Summary
Background
Cetuximab is a chimeric monoclonal antibody targeting the epidermal growth factor receptor (EGFR). The recommended dosage is an initial load of 400 mg/m2 intravenously (IV) followed by a weekly maintenance dose of 250 mg/m2. It has been reported retrospectively that cetuximab efficacy was correlated with dose-related severity of skin rash. This study was prospectively designed to examine the safety and feasibility of escalating weekly doses of cetuximab, testing the hypothesis of the relationship of dose-dependent skin toxicity and efficacy.
Methods
Four dose levels were tested: Cetuximab 400 mg/m2 IV loading dose and 250, 300, 350, 400 mg/m2 weekly IV maintenance. There was no intra-patient dose escalation. Standard dose limiting toxicity criteria were used. Rash was evaluated using two additional validated dermatology methods: global acne grading scale (GAGS) and acne lesion counting (ALC). Tumor specimens and blood samples were obtained for correlative analyses.
Results
Twenty seven patients with solid tumors were enrolled: five head and neck, three pancreas, four gall bladder, two each of prostate, breast, colorectal, lung, and esophagus, and five others. Planned dose escalation was completed without reaching dose-limiting toxicity (DLT) or the maximum tolerated dose (MTD). The highest dose level was expanded to a total of 17 patients. Gr 3/4 toxicities included: lymphopenia (2), fatigue (2), and hypomagnesemia (2). One patient experienced a grade 3 rash (350 mg/m2). Sixty five percent of pts had a ≥ Gr 2 rash that was not dose dependent. In 22 evaluable patients, there was one partial response (PR) in a patient with cholangiocarcinoma (400 mg/m2) and seven patients had stable disease (SD). ALC and GAGS demonstrated no correlation with dose or response. Correlative studies evaluating k-ras, EGFR FISH status and immunologic correlatives were conducted on available tumor samples.
Conclusions
Cetuximab administered at 400 mg/m2 IV as a loading dose with weekly maintenance dose of 400 mg/m2 is feasible and well tolerated. There was no direct correlation of the grade of rash with dose in this group of patients with heterogenous solid tumors.
Keywords: Cetuximab, Acneiform rash, Solid tumors, Global acne grading scale, Acne lesion counting
Introduction
The epidermal growth factor receptor (EGFR) is implicated in tumor cell proliferation, differentiation, and angiogenesis [1]. EGFR is a commonly expressed transmembrane glycoprotein of the tyrosine kinase growth factor receptor family. The extracellular domain of EGFR can bind to a variety of ligands, including epidermal growth factor (EGF), transforming growth factor beta (TGFβ), amphiregulin, and epiregulin. Upon ligand binding, the intracellular domain of EGFR is activated, thereby triggering downstream cellular pathways regulating cell growth. EGFR is found in normal human tissues, but is over-expressed, or inappropriately activated, in many solid tumors including: esophagus, head and neck, colorectal, bladder, ovarian, pancreatic, renal cell, and lung [2].
Cetuximab is a chimeric IgG1 monoclonal antibody that targets EGFR. It inhibits ligand binding, induces receptor endocytosis and causes antigen-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity. The US Food and Drug Administration (FDA) has approved cetuximab for head and neck as a single agent in advanced or recurrent disease and in combination with radiation. It is also indicated in advanced colorectal cancer in combination with chemotherapy or alone in chemo-refractory or poor PS patients. The recommended dose is 400 mg/m2 administered intravenously as a loading dose in the first week followed by weekly doses of 250 mg/m2.
A maximum tolerated (MTD) has not been established for cetuximab. The optimal dose of cetuximab is hypothesized to be one where complete and prolonged receptor saturation occurs [3, 4]. Pharmacodynamic studies have demonstrated maximal inhibition of EGFR expression across the 250–500 mg/m2 dose range. Doses below 250 mg/m2 are linked with an increase in EGFR protein expression, suggesting the most favorable therapeutic activity would be best maintained at or above 250 mg/m2.
The most common adverse event caused by cetuximab is skin rash. This acneiform rash is characterized by monomorphous pustular lesions that occur primarily on the face, neck, and upper torso [5]. The etiology is unclear, but one theory speculates the rash results from inhibition of EGFR expressed on normal keratinocytes on the basal layer of the epidermis.
