Abstract
pDC are the most potent IFN-α-producing cells in the body and serve as a vital link between innate and adaptive immunity. Deficiencies in pDC function were among the earliest observations of immune dysfunction in HIV-1 infection. Herein, we review the status of pDC in individuals with HIV-1 infection and the potential role of these cells in pathogenesis. We begin by reviewing the basic properties of pDC and then discuss the compromise in circulating pDC numbers and function in early and viremic HIV-1 infection and mechanisms that might account for their depletion in HIV-infected patients. In addition, we review the evidence that chronic production of IFN-α, probably through the chronic activation of pDC, is central to the immune activation that is so detrimental in HIV infection. Finally, we discuss the importance of balance in pDC numbers and function and the potential value of using absolute pDC counts and function as a biomarker, along with CD4+ cell counts and VL in HIV-1-infected patients.
Keywords: IFN-α, natural IFN-producing cells, TRAIL, IDO, HAART
pDC, THE NIPC
Human pDC are low-frequency cells found predominantly in peripheral blood and lymphoid tissues that are best known for their ability to produce large quantities of IFN-α in response to stimulation with DNA or RNA viruses (reviewed in ref. [1]). These cells, which were known originally as natural interferon-producing cells (NIPC), were described by our group [1] and the groups of Gunnar Alm, Giorgio Trinchieri, and Charles Rinaldo in the 1980s and early 1990s as HLA-DR+ cells that lacked markers characteristic of traditional PBMC lineages (T cells, B cell, monocytes, and NK cells; reviewed in ref. [1]). Some of the earliest studies of the cells that produce IFN-α were prompted initially by observations that the ability of PBMC from AIDS patients was compromised severely in their ability to produce IFN-α in vitro in response to stimulation with virus (see below). Although Rinaldo’s [2] and our group [3, 4] reported that the NIPC, including those that respond to HIV-1, belong to the DC lineage, other early evidence indicated that these cells were distinct from classical, mature DC [5]. Further characterization of the NIPC had to await the recognition that there are subsets of human DC and that these cells have distinct phenotypes as well as maturation states. Evidence of such heterogeneity was provided, for example, by O’Doherty et al. [6], who demonstrated that there were at least two phenotypically distinct subpopulations of DC circulating in peripheral blood [6]. Recognition that NIPC were the same cell type as a population that had been described alternatively as plasmacytoid monocytes [7] or pDC [8] finally came in 1999 when Dr. Frederick Siegal and colleagues [9] noted similarities in the cells that we had been studying in the context of HIV infection and the cells that had been described by Y. J. Liu and colleagues [9]; this finding was rapidly confirmed by Cella and colleagues [7]. The term “plasmacytoid” refers to their plasma cell-like morphology, resulting from an abundant cytoplasm with a well-developed endoplasmic reticulum.
Although originally thought to be in the lymphoid lineage, there is now evidence that there is plasticity in the pDC lineage and that these cells can potentially be derived from lymphoid or myeloid precursors [10, 11]. A number of transcription factors have been implicated in pDC development, including IRF-4, IRF-8 [12], and Ikaros [13]. In humans, a role for the ETS transcription factor SpiB has been reported for the development of pDC [14] Recently, the basic helix–loop–helix transcription factor E2-2 has been shown to be expressed preferentially in murine and human pDC [15, 16]; deletion of murine E2-2 blocked the development of pDC [15]. In humans, E2-2 was shown to cooperate with SpiB for the development of pDC [16], and E2-2 haploinsufficiency in humans with Pitt-Hopkins syndrome was found to be associated with an aberrant pDC expression profile and impaired type I IFN responses [15]. E2-2 was further found to activate multiple pDC-enriched genes directly, including transcription factors involved in pDC development, such as SpiB and IRF-8, as well as the transcription factor IRF-7, which is required for IFN-α production [17]. The transcription factors Id2 and Id3 have been reported to be negative regulators of pDC but not cDC development [18].
Originally described in humans, pDC have also been characterized in a number of other mammalian species including mice, rats, and monkeys [19,20,21,22,23]. The characterization of pDC in mice was of particular importance, as the ability to knock out specific genes in mice provides essential tools to probe important pathways in these cells, and their characterization in macaques is important for the study of these cells in nonhuman primate models of SIV-1 infection. It should be noted, however, that murine and human pDC are not identical in either phenotype or function; for example, although highly purified human and murine pDC produce large quantities of type I IFNs, murine but not human pDC readily produce IL-12 in response to viral or CpG stimuli [19, 24]. Moreover, murine pDC express CD11c, B220 and SiglecH [20, 25, 26], and human pDC do not. Instead, human pDC can be identified by their expression of CD123 (IL-3Rα chain) as well as BDCA-2 (CD303). Nonhuman primate pDC can be identified by many of the same reagents used to identify human pDC [21, 27]. However, some of the available antibodies used to identify human pDC, such as human BDCA-2 (CD303), a C-type lectin receptor expressed by pDC that has endocytic and signaling capability, fail to react with macaque pDC. An improved set of antibody reagents that identify nonhuman primate pDC is needed and would facilitate SIV research.
In humans, pDC represent 0.2–0.5% of the circulating PBMC, but each pDC is potent, being able to produce as much as 3–10 pg IFN-α in response to HSV-1, a prototypic inducer of IFN-α in these cells [28]; thus, although many cells in the body have the potential to produce IFN-α, pDC produce ten- to 1000-fold more IFN-α than these other cell types [9]. pDC respond to a wide range of enveloped viruses, including HIV-1, with production of a broad range of IFN-α subtypes and also produce other type 1 IFNs (IFN-β, -κ, and -ω, which signal through the type I IFNR) as well as type III IFN (IFN-λ or CD28/CD29, which signal through a distinct receptor; reviewed in ref. [1]). In addition, pDC produce proinflammatory cytokines TNF-α and IL-6 in response to viral stimulation, as well as a number of chemokines, including CXCL10, CCL4, and CCL5 [29,30,31]. The actual induction of IFN-α by viruses in pDC is a two-step process: The recognition of viral envelopes by C-type lectin receptors on pDC is important for viral uptake [4, 32], but actual signaling for IFN-α production occurs in the endosome, where TLR9 is the receptor for viral DNA [33,34,35,36], and TLR7 is the receptor for viral ssRNA [37, 38]. IFN-α production can also be induced in pDC by type A CpG, which interacts with endosomal TLR9 [39]; these nonmethylated CpG sequences are prominent in bacteria and viruses but are suppressed in the mammalian genome. In addition to RNA viruses, some synthetic ssRNA and imidazoquinolones can induce IFN-α production in pDC through TLR7 [37, 38]. In addition to cell-free viruses, virus-infected cells can be potent inducers of IFN production by pDC [40, 41].
The production of IFN-α by pDC depends on the transcription factor IRF-7, which we demonstrated to be expressed at high constitutive levels in pDC but not other PBMC populations [42, 43]. Although many other cell types can make type I IFNs, albeit at much lower levels than pDC, this constitutive expression of IRF-7 and the lack of requirement for IRF-3 participation are central to what makes pDC the “professional IFN-α-producing cells” [17, 44]. In contrast, IRF-7 expression must be up-regulated prior to the production of IFN-α in other cell types, such as monocytes or fibroblasts, with initial signaling through IRF-3 required before these cells can produce significant levels of IFN [45]. Following stimulation with TLR7 or -9 agonists, IRF-7 is translocated rapidly to the nucleus in pDC [43, 46]. In a recent study, failure of cord blood pDC to produce IFN-α in response to stimulation with HSV or CpGA was associated with a failure to translocate IRF-7 [47]. Translocation of IRF-7 (but not NF-κB) in pDC is dependent on PI3K; interestingly, inhibition of PI3K results in inhibition of IFN-α but not proinflammatory cytokine production by pDC [48]. In collaboration with Betsy Barnes [42, 49], we demonstrated that IRF-5 is also constitutively expressed in pDC, and IRF-5 has been implicated in the cytokine induction pathway in pDC [50, 51]; moreover, recent evidence suggests that IRF-5 is involved in IFN-α production as well [51, 52].
pDC: UNIQUE EFFECTORS AT THE INTERFACE OF INNATE AND ADAPTIVE IMMUNITY
As noted above, many cell types in the body have the ability to produce IFN-α in response to acute viral infection, leading to cytoplasmic expression of viral DNA or RNA and their intermediates; these replicative intermediates are recognized by intracellular sensors of RNA such as PKR, RIG-I, MDA-5, and the intracellular DNA sensor, DAI (reviewed in ref. [1]). The IFN-α produced in response to these stimuli results in the establishment of an antiviral program in neighboring, uninfected cells, thus limiting the spread of virus. Although the majority of these other cell types requires viral gene expression and formation of replicative intermediates before the presence of the virus can be detected, pDC produce IFN-α in response to many viruses in the absence of viral gene expression, including, for example, inactivated HSV-1, influenza virus, and HIV-1. In addition, we have demonstrated that pDC can produce IFN-α through preferential sampling of portions of live, virus-infected cells through a process known as “nibbling” (ref. [53] and Wnek et al., manuscript in preparation). Although monocyte-derived DC and circulating cDC can also acquire cellular material from live cells [54], there is no selective uptake of material from virus-infected versus uninfected cells by these DC (ref. [55] and Wnek et al., manuscript in preparation).
The ability to respond to viruses in the absence of virus replication confers a clear advantage to the pDC over other potential IFN-producing cells, as many viruses express gene products with the ability to block IFN-α production in the cells that they actively infect; these anti-IFN mechanisms are widespread and diverse, underscoring the importance of the type I IFN response in host defense (see Haller et al. [56] for an excellent review about these virus-subversive measures). Direct endosomal delivery of live or noninfectious virus to pDC largely bypasses these viral subversive measures, as de novo gene expression is not typically required for the induction of IFN-α in pDC. On the other hand, the ability of pDC to respond to inactivated virus leads to their potential overactivation in viremic HIV disease, where a circulating defective virus is present at high levels (see Conclusions and Perspectives). In some instances where virus actively infects the pDC, such as with vesicular stomatitis virus, pDC are able to deliver viral RNA to endosomal compartments containing TLR7 through the process of autophagy [57]; the degree to which this is a commonly used pathway by pDC is not yet known. The endosomal location of DNA- and RNA-sensing TLRs is important for pDC: Extracellular host DNA or RNA, which might be released by dying host cells, is degraded rapidly by extracellular nucleases; only material that is actively endocytosed is able to stimulate pDC through the TLR7 and -9 interactions.
pDC produce copious amounts of type I IFNs after viral stimulation, all of which signal through the shared type I IFNR, providing an antiviral effect that can be local and systemic. Although the antiviral effects of pDC-derived type I IFNs are clearly important, an equally, if not more important, role of pDC is to provide an interface between these innate immune effectors and other cells of the immune response. Activated pDC acquire the ability to migrate to LN in response to chemotactic signals delivered to CCR7, which is up-regulated on pDC upon activation [7]. The pDC move from the peripheral blood to the LN through interaction of their surface CD62L with receptors in the high endothelial venules. In addition to this recruitment of activated pDC, we have demonstrated that nonactivated pDC reside in secondary lymphoid tissues, including spleen, LN, and tonsil, where they can interact with infected cells and virus that might be brought into the LN through other cell types, such as migrating cDC [53]. In the absence of infection, these lymphoid tissue-resident pDC are not preactivated and are fully capable of producing IFN-α upon stimulation with virus. Within these secondary lymphoid tissues, pDC can interact with T cells and NK cells, which they can chemoattract through pDC-derived chemokines [29, 30], and may be involved in stimulation of antibody responses as well. pDC-derived IFN-α activates NK cells as well as CD4+ and CD8+ T cells; this activation as well as direct cell:cell contact between pDC and NK cells and T cells give rise to enhanced effector functions. Moreover, activated pDC can also migrate to sites of tissue inflammation [7, 58]. IFN-α produced by pDC has direct autocrine effects, leading to “priming” of enhanced IFN-α production, in part, through the up-regulation of IRF-7 [43]. Additionally, IFN-α is an autocrine survival factor for pDC, which are normally labile [59, 60]. pDC, through their production of IFN-α and other cytokines, also enhance the maturation of human CD11c+ cDC in a bystander manner, which has potentially important implications in the pathogenesis of lupus [61] and HIV-1 [62]. We [63] and others [36, 64] have shown that pDC provide an accessory function that licenses NK cells to kill virus-infected target cells, including HIV-infected cells [65, 66]; in the absence of this stimulatory signal, which is mediated by IFN-α as well as cell:cell interactions, the NK cells fail to lyse virus-infected cells, including cells infected with HIV-1 [66].
