Abstract
Personalized peptide vaccination (PPV) combined with chemotherapy could be a novel approach for many cancer patients. In this randomized study, we evaluated the anti-tumor effect and safety of PPV plus low-dose estramustine phosphate (EMP) as compared to standard-dose EMP for HLA-A2- or -A24-positive patients with castration resistant prostate cancer. Patients were randomized into groups receiving either PPV plus low-dose EMP (280 mg/day) or standard-dose EMP (560 mg/day). After disease progression, patients were switched to the opposite regime. The primary end point was progression-free survival (PFS). We randomly assigned 28 patients to receive PPV plus low-dose EMP and 29 patients to receive standard-dose EMP. Nineteen events in the PPV group and 20 events in the EMP group occurred during the first treatment. Median PFS for the first treatment was 8.5 months in the PPV group and 2.8 months in the EMP group with a hazard ratio (HR) of 0.28 (95% CI, 0.14–0.61; log-rank P = 0.0012), while there was no difference for median PFS for the second treatment. The HR for overall survival was 0.3 (95% CI, 0.1–0.91) in favor of the PPV plus low-dose EMP group (log-rank, P = 0.0328). The PPV plus low-dose EMP was well tolerated without major adverse effects and with increased levels of IgG and cytotoxic-T cell responses to the vaccinated peptides. PPV plus low-dose EMP was associated with an improvement in PSA-based PFS as compared to the standard-dose EMP alone.
Keywords: Immunotherapy, Randomized trial, Peptide vaccine, Prostatic neoplasms
Introduction
Prostate cancer remains the most common type of cancer and the second highest cause of cancer-related deaths among men in the United States. In 2007, an estimated 218,890 cases of prostate cancer were diagnosed, with 27,050 deaths being attributed to the disease [1]. Despite improvements in early detection and treatment for localized disease, many patients still present with advanced disease; attempted curative local therapy also frequently fails. Although hormone therapy is initially successful in the vast majority of patients, most develop castration resistant prostate cancer (CRPC) after 14–30 months [2]. Chemotherapy has played only a palliative role in the treatment of prostate cancer, although two docetaxel-based randomized clinical trials demonstrated a survival benefit of 2.4 months as compared to mitoxanthrone and prednisone in CRPC patients [3, 4]. Therefore, the development of new treatment modalities is needed, one of which could be immunotherapy. Indeed, several immunotherapy strategies for prostate cancer, such as single-peptide-based vaccine [5], multiple-peptide-based vaccine [6–8], cell-based vaccine [9–11], viral vaccine [12], antibody-based therapy [13] and their combination with other therapies [7, 8, 14], have been evaluated. We also reported the clinical outcome along with the safety and immune responses of the personalized peptide vaccination (PPV) [15–17] alone as well as combined with other therapies [7, 8]. Under PPV treatment, each patient was tested for their immunological reactivity to many different peptides capable of inducing cytotoxic-T lymphocyte (CTL) responses. The peptides were derived from a number of targets, including prostate-specific antigen (PSA), prostatic acid phosphatase (PAP), prostate-specific membrane antigen (PSMA), multidrug resistance protein and a variety of other epithelial tumor antigens. Each patient was immunized with four peptides on the basis of the reactivity panel. Fifty-eight patients with HLA-A2 or HLA-A24 with CRPC were treated with a combination of PPV and low-dose EMP [8]. As a result, the majority (76%) of patients showed a decreased serum PSA level, along with a median survival time of 17 months for the 58 patients (95% confidence interval, 12–25 months). To further examine the clinical efficacy of PPV, we conducted a randomized phase II study, and report herein the results.
Patients and methods
Patients
Patients who had a histological diagnosis of prostate adenocarcinoma, and exhibited disease progression by clinical, radiological or PSA-based criteria after both androgen deprivation therapy with luteinizing hormone-releasing hormone (LHRH) agonist and anti-androgen therapy were eligible. Patients were required to wait at least 4 weeks for entry into the study after the completion of prior chemotherapy, radiation therapy or a change in hormonal therapy. Anti-androgen therapy was discontinued for at least 4 weeks before enrolment for patients receiving flutamide, and 6 weeks for those receiving bicalutamide. All patients had an Eastern Cooperative Oncology Group performance status of 0 or 1, an HLA-A24- or HLA-A2-positive type, a serum PSA level ≥4 ng/ml and a serum testosterone level ≤50 ng/ml, and were maintained on LHRH agonist therapy or had been castrated. Patients needed to be IgG reactive to at least one of the peptide candidates. Adequate organ functions were required as was a lymphocyte count ≥1,200/mm3. Patients who had undergone radiation therapy or immunosuppressive treatment using a steroid within the last 1 year were not permitted to enroll.
This study was undertaken at four institutions and approved by the ethics review committee of each. All patients gave a written informed consent according to institutional guidelines.