The primary objective of this phase I trial was to determine the MTD of cetuximab in patients with advanced solid malignancies. This study examined the safety and feasibility of escalating weekly doses of cetuximab to test the hypothesis that escalating doses would correlate with increased skin toxicity. Given the limitations of the current National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events, Version 3.0 (CTCAE V3.0) criteria for grading rash, additional validated dermatologic rash assessments were utilized. In addition, correlative studies examining molecular markers (in both blood and tissue) predictive for response to cetuximab were evaluated.
Patients and methods
Patient selection
Eligibility included patients with histologically or cytologically confirmed advanced solid tumors; age greater than 18 years; life expectancy of greater than 12 weeks; and performance status 0–2. Prior chemotherapy and/or radiotherapy must have been completed at least 4 weeks and 2 weeks respectively before study entry and all significant previous treatment-related toxicities had to be resolved. Patients with asymptomatic treated brain metastasis (surgical resection or radiotherapy) were eligible for this trial if they were neurologically stable and had been off steroids for at least 4 weeks. Patients who had previously received EGFR targeted therapy were excluded.
Required laboratory tests included adequate hematologic (absolute neutrophil count ≥1,500/uL, platelet count ≥100,000/uL), renal (serum creatinine ≤1.6 mg/dl or a calculated creatinine clearance ≥40 mL/min) and hepatic (serum bilirubin ≤2 mg/dL and aspartate amino-transferase [AST] within three times the institutional upper limit of normal) function.
The institutional review board at University of California Davis Campus approved this study. All patients gave written informed consent to participate.
Dose limiting toxicity (DLT) and maximum tolerated dose (MTD) definitions
Toxicity was graded according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0. A DLT was defined as any of the following that occurred within the first cycle of treatment: Hematologic DLT: Grade 4 thrombocytopenia or grade 3 thrombocytopenia associated with bleeding or requirement for transfusion or febrile neutropenia. Patients could be transfused to prevent grade 4 anemia. Non-hematologic DLT was defined as: grade 4 cutaneous toxicity or grade 3 cutaneous toxicity necessitating holding cetuximab for greater than 4 weeks, grade 4 diarrhea or grade 3 diarrhea that did not resolve after holding the drug for 2 weeks or that recurred despite adequate treatment with anti-diarrheal agents, or any other ≥ grade 3 non-hematologic toxicity considered by the investigator to be related to study drug with the exception of inadequately treated nausea and vomiting.
The MTD was defined as the highest dose level at which no more than one patient experienced a DLT, when at least six patients were treated at that dose and were evaluable for toxicity.
Dose escalation
The dose escalation scheme is summarized in Table 2. This dose-escalation study was designed to enroll successive cohorts of patients (3 patients/cohort) per a standard phase I design. All patients treated were observed for a minimum of 28 days (1 cycle) before accrual to the next dose level was allowed. No intra-patient dose escalation was allowed.
Table 2.
Dose escalation schema and dose limiting toxicities (DLT)
| Dose level | Dose Cetuximab 400 mg/m2 loading dose |
Number of patient enrolled | DLT |
|---|---|---|---|
| 0 | 250 mg/m2 weekly | 3 | None |
| 1 | 300 mg/m2 weekly | 4a | One patient removed mid cycle for disease progression and replaced |
| 2 | 350 mg/m2 weekly | 3 | None |
| 3 | 400 mg/m2 weekly | 6 expanded to 17 patients total | None |
One patient received 350 mg/m2 instead of 300 mg/m2 with no DLT
Complete blood counts and toxicity assessments were obtained weekly. Serum chemistries including creatinine, magnesium and liver function tests were performed every cycle. Inter-current toxicities were also assessed every 4 weeks. Patients completing at least one treatment course or those experiencing a DLT were assessable for toxicity.
