Abstract
Results from functional magnetic resonance imaging (fMRI) have strongly supported the idea that the ventrolateral prefrontal cortex (VLPFC) contributes to successful memory formation, but the role the dorsolateral prefrontal cortex (DLPFC) in memory encoding is more controversial. Some findings suggest that the DLPFC is recruited when one is processing relationships between items in working memory, and this processing specifically promotes subsequent memory for these relationships. However, previous studies could not rule out the possibility that DLPFC promotes memory during all elaborative encoding conditions and contributes to memory on all subsequent associative memory tests. To address this question directly, we used functional magnetic resonance imaging (fMRI) to examine activity during two encoding tasks which prompted participants to encode either relational or item-specific information. On relational trials, participants imagined pairs of items interacting, whereas on item-specific trials, participants imagined the items spatially separated and in different sizes. After scanning we examined memory for relational information and item-specific information. FMRI results showed that DLPFC activity specifically promoted memory for relational information during relational encoding and not memory for item-specific information during item-specific encoding. In contrast, activity in the VLPFC predicted memory for both relational and item-specific information. The present results are consistent with the idea that the DLPFC specifically contributes to successful memory formation through its role in building relationships amongst items.
Keywords: prefrontal, encoding, long-term memory, DLPFC, relational, item-specific, cognitive control, executive processing
Neuropsychological studies have indicated that lateral prefrontal cortex (PFC) is critical for implementing cognitive control processes required for successful long-term memory (Shimamura, 1995; Stuss and Benson, 1984 for review). Consistent with these results, functional magnetic resonance imaging (fMRI) studies routinely report that ventrolateral PFC (VLPFC: BA 44,45,47/12) activity during encoding of specific items is associated with subsequent memory (e.g. Wagner et al., 1998). In addition, the dorsolateral PFC (DLPFC: BA 46,9/46) also appears to be involved in encoding, but its role is less clear. Early work indicated that DLPFC activity was either uncorrelated or negatively correlated with subsequent memory performance (e.g. Otten and Rugg, 2001; Wagner and Davachi, 2001; Daselaar et al., 2004), whereas more recent studies have reported that DLPFC activity was correlated with successful encoding (Blumenfeld and Ranganath, 2006; Staresina and Davachi, 2006; Summerfield et al., 2006; Murray and Ranganath, 2007; Qin et al., 2007).
According to one account, the VLPFC and DLPFC contribute to successful memory encoding, but in different ways (Blumenfeld and Ranganath, 2006, 2007). According to this view, VLPFC guides selection of goal-relevant, detailed item information in working memory and thereby promotes long term memory for this item information. In contrast, the DLPFC and VLPFC are jointly recruited to guide the processing of inter-item relational information in working memory, which promotes long-term memory for this relational information. The distinction underlying this theory is related to the distinction between ‘item-specific/relational encoding’ proposed by Hunt and Einstein (1981), and was guided by findings that have implicated the VLPFC in the active maintenance, selection and retrieval of goal-relevant item information (Thompson-Schill et al., 1998; D’Esposito et al., 1999; Badre and Wagner, 2004), and findings implicating DLPFC and VLPFC in the manipulation (D’Esposito et al., 1999; Postle et al., 1999), monitoring (Petrides, 2000; Champod and Petrides, 2007) or restructuring of inter-item relational information (Bor et al., 2003; see also Hampshire et al., 2007). Consistent with this theory, a recent study has shown that DLPFC activity predicts high levels of subsequent recollection following relational encoding1 but is not correlated with subsequent item recognition following item-specific encoding (Blumenfeld and Ranganath, 2006). In addition, DLPFC activity has been found to predict subsequent inter-item associative recognition but not item recognition memory (Addis and McAndrews, 2006; Murray and Ranganath, 2007; Qin et al., 2007).
Although the studies described above are consistent with a role for the DLPFC in relational encoding, one important limitation is that they used tests of item recognition (e.g., is the item old or new?) to examine memory for item-specific information, whereas they used tests of inter-item associative recognition (e.g., was A paired with B?) to examine memory for relational information. Associative tests are inherently more complex than item tests because they require memory for the individual items as well as the associations among those items, so DLPFC activity observed in those studies could reflect more effective or more elaborate encoding than that supported by VLPFC. Furthermore, associative recognition is expected to rely heavily on the subject’s ability to recollect qualitative information about the study event, whereas item recognition can be based on assessments of stimulus familiarity (Mandler, 1980; Yonelinas et al., 2002). It is therefore possible that the DLPFC may simply be involved in encoding any associative information that supports recollection, rather than being involved specifically in the encoding of relational information.