In addition to induction of IFN-α, activation of pDC through TLR7 ligands sets into motion a maturation process in these cells; upon viral stimulation, pDC up-regulate the expression of costimulatory molecules CD80 and CD86 as well as MHC class I and class II molecules and also acquire CD40 and CD83. Although initially considered to be inferior APCs compared with cDC as a result of their lower rate of endocytosis and lower expression of MHC class II [8], pDC are clearly endocytic [32, 46], and numerous studies have demonstrated the ability of pDC to function as APC for CD4- and CD8-positive cells (reviewed in ref. [1]).
The type of stimulus received by the pDC is an important determinant in the outcome of the response: In response to viruses and TLR7/9 ligands, pDC produce large amounts of IFN-α and induce Th1 differentiation [60], and IFN-α has been shown to bias CD4+ cells to a Th1 phenotype [67], although this biasing is not as strong as for IL-12 [68]. The type of T cell responses induced by pDC are strongly influenced by the signals received by the pDC; virus-stimulated pDC stimulate naive T cells to produce IFN-γ and IL-10 [69], and stimulation of pDC through CD40:CD40L interactions, in the presence of IL-3, was found to induce Th2 differentiation [8]. In addition to their ability to influence the development of Th1 and Th2 responses, pDC have the potential to induce tolerance or Tregs (reviewed in ref. [70]), in part, through their expression of IDO. As discussed below, pDC-derived IDO has also been associated with HIV pathogenesis [71]. In addition to their roles in directing Th cell subsets, virus-stimulated pDC have been reported to have a role in the priming of CD8+ T cells [72,73,74,75,76]. Most recently, the ability of pDC to cross-present vaccinal lipopeptides and HIV-1 antigens from apoptotic cells to CD8+ T cells has been demonstrated [77]. This cross-presentation was amplified by exposure to influenza virus and occurred at levels comparable with that of cDC, thus implicating cross-presentation as a potentially important in vivo function of pDC. Supporting a potential role of IFN-α in this process is the requirement for IFN-α signaling for the induction of CD8+ cytotoxic T cells [74].
In addition to their effects on T cell polarization, pDC and their production of IFN-α as well as IL-6 have been found to participate in humoral immunity, having important roles in class-switching and development of virus-specific antibodies [78,79,80,81]. Thus, pDC are clearly multifunctional cells at the interface of innate and adaptive immunity.
pDC IN HIV INFECTION
Deficient IFN-α production in patients infected with HIV-1
The observation of deficient in vitro IFN-α production in HIV-infected individuals was made early in the AIDS epidemic: We observed that there was defective production of IFN-α by PBMC of patients with this novel syndrome in response to HSV-infected fibroblasts or cell-free HSV virions, even when corrected for the white blood count [82, 83]. This failure to produce IFN-α was strongly associated with and predictive of opportunistic infections in the immediate follow-up period [83]. In fact, we reported that in this pre-HAART era, progression to opportunistic infections did not occur until absolute CD4+ cell counts and IFN-α production by PBMC fell below critical levels (defined in that early study as CD4+ cells<250 and IFN-α<300 IU/ml). Other investigators later confirmed the association of deficient in vitro production of IFN-α production with opportunistic infections. As a distinct phenotypic description for the NIPC was lacking, we used IFN-α bioassays and later, ELISpot assays to assess functional capacity of the NIPC and determined that not only did the patients have a lower number of IFN-producing cells, but also, each cell made less IFN-α on a per-cell basis in response to HSV-1 than did healthy donors [28].
It was not until the definition of the NIPC/pDC phenotype that it was possible to evaluate this functional deficiency in NIPC with a phenotypic correlation. Beginning in 2001, we [84] and several other groups [85,86,87] described a numerical depletion in pDC in the peripheral blood of patients with HIV-1 infection. In these studies, pDC numbers were found to correlate with CD4 numbers and/or were inversely correlated with VL. Our initial data suggested a functional, in addition to numerical, deficiency in pDC in several patients with high VL and low CD4+ cell numbers; in these individuals, the proportion of pDC that produced IFN-α in response to HSV-1, as determined by intracellular staining for IFN-α, was diminished compared with noninfected individuals or HIV-infected individuals with low VL and reasonably high CD4 counts [84]. A protective role for pDC in HIV infection was suggested by Levy and colleagues [86], who reported higher levels of pDC in LTNP than even found in healthy, noninfected controls, and Almeida et al. [88] described normal levels of pDC in a small cohort group of LTNP. Although several studies clearly demonstrated a correlation between deficient pDC numbers and IFN-α production and progression of HIV infection, pDC numbers were also found to be affected, albeit transiently, in primary HIV infection as well [89, 90]. Moreover, in the latter paper, remaining pDC were found to be refractory to produce IFN-α in response to HSV-1.
Effect of HAART on pDC numbers and IFN-α production in patients with HIV infection
We reported that IFN-α production normalized in individuals receiving HAART and restoration of IFN-α production preceded restoration of CD4+ T cell numbers [91]; however, other groups demonstrated that at the cellular level, this recovery of pDC is incomplete, and absolute pDC numbers never fully normalized [92,93,94,95,96]. The failure of individuals on HAART to fully recover their pDC numbers is consistent with the possibility that chronic HIV replication leads to a chronic stimulation that is detrimental to maintaining pDC homeostasis. Recently, much attention has been given to the accelerated immunosenescence in patients with HIV infection [97]; in CD4+ cells, this is associated with replicative senescence and telomere shortening [98]. It is possible that the delayed start of HAART, which has been the clinical norm, may be encouraging senescence not only in the CD4+ T cells but also in the pDC compartment as well. In support of this possibility were the observations of Chehimi et al. [92], who reported that recovery of pDC in HAART patients was more complete in those patients with the lowest baseline VL versus those with higher VL, despite full suppression of virus in both groups, as well as Kamga et al. [89], who reported the best reconstitution of pDC and IFN production in patients treated with HAART starting in primary infection. Likewise, Lichtner et al. [99] reported that pDC levels in patients treated with HAART are predictive of VL, independent of levels of CD4 T cell recovery.
In vivo and in vitro infection of pDC by HIV-1
pDC express the primary HIV-1 receptor, CD4, as well as the two main coreceptors, CXCR4 and CCR5, and in vitro, they can be infected with R5 and X4 strains of HIV-1 [100]. In vitro, HIV-1 replication in pDC is enhanced by maturation of the pDC with CD40L, as well as by inclusion of anti-IFN-α antibodies in the culture media to prevent autocrine or paracrine inhibition of viral replication [101, 102]. A recent study demonstrated that IFN-α up-regulation of APOBEC3G in pDC is involved in restricting HIV-1 infection in pDC [103, 104], and pDC infected in vitro with HIV-1 are able to transmit the infection to CD4+ T cells [105], suggesting a mechanism by which infected pDC could move to the LN, where they transmit virus to CD4+ cells. Whether pDC are infected with HIV-1 in vivo has been controversial, and purity of the pDC preps was examined clearly as an important factor. For example, in one study, pDC were reported to harbor HIV-1 provirus at frequencies similar to that of CD4+ T cells [106], and in another study of patients on HAART, pDC were not found to harbor HIV-1, leading to the conclusion that circulating pDC are not a major reservoir for HIV-1 [107]. In an experimental SCID-hu model for HIV-1 infection, pDC were clearly demonstrated to become productively infected and produce IFN-α in the implanted human thymus [108]. It is possible, however, that pDC are not resident in the blood long enough to become latently infected with HIV-1.
Induction of IFN-α by HIV-1
Along with the many viruses that induce IFN-α in pDC, cell-free HIV-1 and HIV-infected cells are able to induce IFN-α production by pDC [38, 40, 62, 109]. Unlike with HSV-1 or influenza virus, however, where low multiplicities of infection are required to induce IFN-α (typically in the range of one infectious particle/cell) [109], for HIV-1, virus in the range of 300–1000 ng/ml p24 is required—as much as 10,000 50% tissue culture infective dose [38, 62]. In contrast to the requirement for high levels of cell-free HIV-1 to induce IFN-α, Schmidt et al. [40] reported that cells acutely infected with low titers of HIV-1 were able to induce IFN-α production efficiently by pDC [40].
For cell-free virus, induction of IFN-α by HIV-1 is dependent on the interaction of gp120 with CD4 on the pDC; this envelope:CD4 interaction results in endocytosis of HIV-1 into chloroquine-sensitive compartments and is not dependent on the interaction of HIV-1 with CXCR4 or CCR5 coreceptors. Once inside the endosomal compartments, HIV-1 nucleic acid signals mainly through TLR7. Further, endosomally delivered HIV-1 RNA rather than DNA in early retrotranscripts was found to induce IFN-α in pDC [38]. Although one study suggested that gp120 itself is sufficient for the induction of IFN-α in pDC [110], the majority of studies, including our own, has failed to confirm this result. Beignon et al. [38], for example, demonstrated that although binding of HIV-1 to surface receptors on pDC was required, internalization of HIV-1 into acidic compartments is required for induction of IFN-α, as is the presence of viral nucleic acid, as viral RNA packaging-deficient HIV-1 was not able to induce IFN-α expression. The importance of the initial interaction of gp120 with CD4, however, is supported by a recent study demonstrating using envelope-recombinant viruses, where affinity of viral gp120 for CD4 rather than viral tropism (i.e., X4 or R5) is the primary determinant in the ability of HIV-1 to induce IFN-α in pDC [111].