Treatment administration
To prevent hypersensitivity reactions, all patients were premedicated with diphenhydramine hydrochloride 50 mg (or an equivalent antihistamine) either by IV given 30–60 min prior to the first dose of cetuximab, or orally given 60–120 min prior to the first dose of cetuximab. The infusion rate of cetuximab was limited to 10 mg/minute (5 mL/min). Patients were monitored for treatment-related adverse events, especially allergic/hypersensitivity reactions, during the infusion and the post-infusion observation hour. Patients were evaluated for adverse events at each 4 week visit. Treatment was continued for 12 cycles in the absence of disease progression or intolerable toxicity.
Dose adjustment criteria
Cetuximab was decreased by 50 mg/m2 per week for grade ≥ 3 rash or toxicity as listed below. If a patient experienced a Grade 2 acne/acneiform rash that was intolerable to the patient or Grade 3 rash, defined as acne/acneiform rash associated with pain, disfigurement, ulceration or desquamation, cetuximab therapy was held for up to four consecutive infusions. If the toxicity resolved to Grade 2 or less and was within patient tolerability by the following treatment period, treatment resumed at the same dose level. For Grade 3 and 4 toxicities attributed to study drug (except inadequately treated nausea/vomiting or diarrhea), treatment was withheld until the toxicity resolved to ≤ grade 2 and was then resumed at the next lower dose level. If treatment was withheld for longer than 4 weeks due to Grade 3/4 toxicity, the patient was withdrawn from the study. Cetuximab dose reductions were permanent. Dose reduction below a weekly dose of 150 mg/m2 resulted in withdraw from protocol therapy.
Treatment of the skin toxicity was determined by patient discomfort and/or severity and patients were evaluated by a dermatologist for management of rash. Mild to moderate acneiform rash was treated with topical antibiotics such as clindamycin and/or benzoyl peroxide. Moderate to severe acneiform was treated with a combination of the previous mentioned therapies and/or oral antibiotics such as tetracycline and minocycline.
Dermatologic evaluation
Acne lesion counting [7, 8]
There were 8 regions selected for acne counting: right and left forehead, nose, right and left cheek, chin, chest and upper back. The hairline and jawline defined the perimeters of the face. The chest was considered the anterior portion of the thorax above the diaphragm and below the neck. The back was considered the posterior portion of the torso above the buttocks and below the neck.
The following lesions were counted in each of the above listed areas. Open comedones (blackheads): small discrete, raised areas of skin centered around the opening of a hair follicle. The lesions contain oxidized sebum and keratin, which is black in color. Closed comedones (whiteheads): small discrete, raised areas of skin centered around the opening of a hair follicle. The lesions contain unoxidized sebum and keratin, which is white in color. Papules: inflammatory lesions less than 5 mm in diameter. Pustules: similar in size to papules but have a visible central core of purulent material. Nodules: spherical papules with a diameter of 5 mm or greater.
A template was used during each evaluation to try and increase reliability of the exam. Digital photographs were taken of the left and right sides of the face, the chest and the back of each patient at baseline and at 28 day follow-up visit intervals. Whenever possible, the same rater was used in this study.
Global acne grading scale [9]
The following areas were assessed: forehead, cheeks, nose, chin, chest and upper back. The same definitions as used in the acne lesion counting scale were employed. The presence of lesions in each of the above body areas was determined. A score was assigned dependent on which type (s) of lesions were present and then multiplied by a location factor. The product was the local score for each site evaluated. The sum of all scores from the evaluated areas was the global score and was used to obtain a global grade and severity.
Response assessments
Efficacy was evaluated every 2 cycles (8 weeks) with computed tomography (CT) scans. Patients with evaluable or measurable tumor who completed 2 cycles of therapy were included in the analysis of tumor response. Patients who had clinical progression after 1 cycle were included in the analysis of tumor response. RECIST criteria for response were used [6].
Correlative studies
Archival tumor tissue was requested for correlative biomarker analyses but not mandatory for participation in this trial. The following analyses were performed on available patient specimens: k-ras mutational status, EGFR by fluorescence in situ hybridization (FISH). Blood samples were collected from patients prior to treatment and after cycle 1 and 2 of cetuximab for immunologic correlative studies.
K-ras
Tumor cells were enriched by microdissection, DNA extracted, and subjected to polymerase chain reaction (PCR). DNA from micro-dissected tumor cells was assessed by a sensitive two-step PCR-RFLP assay that detects all possible 12th codon-activating mutations in k-ras.