To more directly characterize the role of lateral prefrontal regions in memory encoding, we used event-related fMRI to examine prefrontal activity during encoding of relational information or item-specific information and subsequently tested memory for this information using two associative recognition memory tests. During all encoding trials, participants were presented with word pairs and asked to form mental images. On interactive imagery trials, participants imagined the two items meaningfully interacting. This task was designed to promote memory for relational information (i.e., the word-pairings). On separation imagery trials, participants imagined the two items spatially separated and in different relative sizes. This task was designed to promote memory for item-specific information (i.e., the location or the size of each item). Following scanning, participants completed two subsequent memory tests that were designed to be differentially sensitive to the relational or item-specific information emphasized during encoding. To measure relational information, an associative recognition test that examined memory for the pairings was used, whereas to measure item-specific information an associative test that measured memory for the location of each item was used. We predicted that DLPFC activity will predict subsequent memory preferentially for the interactive encoding condition compared to the separate encoding condition, whereas VLPFC activity will predict subsequent memory during both the interactive and separate conditions.
Methods
Participants
Sixteen (10 females, mean age 27) participants were recruited from the University of California at Davis community. Participants gave informed consent and were paid for their participation. One participant’s data was excluded because of a technical issue during the recognition phase of the experiment.
Materials
Stimuli consisted of 560 concrete and imageable nouns (concreteness 588.7, imagability 582.6, Kucera-Francis frequency 35.5, number of phonemes 4.3). Two lists of 140 unrelated word pairs were constructed from this set of stimuli. Each word pair was constructed such that there was zero forward or backward association strength amongst the items (USF Free association norms: Nelson, D. L. et al., 1998). The two lists were counterbalanced with respect to subject and condition.
Behavioral Procedure
During scanning, participants encoded word pairs in the context of two mental imagery tasks which were designed to differentially promote memory for relational and item-specific information.
Study Phase
Participants were instructed that the color of the stimuli on each trial would determine which kind of task was to be performed. All stimuli on interact trials were displayed in green, whereas all stimuli on separate trials were displayed in a yellow-orange color. On each interact trial, a word pair was shown for 3s. During this time, participants were instructed to form mental images of the object-referents of these nouns interacting in some meaningful way (see fig. 1a). This relational encoding task prompted participants to encode distinctive relational information (i.e. the word pairings). For example with the word pair “ANT COMB”, participants could imagine an ant walking over the teeth of a comb. Next, the numbers “1” through “4” were shown under the word pair for 1s, during which time participants were instructed to rate the vividness of the image they had formed during the trial. The numbers were replaced with a colored fixation cross appeared for 500 ms that served as a reminder to the participant to make a button response on a 4-button response box. On each separate trial, a word pair was shown for 3 seconds, and during this time, participants were instructed to imagine the object-referents of each noun simultaneously as if the objects were on different sides of a room. Participants were instructed to imagine the object-word on the left side of the triad on the left side of the room and the object-word on the right side of the triad on the right side of the room. Additionally they were instructed to imagine the object on the left very large and the object on the right very small (see fig. 1a). This item-specific encoding task prompted participants to meaningfully encode distinctive information about each item and to link these attributes with the side of the screen on which each word was shown. In the previous example, “ANT COMB”, participants would be instructed to imagine a very large ant on the left side of a room and a very small comb on the right side of the right side of the same room. They were further instructed to ensure that they always keep these objects separated in their mind’s eye. The relative size instruction was counterbalanced with respect to side of screen and participant. Next, the numbers “1” through “4” were shown under the word pair for 1s, during which time participants were instructed to rate the vividness of the image they had formed during the trial. The numbers were then replaced with a colored fixation cross appeared for 500 ms that served as a reminder to the participant to make a button response on a 4-button response box.
Figure 1.
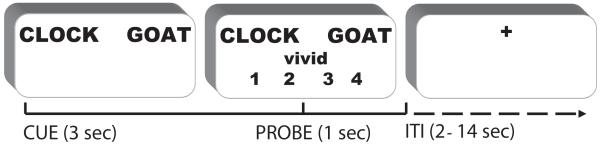
Trial structure and timing.
On each trial, participants were presented with a noun pair for 3.5s (cue) during which time they were instructed to perform one of two different mental imagery tasks.. During interact trials participants imagined the object referents interacting in a meaningful way (e.g. goat kicking the clock). During separate trials participants imagined the object referents separated as if on different sides of a room and in different relative sizes (e.g. a small clock on the left side of the room and a large goat on the right side of the room). A probe was then presented for 1s which prompted participants to rate the vividness of their mental image that they formed during the cue period. A white fixation cross was presented as the ITI (jittered 2-14 sec.)
A white fixation cross was shown during the inter-trial interval (ITI), which was jittered from 2 to 14s (mean= 4.16). One block of 20 interact trials and one block of 20 separate trials was presented during each scanning run and a total 7 scanning runs were acquired for the mental imagery tasks. Thus, each participant encoded a total of 140 pairs in the interact condition and 140 pairs in the separate condition. After the mental imagery tasks, participants performed a motor response task during the final functional scanning run that was used to estimate a participant-specific hemodynamic response function (HRF: Handwerker et al., 2004). After the 8 functional runs were acquired, a high-resolution anatomical image was acquired. Participants were removed from the MRI scanner.
