulating Factor (GM-CSF) for survival and proliferation A strain of Mo7e cells were engineered to express the D816H mutation of c-Kit as described elsewhere(11). U937 and THP-1 are factor-independent myeloid leukemia cell lines and were obtained from the ATCC and Yvon Cayre. K562 cells were derived from a patient with CML myeloid blast crisis, which were obtained from Dr. Joya Chandra, MD Anderson Cancer Center. MV4-11 cells were grown in Iscove’s media supplemented with 10% fetal calf serum (FCS), 2 mM L-glutamine (GIBCO, Gaithersburg, MD), 100 u/ml penicillin/streptomycin (GIBCO), and 5 ng/mL rhGM-CSF (Berlex, Richmond, CA). Mo7e cell lines were grown in RPMI-1640 supplemented with 10% FCS, 2 mM L-glutamine, 100 u/ml penicillin/streptomycin, and 5 ng/mL recombinant human SCF. Ba/F3-Flt3ITD cells were maintained in medium containing 2 ng/ml murine IL-3 (Peprotech Inc, Rocky Hill, NJ), under these conditions their growth is not dependent on expression of mutant Flt3. When dasatinib and other compounds were tested, the IL-3 was washed from the Ba/F3-Flt3ITD cells; in the absence of added growth factors, the survival and proliferation of Ba/F3-Flt3ITD are dependent on their expression of constitutively active Flt3 mutant(9). K562, THP-1, and U937 were grown in RPMI medium supplemented with 10% FCS, 2 mM L-glutamine and 100 u/ml penicillin-streptomycin. Primary AML blast cells were obtained prior to therapy from bone marrow aspirates or peripheral blood after informed consent based on an IRB approved protocol at MD Anderson Cancer Center. These samples were classified according to the FAB system and M3, M6 and M7 samples were excluded. A mononuclear cell fraction was prepared following ficoll-hypaque separation and cryopreserved. Aliquots of blast cells were rapidly thawed at 37°C, washed with RPMI, and resuspended at 600,000 cells/ml in RPMI supplemented with 20% FCS. Cell viability ranged from 50 to 80% and represented specimens containing at least 70% blasts.
Lyn siRNA treatment of Mo7e cells
Scrambled or siRNA to human Lyn was purchased from Dharmacon (Littleton, CO). Cell line nucleofector kit V was purchased from Amaxa Biosystems (Gaithersburg, MD), and nucleofection of Mo7e cells was performed according to the manufacturer’s optimized protocol. Briefly, 2 ×106 cells per nucleofection sample were harvested after cells were freshly split, and the cell pellet resuspended in prewarmed Nucleofector Solution V. Cell suspension (100 μL) was incubated with different concentration of siRNA or control (200 nM each) and transferred into Amaxa-certified cuvettes. Nucleofection was carried out according to program X-01. Cells were harvested after 24, 48 and 72 hours after nucleofection for analysis of protein levels of Lyn by Western blot.
Growth Inhibition Assays
Cellular growth was analyzed by both Trypan blue exclusion and tetrazolium-based metabolic assay. Cell lines had been split before addition of dasatinib or DMSO as control diluent (both at 0.1% vol/vol) for indicated times. They were then collected, diluted in trypan blue dye (Sigma) for counting with hemocytometer. Alternatively, the methylthiazoletetrazolium (MTT) cell proliferation assay was performed according to manufacturer’s instructions. Cells were cultured in 96 well- plates using same growth media and drug concentrations as in trypan blue assay. At indicated time points, measurement of MTT activity was performed on a Spectramax Plus 384 spectrophotometer (Molecular Devices, Sunnyvale, CA). GI50 values were calculated using Calcusyn software (Biosoft, Ferguson, MO).
Cell cycle analysis
After cells were treated with dasatinib (or DMSO control) for 24 hours, 106 cells were collected for each condition, washed with PBS and fixed in 70% ethanol for at least 1 hour at 4°C. Cells were then washed twice with PBS and stained with 0.05 mg/ml propidium iodide solution for 2 hours on ice in the dark. RNase (20 ug/ml) was added before samples were read with FACSCalibur (Becton Dickinson, San Jose, CA) with the Channel FL-2H. Histograms of cell numbers vs. linear integrated red fluorescence were recorded at low settings and analyzed with ModFit Software LT3.0 (Verity Software House, Topsham, ME).