EGFR FISH
Cell copy number was investigated by FISH using the LSI EGFR SpectrumOrange/CEP 7 SpectrumGreen probe and the PathVysion HER-2 DNA probe Kit (Vysis, Abbott Laboratories, Illinois, USA). Using the reference HE-stained slide of the adjacent section where dominant tumor foci were identified, copy numbers of the EGFR and HER2 genes and chromosome 7 and 17 probes as controls were assessed and recorded independently in at least 100 non-overlapping nuclei with intact morphology. The FISH analysis was performed independently by two observers who were blinded to the patients’ clinical characteristics. According to the frequency of tumor cells with specific number of copies of the EGFR or HER2 genes and chromosome 7 and 17 centromeres, patients were classified into two strata: FISH negative, with no or low genomic gain (≥4 copies of the gene in <40% of cells) and FISH positive, with high level of polysomy (≥4 copies of the gene in ≥40% of cells) or gene amplification, defined by the presence of tight gene clusters and a gene/chromosome ratio per cell ≥2, or ≥15 copies of the genes per cell in ≥10% of analyzed cells.
Immunologic correlative studies
Cytokines and chemokines were measured in plasma samples. Non-coagulated blood was collected in standard Vacutainer EDTA plastic tubes. The contents were centrifuged (1,500×g, 15 min). Clear supernatant was collected to assess immunologic correlates (cytokines and chemokines). The multiplex system, Multi-Analyte Profiling (MAP), by Luminex Corp. (Austin, TX) was used to measure several cytokines and chemokines simultaneously in each sample. MAP system is based on unique populations of a 100 different, individually identifiable microbead sets. Each bead set contains a capture antibody to a specific cytokine or chemokine. Commercially available multiplex cytokine kits (23-plex and 27-plex) were used (Bio-Plex by Bio-Rad, Hercules, CA). Detailed lists of cytokine and chemokine analytes are posted on BioRad’s official website: https://www.biorad.com/evportal/evolutionPortal.portal?_nfpb=true&country=US&_pageLabel=productsPage&lang=en&javascriptDisabled=true&catID=85824182-db2a-4c6e-ac82-4e07dd9ab904
These kits (used according to manufacturer’s instructions) allowed simultaneous measurements, in picogram quantities, of human cytokines and chemokines.
Statistical analysis
Results were summarized by descriptive statistics (frequencies, median and median survival).
Dose response relationship with skin lesion measures was assessed by Spearman rank correlation coefficient and by linear regression with dose level; acne lesion count was considered both on the original scale and log-transformed. Tests were two-sided at level 0.05.
Multiplex, cytokine/chemokines data for immunological correlates were analyzed by multivariate analysis as previously described [10]. Briefly, data were adjusted for median- and inter-quantile range. Analytes elevated in patients were identified as fold-change (log2 > 2; p.value < 0.01) in each analyte between healthy and patient samples. Data for these selected analytes are presented as composite values (pg/ml) of each cytokine/chemokine in healthy samples compared to patient samples.
Results
Patient demographics
Twenty seven eligible patients were accrued between January 2005 and January 2007. Patient characteristics are summarized in Table 1. Six patients were chemonaive and the median number of prior chemotherapy treatments was 2. Twelve patients had a PS ≤ 70. The most common tumor type enrolled was head and neck malignancy (n=5, 19%) but a wide variety of tumor types were represented.
Table 1.
Baseline patient demographics
| Number of patients | 27 |
| Median age, years (range) | 59 (43–83) |
| Gender male/female | 20/7 |
| Performance status ≤70/>70 | 12/15 |
| No prior chemotherapy/prior chemotherapy | 6/21 |
| Tumor types | |
| Head and neck | 5 |
| Cholangiocarcinoma | 4 |
| Pancreas | 3 |
| Breast | 2 |
| Colorectal | 2 |
| Esophageal | 2 |
| NSCLC | 2 |
| Prostate | 2 |
| Anal carcinoma, bladder, fibrosarcoma, mesothelioma, ovary | 1 each |
Drug administration and dose levels
At dose level 0, three patients were treated with no DLTs in cycle 1 (Table 2). In dose level 1, four patients were treated. One developed grade 3 hyperglycemia secondary to underlying diabetes and grade 3 confusion attributed to use of narcotics for pain control and was therefore determined not to be a DLT. The second patient had disease progression during cycle 1 and was removed from study mid-cycle and was replaced by another patient for the purpose of DLT evaluation. Patient 3 did not have any significant toxicity in cycle 1. The fourth patient, intended to replace the second, was unintentionally treated at level 2 but did not experience a DLT. Three patients accrued to dose level 2 did not have DLTs. Accrual to the final dose level 3 demonstrated no DLTs in the first cohort of three patients. The level was expanded to include six patients and there were no DLTs. Eleven additional patients were accrued at dose level 3 for a total of 17.