Test phase
Following the scanning phase of the experiment, participants were given two tests, each assessing memory for different kinds of information. The first test was designed to examine memory for the item-specific information that was encoded during separate trials. Given that separate trials emphasized encoding the location and/or size information that was specific to each item in a pair, we gave participants a test that probed memory for the side of the screen on which each item was presented during the study phase. In this test, items were presented individually in the center of the screen. Participants were instructed to decide whether each item was originally presented on the left side of the screen or the right side of the screen using 4 response alternatives (1: left side with high confidence. 2: left side with low confidence, 3: right side with low confidence, 4: right side with high confidence). All the studied items from both encoding conditions were tested, but items studied during separate trials were of primary interest. We tested memory for item-specific information for items studied during the interact task in order to assess whether our behavioral manipulation of item-specific encoding was successful, that is that the separate condition promoted superior memory for item-specific information compared to the interact condition.
The second test was designed to examine memory for the relational information that was encoded during interact trials. Because the interact condition emphasized encoding relational information shared by items within a trial, we administered a test that probed memory for the word pairings. In this test, the first word (cue) in each pair (e.g., ANT) was presented at the center of the screen and two words were presented on the lower left and right sides of the screen. One of these words was the item that was originally paired with the centered item (target) during the study phase and the other had been originally paired with a different word during the study phase (foil). Participants were instructed to decide which of the two alternative items, the item appearing on the left or the right side of the screen during test, was originally paired with the centered item using 4 response alternatives (1: left item with high confidence. 2: left item with low confidence, 3: right item with low confidence, 4: right item with high confidence). Both the target and foil words on each test trial had been previously studied in the same encoding condition. All studied pairs from both encoding conditions were tested, but memory for pairs studied during interact trials was of primary interest. The sides of the screen on which the target and foil items were shown were pseudo-randomized and counterbalanced with respect to subject and encoding condition. All the studied pairs from both encoding conditions were tested, but pairs studied during separate trials were of primary interest. We tested memory for relational information for pairs studied during the separate task in order to determine whether the interact condition promoted superior memory for relational information compared to the separate condition.
MRI data acquisition and processing
MRI data were collected on a 3T Siemens (Erlangen, Germany) Trio scanner at the UC Davis Imaging Research Center, Functional images sensitive to blood oxygen level dependent (BOLD) contrast were acquired using a gradient echo-planar imaging (EPI) sequence [repetition time (TR), 2000 ms; echo time (TE), 25 ms; flip angle, 90° field of view (FOV), 220 mm; matrix size, 64 × 64]. Each volume consisted of 24, 3.4-mm-thick axial slices, resulting in a voxel size of 3.4375 × 3.4375 × 3.4 mm. Coplanar T2-weighted images were acquired using a spin-echo sequence (TR, 4000 ms; TE, 109 ms; flip angle 90°; FOV 220mm matrix size, 512×512; slice thickness, 3.4 mm) and high-resolution, T1-weighted images were also acquired using an MP-RAGE (magnetization-prepared rapid acquisition gradient-echo) sequence (TR, 1750 ms; TE, 2.93 ms; flip angle, 12°; matrix size, 256 × 256).
Data were pre-processed using Statistical Parametric Mapping (SPM) 5. EPI images were sinc interpolated in time to correct for between-slice timing differences in image acquisition, realigned using a six-parameter, rigid-body transformation algorithm, spatially normalized to the template from the International Consortium for Brain Mapping Project (Cocosco et al., 1997) resliced into 3.5 mm isotropic voxels, and spatially smoothed with an 8 mm full-width at half-maximum Gaussian filter.
MRI data analysis
Activity changes during each trial were deconvolved using a modified general linear model (GLM: Worsley and Friston, 1995) as implemented in the VoxBo software package (available at www.voxbo.org). Covariates that modeled BOLD signal for each task (interact and separate) were constructed by convolving vectors of expected neural activity on each trial with a subject-specific HRF estimated from responses in the central sulcus during the visuomotor response task (Aguirre et al., 1997; Handwerker et al., 2004). Each trial was modeled as an event that lasted 2 TRs. Data from the visuomotor response task were not available for 3 subjects, and for these subjects, covariates were constructed by convolving the vector of expected neural activity with an average of 22 HRFs estimated from healthy young participants on the 3T Siemens trio at the UC Davis Imaging Research Center. Additional covariates modeled global signal changes that could not be accounted for by variables in the design matrix (Desjardins et al., 2001), baseline shifts across scanning runs, and an intercept.