Western Blotting
Approximately 5 × 106 cells per aliquot of the cell lines were incubated with varying concentrations of dasatinib, rapamycin, and cytarabine for 60 minutes at 37°C. DMSO served as the diluent control. Ba/F3-Flt3ITD cells were washed free of IL-3, before being incubated with dasatinib. The other lines were incubated in similar conditions as in the cellular proliferation assays. After the incubation, cells were washed twice in PBS and lyzed with radioimmunoprecipitation assay (RIPA) lysis buffer supplemented with protease inhibitors (PI-cocktail, PMSF, and EDTA) and phosphatase inhibitors (NaF, Na3VO4). Lysates (30 ug/lane) were electrophoresed on SDS-PAGE gels. Gels were transferred onto Immobilon-P Transfer membrane (Millipore Corp., Bedford, MA). The membranes were blocked 1 hour at 4°C with blocking buffer, PBS 0.1% Tween-20 supplemented with either 5% BSA or milk, depending on the antibody. Immunoblotting was performed with polyclonal phospho-Src (Tyr416) antibody (#2101, Cell Signaling, Beverly, MA) phospho-Lyn (Tyr396) rabbit monoclonal antibody (Epitomics, Burlingame, CA), and phospho-Lyn (Tyr507) antibody (Cell Signaling). Lyn expression was detected with a polyclonal antibody (#sc-15, Santa Cruz Biotechnology, Santa Cruz, CA). Cbl was immunoprecipitated or blotted with polyclonal Cbl (C-15) antibody (#sc-170 Santa Cruz Biotechnology). Flt3 was immunoprecipitated or blotted with polyclonal Flt3 antibody (#sc-479, Santa Cruz Biotechnology) and c-Kit was immunoprecipitated or blotted with a rabbit polyclonal antibody (Cell Signaling). Lysates from immunoprecipitates were probed with monoclonal antibody (Clone 4G10, #05-321, Upstate, Lake Placid, NY). CrkL was detected with a polyclonal antibody (#sc-319), and CrkL phosphorylation (Tyr207) with a polyclonal antibody from Cell Signaling (#3181). Blotting for cyclin-dependent kinase inhibitors was done with p27 antibody from Santa Cruz (sc-527) or p21 antibody from BD Transduction laboratories (# 610233). After the incubation with the primary antibody for one hour at room temperature or overnight at 4°C, the blots were incubated with the secondary antibody for one hour, at room temperature. Apoptosis was determined by blotting for caspase 3 (Cell Signaling, #9665) and cleaved caspase 3 (Cell Signaling, #9664). Immunoreactive bands were visualized by using a developing solution (Western Lightening, Perkin Elmer LAS, NEL104, Boston). Membranes were restored using a stripping buffer (Pierce, Rockford, IL) for 30 minutes at 37°C, reblocked and reprobed for actin (actin antibody, #sc-1615, Santa Cruz Biotechnology) or total protein as a loading control. Densitometric analysis was performed with NIH ImageJ software1. Sensitivity analysis on Lyn tyrosine phosphorylation patterns was performed with Microsoft Excel™ software.
Phosphoproteome analysis
High throughput analysis of phospoprotein content was performed using the Kinex™ Antibody microarray (Kinexus Bioinformatics Corporation, Vancouver, Canada) following treatment with low (10−9 M) and high (10−5 M) dose dasatinib. Mo7e cells were treated 1 hour with dasatinib or its vehicle DMSO as a control. After treatment, cells were collected and total protein lysates were prepared according to the protocols recommended by Kinexus. Protein lysates were then labeled and incubated on the Kinex™ Antibody microarrays on which approximately 500 pan-specific and 300 phosphosite specific antibodies were immobilized. Following the complete quantification of all detected spots, we extracted information of the Percent CFC (Change from Control) reflecting a measure of change in normalized signal intensity averages between the treated and the control samples.
Combination Studies
Dose-effect curves for the individual drugs were done for Ba/F3 FLT3 ITD cell lines. The experiment was repeated using the drugs alone and in combination Approximately equipotent concentrations of the individual drugs were used in the combination study to assess drug interactions (Supplementary Table 1). The combination index (CI) provides a numerical value for synergism or antagonism between two drugs. The data for combination index was generated using the Calcusyn Software (Biosoft, Cambridge, UK). CI <1, CI=1, and CI >1 indicate synergism, additive effect, and antagonism between the medicines respectively(25). No sigmoidal dose-effect curves could be generated for rapamycin, so the median effect method could not be used in the combination studies involving rapamycin. Instead, analysis of potentiation versus inhibition was performed(26). Ba/F3 FLT3 ITD cells were grown at escalating concentrations of dasatinib along with fixed concentrations of rapamycin (10−9M, 10−8M, and 10−7 M). The GI50 of dasatinib in combination with fixed concentration of rapamycin was calculated using Calcusyn and was compared with the GI50 of dasatinib alone on the same cell line.
Statistical Analysis
Means between 2 or 3 groups were calculated using a two-sided Student’s t-test or analysis of variance (ANOVA) with PRISM (GraphPad Software, San Diego, CA).
RESULTS
Targeting Lyn in myeloid cell growth
Because we and others have shown that Lyn plays a role in myelopoiesis, we hypothesized that specific targeting of Lyn by siRNA would result in decreased cell growth of AML cells. Mo7e cells require the presence of a hematopoietic growth factor, such as IL-3, GM-CSF or SCF, to survive and proliferate. Treatment of Mo7e cells with siRNA to Lyn resulted in decreased Lyn protein levels, compared to scrambled control RNA or mock-nucleofected cells, as well as decreased cell growth (Fig. 1). This is consistent with our previous report of constitutive Lyn activation in myeloid cell lines and inhibition of myeloid cell growth by Lyn antisense (27). However, since RNA interference is not currently used as a therapeutic tool, we further studied the efficacy of the Src kinase inhibitor dasatinib.
Figure 1. Effect of Lyn inhibition on M-o7e cells.
Whole cell lysates were prepared from the mock transfected cells and cells transfected by Lyn siRNA (100 nM) or scrambled siRNA (100 nM) after 48 hours. Western blotting confirmed effective silencing of Lyn (insert). Effect of suppressing Lyn expression on Mo7e cell growth was assessed at indicated time points by counting cells using the trypan blue exclusion method.