Study design
This was a randomized (1:1), open-labeled, crossover study of patients with CRPC. Patients were randomized to receive either PPV plus low-dose EMP (280 mg/day) or standard-dose EMP (560 mg/day) according to age and PSA levels. In the treatment with PPV plus low-dose EMP, after a pre-vaccination measurement of peptide-specific IgG in the plasma of patients reactive to 26 kinds of vaccine candidates (12 for HLA-A2 and 14 for HLA-A24) with the ability to induce CTLs, patients were treated by weekly subcutaneous administration of only reactive peptides (up to four peptides) showing the strongest antibody responses until progressive disease (PD), along with orally administered EMP at a dose of 280 mg daily. In the treatment with standard-dose EMP, patients were orally administered only 560 mg of EMP daily until PD. After PD, each treatment was switched. All patients were treated at an outpatient clinic. All peptide candidates were prepared under conditions of Good Manufacturing Practice using a Multiple Peptide System (San Diego, CA). The peptide candidates consisted of SART293–101, SART2161–169, SART3109–118, Lck208–216, Lck486–494, Lck488–497, MRP3503–511, MRP31293–1302, PAP213–221, PSA248–257, PSMA624–624, EZH2735–743, EGF-R800–809 and PTH-rP102–111 for patients with HLA-A24, and SART3302–310, SART3309–317, CypB129–138, Lck246–254, Lck422–430, ppMAPkkk432–440, WHSC2103–111, WHSC2141–149, UBE2V43–51, UBE2V85–93, HNRPL140–148 and HNRPL501–510 for patients with HLA-A2. Selected peptides were mixed with incomplete Freund’s adjuvant (Montanide ISA-51VG; Seppic, Paris, France), and four peptides of 1.5 ml emulsion each at a dose level of 3 mg/peptide were injected subcutaneously into the thigh or armpit area.
The primary objective was to assess the effect of PPV plus low-dose EMP on progression-free survival (PFS) during the first treatment. The PFS during the second treatment, overall survival, tolerability and immune response were also assessed.
Pretreatment and follow-up studies
A complete survey of medical history, physical examination, routine laboratory studies, and serum PSA tests were performed prior to treatment, and the tests were repeated every 2 weeks. All the patients underwent relevant radiologic studies and bone scans every 6 months. Outcomes were assessed by post-therapy changes in serum PSA and by CT or MRI of measurable disease symptoms if present at the baseline. Post-therapy decreases in PSA level of ≥50% were defined as partial responses (PR) and confirmed by two separate measurements ≥4 weeks apart. Post-therapy decreases of <50% or increases of <25% from the baseline were interpreted as stable disease (SD) [18]. For measurable disease symptoms, Response Evaluation Criteria in Solid Tumors were used [19]. PD was defined as radiological progression, or if defined using PSA level alone, three consecutive increases in PSA level and 125% of the baseline PSA value. Toxicity was graded according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events version 3.0.
Immune response
For selection of peptides and evaluation of immune responses during the treatment, peptide-specific CTL precursors in peripheral blood mononuclear cells (PBMCs) and serum levels of peptide-specific IgG were measured as described previously [20, 21]. Blood samples were collected at screening and following every sixth vaccination. Peptides were chosen based on the evaluation of peptide-specific IgG levels in plasma. Peptide-specific CTL precursors in PBMCs were detected using a previously reported culture method [21]. Before the first vaccination and 7 days after every sixth vaccination, 30 ml of peripheral blood was obtained, and PBMCs were isolated by means of Ficoll–Conray density gradient centrifugation. Briefly, PBMCs (1 × 105 cells/well) were incubated with 10 μM of a peptide in 200 μl of culture medium in U-bottomed 96-well microculture plates (Nunc, Roskilde, Denmark). The culture medium consisted of 45% RPMI-1640 medium, 45% AIM-V medium (GIBCO BRL), 10% FCS, 100 U/ml interleukin-2 (IL-2), and 0.1 μM MEM nonessential amino acid solution (GIBCO BRL). Half of the medium was removed and replaced with a new medium containing a corresponding peptide (20 μM) every 3 days. After incubation for 14 days, these cells were harvested and tested for their ability to produce IFN-γ in response to CIR-A2402 or T2 cells that were preloaded with either a corresponding peptide or HIV peptides (RYLRQQLLGI for HLA-A24 and LLFGYPVYV for HLA-A2) as a negative control. The level of IFN-γ was determined by enzyme-linked immunosorbent assay (ELISA) (limit of sensitivity 10 pg/ml). All assays were performed in quadruplicate. A two-tailed Student’s t test was employed for the statistical analyses. A well was considered positive when the level of IFN-γ production in response to a corresponding peptide was significantly higher (P < 0.05) than that in response to an HIV peptide, and when the mean amount of IFN-γ production in response to a corresponding peptide was >50 ng/ml as compared to that in response to an HIV peptide.
The levels of anti-peptide immunoglobulin G (IgG) were measured using the LuminexTM system, as previously reported [20]. In brief, plasma was incubated with 25 μl of peptide-coupled color-coded beads for 2 h at room temperature on a plate shaker. After incubation, the mixture was washed with a vacuum manifold apparatus and incubated with 100 μl of biotinylated goat anti-human IgG (chain-specific) for 1 h at room temperature. The plate was then washed, followed by the addition of 100 μl of streptavidin-PE to wells, and was incubated for 30 min at room temperature on a plate shaker. The bound beads were washed three times followed by the addition of 100 μl of Tween-PBS to each well. Fifty microliters of sample was detected using the Luminex system. Positive immune responses were defined as pre-IgG levels/post-IgG levels ≥1.5 or pre-IFN-γ levels/post-IFN-γ levels ≥1.5.