Two GLM analyses were performed individually on each participant’s data. The first analysis examined activity as a function of subsequent performance on the test for relational information. Specifically, for each encoding task, one covariate modeled activity during trials that led to high confidence memory for the pairing and another modeled trials that led to either low confidence memory for the pairing or misses. In this analysis, activity only during interact trials was of theoretical interest, because only this condition explicitly required relational encoding. Moreover, there was an insufficient number of separate trials (<10) that led to a high confidence hit on the subsequent relational information test to permit a reliable analysis. The second analysis examined activity related to subsequent performance on the test of item-specific information. For each task, one covariate modeled activity during trials for which the location of both items were subsequently remembered with high confidence, a second modeled trials for which the location of only one item was subsequently remembered with high confidence and the third modeled trials for the location of neither item was subsequently remembered with high confidence.. In this analysis, activity only during separate trials was of theoretical interest, because only this condition explicitly required item-specific encoding. Moreover, there was an insufficient number of interact trials (<10) in which the locations of both items were remembered with high confidence to permit a reliable analysis.
For group analyses, images of parameter estimates were first calculated for each contrast of interest. These contrast images were then entered into a second-level one-sample t-test, in which the mean estimate across participants at each voxel was tested against zero. Significant regions of activation within PFC were identified using an uncorrected statistical threshold of p<.005, with a cluster size threshold chosen to control for multiple comparisons in the PFC (Forman et al., 1995). A cluster threshold of 17 contiguous voxels was chosen, as Monte-Carlo simulations using the AlphaSim program indicated that this threshold would control the family-wise error rate at p < 0.05. This threshold was also used to report significant activity in exploratory analyses examining the contributions of regions outside PFC to item-specific and relational encoding. For visualization purposes, thresholded statistical parametric maps were overlaid on fiducial surface atlas images using Caret 5 (Van Essen et al., 2001).
Results
Behavioral Results
The interact and separate encoding tasks were designed to effectively promote memory for relational and item-specific information respectively, and the analysis of recognition performance indicated that the encoding manipulations were effective at doing so. Discriminability (d’) on the associative recognition test was higher for pairs encoded on interact trials than for pairs encoded on separate trials, both when considering only high confidence hits and false alarms (interact mean = 3.00, s.d. = 1.29, separate mean =1.38, s.d. = 0.78; t(14)=4.32; p < 0.001), and when using all levels of confidence to determine d’ (interact mean = 2.66 s.d. = 1.34, separate mean = 0.84, s.d. = 0.39; t(14)=5.82; p < 0.001). Discriminability on the item-location recognition test was higher for words encoded on separate trials than for words encoded on interact trials, both when only high confidence responses (interact mean = 0.67 s.d. = 0.45, separate mean = 1.71 s.d. = 0.65; t(13)=6.18; p < 0.001 see) were considered and when responses were collapsed across confidence levels (interact mean = 0.53 s.d. = 0.51, separate mean = 1.31, s.d. = 0.57; t(14)=4.88; p < 0.001).
fMRI results
To test whether DLPFC activity supports successful relational encoding, we examined activity during interact trials as a function of subsequent memory for relational information. Specifically, on a map-wise level, we contrasted activity during interact trials that subsequently led to high confidence hits on the relational information memory test against activity during interact trials that subsequently led to either low confidence hits or misses on the relational information memory test. Consistent with our predictions, this contrast revealed significant clusters of suprathreshold voxels in bilateral DLPFC (approx. BA 9/46, see figure 2) and VLPFC (left approx. BA 47/12, 45, 44, right approx. BA 47/12 see figure 2). These findings suggest that trial-to-trial variation in activity in the DLPFC and VLPFC was associated with relational encoding. Outside of the PFC, the same contrast revealed that activity in the right hippocampus, a region in left lateral temporal cortex, regions of occipital cortex, the caudate nucleus, ventral striatum, cerebellum and brainstem were increased during interact trials that led to high confidence associative hits compared to those that led to low confidence associative hits and misses (see Table 1).
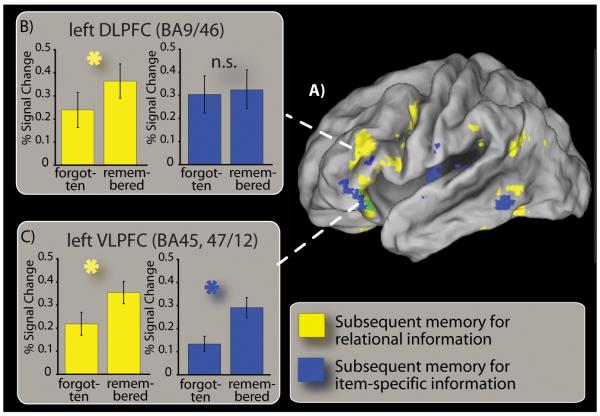
Figure 2.