Src/Abl kinase inhibition
An in vitro kinase screen led to the identification of dasatinib (BMS-354825) as a potent Src/Abl kinase inhibitor with an IC50 0.2 to 1.1 × 10−9 M and 3 × 10−9 M for SFK and Bcr-Abl respectively. Of the nine mammalian SFK, Lyn has the greatest expression in myeloid cells(6). A 60 minute incubation with dasatinib inhibited total SFK phosphorylation at nanomolar concentrations in all cell lines studied, ranging from 10−9 M in Mo7e, and MV4-11 cells, 10−8 M in Ba/F3-Flt3ITD, U937, THP-1, to 10−7 M in Mo7e-KitD816H (Fig. 2A). Phospho-Src inhibition was also investigated in the AML specimen #2956294, and was found to occur at 10−8 M. With the exception of BcrAbl+ K562 cells as a positive control, phosphorylation of Abl could not be detected in any of the cell lines studied (data not shown). We compared the status of phospho-Lyn (Tyr396) and phospho-Lyn (Tyr 507) content with that of phospho-Src (Tyr416) following dasatinib treatment, there was an approximate correlation (Supplemental Fig. 1A). Differences in antibody characteristics (rabbit polyclonal vs. rabbit monoclonal) may account for variance. Densitometric analysis also suggested that net Lyn activity reflected competing effects of dasatinib-induced inhibition on Csk/Chk, which phosphorylates the negative regulatory site at Tyr 507, and Lyn itself at its positive regulatory site (Tyr 396) Supplemental Fig. 1B). Sensitivity analysis demonstrated that trend in net Lyn activity was independent of antibody binding efficiency (Supplemental Fig. 1C). We found that the 10−7 M dasatinib inhibited the tyrosine phosphorylation of Src/Abl downstream targets CrkL and Cbl in Ba/F3-ITD and MV4-11 (Supplemental Fig. 2). Dasatinib also inhibited the tyrosine phosphorylation of oncogenic forms of both c-Kit and Flt3 (Fig. 2B and 2C). Phosphoproteome analysis of dasatinib-treated Mo7e cells showed two patterns. At the low dose (10−9 M), there was inhibition of protein kinase Cδ̤ Aktı̤ anḍfocal adhesion kinase, and at the high dose (10-5 M), ErbB2 and vascular endothelial growth factor receptor 2 (Supplemental Table 2). These data suggest that there are off-target effects of dasatinib, which may account for the difference between the observed IC50 of dasatinib on SFK and the GI50 of various AML cell lines (see below).
Figure 2.
(A) Inhibition of SFK phosphorylation by dasatinib
5 ×106/ml cells were incubated for 60 minutes in growth medium with escalating doses of dasatinib. Whole cell lysates were separated by SDS-PAGE 10% gels. 30 ug of protein was loaded per lane. Western blotting was performed with polyclonal phospho-Src (Tyr416) antibody with polyclonal anti-actin antibody for loading control.
(B) Inhibition of c-Kit by dasatinib (C) Inhibition of Flt3 by dasatinib
5 ×106/ml cells were incubated for 60 minutes in growth medium with escalating doses of dasatinib. Whole cell lysates were prepared and immunoprecipitation was performed with anti-c-Kit or anti-Flt3 antibodies at 4°C with protein A-sepharose beads. After bead complexes were washed, samples were boiled, and resolved by SDS-PAGE 7.5% gels. 30 ug of protein was loaded per lane. Western blotting was performed with monoclonal anti-phosphotyrosine 4G10 antibody and then reprobed for total Flt3 or c-Kit.
Dasatinib inhibition of primary myeloid leukemia cell growth
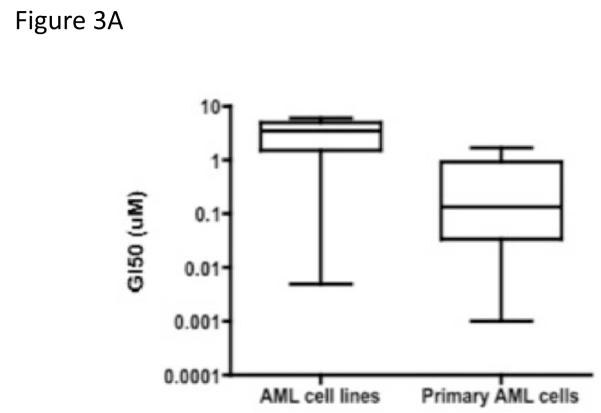
We used two different assays to determine the effects of dasatinib on cell growth and viability: trypan blue exclusion and MTT activity. As shown in Supplemental Table 3, dasatinib treatment resulted in 50% growth inhibition at 3 to 6 × 10−6 M for both Flt3ITD+ and factor-independent cell lines. The degree of growth inhibition was similar to that observed in cells treated with a general Src kinase inhibitor PP1 (data not shown). Mo7e-KitD816H cells were most sensitive to dasatinib (GI50 = 5 × 10−9 M). Fourteen patient samples were treated with dasatinib or DMSO control, and viability was determined in triplicate at 48 hours. As shown in Fig. 3A, dasatinib inhibited the growth of primary AML blasts more strongly than the leukemic cell lines (Mann-Whitney test, P = 0.0188). The lowest GI50 at 24 hours was observed for an AML M4 specimen that carried a gain-of-function mutation in NRAS (10−9 M). The GI50 at 48 hours ranged between 10−9 to 1.7 × 10−6 M (Supplemental table 4). Similar values were obtained at 72 and 96 hours (data not shown). Next, we correlated inhibition of Lyn activation with that of growth. Viability was measured in primary myeloid leukemic cells treated for 48 hours in the presence of varying concentrations of dasatinib. An aliquot of these cells were also treated with dasatinib for 1 hour and analyzed for anti-phospho-Lyn (Tyr396) content by western blotting. We identified dasatinib high sensitive and low sensitive primary AML specimens (Fig. 3B) and observed a positive correlation in the dose-responsiveness of dasatinib-induced Lyn inhibition and cell growth.