The reasons for the requirement of such large quantities of cell-free HIV-1 for the induction of IFN-α by HIV-1 are unclear. A number of studies have demonstrated that pDC can be productively infected with HIV-1 and that this infection leads to cell death [102, 112,113,114]; this observation probably explains why aldrithiol-2-inactivated HIV-1 is somewhat more efficient at inducing IFN-α than infectious virus. However, even nonchemically inactivated HIV-1 preparations contain a large preponderance of noninfectious to infectious viral particles, and live virus is estimated to make up only 0.1% of the virion particles in a typical HIV-1 prep, and in vivo, noninfectious particles are estimated to outnumber infectious particles by a factor of 102–104 [115, 116]. Autocrine antiviral effects of HIV-induced IFN on the pDC are supported by the observation that increased HIV-1 replication occurs when neutralizing anti-IFN antibodies are included in the cultures. Direct suppressive effects of several different HIV-1 gene products have been suggested. Martinelli et al. [117] reported that not only does soluble HIV-1 gp120 not induce IFN-α in pDC, but also, it can inhibit the production of IFN-α as well as subsequent maturation in response to subsequent stimulation with the TLR9 agonists CpGA or HSV-2. Interestingly, they found no inhibition of IFN-α production in response to imiquimod, which activates pDC through TLR7. An even stronger effect of inhibition was seen with trimeric gp120 as compared with monomeric gp120. These results are reminiscent of our report that cross-linking CD4 with antibodies delivered a strong inhibitory signal for pDC IFN-α production [46]. Likewise Vpr, a small accessory HIV gene product that can be detected in plasma of HIV-1-seropositive individuals, strongly inhibited type 1 IFN production by pDC without inducing their apoptosis [118]. These authors also found that Vpr inhibited subsequent pDC/NK interactions. A role for HIV-1 matrix protein p17 in pDC dysregulation has also been reported; p17 treatment was found to up-regulate CCR7, which should then confer upon them a migratory phenotype, but failed to up-regulate maturation and costimulatory molecules on the pDC, which would usually accompany CCR7 expression. The authors propose that circulating pDC would be induced to migrate prematurely to the LN, where they would retain the capacity to produce IFN-α but would not be mature [119]. Finally, multiple effects of HIV-1 Nef on monocyte-derived DC and bone marrow-derived pDC biology have been described, but specific effects of Nef on pDC function are not known [120].
Evidence of in vivo production of IFN-α in HIV infection
The presence of circulating IFN-α in the serum of some HIV-infected individuals was one of the earliest immune alterations discovered in AIDS [121]; like deficient in vitro production of IFN-α by NIPC, the presence of serum IFN was associated with a poor prognosis. Curiously, the circulating IFN-α in patients with HIV was found to be sensitive to treatment at pH 2, although none of the individual IFN-α subtypes are themselves intrinsically acid-labile; furthermore, Gendelman and colleagues [122] isolated the IFN-α from plasma from HIV-infected individuals and found that the purifed IFN-α was not acid-labile [123]; instead, a serum factor found in HIV-infected individuals as well as individuals with lupus is able to confer the lability [124, 125]. Moreover, although presumed by many to be the pDC, the source of the circulating IFN-α has not been described definitively.
Evidence that pDC produce IFN-α in situ in response to HIV-1 comes from a study by Gurney et al. [108], who were studying pDC in thymus in a SCID-hu model. This pDC-derived IFN inhibited HIV-1 production directly in the HIV-infected thymus, and their depletion augmented HIV-1 replication dramatically. Although the majority of reports indicates that circulating pDC in HIV-infected individuals do not constitutively produce IFN-α, as detected by intracellular flow cytometry or IFN bioassay (see, for example, ref. [84]), Lehmann and colleagues [126] found an increased expression of IFN-α mRNA in PBMC and pDC when isolated directly from CDC Stage C HIV-infected patients relative to controls and CDC Stage A patients; interestingly, there were no differences in IFN-α mRNA in lymphoid tissue between controls and patients. Further evidence for an IFN “signature” was found in an increased MxA gene expression in PBMC but not lymphoid tissue. These investigators also reported extremely high constitutive levels of intracellular IFN-α expressed by all of the pDC from patients and controls, a result that contradicts others. Two other reports suggest a strong IFN signature in vivo: Tilton et al. [127] demonstrated a reduced capacity of pDC from individuals who were on short-term interruption of HAART-treated to produce IFN-α, which was accompanied by an up-regulation of type 1 IFN response genes in PBMC and lymphoid tissues. These investigators suggest that the failure of pDC from these patients to produce IFN-α in vitro may not reflect an intrinsic deficiency in the pDC but rather, a refractory period for the pDC after they have been stimulated in vivo with virus or type 1 IFNs; they demonstrate further that a similar refractoriness occurs in pDC of HCV patients treated with IFN-α2B. In the second paper, Badolato report [128] that in children who were infected perinatally with HIV-1, there is MxA expression, even at times when serum IFN-α is low. It is possible that given the ubiquitous expression of IFN-α receptors and the short half-life of this cytokine, measurements of serum IFN-α might not fully reflect its presence and activity.
Mechanisms of depletion of circulating pDC
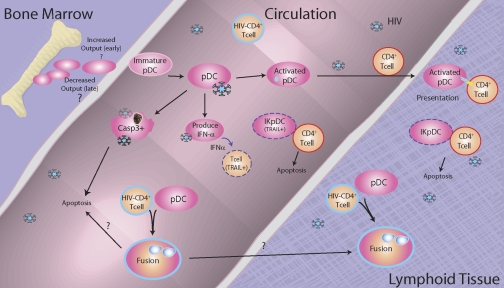
The mechanisms that account for the decreased circulating pDC and their dysfunction in HIV-infected patients are currently a topic of intense investigation. It has been suggested that in HIV infection, pDC are activated in the periphery and are then recruited to the LN, thus accounting for the lower circulating numbers of pDC; indeed, the depletion of pDC in the peripheral blood of patients with systemic lupus has been associated with redistribution of these cells to lesions in the skin [129]. Such redistribution of pDC was observed early after SIV infection by Barratt-Boyes and colleagues [130, 131] and others [132], and Dillon et al. [133] have reported that cDC and pDC accumulated in the LN of asymptomatic, untreated, HIV-1-infected subjects at a point when pDC were only depleted modestly from the peripheral blood. pDC have also been reported to accumulate in the lymphoid tissues, including spleen, of chronically infected individuals [134, 135]. The accumulation of the pDC in the spleen was associated with a high pro-VL; interestingly, however, in that study, although IFN-α was found in situ in the spleens from HIV-infected individuals, pDC were not the major source for the IFN-α [134]. In contrast to the reports of accumulation of pDC in lymphoid tissue, a parallel depletion of cDC (or myeloid) and pDC from the blood and LN of chronically infected monkeys was reported [136]. Likewise, Biancotto et al. [137] have recently found a dramatic depletion of cDC and pDC in LN of HIV+ individuals, and Badolato et al. [128] found dramatic depletion of pDC in tonsils of HIV-infected children. Thus, it appears that redistribution of pDC to the LN in HIV-infected individuals is insufficient to explain the peripheral depletion of these cells, especially in viremic individuals, although it is possible that the pDC, which do get recruited to the LN, die rapidly [132, 138]. Complicating further the issue of the fate of circulating pDC in HIV-infected patients are the observations of phenotypic changes in circulating pDC from patients chronically infected with HIV-1. Dillon et al. [133] have reported that circulating pDC and those that accumulate in the LN of asymptomatic subjects have what they termed a “partial activation phenotype” with a significant increase in the expression of CD40; these partially activated pDC also demonstrated a reduced capacity to migrate in response to CXCL12. In recent studies, our lab has found evidence for multifactorial changes in circulating pDC from patients; we observed subsets of pDC undergoing apoptosis (as evidenced by increased activated caspase 3+ pDC), increased pDC expressing activation and maturation markers, as well as replacement of dying or migrating pDC with less-mature pDC that express lower levels of IRF-7 (Feng et al., submitted). Moreover, we observe that coculture of pDC with acutely HIV-infected autologous or heterologous CD4+ T cells or with chronically HIV-infected H9 cells leads to pDC:CD4+ T cell fusion; inhibition of cell:cell fusion was found to rescue pDC IFN-α production (E. S. Jacobs et al., manuscript in preparation). The possible contribution of cell:cell fusion to pDC loss has also been proposed by Fauci and colleagues [139]. An additional possibility that needs to be addressed is whether deficient pDC in advanced HIV infection could, in part, result from a failure of the bone marrow to produce adequate numbers of pDC. A summary of some of the contributory factors to and consequences of pDC depletion is shown in Figure 1.
Figure 1.
Fate of pDC in HIV-infected individuals. pDC enter the circulation from the bone marrow, where they migrate to the lymphoid tissue directly or circulate until they encounter live or defective HIV-1 or other pathogens. Encounter with HIV-1 leads to pDC activation and production of IFN-α, partial or full maturation of the pDC, apoptosis of the pDC, or fusion of the pDC with HIV-infected CD4+ cells. This can result in migration of the pDC to the LN and replacement with immature pDC from the bone marrow. At later times, we hypothesize that bone marrow output of nascent pDC may decline as a result of replicative exhaustion. High levels of IFN-α induce TRAIL expression on pDC, making them into IKpDC as well as DR expression CD4+ T cells. This in turn is hypothesized to lead to killing and depletion of infected T cells and T cells that have encountered defective virus as well as death of the pDC.
Effects of HIV infection on other functions of pDC
In addition to their well-known role as IFN-producing cells, as described above, pDC produce other cytokines and interact with other cell types. A number of studies have addressed some of these other functions of pDC from HIV-infected individuals. Defective stimulation by pDC in the allogeneic mixed lymphocyte reaction has been described [106], as well as a partial activation rather than full activation phenotype [133]. Defects have also been reported in the ability of TLR7/9 agonists to up-regulate CCR7 expression and maturation markers in pDC from HIV-infected donors [140]. Interestingly, Sachdeva et al. [141] reported that the IFN-α and proinflammatory cytokine (IL-6 and TNF-α) production by pDC are not necessarily coupled: In HAART-treated patients with poor CD4 reconstitution, pDC production of IFN-α but not TNF-α and IL-6 was impaired. In fact, spontaneous production of proinflammatory cytokines by pDC from HIV-infected individuals in the absence of additional stimuli has been reported [88]. Another area of interest is the potential defect in the interaction of pDC with NK cells. In a recent study, defective NK activity in viremic HIV infection was attributed to NK cells and defects in pDC [142, 143]. HIV gene products may have a direct role in these defective interactions: Along with inhibiting IFN-α production, trimeric gp120 was found to interfere with pDC-induced NK cytolytic activity [117].
pDC as mediators of overzealous immune activation in HIV-1 infection
Although as described above, there is significant evidence for association of loss of circulating pDC with HIV disease progression, other evidence suggests that chronic production of IFN-α can have detrimental effects in HIV- or SIV-infected individuals. Whether IFN-α is beneficial or detrimental in HIV infection remains somewhat controversial. As reviewed by Herbeuval and Shearer [116], some early trials of IFN therapy in HIV-infected subjects showed only modest beneficial effects, whereas other studies suggested a negative association of the presence of IFN-α on HIV infection. In fact, these same authors have reported that IFN-α, which is produced in response to the high levels of HIV-1 virions found in patients, leads to the up-regulation of the TRAIL death molecule on uninfected T cells. In addition, they demonstrated that in vitro exposure of pDC to HIV-1 leads to up-regulation of TRAIL on the pDC themselves, turning them into IKpDC capable of killing HIV-infected SupT1 cells through the TRAIL pathway [116, 144,145,146]. Moreover, Stingl and colleagues [147] reported recently that TRAIL is expressed on as many as half of the circulating pDC in HIV-infected individuals and that these TRAIL-expressing pDC can kill HIV-infected T cells and even T cells associated with noninfectious HIV particles. They propose that migration of these killer pDC to the LN could be responsible for the depletion of uninfected CD4 cells. Interestingly, they did not find similar generation of IKpDC in patients with varicella zoster virus or hepatitis C virus infection [147]. However, Chehimi et al. [148] has questioned recently whether lysis of HIV-infected T cells by TRAIL-expressing pDC has physiological significance. These investigators evaluated blood from HIV-infected subjects and confirmed the expression of TRAIL on circulating pDC; however, although the activated pDC from these donors were able to kill DR5-expressing, HIV-infected SupT1 cells (in agreement with Shearer and colleagues [116, 144–146]), these pDC did not kill autologous, HIV-infected CD4+ T cells, which did not express DR5. Moreover, comparable levels of type I IFN were found in plasma of viremic, HAART-treated vs. control subjects, potentially arguing against a sustained type I IFN signaling specific to chronic HIV infection.