Toxicity
Weekly cetuximab was generally well tolerated, toxicities experienced throughout treatment cycles are summarized in Tables 3 and 4. There were no infusion related allergic reactions. Grade 3 hematologic toxicity included lymphopenia in two patients. Grade ≥ 3 toxicities included hypomagnesemia (n=2), acneiform rash (1) and fatigue (2). There were no grade 4 toxicity or treatment related deaths.
Table 3.
All grades of toxicity possibly related to cetuximab
| All grades (occurring in ≥2 patients) | Number of patients (n=27) |
|---|---|
| Hematologic | |
| Anemia | 8 |
| Thrombocytopenia | 2 |
| Leukopenia | 5 |
| Lymphopenia | 8 |
| Non-hematologic | |
| Acneiform rash | 21 |
| Fatigue | 16 |
| Headache | 12 |
| Dry Skin | 11 |
| Nausea | 9 |
| Anorexia | 6 |
| Hypomagnesemia | 5 |
| Constipation | 4 |
| Diarrhea | 4 |
| Fever | 4 |
| Pruritus | 4 |
| Increased liver enzymes | 4 |
| Infection | 3 |
| Mucositis | 3 |
| Hypoalbuminemia | 3 |
| Dizziness | 2 |
| Nail changes | 2 |
| Pain | 2 |
| Vomiting | 2 |
| Hypocalcemia | 2 |
Table 4.
Grade ≥ 3 toxicity possibly related to cetuximab
| Grade ≥ 3 toxicity | Number of patients |
|---|---|
| Hematologic | |
| Lymphopenia | 2 |
| Non-hematologic | |
| Acneiform rash | 1 |
| Fatigue | 2 |
| Hypomagnesemia | 2 |
There were no grade 4 toxicities or treatment related deaths
Dermatologic evaluation
Of the 27 patient enrolled, 24 had baseline dermatologic evaluations, 19 were seen after cycle 1 and four patients had two or more follow up exams. Dermatological assessments were completed in 15 of the 22 patients evaluable for response (Table 5). Acne lesion counting and the global acne grading scale were applied at baseline and after each cycle of cetuximab. The CTCAE V 3.0 scale was utilized after every cycle of cetuximab by the treating physician. There was no statistically significant correlation between the severity of rash and dose or response to therapy, and regression models did not suggest an increase in lesions or severity of rash with dose (results not shown).
Table 5.