DLPFC activity specifically correlates with subsequent memory for relational information. (A) At upper right, a cortical surface rendering shows regions (colored in yellow) for which activity was increased during relational encoding (interact) trials for which the relational information was remembered with high confidence compared to relational trials for which this information was forgotten (see methods for details). Also depicted are regions (colored in blue) for which activity was increased on item-specific encoding (separate) trials for which the location of both items were subsequently remembered with high confidence compared to trials for which the location the items were subsequently forgotten (see methods for details). Regions colored in green exhibited suprathreshold responses in both contrasts. Note that green colored regions fall exclusively within VLPFC in the PFC. (B-C): Activity in two prefrontal regions of interest are separately plotted for interact trials (yellow bars) as a function of subsequent memory for relational information and for separate trials (blue bars) as a function of subsequent memory for item-specific information. (B) Activity in a region of left DLPFC (approximate BA 9/46) was correlated with memory for relational information on interact trials, and was not correlated with memory for item-specific information on separate trials in either map-wise or ROI analyses. (BC) Activity in left aVLPFC (approximate BA 45, 47/12) was correlated with memory relational information and item-specific information. Error bars depict the standard error of the mean.
Table 1.
subsequent memory local maxima
Plot of the peak-voxels from each statistically significant cluster of activity
| SUBSEQUENT INTER-ITEM ASSOCIATIVE MEMORY | ||||
|---|---|---|---|---|
| interact trials: high
confidence subsequent inter-item associative
memory>low-confidence and misses Lateral Prefrontal Regions | ||||
| x | y | z | T-value | |
| −44 | 35 | 26 | 3.22 | left middle frontal gyrus (DLPFC: ~BA 9/46) |
| −44 | 32 | 11 | 4.16 | left inferior frontal gyrus (VLPFC: BA 45) |
| 23 | 34 | 17 | 3.2 | right middle frontal gyrus (DLPFC: ~BA 9/46) |
| 43 | 41 | 5 | 4.33 | right inferior frontal sulcus (BA 45, 9/46) |
| Outside Lateral Prefrontal Cortex | ||||
| x | y | z | T-value | |
|
| ||||
| 37 | −30 | −13 | 4.5 | right hippocampus |
| −17 | −19 | 20 | 5.01 | left caudate nucleus |
| 25 | −32 | 29 | 6.85 | right caudate nucleus |
| −39 | −31 | −16 | 4.62 | left collateral sulcus (~BA 20,30) |
| 38 | −31 | −13 | 4.34 | right collateral sulcus (~BA 20,30) |
| 45 | −51 | −7 | 5.41 | right temporo-occipital incisure (~BA 21) |
| −44 | −46 | 0 | 4.7 | left temporo-occipital incisure (~BA 21) |
| −38 | −54 | −26 | 5.73 | left cerebellum |
| 40 | −49 | −31 | 4.94 | right cerebellum |
| SUBSEQUENT ITEM-LOCATION MEMORY | ||||
|---|---|---|---|---|
| separate trials: 2
item-locations subsequent remembered with high confidence > 0
item-locations remembered with high confidence | ||||
| x | y | z | T-value | |
| −47 | 29 | 14 | 5.23 | left inferior frontal gyrus (VLPFC: BA 45) |
| Outside Lateral Prefrontal Cortex | ||||
| x | y | z | T-value | |
|
| ||||
| −53 | −58 | −6 | 5.34 | temporo-occipital incisure (~BA 21) |
| −45 | −26 | 11 | 4.8 | left posterior insula |
| 34 | −4 | 11 | 4.01 | right posterior insula |
| −42 | 32 | −13 | 3.74 | left orbital frontal cortex (BA: 47/12) |
Our next analysis was aimed at identifying regions involved in encoding item-specific information. To this end, we contrasted activity during separate trials for which the locations of both items were recognized with high confidence against activity during separate trials for which the location of neither item was confidently recognized. This contrast revealed a cluster of suprathreshold voxels in a region of left VLPFC (approx. BA 47/12). As shown in Figure 2, this VLPFC region partially overlapped with the left VLPFC region in which activity was associated with successful relational encoding. This finding suggests that VLPFC activity supports successful item-specific and relational encoding. In contrast, no suprathreshold voxels were observed in DLPFC2.To further investigate whether DLPFC activity was sensitive to successful item-specific encoding, we interrogated the regions in left and right DLPFC that showed activity correlated with successful relational encoding in the analyses described above. These analyses revealed no relationship between activity in the ROIs and subsequent item-location memory during separate trials (left DLPFC: t(12,1)=0.98, p > 0.05 see figure 3, right DLPFC: t(12,1)=1.15, p > 0.05). Outside of PFC, activation was also observed in a region of left lateral temporal cortex and bilateral regions of posterior insula (see Table 1).
Discussion
The present study used fMRI to investigate the role of DLPFC in successful encoding of relational and item-specific information. Results showed that DLPFC activity was correlated with subsequent memory for relational information during relational encoding, but did not differ as a function of memory for item-specific information during item-specific encoding. In contrast, VLPFC activity was correlated with memory for relational as well as item-specific information. The present results suggest that DLPFC is involved specifically in encoding of inter-item relational information.