A role for IDO production by activated pDC in response to HIV-1 in vivo or in vitro, leading to T cell activation and dysfunction, has also been reported [71, 149, 150], and Manches et al. [151] have demonstrated the pDC-derived, IDO-dependent induction of Tregs, which may serve to regulate and potentially limit protective anti-HIV immune responses [132].
Further evidence for a potentially detrimental role for pDC in lentiviral infection is the recent observation that although in the rhesus macaque, SIV infection leads to IFN-α production by pDC and chronic immune activation, in the natural host for SIV—the Sooty Mangabey, where the virus is nonpathogenic despite ongoing replication—there is a failure of the pDC to produce high levels of IFN-α as a result of an endogenous defect in IRF-7 [152]. It is speculated that in the absence of strong production of IFN-α, strong detrimental immune activation triggered by IFN-α does not occur. However, a recent paper by Campillo-Giminez et al. [153] draws different conclusions regarding nonpathogenic SIV infection: These authors found that rhesus macaques and African green monkeys produce similar levels of IFN-α in response to SIV infection and instead, associated pathogenesis with an enhanced recruitment of pDC to the LN and an inflammatory environment early after infection. A further association of an overzealous production of IFN-α and HIV progression was reported recently by Altfeld and colleagues [154]; these investigators found that women, who progress more rapidly to AIDS than men, produced more IFN-α in response to TLR7 agonists and had higher levels of CD8+ cell activation than men when corrected for VL. Interestingly, there were no differences in response to CpG-A (a TLR9 agonist) or in TNF-α production between men and women. This higher level of IFN-α production in females in response to TLR7 stimulation has been proposed to reflect escape of X-chromosome inactivation of TLR7 (which is encoded on the X chromosome), leading to higher levels of IFN-α production in females; moreover, this higher production of IFN-α in females may be associated with the much higher levels of lupus, a disease that is clearly linked to pDC and IFN-α, seen in females [155]. In the case of HIV-1 infection, these higher levels of activation could be responsible initially for the lower VL seen in women earlier in infection, but relentless, chronic activation may lead to accelerated progression to AIDS.
CONCLUSIONS AND PERSPECTIVES
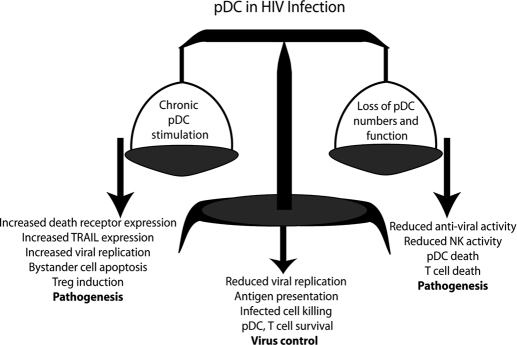
The status and role of pDC in HIV-1 infection are clearly complex; as outlined above, the loss of pDC function is strongly associated with disease progression in HIV and insufficient pDC interaction with other cell types. Additionally, the presence of a strong pDC response is seen in virus controllers and LTNP. On the other hand, chronic activation of pDC, particularly through TLR7, can lead to detrimental effects of IFN-α, including induction of IDO and up-regulation of TRAIL on T cells and pDC, leading to increased killing of CD4+ T cells as well as killing of uninfected T cells by so-called “killer” pDC. These seemingly contradictory observations underlie the importance of maintaining balance in the IFN-α system: Underproduction and chronic overproduction of IFN-α by pDC can be expected to have detrimental effects to the host (Fig. 2). Evidence of immune exhaustion of pDC and better reconstitution of pDC in patients treated early on with antiretroviral therapy argue that an earlier start to HAART might result not only in lowering of the viral set-point but also preservation of pDC numbers and function [89, 92]. We had suggested previously that given the discordance of CD4 counts with pDC numbers that occur frequently early and late in disease, monitoring pDC numbers along with CD4 counts will provide the clinician with better information about the innate and adaptive immune system in HIV infection [156], which has also been proposed by Hosmalin and colleagues [89].
Figure 2.
Keeping the balance of pDC activity in HIV infection. Loss of a balanced pDC response is of great importance in HIV-1 pathogenesis. Depletion of pDC numbers and their functional activity can have a number of effects that lead to HIV pathogenesis. In addition to limiting virus replication through the production of IFN-α, pDC function is critical for licensing effective NK and for preventing pDC and T cell apoptosis. Loss of pDC in viremic HIV-1 infection (right pan in the balance) results in increased virus replication, decreased killing of virus-infected cells, as well as pDC and CD4+ T cell death, all leading to HIV pathogenesis. On the contrary, chronic stimulation of pDC leads to expression of TRAIL on CD4 T cells and pDC and the expression of DRs on CD4+ T cells, which contributes to the induction of apoptosis of uninfected CD4+ T cells through IKpDC (left pan of the balance). Additionally, chronic stimulation of pDC driven by continual HIV production can lead to the generation of Tregs through the induction of IDO, which attenuates the immune response when it is most critical and fosters an increase in viral replication. However, the correct balance of pDC activation and the subsequent functions of pDC will result in proper antigen presentation and elimination of HIV-infected cells and decreased viral replication and allow for the survival of pDC and CD4 T cells that are required for an effective immune response (“balance”). We hypothesize that steps to prevent this chronic stimulation of the pDC, perhaps by early antiretroviral therapy, will preserve normal, protective pDC function.
Acknowledgments
This work was supported by AI26806 to P. F-B.; E. S. J. was supported in part by a fellowship from the UMDNJ-Graduate School of Biomedical Sciences. We thank Dr. Sukhwinder Singh for help with preparing the figures.
Footnotes
Abbreviations: BDCA-2=blood DC antigen-2, cDC=conventional (myeloid) DC(s), CDC=U.S. Centers for Disease Control and Prevention, DC=dendritic cell(s), DR5=death receptor 5, HAART=highly active anti-retroviral therapy, IDO=indoleamine (2,3)dioxygenase, IFN-α=interferon-α, IKpDC=IFN-producing killer pDC(s), IRF=IFN response factor, L=ligand, LN=lymph node(s), LTNP=long-term nonprogressors, MxA=myxovirus-resistance protein A, NIPC=natural IFN-producing cells, pDC=plasmacytoid DC(s), SCID-hu=SCID-human, ssRNA=single-stranded RNA, TRAIL=TNF-related apoptosis-inducing ligand, Treg=T regulatory cell, VL=viral load(s)
References
- Fitzgerald-Bocarsly P, Dai J, Singh S. Plasmacytoid dendritic cells and type I IFN: 50 years of convergent history. Cytokine Growth Factor Rev. 2008;19:3–19. doi: 10.1016/j.cytogfr.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbas J J, Toso J F, Logar A J, Navratil J S, Rinaldo C R., Jr CD4+ blood dendritic cells are potent producers of IFN-α in response to in vitro HIV-1 infection. J Immunol. 1994;152:4649–4662. [PubMed] [Google Scholar]
- Feldman M, Fitzgerald-Bocarsly P. Sequential enrichment and immunocytochemical visualization of human interferon-α producing cells. J Interferon Res. 1990;10:435–446. doi: 10.1089/jir.1990.10.435. [DOI] [PubMed] [Google Scholar]
- Milone M C, Fitzgerald-Bocarsly P. The mannose receptor mediates induction of IFN-α in peripheral blood dendritic cells by enveloped RNA and DNA viruses. J Immunol. 1998;161:2391–2399. [PubMed] [Google Scholar]
- Chehimi J, Starr S E, Kawashima H, Miller D S, Trinchieri G, Perussia B, Bandyopadhyay S. Dendritic cells and IFN-α producing cells are two functionally distinct non-B, non-monocytic HLA-DR+ cell subsets in human peripheral blood. Immunology. 1989;68:486–490. [PMC free article] [PubMed] [Google Scholar]
- O'Doherty U, Peng M, Gezelter S, Swiggard W J, Betjes M, Bhardwaj N, Steinman R M. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology. 1994;82:487–493. [PMC free article] [PubMed] [Google Scholar]
- Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- Grouard G, Rissoan M, Filguiera L, Durand I, Banchereau J, Liu J. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin-3 and CD40 ligand. J Exp Med. 1997;185:1101–1111. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal F P, Kadowaki N, Shodell M, Fitzgerald-Bocarsly P, Shah K, Ho S, Antonenko A, Liu Y J. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- Zuniga E I, McGavern D B, Pruneda-Paz J L, Teng C, Oldstone M B. Bone marrow plasmacytoid dendritic cells can differentiate into myeloid dendritic cells upon virus infection. Nat Immunol. 2004;5:1227–1234. doi: 10.1038/ni1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Liu Y J, Kadowaki N. Functional diversity and plasticity of human dendritic cell subsets. Int J Hematol. 2005;81:188–196. doi: 10.1532/IJH97.05012. [DOI] [PubMed] [Google Scholar]
- Tamura T, Tailor P, Yamaoka K, Kong H J, Tsujimura H, O'Shea J J, Singh H, Ozato K. IFN regulatory factor-4 and -8 govern dendritic cell subset development and their functional diversity. J Immunol. 2005;174:2573–2581. doi: 10.4049/jimmunol.174.5.2573. [DOI] [PubMed] [Google Scholar]
- Allman D, Dalod M, Asselin-Paturel C, Delale T, Robbins S H, Trinchieri G, Biron C A, Kastner P, Chan S. IKAROS is required for plasmacytoid dendritic cell differentiation. Blood. 2006;108:4025–4034. doi: 10.1182/blood-2006-03-007757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotte R, Rissoan M C, Bendriss-Vermare N, Bridon J M, Duhen T, Weijer K, Briere F, Spits H. The transcription factor Spi-B is expressed in plasmacytoid DC precursors and inhibits T-, B-, and NK-cell development. Blood. 2003;101:1015–1023. doi: 10.1182/blood-2002-02-0438. [DOI] [PubMed] [Google Scholar]