Acne lesion count (ALC) and global acne grading system (GAGs) and response
| Dose level | ALC baseline/cycle 1/cycle 2 | GAGs baseline/cycle 1/cycle 2 | CTCAE V3.0 grade of rash cycle 1/cycle 2 | Best response |
|---|---|---|---|---|
| 0 | 0/149 | 0/22 | 2 | PD |
| 0 | 0/301/275 | 0/32/33 | 2/2 | SD |
| 0 | 0/46 | 0/23 | 2 | PD |
| 1 | 0 | 0 | 0 | PD |
| 1 | 0 | 0 | 1 | PD |
| 1 | 0/69/119 | 0/6/33 | 2/2 | SD |
| 2 | 0/59 | 0/27 | 2 | NE |
| 2 | 159/998/1773 | 9/33/33 | 3/3 | PD |
| 2 | 0/137/418 | 0/33/33 | 2/2 | PD |
| 3 | 0/67 | 0/27 | 2 | PD |
| 3 | 0 | 0 | 2 | SD |
| 3 | 0/0 | 0/0 | 2 | PD |
| 3 | 0/83 | 0/24 | 2 | PD |
| 3 | 0/223 | 0/21 | 2 | PD |
| 3 | 0/26 | 0/2 | 2 | PD |
| 3 | 0 | 0 | 1 | SD |
| 3 | 0/20 | 0/24 | 1 | PD |
| 3 | 0 | 0 | 1 | PD |
| 3 | 0/36 | 0/21 | 2 | SD |
| 3 | 0/3 | 0/3 | 2 | NE |
| 3 | 0 | 0 | 0 | NE |
| 3 | 0/216 | 0/27 | 1 | NE |
| 3 | 0/0 | 0/0 | 0 | SD |
| 3 | 0/87 | 0/33 | 2 | SD |
Efficacy
Of the 22 patients evaluable for radiographic response, there was 1 objective response, seven patients had stable disease and 14 patients had progressive disease. Five patients did not complete 2 cycles; three due to clinical progression and two patients chose to discontinue therapy. The patient who had a response had cholangiocarcinoma and had received two prior lines of chemotherapy. She was treated with cetuximab for 7 cycles. Disease stabilization was seen in one patient with pancreatic carcinoma for 8 cycles.
Correlative studies
Of the 27 patients in the study, 21 had archival tissue samples and 16 had sufficient tumor tissue for correlative studies.
k-ras mutational analysis of tumor tissue was attempted in 16 patients; 13 had k-ras wild type, 1 had a point mutation resulting in Gly 12 to Val 12 and 2 had insufficient tumor samples. In the 11 sequenced patients evaluable for response the following was observed; k ras wild type—4 PD, 5 SD and 1 PR and K-ras mutation—PD. The median survival in k-ras wild type patients was 7.3 months. The k-ras mutation patient had pancreatic carcinoma with a best response of PD and survival of 1.9 months.
Evaluation of EGFR amplification by FISH was attempted in 16 patients; 11 had normal EGFR levels, one demonstrated amplification and four were not evaluable. Of the 12 patients with EGFR FISH data, nine were evaluable for response; normal EGFR levels 1 PR, 2 SD and 5 PD and the single patient with EGFR amplification had stable disease. The patient with EGFR amplification had heavily pre-treated breast cancer (six lines of therapy), and remained on cetuximab for 4 cycles with a best response of SD and survival of 10.4 months.
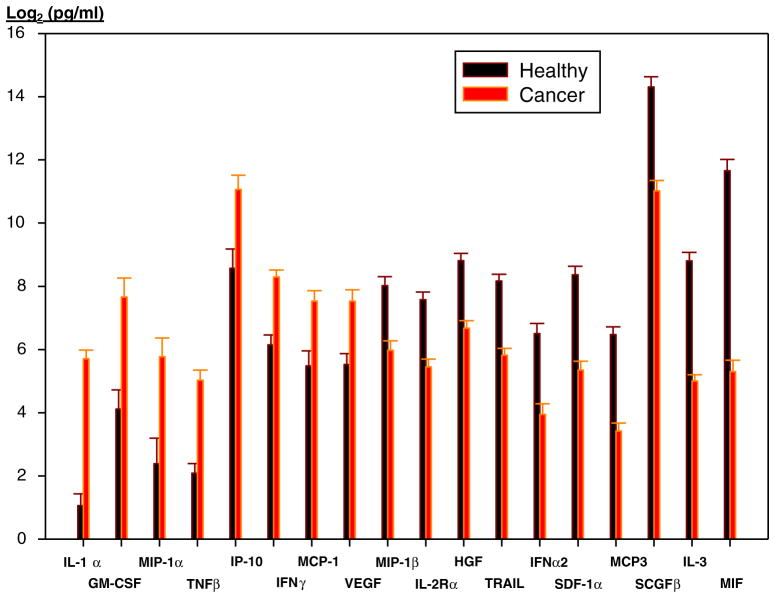
Immunologic correlative studies using the multiplex microbead cytokine detection kits were performed on 22 patients, in comparison to 17 healthy individuals. Results of these analyses are shown as composite levels (pg/ml) of each analyte in healthy and patient samples (Fig. 1). Values for individual cytokines/chemokines in each individual patient are not shown. Eighteen of fifty cytokines/chemokines were significantly elevated in patients compared to healthy individuals (Fig. 1). Due to the limited sample size and disparity of tumor types, no conclusions could be drawn concerning the relationship between cytokine/chemokine levels and treatment efficacy. Anecdotally, a patient with bladder cancer who progressed rapidly on treatment had substantially elevated inflammatory cytokines (IL-1beta, IL-2, IL-6, IFN-gamma, and TNF-alpha; 250 to 1000 fold compared to healthy controls). In addition, in this patient, several other cytokines/chemokines were similarly elevated (IL-5, IL-9, IL-10, IL-12 (p70), IL-13, IL-15, Eotaxin, G-CSF, GM-CSF, IP-10 and VEGF). In contrast, the cholangiocarcinoma patient who exhibited a partial response to treatment had cytokine/chemokine levels generally similar to healthy volunteers, with the exception of moderately elevated IL-3, IL-12 (p40), GMCSF and IFN-gamma.