Statistical analysis
The determination of sample size was guided by the assumption that median PFS will be 6 months with standard-dose EMP alone and 12 months with PPV plus low-dose EMP. Assuming a dropout rate of 5%, the approximate number of patients to be recruited was 80, with 12 months accrual time and 12 months follow-up time. PFS during the first treatment was assessed from the date of randomization to the date of PD events. PFS during the second treatment was from the date of switching treatment to the date of PD events. Patients discontinued study treatment after PD, but continued in the study with the best supportive care for follow-up. Patients who had no progression at the time of the analysis were censored using the last available assessment date. Overall survival was assessed from the date of randomization to the date of a patient’s death irrespective of cause. Kaplan–Meier curves were plotted for PFS and overall survival for both treatment groups, and the log-rank test was used to assess the difference between the two treatment groups with a two-sided significance level of 5%. All patients undergoing the first treatment were included in the safety analysis. The primary statistical analysis was for PFS during the first treatment and was planned after 37 progression events, which was estimated to have at least 80% statistical power. Nineteen events in the PPV group and 20 events in the EMP group occurred during the first treatment. Actual median PFS was 2.8 months with standard-dose EMP alone and 8.5 months with PPV plus low-dose EMP. On the basis of this significant difference, early termination of the trial was recommended by the Safety and Efficacy Assessment Committee. We closed the trial on 1 June 2009.
Results
Patient characteristics
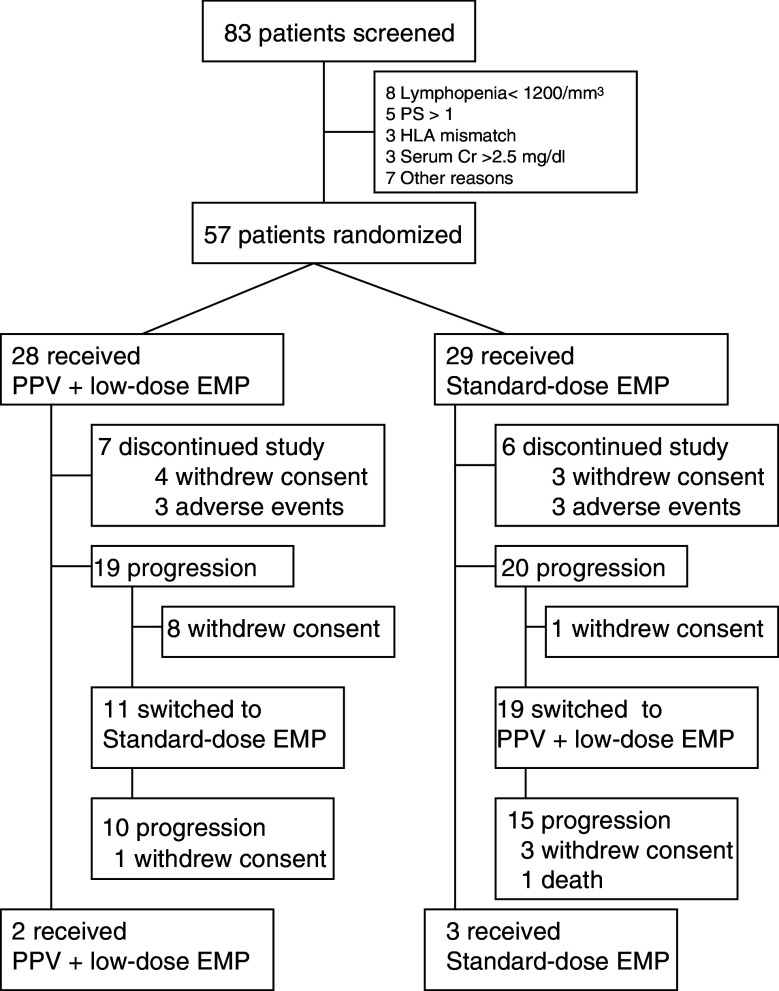
A total of 57 patients were randomized in a 1:1 ratio (28 PPV plus low-dose EMP, 29 standard-dose EMP) between April 2006 and March 2009 (Fig. 1). The demographic and baseline disease characteristics are listed in Table 1. Before the randomization, all the 57 patients received maximum androgen blockade using an LHRH analog (or had previously undergone castration) and anti-androgen as an initial or secondary hormonal therapy; 35 patients had previously undergone estramustine-based chemotherapy and 7 had docetaxel-based chemotherapy. Seven patients had previously undergone radiation therapy for bone metastases. Median durations of PSA failure and the randomization from initial treatments were 13 and 28 months, respectively.
Fig. 1.
Trial profile. PPV personalized peptide vaccination, EMP estramustine phosphate
Table 1.
Baseline patient characteristics
| Peptide + low-dose EMP | EMP alone | |
|---|---|---|
| No. of patients | 28 | 29 |
| Age (year) | ||
| Median | 70 | 69 |
| Range | 60–80 | 52–80 |
| ECOG performance status | ||
| 0 | 28 | 28 |
| 1 | 0 | 1 |
| HLA typing | ||
| A24 (+) | 12 | 15 |
| A2 (+) | 8 | 8 |
| A24 (+), A2 (+) | 8 | 6 |
| PSA (ng/ml) | ||
| Median | 29.3 | 48.8 |
| Range | 4–1,086 | 5.1–906 |
| Gleason score | ||
| 6 | 0 | 1 |
| 7 | 10 | 6 |
| 8 | 6 | 8 |
| 9 | 9 | 11 |
| 10 | 3 | 3 |
| Site of metastasis | ||
| No | 2 | 4 |
| Bone only | 14 | 14 |
| Bone and nodal/organ | 9 | 9 |
| Nodal/organ | 3 | 2 |
| Prior use of EMP | ||
| Yes | 19 | 14 |
| No | 9 | 15 |
Toxicity
There were no grade 4 toxicities or treatment-related deaths. The overall rate of grade 3 toxicities was higher in the standard-dose EMP group than the PPV plus low-dose EMP group (41 vs. 21%) during the first treatment. Toxicities occurring in ≥4% of patients during the first treatment are listed in Table 2. The patients who changed treatment to standard-dose EMP (11 patients) had additional ≤grade 2 toxicities including nausea, vomiting or both (three patients) and peripheral edema (two patients). All the patients who changed treatment to PPV plus low-dose EMP had a local skin reaction. These toxicities were manageable through routine interventions.