- Cisse B, Caton M L, Lehner M, Maeda T, Scheu S, Locksley R, Holmberg D, Zweier C, den Hollander N S, Kant S G, Holter W, Rauch A, Zhuang Y, Reizis B. Transcription factor E2–2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell. 2008;135:37–48. doi: 10.1016/j.cell.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa M, Schmidlin H, Hazekamp M G, Schotte R, Blom B. Development of human plasmacytoid dendritic cells depends on the combined action of the basic helix-loop-helix factor E2–2 and the Ets factor Spi-B. Eur J Immunol. 2008;38:2389–2400. doi: 10.1002/eji.200838470. [DOI] [PubMed] [Google Scholar]
- Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, Taniguchi T. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- Spits H, Couwenberg F, Bakker A Q, Weijer K, Uittenbogaart C H. Id2 and Id3 inhibit development of CD34(+) stem cells into predendritic cell (pre-DC)2 but not into pre-DC1. Evidence for a lymphoid origin of pre-DC2. J Exp Med. 2000;192:1775–1784. doi: 10.1084/jem.192.12.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselin-Paturel C. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol. 2001;2:1144–1150. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- Gilliet M, Boonstra A, Paturel C, Antonenko S, Xu X L, Trinchieri G, O'Garra A, Liu Y J. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J Exp Med. 2002;195:953–958. doi: 10.1084/jem.20020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E, Amrute S B, Abel K, Gupta G, Wang Y, Miller C J, Fitzgerald-Bocarsly P. Characterization of virus-responsive plasmacytoid dendritic cells in the rhesus macaque. Clin Diagn Lab Immunol. 2005;12:426–435. doi: 10.1128/CDLI.12.3.426-435.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano H, Yanagita M, Gunn M D. CD11c(+)B220(+)Gr-1(+) cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J Exp Med. 2001;194:1171–1178. doi: 10.1084/jem.194.8.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorck P. Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated mice. Blood. 2001;98:3520–3526. doi: 10.1182/blood.v98.13.3520. [DOI] [PubMed] [Google Scholar]
- Ito T, Kanzler H, Duramad O, Cao W, Liu Y J. Specialization, kinetics, and repertoire of type 1 interferon responses by human plasmacytoid predendritic cells. Blood. 2006;107:2423–2431. doi: 10.1182/blood-2005-07-2709. [DOI] [PubMed] [Google Scholar]
- Blasius A L, Colonna M. Sampling and signaling in plasmacytoid dendritic cells: the potential roles of Siglec-H. Trends Immunol. 2006;27:255–260. doi: 10.1016/j.it.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Zhang J, Raper A, Sugita N, Hingorani R, Salio M, Palmowski M J, Cerundolo V, Crocker P R. Characterization of Siglec-H as a novel endocytic receptor expressed on murine plasmacytoid dendritic cell precursors. Blood. 2006;107:3600–3608. doi: 10.1182/blood-2005-09-3842. [DOI] [PubMed] [Google Scholar]
- Brown K N, Barratt-Boyes S M. Surface phenotype and rapid quantification of blood dendritic cell subsets in the rhesus macaque. J Med Primatol. 2009;38:272–278. doi: 10.1111/j.1600-0684.2009.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell D M, Feldman S, Kloser P, Fitzgerald-Bocarsly P. Decreased frequency of natural interferon producing cells in peripheral blood of patients with the acquired immune deficiency syndrome. Clin Immunol Immunopathol. 1994;71:223–230. doi: 10.1006/clin.1994.1076. [DOI] [PubMed] [Google Scholar]
- Megjugorac N J, Young H A, Amrute S, Olshalsky S, Fitzgerald-Bocarsly P. Virally stimulated plasmacytoid dendritic cells produce chemokines and induce migration of T and NK cells. J Leukoc Biol. 2004;75:504–514. doi: 10.1189/jlb.0603291. [DOI] [PubMed] [Google Scholar]
- Penna G, Vulcano M, Roncari A, Facchetti F, Sozzani S, Adorini L. Cutting edge: differential chemokine production by myeloid and plasmacytoid dendritic cells. J Immunol. 2002;169:6673–6676. doi: 10.4049/jimmunol.169.12.6673. [DOI] [PubMed] [Google Scholar]
- Penna G, Vulcano M, Sozzani S, Adorini L. Differential migration behavior and chemokine production by myeloid and plasmacytoid dendritic dells. Hum Immunol. 2002;63:1164–1171. doi: 10.1016/s0198-8859(02)00755-3. [DOI] [PubMed] [Google Scholar]
- Dzionek A, Sohma Y, Nagafune J, Cella M, Colonna M, Facchetti F, Gunther G, Johnston I, Lanzavecchia A, Nagasaka T, Okada T, Vermi W, Winkels G, Yamamoto T, Zysk M, Yamaguchi Y, Schmitz J. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon {α}/{β} induction. J Exp Med. 2001;194:1823–1834. doi: 10.1084/jem.194.12.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug A, French A R, Barchet W, Fischer J A, Dzionek A, Pingel J T, Orihuela M M, Akira S, Yokoyama W M, Colonna M. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–119. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003;198:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochrein H, Schlatter B, O'Keeffe M, Wagner C, Schmitz F, Schiemann M, Bauer S, Suter M, Wagner H. Herpes simplex virus type-1 induces IFN-α production via Toll-like receptor 9-dependent and -independent pathways. Proc Natl Acad Sci USA. 2004;101:11416–11421. doi: 10.1073/pnas.0403555101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug A, Luker G D, Barchet W, Leib D A, Akira S, Colonna M. Herpes simplex virus type 1 activates murine natural interferon-producing cells through Toll-like receptor 9. Blood. 2004;103:1433–1437. doi: 10.1182/blood-2003-08-2674. [DOI] [PubMed] [Google Scholar]
- Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- Beignon A S, McKenna K, Skoberne M, Manches O, DaSilva I, Kavanagh D G, Larsson M, Gorelick R J, Lifson J D, Bhardwaj N. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug A, Towarowski A, Britsch S, Rothenfusser S, Hornung V, Bals R, Giese T, Engelmann H, Endres S, Krieg A M, Hartmann G. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur J Immunol. 2001;31:3026–3037. doi: 10.1002/1521-4141(2001010)31:10<3026::aid-immu3026>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Ashlock B M, Foster H, Fujimura S H, Levy J A. HIV-infected cells are major inducers of plasmacytoid dendritic cell interferon production, maturation, and migration. Virology. 2005;343:256–266. doi: 10.1016/j.virol.2005.09.059. [DOI] [PubMed] [Google Scholar]
- Fitzgerald-Bocarsly P, Feldman M, Mendelsohn M, Curl S, Lopez C. Human mononuclear cells which produce interferon-α during NK (HSV-FS) assays are HLA-DR positive cells distinct from cytolytic natural killer effectors. J Leukoc Biol. 1988;43:323–334. doi: 10.1002/jlb.43.4.323. [DOI] [PubMed] [Google Scholar]
- Izaguirre A, Barnes B, Amrute S, Yeow Y-Z, Megjugorac N, Dai J, Feng D, Chung E, Pitha P, Fitzgerald-Bocarsly P. Comparative analysis of IRF and IFN-α expression in human plasmacytoid and monocyte derived dendritic cells. J Leukoc Biol. 2003;74:1125–1138. doi: 10.1189/jlb.0603255. [DOI] [PubMed] [Google Scholar]
- Dai J, Megjugorac N J, Amrute S B, Fitzgerald-Bocarsly P. Regulation of IFN regulatory factor-7 and IFN-α production by enveloped virus and lipopolysaccharide in human plasmacytoid dendritic cells. J Immunol. 2004;173:1535–1548. doi: 10.4049/jimmunol.173.3.1535. [DOI] [PubMed] [Google Scholar]
- Liu Y J. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- Yeow W-S, Au W-C, Juang Y-T, Fields C D, Dent C L, Gewert D R, Pitha P M. Reconstitution of virus-mediated expression of interferon α genes in human fibroblast cells by ectopic interferon regulatory factor-7. J Biol Chem. 2000;275:6313–6320. doi: 10.1074/jbc.275.9.6313. [DOI] [PubMed] [Google Scholar]
- Fanning S L, George T C, Feng D, Feldman S B, Megjugorac N J, Izaguirre A G, Fitzgerald-Bocarsly P. Receptor cross-linking on human plasmacytoid dendritic cells leads to the regulation of IFN-α production. J Immunol. 2006;177:5829–5839. doi: 10.4049/jimmunol.177.9.5829. [DOI] [PubMed] [Google Scholar]
- Danis B, George T C, Goriely S, Dutta B, Renneson J, Gatto L, Fitzgerald-Bocarsly P, Marchant A, Goldman M, Willems F, De Wit D. Interferon regulatory factor 7-mediated responses are defective in cord blood plasmacytoid dendritic cells. Eur J Immunol. 2008;38:507–517. doi: 10.1002/eji.200737760. [DOI] [PubMed] [Google Scholar]
- Guiducci C, Ghirelli C, Marloie-Provost M A, Matray T, Coffman R L, Liu Y J, Barrat F J, Soumelis V. PI3K is critical for the nuclear translocation of IRF-7 and type I IFN production by human plasmacytoid predendritic cells in response to TLR activation. J Exp Med. 2008;205:315–322. doi: 10.1084/jem.20070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancl M E, Hu G, Sangster-Guity N, Olshalsky S L, Hoops K, Fitzgerald-Bocarsly P, Pitha P M, Pinder K, Barnes B J. Two discrete promoters regulate the alternatively spliced human interferon regulatory factor-5 isoforms. Multiple isoforms with distinct cell type-specific expression, localization, regulation, and function. J Biol Chem. 2005;280:21078–21090. doi: 10.1074/jbc.M500543200. [DOI] [PubMed] [Google Scholar]
- Coccia E M, Severa M, Giacomini E, Monneron D, Remoli M E, Julkunen I, Cella M, Lande R, Uze G. Viral infection and Toll-like receptor agonists induce a differential expression of type I and λ interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur J Immunol. 2004;34:796–805. doi: 10.1002/eji.200324610. [DOI] [PubMed] [Google Scholar]
- Barnes B J, Field A E, Pitha-Rowe P M. Virus-induced heterodimer formation between IRF-5 and IRF-7 modulates assembly of the IFNA enhanceosome in vivo and transcriptional activity of IFNA genes. J Biol Chem. 2003;278:16630–16641. doi: 10.1074/jbc.M212609200. [DOI] [PubMed] [Google Scholar]
- Yanai H, Chen H M, Inuzuka T, Kondo S, Mak T W, Takaoka A, Honda K, Taniguchi T. Role of IFN regulatory factor 5 transcription factor in antiviral immunity and tumor suppression. Proc Natl Acad Sci USA. 2007;104:3402–3407. doi: 10.1073/pnas.0611559104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megjugorac N J, Jacobs E S, Izaguirre A G, George T C, Gupta G, Fitzgerald-Bocarsly P. Image-based study of interferongenic interactions between plasmacytoid dendritic cells and HSV-infected monocyte-derived dendritic cells. Immunol Invest. 2007;36:739–761. doi: 10.1080/08820130701715845. [DOI] [PubMed] [Google Scholar]