Figure 3.
(A) Growth inhibition of primary AML cell by dasatinib
AML cell lines or AML specimens were treated for 48 hours with dasatinib. The growth inhibition was assessed by trypan blue exclusion and compared to the DMSO control. GI50 were calculated using Calcusyn software. The data were plotted in PRISM and showed significance with a Mann-Whitney test, P=0.0188.
(B) Correlation of growth inhibition and Lyn activity
AML primary cells (1×106/ml) were cultured in RPMI medium containing 20% FBS and dasatinib (0 to 10−6 M) for 48 hours. Cell number was determined using trypan blue dye. An aliquot of the same AML primary cells were treated with different concentrations of dasatinib (0 to 10−6 M) for 60 min at hour 0. Cells were lysed, and 40 μg of protein lysates was electrophoresed and subsequently blotted with anti-phospho-Lyn (Tyr396) or anti-actin antibodies. The western blot film was scanned and the band intensity of phospho-Lyn (Tyr396) was quantified by densitometric analysis using ImageJ 1.42 software (National Institutes of Health, Bethesda, MD). The scatter diagram shows the correlation between phospho-Lyn (Tyr396) levels and cell numbers after treatment with dasatinib, delineating high sensitive and low sensitive AML patients. Control specimens maintained viability >90 %. Positive correlation between inhibition of Lyn activity and cell growth was observed.
Dasatinib induces G1 arrest with accumulation of p21 and p27 or apoptosis in sensitive cells
Because we observed little cytotoxicity, we hypothesized that dasatinib caused growth arrest. Therefore, cell cycle analysis was performed on cells stained with propidium iodide and then measured by flow cytometry. The proportion of Ba/F3-Flt3ITD, THP-1, and Mo7e cells in G1 phase increased after 24 hours of dasatinib treatment (Table 1). Exposure of THP-1 cells to dasatinib resulted in a dose-dependent upregulation of the cyclin-dependent kinase inhibitors p21 (Waf1) and p27 at 48 hours (Fig. 4A). At 10−5 M, we detected minor cleavage of caspase 3 but no PARP cleavage in THP-1 cells (data not shown). However, at 24 hours exposure to dasatinib, we observed significant caspase activity, indicative of apoptosis, in cells expressing oncogenic Kit (Fig. 4B).
Table 1. Dasatinib induces growth arrest in THP-1, Ba/F3-ITD, and Mo7e cells.
Cells were treated with dasatinib at the indicated concentrations for 24 hours and then stained with propidium iodide and cell cycle distribution was analysed by flow cytometry. Percentage of cells was determined by ModFit software.
| Cell type | concentration | G1 | S | G2/M |
|---|---|---|---|---|
| THP-1 | 0 μM | 47 | 39 | 14 |
| 0.1 μM | 46 | 35 | 20 | |
| 1 μM | 50 | 34 | 17 | |
| 10 μM | 57 | 30 | 14 | |
| BaF3-ITD | 0 μM | 49 | 45 | 7 |
| 0.1 μM | 50 | 38 | 12 | |
| 1 μM | 58 | 33 | 10 | |
| 10 μM | 65 | 23 | 13 | |
| Mo7e | 0 μM | 35 | 44 | 22 |
| 0.1 μM | 34 | 45 | 22 | |
| 1 μM | 33 | 49 | 19 | |
| 10 μM | 44 | 49 | 7 |
Fig. 4. Effect of dasatinib on tyrosine phosphorylation of p27 and p21 in THP-1 cells and caspase cleavage in Mo7e-KitD816H cells.
(A) Dasatnib induced cell cycle arrest. Dasatinib induced growth arrest, shown as a cell cycle distribution graph in the lower panel, correlated with increased p27 and p21 levels. Western blotting for levels of p27 and p21 was performed on protein lysates of THP-1 cells treated for 48 hours with indicated doses of dasatinib or DMSO. Detection of actin served as control for comparable protein loading.
(B) Dasatinib induced apoptosis. Dasatinib causes apoptosis in Mo7e-KitD816H cells as detected by cleavage of caspase 3. Whole cell lysates were prepared after 24 hour treatment with dasatinib. Caspase 3 and cleaved capase 3 were analyzed by western blotting. Actin serves as loading control.
(C) Synergy between dasatinib and rapamycin in Ba/F3-Flt3ITD: potentiation versus inhibition analysis. Ba/F3-Flt3ITD cells were treated with dasatinib in combination with fixed doses of rapamycin 10−7, 10−8, and 10−9 M versus dasatinib alone. Cells were collected at 48 hours, and the growth inhibition was determined by Trypan blue exclusion. Each data point represents the average of triplicate cultures. Values of >1, 1, and <1 denote inhibition, no effect, and potentiation, respectively.
Synergy with targeted and cytotoxic agents
We hypothesized that dasatinib’s effects could be synergistic with either other targeted agents or cytotoxic drugs. In Ba/F3-FLT3ITD cells, dasatinib and rapamycin were synergistic. Rapamycin inhibited the growth of Ba/F3-FLT3ITD cells by 14-31% when used alone at concentrations 10−9 to 10−7 M, and the GI50 exceeded 10−6 M. The results of the combination studies of rapamycin plus dasatinib are shown in the form of relative GI50 value, i.e. the ratio of the GI50 of dasatinib in combination with fixed concentrations of rapamycin versus the GI50 of dasatinib alone. The addition of rapamycin at concentrations of 10−9 and 10−8 M decreased the GI50 of dasatinib by 25%. The addition of 10−7 M rapamycin decreased the GI50 of dasatinib by 55% (Fig. 4C). As shown in Table 2, synergism was observed when dasatinib is combined with cytarabine (A) or etoposide (B) at concentrations equal to their GI50. A stronger synergy was observed at concentrations exceeding their respective GI50.