Table 2.
Treatment-related adverse events in the first treatment
| Adverse events | Peptide + low-dose EMP (n = 28) | Standard-dose EMP (n = 29) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | Total (%) | G1 | G2 | G3 | G4 | Total (%) | |
| Local skin reaction | 18 | 10 | 28 (100) | |||||||
| Electrolyte disorders | 8 | 8 | 4 | 20 (71) | 11 | 3 | 2 | 16 (55) | ||
| Low albuminemia | 4 | 3 | 7 (25) | 6 | 4 | 10 (34) | ||||
| Liver dysfunction | 4 | 2 | 1 | 7 (25) | 4 | 2 | 2 | 8 (28) | ||
| Anemia | 3 | 3 | 1 | 7 (25) | 1 | 2 | 1 | 4 (14) | ||
| Lymphopenia | 5 | 1 | 6 (21) | 4 | 1 | 5 (17) | ||||
| Nausea, vomiting or both | 5 | 5 (18) | 5 | 4 | 4 | 13 (45) | ||||
| Fatigue | 4 | 1 | 5 (18) | 2 | 1 | 1 | 4 (14) | |||
| Bone pain | 1 | 2 | 3 (11) | 1 | 4 | 5 (17) | ||||
| Urinary retention | 1 | 2 | 3 (11) | 1 | 1 | 2 (7) | ||||
| Peripheral edema | 1 | 1 | 2 (7) | 6 | 4 | 10 (34) | ||||
| Diarrhea | 1 | 1 (4) | 1 | 1 | 2 (7) | |||||
| Hypertension | 1 | 1 | 2 (7) | |||||||
| Headache | 1 | 1 (4) | ||||||||
Efficacy
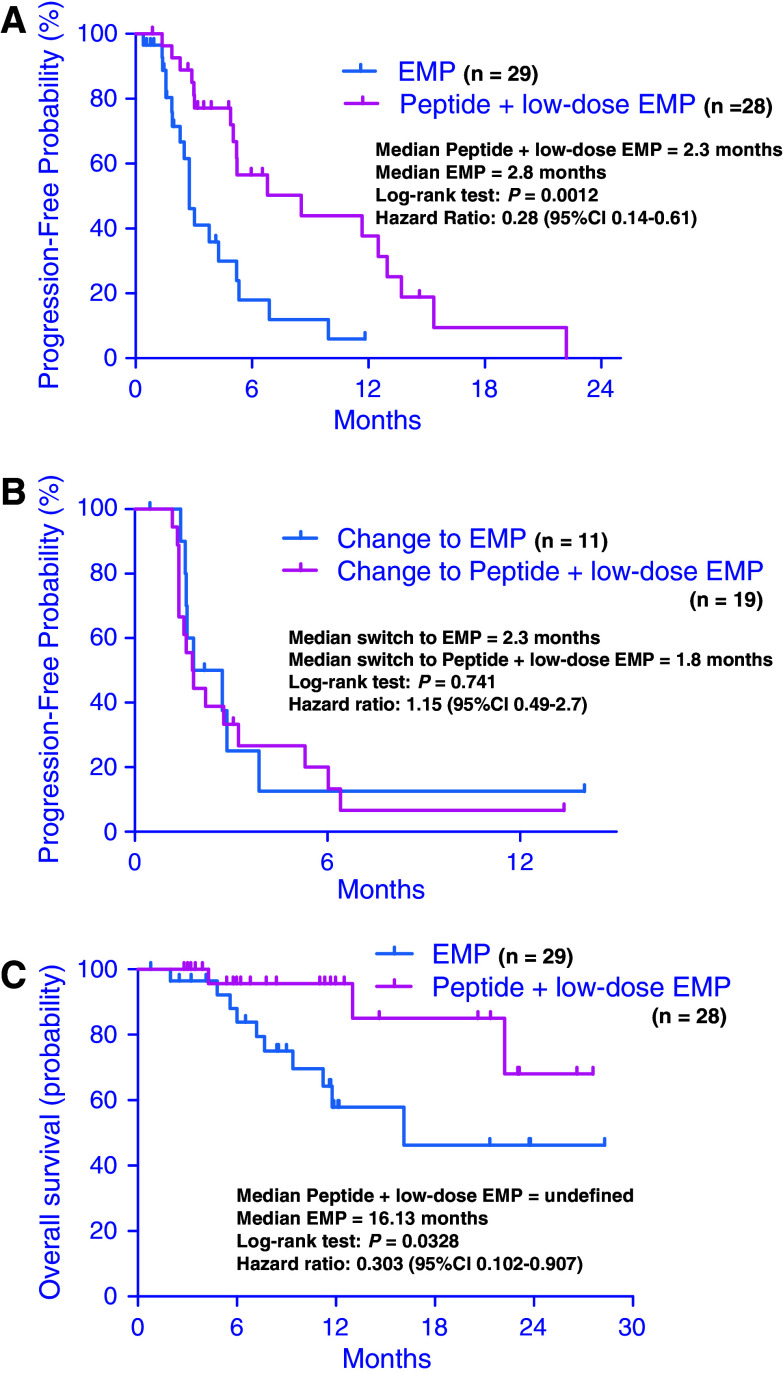
The response to the first treatment was assessed for 27 patients in the PPV plus low-dose EMP group and 28 patients in the standard-dose EMP group. No complete responses (CR) were observed in either group. Six patients (21%) had PR and 15 patients (54%) had SD in the PPV plus low-dose EMP group, while 6 patients (20%) had PR and 8 patients (28%) had SD in the standard-dose EMP group as best responses. Seven patients (21%) in the PPV plus low-dose EMP group and 14 patients (48%) in the standard-dose EMP group had progression without responses. During a median follow-up of 3 months (95% CI, 3.7–6.1 months) in the first treatment, 19 events occurred in the PPV plus low-dose EMP group; 17 patients had a PSA progression, 1 patient had a new lesion on bone scan and 1 patient had lymph node metastasis on CT scan, and 20 events occurred in the standard-dose EMP group; 18 patients had a PSA progression and 2 patients had a new lesion on bone scan. Three of the 17 patients in the PPV plus low-dose EMP group and 1 of the 20 patients in the standard-dose EMP had three consecutive increases in the PSA level and 125% of the baseline PSA value after two rises and a small drop in the PSA level. The median PFS in the first treatment was 8.5 months (95% CI, 4.7–8.8 months) for patients treated with PPV plus low-dose EMP and 2.8 months for those treated with standard-dose EMP (Fig. 2a, P = 0.0012). The hazard ratio (HR) for PD was 0.28 in favor of the PPV plus low-dose EMP group. The response to switching treatment was assessed in 11 patients who switched to standard-dose EMP from PPV plus low-dose EMP and 19 patients who switched to PPV plus low-dose EMP from standard-dose EMP. After the switch, there was no CR or PR in either group, and three patients (16%) who switched to PPV