DLPFC specifically promotes memory for relational information
Imaging studies have often failed to find a link between DLPFC activity and successful LTM encoding. Of the studies that reported significant positive correlations between DLPFC activity and subsequent memory, most used relational encoding tasks and found that DLPFC activity was correlated with subsequent memory for relational information (Blumenfeld and Ranganath, 2006; Staresina and Davachi, 2006; Summerfield et al., 2006; Murray and Ranganath, 2007; Qin et al., 2007). A limitation of these studies, however, is that relational encoding tasks additionally tend to require higher levels of executive control or elaborative encoding and disproportionately lead to strong memory or recollection compared to many the item tasks. Furthermore, these studies used associative recognition to test memory for relational information but none of these studies have examined memory for any other form of associative information. Thus, it is could be argued that the DLPFC supports memory on all forms
The present results are difficult to reconcile with such accounts for two main reasons. First we found that DLPFC does not support all types of effortful elaborative encoding. Given that both interact and separate trials required elaborative encoding, if DLPFC supports memory during all types of elaborative encoding, we would have expected DLPFC to support memory on both tasks (as was observed in VLPFC). In contrast, we found that DLPFC activity was linked to memory for relational information during interact trials but did not predict memory for item-specific information during separate trials. Second, we found that DLPFC activity is not generally correlated with subsequent associative memory. In our study, we used two associative tests; one test that examined memory for relational information and one that examined memory for item-specific information. Both of these tests are more complex and are thought of as requiring more strong memory or recollection compared to a standard item recognition test. We found that DLPFC activity was correlated with performance on the subsequent test of relational information and not item-specific information. Accordingly, the present results are most consistent with the idea that DLPFC processes distinct relationships in working memory (D’Esposito et al., 1999; Bor et al., 2003; Champod and Petrides, 2007; see also Blumenfeld and Ranganath, 2007) and thereby strengthens inter-item associative information in LTM.
VLPFC contributes to item-specific and relational encoding
Unlike DLPFC which showed a selective pattern of subsequent memory activity, VLPFC activity predicted subsequent memory both for relational information during interact trials and predicted subsequent item-specific information during separate. Evidence from fMRI studies of working memory strongly point to the notion that VLPFC regions are involved in the controlled processing of goal-relevant item information. For example, VLPFC but not DLPFC activity has been reported during tasks that require maintaining, retrieving and/or selecting detailed item information (Thompson-Schill et al., 1998; M. D’Esposito, Postle, Ballard, & Lease, 1999; D. Badre & A. D Wagner, 2004),. Furthermore, more recently, it has been shown that VLPFC but not DLPFC is finely tuned to represent the specific details of a target item (Hampshire, Duncan, & Owen, 2007) and that the level abstractness of goal-relevant item information may be represented differentially along the rostro-caudal axis of VLPFC (Race, Shanker, & Anthony D Wagner, 2009). Critically, goal-relevant selection of item information is necessary for successfully encoding item-specific as well as relational information. For example, during separate trials, information relevant to each item’s size and location must be selected and retrieved on each trial, and this information is critical for the item-specific information that was tested (the location of each item). During interact trials, some degree of information about each item must be selected in order for the second-order relational processing to proceed, and therefore this item information supports relational encoding. Thus the present results are consistent with the view that VLPFC subserves the controlled selection of goal-relevant item information during encoding and this processing supports subsequent memory for both item-specific and relational information. Furthermore, taken as a whole, the present results are consistent with a hierarchical model of PFC function (c.f. David Badre & Mark D’Esposito, 2009; Fuster, 2009) which posits that VLPFC activity contributes to LTM encoding through its role in controlled selection and/or retrieval of item information and DLPFC and VLPFC collectively support the encoding of inter-item relationships (Blumenfeld & Charan Ranganath, 2007; Charan Ranganath & Blumenfeld, 2007).
Involvement of regions outside of PFC in relational and item-specific encoding
Several regions outside PFC demonstrated significant correlations with subsequent memory. Indeed the role of PFC activity in LTM encoding may be to modulate processing in these posterior sites that more closely represent the information that is to be encoded (Ranganath, 2006; Davidson et al., 2006; Gazzaley et al., 2007). For instance, we found that along with PFC, a region of right hippocampus demonstrated activity predictive of subsequent memory for relational but not item-specific information. This finding is consistent with neuropsychological and neuroimaging evidence implicating hippocampus in relational binding (e.g. Konkel et al., 2008; Hannula and Ranganath, 2009) and recollection of inter-item associative information (Eichenbaum et al., 2007; Diana et al., 2007). Additionally, we found that activity in a region in the left posterior middle temporal gyrus was correlated with both successful item-specific and relational encoding. Activation in this region, along with VLPFC, is often observed in studies that require processing of semantic information (e.g. Wagner et al., 1998; Baker et al., 2001; Chee et al., 2003) or the retrieval of information about concrete visual objects (Donohue et al., 2005; Souza et al., 2009) that guides their usage. We speculate that this region might have contributed to memory encoding in both encoding conditions of the present study via its role in the storage and/or retrieval of semantic information about concrete visual objects.