Table 2.
(A) Combination study of dasatinib and cytarabine.
The GI50 for cytarabine is 2 × 10−6 M and for dasatinib is 3 × 10−6 M in Ba/F3-Flt3ITD cells. CI: combination index where CI <1, CI=1, and CI >1 indicate synergism, additive effect, and antagonism between dasatinib and cytarabine, respectively.
(B) Combination study of dasatinib and etoposide
The GI50 for etoposide is 2.5 × 10−7 and for dasatinib is 3 × 10−6 M in Ba/F3-Flt3ITD cells. CI: combination index where CI <1, CI=1, and CI >1 indicate synergism, additive effect, and antagonism between dasatinib and etoposide, respectively.
| dasatinib | ||||||
|---|---|---|---|---|---|---|
| 0 μM | 0.75 μM | 1.5 μM | 3 μM | 6 μM | ||
| cytarabine | 0 | Control | ||||
| 0.5 μM | CI > 10 | |||||
| 1 μM | CI > 10 | |||||
| 2 μM | CI: 0.8 | |||||
| 4 μM | CI: 0.05 | |||||
| dasatinib | ||||||
|---|---|---|---|---|---|---|
| 0 μM | 0.75 μM | 1.5 μM | 3 μM | 6 μM | ||
| etoposide | 0 | Control | ||||
| 0.062 μM | CI: 1.0 | |||||
| 0.125 μM | CI: 1.4 | |||||
| 0.25 μM | CI: 0.8 | |||||
| 0.5 μM | CI: 0.4 | |||||
DISCUSSION
Because SFK activate many of the same growth-promoting signaling molecules associated with Bcr-Abl, and data from our group and other investigators on the aberrant activation of SFK, primarily Lyn, in AML cells (27-29), we studied the role of dasatinib in a variety of AML cell lines and primary AML blasts. Src kinases, primarily Lyn, were active in all of these cell lines and blasts cells. In our studies, phosphorylation of Src Tyr416, used to monitor an activating autophosphorylation site, was decreased by dasatinib (30). Our preclinical studies demonstrated that the Src-Abl inhibitor dasatinib caused growth arrest at ≤10−6 M in molecularly heterogeneous AML cell lines and primary AML blasts. Oral dosing of dasatinib in human clinical trials has achieved micromolar concentrations (31). Dasatinib directly inhibits c-Kit and was highly effective in inhibiting the growth of Mo7e cells expressing an activating mutation of Kit at 10−9 M. It is unlikely that Abl was the drug target in these cell lines, because it was not constitutively activated (data not shown). The difference in IC50 for SFK and GI50 suggests that a negative feedback loop exists to buffer inhibition of SFK, as has been shown with inhibition of Akt (32). Dasatinib also inhibited Csk/Chk-mediated phosphorylation of the Src’s autoinhibitory phosphorylation site (e.g. phospho-Src Tyr527), which would result in an activated Src kinase (Supplemental Fig. 1A). Higher doses of dasatinib may be required for a complete inhibition of Src kinase activity (Supplemental Fig. 1B). The predominant Src kinase in myeloid cells, Lyn produces both stimulatory and inhibitory effects (33), and the biologically optimal dose for growth inhibition may occur at a greater concentration. Finally, dasatinib may have off-target effects on kinases not currently known to play a role in AML growth. Dasatinib, however, does not inhibit the Janus, Syk, fibroblast growth factor receptor, vascular endothelial growth factor (VEGF) receptor, or epidermal growth factor receptor kinase activities, which have been associated with various forms of AML cell growth (34-37). Proteomic profiling of 52 signaling proteins in primary AML blasts reveals considerable diversity in their levels and activation states (38). We did not observe any dose-inhibition of phospho-ERK1/2 (a downstream effector for c-Raf) or phospho-STAT5 (data not shown).
A likely mechanism for growth inhibition without apoptosis lies in the observed cell cycle arrest of AML cells in G1 phase. We observed a dose-dependent accumulation of the cyclin-dependent kinase inhibitors p21 and p27. Src-activated breast cancer cell lines display reduced p27(39). By phosphorylating Tyr88 in p27, the Src kinase Lyn promotes Cdk2-mediated phosphorylation of Thr187, which in turn promotes its SCF-Skp2-dependent degradation.(40). Dasatinib caused little inhibition of phospho-Erk, phospho-Akt, or phospho-STAT3 in Mo7e-KitD816H, Ba/F3-Flt3ITD, U937, and THP-1 cells (data not shown). On the other hand, treatment led to caspase activation in cells expressing an activating mutation of Kit. Dasatinib may thus be effective at lower concentrations in those leukemic cells displaying Src, Abl, or Kit oncogene addiction (41).