plus low-dose EMP had SD and three patients (27%) who switched to standard-dose EMP had SD. During a median follow-up of 1.8 months (95% CI, 1.8–4.3 months) in the second treatment, 16 events occurred among those who switched to PPV plus low-dose EMP and 8 events occurred among those who switched to standard-dose EMP. The median PFS in the second treatment period was 1.8 months (95% CI, 1.5–4.5 months) for patients who switched to PPV plus low-dose EMP and 2.3 months (95% CI, 0.6–5.6 months) for those who switched to standard-dose EMP (Fig. 2b, P = 0.741). There was no significant difference in PFS in the second treatment between the two groups.
Fig. 2.
Duration of (a) progression-free survival in the first treatment, b progression-free survival in the second treatment and (c) overall survival of patients treated with personalized peptide vaccination (PPV) plus low-dose estramustine phosphate (EMP) and standard-dose EMP
All the 57 patients were analyzed for overall survival and the median follow-up was 11.7 months (95% CI, 10.6–14.5 months). The median overall survival for the PPV plus low-dose EMP group was undefined (95% CI, 11.7–17.4 months) at the time of this analysis and the median overall survival for the standard-dose EMP group was 16.1 months (95% CI, 8.0–13.4 months) (Fig. 2c, P = 0.0328). The HR for overall survival was 0.3 in favor of the PPV plus low-dose EMP group.
Immunological response
Before the peptide vaccination, anti-peptide IgG levels were examined in 47 patients, and three to four peptides were selected for each patient in the PPV plus low-dose EMP group. The most frequently selected peptides were SART3109–118 (22/47), Lck488–497 (18/47), PAP213–221 (18/47), UBE2V43–51 (17/47), Lck486–494 (11/47) and SART3302–310 (11/47). For the 11 patients that were HLA–A24 and -A2 positive, both candidate peptides for HLA-A24 and -A2 were selected. Three peptides derived from EZH2, PSMA and EGF-R were not selected in this trial.
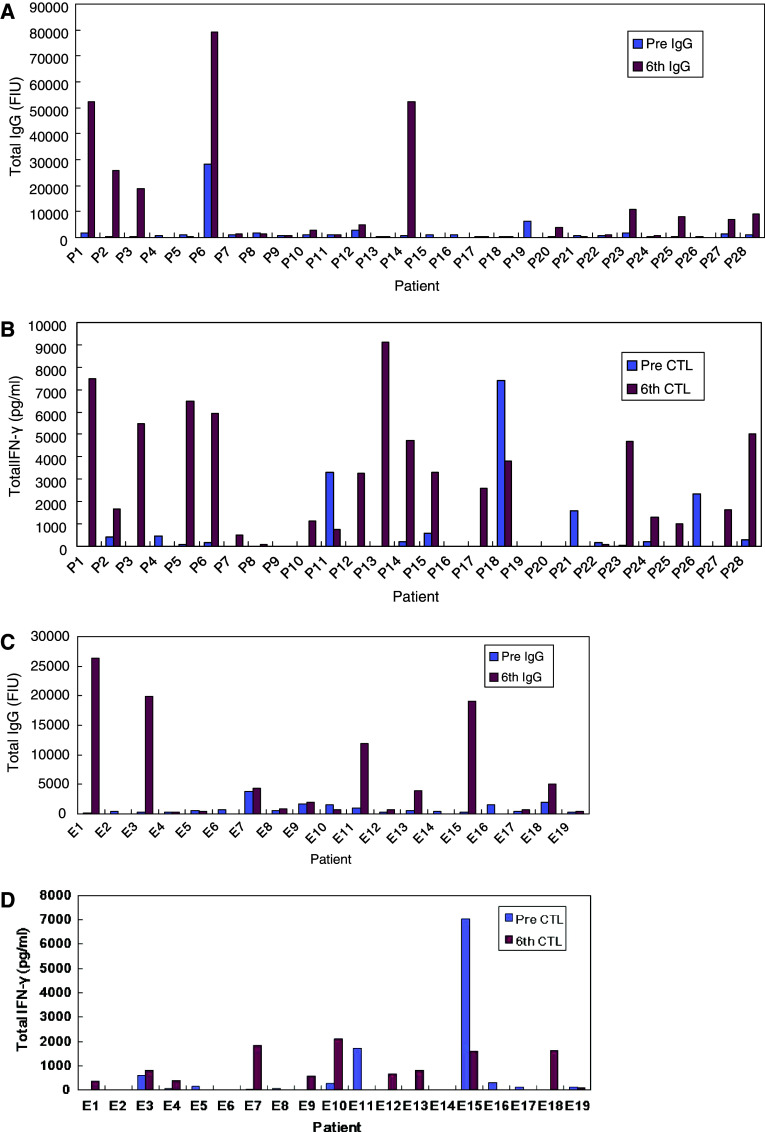
Immune responses of both IgG and CTL during PPV plus low-dose EMP treatment were evaluated in 25 patients in the first treatment and in 14 patients in the second treatment. Figure 3 shows the total levels of IgG and CTL in each patient prior to the vaccinations and at the sixth vaccination. In the first treatment, IgG responses were boosted in 16 of 25 (64%) patients and CTL responses measured by IFN-γ release assay were boosted in 18 of 25 (72%) patients. Both IgG and CTL responses were observed in 13 of 25 (52%) patients. In the second treatment, IgG responses were boosted in 9 of 14 (64%) patients and CTL responses were boosted in 7 of 14 (50%) patients. Both IgG and CTL responses were observed in 4 of 14 (29%) patients. However, there was no correlation between clinical outcome and immunological responses during PPV plus low-dose EMP treatment.