Fig. 1.
Cytokine and chemokine levels in healthy and patient plasma samples. Significantly altered cytokines/chemokines in plasma samples of cancer patients as compared to healthy individuals. Results for each cytokine/chemokine represent composite values (pg/ml) for all healthy individuals (n=17; black bars) and all cancer patients (n=22; orange)
Discussion
This phase I study was conducted to determine the safety and feasibility of escalating doses of cetuximab, testing the hypothesis of the relationship of dose-dependent skin toxicity. There were no dose limiting toxicities up to a weekly dose of cetuximab 400 mg/m2 and the maximum tolerated dose was not reached. There was no direct correlation of the grade of rash with tumor response or the level of dosage tested in this group of patients with heterogenous solid tumors.
EGFR targeted therapies are associated with the development of cutaneous toxicity, predominantly an acneiform rash. The current tools for assessing rash are basic; the CTCAE v3.0 categorizes acneiform rash as; grade 1 intervention not indicated, grade 2 intervention indicated and grade 3 associated with pain, disfigurement, ulceration or desquamation [11]. This trial implemented two dermatologic assessment scales to characterize the rash; acne lesion counting (ALC) and the global acne grading scale (GAGS). While the degree of rash did not correlate with dose in this heavily pre-treated mixed solid tumor population, ALC and GAGS provided a more detailed description of the severity of the cetuximab-associated rash [7, 8]. The drawbacks to these methods however, include inter-rater variability and time required to count the lesions. An instrument to assess rash with EGFR targeted therapies that echoes the ease of the CTCAE v3.0 and incorporates a degree of the accuracy of the ALC and GAGS would facilitate documentation and management of this toxicity.
Clinical trials in colorectal, lung and head and neck cancers have demonstrated a correlation between rash and overall survival [12–15]. The degree of cetuximab efficacy has also been correlated with grade of skin rash, which is reported to be dose dependent. The ability to deliver higher doses of cetuximab safely allows treatment to rash and may result in increased efficacy in selected patients.
Our study did not find a correlation between rash and response to cetuximab therapy likely due to our heterogeneous, heavily pre-treated patient population. In a uniform patient group known to benefit from single agent cetuximab (such as colorectal or head and neck cancer), the ability to correlate rash with efficacy may be more pronounced. The patient population may also have impacted the ability to deliver an adequate amount of cetuximab to allow for the assessment of rash effect. The rash data may also have been confounded by the fact that our patients were seen routinely by dermatology and therefore received prompt therapy for their cutaneous toxicity that may have influenced the severity of rash observed.
In this phase I trial, high dose weekly cetuximab at 400 mg/m2 was safe and tolerable and the MTD for single agent cetuximab was not found. No correlation was observed between dose and rash intensity. High dose cetuximab can be delivered in a safe and tolerable manner in a heterogenous solid tumor population.
Acknowledgments
Sources of Support: Bristol-Myers Squibb.
Contributor Information
Cheryl Ho, British Columbia Cancer Agency, Vancouver, BC, Canada.
Randeep Sangha, Cross Cancer Institute, Edmonton, AB, Canada.
Laurel Beckett, University of California, Davis Campus, Sacramento, CA, USA.