- Harshyne L A, Watkins S C, Gambotto A, Barratt-Boyes S M. Dendritic cells acquire antigens from live cells for cross-presentation to CTL. J Immunol. 2001;166:3717–3723. doi: 10.4049/jimmunol.166.6.3717. [DOI] [PubMed] [Google Scholar]
- Maranon C, Desoutter J F, Hoeffel G, Cohen W, Hanau D, Hosmalin A. Dendritic cells cross-present HIV antigens from live as well as apoptotic infected CD4+ T lymphocytes. Proc Natl Acad Sci USA. 2004;101:6092–6097. doi: 10.1073/pnas.0304860101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller O, Kochs G, Weber F. The interferon response circuit: induction and suppression by pathogenic viruses. Virology. 2006;344:119–130. doi: 10.1016/j.virol.2005.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H K, Lund J M, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- Yoneyama H, Matsuno K, Toda E, Nishiwaki T, Matsuo N, Nakano A, Narumi S, Lu B, Gerard C, Ishikawa S, Matsushima K. Plasmacytoid DCs help lymph node DCs to induce anti-HSV CTLs. J Exp Med. 2005;202:425–435. doi: 10.1084/jem.20041961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Amakawa R, Inaba M, Ikehara S, Inaba K, Fukuhara S. Differential regulation of human blood dendritic cell subsets by IFNs. J Immunol. 2001;166:2961–2969. doi: 10.4049/jimmunol.166.5.2961. [DOI] [PubMed] [Google Scholar]
- Kadowaki N, Antonenko S, Lau J Y, Liu Y J. Natural interferon α/β-producing cells link innate and adaptive immunity. J Exp Med. 2000;192:219–226. doi: 10.1084/jem.192.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucka A K, Banchereau J, Blanco P, Pascual V. The interplay of dendritic cell subsets in systemic lupus erythematosus. Immunol Cell Biol. 2002;80:484–488. doi: 10.1046/j.1440-1711.2002.01112.x. [DOI] [PubMed] [Google Scholar]
- Fonteneau J F, Larsson M, Beignon A S, McKenna K, Dasilva I, Amara A, Liu Y J, Lifson J D, Littman D R, Bhardwaj N. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J Virol. 2004;78:5223–5232. doi: 10.1128/JVI.78.10.5223-5232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald-Bocarsly P, Feldman M, Curl S, Schnell J, Denny T. Positively selected Leu-11a (CD16+) cells require the presence of accessory cells or factors for the lysis of HSV-infected fibroblasts but not HSV-infected Raji. J Immunol. 1989;143:1318–1326. [PubMed] [Google Scholar]
- Gerosa F, Gobbi A, Zorzi P, Burg S, Briere F, Carra G, Trinchieri G. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J Immunol. 2005;174:727–734. doi: 10.4049/jimmunol.174.2.727. [DOI] [PubMed] [Google Scholar]
- Tomescu C, Chehimi J, Maino V C, Montaner L J. NK cell lysis of HIV-1-infected autologous CD4 primary T cells: requirement for IFN-mediated NK activation by plasmacytoid dendritic cells. J Immunol. 2007;179:2097–2104. doi: 10.4049/jimmunol.179.4.2097. [DOI] [PubMed] [Google Scholar]
- Mavilio D, Lombardo G, Kinter A, Fogli M, La Sala A, Ortolano S, Farschi A, Follmann D, Gregg R, Kovacs C, Marcenaro E, Pende D, Moretta A, Fauci A S. Characterization of the defective interaction between a subset of natural killer cells and dendritic cells in HIV-1 infection. J Exp Med. 2006;203:2339–2350. doi: 10.1084/jem.20060894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Geiger T, Alkan S, Heusser C H. Interferon α increases the frequency of interferon γ-producing human CD4+ T cell. J Exp Med. 1993;178:1655–1663. doi: 10.1084/jem.178.5.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos H J, Davis A M, George T C, Farrar J D. IFN-α is not sufficient to drive Th1 development due to lack of stable T-bet expression. J Immunol. 2007;179:3792–3803. doi: 10.4049/jimmunol.179.6.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat Immunol. 2000;1:305–310. doi: 10.1038/79747. [DOI] [PubMed] [Google Scholar]
- Colonna M. Toll-like receptors and IFN-α: partners in autoimmunity. J Clin Invest. 2006;116:2319–2322. doi: 10.1172/JCI29879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boasso A, Hardy A W, Anderson S A, Dolan M J, Shearer G M. HIV-induced type I interferon and tryptophan catabolism drive T cell dysfunction despite phenotypic activation. PLoS One. 2008;3:e2961. doi: 10.1371/journal.pone.0002961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villadangos J A, Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity. 2008;29:352–361. doi: 10.1016/j.immuni.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Gray R C, Kuchtey J, Harding C V. CpG-B ODNs potently induce low levels of IFN-αβ and induce IFN-αβ-dependent MHC-I cross-presentation in DCs as effectively as CpG-A and CpG-C ODNs. J Leukoc Biol. 2007;81:1075–1085. doi: 10.1189/jlb.1006606. [DOI] [PubMed] [Google Scholar]
- Rothenfusser S, Hornung V, Ayyoub M, Britsch S, Towarowski A, Krug A, Sarris A, Lubenow N, Speiser D, Endres S, Hartmann G. CpG-A and CpG-B oligonucleotides differentially enhance human peptide-specific primary and memory CD8+ T-cell responses in vitro. Blood. 2004;103:2162–2169. doi: 10.1182/blood-2003-04-1091. [DOI] [PubMed] [Google Scholar]
- Mouries J, Moron G, Schlecht G, Escriou N, Dadaglio G, Leclerc C. Plasmacytoid dendritic cells efficiently cross-prime naive T cells in vivo after TLR activation. Blood. 2008;112:3713–3722. doi: 10.1182/blood-2008-03-146290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlecht G, Garcia S, Escriou N, Freitas A A, Leclerc C, Dadaglio G. Murine plasmacytoid dendritic cells induce effector/memory CD8+ T-cell responses in vivo after viral stimulation. Blood. 2004;104:1808–1815. doi: 10.1182/blood-2004-02-0426. [DOI] [PubMed] [Google Scholar]
- Hoeffel G, Ripoche A C, Matheoud D, Nascimbeni M, Escriou N, Lebon P, Heshmati F, Guillet J G, Gannage M, Caillat-Zucman S, Casartelli N, Schwartz O, De la Salle H, Hanau D, Hosmalin A, Maranon C. Antigen crosspresentation by human plasmacytoid dendritic cells. Immunity. 2007;27:481–492. doi: 10.1016/j.immuni.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Proietti E, Bracci L, Puzelli S, Di Pucchio T, Sestili P, De Vincenzi E, Venditti M, Capone I, Seif I, De Maeyer E, Tough D, Donatelli I, Belardelli F. Type I IFN as a natural adjuvant for a protective immune response: lessons from the influenza vaccine model. J Immunol. 2002;169:375–383. doi: 10.4049/jimmunol.169.1.375. [DOI] [PubMed] [Google Scholar]
- Jego G, Palucka A K, Blanck J P, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- Le Bon A, Tough D F. Links between innate and adaptive immunity via type I interferon. Curr Opin Immunol. 2002;14:432–436. doi: 10.1016/s0952-7915(02)00354-0. [DOI] [PubMed] [Google Scholar]
- Le Bon A, Schiavoni G, D'Agostino G, Gresser I, Belardelli F, Tough D F. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14:461–470. doi: 10.1016/s1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- Lopez C, Fitzgerald P A, Siegal F P. Severe acquired immune deficiency syndrome in male homosexuals: diminished capacity to make interferon-α in vitro associated with severe opportunistic infections. J Infect Dis. 1983;148:962–966. doi: 10.1093/infdis/148.6.962. [DOI] [PubMed] [Google Scholar]
- Siegal F P, Lopez C, Fitzgerald P A, Shah K, Baron P, Leiderman I Z, Imperato D, Landesman S. Opportunistic infections in acquired immune deficiency syndrome result from synergistic defects of both natural and adaptive components of cellular immunity. J Clin Invest. 1986;78:115–123. doi: 10.1172/JCI112539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman S, Stein D, Amrute S, Denny T, Garcia Z, Kloser P, Sun Y, Megjugorac N, Fitzgerald-Bocarsly P. Decreased interferon-α production in HIV-infected patients correlates with numerical and functional deficiencies in circulating type 2 dendritic cell precursors. Clin Immunol. 2001;101:201–210. doi: 10.1006/clim.2001.5111. [DOI] [PubMed] [Google Scholar]
- Pacanowski J, Kahi S, Baillet M, Lebon P, Deveau C, Goujard C, Meyer L, Oksenhendler E, Sinet M, Hosmalin A. Reduced blood CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood. 2001;98:3016–3021. doi: 10.1182/blood.v98.10.3016. [DOI] [PubMed] [Google Scholar]
- Soumelis V, Scott I, Gheyas F, Bouhour D, Cozon G, Cotte L, Huang L, Levy J A, Liu Y J. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood. 2001;98:906–912. doi: 10.1182/blood.v98.4.906. [DOI] [PubMed] [Google Scholar]
- Donaghy H, Pozniak A, Gazzard B, Qazi N, Gilmour J, Gotch F, Patterson S. Loss of blood CD11c(+) myeloid and CD11c(–) plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood. 2001;98:2574–2576. doi: 10.1182/blood.v98.8.2574. [DOI] [PubMed] [Google Scholar]
- Almeida M, Cordero M, Almeida J, Orfao A. Different subsets of peripheral blood dendritic cells show distinct phenotypic and functional abnormalities in HIV-1 infection. AIDS. 2005;19:261–271. [PubMed] [Google Scholar]
- Kamga I, Kahi S, Develioglu L, Lichtner M, Maranon C, Deveau C, Meyer L, Goujard C, Lebon P, Sinet M, Hosmalin A. Type I interferon production is profoundly and transiently impaired in primary HIV-1 infection. J Infect Dis. 2005;192:303–310. doi: 10.1086/430931. [DOI] [PubMed] [Google Scholar]
- Killian M S, Fujimura S H, Hecht F M, Levy J A. Similar changes in plasmacytoid dendritic cell and CD4 T-cell counts during primary HIV-1 infection and treatment. AIDS. 2006;20:1247–1252. doi: 10.1097/01.aids.0000232231.34253.bd. [DOI] [PubMed] [Google Scholar]
- Siegal F P, Fitzgerald-Bocarsly P, Holland B, Shodell M. Interferon-α generation and immune reconstitution during antiretroviral therapy for human imunodeficiency virus infection. AIDS. 2001;15:1603–1612. doi: 10.1097/00002030-200109070-00002. [DOI] [PubMed] [Google Scholar]
- Chehimi J, Azzoni L, Farabaugh M, Creer S A, Tomescu C, Hancock A, Mackiewicz A, D'Alessandro L, Ghanekar S, Foulkes A S, Mounzer K, Kostman J, Montaner L J. Baseline viral load and immune activation determine the extent of reconstitution of innate immune effectors in HIV-1-infected subjects undergoing antiretroviral treatment. J Immunol. 2007;179:2642–2650. doi: 10.4049/jimmunol.179.4.2642. [DOI] [PubMed] [Google Scholar]