Fig. 3.
Immune responses in each patient. a Total levels of IgG and b CTL prior to vaccination and at the sixth vaccination in the first treatment, and c total levels of IgG and d CTL prior to vaccination and at the sixth vaccination in the second treatment
Discussion
Presently, the most advanced vaccines for patients with CRPC are made from whole tumor cells or use dendritic cells (DCs) loaded with antigens. Sipuleucel-T (APC8015; Provenge®, Dendreon, Seattle, WA) is one of the most extensively studied DC modalities. It consists of autologous dendritic cells, which are harvested by leukophoresis. The cells are loaded by coculture with PA2024, a recombinant fusion protein of PAP and human granulocyte–macrophage colony-stimulating factor (GM-CSF). Small et al. [11] recently reported the results of a randomized, placebo-controlled phase III study of DC-based immunotherapy using sipuleucel-T (APC8015) in 127 patients with metastatic CRPC who were similar to the population in the present study. This study did not reveal prolonged TTP defined as disease progression on serial radiologic imaging tests without PSA progression (11.7 vs. 10 weeks; HR, 1.45; 95% CI, 0.99–2.11; P = 0.052). On the other hand, follow-up survival data showed that the median overall survival was 25.9 months for patients in the vaccine group versus 21.4 months for patients in the placebo group (P = 0.02). However, the DC-based vaccine was not approved by the US Food and Drug Administration for various reasons.
This is the first randomized study investigating the role of PPV plus low-dose EMP in patients with CRPC. This trial demonstrated that median PFS in the PPV plus low-dose EMP group was significantly longer than that in the standard-dose EMP group during the first treatment, while PFS during the second treatment was similar in both groups. Although overall survival analyses in the present study were confounded by the switching of study participants from standard-dose EMP to PPV plus low-dose EMP, prolongation of overall survival was observed in the PPV plus low-dose EMP group, suggesting a potential clinical benefit of first-line PPV plus low-dose EMP as compared to standard-dose EMP. Prolongation of PSA rising time during the treatment for CRPC patients is a strong prognostic parameter used in many trials, and proposed by the PSA Working Group to measure therapeutic activity [22]. To mention this proposal, we used PD criteria as three consecutive increases in PSA level and 125% of the baseline PSA value instead of two consecutive increases in PSA level. These results also suggest that this combination produces additional anti-tumor effects. Basal autopriming by immunotherapy alone is unlikely to be enough to stimulate a sufficient immune response to generate enough anti-tumor activity to control disease. Therefore, multiple approaches to enhancing the immune response are being evaluated. Combinations of immunotherapy with radiotherapy, hormonal therapy or chemotherapy look promising. Radiation has been found to augment the immune response to prostate cancer [23]. Anti-hormonal therapy was also followed by infiltration of the prostate by activated T lymphocytes [24]. Low-dose EMP was chosen for combination chemotherapy with PPV, because of our previous study showing that low-dose EMP causes minimal immune system suppression in patients with the personalized vaccination, and benefits patients with metastatic CRPC [7]. To our knowledge, there are no preclinical studies on immunotherapy with EMP. However, in many cases of combination therapies using two or more chemotherapeutic agents or a chemotherapeutic agent and a targeted therapy, each agent works individually with the goal of additive anti-tumor effects. This assumption has been shown in numerous preclinical models using vaccines in combination with chemotherapeutic agents. For example, in preclinical studies, it has been shown that the cyclooxygenase-2 inhibitor celecoxib, an established anti-inflammatory, had no adverse effect on immune responses to vaccine and worked well in combination with vaccine to enhance anti-tumor effect [25].
PPV plus low-dose EMP was well tolerated, and most adverse events were grade 1 or 2 local redness and swelling at the injection site. Some adverse events were even related to the EMP rather than PPV itself. Indeed, the overall rate of grade 3 toxicities was higher in the standard-dose EMP group than the PPV plus low-dose EMP group (41 vs. 21%) during the first treatment. The toxicity of the combination of PPV plus low-dose EMPs reported here was tolerable and considered acceptable in the treatment of the vast majority of metastatic CRPC cases.