Our studies demonstrate that dasatinib has an anti-leukemic activity and advance its potential use for new therapeutic approaches in AML. The optimal scheduling and combination regimen for dasatinib in AML will be determined from clinical trials. Despite its short plasma half-life, dasatinib was effective with a once-daily regimen in CML patients (31, 42). We did not observe greater inhibition when the drug was added daily to cell cultures (data not shown). Snead et al have reported that dasatinib treatment of CML patient samples for 4 hours followed by washout induced apoptosis and inhibited proliferation. Thus, continuous inhibition of kinases may not be necessary for clinical efficacy (43). Plasma concentrations of dasatinib in patients receiving the 70 mg twice daily or the 140 mg once daily regimens were reported to be greater than 14.6 ng/mL (~30 nM) for 8 to 10 hours (31). These reports suggest that short exposure to dasatinib may be sufficient, and so AML patients with low GI50 (Supplemental Table 4) might respond to clinically achievable dose of dasatinib alone. Preliminary results from a European phase I/II study of dasatinib in children and adolescents show that two of 12 patients with non-mutated c-Kit, Bcr-Abl− relapsed AML responded (44). Although our results show growth inhibition, doses required for dasatinib alone are high for the majority of patients, suggesting that dasatinib will be most effective in combination with traditional chemotherapy agents. Combination of dasatinib and high-dose cytarabine was effective and well tolerated in a 50 year-old man with systemic mastocytosis and AML(45). Synergistic activity of dasatinib with rapamycin or cytotoxic agents results in an effective concentration of dasatinib achieved through current oral dosing and provides rationale for inclusion of dasatinib in multi-drug regimens. Given our previous findings that activation of Flt3 leads to SFK Lyn activation (9), Src kinase inhibition may have an adjuvant role in treating leukemias harboring gain-of-function Flt3 or Kit mutations. Therefore, there are two mechanisms whereby dasatinib may contribute to anti-leukemia growth. In leukemic cells driven by oncogenic forms of Src, Abl, or Kit, dasatinib may induce apoptosis. In cells that depend on Src, Abl, or Kit for salvage or accessory growth promoting pathways, dasatinib may induce growth arrest. In the phosphoproteome analysis of dasatinib treated cells, we observed differences in a number of potential mediators of leukemic cell growth, including serine/threonine kinases, receptor tyrosine kinases, and cell cycle proteins (Supplemental Table 2), Clinical trials with combination therapy including dasatinib are warranted in AML. Future studies should identify additional targets for dasatinib and biomarkers for sensitivity.
Translational Relevance.
Acute myeloid leukemia (AML) remains one of the most difficult hematologic malignancies to treat. Improvements in therapy will come from identification of new molecularly targeted agents and their incorporation into multi-drug regimens. The dual Src/Abl kinase inhibitor dasatinib has been approved for use in chronic myeloid leukemia. Dasatinib also has efficacy against the receptor tyrosine kinase c-Kit, sometimes mutated in AML. In this study, we observed that dasatinib significantly inhibited growth in a variety of AML cell lines and primary blasts. We also found that the effects of dasatinib could be enhanced with the addition of other cytotoxic or molecular targeted agents. Our results provide rationale for evaluating dasatinib in a multi-drug regimen for AML with a variety of molecular lesions.
Supplementary Material
ACKNOWLEDGMENTS
We thank Sean Hartig, Daniel Lee, and Kevin Hwang for technical advice and comments.
Financial support This work was supported in part by grants RO1-CA108922, Leukemia SPORE P50, JP McCarthy Development Fund, Ladies Leukemia League, Gilson-Longenbaugh, and AA/MDS Foundation to SJC; “Moelle, Partage et Vie” to YEC, the Lyles Parachini Fund, the Michael Corb Fund, the Michael Garil Leukemia Survivors Program and RO1-CA120535 to RJA. Foundation for Pediatric Research, Vare Foundation, Academy of Finland, Finnish Medical Foundation, Finnish Association of Hematology, and the Kuistila Memorial Foundation to JK, and Association MOELLE PARTAGE ET VIE (France) to YEC. FL is an employee of Bristol-Myers Squibb. SC is a recipient of a research grant from Bristol-Myers Squibb.
Footnotes
REFERENCES
- 1.Tallman MS. New agents for the treatment of acute myeloid leukemia. Best Pract Res Clin Haematol. 2006;19:311–20. doi: 10.1016/j.beha.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Bullinger L, Dohner K, Bair E, et al. Use of gene-expression profiling to identify prognostic subclasses in adult acute myeloid leukemia. N Engl J Med. 2004;350:1605–16. doi: 10.1056/NEJMoa031046. [DOI] [PubMed] [Google Scholar]
- 3.Gilliland DG. Molecular genetics of human leukemias: new insights into therapy. Semin Hematol. 2002;39:6–11. doi: 10.1053/shem.2002.36921. [DOI] [PubMed] [Google Scholar]