- Chehimi J, Campbell D E, Azzoni L, Bacheller D, Papasavvas E, Jerandi G, Mounzer K, Kostman J, Trinchieri G, Montaner L J. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J Immunol. 2002;168:4796–4801. doi: 10.4049/jimmunol.168.9.4796. [DOI] [PubMed] [Google Scholar]
- Finke J S, Shodell M, Shah K, Siegal F P, Steinman R M. Dendritic cell numbers in the blood of HIV-1 infected patients before and after changes in antiretroviral therapy. J Clin Immunol. 2004;24:647–652. doi: 10.1007/s10875-004-6250-5. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Fujimura S H, Martin J N, Levy J A. Variations in plasmacytoid dendritic cell (PDC) and myeloid dendritic cell (MDC) levels in HIV-infected subjects on and off antiretroviral therapy. J Clin Immunol. 2006;26:55–64. doi: 10.1007/s10875-006-8401-3. [DOI] [PubMed] [Google Scholar]
- Azzoni L, Chehimi J, Zhou L, Foulkes A S, June R, Maino V C, Landay A, Rinaldo C, Jacobson L P, Montaner L J. Early and delayed benefits of HIV-1 suppression: timeline of recovery of innate immunity effector cells. AIDS. 2007;21:293–305. doi: 10.1097/QAD.0b013e328012b85f. [DOI] [PubMed] [Google Scholar]
- Effros R B, Fletcher C V, Gebo K, Halter J B, Hazzard W R, Horne F M, Huebner R E, Janoff E N, Justice A C, Kuritzkes D, Nayfield S G, Plaeger S F, Schmader K E, Ashworth J R, Campanelli C, Clayton C P, Rada B, Woolard N F, High K P. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis. 2008;47:542–553. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Jamieson B D, Hultin L E, Hultin P M, Effros R B, Detels R. Premature aging of T cells is associated with faster HIV-1 disease progression. J Acquir Immune Defic Syndr. 2009;50:137–147. doi: 10.1097/QAI.0b013e3181926c28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtner M, Rossi R, Rizza M C, Mengoni F, Sauzullo I, Massetti A P, Luzi G, Hosmalin A, Mastroianni C M, Vullo V. Plasmacytoid dendritic cells count in antiretroviral-treated patients is predictive of HIV load control independent of CD4+ T-cell count. Curr HIV Res. 2008;6:19–27. doi: 10.2174/157016208783571937. [DOI] [PubMed] [Google Scholar]
- Patterson S, Rae A, Hockey N, Gilmour J, Gotch F. Plasmacytoid dendritic cells are highly susceptible to human immunodeficiency virus type 1 infection and release infectious virus. J Virol. 2001;75:6710–6713. doi: 10.1128/JVI.75.14.6710-6713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B, Scott I, Whitmore R G, Foster H, Fujimura S, Schmitz J, Levy J A. Low-level HIV infection of plasmacytoid dendritic cells: onset of cytopathic effects and cell death after PDC maturation. Virology. 2004;329:280–288. doi: 10.1016/j.virol.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Fong L, Mengozzi M, Abbey N W, Herndier B G, Engleman E G. Productive infection of plasmacytoid dendritic cells with human immunodeficiency virus type 1 is triggered by CD40 ligation. J Virol. 2002;76:11033–11041. doi: 10.1128/JVI.76.21.11033-11041.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F X, Huang J, Zhang H, Ma X, Zhang H. APOBEC3G upregulation by α interferon restricts human immunodeficiency virus type 1 infection in human peripheral plasmacytoid dendritic cells. J Gen Virol. 2008;89:722–730. doi: 10.1099/vir.0.83530-0. [DOI] [PubMed] [Google Scholar]
- Peng G, Lei K J, Jin W, Greenwell-Wild T, Wahl S M. Induction of APOBEC3 family proteins, a defensive maneuver underlying interferon-induced anti-HIV-1 activity. J Exp Med. 2006;203:41–46. doi: 10.1084/jem.20051512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot F, van Capel T M, Kapsenberg M L, Berkhout B, de Jong E C. Opposing roles of blood myeloid and plasmacytoid dendritic cells in HIV-1 infection of T cells: transmission facilitation versus replication inhibition. Blood. 2006;108:1957–1964. doi: 10.1182/blood-2006-03-010918. [DOI] [PubMed] [Google Scholar]
- Donaghy H, Gazzard B, Gotch F, Patterson S. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood. 2003;101:4505–4511. doi: 10.1182/blood-2002-10-3189. [DOI] [PubMed] [Google Scholar]
- Otero M, Nunnari G, Leto D, Sullivan J, Wang F X, Frank I, Xu Y, Patel C, Dornadula G, Kulkosky J, Pomerantz R J. Peripheral blood dendritic cells are not a major reservoir for HIV type 1 in infected individuals on virally suppressive HAART. AIDS Res Hum Retroviruses. 2003;19:1097–1103. doi: 10.1089/088922203771881194. [DOI] [PubMed] [Google Scholar]
- Gurney K B, Colantonio A D, Blom B, Spits H, Uittenbogaart C H. Endogenous IFN-α production by plasmacytoid dendritic cells exerts an antiviral effect on thymic HIV-1 infection. J Immunol. 2004;173:7269–7276. doi: 10.4049/jimmunol.173.12.7269. [DOI] [PubMed] [Google Scholar]
- Feldman S B, Ferraro M, Zheng H M, Patel N, Gould-Fogerite S, Fitzgerald-Bocarsly P. Viral induction of low frequency interferon-α producing cells. Virology. 1994;204:1–7. doi: 10.1006/viro.1994.1504. [DOI] [PubMed] [Google Scholar]
- Del Corno M, Gauzzi M C, Penna G, Belardelli F, Adorini L, Gessani S. Human immunodeficiency virus type 1 gp120 and other activation stimuli are highly effective in triggering α interferon and CC chemokine production in circulating plasmacytoid but not myeloid dendritic cells. J Virol. 2005;79:12597–12601. doi: 10.1128/JVI.79.19.12597-12601.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt S, Donhauser N, Chaipan C, Schuster P, Puffer B, Daniels R S, Greenough T C, Kirchhoff F, Schmidt B. CD4 binding affinity determines human immunodeficiency virus type 1-induced α interferon production in plasmacytoid dendritic cells. J Virol. 2008;82:8900–8905. doi: 10.1128/JVI.00196-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smed-Sorensen A, Lore K, Vasudevan J, Louder M K, Andersson J, Mascola J R, Spetz A L, Koup R A. Differential susceptibility to human immunodeficiency virus type 1 infection of myeloid and plasmacytoid dendritic cells. J Virol. 2005;79:8861–8869. doi: 10.1128/JVI.79.14.8861-8869.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa A, Morita R, Takaori-Kondo A, Kadowaki N, Kitawaki T, Hori T, Uchiyama T. Natural α interferon-producing cells respond to human immunodeficiency virus type 1 with α interferon production and maturation into dendritic cells. J Virol. 2003;77:3777–3784. doi: 10.1128/JVI.77.6.3777-3784.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An D S, Poon B, Ho Tsong Fang R, Weijer K, Blom B, Spits H, Chen I S, Uittenbogaart C H. Use of a novel chimeric mouse model with a functionally active human immune system to study human immunodeficiency virus type 1 infection. Clin Vaccine Immunol. 2007;14:391–396. doi: 10.1128/CVI.00403-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatak M, Jr, Saag M S, Yang L C, Clark S J, Kappes J C, Luk K C, Hahn B H, Shaw G M, Lifson J D. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science. 1993;259:1749–1754. doi: 10.1126/science.8096089. [DOI] [PubMed] [Google Scholar]
- Herbeuval J P, Shearer G M. HIV-1 immunopathogenesis: how good interferon turns bad. Clin Immunol. 2007;123:121–128. doi: 10.1016/j.clim.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli E, Cicala C, Van Ryk D, Goode D J, Macleod K, Arthos J, Fauci A S. HIV-1 gp120 inhibits TLR9-mediated activation and IFN-{α} secretion in plasmacytoid dendritic cells. Proc Natl Acad Sci USA. 2007;104:3396–3401. doi: 10.1073/pnas.0611353104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H S, Bhatnagar N, Ballmaier M, Schubert U, Henklein P, Volgmann T, Heiken H, Schmidt R E, Meyer-Olson D. Exogenous HIV-1 Vpr disrupts IFN-α response by plasmacytoid dendritic cells (pDCs) and subsequent pDC/NK interplay. Immunol Lett. 2009;125:100–104. doi: 10.1016/j.imlet.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Fiorentini S, Riboldi E, Facchetti F, Avolio M, Fabbri M, Tosti G, Becker P D, Guzman C A, Sozzani S, Caruso A. HIV-1 matrix protein p17 induces human plasmacytoid dendritic cells to acquire a migratory immature cell phenotype. Proc Natl Acad Sci USA. 2008;105:3867–3872. doi: 10.1073/pnas.0800370105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaranta M G, Mattioli B, Giordani L, Viora M. The immunoregulatory effects of HIV-1 Nef on dendritic cells and the pathogenesis of AIDS. FASEB J. 2006;20:2198–2208. doi: 10.1096/fj.06-6260rev. [DOI] [PubMed] [Google Scholar]
- Buimovici-Klein E, Lange M, Klein J, Grieco M, Cooper L. Long-term follow-up of serum-interferon and its acid-stability in a group of homosexual men. AIDS Res. 1986;2:99–108. doi: 10.1089/aid.1.1986.2.99. [DOI] [PubMed] [Google Scholar]
- Swindells S, Baldwin T, Kelly C, Baca-Regen L, Loomis L, Post D, Brichacek B, Stevenson M, Dominguez E A, Reddy R, Klein R, Liao M J, Testa D, McDonald T, Bellanti J, Skurkovich S, Gendelman H E. Regulation and characterization of the interferon-α present in patients with advanced human immunodeficiency virus type 1 disease. J Interferon Cytokine Res. 1996;16:127–137. doi: 10.1089/jir.1996.16.127. [DOI] [PubMed] [Google Scholar]
- Eyster M E, Goedert J J, Poon M C, Preble O T. Acid-labile α interferon. A possible preclinical marker for the acquired immunodeficiency syndrome in hemophilia. N Engl J Med. 1983;309:583–586. doi: 10.1056/NEJM198309083091003. [DOI] [PubMed] [Google Scholar]
- Yee A M, Yip Y K, Fischer H D, Buyon J P. Serum activity that confers acid lability to α-interferon in systemic lupus erythematosus: its association with disease activity and its independence from circulating α-interferon. Arthritis Rheum. 1990;33:563–568. doi: 10.1002/art.1780330414. [DOI] [PubMed] [Google Scholar]
- Capobianchi M R, Mattana P, Mercuri F, Conciatori G, Ameglio F, Ankel H, Dianzani F. Acid lability is not an intrinsic property of interferon-α induced by HIV-infected cells. J Interferon Res. 1992;12:431–438. doi: 10.1089/jir.1992.12.431. [DOI] [PubMed] [Google Scholar]