Although CTL responses measured by IFN-γ release assay and IgG responses were boosted in the majority of patients during the PPV plus low-dose EMP administration, both IgG and CTL responses were higher in the first treatment, and a significant improvement in PFS was only observed in the first treatment. These findings may have been caused by the different population of patients in the second treatment after switching or immune suppression by administration of standard-dose EMP. These results suggest that the administration of first-line PPV plus low-dose EMP for patients with CRPC may convey clinical benefits.
The currently conducted peptide vaccine trials for cancer patients, including our trial, generally contained peptides encompassing CD8 + CTL epitopes, but not CD4+ helper T cell epitopes. The use of such peptides would be one of the demerits to obtain better clinical responses, primarily because activation of helper T cells is absolutely required for optimal CTL responses. Indeed, the clinical benefits of therapeutic cancer vaccine using such CTL peptides do not seem to be sufficient to obtain drug approval as far as searched literature reveals. Therefore, longer peptides containing both helper and CTL epitopes should be used for future clinical trials to obtain much better clinical responses in cancer patients and drug approval. We previously reported that personalized peptide vaccination induced infiltration of CD45RO positive cells into prostate cancer tissues, rather than CD8 + CTLs or CD20 + B cells [17]. These results suggest that the personalized peptide vaccination firstly activated CD4+ helper T cells, which in turn facilitated CD8 + CTL activation. We are currently conducting studies to clarify mechanisms involved in this phenomenon along with developing a personalized peptide vaccination using both helper and CTL epitopes, which in turn should promise a greater chance of success of therapeutic cancer vaccine.
The vaccinated peptides to the subcutaneous regions were captured by antigen presenting cells followed by presentation to T cells in the lymph nodes. These peptides were undetectable in circulation. Therefore, it is unlikely that the vaccinated peptides have an impact on the measurement of PSA. It is also unlikely that the personalized peptide vaccination selectively eliminated high PSA-producing cells, which in turn resulted in decrease of serum levels of PSA associated with increase of tumor cells producing no or lower amounts of PSA.
One potential weakness of this study is that the crossover study design may result in the analysis of overall survival not accurately reflecting the results because different numbers of patients in the two groups were treated by PPV plus low-dose EMP. A second potential weakness is the small number of patients for a phase II study setting.
In summary, PPV plus low-dose EMP was safe and feasible, and was associated with an improvement in PSA-based PFS in metastatic CRPC patients as compared to standard-dose EMP alone. These results suggest the need for additional clinical trials of PPV plus low-dose EMP for the treatment of CRPC.
Acknowledgments
This study was supported in part by Grants-in-Aid (KAKENHI) (no.12213134 to KI), “TOSHI aria jigyo to Kurume City”, and by “High-Tech Research Center” Project for Private Universities: matching fund subsidy from Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan.
Conflict of interest statement
The authors indicated no potential conflict of interest except for Yamada and Itoh received a research grant from the Green Peptide Co., Ltd; Yamada and Itoh own stock in the Green Peptide Co.; Yamada is a part-time executive of the Green Peptide Co.
Abbreviations
- CR
Complete response
- CT
Computed tomography
- CTL
Cytotoxic T lymphocytes
- EBV
Epstein–Barr virus
- EMP
Estramustine phosphate
- ECOG
Eastern Cooperative Oncology Group
- HLA
Human leukocyte antigen
- IFN-γ
Interferon-γ
- PBMC
Peripheral blood mononuclear cells
- HIV
Human immunodeficiency virus
- IgG
Immunoglobulin G
- PSA
Prostate-specific antigen
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 3.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA, TAX 327 Investigators Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1488–1490. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 4.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 5.Ressing ME, van Driel WJ, Brandt RM, Kenter GG, de Jong JH, Bauknecht T, Fleuren GJ, Hoogerhout P, Offringa R, Sette A, Celis E, Grey H, Trimbos BJ, Kast WM, Melief CJ. Detection of T helper responses, but not of human papillomavirus-specific cytotoxic T lymphocyte responses, after peptide vaccination of patients with cervical carcinoma. J Immunother. 2000;23:255–266. doi: 10.1097/00002371-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Mine T, Sato Y, Noguchi M, Gouhara R, Tsuda N, Tanaka S, Shomura H, Katagiri K, Rikimaru T, Shichijo S, Kamura T, Hashimoto T, Shirouzu K, Yamada A, Todo S, Itoh K, Yamana H. Humoral responses to peptides correlate with overall survival in advanced cancer patients vaccinated with peptides based on pre-existing peptide-specific cellular responses. Clin Cancer Res. 2004;10:929–937. doi: 10.1158/1078-0432.CCR-1117-3. [DOI] [PubMed] [Google Scholar]
- 7.Noguchi M, Itoh K, Yao A, Mine T, Yamada A, Obata Y, Furuta M, Harada M, Suekane S, Matsuoka K. Immunological evaluation of individualized peptide vaccination with a low-dose of estramustine for HLA-A24+ HRPC patients. Prostate. 2005;63:1–12. doi: 10.1002/pros.20157. [DOI] [PubMed] [Google Scholar]