Michael Tanaka, University of California, Davis Campus, Sacramento, CA, USA.
Derick H. Lau, University of California, Davis Campus, Sacramento, CA, USA
Daniel B. Eisen, University of California, Davis Campus, Sacramento, CA, USA
Rachel A. Burich, University of California, Davis Campus, Sacramento, CA, USA
Paul Luciw, University of California, Davis Campus, Sacramento, CA, USA.
Imran Khan, University of California, Davis Campus, Sacramento, CA, USA.
Philip C. Mack, University of California, Davis Campus, Sacramento, CA, USA
David R. Gandara, University of California, Davis Campus, Sacramento, CA, USA
Angela M. Davies, Email: adavies@osip.com, OSI Pharmaceuticals, Inc., 2860 Wilderness Place, Boulder, CO 80301, USA
References
- 1.Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. 2003;21:2787–2799. doi: 10.1200/JCO.2003.01.504. [DOI] [PubMed] [Google Scholar]
- 2.Tos APD, Ellis I. Assessing epidermal growth factor receptor expression in tumours: what is the value of current test methods? Eur J Cancer. 2005;41:1383–1392. doi: 10.1016/j.ejca.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 3.Fan Z, Masui H, Altas I, et al. Blockade of epidermal growth factor receptor function by bivalent and monovalent fragments of 225 anti-epidermal growth factor receptor monoclonal antibodies. Cancer Res. 1993;53:4322–4328. [PubMed] [Google Scholar]
- 4.Sato JD, Kawamoto T, Le AD, et al. Biological effects in vitro of monoclonal antibodies to human epidermal growth factor receptors. Mol Biol Med. 1983;1:511–529. [PubMed] [Google Scholar]
- 5.Perez-Soler R, Saltz L. Cutaneous adverse effects with HER1/EGFR-targeted agents: is there a silver lining? J Clin Oncol. 2005;23:5235–5246. doi: 10.1200/JCO.2005.00.6916. [DOI] [PubMed] [Google Scholar]
- 6.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the United States, national cancer institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 7.Witkowski JA, Parish LC. From other ghosts of the past: acne lesion counting. J Am Acad Dermatol. 1999;40:131. doi: 10.1016/s0190-9622(99)70552-9. [DOI] [PubMed] [Google Scholar]
- 8.Lucky AW, Barber BL, Girman CJ, et al. A multirater validation study to assess the reliability of acne lesion counting. J Am Acad Dermatol. 1996;35:559–565. doi: 10.1016/s0190-9622(96)90680-5. [DOI] [PubMed] [Google Scholar]
- 9.Doshi A, Zaheer A, Stiller MJ. A comparison of current acne grading systems and proposal of a novel system. Int J Dermatol. 1997;36:416–418. doi: 10.1046/j.1365-4362.1997.00099.x. [DOI] [PubMed] [Google Scholar]
- 10.Khan IH, Krishnan VV, Ziman M, et al. A comparison of multiplex suspension array large-panel kits for profiling cytokines and chemokines in rheumatoid arthritis patients. Cytometry B Clin Cytom. 2009;76:159–168. doi: 10.1002/cyto.b.20452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE) version 3.0. National Institutes of Health, National Cancer Institute; 2003. [Google Scholar]
- 12.Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 13.Koza I, Sr, Wrba F, Vrbanec D, et al. Correlation of KRAS status with clinical outcome in patients (pts) with metastatic colorectal cancer (mCRC) treated first-line with FOLFOX6 + cetuximab (FX+C) or FOLFIRI + cetuximab (FF+C): The CECOG/CORE1.2.001 trial. ASCO Meeting Abstracts. 2009;27:4055. [Google Scholar]
- 14.Burtness B, Goldwasser MA, Flood W, et al. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in Metastatic/Recurrent head and neck cancer: an eastern cooperative oncology group study. J Clin Oncol. 2005;23:8646–8654. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- 15.Saltz L, Kies M, Abbruzzese L, et al. The presence and intensity of the cetuximab-induced acne-like rash predicts increased survival in studies across multiple malignancies. Proc Am Soc Clin Oncol. 2003;22:204. [Google Scholar]