- 4.Kelly LM, Gilliland DG. Genetics of myeloid leukemias. Annu Rev Genomics Hum Genet. 2002;3:179–98. doi: 10.1146/annurev.genom.3.032802.115046. [DOI] [PubMed] [Google Scholar]
- 5.Wadleigh M, DeAngelo DJ, Griffin JD, Stone RM. After chronic myelogenous leukemia: tyrosine kinase inhibitors in other hematologic malignancies. Blood. 2005;105:22–30. doi: 10.1182/blood-2003-11-3896. [DOI] [PubMed] [Google Scholar]
- 6.Corey SJ, Anderson SM. Src-related protein tyrosine kinases in hematopoiesis. Blood. 1999;93:1–14. [PubMed] [Google Scholar]
- 7.Tomasson MH, Xiang Z, Walgren R, et al. Somatic mutations and germline sequence variants in the expressed tyrosie kinase genes of patients with de novo acute myeloid leukemia. Blood. 2008;111:47797–808. doi: 10.1182/blood-2007-09-113027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loriaux MM, Levine RL, Tyner JW, et al. High throughout sequence analysis of the tyrosine kinome in acute myeloid leukemia. Blood. 2008;111 doi: 10.1182/blood-2007-07-101394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson LJ, Xue J, Corey SJ. Src family tyrosine kinases are activated by Flt3 and are involved in the proliferative effects of leukemia-associated Flt3 mutations. Exp Hematol. 2005;33:469–79. doi: 10.1016/j.exphem.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Okamoto M, Hayakawa F, Miyata Y, et al. Lyn is an important component of the signal transduction pathway specific to FLT3/ITD and can be a therapeutic target in the treatment of AML with FLT3/ITD. Leukemia. 2007;21:403–10. doi: 10.1038/sj.leu.2404547. [DOI] [PubMed] [Google Scholar]
- 11.Ning ZQ, Li J, McGuinness M, Arceci RJ. STAT3 activation is required for Asp(816) mutant c-Kit induced tumorigenicity. Oncogene. 2001;20:4528–36. doi: 10.1038/sj.onc.1204590. [DOI] [PubMed] [Google Scholar]
- 12.Care RS, Valk PJ, Goodeve AC, et al. Incidence and prognosis of c-KIT and FLT3 mutations in core binding factor (CBF) acute myeloid leukaemias. Br J Haematol. 2003;121:775–7. doi: 10.1046/j.1365-2141.2003.04362.x. [DOI] [PubMed] [Google Scholar]
- 13.Schittenhelm MM, Shiraga S, Schroeder A, et al. Dasatinib (BMS-354825), a dual SRC/ABL kinase inhibitor, inhibits the kinase activity of wild-type, juxtamembrane, and activation loop mutant KIT isoforms associated with human malignancies. Cancer Res. 2006;66:473–81. doi: 10.1158/0008-5472.CAN-05-2050. [DOI] [PubMed] [Google Scholar]
- 14.Shimada A, Taki T, Tabuchi K, et al. KIT mutations, and not FLT3 internal tandem duplication, are strongly associated with a poor prognosis in pediatric acute myeloid leukemia with t(8;21): a study of the Japanese Childhood AML Cooperative Study Group. Blood. 2006;107:1806–9. doi: 10.1182/blood-2005-08-3408. [DOI] [PubMed] [Google Scholar]
- 15.Schnittger S, Kohl TM, Haferlach T, et al. KIT-D816 mutations in AML1-ETO-positive AML are associated with impaired event-free and overall survival. Blood. 2006;107:1791–9. doi: 10.1182/blood-2005-04-1466. [DOI] [PubMed] [Google Scholar]
- 16.O’Laughlin-Bunner B, Radosevic N, Taylor ML, et al. Lyn is required for normal stem cell factor-induced proliferation and chemotaxis of primary hematopoietic cells. Blood. 2001;98:343–50. doi: 10.1182/blood.v98.2.343. [DOI] [PubMed] [Google Scholar]
- 17.Linnekin D, DeBerry CS, Mou S. Lyn associates with the juxtamembrane region of c-Kit and is activated by stem cell factor in hematopoietic cell lines and normal progenitor cells. J Biol Chem. 1997;272:27450–5. doi: 10.1074/jbc.272.43.27450. [DOI] [PubMed] [Google Scholar]
- 18.Knapper S, Burnett AK, Littlewood T, et al. A phase 2 trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line treatment for older patients with acute myeloid leukemia not considered fit for intensive chemotherapy. Blood. 2006;108:3262–70. doi: 10.1182/blood-2006-04-015560. [DOI] [PubMed] [Google Scholar]
- 19.Kelly LM, Liu Q, Kutok JL, Williams IR, Boulton CL, Gilliland DG. FLT3 internal tandem duplication mutations associated with human acute myeloid leukemias induce myeloproliferative disease in a murine bone marrow transplant model. Blood. 2002;99:310–8. doi: 10.1182/blood.v99.1.310. [DOI] [PubMed] [Google Scholar]
- 20.Burgess MR, Skaggs BJ, Shah NP, Lee FY, Sawyers CL. Comparative analysis of two clinically active BCR-ABL kinase inhibitors reveals the role of conformation-specific binding in resistance. Proc Natl Acad Sci U S A. 2005;102:3395–400. doi: 10.1073/pnas.0409770102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- 22.Hu Y, Swerdlow S, Duffy TM, Weinmann R, Lee FY, Li S. Targeting multiple kinase pathways in leukemic progenitors and stem cells is essential for improved treatment of Ph+ leukemia in mice. Proc Natl Acad Sci U S A. 2006;103:16870–5. doi: 10.1073/pnas.0606509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans TR, Morgan JA, van den Abbeele AD, et al. Phase I dose-escalation study of the SRC and multi-kinase inhibitopr BMS-354825 in patients with GISTand other solid tumors. J Clin Oncol. 2005;23:3034A. [Google Scholar]
- 24.Shah NP, Lee FY, Luo R, Jiang Y, Donker M, Akin C. Dasatinib (BMS-354825) inhibits KITD816V, an imatinib-resistant activating mutation that triggers neoplastic growth in most patients with systemic mastocytosis. Blood. 2006;108:286–91. doi: 10.1182/blood-2005-10-3969. [DOI] [PubMed] [Google Scholar]