- 8.Noguchi M, Mine T, Yamada A, Obata Y, Yoshida K, Mizoguchi J, Harada M, Suekane S, Itoh K, Matsuoka K. Combination therapy of personalized peptide vaccination and low-dose estramustine phosphate for metastatic hormone refractory prostate cancer patients: an analysis of prognostic factors in the treatment. Oncol Res. 2007;16:341–349. doi: 10.3727/000000006783980955. [DOI] [PubMed] [Google Scholar]
- 9.Simons JW, Carducci MA, Mikhak B, Lim M, Biedrzycki B, Borellini F, Clift SM, Hege KM, Ando DG, Piantadosi S, Mulligan R, Nelson WG. Phase I/II trial of an allogeneic cellular immunotherapy in hormone-naïve prostate cancer. Clin Cancer Res. 2006;12:3394–3401. doi: 10.1158/1078-0432.CCR-06-0145. [DOI] [PubMed] [Google Scholar]
- 10.Small EJ, Sacks N, Nemunatitis J, Urba WJ, Dula E, Centeno AS, Nelson WG, Ando D, Howard C, Borellini F, Nguyen M, Hege K, Simons JW. Granulocyte macrophage colony-stimulating facto-secreting allogeneic cellular immunotherapy for hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:3883–3891. doi: 10.1158/1078-0432.CCR-06-2937. [DOI] [PubMed] [Google Scholar]
- 11.Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, Verjee SS, Jones LA, Hershberg RM. Placebo-controlled phase III trial of immunologic therapy with sipueucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman HI, Wang W, Manola J, DiPaola RS, Ko YJ, Sweeney C, Whiteside TL, Schlom J, Wilding G, Weiner LM. Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2003;22:2122–2132. doi: 10.1200/JCO.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 13.Small EJ, Tchekmedyian NS, Rini BI, Fong L, Lowy I, Allison JP. A pilot study of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:1810–1815. doi: 10.1158/1078-0432.CCR-06-2318. [DOI] [PubMed] [Google Scholar]
- 14.Rini BI, Weinberg V, Fong L, Conry S, Hershberg RM, Small EJ. Combination immunotherapy with prostatic acid phosphatase pulsed antigen-presenting cells (Provenge) plus bevacizumab in patients with serologic progression of prostate cancer after definitive local therapy. Cancer. 2006;107:67–74. doi: 10.1002/cncr.21956. [DOI] [PubMed] [Google Scholar]
- 15.Noguchi M, Kobayashi K, Suetsugu N, Tomiyasu K, Suekane S, Yamada A, Itoh K, Noda S. Induction of cellular and humoral immune responses to tumor cells and peptides in HLA-A24 positive hormone-refractory prostate cancer patients by peptide vaccination. Prostate. 2003;57:80–92. doi: 10.1002/pros.10276. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi K, Noguchi M, Itoh K, Harada M. Identification of a prostate-specific membrane antigen-derived peptide capable of eliciting both cellular and humoral immune responses in HLA-A24+ prostate cancer patients. Cancer Sci. 2003;94:622–627. doi: 10.1111/j.1349-7006.2003.tb01493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noguchi M, Yao A, Harada M, Nakashima O, Komohara Y, Yamada S, Itoh K, Matsuoka K. Immunological evaluation of neoadjuvant peptide vaccination before radical prostatectomy for patients with localized prostate cancer. Prostate. 2007;67:933–942. doi: 10.1002/pros.20572. [DOI] [PubMed] [Google Scholar]
- 18.Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, Eisenberger M, Figg WD, Freidlin B, Halabi S, Hudes G, Hussain M, Kaplan R, Myers C, Oh W, Petrylak DP, Reed E, Roth B, Sartor O, Scher H, Simons J, Sinibaldi V, Small EJ, Smith MR, Trump DL, Vollmer R, Wilding G. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–3467. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 19.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Glabbeke MV, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumor: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 20.Komatsu N, Shichijo S, Nakagawa M, Itoh K. New multiplexed flow cytometric assay to measure anti-peptide antibody: a novel tool for monitoring immune responses to peptides used for immunization. Scand J Cin Lab Invest. 2004;64:1–11. doi: 10.1080/00365510310003391. [DOI] [PubMed] [Google Scholar]
- 21.Hida N, Maeda Y, Katagiri K, Takasu H, Harada M, Itoh K. A simple culture protocol to detect peptide-specific cytotoxic T lymphocyte precursors in circulation. Cancer Immunol Immunotherapy. 2002;51:219–228. doi: 10.1007/s00262-002-0273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arlen PM, Bianco F, Dahut WL, D’Amico A, Figg WD, Freedland SJ, Gulley JL, Kantoff PW, Kattan MW, Lee A, Regan MM, Sartor O, Prostate Specific Antigen Working Group Prostate Specific Antigen Working Group guidelines on prostate specific antigen doubling time. J Urol. 2008;179:2185–2186. doi: 10.1016/j.juro.2008.01.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris TJ, Hipkiss EL, Borzillary S, Wada S, Grosso JF, Yen HR, Getnet D, Bruno TC, Goldberg MV, Pardoll DM, DeWeese TL, Drake CG. Radiotherapy augments the immune response to prostate cancer in a time-dependent manner. Prostate. 2008;68:1319–1329. doi: 10.1002/pros.20794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adler AJ. Mechanisms of T cell tolerance and suppression cancer mediated by tumor-associated antigens and hormones. Curr Cancer Drug Targets. 2007;7:3–14. doi: 10.2174/156800907780006931. [DOI] [PubMed] [Google Scholar]
- 25.Zeytin HE, Patel AC, Rogers CJ, Canter D, Hursting SD, Schlom J, Greiner JW. Combination of a poxvirus-based vaccine with a cyclooxygenase-2 inhibitor (celecoxib) elicits antitumor immunity and long-term survival in CEA Tg/MIN mice. Cancer Res. 2004;64:3668–3678. doi: 10.1158/0008-5472.CAN-03-3878. [DOI] [PubMed] [Google Scholar]