Conclusion
The present results are consistent with the idea that the DLPFC specifically supports the encoding of relational information and does not simply contribute to subsequent memory during all elaborative tasks nor does it contribute to memory for all associative information. These findings add to accumulating evidence suggesting that the DLPFC plays a specific and important role in the encoding of relational information that is critical for episodic memory formation.
Acknowledgments
We thank Linda Murray, Craig Brozinsky, Deborah Hannula, Rachel Diana, Dan Ragland and Silvia Bunge for their insightful comments. This work was supported by National Institutes of Health Grant R01 MH068721 and RB was supported by National Institutes of Health Predoctoral Fellowship 1F31 MH079776-01A1
Footnotes
We use the term “relational” to refer solely to inter-item relationships and not other types of relationships (e.g. item-source or intra-item relationships)
A similar pattern emerged in VLPFC and DLPFC when contrasting activity during separate trials for which the location of both items were recognized with high confidence against activity during separate trials for which the location of neither or one of the items was confidently recognized
References
- Addis DR, McAndrews MP. Prefrontal and hippocampal contributions to the generation and binding of semantic associations during successful encoding. Neuroimage. 2006;33:1194–206. doi: 10.1016/j.neuroimage.2006.07.039. [DOI] [PubMed] [Google Scholar]
- Aguirre GK, Zarahn E, D’Esposito M. Empirical analyses of BOLD fMRI statistics. II. Spatially smoothed data collected under null-hypothesis and experimental conditions. Neuroimage. 1997;5:199–212. doi: 10.1006/nimg.1997.0264. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Selection, integration, and conflict monitoring; assessing the nature and generality of prefrontal cognitive control mechanisms. Neuron. 2004;41:473–87. doi: 10.1016/s0896-6273(03)00851-1. [DOI] [PubMed] [Google Scholar]
- Badre D, D’Esposito M. Is the rostro-caudal axis of the frontal lobe hierarchical? Nat. Rev. Neurosci. 2009;10:659–669. doi: 10.1038/nrn2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JT, Sanders AL, Maccotta L, Buckner RL. Neural correlates of verbal memory encoding during semantic and structural processing tasks. Neuroreport. 2001;12:1251–6. doi: 10.1097/00001756-200105080-00039. [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C. Dorsolateral prefrontal cortex promotes long-term memory formation through its role in working memory organization. J Neurosci. 2006;26:916–25. doi: 10.1523/JNEUROSCI.2353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C. Prefrontal cortex and long-term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. The Neuroscientist. 2007;13:280–91. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- Bor D, Duncan J, Wiseman RJ, Owen AM. Encoding strategies dissociate prefrontal activity from working memory demand. Neuron. 2003;37:361–7. doi: 10.1016/s0896-6273(02)01171-6. [DOI] [PubMed] [Google Scholar]
- Champod AS, Petrides M. Dissociable roles of the posterior parietal and the prefrontal cortex in manipulation and monitoring processes. Proc. Natl. Acad. Sci. U.S.A. 2007;104:14837–42. doi: 10.1073/pnas.0607101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MW, Westphal C, Goh J, Graham S, Song AW. Word frequency and subsequent memory effects studied using event-related fMRI. Neuroimage. 2003;20:1042–51. doi: 10.1016/S1053-8119(03)00335-5. [DOI] [PubMed] [Google Scholar]
- Cocosco C, Kollokian V, Kwan R, Evans A. Brainweb: online interface to a 3D MRI simulated brain database. Neuroimage. 1997;5:s425. [Google Scholar]
- Daselaar SM, Prince SE, Cabeza R. When less means more: deactivations during encoding that predict subsequent memory. Neuroimage. 2004;23:921–7. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Davidson PSR, Troyer AK, Moscovitch M. Frontal lobe contributions to recognition and recall: linking basic research with clinical evaluation and remediation. J Int Neuropsychol Soc. 2006;12:210–223. doi: 10.1017/S1355617706060334. [DOI] [PubMed] [Google Scholar]
- Desjardins AE, Kiehl KA, Liddle PF. Removal of confounding effects of global signal in functional MRI analyses. Neuroimage. 2001;13:751–8. doi: 10.1006/nimg.2000.0719. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain Cogn. 1999;41:66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 2007;11:379–86. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Donohue SE, Wendelken C, Crone EA, Bunge SA. Retrieving rules for behavior from long-term memory. Neuroimage. 2005;26:1140–9. doi: 10.1016/j.neuroimage.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu. Rev. Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Cortex and memory: emergence of a new paradigm. J Cogn Neurosci. 2009;21:2047–2072. doi: 10.1162/jocn.2009.21280. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Rissman J, Cooney J, Rutman A, Seibert T, Clapp W, D’Esposito M. Functional interactions between prefrontal and visual association cortex contribute to top-down modulation of visual processing. Cereb. Cortex. 