- Lehmann C, Harper J M, Taubert D, Hartmann P, Fatkenheuer G, Jung N, van Lunzen J, Stellbrink H J, Gallo R C, Romerio F. Increased interferon α expression in circulating plasmacytoid dendritic cells of HIV-1-infected patients. J Acquir Immune Defic Syndr. 2008;48:522–530. doi: 10.1097/QAI.0b013e31817f97cf. [DOI] [PubMed] [Google Scholar]
- Tilton J C, Manion M M, Luskin M R, Johnson A J, Patamawenu A A, Hallahan C W, Cogliano-Shutta N A, Mican J M, Davey R T, Jr, Kottilil S, Lifson J D, Metcalf J A, Lempicki R A, Connors M. Human immunodeficiency virus viremia induces plasmacytoid dendritic cell activation in vivo and diminished α interferon production in vitro. J Virol. 2008;82:3997–4006. doi: 10.1128/JVI.01545-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badolato R, Ghidini C, Facchetti F, Serana F, Sottini A, Chiarini M, Spinelli E, Lonardi S, Plebani A, Caimi L, Imberti L. Type I interferon-dependent gene MxA in perinatal HIV-infected patients under antiretroviral therapy as marker for therapy failure and blood plasmacytoid dendritic cells depletion. J Transl Med. 2008;6:49. doi: 10.1186/1479-5876-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccala R, Hoebe K, Kono D H, Beutler B, Theofilopoulos A N. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat Med. 2007;13:543–551. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- Brown K N, Wijewardana V, Liu X, Barratt-Boyes S M. Rapid influx and death of plasmacytoid dendritic cells in lymph nodes mediate depletion in acute simian immunodeficiency virus infection. PLoS Pathog. 2009;5:e1000413. doi: 10.1371/journal.ppat.1000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijewardana V, Brown K N, Barratt-Boyes S M. Studies of plasmacytoid dendritic cell dynamics in simian immunodeficiency virus infection of nonhuman primates provide insights into HIV pathogenesis. Curr HIV Res. 2009;7:23–29. doi: 10.2174/157016209787048483. [DOI] [PubMed] [Google Scholar]
- Malleret B, Maneglier B, Karlsson I, Lebon P, Nascimbeni M, Perie L, Brochard P, Delache B, Calvo J, Andrieu T, Spreux-Varoquaux O, Hosmalin A, Le Grand R, Vaslin B. Primary infection with simian immunodeficiency virus: plasmacytoid dendritic cell homing to lymph nodes, type I interferon, and immune suppression. Blood. 2008;112:4598–4608. doi: 10.1182/blood-2008-06-162651. [DOI] [PubMed] [Google Scholar]
- Dillon S M, Robertson K B, Pan S C, Mawhinney S, Meditz A L, Folkvord J M, Connick E, McCarter M D, Wilson C C. Plasmacytoid and myeloid dendritic cells with a partial activation phenotype accumulate in lymphoid tissue during asymptomatic chronic HIV-1 infection. J Acquir Immune Defic Syndr. 2008;48:1–12. doi: 10.1097/QAI.0b013e3181664b60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimbeni M, Perie L, Chorro L, Diocou S, Kreitmann L, Louis S, Garderet L, Fabiani B, Berger A, Schmitz J, Marie J P, Molina T J, Pacanowski J, Viard J P, Oksenhendler E, Beq S, Abehsira-Amar O, Cheynier R, Hosmalin A. Plasmacytoid dendritic cells accumulate in spleens from chronically HIV-infected patients but barely participate in interferon-α expression. Blood. 2009;113:6112–6119. doi: 10.1182/blood-2008-07-170803. [DOI] [PubMed] [Google Scholar]
- Foussat A, Bouchet-Delbos L, Berrebi D, Durand-Gasselin I, Coulomb-L'Hermine A, Krzysiek R, Galanaud P, Levy Y, Emilie D. Deregulation of the expression of the fractalkine/fractalkine receptor complex in HIV-1-infected patients. Blood. 2001;98:1678–1686. doi: 10.1182/blood.v98.6.1678. [DOI] [PubMed] [Google Scholar]
- Brown K N, Trichel A, Barratt-Boyes S M. Parallel loss of myeloid and plasmacytoid dendritic cells from blood and lymphoid tissue in simian AIDS. J Immunol. 2007;178:6958–6967. doi: 10.4049/jimmunol.178.11.6958. [DOI] [PubMed] [Google Scholar]
- Biancotto A, Grivel J C, Iglehart S J, Vanpouille C, Lisco A, Sieg S F, Debernardo R, Garate K, Rodriguez B, Margolis L B, Lederman M M. Abnormal activation and cytokine spectra in lymph nodes of people chronically infected with HIV-1. Blood. 2007;109:4272–4279. doi: 10.1182/blood-2006-11-055764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stary G, Klein I, Kohlhofer S, Koszik F, Scherzer T, Mullauer L, Quendler H, Kohrgruber N, Stingl G. Plasmacytoid dendritic cells express TRAIL and induce CD4+ T-cell apoptosis in HIV-1 viremic patients. Blood. 2009;114:3854–3863. doi: 10.1182/blood-2009-04-217927. [DOI] [PubMed] [Google Scholar]
- Meyers J H, Justement J S, Hallahan C W, Blair E T, Sun Y A, O'Shea M A, Roby G, Kottilil S, Moir S, Kovacs C M, Chun T W, Fauci A S. Impact of HIV on cell survival and antiviral activity of plasmacytoid dendritic cells. PLoS One. 2007;2:e458. doi: 10.1371/journal.pone.0000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinson J A, Roman-Gonzalez A, Tenorio A R, Montoya C J, Gichinga C N, Rugeles M T, Tomai M, Krieg A M, Ghanekar S, Baum L L, Landay A L. Dendritic cells from HIV-1 infected individuals are less responsive to Toll-like receptor (TLR) ligands. Cell Immunol. 2007;250:75–84. doi: 10.1016/j.cellimm.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdeva N, Asthana V, Brewer T H, Garcia D, Asthana D. Impaired restoration of plasmacytoid dendritic cells in HIV-1-infected patients with poor CD4 T cell reconstitution is associated with decrease in capacity to produce IFN-α but not proinflammatory cytokines. J Immunol. 2008;181:2887–2897. doi: 10.4049/jimmunol.181.4.2887. [DOI] [PubMed] [Google Scholar]
- Conry SJ, Milkovich KA, Yonkers NL, Rodriguez B, Bernstein HB, Asaad R, Heinzel FP, Tary-Lehmann M, Lederman MM, Anthony DD. Impaired plasmacytoid dendritic cell (PDC)-NK cell activity in viremic human immunodeficiency virus infection attributable to impairments in both PDC and NK function. J Virol. 2009;83:11175–11187. doi: 10.1128/JVI.00753-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitano K N, Kottilil S, Gille C M, Zhang X, Yan M, O'Shea M A, Roby G, Hallahan C W, Yang J, Lempicki R A, Arthos J, Fauci A S. Defective plasmacytoid dendritic cell-NK cell cross-talk in HIV infection. AIDS Res Hum Retroviruses. 2009;25:1029–1037. doi: 10.1089/aid.2008.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbeuval J P, Nilsson J, Boasso A, Hardy A W, Vaccari M, Cecchinato V, Valeri V, Franchini G, Andersson J, Shearer G M. HAART reduces death ligand but not death receptors in lymphoid tissue of HIV-infected patients and simian immunodeficiency virus-infected macaques. AIDS. 2009;23:35–40. doi: 10.1097/QAD.0b013e32831cb907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbeuval J P, Nilsson J, Boasso A, Hardy A W, Kruhlak M J, Anderson S A, Dolan M J, Dy M, Andersson J, Shearer G M. Differential expression of IFN-α and TRAIL/DR5 in lymphoid tissue of progressor versus nonprogressor HIV-1-infected patients. Proc Natl Acad Sci USA. 2006;103:7000–7005. doi: 10.1073/pnas.0600363103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbeuval J P, Boasso A, Grivel J C, Hardy A W, Anderson S A, Dolan M J, Chougnet C, Lifson J D, Shearer G M. TNF-related apoptosis-inducing ligand (TRAIL) in HIV-1-infected patients and its in vitro production by antigen-presenting cells. Blood. 2005;105:2458–2464. doi: 10.1182/blood-2004-08-3058. [DOI] [PubMed] [Google Scholar]
- Stary G, Klein I, Kohlhofer S, Koszik F, Scherzer T, Mullauer L, Quendler H, Kohrgruber N, Stingl G. Plasmacytoid dendritic cells express TRAIL and induce CD4+ T cell apoptosis in HIV-1 viremic patients. Blood. 2009;114:3854–3863. doi: 10.1182/blood-2009-04-217927. [DOI] [PubMed] [Google Scholar]
- Chehimi J, Papasavvas E, Tomescu C, Gekonge B, Abdulhaqq S, Raymond A, Hancock A, Vinekar K, Carty C, Reynolds G, Pistilli M, Mounzer K, Kostman J, Montaner LJ. Inability of PDC to directly lyse HIV-infected autologous CD4+ T cells despite TRAIL induction. J Virol. 2009 doi: 10.1128/JVI.01350-09. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boasso A, Shearer G M. Chronic innate immune activation as a cause of HIV-1 immunopathogenesis. Clin Immunol. 2008;126:235–242. doi: 10.1016/j.clim.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boasso A, Herbeuval J P, Hardy A W, Anderson S A, Dolan M J, Fuchs D, Shearer G M. HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood. 2007;109:3351–3359. doi: 10.1182/blood-2006-07-034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manches O, Munn D, Fallahi A, Lifson J, Chaperot L, Plumas J, Bhardwaj N. HIV-activated human plasmacytoid DCs induce Tregs through an indoleamine 2,3-dioxygenase-dependent mechanism. J Clin Invest. 2008;118:3431–3439. doi: 10.1172/JCI34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl J N, Barry A P, Vanderford T H, Kozyr N, Chavan R, Klucking S, Barrat F J, Coffman R L, Staprans S I, Feinberg M B. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat Med. 2008;14:1077–1087. doi: 10.1038/nm.1871. [DOI] [PubMed] [Google Scholar]
- Campillo-Giminez L, Laforge M, Fay M, Brussel A, Cumont M, Monceaux V, Diop A, Levy Y, Hurtel B, Zaunders J, Corbeil J, Elbim J, Estaquier J. Non pathogenesis of SIV infection is associated with reduced inflammation and recruitment of plasmacytoid dendritic cells to lymph nodes, not to lack of an interferon type I response, during acute phase. J Virol. 2010 doi: 10.1128/JVI.01496-09. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier A, Chang J J, Chan E S, Pollard R B, Sidhu H K, Kulkarni S, Wen T F, Lindsay R J, Orellana L, Mildvan D, Bazner S, Streeck H, Alter G, Lifson J D, Carrington M, Bosch R J, Robbins G K, Altfeld M. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med. 2009;15:955–959. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghofer B, Frommer T, Haley G, Fink L, Bein G, Hackstein H. TLR7 ligands induce higher IFN-α production in females. J Immunol. 2006;177:2088–2096. doi: 10.4049/jimmunol.177.4.2088. [DOI] [PubMed] [Google Scholar]
- Fitzgerald-Bocarsly P. Washington, DC: NIH Conference on Biomarkers in HIV infection; Plasmacytoid DC in HIV infection. 2004 [Google Scholar]