- 25.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–81. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 26.Topaly J, Zeller WJ, Fruehauf S. Synergistic activity of the new ABL-specific tyrosine kinase inhibitor STI571 and chemotherapeutic drugs on BCR-ABL-positive chronic myelogenous leukemia cells. Leukemia. 2001;15:342–7. doi: 10.1038/sj.leu.2402041. [DOI] [PubMed] [Google Scholar]
- 27.Roginskaya V, Zuo S, Caudell E, Nambudiri G, Kraker AJ, Corey SJ. Therapeutic targeting of Src-kinase Lyn in myeloid leukemic cell growth. Leukemia. 1999;13:855–61. doi: 10.1038/sj.leu.2401429. [DOI] [PubMed] [Google Scholar]
- 28.Dos Santos C, Demur C, Bardet V, Prade-Houdellier N, Payrastre B, Recher C. A critical role for Lyn in Acute Myeloid Leukemia. Blood. 2007;111:2269–79. doi: 10.1182/blood-2007-04-082099. [DOI] [PubMed] [Google Scholar]
- 29.Ozawa Y, Williams AH, Estes ML, et al. Src family kinases promote AML cell survival through activation of signal transducers and activators of transcription (STAT) Leuk Res. 2008;32:893–903. doi: 10.1016/j.leukres.2007.11.032. [DOI] [PubMed] [Google Scholar]
- 30.Donella-Deana A, Cesaro L, Russene M, Brunatim AN, Marin O, Pinna LA. Spontaneous autophosphorylation of Lyn tyrosine kinase at both activation sement and C-terminal tail confers altered substrate specificity. Biochemistry. 1998;37:1438–46. doi: 10.1021/bi971332s. [DOI] [PubMed] [Google Scholar]
- 31.Luo FR, Yang Z, Camuso A, et al. Dasatinib (BMS-354825) pharmacokinetics and pharmacodynamic biomarkers in animal models predict optimal clinical exposure. Clin Cancer Res. 2006;12:7180–6. doi: 10.1158/1078-0432.CCR-06-1112. [DOI] [PubMed] [Google Scholar]
- 32.Han EK, Leverson JD, McGonigal T, et al. Akt inhibitor A-443654 induces rapid Akt Ser-473 phosphorylation independent of mTORC1 inhibition. Oncogene. 2007;26:5655–61. doi: 10.1038/sj.onc.1210343. [DOI] [PubMed] [Google Scholar]
- 33.Nishizumi H, Horikawa K, Mlinaric-Rascan I, Yamamoto T. A double-edged kinase Lyn: a positive and negative regulator for antigen receptor-mediated signals. J Exp Med. 1998;187:1343–8. doi: 10.1084/jem.187.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kulimova E, Oelmann E, Bisping G, et al. Growth inhibition and induction of apoptosis in acute myeloid leukemia cells by new indolinone derivatives targeting fibroblast growth factor, platelet-derived growth factor, and vascular endothelial growth factor receptors. Mol Cancer Ther. 2006;5:3105–12. doi: 10.1158/1535-7163.MCT-06-0323. [DOI] [PubMed] [Google Scholar]
- 35.Stegmaier K, Corsello SM, Ross KN, Wong JS, Deangelo DJ, Golub TR. Gefitinib induces myeloid differentiation of acute myeloid leukemia. Blood. 2005;106:2841–8. doi: 10.1182/blood-2005-02-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishii K, Nanbu R, Lorenzo VF, et al. Expression of the JAK2 V617F mutation is not found in de novo AML and MDS but is detected in MDS-derived leukemia of megakaryoblastic nature. Leukemia. 2007;21:1337–8. doi: 10.1038/sj.leu.2404626. [DOI] [PubMed] [Google Scholar]
- 37.Balaian L, Zhong RK, Ball ED. The inhibitory effect of anti-CD33 monoclonal antibodies on AML cell growth correlates with Syk and/or ZAP-70 expression. Exp Hematol. 2003;31:363–71. doi: 10.1016/s0301-472x(03)00044-4. [DOI] [PubMed] [Google Scholar]
- 38.Kornblau SM, Tibes R, Qiu YH, et al. Functional proteomic profiling of AML predicts response and survival. Blood. 2009;113:154–64. doi: 10.1182/blood-2007-10-119438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu I, Sun J, Arnaout A, et al. p27 phosphorylation by Src regulates inhibition of cyclin E-Cdk2. Cell. 2007;128:281–94. doi: 10.1016/j.cell.2006.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grimmler M, Wang Y, Mund T, et al. Cdk-inhibitory activity and stability of p27Kip1 are directly regulated by oncogenic tyrosine kinases. Cell. 2007;128:269–80. doi: 10.1016/j.cell.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 41.Sharma SV, Settleman J. Oncogene addiction: setting the stage for molecularly targeted cancer therapy. Genes Dev. 2007;21:3214–31. doi: 10.1101/gad.1609907. [DOI] [PubMed] [Google Scholar]
- 42.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–41. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 43.Snead JL, O’Hare T, Adrian LT, et al. Acute dasatinib exposure commits Bcr-Abl-dependent cells to apoptosis. Blood. 2009;114:3459–63. doi: 10.1182/blood-2007-10-113969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zwaan CM, de Boer ML, Beverloo HB, et al. Dasatinib (sprycel) in children and adolescents with relapsed or refractory leukemia: preliminary resultas of the CA180019 phase I/II study from the ITCC consortium. Blood. 2007;110:318a. [Google Scholar]
- 45.Ustun C, Corless CL, Savage N, et al. Chemotherapy and dasatinib induce long-term hematologic and molecular remission in systemic mastocytosis with acute myeloid leukemia with KIT D816V. Leuk Res. 2009;33:735–41. doi: 10.1016/j.leukres.2008.09.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.