2007;17(Suppl 13):i125–135. doi: 10.1093/cercor/bhm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Duncan J, Owen AM. Selective tuning of the blood oxygenation level-dependent response during simple target detection dissociates human frontoparietal subregions. J. Neurosci. 2007;27:6219–23. doi: 10.1523/JNEUROSCI.0851-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerker DA, Ollinger JM, D’Esposito M. Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. Neuroimage. 2004;21:1639–51. doi: 10.1016/j.neuroimage.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Hannula DE, Ranganath C. The Eyes Have It: Hippocampal Activity Predicts Expression of Memory in Eye Movements. Neuron. 2009;63:8. doi: 10.1016/j.neuron.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel A, Warren DE, Duff MC, Tranel DN, Cohen NJ. Hippocampal amnesia impairs all manner of relational memory. Front Hum Neurosci. 2008;2:15. doi: 10.3389/neuro.09.015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandler G. Recognizing - the Judgment of Previous Occurrence. Psychological Review. 1980;87:252–271. [Google Scholar]
- Murray LJ, Ranganath C. The dorsolateral prefrontal cortex contributes to successful relational memory encoding. J. Neurosci. 2007;27:5515–22. doi: 10.1523/JNEUROSCI.0406-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DL, McEvoy CL,, Schreiber TA. The University of South Florida word association, rhyme, and word fragment norms. 1998 doi: 10.3758/bf03195588. Available at: http://www.usf.edu/FreeAssociation/ [DOI] [PubMed]
- Otten LJ, Rugg MD. When more means less: neural activity related to unsuccessful memory encoding. Curr Biol. 2001;11:1528–30. doi: 10.1016/s0960-9822(01)00454-7. [DOI] [PubMed] [Google Scholar]
- Petrides M. The role of the mid-dorsolateral prefrontal cortex in working memory. Exp Brain Res. 2000;133:44–54. doi: 10.1007/s002210000399. [DOI] [PubMed] [Google Scholar]
- Postle BR, Berger JS, D’Esposito M. Functional neuroanatomical double dissociation of mnemonic and executive control processes contributing to working memory performance. Proc Natl Acad Sci U S A. 1999;96:12959–64. doi: 10.1073/pnas.96.22.12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Piekema C, Petersson KM, Han B, Luo J, Fernández G. Probing the transformation of discontinuous associations into episodic memory: an event-related fMRI study. Neuroimage. 2007;38:212–22. doi: 10.1016/j.neuroimage.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Ranganath C. Working memory for visual objects: Complementary roles of inferior temporal, medial temporal, and prefrontal cortex. Neuroscience. 2006;139:277–89. doi: 10.1016/j.neuroscience.2005.06.092. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Blumenfeld RS. Prefrontal cortex and human memory: an integrated account of results from neuropsychological and neuroimaging studies of working and long-term memory. In: Eichenbaum H, editor. Learning and memory: a comprehensive reference. Elsevier; Oxford, England: 2007. [Google Scholar]
- Shimamura AP. Memory and the prefrontal cortex. Ann N Y Acad Sci. 1995;769:151–9. doi: 10.1111/j.1749-6632.1995.tb38136.x. [DOI] [PubMed] [Google Scholar]
- Souza MJ, Donohue SE, Bunge SA. Controlled retrieval and selection of action-relevant knowledge mediated by partially overlapping regions in left ventrolateral prefrontal cortex. Neuroimage. 2009;46:299–307. doi: 10.1016/j.neuroimage.2009.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Differential encoding mechanisms for subsequent associative recognition and free recall. The Journal of neuroscience. 2006;26:9162–72. doi: 10.1523/JNEUROSCI.2877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Benson DF. Neuropsychological studies of the frontal lobes. Psychol Bull. 1984;95:3–28. [PubMed] [Google Scholar]
- Summerfield C, Greene M, Wager T, Egner T, Hirsch J, Mangels J. Neocortical connectivity during episodic memory formation. PLoS biology. 2006;4:e128. doi: 10.1371/journal.pbio.0040128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, Swick D, Farah MJ, D’Esposito M, Kan IP, Knight RT. Verb generation in patients with focal frontal lesions: a neuropsychological test of neuroimaging findings. Proc Natl Acad Sci U S A. 1998;95:15855–60. doi: 10.1073/pnas.95.26.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH. An integrated software suite for surface-based analyses of cerebral cortex. J Am Med Inform Assoc. 2001;8:443–59. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Davachi L. Cognitive neuroscience: forgetting of things past. Curr Biol. 2001;11:R964–7. doi: 10.1016/s0960-9822(01)00575-9. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–91. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited—again. Neuroimage. 1995;2:173–81. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NE, Quamme JR, Lazzara MM, Sauve MJ, Widaman KF, Knight RT. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nat Neurosci. 2002;5:1236–41. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]